Abstract
The proprotein convertase subtilisin kexin-9 (PCSK9) circulates in plasma as mature and furin-cleaved forms. A polyclonal antibody against human PCSK9 was used to develop an ELISA that measures total plasma PCSK9 rather than only the mature form. A cross-sectional study evaluated plasma levels in normal (n = 254) and hypercholesterolemic (n = 200) subjects treated or untreated with statins or statin plus ezetimibe. In controls, mean plasma PCSK9 (89.5 ± 31.9 ng/ml) correlated positively with age, total cholesterol, LDL-cholesterol (LDL-C), triglycerides, and fasting glucose. Sequencing PCSK9 from individuals at the extremes of the normal PCSK9 distribution identified a new loss-of-function R434W variant associated with lower levels of circulating PCSK9 and LDL-C. In hypercholesterolemic subjects, PCSK9 levels were higher than in controls (99.3 ± 31.7 ng/ml, P < 0.04) and increased in proportion to the statin dose, combined or not with ezetimibe. In treated patients (n = 139), those with familial hypercholesterolemia (FH; due to LDL receptor gene mutations) had higher PCSK9 values than non-FH (147.01 ± 42.5 vs. 127.2 ± 40.8 ng/ml, P < 0.005), but LDL-C reduction correlated positively with achieved plasma PCSK9 levels to a similar extent in both subsets (r = 0.316, P < 0.02 in FH and r = 0.275, P < 0.009 in non-FH). The detection of circulating PCSK9 in both FH and non-FH subjects means that this assay could be used to monitor response to therapy in a wide range of patients.
Keywords: proprotein convertase subtilisin/kexin type 9, LDL-cholesterol, hypercholesterolemia, hypocholesterolemia, ELISA, novel natural mutation, cardiovascular disease
The familial hypercholesterolemia (FH) phenotype is characterized by elevated plasma LDL-cholesterol (LDL-C) levels, xanthomas, and premature atherosclerosis associated with increased risk of coronary artery disease (CAD). FH is caused primarily by loss-of-function (LOF) mutations in the LDL receptor gene (LDLR) responsible for the removal of plasma cholesterol, which is mainly found in LDL particles (1) or in the apolipoprotein B gene (2), the main protein component of LDL. In 2003, Abifadel et al. (3) identified another protein associated with this phenotype, the proprotein convertase subtilisin/kexin-9 (PCSK9) (4).
The human PCSK9 gene (PCSK9) located on chromosome 1p32.3 is ∼22 kb long and comprises 12 exons encoding a 692 amino acid protein (5). PCSK9 is expressed mainly in the liver, small intestine, and kidney (4) and is thought to accelerate the degradation of hepatic LDLR in endosomes/lysosomes (6) by direct binding to the epidermal growth factor-like repeat A (EGF-A) domain of the LDLR (7, 8). PCSK9 overexpression in cell lines and mice has been shown to reduce LDLR levels and increase plasma LDL-C (9–12). Similarly, transgenic overexpression of mouse (13) and human PCSK9 (14) in hepatocytes or human PCSK9 in kidney (15) also results in a dramatic reduction of hepatic LDLR. Furthermore, parabiotic transfer of plasma PCSK9 from human PCSK9 transgenic mice to wild-type (WT) mice causes a dramatic reduction in hepatic LDLR levels (14). Conversely, inactivation of the mouse Pcsk9 gene leads to increased LDLR protein and decreased plasma LDL-C (13, 16). During secretion, PCSK9 may be cleaved by a furin-like proprotein convertase(s), curtailing its action on cell surface LDLR (17).
To date, more than 40 amino acid variants of PCSK9 have been shown to affect plasma cholesterol levels in humans (5, 18–20). These changes are classified as gain-of-function (GOF) mutations when they are associated with high levels of LDL-C and as LOF mutations when associated with low LDL-C. GOF mutations result in mild to severe hypercholesterolemia. In the most severe Anglo-Saxon mutation, D374Y, total cholesterol (TC) values reach as high as 13.1 mmol/L (21). The onset of CAD in patients with D374Y may be 10 years sooner than in heterozygous FH patients with severe LDLR mutations (22). On the other hand, a retrospective study has shown a significantly reduced risk of CAD in carriers of PCSK9 LOF variants R46L (partial LOF) and Y142× or C679× (complete LOF). Together, the latter two nonsense mutations were associated with a 28% reduction of plasma LDL-C and an 88% reduction in the frequency of coronary events (23). While that study supported the cardioprotective role of long-term reduction of cholesterol levels, a direct protective effect of reduced PCSK9 was not excluded. Furthermore, a compound heterozygote for two inactivating mutations (Y142× and ΔR97) in PCSK9 had a strikingly low plasma level of LDL-C (0.36 mmol/L) and no immunodetectable circulating PCSK9 (18). Another individual homozygous for the C679× mutation had a plasma LDL-C of 0.41 mmol/L (24). All these findings support the hypothesis that levels and/or higher activity of plasma PCSK9 modulate the levels of LDL-C and TC, suggesting that long-term lowering of PCSK9 might be beneficial in reducing the incidence of CAD (25).
PCSK9, like the LDLR, is regulated by sterol regulatory element-binding protein-2 (SREBP-2), a transcription factor involved in activation of many genes implicated in cholesterol metabolism (26, 27). This finding is supported by our previous work, in which we showed that in HepG2 cells (a human hepatoma cell line) and human primary hepatocytes, PCSK9 mRNA levels were increased by statins, likely via SREBP-2 (28). Preliminary data on the response of PCSK9 to cholesterol-lowering therapy revealed that statins and fibrates can significantly modify plasma PCSK9 levels (29–31).
In the present study, we measured plasma PCSK9 by ELISA in 254 volunteers and 200 hypercholesterolemic patients. We demonstrated that plasma PCSK9 levels are correlated significantly with age and with levels of TC, LDL-C, triglycerides (TG), and fasting glucose. Moreover, we show that PCSK9 levels are markedly higher in hypercholesterolemic patients than in controls and higher still in patients receiving cholesterol-lowering therapy. Finally, we identified a novel LOF R434W mutant exhibiting lower plasma levels of LDL-C and PCSK9. The mechanism behind such observations is shown to be related to a 3-fold lower secretion rate of PCSK9-R434W from cells and ∼70% LOF on its effect on cell surface LDLR.
MATERIALS AND METHODS
Production and purification of anti-PCSK9 antibodies
Recombinant truncated human PCSK9 (rPCSK9; Met-amino acids 31–454) was produced in bacteria and purified as described (6). It was injected into two rabbits by a standard protocol to generate a polyclonal antibody to human PCSK9 (hPCSK9-Ab). The antibodies were first prepurified by precipitation with ammonium sulfate (50% final concentration). After solubilization and dialysis of the precipitate, the antibodies were purified by affinity chromatography using a CNBr-activated Sepharose 4B column (GE Healthcare Bio-Sciences AB, Sweden) coupled with the purified antigen (rPCSK9). A fraction of this purified antibody was conjugated with horseradish peroxidase (hPCSK9- Ab-HRP) using the EZ-Link™ Plus Activated Peroxidase protocol from Pierce (Rockford, IL). Finally, the conjugated antibody was purified from excess free HRP using the FreeZyme Conjugate Purification kit (Pierce prod no. 44920).
Immunoprecipitation and immunoblotting
Immunoprecipitations were carried out as previously described (17) using the hPCSK9-Ab and activated agarose beads coupled with goat anti-rabbit IgG (Trueblot™ eBioscience, San Diego, CA) according to the manufacturer's instructions. Immunoprecipitated proteins were separated on 4–20% gradient acrylamide gels and transferred to a polyvinylidene difluoride membrane (Immobilon-P™, Millipore, Billerica, MA). For immunoblotting, hPCSK9-Ab-HRP was used at a dilution of 1:500. The blots were revealed by chemiluminescence with Pierce SuperSignal™ West Dura on Amersham Hyperfilm™ ECL (GE Healthcare Limited, UK). HepG2 and HuH7 cells were cultured as previously described (17, 28).
Subjects, sample handling, and sequencing
Blood was collected into EDTA-Vacutainer™ tubes after a 12 h fast. Samples were taken from 254 healthy volunteers over 18 years of age who were not taking any medication for hyperlipidemia, hypertension, or diabetes, and from 200 hyperlipidemic patients attending our lipid clinic. Plasma and blood leukocytes were obtained by centrifugation at 3,000 rpm for 15 min at 4°C. Total and lipoprotein cholesterol and TGs were quantitated at the laboratory of the Centre Hospitalier de l'Université de Montréal using a standard enzymatic method on a Bayer Advia multi-analyzer. LDL-C was calculated using the Friedewald equation, except if TG were ≥4.5 mmol/L (n = 6) (32). All subjects gave informed written consent and the Institut de recherches cliniques de Montréal (IRCM) ethics committee approved this protocol. DNA was extracted from white blood cells using QIAmp Blood Maxi kit (Qiagen, Missisauga, ON) according to the manufacturer's instructions. The sequences of the primers used for amplifying all 12 exons were obtained from NCBI at http://www.ncbi.nlm.nih.gov/genome/probe/ using the resequencing amplicons for PCSK9. The amplified fragments were purified from agarose gels using QIAquick Gel Extraction kit (Qiagen, Missisauga, ON) and sequenced on a 3130 XL Genetic analyzer from ABI (Applied Biosystems, Foster City, CA) using M13 sequencing primers. Sequences were analyzed with SequencherTM software (Gene Codes Corporation, Ann Arbor, MI).
ELISA assay
LumiNunc Maxisorp white assay plates (Nunc, Denmark) were coated with 0.5 μg/well of hPCSK9-Ab by incubation at 37°C for 3 h in PBS (NaPO4 10 mM, NaCl 0.15 M, pH 7.4) containing Na azide (1 g/L) then stored at 4°C. The plates were washed six times before use with PBS containing Tween 20 (0.5 ml/L) and then incubated for 1 h at room temperature with blocking buffer (PBS, casein 0.1%, merthiolate 0.01%). Calibrators were prepared using serial dilutions of rPCSK9 in dilution buffer (PBS, urea 1.8 M, BSA 0.25%, Tween 20 0.5 ml/L, and merthiolate 0.01%). Samples were diluted 1:20 in dilution buffer without BSA. Calibrators and samples were incubated for 30 min in a water bath at 46°C prior to plate addition (100 μl) in duplicate. The plates were incubated overnight at 37°C with shaking. After washing, 100 μl of hPCSK9-Ab-HRP diluted 1:750 was added for 3 h at 37°C with shaking. Finally, after washing, 100 µl of substrate (SuperSignal™ ELISA Femto Substrate, Pierce) was applied to each well. Chemiluminescence was quantitated on a Pherastar luminometer (BMG Labtech). A standard curve was established using a conditioned medium containing recombinant human PCSK9 as described previously (33). This standard medium was calibrated by comparison to a full-length secreted and purified PCSK9 from a Baculovirus system in HiFive cells (kind gift from Rex Parker, Bristol-Myers Squibb). The peptide purity and concentration were determined by quantitative amino acid analysis following 18–24 h hydrolysis in the presence of 5.7 N HCl in vacuo at 110°C on a Beckman autoanalyzer (model 6300) with a postcolumn ninhydrin detection system coupled to a Varian DS604 data station (performed by Dr C. Lazure, IRCM). Plasma PCSK9 concentration was calculated by comparing sample luminescence to the standard luminescence curve. It was measured on frozen plasma samples.
Statistical analysis
Spearman correlation coefficients (r) were used to assess the relationship between variables. Data were analyzed with GraphPad Prism software and significance defined as P < 0.05 (two-sided). Stepwise regression analysis was performed by the statistical department of the Université de Montréal using SPSS software, version 15. ANOVA was used to determine drug dosage effect on PCSK9 levels. No correlations were performed in patients who had TG ≥ 4.5 mmol/L.
Functional analysis of the novel natural mutant R434W
HEK293 cells were transiently transfected with pIRES-cDNAs coding for WT PCSK9, or the novel variant R434W, tagged at the C terminus with a V5-antigen (4). Forty-eight hours posttransfection, the cells were pulsed for 4 h with 35S-(Cys+Met). Cell lysates and media were then immunoprecipitated with a V5 mAb and the precipitates separated by SDS-PAGE (8%) and analyzed by autoradiography, as reported (4, 10). To define the ability of the R434W mutant to degrade LDLR, 24 h spent media were prepared from HEK293 cells transiently transfected with each construct. An ELISA assay of the media defined the amount of PCSK9 secreted for each construct. The media were then incubated with HuH7 cells for 1 h or overnight and the cells were washed, detached in 0.5 mM EDTA (Versene, Gibco), and subjected to fluorescence-activated cell sorting (FACS) analysis using an LDLR-specific monoclonal antibody (C-7 mAb, 1:100 dilution, Santa-Cruz, CA), thus quantitating the levels of cell-surface LDLR.
RESULTS
Anti-PCSK9 antibody recognizes both mature and furin-cleaved PCSK9 in human plasma
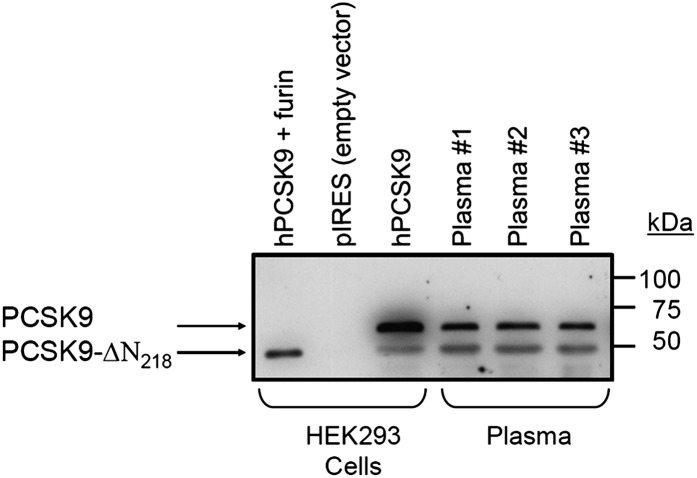
Using affinity-purified hPCSK9-Ab, Western blot analysis of human PCSK9 in the culture media of HEK293 cells overexpressing hPCSK9 revealed the mature (∼60 kDa) and furin-cleaved PCSK9 (PCSK9-ΔN218; ∼53 kDa) forms (17), none of which were observed in control cells expressing an empty pIRES vector. Similar immunoreactive PCSK9 forms are also observed in the plasma of three different individuals (Fig. 1).
Fig. 1.
Specificity of hPCSK9-Ab for native PCSK9 in culture medium and plasma. Immunoprecipitation was carried out with polyclonal hPCSK9-Ab against culture media of HEK293 cells respectively transfected with vector alone (pIRES), PCSK9, PCSK9 and furin, and against plasma from three different individuals. Immunoprecipitates were separated by a 4–20% polyacrylamide gradient SDS-PAGE, transferred to a polyvinylidene difluoride membrane, and revealed with hPCSK9-Ab-HRP. PCSK9-ΔN218 represents PCSK9 cleaved by furin (17).
Validation of the ELISA to measure PCSK9 in human plasma
We designed an ELISA to measure circulating levels of PCSK9 in human plasma samples using an affinity-purified hPCSK9-Ab polyclonal antibody. Part of this antibody was conjugated to HRP and excess HRP was removed by affinity chromatography. A linear standard curve was established with culture media and serial 1:2 dilutions of the media of HEK293 cells overexpressing recombinant human PCSK9 (r2 = 0.997). This culture medium was calibrated with respect to purified rPCSK9 characterized by quantitative amino acid analysis. Intra- and inter-assay variation coefficients of plasma samples were 1.6% (n = 33) and 7.5% (n = 48), respectively. A spike and recovery assessment was performed in two different plasma samples containing very low endogenous PCSK9. Five different quantities of recombinant PCSK9 (1–10 ng) were added to plasma and mean recoveries were 90, 88, 88, 94, and 98%, respectively. A plasma sample having a high PCSK9 concentration presented a 10% variation within a dilution range of 1:20 to 1:80. Three different plasma samples were subjected to three freeze-thaw cycles (−80°C to room temperature) and their PCSK9 concentration varied by ∼3%, which is within the variability range of the assay. Interestingly, no detectable immunoreactive PCSK9 was present in urine or saliva.
ELISA assay of PCSK9: identification of a novel R434W LOF variant
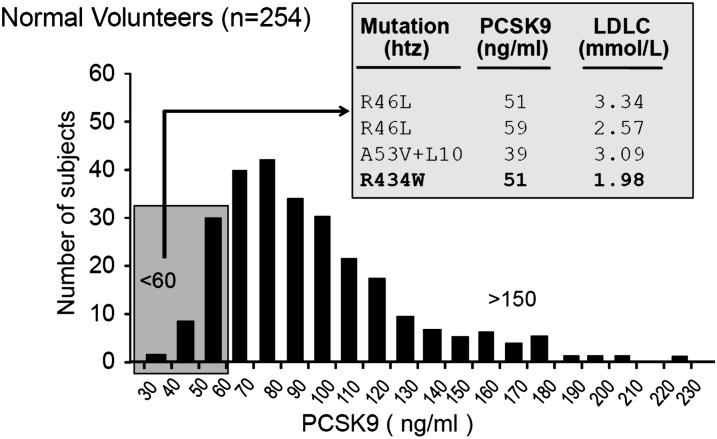
We collected plasma from 254 healthy volunteers, 117 males and 137 females. Clinical characteristics, fasting plasma lipids, and PCSK9 levels are shown in Table 1. There was no significant difference in plasma PCSK9 levels or in any other variables measured between men and women in this sample (Mann Whitney U test). Distribution of plasma PCSK9 levels measured by ELISA was skewed toward higher values in both men and women (supplementary Fig. I). The mean concentration was 89 ± 32 ng/ml (range, 35–225 ng/ml), and it did not differ significantly between men and women (85 ± 27 ng/ml and 93 ± 35 ng/ml, respectively) (supplementary Fig. I). Combining both genders, we sequenced PCSK9 in subjects whose plasma values were at the extreme of the frequency distribution, i.e., values < 60 ng/ml or > 150 ng/ml (Fig. 2). This revealed that 5/37 persons exhibiting low PCSK9 levels also showed either a known hypocholesterolemic variation/polymorphism [R46L (3, 19) and double A53V + L10 (19, 34)] or a new, previously unreported variant R434W with no other mutations in the sequence (Fig. 2).
TABLE 1.
Clinical characteristics, fasting plasma lipids, and PCSK9 levels of 254 healthy volunteers (mean ± SD)
| All Subjects (min–max) n = 254 | Men (min–max) n = 117 | Women (min–max) n = 137 | |
|---|---|---|---|
| Age (years) | 42 ± 13 (20–77) | 41 ± 13 (20–77) | 43 ± 12 (21–69) |
| BMI (kg/m2) | 24.5 ± 4.3 (16.2–41.6) | 25.6 ± 4.0 (17.6–38.8) | 23.4 ± 4.4 (16.2–41.6) |
| PCSK9 (ng/ml) | 89.5 ± 31.9 (35.3–225.2) | 85.3 ± 26.9 (35.3–172.1) | 93.1 ± 35.4 (47.4–225.2) |
| TC (mmol/L) | 4.86 ± 0.90 (2.83–7.30) | 4.90 ± 0.89 (3.11–7.05) | 4.83 ± 0.91 (2.83–7.30) |
| TG (mmol/L) | 1.17 ± 0.62 (0.34–4.30) | 1.22 ± 0.64 (0.45–4.30) | 1.12 ± 0.59 (0.34–3.86) |
| LDL-C (mmol/L) | 2.84 ± 0.80 (1.17–4.72) | 3.01 ± 0.80 (1.17–4.72) | 2.69 ± 0.78 (1.36–4.59) |
| HDL-C (mmol/L) | 1.50 ± 0.38 (0.80–2.60) | 1.33 ± 0.32 (0.80–2.36) | 1.64 ± 0.36 (0.83–2.60) |
| TC-HDL-C | 3.44 ± 1.02 (1.70–6.79) | 3.85 ± 1.03 (2.00–6.79) | 3.08 ± 0.88 (1.70–6.19) |
| Glucose (mmol/L) | 4.84 ± 0.61 (3.60–6.70) | 4.89 ± 0.63 (3.60–6.70) | 4.79 ± 0.58 (3.60–6.70) |
Fig. 2.
Sequencing PCSK9 in individuals from extremes of distribution of plasma levels revealed a novel R434W mutation. The cohort consisted of 117 males and 137 females ranging from 20 to 77 years of age. Plasma was diluted 1:20 and PCSK9 measured by sandwich ELISA as described. DNA from subjects exhibiting low (<60 ng/ml) and high (>150 ng/ml) PCSK9 plasma levels was subjected to exon sequencing. The new mutation R434W is emphasized in bold.
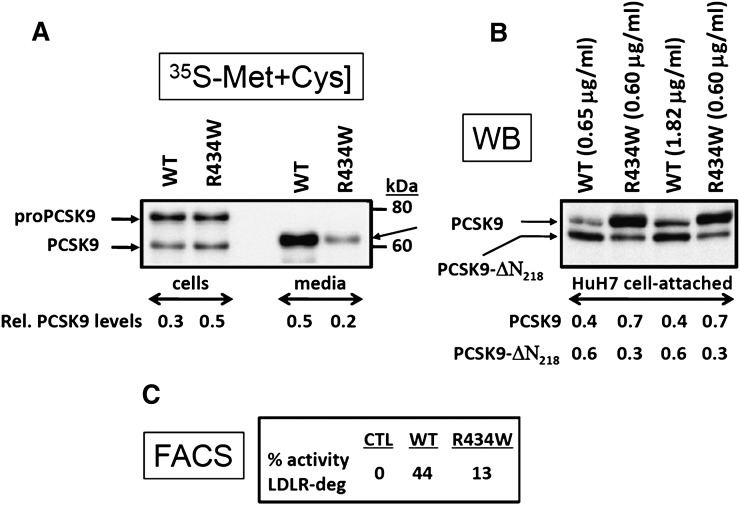
Biosynthetic analysis using a 4 h pulse with 35S-[Met+Cys] of HEK293 cells overexpressing the R434W mutant or the WT sequence revealed that the autocatalytic zymogen cleavage of proPCSK9 to PCSK9 is slightly reduced and the PCSK9 secretion was lower in the R434W mutant (Fig. 3A). Calculations revealed an ∼2.5-fold lower level of PCSK9 in the 4 h medium. These data were further confirmed by ELISA assays, which showed at steady state a 3-fold lower level of secreted PCSK9-R434W (0.60 µg/ml) compared with the WT protein (1.82 µg/ml) (Fig. 3B). To compare the ability of PCSK9 to that of the R434W variant to degrade LDLR, we incubated overnight human hepatic HuH7 cells with either equivalent amounts of immunoreactive PCSK9 resulting from spent media of HEK293 cells overexpressing these constructs or 3-fold higher levels of the WT protein. The cells were then detached in EDTA and the media analyzed by Western blot (Fig. 3B), whereas the cells were immediately subjected to FACS analysis to estimate the levels of cell surface LDLR remaining after the incubation (Fig. 3C). The results showed that after 1 h (not shown) or overnight incubation (Fig. 3B) the levels of cell-associated PCSK9-R434W were ∼2-fold higher than those of the WT protein and that the furin cleavage that occurs during the overnight incubation was ∼3-fold lower with the R434W variant. These data suggest that this mutation must have a profound influence on the conformation of PCSK9, because it partially delays the zymogen cleavage of proPCSK9 into PCSK9, the secretion of the latter, and the furin processing. Furthermore, the lower level of secreted PCSK9-R434W mutant with a minimal effect on its intracellular levels suggests that a fraction of this mutant protein is rapidly degraded in the cell. As a quantitative measure of the level of cell surface LDLR, FACS analysis of HuH7 cells revealed that compared with WT PCSK9, the R434W variant had ∼30% of the LDLR-lowering activity, suggesting a partial LOF (Fig. 3C). Thus, the heterozygote variant PCSK9-R434W results in ∼2-fold lower circulating levels of PCSK9 (Fig. 2), rationalized by a ∼3-fold lower secretion rate of the protein (Fig. 3B) and a ∼40% lower LDL-C (Fig. 2) likely related to its LOF on LDLR degradation (Fig. 3C).
Fig. 3.
Functional analysis of the PCSK9-R434W variant. (A) HEK293 cells transiently transfected with cDNAs coding for either the V5-tagged PCSK9 (WT) or its R434W variant were pulse labeled with 35S-[Met+Cys] for 4 h and the cell extract and media immunoprecipitated with a V5-mAb and the precipitate separated by 8% SDS-PAGE in 3% Tricine. Autoradiography allowed the identification of the various forms of PCSK9 and image analysis allowed the quantification of the relative amounts of mature PCSK9 in cells and media versus the total levels (17). (B) Spent media from HEK293 cells containing either 0.65 or 1.82 µg/ml of PCSK9 or 0.6 µg/ml of PCSK9-R434W were incubated overnight with naïve HuH7 cells. Following washes, the cells were then detached by EDTA and analyzed by Western blot (WB) using the V5-mAb. Notice that the R434W variant is somewhat resistant to furin cleavage, as evidenced by the lower levels of the PCSK9-ΔN218 form. The relative quantitation of the two forms is presented at the bottom of Figure 3B. (C) The detached HuH7 cells were also analyzed by FACS using a C-7 mAb directed against the LDLR. The levels of the remaining cell-surface LDLR are then expressed as compared with cells incubated with media of HEK293 cells transfected with an empty vector.
Plasma PCSK9 levels correlate with cholesterol
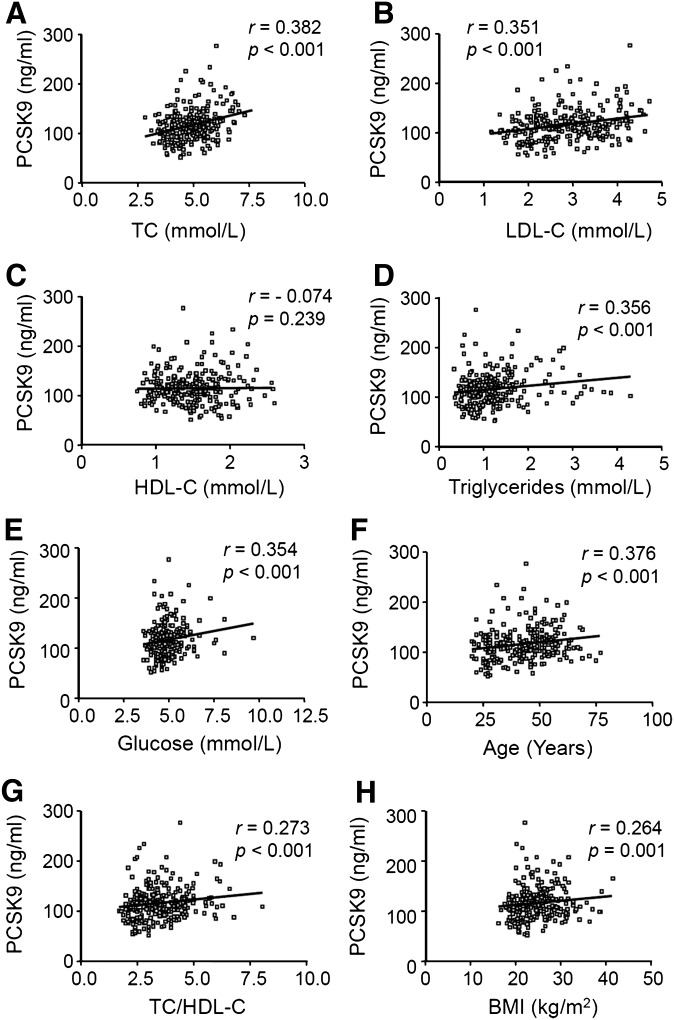
Statistical analysis showed significant correlations between PCSK9 levels and TC (r = 0.382, P < 0.001), LDL-C (r = 0.351, P < 0.001), TG (r = 0.356, P < 0.001), fasting glucose (r = 0.354, P < 0.001), age (r = 0.376, P < 0.001), and body mass index (BMI) (r = 0.264, P < 0.001) (Fig. 4). No significant correlation was observed between PCSK9 levels and HDL-cholesterol (HDL-C) (r = −0.074, P = 0.239). Hormone treatment such as oral contraceptives (n = 19) or hormone replacement therapy (n = 7) had no detectable effect on plasma PCSK9 levels (not shown).
Fig. 4.
Relationship between plasma PCSK9 and (A) TC, (B) LDL-C, (C) HDL-C, (D) TG, (E) fasting glucose, (F) age, (G) TC/HDL-C ratio and (H) BMI. r and P were determined using GraphPad Prism software.
Stepwise regression: a model to predict PCSK9 value
Because many of the parameters studied are colinear, such as TC, LDL-C, and HDL-C, we performed a multiple stepwise regression using SPSS software. This stepwise regression selects variables to include in a regression model for the purpose of identifying an optimal subset of predictors. The best model to predict PCSK9 levels showed that TC (β = 8.84), fasting glucose (β = 11.58), TG (β = 9.19), gender (β = −10.14 for men), and age (β = 0.18) were parameters that had a significant influence on PCSK9 levels in this multiple regression model. This model explains ∼27% of the PCSK9 variability among individuals in our sample.
Statins and ezetimibe upregulate plasma PCSK9
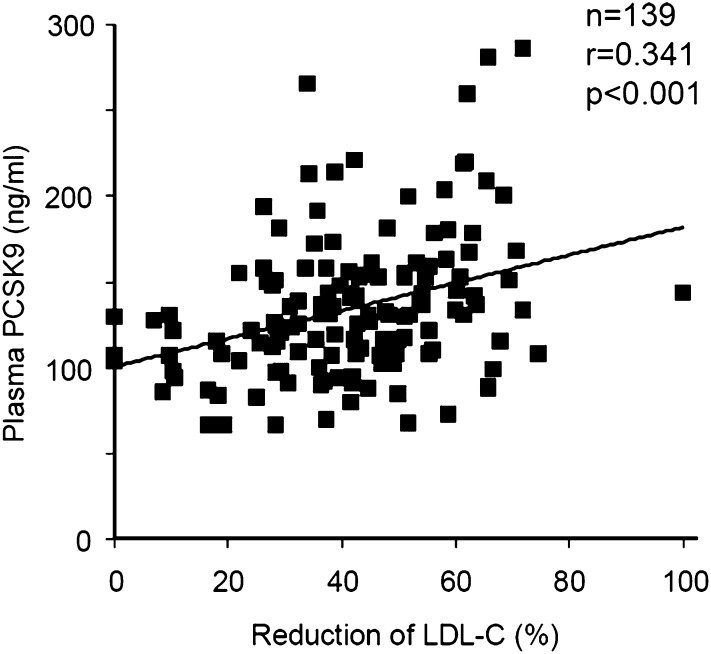
We measured plasma PCSK9 in 200 patients attending the lipid clinic at the IRCM, a tertiary care lipidology reference center. Fifty-nine patients were not on medication, 98 were on statin treatment (55 on atorvastatin, 27 on rosuvastatin, 14 on simvastatin, and 2 on pravastatin), 39 were on a statin-ezetimibe combination, and 4 were on ezetimibe treatment only. All patients had been treated for at least 3 months. There was no significant difference in the correlation between PCSK9 and LDL-C changes according to statin type, nor was there a gender difference in the relationship between PCSK9 and the reduction in LDL-C. However, a significant correlation between plasma PCSK9 and the percent reduction of LDL-C from baseline (before drug treatment) was observed (r = 0.341, P < 0.0001) (Fig. 5). A positive correlation between PCSK9 plasma levels and change in LDL-C was also seen when we looked at each statin separately (r = 0.21, 0.28, and 0.4 for atorvastatin, rosuvastatin, and simvastatin, respectively) (not shown). For the two statins (atorvastatin and rosuvastatin) for which we had a sufficient number of patients, we observed a significant increase in PCSK9 with increasing statin dose in the absence of ezetimibe. When atorvastatin was increased from 5 to 80 mg, plasma PCSK9 levels increased from 109 ± 33 to 142 ± 35 ng/ml (n = 53, P for trend = 0.0001 by ANOVA) and for rosuvastatin from 5 to 40 mg, plasma PCSK9 increased from 123 ± 23 to 168 ± 84 ng/ml (n = 28, P for trend = 0.0001 by ANOVA). In treated patients (n = 139) FH (n = 51) subjects had higher PCSK9 values than non-FH (n = 88, 147 ± 42 vs. 127 ± 41 ng/ml, P < 0.005). However, plasma PCSK9 levels and LDL-C reduction correlated positively to a similar extent in both subsets (r = 0.316, P < 0.02 in FH and r = 0.275, P < 0.009 in non-FH). These data support the hypothesis that treatments with statins, or a statin combined with ezetimibe, are significantly associated with an increase in circulating levels of PCSK9.
Fig. 5.
Statins and ezetimibe are associated with high levels of human plasma PCSK9 as a function of LDL-C reduction. Plasma PCSK9 levels of 139 hypercholesterolemic subjects were measured by ELISA as described. Hypercholesterolemic patients were being treated with one of the following: statins, ezetimibe, or a combination of both. The percent reduction in LDL-C was then plotted against PCSK9 levels (ng/ml). r and P were determined with GraphPad Prism software.
Effects of hypercholesterolemia, statins, and statin-ezetimibe combination on plasma PCSK9 levels
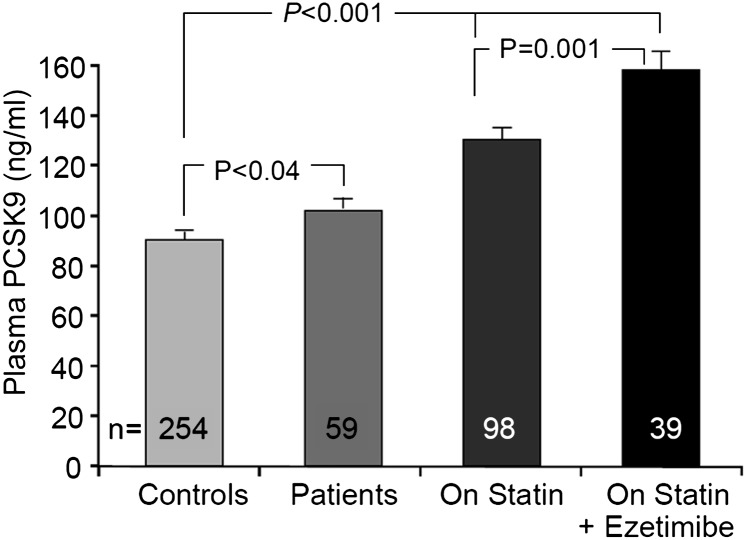
We next stratified patients according to their LDL-C state and/or drug regimen. Thus, we compared the mean plasma PCSK9 levels of 254 healthy controls, 59 hypercholesterolemic patients without medication, 98 patients on statin treatment, and 39 on a statin-ezetimibe combination treatment. We first observed significantly higher plasma PCSK9 levels (∼11%, P < 0.04) in hyperlipidemic patients compared with controls. Furthermore, patients on statin treatment presented a ∼45% (P < 0.001) higher plasma PCSK9, whereas those on a combined statin-ezetimibe treatment showed ∼77% (P < 0.001) higher levels (Fig. 6). We also observed that combined treatment was associated with ∼22% higher mean plasma PCSK9 levels when compared with equivalent doses of statin treatment alone (P = 0.001). We concluded that hyperlipidemia is associated with higher levels of PCSK9 than those observed in controls and that patients on treatments to lower LDL-C levels exhibited even higher plasma PCSK9 levels. In view of the upregulation of LDL-C by PCSK9, this could potentially result in an otherwise dampened response to these treatments.
Fig. 6.
Relationship of hypercholesterolemia, statins, and statin-ezetimibe combination with plasma PCSK9. Mean ± SEM. Significance was determined by Student's t-test.
DISCUSSION
PCSK9 is now recognized as an important contributor in cholesterol homeostasis and has become a promising target for cholesterol-lowering therapy and CAD prevention (25, 35, 36). Herein, we report a new sandwich ELISA to measure human plasma PCSK9 concentrations using a polyclonal antibody. With this method, plasma PCSK9 levels in a healthy sample of population average ∼90 ng/ml (ranging from 35.3 to 225.2 ng/ml) (Fig. 2) and are significantly correlated with both TC and LDL-C levels (Fig. 4A, B).
The significant correlation with TGs, though intriguing, agrees with a very recent report on a large multiethnic cohort analysis of plasma PCSK9 (37). With respect to glucose, it seems that factors that contribute to insulin resistance such as TGs and BMI are associated with higher circulating levels PCSK9 (37). Furthermore, it was also recently reported that in mice, PCSK9 deficiency reduces postprandial triglyceridemia and enhances the hepatic clearance rate of chylomicrons (38). The underlying mechanism(s) requires more extensive studies. Three other groups also developed ELISAs for PCSK9 using different approaches, species, and/or antigens to produce the polyclonal antibodies (14, 30, 39). One report presented a mean value of ∼200 ng/ml in plasma with a range of 50–600 ng/ml (14), another a range of 11–115 ng/ml in serum (no mean value given) (39), and the third, a mean value of ∼4 μg/ml and a range of 0.1–9.3 μg/ml (30). Using a different method involving immunoprecipitation, immunoblotting, and densitometry, Mayne et al. (40) obtained a mean concentration of plasma PCSK9 of 6.1 μg/ml, which is about 50 times the value found in this study but similar to that reported by Lambert et al. (30). The differences are likely due to sample, methodology, antibody specificities, and the standard used for the absolute quantitation of PCSK9. We used purified full-length human PCSK9 as our reference standard; this was confirmed by amino acid sequence analysis and mass spectrometry and quantitated by amino acid composition of a known weighed sample. Only our assay recognizes both active and furin-inactivated forms of PCSK9 (Fig. 1), offering the potential to measure the ratio of both forms in relation to drug effects and in response to physiological modulators.
While Mayne et al. (40) found significant correlations between PCSK9 and cholesterol levels in men only within a cohort of 182 individuals, we did not confirm this finding in our cohort of 254 individuals, because we found a significant correlation with cholesterol levels in both men (P < 0.001) and women (P < 0.001) (Table 2). This difference may be in part related to the age of the population sampled [mean age of 53 (40) or 42 in the present study]. However, in a much larger cohort comprising 3,138 individuals, Lakoski et al. (37) reported that circulating PCSK9 levels are slightly higher in premenopausal women than in postmenopausal women and that it is ∼15% higher in women versus men. Given the physiological role of PCSK9 in degrading LDLR, the observed correlation with LDL-C was expected. Cholesterol metabolism is profoundly modified during aging, and LDL-C increases by ∼40% from 20 to 60 years of age (41). Other physiological changes occur with age, such as reduced physical activity, redistribution of body fat with a relative increase in visceral adipose mass, decreased insulin sensitivity, and increased blood pressure. Thus, a correlation between PCSK9 and age may explain, in part, correlations with other parameters modulated by age.
TABLE 2.
Relationship between plasma PCSK9 and individual variables in men and women
| All Subjects (n = 254) |
Men (n = 117) |
Women (n = 137) |
||||
|---|---|---|---|---|---|---|
| r | P | r | P | r | P | |
| Age (years) | 0.376 | <0.001 | 0.276 | 0.003 | 0.448 | <0.001 |
| BMI (kg/m2) | 0.264 | <0.001 | 0.280 | 0.002 | 0.347 | <0.001 |
| TC (mmol/L) | 0.382 | <0.001 | 0.404 | <0.001 | 0.381 | <0.001 |
| TG (mmol/L) | 0.356 | <0.001 | 0.304 | <0.001 | 0.411 | <0.001 |
| LDL-C (mmol/L) | 0.351 | <0.001 | 0.370 | <0.001 | 0.372 | <0.001 |
| HDL-C (mmol/L) | −0.074 | 0.239 | −0.037 | 0.691 | −0.174 | 0.042 |
| TC-HDL-C | 0.273 | <0.001 | 0.303 | <0.001 | 0.422 | <0.001 |
| Glucose (mmol/L) | 0.354 | <0.001 | 0.230 | 0.013 | 0.466 | <0.001 |
This study first demonstrated that measurements of plasma PCSK9 levels can help in the identification of novel PCSK9 variants using our strategy. Indeed, we identified a new variant, R434W (Fig. 2). Biosynthetic analysis of this variant revealed that it results in a slightly reduced zymogen activation of proPCSK9, a lower secretion rate by ∼50%, and a ∼70% lower activity of the PCSK9- enhanced degradation of LDLR in HuH7 cells (Fig. 3). The ∼3-fold lower secretion rate observed in HEK293 cells (Fig. 3A, B) correlate with the ∼50% lower levels of circulating PCSK9-R434W in this heterozygote subject, which is 51 ng/ml versus the mean value of 89 ± 32 ng/ml in normal subjects (Fig. 2). The LOF of the PCSK9-R434W is intriguing, as this residue does not seem to be implicated in the direct interaction of the catalytic domain of PCSK9 with the EGF-A domain of the LDLR (8). The R434W variant occurs in a loop structure occurring in a hinge region between the catalytic domain and the Cys-His rich domain of PCSK9 (42) (supplementary Fig. II). However, because the Cys-His rich domain is critical for the sorting of the complex LDLR-PCSK9 toward endosomes/ lysosomes for degradation (7), the R434W mutation may hamper such a process and hence result in a LOF (supplementary Fig. II).
The Leu insertion in the signal peptide of PCSK9 (p.L15ins1L or L10) is often associated with another variation, namely A53V, and this double variant has been reported to be associated with low plasma PCSK9 levels, a finding that supports the hypothesis that a modification in the signal peptide could subtly interfere with protein folding, processing, and/or secretion (43). A high level of PCSK9 (222 ng/ml) was observed in an hypercholesterolemic patient of African descent exhibiting the single variant A443T. Although rare in the Caucasian population, the A443T mutation is relatively frequent in African-Americans, where the heterozygous condition is associated with a wide range of cholesterol values (19). The PCSK9 mutation A443T has been reported in a case of mild and variable hypercholesterolemia (GOF), which is sensitive to diet, but the mutation did not segregate with the phenotype in the family (44). However, in homozygotes, it is associated with low levels of LDL-C (19, 44). Indeed, Benjannet et al. (17) showed in vitro that the A443T mutation resulted in an O-glycosylated protein that was more susceptible to furin cleavage, suggesting a LOF.
One of the main findings in this study was the high levels of PCSK9 in patients treated with two widely used cholesterol-lowering agents, statins and ezetimibe. We have already demonstrated that PCSK9 mRNA is upregulated in vitro by statins (28) via a pathway involving reduced intracellular cholesterol concentration and increased SREBP-2 levels (26, 27, 31). The present work supports the hypothesis of the in vivo upregulation of PCSK9 protein in plasma in response to statins. This is in line with the observation of Careskey et al. (31) that plasma PCSK9 increases as LDL-C goes down during 16 weeks of atorvastatin therapy. We also showed that plasma PCSK9 is even higher when ezetimibe and statin are used in combination (Fig. 6).
We surmise that the correlation between PCSK9 and the percent reduction in LDL-C is driven by the inhibition of cholesterol synthesis, as seen in vitro. One group reported the effect of statins when the PCSK9 gene was deleted in the mouse (16) and another when the gene was disrupted by a LOF mutation in humans (45). Both groups observed increased sensitivity to statin treatment when PCSK9 was either not expressed or expressed at low levels. Accordingly, to counteract the effect of cholesterol-lowering agents on PCSK9, it is likely that a potent inhibitor of PCSK9 used in combination with statins would greatly enhance LDL-C lowering.
Addition of ezetimibe, an inhibitor of intestinal cholesterol absorption, was associated with still higher PCSK9 levels (Fig. 6). Although PCSK9 mRNA is expressed in the small intestine (4), nothing is yet known of the function of PCSK9 in this tissue, except that, at least in the mouse, it is not a major contributor to circulating PCSK9 (13). Therefore, we cannot determine whether the high levels of plasma PCSK9 associated with ezetimibe treatment arise from increased production in the intestine or in the liver, the main PCSK9 producer. One possibility would be to test this drug on tissue-specific knockout mice lacking PCSK9 expression specifically in hepatocytes (13). Another group measured PCSK9 mRNA in peripheral blood mononuclear cells in 24 subjects and found a 5.5% increase after 2 weeks of combination treatment with 40 mg simvastatin and 10 mg ezetimibe versus baseline (46). In accordance with this observation, four of our patients who were on ezetimibe alone had a mean PCSK9 concentration of 105 ng/ml, equivalent to that of untreated hypercholesterolemic patients. Finally, it was recently shown that monotherapy with fibrates also resulted in a significant downregulation of plasma PCSK9 by ∼8.5%, paralleling the efficacy of fibrates in decreasing LDL-C and TG (29). It was also suggested that treatment with fibrates can suppress the induction of PCSK9 by statins while still resulting in lower LDL-C (47).
In conclusion, we report for the first time an ELISA method that measures total PCSK9 in plasma, which also allows the estimation of the relative proportion of the mature protein and the fragment cut by furin by Western blot. This method allowed the identification of a novel R434W variant exhibiting a partial LOF on LDLR degradation. This report is also the first demonstration that ezetimibe administration in combination with a statin is associated with a markedly high PCSK9 level, consistent with the ability of ezetimibe to significantly enhance the statin effect on LDL-C, as evidenced by a meta-analysis (48). If the high LDL-C concentrations still observed in patients during treatment reflect an increase in PCSK9 levels, drugs aimed at reducing PCSK9 expression would be expected to greatly enhance current cholesterol-lowering therapies. With the rapid pace of discoveries in the field, it is hoped that within a few years lead molecules reducing the level and/or activity of PCSK9 will be uncovered and that these will emerge for therapeutic use after clinical trials to assess their potency and safety. In that context, it was recently shown that administration of a monoclonal antibody that blocks the PCSK9-LDLR interaction to either mice or cynomolgus monkeys results in an ∼80% drop in LDL-C lasting 10 days and that in mice, such an effect enhances the LDL-C lowering by the statin mevinolin (49).
Acknowledgments
The authors acknowledge the invaluable assistance of the nursing staff, especially Lise Patenaude and Rina Casoni Riberdy, and excellent technical assistance of Lise St-Germain and secretarial assistance of Carole Tremblay. The authors also thank Dr. Delia Susan-Resiga for modeling the PCSK9 structure including its mutant R434W.
Footnotes
Abbreviations:
- BMI
- body mass index
- CAD
- coronary artery disease
- FACS
- fluorescence-activated cell sorting
- FH
- familial hypercholesterolemia
- GOF
- gain-of-function
- HDL-C
- HDL-cholesterol
- LDL-C
- LDL-cholesterol
- LDLR
- LDL receptor
- LOF
- loss-of-function
- PCSK9
- proprotein convertase subtilisin/kexin type 9
- rPCSK9
- recombinant truncated human PCSK9
- SREBP-2
- sterol regulatory element binding protein-2
- TC
- total cholesterol
- TG
- triglyceride
- WT
- wild-type
This work was funded in part by the first Pfizer Jean Davignon Distinguished Cardiovascular and Metabolic Research Award and by CIHR grant no. MOP 36496 to N.G.S., no. CTP 82946, and Canada chair no. 20652. G.D. is a recipient of a doctoral award from Fonds de la Recherche en Santé du Québec.
REFERENCES
- 1.Goldstein J. L., Hobbs H. H., Brown M. S. Familial hypercholesterolemia. 2001. The metabolic and molecular bases of inherited disease. Valle D., Scriver C. R., Beaudet A. L., Sly W. S., Childs B., Volgestein B., editors. Chapter 120, 8th edition McGraw Hill, New York: 2863–2913. [Google Scholar]
- 2.Innerarity T. L., Weisgraber K. H., Arnold K. S., Mahley R. W., Krauss R. M., Vega G. L., Grundy S. M. 1987. Familial defective apolipoprotein B-100: low density lipoproteins with abnormal receptor binding. Proc. Natl. Acad. Sci. USA. 84: 6919–6923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abifadel M., Varret M., Rabes J. P., Allard D., Ouguerram K., Devillers M., Cruaud C., Benjannet S., Wickham L., Erlich D., et al. 2003. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat. Genet. 34: 154–156. [DOI] [PubMed] [Google Scholar]
- 4.Seidah N. G., Benjannet S., Wickham L., Marcinkiewicz J., Jasmin S. B., Stifani S., Basak A., Prat A., Chretien M. 2003. The secretory proprotein convertase neural apoptosis-regulated convertase 1 (NARC-1): liver regeneration and neuronal differentiation. Proc. Natl. Acad. Sci. USA. 100: 928–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seidah N. G., Prat A. 2007. The proprotein convertases are potential targets in the treatment of dyslipidemia. J. Mol. Med. 85: 685–696. [DOI] [PubMed] [Google Scholar]
- 6.Nassoury N., Blasiole D. A., Tebon O. A., Benjannet S., Hamelin J., Poupon V., McPherson P. S., Attie A. D., Prat A., Seidah N. G. 2007. The cellular trafficking of the secretory proprotein convertase PCSK9 and its dependence on the LDLR. Traffic. 8: 718–732. [DOI] [PubMed] [Google Scholar]
- 7.Zhang D. W., Garuti R., Tang W. J., Cohen J. C., Hobbs H. H. 2008. Structural requirements for PCSK9-mediated degradation of the low-density lipoprotein receptor. Proc. Natl. Acad. Sci. USA. 105: 13045–13050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kwon H. J., Lagace T. A., McNutt M. C., Horton J. D., Deisenhofer J. 2008. Molecular basis for LDL receptor recognition by PCSK9. Proc. Natl. Acad. Sci. USA. 105: 1820–1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park S. W., Moon Y. A., Horton J. D. 2004. Post-transcriptional regulation of low density lipoprotein receptor protein by proprotein convertase subtilisin/kexin type 9a in mouse liver. J. Biol. Chem. 279: 50630–50638. [DOI] [PubMed] [Google Scholar]
- 10.Benjannet S., Rhainds D., Essalmani R., Mayne J., Wickham L., Jin W., Asselin M. C., Hamelin J., Varret M., Allard D., et al. 2004. NARC-1/PCSK9 and its natural mutants: zymogen cleavage and effects on the low density lipoprotein (LDL) receptor and LDL cholesterol. J. Biol. Chem. 279: 48865–48875. [DOI] [PubMed] [Google Scholar]
- 11.Maxwell K. N., Fisher E. A., Breslow J. L. 2005. Overexpression of PCSK9 accelerates the degradation of the LDLR in a post-endoplasmic reticulum compartment. Proc. Natl. Acad. Sci. USA. 102: 2069–2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cameron J., Holla O. L., Ranheim T., Kulseth M. A., Berge K. E., Leren T. P. 2006. Effect of mutations in the PCSK9 gene on the cell surface LDL receptors. Hum. Mol. Genet. 15: 1551–1558. [DOI] [PubMed] [Google Scholar]
- 13.Zaid A., Roubtsova A., Essalmani R., Marcinkiewicz J., Chamberland A., Hamelin J., Tremblay M., Jacques H., Jin W., Davignon J., et al. 2008. Proprotein convertase subtilisin/kexin type 9 (PCSK9): hepatocyte-specific low-density lipoprotein receptor degradation and critical role in mouse liver regeneration. Hepatology. 48: 646–654. [DOI] [PubMed] [Google Scholar]
- 14.Lagace T. A., Curtis D. E., Garuti R., McNutt M. C., Park S. W., Prather H. B., Anderson N. N., Ho Y. K., Hammer R. E., Horton J. D. 2006. Secreted PCSK9 decreases the number of LDL receptors in hepatocytes and inlivers of parabiotic mice. J. Clin. Invest. 116: 2995–3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luo Y., Warren L., Xia D., Jensen H., Sand T., Petras S., Qin W., Miller K. S., Hawkins J. 2009. Function and distribution of circulating human PCSK9 expressed extrahepatically in transgenic mice. J. Lipid Res. 50: 1581–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rashid S., Curtis D. E., Garuti R., Anderson N. N., Bashmakov Y., Ho Y. K., Hammer R. E., Moon Y. A., Horton J. D. 2005. Decreased plasma cholesterol and hypersensitivity to statins in mice lacking Pcsk9. Proc. Natl. Acad. Sci. USA. 102: 5374–5379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benjannet S., Rhainds D., Hamelin J., Nassoury N., Seidah N. G. 2006. The proprotein convertase PCSK9 is inactivated by furin and/or PC5/6A: Functional consequences of natural mutations and post-translational modifications. J. Biol. Chem. 281: 30561–30572. [DOI] [PubMed] [Google Scholar]
- 18.Zhao Z., Tuakli-Wosornu Y., Lagace T. A., Kinch L., Grishin N. V., Horton J. D., Cohen J. C., Hobbs H. H. 2006. Molecular characterization of loss-of-function mutations in PCSK9 and identification of a compound heterozygote. Am. J. Hum. Genet. 79: 514–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kotowski I. K., Pertsemlidis A., Luke A., Cooper R. S., Vega G. L., Cohen J. C., Hobbs H. H. 2006. A spectrum of PCSK9 alleles contributes to plasma levels of low-density lipoprotein cholesterol. Am. J. Hum. Genet. 78: 410–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abifadel M., Rabes J. P., Boileau C., Varret M. 2007. [After the LDL receptor and apolipoprotein B, autosomal dominant hypercholesterolemia reveals its third protagonist: PCSK9.] Ann. Endocrinol. (Paris). 68: 138–146. [DOI] [PubMed] [Google Scholar]
- 21.Timms K. M., Wagner S., Samuels M. E., Forbey K., Goldfine H., Jammulapati S., Skolnick M. H., Hopkins P. N., Hunt S. C., Shattuck D. M. 2004. A mutation in PCSK9 causing autosomal-dominant hypercholesterolemia in a Utah pedigree. Hum. Genet. 114: 349–353. [DOI] [PubMed] [Google Scholar]
- 22.Naoumova R. P., Tosi I., Patel D., Neuwirth C., Horswell S. D., Marais A. D., van Heyningen C., Soutar A. K. 2005. Severe hypercholesterolemia in four British families with the D374Y mutation in the PCSK9 gene: long-term follow-up and treatment response. Arterioscler. Thromb. Vasc. Biol. 25: 2654–2660. [DOI] [PubMed] [Google Scholar]
- 23.Cohen J. C., Boerwinkle E., Mosley T. H., Jr, Hobbs H. H. 2006. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N. Engl. J. Med. 354: 1264–1272. [DOI] [PubMed] [Google Scholar]
- 24.Hooper A. J., Marais A. D., Tanyanyiwa D. M., Burnett J. R. 2007. The C679X mutation in PCSK9 is present and lowers blood cholesterol in a Southern African population. Atherosclerosis. 193: 445–448. [DOI] [PubMed] [Google Scholar]
- 25.Horton J. D., Cohen J. C., Hobbs H. H. 2009. PCSK9: a convertase that coordinates LDL catabolism. J. Lipid Res. 50: S172–S177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maxwell K. N., Soccio R. E., Duncan E. M., Sehayek E., Breslow J. L. 2003. Novel putative SREBP and LXR target genes identified by microarray analysis in liver of cholesterol-fed mice. J. Lipid Res. 44: 2109–2119. [DOI] [PubMed] [Google Scholar]
- 27.Horton J. D., Shah N. A., Warrington J. A., Anderson N. N., Park S. W., Brown M. S., Goldstein J. L. 2003. Combined analysis of oligonucleotide microarray data from transgenic and knockout mice identifies direct SREBP target genes. Proc. Natl. Acad. Sci. USA. 100: 12027–12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dubuc G., Chamberland A., Wassef H., Davignon J., Seidah N. G., Bernier L., Prat A. 2004. Statins upregulate PCSK9, the gene encoding the proprotein convertase neural apoptosis-regulated convertase-1 implicated in familial hypercholesterolemia. Arterioscler. Thromb. Vasc. Biol. 24: 1454–1459. [DOI] [PubMed] [Google Scholar]
- 29.Mayne J., Dewpura T., Raymond A., Cousins M., Chaplin A., Lahey K. A., Lahaye S. A., Mbikay M., Ooi T. C., Chretien M. 2008. Plasma PCSK9 levels are significantly modified by statins and fibrates in humans. Lipids Health Dis. 7: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lambert G., Ancellin N., Charlton F., Comas D., Pilot J., Keech A., Patel S., Sullivan D. R., Cohn J. S., Rye K. A., et al. 2008. Plasma PCSK9 concentrations correlate with LDL and total cholesterol in diabetic patients and are decreased by fenofibrate treatment. Clin. Chem. 54: 1038–1045. [DOI] [PubMed] [Google Scholar]
- 31.Careskey H. E., Davis R. A., Alborn W. E., Troutt J. S., Cao G., Konrad R. J. 2008. Atorvastatin increases human serum levels of proprotein convertase subtilisin/kexin type 9. J. Lipid Res. 49: 394–398. [DOI] [PubMed] [Google Scholar]
- 32.Demacker P. N., Veerkamp M. J., Bredie S. J., Marcovina S. M., de Graaf J., Stalenhoef A. F. 2000. Comparison of the measurement of lipids and lipoproteins versus assay of apolipoprotein B for estimation of coronary heart disease risk: a study in familial combined hyperlipidemia. Atherosclerosis. 153: 483–490. [DOI] [PubMed] [Google Scholar]
- 33.Mayer G., Poirier S., Seidah N. G. 2008. Annexin A2 is a C-terminal PCSK9-binding protein that regulates endogenous low density lipoprotein receptor levels. J. Biol. Chem. 283: 31791–31801. [DOI] [PubMed] [Google Scholar]
- 34.Yue P., Averna M., Lin X., Schonfeld G. 2006. The c.43_44insCTG variation in PCSK9 is associated with low plasma LDL-cholesterol in a Caucasian population. Hum. Mutat. 27: 460–466. [DOI] [PubMed] [Google Scholar]
- 35.Seidah N. G., Mayer G., Zaid A., Rousselet E., Nassoury N., Poirier S., Essalmani R., Prat A. 2008. The activation and physiological functions of the proprotein convertases. Int. J. Biochem. Cell Biol. 40: 1111–1125. [DOI] [PubMed] [Google Scholar]
- 36.Lambert G. 2007. Unravelling the functional significance of PCSK9. Curr. Opin. Lipidol. 18: 304–309. [DOI] [PubMed] [Google Scholar]
- 37.Lakoski S. G., Lagace T. A., Cohen J. C., Horton J. D., Hobbs H. H. 2009. Plasma levels of PCSK9 in a large multiethnic population. J. Clin. Endocrinol. Metab. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Le May C., Kourimate S., Langhi C., Chetiveaux M., Jarry A., Comera C., Collet X., Kuipers F., Krempf M., Cariou B., et al. 2009. Proprotein convertase subtilisin kexin type 9 null mice are protected from postprandial triglyceridemia. Arterioscler. Thromb. Vasc. Biol. In press. [DOI] [PubMed] [Google Scholar]
- 39.Alborn W. E., Cao G., Careskey H. E., Qian Y. W., Subramaniam D. R., Davies J., Conner E. M., Konrad R. J. 2007. Serum proprotein convertase subtilisin kexin type 9 is correlated directly with serum LDL cholesterol. Clin. Chem. 53: 1814–1819. [DOI] [PubMed] [Google Scholar]
- 40.Mayne J., Raymond A., Chaplin A., Cousins M., Kaefer N., Gyamera-Acheampong C., Seidah N. G., Mbikay M., Chretien M., Ooi T. C. 2007. Plasma PCSK9 levels correlate with cholesterol in men but not in women. Biochem. Biophys. Res. Commun. 361: 451–456. [DOI] [PubMed] [Google Scholar]
- 41.Wilhelmsen L., Johansson S., Rosengren A., Wallin I., Dotevall A., Lappas G. 1997. Risk factors for cardiovascular disease during the period 1985–1995 in Goteborg, Sweden. The GOT-MONICA Project. J. Intern. Med. 242: 199–211. [DOI] [PubMed] [Google Scholar]
- 42.Cunningham D., Danley D. E., Geoghegan K. F., Griffor M. C., Hawkins J. L., Subashi T. A., Varghese A. H., Ammirati M. J., Culp J. S., Hoth L. R., et al. 2007. Structural and biophysical studies of PCSK9 and its mutants linked to familial hypercholesterolemia. Nat. Struct. Mol. Biol. 14: 413–419. [DOI] [PubMed] [Google Scholar]
- 43.Abifadel M., Bernier L., Dubuc G., Nuel G., Rabes J. P., Bonneau J., Marques A., Marduel M., Devillers M., Erlich D., et al. 2008. A PCSK9 variant and familial combined hyperlipidemia. J. Med. Genet. 45: 780–786. [DOI] [PubMed] [Google Scholar]
- 44.Allard D., Amsellem S., Abifadel M., Trillard M., Devillers M., Luc G., Krempf M., Reznik Y., Girardet J. P., Fredenrich A., et al. 2005. Novel mutations of the PCSK9 gene cause variable phenotype of autosomal dominant hypercholesterolemia. Hum. Mutat. 26: 497–506. [DOI] [PubMed] [Google Scholar]
- 45.Berge K. E., Ose L., Leren T. P. 2006. Missense mutations in the PCSK9 gene are associated with hypocholesterolemia and possibly increased response to statin therapy. Arterioscler. Thromb. Vasc. Biol. 26: 1094–1100. [DOI] [PubMed] [Google Scholar]
- 46.Gouni-Berthold I., Berthold H. K., Gylling H., Hallikainen M., Giannakidou E., Stier S., Ko Y., Patel D., Soutar A. K., Seedorf U., et al. 2008. Effects of ezetimibe and/or simvastatin on LDL receptor protein expression and on LDL receptor and HMG-CoA reductase gene expression: a randomized trial in healthy men. Atherosclerosis. 198: 198–207. [DOI] [PubMed] [Google Scholar]
- 47.Kourimate S., Le May C., Langhi C., Jarnoux A. L., Ouguerram K., Zair Y., Nguyen P., Krempf M., Cariou B., Costet P. 2008. Dual mechanisms for the fibrate-mediated repression of proprotein convertase subtilisin/kexin type 9. J. Biol. Chem. 283: 9666–9673. [DOI] [PubMed] [Google Scholar]
- 48.Mikhailidis D. P., Sibbring G. C., Ballantyne C. M., Davies G. M., Catapano A. L. 2007. Meta-analysis of the cholesterol-lowering effect of ezetimibe added to ongoing statin therapy. Curr. Med. Res. Opin. 23: 2009–2026. [DOI] [PubMed] [Google Scholar]
- 49.Chan J. C., Piper D. E., Cao Q., Liu D., King C., Wang W., Tang J., Liu Q., Higbee J., Xia Z., et al. 2009. From the cover: a proprotein convertase subtilisin/kexin type 9 neutralizing antibody reduces serum cholesterol in mice and nonhuman primates. Proc. Natl. Acad. Sci. USA. 106: 9820–9825. [DOI] [PMC free article] [PubMed] [Google Scholar]