Abstract
Apolipoprotein (apo) C-III plays a regulatory role in VLDL lipolysis and clearance. In this study, we determined a potential intracellular role of apoC-III in hepatic VLDL assembly and secretion. Stable expression of recombinant apoC-III in McA-RH7777 cells resulted in increased secretion efficiency of VLDL-associated triacylglycerol (TAG) and apoB-100 in a gene-dosage-dependent manner. The stimulatory effect of apoC-III on TAG secretion was manifested only when cells were cultured under lipid-rich (i.e., media supplemented with exogenous oleate) but not lipid-poor conditions. The stimulated TAG secretion was accompanied by increased secretion of apoB-100 and apoB-48 as VLDL1. Expression of apoC-III also increased mRNA and activity of microsomal triglyceride transfer protein (MTP). Pulse-chase experiments showed that apoC-III expression promoted VLDL1 secretion even under conditions where the MTP activity was inhibited immediately after the formation of lipid-poor apoB-100 particles, suggesting an involvement of apoC-III in the second-step VLDL assembly process. Consistent with this notion, the newly synthesized apoC-III was predominantly associated with TAG within the microsomal lumen that resembled lipid precursors of VLDL. Introducing an Ala23-to-Thr mutation into apoC-III, a naturally occurring mutation originally identified in two Mayan Indian subjects with hypotriglyceridemia, abolished the ability of apoC-III to stimulate VLDL secretion from transfected cells. Thus, expression of apoC-III in McA-RH7777 cells enhances hepatic TAG-rich VLDL assembly and secretion under lipid-rich conditions.
Keywords: triacylglycerol, hypotriglyceridemia, hypertriglyceridemia, microsomal triglyceride transfer protein, hepatocyte
Apolipoprotein (apo) C-III is a small exchangeable apolipoprotein (79 amino acids) that is synthesized mainly in the liver and intestine from the APOA5-APOA4-APOC3-APOA1 gene locus and is secreted into the plasma as a component of VLDL and HDL (1). Elevated plasma apoC-III concentration is commonly observed in human subjects with insulin resistance and central obesity and is positively correlated with plasma triacylglycerol (TAG) concentrations in hypertriglyceridemia subjects (2, 3). Early studies have shown that mutations within the APOA4-APOC3-APOA1 gene locus were associated with patients of premature atherosclerosis (4, 5). However, the close proximity of apoA-IV, apoC-III, and apoA-I encoded within this gene cluster makes it difficult to ascertain the contribution of apoC-III deficiency per se to the development of atherosclerosis. Recently, a genome-wide association study has discovered an apoC-III null allele (R19×) in Lancaster Amish population and shown that individuals heterozygous of the R19× allele have a favorable plasma lipid profile (i.e., lowered fasting and postprandial serum TAG) and apparent cardioprotection (6). Studies with genetically modified mice have shown that overexpression of human APOC3 resulted in severe hypertriglyceridemia and in some cases hepatosteatosis (7, 8), whereas lowered concentration of fasted plasma TAG (70% of normal) was observed in apoc3-knockout mice (apoc3−/−) (9). The positive correlation between apoC-III and plasma TAG is mainly attributable to the roles of apoC-III in i) inhibiting lipolytic activity of LPL (10, 11), and ii) attenuating the binding of TAG-rich lipoproteins to heparin sulfate proteoglycans (12) and LDL receptor (13) and thus preventing uptake.
Although the majority of plasma apoC-III is associated with HDL, a significant amount of the protein exists as a component of VLDL, and the VLDL-bound apoC-III is further increased in hypertriglyceridemia (14, 15). This correlation between VLDL-apoC-III and hypertriglyceridemia begs the question as to whether or not apoC-III plays a role in hepatic VLDL assembly or secretion. To date, results from studies with genetically modified mice are inconclusive regarding the impact of apoC-III expression on the rate of VLDL production in vivo. While transgenic mice overexpressing APOC3 exhibited a 2-fold increase in VLDL production (7, 8), no change in VLDL production was observed with the apoC3−/− mice (16). Similarly, there is no clear conclusion regarding apoC-III expression affecting VLDL production in humans. Although a positive correlation between in vivo production rates of VLDL-associated apoC-III and VLDL-TAG has been observed in a wide variety of normolipidemic or hyperlipidemic human subjects under different metabolic or diet conditions (2, 3, 17–19), such correlation could not be confirmed in other studies (20). The exchangeability of apoC-III among plasma lipoproteins makes it challenging to draw definite conclusions from these in vivo studies as to whether the positive correlation between plasma apoC-III and VLDL-TAG is attributable to apoC-III promoting VLDL production (21). Moreover, because many human subjects used in the above clinical studies had various stages of insulin resistance and/or different forms of hypertriglyceridemia that are known factors contributing to hepatic VLDL overproduction, the role of apoC-III expression in promoting VLDL assembly/secretion could not be independently discerned.
The assembly of hepatic VLDL is achieved through multiple steps during and after translation and translocation of apoB-100 across the endoplasmic reticulum (ER) membranes (22). The initial step occurs during apoB-100 translation and translocation across the ER membrane, forming primordial VLDL precursor particles with limited amount of TAG. The activity of the abetalipoproteinemia gene (Mttp) product microsomal triglyceride-transfer protein (MTP) is required for the process (23, 24). Additional lipid recruitment steps involve incorporation of bulk TAG, presumably in the form of lipid droplets, into the VLDL precursor particles. The molecular details involved in the formation and incorporation of lipid droplets during VLDL assembly is not completely elucidated. In this study, we determined the effect of hepatic apoC-III expression on VLDL assembly/secretion using rat hepatoma McA-RH7777 cells. These cells, unlike the human hepatoblstoma HepG2 cells that are unable to synthesize VLDL, retain the ability to assemble and secrete large TAG-rich apoB-100-VLDL1 (Sf > 100) when cultured in lipid-rich medium (24). The present data suggest that apoC-III expression enhances VLDL assembly/secretion in a gene-dosage-dependant manner, and the enhancement is manifested only when the transfected cells were cultured in lipid-rich media (i.e., in the presence of exogenous FFA) to mimic the increased FFA supply to the liver under stress or other aberrant metabolic conditions (25). Thus, hepatic expression of apoC-III may exacerbate hypertriglyceridemia under abnormal metabolic conditions by promoting hepatic VLDL assembly and secretion, in addition to its known function in attenuating VLDL hydrolysis and clearance in the plasma.
MATERIALS AND METHODS
Materials
Cell culture reagents were purchased from Invitrogen Canada (Burlington, ON). Reagents for recombinant DNA experiments were obtained from New England Biolabs (Pickering, ON). [2-3H]glycerol (9.6 Ci/mmol), [14C]oleate (80 mCi/mmol), and [35S]methionine/cysteine (1,000 Ci/mmol) were obtained from GE Healthcare (Mississauga, ON). Heparin, heparinase I, and horseradish-peroxidase-linked anti-goat antibody was obtained from Sigma-Aldrich (Oakville, ON). Oleate, TAG, and phospholipid standards were from Avanti Polar Lipids (Albaster, AL). Antibodies against human apoC-III or apoE (used for immunoblot analysis) were purchased from Academy Biomedical (Houston, TX). Rabbit anti-mouse apoE antibody (used for immunoprecipitation) was obtained from BioDesign (Saco, ME). Polyclonal antiserum against rat VLDL proteins (used for immunoprecipitation of rat apoB-100) or human apoC-III (used for immunoprecipitation) was produced in our laboratory. Horseradish-peroxidase- conjugated anti-mouse and anti-rabbit IgG antibodies were obtained from Amersham Biosciences (Baie d'Urfe, PQ). Chemiluminescent substrates were purchased from Roche Diagnostics (Laval, PQ).
Human apoC-III expression plasmids and transfection
Total RNA was isolated from HepG2 cells using Trizol™ reagent (Invitrogen) and reverse-transcribed into cDNAs using MMLV reverse transcriptase and an oligo-dT primer. Coding sequences of apoC-III were amplified from the cDNAs by PCR using primers listed in Table 1. The resulting APOC3 cDNA fragments were digested with BamHI and HindIII, respectively, and inserted into pCMV5 vector between the BglII and HindIII sites. The site-specific mutagenesis Ala-to-Thr mutation at residue 23 of mature apoC-III (26) was performed using the QuikChangeTM kit (Stratagene, Ann Arbor, MI) with the appropriate forward and reverse primers (Table 1). The coding sequences of all of the cDNA constructs were verified by sequencing. Transfection of the expression plasmids into McA-RH7777 cells was achieved by the calcium phosphate precipitation method, and stable transformants were obtained by cotransfection with pSV2neo and selected with G418 as described previously (27).
TABLE 1.
Nucleotide sequences of the PCR primers
| C3 forward | CAGTGGATCCTAGAGGCAGC |
| C3 reverse | CCCTGAAGCTTGCAGGACCCA |
| A23T forward | CCACCAAGACCaCaAAGGATGCA |
| A23T reverse | TGCATCCTTtGtGGTCTTGGTGG |
All sequences are 5′ to 3′ direction. The BamHI and HindIII restriction sites included in the forward and reverse PCR primers are indicated as underlined. In mutagenesis primers for C3A23T, the underlined sequences containing lowercased nucleotides denote the positions of respective mutations.
ApoC-III knockdown
Short interfering RNAs (siRNAs) specific for human apoC-III were obtained from Ambion (Austin, TX). HepG2 cells were cultured in DMEM supplemented with 10% FBS. The cells (3 ×105 in 6-well plate) were transfected with 40 nM apoC-III siRNA according to the manufacturer's instructions. The transfection medium was replaced with DMEM (10% FBS) 24 h posttransfection and cultured for additional 24 h prior to experimentation. Expression of apoC-III in the transfected cells was determined by immunoblotting as described below.
Immunoblot analysis of apolipoproteins
Transfected McA-RH7777 cells were incubated with serum-free DMEM for 16 h. Secreted apoC proteins in the conditioned medium were absorbed onto hydrated colloidal silica as previously described (28). The silica-bound apoC proteins were eluted into 200 µl SDS-PAGE sample buffer (8 M Urea, 2% SDS). Sample containing equal amount of cell proteins were resolved by SDS-PAGE (12% gel) using Tris-Tricine-SDS running buffer system and transferred onto nitrocellulose membranes for immunoblot analysis using appropriate polyclonal antibodies against human apoC-III. Endogenous apoE secreted from the transfected cells were detected using polyclonal rabbit anti-mouse apoE antibody (BioDesign International).
Metabolic labeling of lipids
Cells were labeled with [3H]glycerol (5 µCi/ml, 2 ml/dish) in DMEM containing 20% FBS ± oleate (0–1.2 mm) for up to 4 h as described previously (24, 29). Reuptake of lipoproteins by cells was prevented by including heparinase I (12 units/ml) in the labeling media. Total lipids were extracted from cells and media, respectively, and separated by TLC. The radioactivity associated with [3H]TAG and phosphatidylcholine (PC) was quantified by scintillation counting. The secretion efficiency of [3H]TAG was calculated as the percentage of [3H]TAG in media over the total [3H]TAG ([3H]TAG in the media + [3H]TAG in cell) at the end of the labeling period. In some experiments, lipid secretion efficiency was determined by pulse-chase experiments in which cells were labeled with [14C]oleate (5 μCi/ml) for 2 h and “chased” for up to 4 h.
Metabolic labeling of proteins
Cells (60 mm dishes) were labeled with [35S]methionine/cysteine (200 μCi) for up to 3 h in methionine/cysteine-free DMEM (2 ml) containing 20% FBS ± 0.4 mm oleate. Posttranslational stability and secretion of apoB-100, apoE, and apoC-III were determined by pulse-chase experiments (30 min pulse followed by 2 h chase) as described previously (24). The respective 35S-labeled apolipoproteins were immunoprecipiated and resolved by SDS-PAGE (5% gel for apoB and 12% gel for apoE and apoC-III). Radioactivity associated with the 35S-labeled protein was quantified by scintillation counting. The secretion efficiency of apoB-100, apoE, and apoC-III was defined as the percent of total labeled respective proteins that was secreted into the media during chase.
Cumulative rate floatation of lipoproteins
Cells were labeled with [3H]glycerol or [35S]methionine/cysteine as described above. The medium lipoproteins secreted from metabolically labeled cells were fractionated by cumulative rate floatation ultracentrifugation into VLDL1 (Sf >100), VLDL2 (Sf 20–100) and other lipoproteins as described previously (24). Lipoproteins within the microsomal lumen were similarly fractionated (24). The lipids were extracted, and apolipoproteins (apoB-100, apoE, and apoC-III) were recovered from each fraction and analyzed by TLC and SDS-PAGE, respectively, as described above.
Quantification of RNA by real-time RT-PCR
Total RNA was isolated from stably transfected cells using the RNeasy Plus Mini Kit (Qiagen) according to the manufacturer's instructions. The RNA samples (1 μg) were primed with random hexamer primers and reversely transcribed into cDNA using SuperScript II reverse transcriptase (Invitrogen Canada). The cDNAs were amplified by TaqMan-based detection chemistry using TaqMan Universal Master Mix (Applied Biosystems) and Assays-on-Demand primer/probe sets (Applied Biosystems) for Mttp (Rn01522970_m1) and ApoB (Mm01545156_m1). All reactions were run in triplicates on a 7900HT Fast Real-Time PCR System (Applied Biosystems) with 384-well plate block module using cycle parameters specified by the manufacturer. Serial dilutions of cDNA were used to generate standard curves of cycle threshold versus logarithms of concentration for the genes of interest and 18s rRNA. The cycle threshold values were obtained using Applied Biosystems SDS 2.3 software and were normalized with 18s rRNA.
Preparation of ApoC-III adenovirus vectors
cDNA encoding wild-type Apo-CIII or the Ala23Thr mutant under the regulation of the human cytomegalovirus immediate early promoter/enhancer and bovine growth hormone polyadenylation sequence were cloned in place of the early region 1 (E1) of an E1/E3-deleted adenovirus vector, using standard methods (30), generating C3wt-Adv and C3AT-Adv, respectively.
Other assays
The in vitro MTP activity in the cell lysate of apoC-III expressing cells was determined as described previously (31, 32). Protein concentrations in the cells were quantified using the Bradford method (33).
RESULTS
Expression of apoC-III in McA-RH7777 cells enhances VLDL-TAG secretion under lipid-rich conditions in a gene-dose-dependent manner
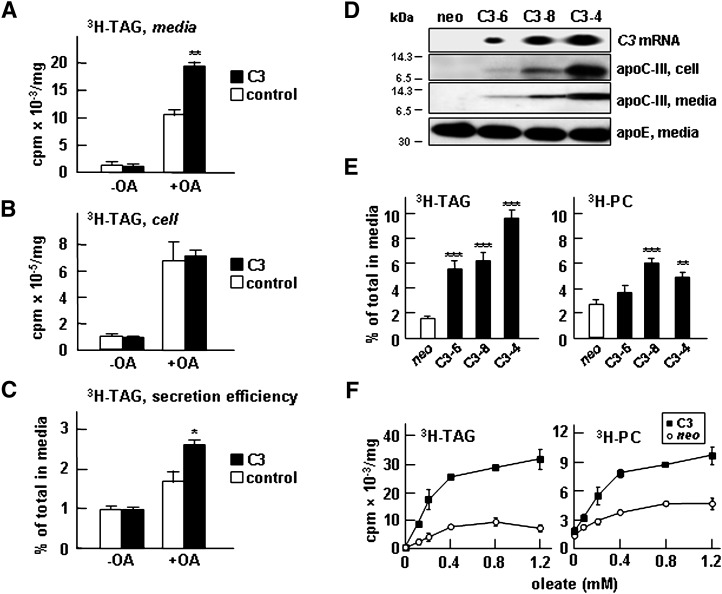
The potential role of apoC-III expression in VLDL assembly and secretion was determined using in vitro cell culture systems under defined lipid substrate conditions. Transient transfection of apoC-III into McA-RH7777 cells, a rat hepatoma cell line that does not express endogenous apoC-III, resulted in increased secretion of [3H]glycerol-labeled TAG compared with CMV5 vector transfected controls under lipid-rich conditions (i.e., media supplemented with exogenous oleate) (Fig. 1A). The effect of apoC-III expression had no effect on [3H]TAG secretion when cells were cultured in the absence of oleate. The lack of an effect on [3H]TAG secretion under oleate-free conditions suggests that apoC-III expression per se has no impact on VLDL secretion. The cell-associated [3H]TAG was comparable between apoC-III-expressing cells and the controls under both lipid-rich and lipid-poor conditions (Fig. 1B). Thus, the secretion efficiency of [3H]TAG, expressed as the proportion of total TAG (i.e., cell + media) that was secreted at the end of labeling, was enhanced by apoC-III expression under lipid-rich conditions (Fig. 1C).
Fig. 1.
Expression of apoC-III in McA-RH7777 cells resulted in enhanced [3H]TAG secretion under lipid-rich conditions. Control or transiently apoC-III (C3) transfected cells (48 h posttransfection) were labeled with [3H]glycerol for 2 h in DMEM containing 20% FBS ± 0.4 mM oleate. At the end of labeling, radioactivity associated with [3H]TAG in media (A) and cells (B) was quantified. C: [3H]TAG secretion efficiency is expressed as percentage of total [3H]TAG (cell + media) that was secreted into media. D: Characterization of three stable clones (C3-6, C3-8, and C3-4) expressing different levels of apoC-III. From top to bottom, Northern blots for APOC3 mRNA, immunoblots for apoC-III in cells, immunoblots for apoC-III in media, and immunoblots for endogenous apoE. E: Secretion efficiency of [3H]TAG and [3H]PC from the stable apoC-III clones. The indicated cells were labeled with [3H]glycerol for 4 h in DMEM containing 20% FBS + 0.4 mM oleate. Statistical significance ***P < 0.001; **P < 0.01; *P < 0.05 (Student's t-test of C3 versus control). Error bars indicate ± SD (n = 3). F: Control (neo) and cells expressing apoC-III (C3) were labeled with [3H]glycerol for 2 h in DMEM containing 20% FBS and increasing concentrations of oleate. At the end of labeling, radioactivity associated with [3H]TAG (left panel) and [3H]PC (right panel) in media and cells was quantified.
The effect of apoC-III expression on TAG secretion was further determined using stable McA-RH7777 cell lines that expressed apoC-III at different levels. The concentrations of cell-associated and medium apoC-III proteins in the stable cell clones were positively correlated to the gene copy numbers (as demonstrated by APOC3 mRNA levels) (Fig. 1D, top three panels). Secretion of apoE was not affected by apoC-III expression and was used as loading controls in immunoblot analysis (Fig. 1D, bottom panel). Among all apoC-III cell lines examined, increased secretion efficiency of [3H]TAG over controls was invariably observed (Fig. 1E), and the enhancement was positively correlated to apoC-III expression levels. Increased secretion efficiency of TAG from apoC-III-expressing cells was also observed when the fatty acyl moieties of TAG were labeled with [14C]oleate (data not shown). Expression of apoC-III also resulted in increased secretion efficiency of [3H]glycerol-labeled PC (Fig. 1E). As was the case in transiently transfected cells, apoC-III expression had no effects on TAG secretion when the stable cell lines were cultured in the absence of exogenous oleate (Fig. 1F). However, the effect of apoC-III expression on lipid secretion (both TAG and PC) was clearly manifested when the media were supplemented with oleate ranging from 0.1–1.2 mM (Fig. 1F). Additional experiments with the stable cell lines showed that the increased TAG secretion was not attributable to reduced reuptake of newly secreted VLDL because metabolic labeling experiments with the cells cultured in the presence of heparinase had no effect on [3H]TAG accumulation in the media (data not shown). We next determined the effect of apoC-III knockdown on TAG secretion using HepG2 cells, a human hepatoblastoma cell line that expresses endogenous apoC-III yet lacks the ability to secrete VLDL even under lipid-rich conditions (34). Treatment of HepG2 cells with apoC-III-specific siRNA effectively decreased the expression of endogenous apoC-III but had little effect on that of apoE or apoB (see supplementary Fig. IA). However, secretion of [3H]TAG or [3H]PC from the siRNA-treated HepG2 cells was not affected by apoC-III downregulation (see supplementary Fig. IB). Thus, the effect of apoC-III expression on TAG secretion was not demonstrated with HepG2 cells that are unable to synthesize VLDL. These results gave the first indication that expression of apoC-III in McA-RH7777 cells could augment TAG secretion in the presence of exogenous oleate, and this effect was manifested in an APOC3 gene-dose- dependent manner.
Expression of apoC-III in McA-RH7777 cells enhances secretion apoB-100 and apoB-48 as VLDL
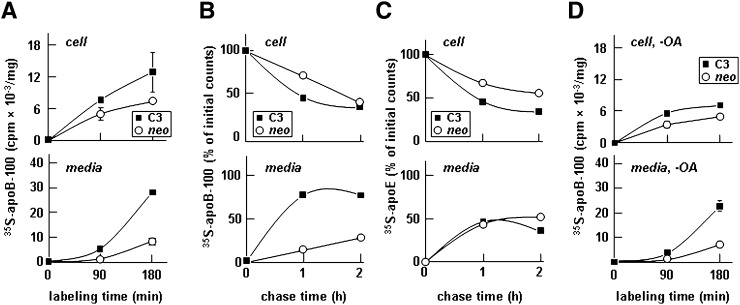
We next determined the effect of apoC-III expression on the secretion of apoB-100 from McA-RH7777 cells stably expressing human apoC-III. Metabolic labeling experiments with [35S]methionine in the presence of 0.4 mM oleate showed that the expression of apoC-III markedly stimulated [35S]apoB-100 secretion into the media, but it had only a marginal effect on cell-associated [35S]apoB-100 (Fig. 2A). Pulse-chase experiments further confirmed that the effect of apoC-III expression promoted [35S]apoB-100 secretion. Thus, the secretion efficiency of newly synthesized [35S]apoB-100 was increased from 25% in controls (neo) to >75% in apoC-III-expressing cells (Fig. 2B). Secretion efficiency of apoE was not affected by apoC-III expression (Fig. 2C). Notably, secretion of [35S]apoB-100 from apoC-III-expressing cells was increased even when the metabolic labeling experiment was conducted in the absence of exogenous oleate (Fig. 2D). This result suggests that apoC-III expression not only promote TAG- rich lipoprotein secretion from transfected McA-RH7777 cells but also increased secretion of lipid-poor apoB-100-containing lipoproteins.
Fig. 2.
Expression of apoC-III-enhanced secretion of [35S]apolipoprotein B-100 (apoB-100). A: Control (neo) and apoC-III-expressing cells (C3) were continuously labeled with [35S]methionine/cysteine for 90 or 180 min in DMEM containing 20% FBS + 0.4 mM oleate. B and C: Cells were pulse labeled for 30 min and “chased” for up to 2 h in DMEM containing 20% FBS + 0.4 mM oleic acid (OA). D: Cells were continuously labeled for 90 or 180 min in the media without OA. At the indicated times, [35S]apoB-100 (A, B, and D) and [35S]apoE (C) were recovered from the cells and media by immunoprecipitation, resolved by SDS-PAGE, and subjected to fluorography. Radioactivity associated with [35S]apoB and [35S]apoE in cells and media was quantified. For the continuous labeling experiments (A and D), data are presented as cpm/mg cell protein. For the pulse-chase experiments (B and C), data are presented as percentage of initial counts, which are the counts associated with cell [35S]apoB-100 or cell [35S]apoE at the end of a 30 min pulse. The experiment was repeated, and similar results were obtained.
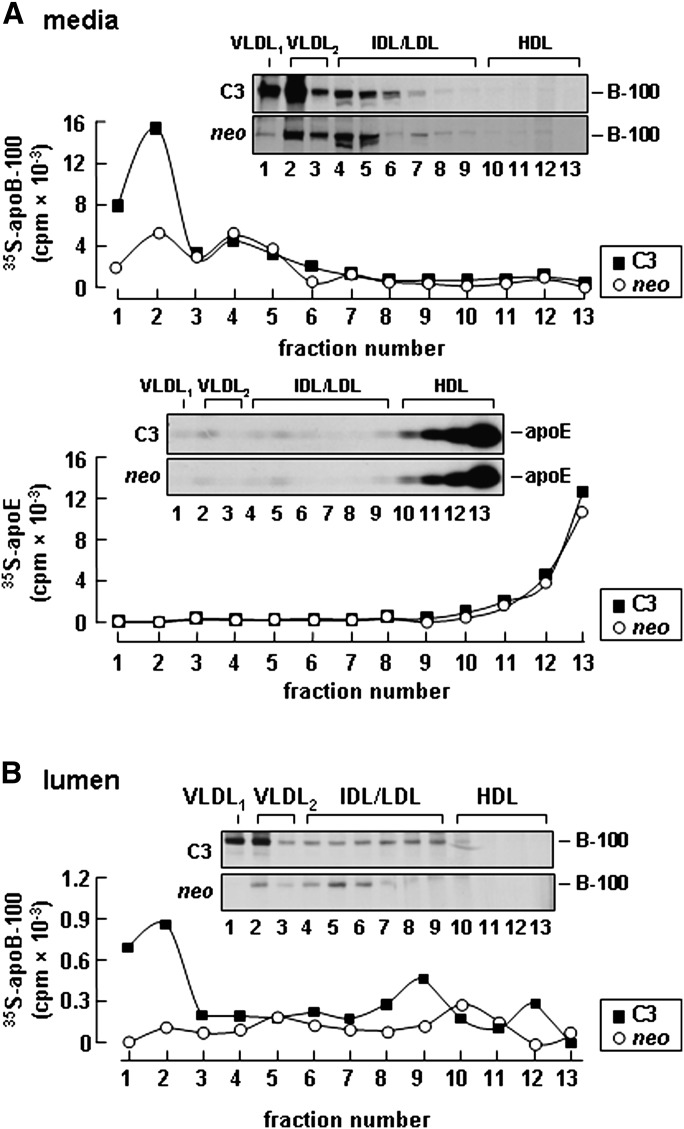
Fractionation of secreted lipoprotein in the media revealed that the increased [35S]apoB-100 secreted from apoC-III expressing cells was predominantly associated with VLDL1 and VLDL2 (Fig. 3A, top panel). Density distribution of apoE among secreted lipoproteins was comparable between control (neo) and apoC-III-expressing cells (Fig. 3A, bottom panel), suggesting that the effect of apoC-III expression is specific to apoB-100 containing VLDL. Inclusion of heparin in the labeling media had no effect on [35S]apoB-100 accumulation in the media (data not shown), indicating that uptake of newly secreted apoB-containing lipoproteins by McA-RH7777 cells was negligible. To determine whether or not the increased VLDL secretion from the apoC-III-expressing cells was a consequence of increased VLDL assembly, we examined association of apoB-100 with lipoproteins within the microsome lumen by continuous labeling for 60 min (24). In control cells, [35S]apoB-100 was found predominately in VLDL2 and IDL/LDL fractions (Fig. 3B), whereas in apoC-III- expressing cells, the majority of [35S]apoB-100 was associated with VLDL1 and VLDL2 fractions (Fig. 3B). These data indicate that apoC-III expression promotes assembly of VLDL containing apoB-100.
Fig. 3.
Expression of apoC-III increased [35S]apolipoprotein B-100 (apoB-100) secretion as VLDL1 and VLDL2. A: Representative fluorograms and quantification of [35S]apoB-100 (top panel) and [35S]apoE (bottom panel) distribution among medium lipoproteins. Control (neo) or apoC-III expressing cells (C3) were labeled with [35S]methionine/cysteine for 3 h in the presence of 20% serum + 0.4 mM oleate. After labeling, the media were subjected to cumulative rate flotation ultracentrifugation. The apoB-100 and apoE proteins associated with each fraction were recovered by immunoprecipitation, resolved by SDS-PAGE, and visualized by fluorography. Radioactivity associated with [35S]apoB-100 and apoE was quantified by scintillation counting. B: Representative fluorograms and quantification of [35S]apoB-100 distribution among lipoproteins within the microsomal lumen. The cells were labeled with [35S]methionine/cysteine for 60 min in the presence of 20% serum + 0.4 mM oleate. The content of microsomal lumen was fractionated by ultracentrifugation, [35S]apoB-100 in each fraction was analyzed by SDS-PAGE, and the associated radioactivity was quantified as described above. The experiment was repeated, and similar results were obtained.
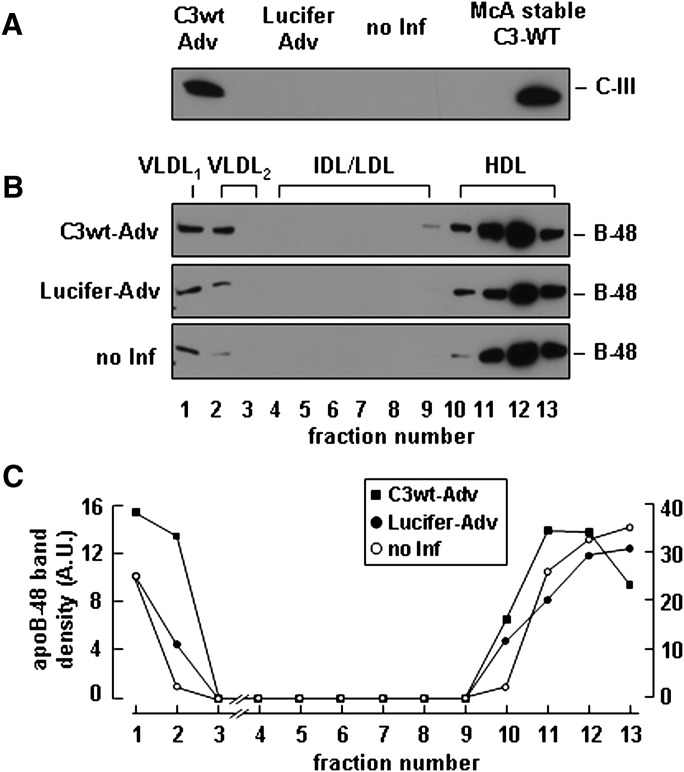
We also determined the effect of apoC-III expression on the secretion of VLDL containing apoB-48. Because McA-RH7777 cells produce low levels of endogenous apoB-48, we used a previously developed stable cell line overexpressing human apoB-48 (35). We infected the apoB-48 expressing cells with a adenovirus vector encoding apoC-III (C3wt-Adv) and achieved an apoC-III expression level comparable to that seen in stable cell lines (Fig. 4A). Under lipid-rich conditions, the virus-mediated apoC-III expression increased secretion of apoB-48 as VLDL1 and VLDL2 compared with controls (i.e., cells transfected with virus encoding luciferase or noninfected cells) (Fig. 4B). Semiquantitative analysis by scanning densitometry showed a 2-fold increase in VLDL-associated B48 from apoC-III expressing cells, whereas secretion of HDL associated B48 was unchanged (Fig. 4C). These data together suggest that expression of apoC-III in McA-RH7777 cells promotes assembly and secretion of TAG-rich VLDL containing either apoB-100 or apoB-48 under lipid-rich conditions.
Fig. 4.
Adenovirus-mediated apoC-III expression stimulated apoB-48 secretion as VLDL. McA-RH7777 cells stably expressing human apoB-48 ( 35 ) were infected with adenovirus vectors encoding C3-wt or luciferase genes for 4 h and cultured for additional 48 h in DMEM supplemented with 20% serum. A: 48 h after infection, the cells were cultured in the same media + 0.4 mM OA for 8 h, and the conditioned media were collected to determine apoC-III secretion. B: The conditioned media were fractioned by cumulative rate flotation, and the apoB-48 proteins were recovered from each fraction by immunoprecipitation, resolved by SDS-PAGE, and detected by immunoblotting. C: Scanning densitometry of apo-B48 bands. (Note the scale for VLDL fractions is enlarged.)
Codistribution of apoC-III and TAG within the microsomal lumen
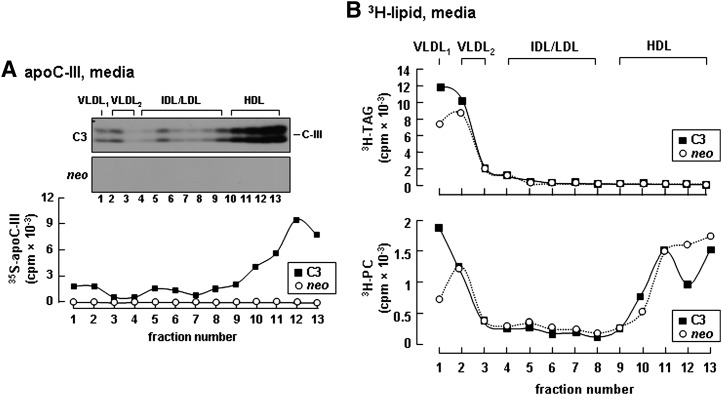
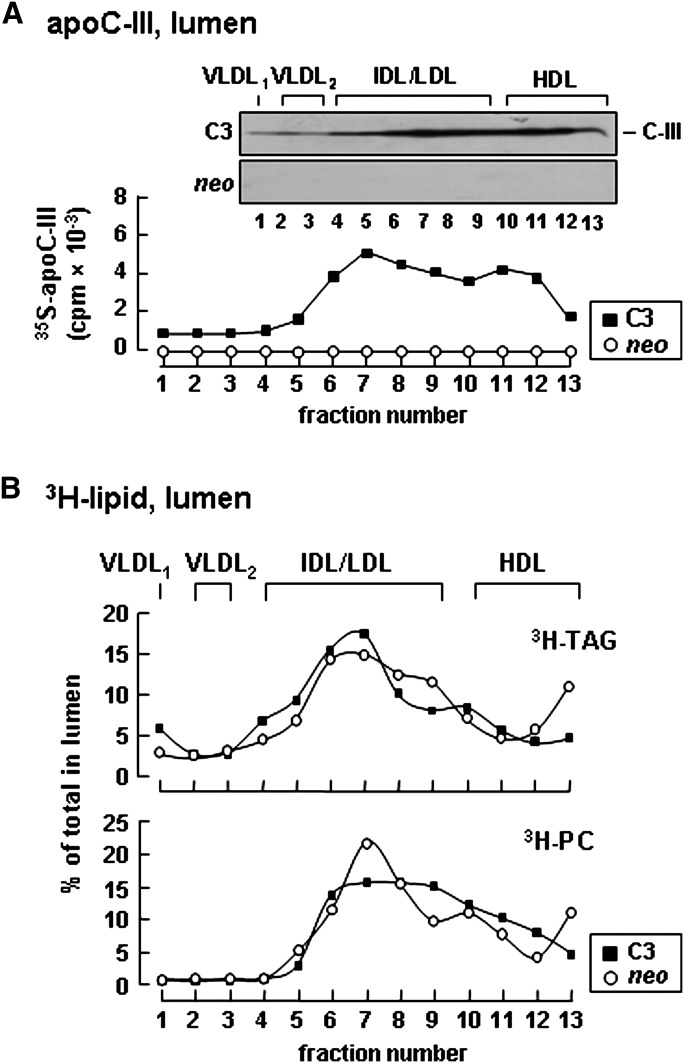
To gain an insight into mechanisms by which apoC-III expression exerts an intracellular role in enhancing VLDL assembly/secretion, we determined distribution of apoC-III among lipoprotein fractions in the media and within the microsomal lumen. In the conditioned media of apoC-III-expressing cells, although the majority of apoC-III was associated with HDL-like particles, a noticeable amount of apoC-III was bound to VLDL1 and VLDL2 (Fig. 5A, fractions 1 and 2). The distribution of apoC-III in VLDL fractions coincided with the distribution of increased TAG/PC in these fractions (Fig. 5B), suggesting that apoC-III has the propensity to associate with the secreted TAG-rich VLDL particles. Examination of apoC-III distribution within the microsomal lumen showed a profile markedly differing from that of medium lipoproteins. Within the microsomal lumen, the majority of [35S]apoC-III was found in fractions of IDL/LDL density, and only a trace amount of [35S]apoC-III presented in VLDL fractions (Fig. 6A). Of note, the microsomal distribution of [35S]apoC-III coincided with that of [3H]TAG, which also showed a prominent enrichment in fractions with IDL/LDL density (Fig. 6B). Similar results were reported previously where >70% of luminal TAG was not associated with apoB in mouse hepatocytes (36). The fact that the majority of luminal [35S]apoC-III and [3H]TAG was not associated with apoB-containing lipoproteins suggests that apoC-III might be associated with TAG-containing particles of IDL/LDL density within the microsomal lumen.
Fig. 5.
Expression of apoC-III increased secretion as VLDL containing [35S]apoC-III and [3H]TAG. A: Control (neo) or apoC-III-expressing cells (C3) were labeled with either [35S]methionine/cysteine for 3 h or [3H]glycerol for 4 h in DMEM containing 20% serum + 0.4 mM oleate. The conditioned media were fractionated by rate flotation ultracentrifugation, and metabolic labeled proteins and lipids were recovered from each fraction. A: Distribution of [35S]apoC-III among the fractionated lipoproteins. B: Distribution of [3H]TAG (top panel) and [3H]PC (bottom panel). The experiment was repeated, and similar results were obtained.
Fig. 6.
Distribution of apoC-III and TAG among lipoproteins within the microsomal lumen. Control (neo) or apoC-III-expressing cells (C3) were labeled with [35S]methionine/cysteine for 60 min or with [3H]glycerol for 60 min in DMEM containing 20% serum + 0.4 mM oleate. The content of microsomal lumen was fractionated by ultracentrifugation as described in Fig. 3B. A: Distribution of [35S]apoC-III among lipoproteins within the microsomal lumen. B: Distribution of [3H]TAG (top panel) and [3H]PC (bottom panel). The [3H]TAG and [3H]PC in each fraction is expressed as percentage of total lumenal [3H]TAG or [3H]PC.
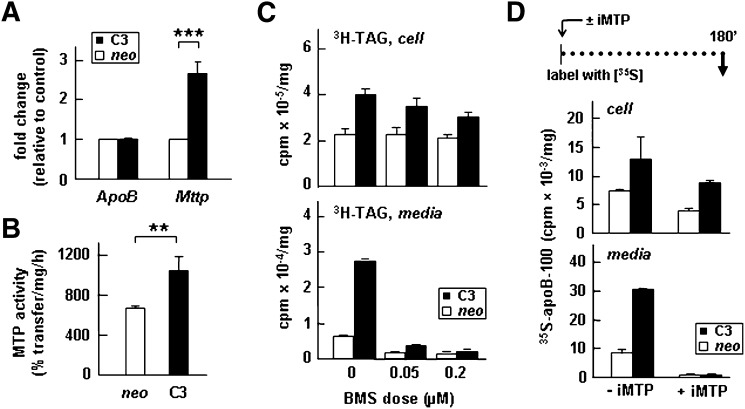
It has been shown previously that partitioning of TAG into microsomal lumen to form lipid droplets requires the normal activity of MTP (23, 24), whereas the addition of core lipid to form mature TAG-rich VLDL is independent of MTP activity (37). We thus determined whether or not apoC-III expression would affect the expression and activity of MTP. Quantitative analysis of mRNA levels using real-time RT-PCR showed that expression of apoC-III resulted in a significant increase (∼2.5-fold) in the Mttp mRNA but exerted no effects on the Apob mRNA (Fig. 7A). Determination of in vitro MTP activity using cell lysate showed that apoC-III expression increased the activity by 30% (P < 0.01) (Fig. 7B). We next determined whether or not inactivation of MTP would abolish the stimulatory effect of apoC-III expression on VLDL secretion. We used the MTP inhibitor BMS-197636 that, as shown previously (24) and confirmed here (Fig. 7C, bottom panel), could effectively inhibit secretion of [3H]TAG when the lipid- labeling media contained 0.05 or 0.2 μM of the inhibitor. Inhibition of MTP activity under these conditions had little effect on cell-associated [3H]TAG (Fig. 7C, top panel). Likewise, inclusion of the MTP inhibitor in the [35S]methionine-labeling media also completely inhibited secretion of [35S]apoB-100 from control and apoC-III-expressing cells (Fig. 7D, bottom panel) with relatively little effect on cell-associated [35S]apoB-100 (Fig. 7D, top panel). These data suggest that apoC-III expression cannot substitute the requirement of MTP activity for VLDL secretion.
Fig. 7.
Expression of apoC-III increased the expression of Mttp gene and MTP activity. A: The relative mRNA concentrations of apoB and MTP (with respect to 18s rRNA) were quantified by real-time RT-PCR. The data are presented as fold changes between apoC-III-expressing cells (C3) and control (neo). B: MTP activity assay. Data are the average of triplicate assays using cell lysate obtained from three different stable apoC-III-expressing clones (C3) or neo controls. ***P < 0.001; **P < 0.01 (Student's t-test of C3 versus control). Error bars indicate ± SD (n = 3). C: Cells were labeled with [3H]glycerol for 3 h as in Fig. 1E in the absence or presence of 0.05 or 0.2 µM BMS-197636 (BMS). At the end of labeling, radioactivity associated with [3H]TAG in media and cells was quantified as described in Fig. 1A, B. D: Control (neo) and apoC-III-expressing cells (C3) were continuously labeled with [35S]methionine/cysteine for 180 min in the presence or absence of 0.2 µM BMS-197636 (± iMTP). The labeling media were supplemented with 0.4 mM OA. The apoB-100 protein was recovered from the cell (top panel) and media (bottom panel) by immunoprecipitation, resolved by SDS-PAGE, and followed by fluorography. Radioactivity associated with [35S]apoB-100 was quantified by scintillation counting.
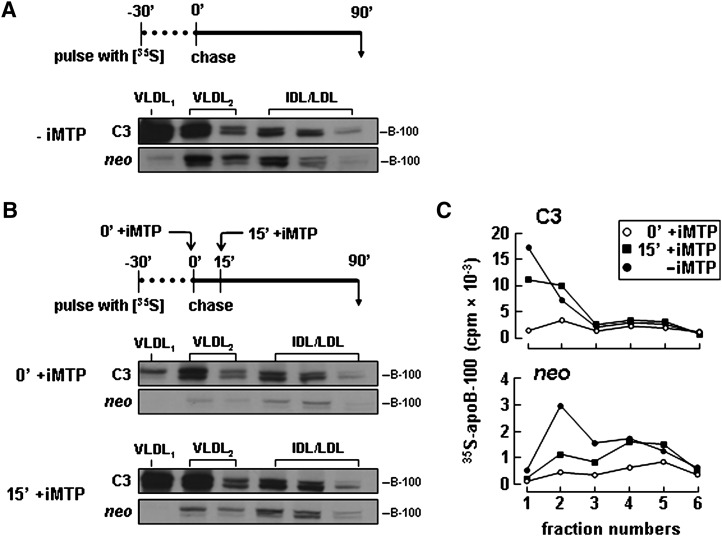
To determine whether or not apoC-III expression may play a role in the recruitment of bulk TAG into VLDL during the late, MTP-independent assembly stage (commonly known as the second-step lipidation), we designed a pulse-chase protocol (30 min pulse, 90 min chase) in which the MTP inhibitor was either introduced immediately at the beginning of chase or with a 15-min delay (Fig. 8). [Since our previous pulse-chase experiments with McA-RH7777 cells have shown that the second-step lipidation occurred ∼15 min after apoB-100 translation (24) and the resulting VLDL1 was detected only in postER compartments (38), this delayed MTP inhibition would allow the second-step lipidation to reach completion.] In the absence of MTP inhibitor, the apoC-III-expressing cells, as expected, secreted increased amount of [35S]apoB-100 as VLDL1 and VLDL2 compared with control (Fig. 8A). When MTP inhibitor was introduced immediately at the end of 30-min pulse, secretion of [35S]apoB-100 as VLDL1 was markedly impaired from both control and apoC-III-expressing cells (Fig. 8B, panels marked 0′ +iMTP). However, when the MTP inhibitor was added to the chase media with a 15 min delay, secretion of [35S]apoB-100 as VLDL1 from apoC-III-expressing cells was nearly normal, whereas that from the control cells was still impaired (Fig. 8B, bottom panels marked 15′ +iMTP). Quantitative analysis clearly indicated that secretion of [35S]apoB-100 as VLDL1 and VLDL2 from apoC-III-expressing cells was relatively insensitive to MTP inhibition (Fig. 8C). Since recruitment of bulk TAG into VLDL during the second-step lipidation is relatively independent of the MTP activity, the current apoB-100 pulse-chase data suggest that apoC-III expression may promote VLDL assembly during the late, MTP-independent stage.
Fig. 8.
Secretion of apoB-100 as VLDL1 from apoC-III-expressing cells was less sensitive to MTP inactivation. A: Cells were pulse labeled for 30 min with [35S]methionine/cysteine in media supplemented with 0.4 mM OA in the absence of MTP inhibitor (-iMTP) and then “chased” for 90 min with DMEM supplemented with 20% FBS and 0.4 mM OA in the absence of MTP inhibitor (-iMTP). B: Cells were pulse-labeled for 30 min as in A. During chase, the MTP inhibitor (+ iMTP) was included either at the beginning of chase (0′+ iMTP) or 15 min after (15′+ iMTP) the start of chase. The media were collected at the end of 90 min chase and subjected to cumulative rate floatation centrifugation. The apoB-100 protein associated with each fraction was recovered by immunoprecipitation, resolved by SDS-PAGE, and visualized by fluorography. C: Plot of the radioactivity associated with [35S]apoB-100 shown in A and B was quantified by scintillation counting. (Note that only fractions 1 through 6 that contained apoB-100 are presented.)
The function of apoC-III in enhancing VLDL secretion is abolished by Ala23Thr mutation
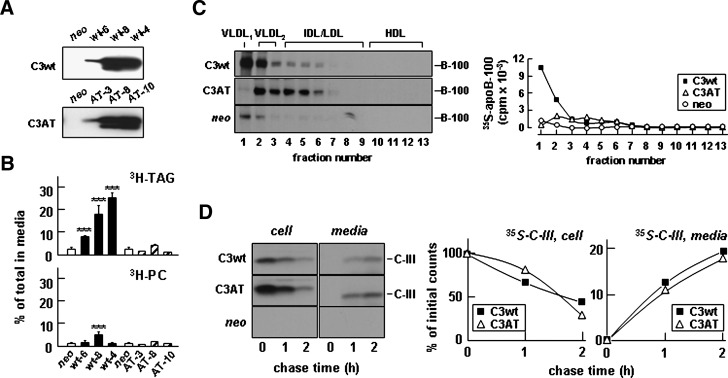
To determine further the involvement of apoC-III in hepatic VLDL assembly/secretion, we examined the effect of a naturally occurring apoC-III mutation Ala23Thr that was discovered in some Mayan Indians. Heterozygote of Ala23Thr carriers had plasma TAG concentrations 25–48% of normal (26). Stable McA-RH7777 cell lines expressing the Ala23Thr mutant were generated, of which the levels of Ala23Thr expression were comparable to that of wild-type apoC-III (Fig. 9A). Lipid metabolic labeling experiments showed that in contrast to that of apoC-III wild type, expression of the Ala23Thr mutant had no effect on [3H]TAG secretion from any of the stable clones of transfected cells (Fig. 9B, top panel). Secretion of [3H]PC was not affected by expression of the Ala23Thr mutant (Fig. 9B, bottom panel). The Ala23Thr mutation also abolished the enhanced secretion of apoB-100 in association with VLDL1 seen in cells expressing apoC-III (Fig. 9C). We also determined the effect of Ala23Thr mutant expression on apoB48-VLDL secretion with McA-RH7777 cells stably expressing human apoB-48. Infecting the cells with A23T-Adv showed that the Ala23Thr mutation also compromised its ability to stimulate apoB48-VLDL secretion compared with apoC-III wild type (data not shown). Thus, unlike in apoC-III wild-type-expressing cells where the secreted [35S]apoB-100 was found in VLDL1 and VLDL2 fractions, in the Ala23Thr mutant cells, the secreted [35S]apoB-100 was associated with VLDL2 and dense IDL/LDL fractions (Fig. 9C). The MTP activity in Ala23Thr mutant cells was increased slightly (by 20%) compared with neo control cells (data not shown). These results provide additional evidence that expression of functional apoC-III can promote the VLDL assembly/ secretion, and the Ala23Thr mutation abolishes the apoC-III function. The lack of an effect of Ala23Thr mutant on TAG secretion was not due to impaired secretion of the apoC-III mutant because pulse-chase experiments showed that the secretion efficiency of Ala23Thr was identical to that of apoC-III (Fig. 9D).
Fig. 9.
The Ala23Thr mutation compromised the ability of apoC-III to stimulate VLDL secretion. A: Immunoblots of apoC-III (C3wt) or Ala23Thr mutant (C3AT) secreted from different stable cell lines. B: Secretion efficiency of [3H]TAG (top panel) and [3H]PC (bottom panel). The experiments were performed as described in the legend of Fig. 1E. C: Representative fluorograms (left panel) and quantification of [35S]apoB-100 (right panel) associated with fractionated medium lipoproteins. The experiment was performed essentially the same as described in the legend of Fig. 3A, except cells expressing C3wt or the C3AT mutant were used. D: Secretion efficiency of apoC-III. The cells were pulse-labeled with [35S]methionine/cysteine for 30 min and “chased” for up to 2 h. [35S]apoC-III was recovered from cell and media, respectively, resolved by SDS-PAGE, and visualized by fluorography. Left panel, fluorograms of cell and medium-associated [35S]apoC-III. Right panel, radioactivity associated with [35S]apoC-III was quantified by scintillation counting. Data are expressed as percentage of initial [35S]apoC-III associated with cell or secreted into media during chase. ***P < 0.001 (Student's t-test of C3wt versus control). Error bars indicate ± SD (n = 3).
DISCUSSION
Variants within the APOA5-APOA4-APOC3-APOA1 cluster are strongly associated with plasma TAG concentration (39), an independent risk factor for cardiovascular disease in humans. An increase in apoC-III levels is closely correlated with the development of hypertriglyceridemia, which has been associated with the ability of apoC-III to inhibit LPL activity and to interfere with receptor-mediated clearance of TAG-rich lipoproteins. The in vitro cell culture studies presented herein, however, have revealed a novel intracellular function of apoC-III in enhancing VLDL assembly/secretion under lipid-rich conditions and thus provided an alternative explanation for the linkage between apoC-III levels and hypertriglyceridemia. Expression of apoC-III not only stimulated the assembly and secretion of TAG-rich VLDL particles under lipid-rich conditions (Fig. 3), but also increased apoB-100 secretion under lipid-poor conditions (Fig. 2). Pulse-chase experiments in conjunction with MTP inactivation suggest that apoC-III may play a role in the late, MTP-independent stage of VLDL assembly (Fig. 8). Consequently, apoC-III expression leads to an increase in the number as well as the TAG content of VLDL particles. The effect of apoC-III is specific because expression of apoC-I or apoC-II, two other exchangeable apolipoproteins rich in amphipathic α-helices, did not have any effect on TAG secretion from McA-RH7777 cells (data not shown). Moreover, experiments with the apoC-III mutant Ala23Thr indicates that the N-terminal region of apoC-III is critical for its function in promoting VLDL assembly/secretion and suggests a causative effect of apoC-III on hypotriglyceridemia associated with the heterozygous carriers of the Ala23Thr mutation (26).
How does the increased apoC-III expression stimulate VLDL assembly/secretion? Data derived from this work have suggested that apoC-III expression exerts little effects on synthesis of apoB because the levels of Apob mRNA (Fig. 7A) or apoB-100 synthesis (Fig. 2A) were relatively unchanged by apoC-III expression. Rather, two pieces of information have suggested that apoC-III expression may exert its effects on lipid substrate utilization for VLDL assembly/secretion. First, apoC-III expression resulted in increased mRNA levels and activity of MTP (Fig. 7A, B), a known factor that facilitates TAG partitioning into the microsomal lumen and promotes TAG assembly with apoB-100 (23, 24). Second, examination of apoC-III distribution within the microsomal lumen revealed that the majority of apoC-III was distributed in IDL/LDL-like fractions (Fig. 5A) that were rich in TAG (Fig. 5B) yet devoid of apoB-100 (Fig. 3B). It is tempting to speculate that these apoC-III-containing entities may represent TAG substrates for VLDL assembly. Notably, the IDL/LDL-like entities rich in TAG were also present in neo control cells (Fig. 5B), indicating that apoC-III is not essential for the formation of these lipid precursor particles. However, the presence of apoC-III on these TAG-rich particles within the microsomal lumen may render them a better substrate for TAG-rich VLDL1 assembly (Fig. 8B), a late process of core lipid addition independent of MTP activity (37). Our coimmunoprecipitation experiments did not detect physical interaction between apoC-III and MTP (data not shown). Mechanisms responsible for enhanced VLDL assembly/secretion upon apoC-III expression remain to be defined.
The molecular nature of apoC-III interaction with the putative TAG-rich particles within the microsomal lumen is unclear. Our present studies with the apoC-III Ala23Thr mutant have provided useful information on the structural requirements for apoC-III function in enhancing VLDL assembly/secretion. Recent NMR analysis of apoC-III in complex with SDS micelles has suggested that apoC-III assumes a curved six-amphipathic-helix (approximately 10 amino acids in each helix) structure with several flexible linker regions (40). The residue Ala-23 is located on the hydrophobic side of a conserved amphipathic α-helix region among mammalian apoC-III proteins (40); hence, it is expected that the Thr-for-Ala substitution will alter the overall hydrophobic moment of the α-helix. Previous studies with the bacterially expressed Ala23Thr proteins showed that the mutant exhibited a reduced affinity for dimyristoylphosphatidylcholine multilamellar vesicles (26). We found that Ala23Thr mutation completely abolished the stimulatory effect of apoC-III in stimulating secretion of VLDL-TAG and apoB-100 (Fig. 9B, C). Thus, the stimulatory effect on TAG secretion was specific to the structural motif of apoC-III, and the loss of function associated with Ala-to-Thr mutation reveals an important functional domain within the second amphipathic α-helix region of apoC-III in enhancing hepatic VLDL assembly/secretion.
Previous human studies showed that increased hepatic production of apoC-III is an important determinant in patients with hypertriglyceridemia (2), as well as subjects with high body weight and lower levels of insulin sensitivity (41). Using a multicompartmental model to trace the production and turnover of TAG-rich lipoproteins in 11 hypertriglyceridemic and normolipidemic subjects, Zheng et al. (3) showed that the increase in VLDL apoC-III levels was positively associated with an increase in VLDL production rather than with slow VLDL turnover. More importantly, hypertriglyceridemic subjects have greater production of apoC-III-containing VLDL as well as prolonged residence time of all particle types (3). These clinical data imply a possible role of apoC-III in promoting VLDL production, thus contributing to hypertriglyceridemia, in addition to its extracellular roles in inhibiting lipase activities and impairing VLDL clearance through receptor-mediated endocytosis. Additional studies with human subjects under different metabolically stressed conditions also showed a positive correlation between in vivo production rates of VLDL-associated apoC-III and VLDL-TAG (2, 3, 17–19). Together, these clinical studies reveal two important factors critical to the apoC-III- stimulated VLDL production: i) enhanced hepatic TAG availability, possibly through elevated FFA influx and/or increased hepatic lipogenesis; and ii) increased hepatic apoC-III production. In hypertriglyceridemia subjects, the apoC-III production (measured in total plasma or in VLDL) is at least 2- to 5-fold higher than that in normolipidemic subjects (2). Thus, the present in vitro study offers a mechanistic explanation for the link between apoC-III overexpression and increased VLDL production in the context of enhanced hepatic TAG availability.
Results of this study, together with the above clinical data, also suggest that certain features of metabolic stress (such as hepatic TAG overload and apoC-III overexpression) must be taken into consideration in assessing the functional role of apoC-III in hepatic VLDL secretion in mouse models. Currently, in the literature derived from studies using transgenic mouse models, there are inconsistent results with respect to apoC-III deficiencies versus apoC-III overexpression on hepatic VLDL-TAG production. Under the chow diet condition, apoc3−/− mice showed no difference in hepatic VLDL production compared with control littermates (16), whereas the APOC3 high-expressing transgenic mice displayed 2-fold increase in VLDL secretion (7). In primary hepatocytes isolated from the APOC3 high-expressing mice, secretion of TAG was normal under oleate-free condition and was increased when the cells were cultured with exogenous oleate (7). Few studies with these mouse models were performed under metabolically stressed conditions. In one study with apoc3−/− mice, 2 weeks of high-fat feeding resulted in no difference in VLDL production (measured after Triton WR1339 injection), yet the mice developed obesity and increased body weight (by 22%) (42). It would be of interest to determine if APOC3-overexpressing mice are protected from diet-induced obesity (42) yet prone to develop hypertriglyceridemia through overproduction of TAG-rich VLDL. Recent kinetics studies in human subjects demonstrate that an acute elevation of plasma FFA stimulates the production of apoC-III and its association with TAG-rich lipoproteins (43). Thus, it is likely that increased FFA flux into hepatocytes in certain metabolically stressed states (such as insulin resistance) not only increased hepatic TAG synthesis but also aggravated apoC-III expression, thus enhancing VLDL assembly/secretion. This study thus suggests a need for reevaluating the role of apoC-III in the development of hypertriglyceridemia by choosing appropriate mouse models that more closely resemble aberrant metabolic conditions in humans. Alternatively, the contribution of VLDL secretion versus catabolism toward hypotriglyceridemic effect of apoC3 deficiency in human (6) should also be carefully reexamined. Unraveling the intracellular role of apoC-III in hepatic VLDL assembly/secretion will provide new understanding of the strong influence of the APOA5-APOA4-APOC3-APOA1 gene locus on plasma TAG concentrations and risk of coronary artery disease.
Supplementary Material
Acknowledgments
We are grateful to Vincent Ngo for excellent technical assistance and Michelle Bamji-Mirza for a critical reading of the manuscript.
Footnotes
Abbreviations:
- apo
- apolipoprotein
- ER
- endoplasmic reticulum
- MTP
- microsomal triglyceride-transfer protein
- OA
- oleic acid
- PC
- phosphatidylcholine
- siRNA
- short interfering RNA
- TAG
- triacylglycerol
This work is supported by Canadian Institutes of Health Research Grant MT-15486. Z.Y. was a recipient of the Career Investigator award from the Heart and Stroke Foundation of Ontario.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of three figures.
REFERENCES
- 1.Jong M. C., Hofker M. H., Havekes L. M. 1999. Role of ApoCs in lipoprotein metabolism: functional differences between ApoC1, ApoC2, and ApoC3. Arterioscler. Thromb. Vasc. Biol. 19: 472–484. [DOI] [PubMed] [Google Scholar]
- 2.Cohn J. S., Tremblay M., Batal R., Jacques H., Rodriguez C., Steiner G., Mamer O., Davignon J. 2004. Increased apoC-III production is a characteristic feature of patients with hypertriglyceridemia. Atherosclerosis. 177: 137–145. [DOI] [PubMed] [Google Scholar]
- 3.Zheng C., Khoo C., Ikewaki K., Sacks F. M. 2007. Rapid turnover of apolipoprotein C–III-containing triglyceride-rich lipoproteins contributing to the formation of LDL subfractions. J. Lipid Res. 48: 1190–1203. [DOI] [PubMed] [Google Scholar]
- 4.Karathanasis S. K., Norum R. A., Zannis V. I., Breslow J. L. 1983. An inherited polymorphism in the human apolipoprotein A-I gene locus related to the development of atherosclerosis. Nature. 301: 718–720. [DOI] [PubMed] [Google Scholar]
- 5.Karathanasis S. K., Ferris E., Haddad I. A. 1987. DNA inversion within the apolipoproteins AI/CIII/AIV-encoding gene cluster of certain patients with premature atherosclerosis. Proc. Natl. Acad. Sci. USA. 84: 7198–7202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pollin T. I., Damcott C. M., Shen H., Ott S. H., Shelton J., Horenstein R. B., Post W., McLenithan J. C., Bielak L. F., Peyser P. A., et al. 2008. A null mutation in human APOC3 confers a favorable plasma lipid profile and apparent cardioprotection. Science. 322: 1702–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aalto-Setala K., Fisher E. A., Chen X., Chajek-Shaul T., Hayek T., Zechner R., Walsh A., Ramakrishnan R., Ginsberg H. N., Breslow J. L. 1992. Mechanism of hypertriglyceridemia in human apolipoprotein (apo) CIII transgenic mice. Diminished very low density lipoprotein fractional catabolic rate associated with increased apo CIII and reduced apo E on the particles. J. Clin. Invest. 90: 1889–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Silva H. V., Lauer S. J., Wang J., Simonet W. S., Weisgraber K. H., Mahley R. W., Taylor J. M. 1994. Overexpression of human apolipoprotein C–III in transgenic mice results in an accumulation of apolipoprotein B48 remnants that is corrected by excess apolipoprotein E. J. Biol. Chem. 269: 2324–2335. [PubMed] [Google Scholar]
- 9.Maeda N., Li H., Lee D., Oliver P., Quarfordt S. H., Osada J. 1994. Targeted disruption of the apolipoprotein C–III gene in mice results in hypotriglyceridemia and protection from postprandial hypertriglyceridemia. J. Biol. Chem. 269: 23610–23616. [PubMed] [Google Scholar]
- 10.Ginsberg H. N., Le N. A., Goldberg I. J., Gibson J. C., Rubinstein A., Wang-Iverson P., Norum R., Brown W. V. 1986. Apolipoprotein B metabolism in subjects with deficiency of apolipoproteins CIII and AI. Evidence that apolipoprotein CIII inhibits catabolism of triglyceride-rich lipoproteins by lipoprotein lipase in vivo. J. Clin. Invest. 78: 1287–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McConathy W. J., Gesquiere J. C., Bass H., Tartar A., Fruchart J. C., Wang C. S. 1992. Inhibition of lipoprotein lipase activity by synthetic peptides of apolipoprotein C–III. J. Lipid Res. 33: 995–1003. [PubMed] [Google Scholar]
- 12.Aalto-Setala K., Weinstock P. H., Bisgaier C. L., Wu L., Smith J. D., Breslow J. L. 1996. Further characterization of the metabolic properties of triglyceride-rich lipoproteins from human and mouse apoC-III transgenic mice. J. Lipid Res. 37: 1802–1811. [PubMed] [Google Scholar]
- 13.Sehayek E., Eisenberg S. 1991. Mechanisms of inhibition by apolipoprotein C of apolipoprotein E-dependent cellular metabolism of human triglyceride-rich lipoproteins through the low density lipoprotein receptor pathway. J. Biol. Chem. 266: 18259–18267. [PubMed] [Google Scholar]
- 14.Fredenrich A., Giroux L. M., Tremblay M., Krimbou L., Davignon J., Cohn J. S. 1997. Plasma lipoprotein distribution of apoC-III in normolipidemic and hypertriglyceridemic subjects: comparison of the apoC-III to apoE ratio in different lipoprotein fractions. J. Lipid Res. 38: 1421–1432. [PubMed] [Google Scholar]
- 15.Schonfeld G., George P. K., Miller J., Reilly P., Witztum J. 1979. Apolipoprotein C–II and C–III levels in hyperlipoproteinemia. Metabolism. 28: 1001–1010. [DOI] [PubMed] [Google Scholar]
- 16.Jong M. C., Rensen P. C., Dahlmans V. E., van der Boom H., van Berkel T. J., Havekes L. M. 2001. Apolipoprotein C–III deficiency accelerates triglyceride hydrolysis by lipoprotein lipase in wild-type and apoE knockout mice. J. Lipid Res. 42: 1578–1585. [PubMed] [Google Scholar]
- 17.Batal R., Tremblay M., Barrett P. H., Jacques H., Fredenrich A., Mamer O., Davignon J., Cohn J. S. 2000. Plasma kinetics of apoC-III and apoE in normolipidemic and hypertriglyceridemic subjects. J. Lipid Res. 41: 706–718. [PubMed] [Google Scholar]
- 18.Chan D. C., Nguyen M. N., Watts G. F., Barrett P. H. 2008. Plasma apolipoprotein C–III transport in centrally obese men: associations with very low-density lipoprotein apolipoprotein B and high-density lipoprotein apolipoprotein A-I metabolism. J. Clin. Endocrinol. Metab. 93: 557–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zheng C., Khoo C., Furtado J., Ikewaki K., Sacks F. M. 2008. Dietary monounsaturated fat activates metabolic pathways for triglyceride-rich lipoproteins that involve apolipoproteins E and C–III. Am. J. Clin. Nutr. 88: 272–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chan D. C., Watts G. F., Nguyen M. N., Barrett P. H. 2006. Apolipoproteins C–III and A-V as predictors of very-low-density lipoprotein triglyceride and apolipoprotein B-100 kinetics. Arterioscler. Thromb. Vasc. Biol. 26: 590–596. [DOI] [PubMed] [Google Scholar]
- 21.Ginsberg H. N., Ramakrishnan R. 2008. Kinetic studies of the metabolism of rapidly exchangeable apolipoproteins may leave investigators and readers with exchangeable results. Arterioscler. Thromb. Vasc. Biol. 28: 1685–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fisher E. A., Ginsberg H. N. 2002. Complexity in the secretory pathway: the assembly and secretion of apolipoprotein B-containing lipoproteins. J. Biol. Chem. 277: 17377–17380. [DOI] [PubMed] [Google Scholar]
- 23.Raabe M., Veniant M. M., Sullivan M. A., Zlot C. H., Bjorkegren J., Nielsen L. B., Wong J. S., Hamilton R. L., Young S. G. 1999. Analysis of the role of microsomal triglyceride transfer protein in the liver of tissue-specific knockout mice. J. Clin. Invest. 103: 1287–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Y., Tran K., Yao Z. 1999. The activity of microsomal triglyceride transfer protein is essential for accumulation of triglyceride within microsomes in McA-RH7777 cells. A unified model for the assembly of very low density lipoproteins. J. Biol. Chem. 274: 27793–27800. [DOI] [PubMed] [Google Scholar]
- 25.Ginsberg H. N. 2000. Insulin resistance and cardiovascular disease. J. Clin. Invest. 106: 453–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu H., Labeur C., Xu C. F., Ferrell R., Lins L., Brasseur R., Rosseneu M., Weiss K. M., Humphries S. E., Talmud P. J. 2000. Characterization of the lipid-binding properties and lipoprotein lipase inhibition of a novel apolipoprotein C–III variant Ala23Thr. J. Lipid Res. 41: 1760–1771. [PubMed] [Google Scholar]
- 27.Chen C., Okayama H. 1987. High-efficiency transformation of mammalian cells by plasmid DNA. Mol. Cell. Biol. 7: 2745–2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vance D. E., Weinstein D. B., Steinberg D. 1984. Isolation and analysis of lipoproteins secreted by rat liver hepatocytes. Biochim. Biophys. Acta. 792: 39–47. [DOI] [PubMed] [Google Scholar]
- 29.Bou Khalil M., Sundaram M., Zhang H. Y., Links P. H., Raven J. F., Manmontri B., Sariahmetoglu M., Tran K., Reue K., Brindley D. N., et al. 2009. The level and compartmentalization of phosphatidate phosphatase-1 (lipin-1) control the assembly and secretion of hepatic VLDL. J. Lipid Res. 50: 47–58. [DOI] [PubMed] [Google Scholar]
- 30.Ross P. J., Parks R. J. 2006. Construction of first-generation adenoviral vectors. Gene Therapy Vectors: A Techniques Manual. Freidman T., Rossi John, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY: 149–165.. [Google Scholar]
- 31.Athar H., Iqbal J., Jiang X. C., Hussain M. M. 2004. A simple, rapid, and sensitive fluorescence assay for microsomal triglyceride transfer protein. J. Lipid Res. 45: 764–772. [DOI] [PubMed] [Google Scholar]
- 32.Chang T. Y., Limanek J. S., Chang C. C. 1981. A simple and efficient procedure for the rapid homogenization of cultured animal cells grown in monolayer. Anal. Biochem. 116: 298–302. [DOI] [PubMed] [Google Scholar]
- 33.Bradford M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72: 248–254. [DOI] [PubMed] [Google Scholar]
- 34.Tsai J., Qiu W., Kohen-Avramoglu R., Adeli K. 2007. MEK-ERK inhibition corrects the defect in VLDL assembly in HepG2 cells: potential role of ERK in VLDL-ApoB100 particle assembly. Arterioscler. Thromb. Vasc. Biol. 27: 211–218. [DOI] [PubMed] [Google Scholar]
- 35.Vukmirica J., Tran K., Liang X., Shan J., Yuan J., Miskie B. A., Hegele R. A., Resh M. D., Yao Z. 2003. Assembly and secretion of very low density lipoproteins containing apolipoprotein B48 in transfected McA-RH7777 cells. Lack of evidence that palmitoylation of apolipoprotein B48 is required for lipoprotein secretion. J. Biol. Chem. 278: 14153–14161. [DOI] [PubMed] [Google Scholar]
- 36.Wang H., Gilham D., Lehner R. 2007. Proteomic and lipid characterization of apolipoprotein B-free luminal lipid droplets from mouse liver microsomes: implications for very low density lipoprotein assembly. J. Biol. Chem. 282: 33218–33226. [DOI] [PubMed] [Google Scholar]
- 37.Pan M., Liang Js J. S., Fisher E. A., Ginsberg H. N. 2002. The late addition of core lipids to nascent apolipoprotein B100, resulting in the assembly and secretion of triglyceride-rich lipoproteins, is independent of both microsomal triglyceride transfer protein activity and new triglyceride synthesis. J. Biol. Chem. 277: 4413–4421. [DOI] [PubMed] [Google Scholar]
- 38.Tran K., Thorne-Tjomsland G., DeLong C. J., Cui Z., Shan J., Burton L., Jamieson J. C., Yao Z. 2002. Intracellular assembly of very low density lipoproteins containing apolipoprotein B100 in rat hepatoma McA-RH7777 cells. J. Biol. Chem. 277: 31187–31200. [DOI] [PubMed] [Google Scholar]
- 39.Willer C. J., Sanna S., Jackson A. U., Scuteri A., Bonnycastle L. L., Clarke R., Heath S. C., Timpson N. J., Najjar S. S., Stringham H. M., et al. 2008. Newly identified loci that influence lipid concentrations and risk of coronary artery disease. Nat. Genet. 40: 161–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gangabadage C. S., Zdunek J., Tessari M., Nilsson S., Olivecrona G., Wijmenga S. S. 2008. Structure and dynamics of human apolipoprotein CIII. J. Biol. Chem. 283: 17416–17427. [DOI] [PubMed] [Google Scholar]
- 41.Cohn J. S., Patterson B. W., Uffelman K. D., Davignon J., Steiner G. 2004. Rate of production of plasma and very-low-density lipoprotein (VLDL) apolipoprotein C–III is strongly related to the concentration and level of production of VLDL triglyceride in male subjects with different body weights and levels of insulin sensitivity. J. Clin. Endocrinol. Metab. 89: 3949–3955. [DOI] [PubMed] [Google Scholar]
- 42.Duivenvoorden I., Teusink B., Rensen P. C., Romijn J. A., Havekes L. M., Voshol P. J. 2005. Apolipoprotein C3 deficiency results in diet-induced obesity and aggravated insulin resistance in mice. Diabetes. 54: 664–671. [DOI] [PubMed] [Google Scholar]
- 43.Pavlic M., Valero R., Duez H., Xiao C., Szeto L., Patterson B. W., Lewis G. F. 2008. Triglyceride-rich lipoprotein-associated apolipoprotein C–III production is stimulated by plasma free fatty acids in humans. Arterioscler. Thromb. Vasc. Biol. 28: 1660– 1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.