Abstract
Cholesterol homeostasis, defined as the balance between absorption and synthesis, influences circulating cholesterol concentrations and subsequent coronary heart disease (CHD) risk. Statin therapy targets the rate-limiting enzyme in cholesterol biosynthesis and is efficacious in lowering CHD events and mortality. Nonetheless, CHD events still occur in some treated patients. To address differences in outcome during pravastatin therapy (40 mg/day), plasma markers of cholesterol synthesis (desmosterol, lathosterol) and fractional cholesterol absorption (campesterol, sitosterol) were measured, baseline and on treatment, in the Prospective Study of Pravastatin in the Elderly at Risk trial participants with (cases, n = 223) and without (controls, n = 257) a CHD event. Pravastatin therapy decreased plasma LDL-cholesterol and triglycerides and increased HDL-cholesterol concentrations to a similar extent in cases and controls. Decreased concentrations of the cholesterol synthesis markers desmosterol (−12% and −11%) and lathosterol (−50% and −56%) and increased concentrations of the cholesterol absorption markers campesterol (48% and 51%) and sitosterol (25% and 26%) were observed on treatment, but the magnitude of change was similar between cases and controls. These data suggest that decreases in cholesterol synthesis in response to pravastatin treatment were accompanied by modest compensatory increases in fractional cholesterol absorption. The magnitude of these alterations were similar between cases and controls and do not explain differences in outcomes with pravastatin treatment.
Keywords: lipoproteins, lathosterol, desmosterol, phytosterols
Elevated plasma LDL-cholesterol and triglyceride concentrations and low HDL-cholesterol concentrations are well-established risk factors for coronary heart disease (CHD) (1, 2). Several primary and secondary prevention trials have documented that statin therapy is efficacious in lowering these lipid risk factors and subsequent CHD events and mortality (3–10). Nonetheless, CHD events still occur in some treated patients. For instance, in the Scandinavian Simvastatin Survival Study, which evaluated the effect of simvastatin on mortality and morbidity in 4444 CHD patients, simvastatin (20–40 mg/day) lowered LDL-cholesterol concentrations by 35% and raised HDL- cholesterol concentrations by 8% relative to placebo (8). These changes were associated with a 42% reduction in the risk of coronary deaths over a 5.4 year median follow-up period. However, there were 431 coronary events and 111 CHD-related deaths in the simvastatin-treated group.
Likewise, the Prospective Study of Pravastatin in the Elderly at Risk (PROSPER) Trial examined the effects of pravastatin therapy (40 mg/day) in an elderly cohort of men and women with a history of or risk factors for vascular disease (11). The primary endpoint was a composite of CHD death, nonfatal myocardial infarct (MI), and fatal or nonfatal stroke over an average treatment period of 3.2 years. In this trial, at 3 months, pravastatin significantly lowered LDL-cholesterol concentrations by 32%, increased HDL-cholesterol concentrations by 5%, and lowered triglyceride concentrations by 12% compared with the placebo group. These changes were associated with a 15% reduction in incidence of primary endpoints and a 19% reduction in the secondary endpoint of CHD death or nonfatal MI. There were 408 events in the pravastatin group compared with 473 events in the placebo group. These results as well as those from the other statin trials suggest that there is a subgroup of individuals in whom coronary events are not reduced by statin treatment (12). The reason for this difference in clinical outcomes has yet to be elucidated.
Statins are inhibitors of 3-hydroxy-3-methylglutaryl CoA reductase, the rate limiting enzyme in the cholesterol biosynthetic pathway. Because circulating cholesterol concentrations are dependent, in part, on changes in cholesterol synthesis and absorption rates, which are key components of cholesterol homeostasis, it has been suggested that the effectiveness of statins depends on an individual's baseline cholesterol metabolism (13). Additionally, because statin therapy has been shown to increase cholesterol absorption marker concentrations (14), it has also been hypothesized that the beneficial effect on LDL-cholesterol-lowering and subsequent CVD risk reduction in response to statin inhibition of cholesterol synthesis would be attenuated in individuals who demonstrated a greater rebound increase in cholesterol absorption. To address these issues, the objective of the present analysis was to quantify the plasma concentrations of selected noncholesterol sterols, reflecting fractional cholesterol absorption efficiency and synthesis rates, in a subset of PROSPER trial participants randomized to pravastatin treatment, with and without a CHD event, baseline and on treatment, and to relate these data to changes in lipoprotein profiles.
METHODS
Study population and experimental design
Details relating to the PROSPER trial have been published previously (11). Briefly, PROSPER was a randomized controlled trial of 2,804 men and 3,000 women aged 70–82 years. All subjects had vascular disease or a CHD risk factor at the time of enrollment: smoking, hypertension, diabetes, or elevated total cholesterol (4.0–9.0 mmol/L). Subjects were randomized to receive either 40 mg/day pravastatin or placebo and were followed for an average of 3.2 years. The present study population included 584 subjects all randomized to pravastatin, 292 cases who experienced an event defined as CHD death or nonfatal MI during the study, and 292 controls who did not have an event during the study. Cases and controls were matched on the basis of age, gender, history of vascular disease, smoking status, antihypertensive treatment, diabetes, and country. Baseline and 6 month (on treatment) plasma samples were available for only 223 cases and 257 controls. All subjects included in the present study were still taking pravastatin medication at 6 months. The institutional ethics review boards of all centers approved the protocol, and all participants gave written informed consent. The protocol was consistent with the Declaration of Helsinki.
Biochemical analyses
Fasting plasma total cholesterol, triglycerides, and HDL-cholesterol concentrations were measured using standard enzymatic methods, as previously described (11). LDL-cholesterol concentrations were calculated according to the Friedewald formula (15). Plasma concentrations of cholesterol homeostasis markers in baseline and on treatment samples were measured using a GC method similar to that previously described (16, 17). Peaks of interest were identified by comparison with authentic standards (Supelco, Bellefonte, PA) and expressed relative to the internal standard. The investigators and laboratory personnel were blinded as to case-control status of the plasma samples. Each case-control set was analyzed in the same run by the same technician in a random sequence under identical conditions. High and low external quality control samples were routinely interspersed and analyzed with study samples. The noncholesterol sterols reported include desmosterol and lathosterol as markers of cholesterol synthesis rates and campesterol and sitosterol as markers of fractional cholesterol absorption efficiency.
Statistical analysis
Baseline characteristics were compared between cases and controls using the two-sample t-test for continuous variables and the chi-square test for categorical variables. The lipid and cholesterol homeostasis marker concentrations at baseline, on treatment and difference (on treatment-baseline) were compared between cases and controls using the two-sample t-test. For lipids the percentage change was analyzed, while for the cholesterol homeostasis markers the absolute change was analyzed. The distribution of triglycerides and the cholesterol homeostasis markers were positively skewed; therefore, a logarithmic transformation was used. The absolute difference in raw values of cholesterol homeostasis markers and percentage difference in triglycerides was analyzed, as these differences were normally distributed. Relationships between the percentage difference in lipids and absolute difference in cholesterol markers were assessed through the use of the Pearson correlation coefficient. Because the noncholesterol sterols are transported in plasma by lipoproteins, it is common practice to express their concentration relative to the concentration of plasma total cholesterol (μmol/mmol of cholesterol) rather than in absolute terms (μmol/L). Analysis was performed using both the corrected as well as uncorrected (absolute) data. The results of the statistical analysis were similar. In the present study, the cholesterol absorption and synthesis marker concentrations have been expressed as a ratio to cholesterol.
RESULTS
Baseline characteristics and plasma lipid and lipoprotein profile
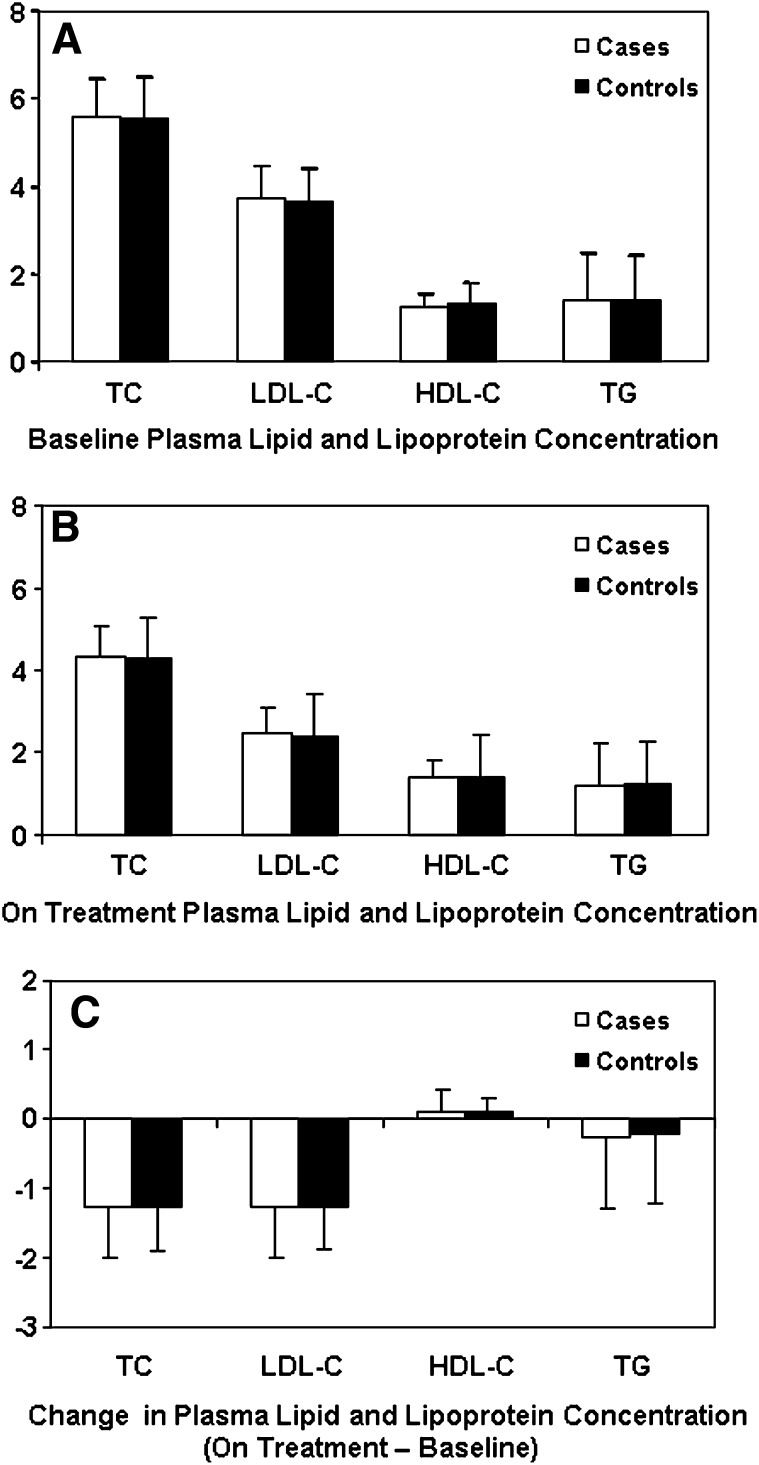
As per the matching criteria, age, body mass index, and gender distribution were similar among cases and controls (Table 1). Likewise, the percentage of cases and controls who were smokers and who had vascular disease, hypertension, and diabetes was also similar. There were no significant differences between cases and controls, irrespective of gender, in baseline and on treatment plasma lipid and lipoprotein profiles (Fig. 1A, B). Reductions in total and LDL-cholesterol and triglyceride concentrations and increases in HDL-cholesterol concentrations with pravastatin treatment were observed in both cases and controls, but there were no statistically significant differences between groups (Fig. 1C).
TABLE 1.
Baseline characteristics
| Variables | Cases N = 223 (128 M/95 F) | Controls N = 257 (150 M/107 F) | Pb |
|---|---|---|---|
| Age (years)a | |||
| Males | 75.3 ± 3.4 | 75.3 ± 3.2 | 0.97 |
| Females | 76.2 ± 3.2 | 76.3 ± 3.4 | 0.78 |
| All | 75.7 ± 3.3 | 75.6 ± 3.3 | 0.86 |
| BMI (kg/m2)a | |||
| Males | 26.7 ± 3.8 | 26.7 ± 3.4 | 0.92 |
| Females | 27.4 ± 5.0 | 27.3 ± 4.7 | 0.85 |
| All | 27.0 ± 4.3 | 26.9 ± 4.0 | 0.92 |
| Vascular disease [n] (%) | |||
| Males | [85] (66) | [88] (59) | 0.18 |
| Females | [43] (45) | [61] (57) | 0.10 |
| All | [128] (57) | [149] (58) | 0.90 |
| Hypertension [n] (%) | |||
| Males | [67] (52) | [71] (47) | 0.40 |
| Females | [75] (79) | [76] (71) | 0.20 |
| All | [142] (64) | [147] (57) | 0.15 |
| Diabetes [n] (%) | |||
| Males | [23] (18) | [23] (15) | 0.56 |
| Females | [15] (16) | [18] (17) | 0.84 |
| All | [38] (17) | [41] (16) | 0.75 |
| Current smoker [n] (%) | |||
| Males | [39] (30) | [54] (36) | 0.33 |
| Females | [18] (19) | [16] (15) | 0.45 |
| All | [57] (26) | [70] (27) | 0.68 |
Values are mean ± SD.
P-values from two-sample t-test or chi-square test.
Fig. 1.
Plasma lipid and lipoprotein profile (mmol/L) in cases and controls at baseline (A), on treatment (B), and difference (on treatment-baseline) (C). The values for total, LDL-, and HDL- cholesterol are untransformed mean (bar is sd). The triglyceride values are geometric means (bar is SD) calculated from the log-transformed values (except for absolute difference). The absolute change values are untransformed mean (bar is SD) (absolute difference of the raw values).
Cholesterol homeostasis marker profile
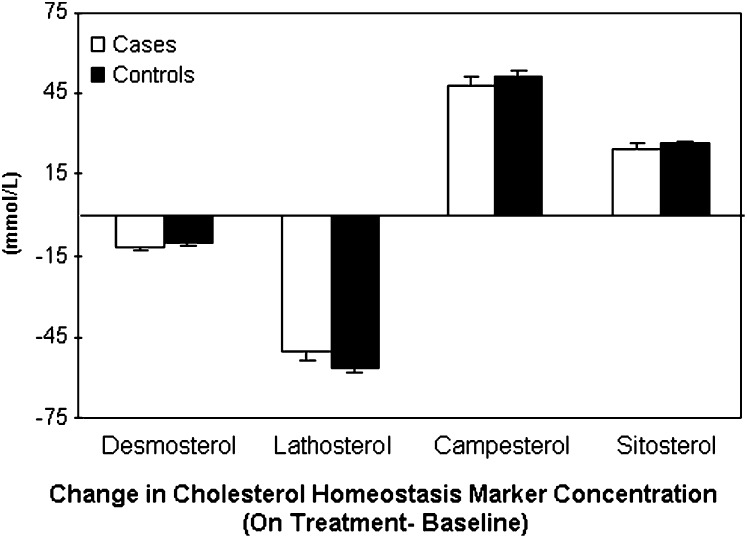
At baseline, cases and controls had similar concentrations of the cholesterol synthesis and absorption markers (Table 2). After 6 months on pravastatin treatment, there was a significant reduction in plasma concentrations of the cholesterol synthesis markers desmosterol (−12% and − 11%) and lathosterol (−50% and −56%) in the cases and controls, respectively, compared with baseline values (Fig. 2). In contrast, plasma concentrations of the cholesterol absorption markers campesterol (48% and 51%) and sitosterol (25% and 26%) significantly increased with pravastatin treatment in both cases and controls. However, there was no significant difference in the magnitude of the change between the cases and controls. Females and males followed similar patterns with respect to cholesterol homeostasis marker concentrations.
TABLE 2.
Plasma cholesterol homeostasis marker profile
| Baseline |
On Treatment |
|||||
|---|---|---|---|---|---|---|
| Variable | Cases (N = 223) | Controls (N = 257) | P | Cases (N = 223) | Controls (N = 257) | Pa |
| Cholesterol synthesis markers (102 mmol/mol of cholesterol) | ||||||
| Desmosterolb | ||||||
| Males | 36.61 ± 1.04 | 37.07 ± 1.03 | 0.81 | 25.41 ± 1.05 | 26.62 ± 1.04 | 0.42 |
| Females | 32.89 ± 1.04 | 30.93 ± 1.04 | 0.30 | 22.08 ± 1.04 | 21.29 ± 1.04 | 0.55 |
| All | 34.97 ± 1.03 | 34.38 ± 1.03 | 0.67 | 23.93 ± 1.03 | 24.26 ± 1.03 | 0.75 |
| Lathosterolb | ||||||
| Males | 117.23 ± 1.04 | 122.81 ± 1.03 | 0.34 | 65.57 ± 1.04 | 66.80 ± 1.04 | 0.72 |
| Females | 110.26 ± 1.04 | 111.43 ± 1.04 | 0.85 | 64.44 ± 1.05 | 63.04 ± 1.05 | 0.75 |
| All | 114.21 ± 1.03 | 117.93 ± 1.03 | 0.38 | 65.09 ± 1.03 | 65.21 ± 1.03 | 0.97 |
| Cholesterol absorption markers (102 mmol/mol of cholesterol) | ||||||
| Campesterolb | ||||||
| Males | 150.01 ± 1.04 | 143.27 ± 1.04 | 0.42 | 197.37 ± 1.04 | 190.68 ± 1.04 | 0.54 |
| Females | 144.93 ± 1.05 | 157.19 ± 1.06 | 0.26 | 181.77 ± 1.05 | 199.88 ± 1.06 | 0.20 |
| All | 147.82 ± 1.03 | 148.91 ± 1.03 | 0.87 | 190.57 ± 1.03 | 194.46 ± 1.03 | 0.65 |
| Sitosterolb | ||||||
| Males | 90.58 ± 1.04 | 89.19 ± 1.04 | 0.78 | 114.77 ± 1.04 | 113.92 ± 1.04 | 0.90 |
| Females | 89.68 ± 1.05 | 99.82 ± 1.05 | 0.13 | 108.46 ± 1.05 | 121.33 ± 1.05 | 0.11 |
| All | 90.19 ± 1.03 | 93.47 ± 1.03 | 0.42 | 112.04 ± 1.03 | 116.95 ± 1.03 | 0.34 |
| Lathosterol/campesterolb | ||||||
| Males | 0.78 ± 1.06 | 0.86 ± 1.06 | 0.28 | 0.33 ± 1.06 | 0.35 ± 1.06 | 0.52 |
| Females | 0.76 ± 1.07 | 0.71 ± 1.08 | 0.50 | 0.35 ± 1.08 | 0.32 ± 1.08 | 0.30 |
| All | 0.77 ± 1.05 | 0.79 ± 1.05 | 0.71 | 0.34 ± 1.05 | 0.34 ± 1.05 | 0.78 |
| Lathosterol/sitosterolb | ||||||
| Males | 1.29 ± 1.06 | 1.38 ± 1.06 | 0.45 | 0.57 ± 1.06 | 0.59 ± 1.06 | 0.75 |
| Females | 1.23 ± 1.07 | 1.12 ± 1.08 | 0.35 | 0.59 ± 1.08 | 0.52 ± 1.08 | 0.22 |
| All | 1.27 ± 1.04 | 1.26 ± 1.05 | 0.96 | 0.58 ± 1.05 | 0.56 ± 1.05 | 0.53 |
P-values from two-sample t-test.
Values are geometric means ± SEM calculated from the log-transformed values.
Fig. 2.
Change in plasma cholesterol homeostasis marker profile (on treatment-baseline) in all subjects. Values are untransformed mean (bar is SEM) of the ratios of the cholesterol homeostasis markers-cholesterol.
Cholesterol homeostasis markers and lipid risk factors
The correlation coefficients between change in the cholesterol synthesis and absorption markers with pravastatin treatment and the corresponding percent change in plasma lipid and lipoprotein parameters are provided in Table 3. In both cases and controls, positive associations were observed between the cholesterol synthesis markers and total and LDL-cholesterol, and the cholesterol absorption markers and HDL-cholesterol concentrations. In the controls only, the cholesterol synthesis markers were positively associated with change in triglycerides. In the cases only, the cholesterol synthesis markers were positively associated with change in HDL-cholesterol concentrations, and the cholesterol absorption markers were negatively associated with change in LDL-cholesterol concentrations.
TABLE 3.
Correlation between cholesterol homeostasis markers and lipid risk factorsa
| Synthesis Markers |
Absorption Markers |
||||
|---|---|---|---|---|---|
| Variable | Δ Desmosterol | Δ Lathosterol | Δ Campesterol | Δ Sitosterol | |
| Total cholesterol (% Δ) | Cases | 0.36 (<0.0001) | 0.36 (<0.0001) | −0.07 (0.28) | −0.09 (0.17) |
| Controls | 0.32 (<0.0001) | 0.29 (<0.0001) | −0.10 (0.13) | −0.08 (0.20) | |
| LDL-cholesterol (% Δ) | Cases | 0.35 (<0.0001) | 0.37 (<0.0001) | −0.16 (0.02) | -0.19 (0.01) |
| Controls | 0.23 (0.0003) | 0.28 (<0.0001) | −0.09 (0.16) | -0.09 (0.18) | |
| HDL-cholesterol (% Δ) | Cases | 0.15 (0.03) | 0.14 (0.05) | 0.12 (0.08) | 0.14 (0.04) |
| Controls | 0.05 (0.44) | −0.05 (0.40) | 0.19 (0.003) | 0.12 (0.05) | |
| Triglycerides (% Δ) | Cases | 0.20 (0.30) | 0.06 (0.36) | 0.04 (0.54) | −0.04 (0.52) |
| Controls | 0.15 (0.01) | 0.12 (0.05) | −0.05 (0.43) | 0.02 (0.74) | |
Pearson correlations were carried out between the percentage difference in lipids and absolute difference in synthesis/absorption markers. The data presented are correlation coefficients (values lie in range of −1 to +1) and the P-value.
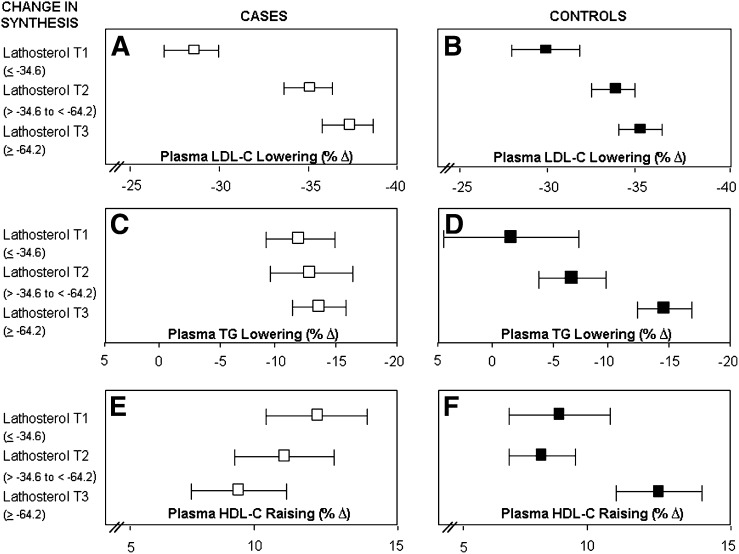
To further explore this relationship, the change in cholesterol homeostasis markers induced by pravastatin treatment was divided into tertiles and the corresponding percent decrease in plasma LDL-cholesterol and triglyceride and the percent increase in HDL-cholesterol concentrations were computed separately for the cases and controls (Figs. 3 and 4). Cases and controls who had the greatest decrease in cholesterol synthesis, as reflected by lathosterol concentrations (Fig. 3A, B), also had the greatest decrease in plasma LDL-cholesterol concentrations (tertile 3 > tertile 2 > tertile 1). Similar results were also observed for desmosterol concentrations (data not shown).
Fig. 3.
Tertiles of change (on treatment-baseline) in cholesterol synthesis, as reflected by plasma lathosterol concentrations (as a ratio to cholesterol) and percent change in plasma LDL-cholesterol lowering (A and B), triglyceride lowering (C and D), and HDL-cholesterol raising (E and F) in cases and controls, respectively. Bar is 95% confidence interval.
Fig. 4.
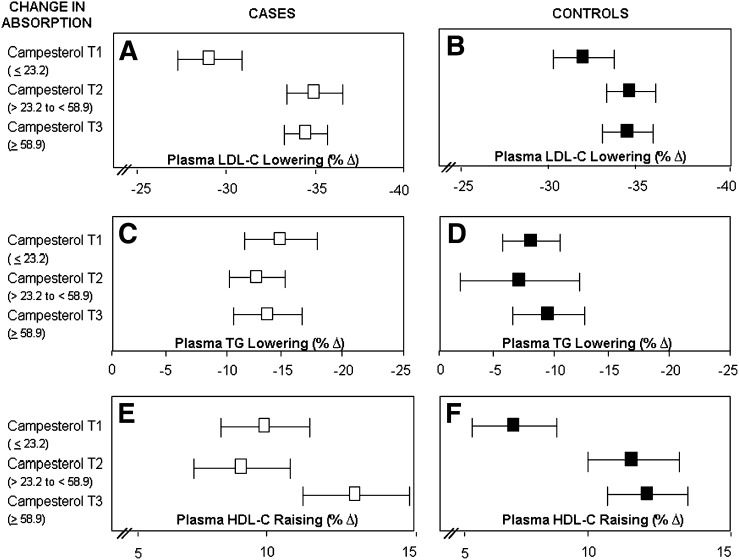
Tertiles of change (on treatment-baseline) in cholesterol absorption, as reflected by plasma campesterol concentrations (as a ratio to cholesterol) and percent change in plasma LDL-cholesterol lowering (A and B), triglyceride lowering (C and D), and HDL-cholesterol raising (E and F) in cases and controls, respectively. Bar is 95% confidence interval.
Cases and controls showed contrasting patterns of response with regard to the association between cholesterol synthesis and triglyceride (Fig. 3C, D) and HDL-cholesterol concentrations (Fig. 3E, F). Controls who had the greatest reduction in lathosterol (tertile 3) demonstrated greater decreases in triglycerides (P = 0.046) and a trend toward greater increases in HDL-cholesterol concentrations (P = 0.091). However, in the cases, greater decreases in lathosterol were associated with lower increases in HDL-cholesterol concentrations. No significant association was observed between cholesterol synthesis and change in triglyceride concentrations in response to pravastatin therapy in the cases (P = 0.918). With regard to the cholesterol absorption markers and plasma LDL-cholesterol, HDL-cholesterol, and triglyceride response, cases and controls showed a similar pattern of response (Fig. 4; campesterol, data not shown for sitosterol but pattern was similar). Taken together, these results suggest that the decrease in cholesterol synthesis in response to pravastatin is related to the magnitude of plasma LDL-cholesterol lowering in both cases and controls. The finding that the decrease in cholesterol synthesis appears to be associated with plasma triglyceride-lowering response in control subjects and HDL-cholesterol-raising response in cases demonstrating the greatest decrease in cholesterol synthesis is interesting, but the correlations and associations are relatively modest and the clinical relevance is unclear.
DISCUSSION
As part of a clinical trial that examined the effects of pravastatin on CVD outcomes in an elderly high risk population, plasma concentrations of noncholesterol sterols (reflecting fractional cholesterol absorption and synthesis) were measured at baseline and after 6 months on treatment in a subset of individuals who experienced a clinical event (cases) and matched controls who did not have an event. The intent was to determine whether changes in cholesterol homeostasis marker concentrations induced by statin treatment predicted plasma lipoprotein response and CVD outcomes. Results of this investigation demonstrate that pravastatin treatment decreased cholesterol synthesis and increased fractional cholesterol absorption and these changes were associated with the magnitude of LDL-cholesterol lowering. However, the pattern of response was similar between cases and controls and does not explain the difference in outcome.
The decrease in plasma total and LDL-cholesterol concentrations observed in patients treated with a statin is ascribed to the inhibition in 3-hydroxy-3-methylglutaryl CoA reductase activity, the rate limiting enzyme in cholesterol biosynthesis. This reduction in cholesterol synthesis upregulates expression of hepatic LDL receptors resulting in the increased clearance of circulating cholesterol (18). The current findings are also consistent with our previous observations and those of other investigations that statin treatment increases the rate of intestinal cholesterol absorption (16, 19, 20). This response may reflect a compensatory increase in absorption efficiency to restore cholesterol homeostasis altered by the suppression of endogenous cholesterol synthesis. Consequently, we hypothesized that the beneficial effect on LDL-cholesterol lowering and subsequent CVD risk reduction produced by statin inhibition of cholesterol synthesis would be attenuated in individuals who demonstrated a greater rebound increase in cholesterol absorption. However, no significant differences were observed between cases and controls in the magnitude of decrease in cholesterol synthesis or increase in fractional cholesterol absorption in response to pravastatin treatment and the subsequent degree of LDL-cholesterol reduction.
Alternatively, it has been suggested that the variability in cholesterol lowering response among individuals treated with statins is due to differences in baseline cholesterol metabolism (21). Gylling and Miettinen (21) reported in a subgroup analysis of coronary patients who did not respond to simvastatin treatment that patients who were classified as high cholesterol absorbers/ low synthesizers (defined by plasma concentrations of the cholesterol homeostasis markers) were less responsive to statins than patients classified as low absorbers/high synthesizers. In the present study, there were no significant differences in the cholesterol homeostasis marker concentrations at baseline between cases and controls. Additionally, a similar pattern of response was observed between cases and controls in the magnitude of decrease in cholesterol synthesis or increase in fractional cholesterol absorption efficiency in response to pravastatin treatment and the subsequent degree of LDL-cholesterol reduction. Thus, alterations in cholesterol homeostasis and LDL-cholesterol do not explain the difference in clinical outcomes in our study population.
The major difference between cases and controls was in the response of triglycerides and HDL-cholesterol to the alterations in cholesterol synthesis. Specifically, the decrease in cholesterol synthesis in response to pravastatin treatment was associated with greater decreases in triglyceride concentrations and a trend toward greater increases in HDL-cholesterol concentrations in the controls. However, in the cases, greater decreases in cholesterol synthesis in response to pravastain treatment were associated with lower increases in HDL-cholesterol concentrations, and there was no difference in triglyceride response. The reason for the difference in response to pravastain treatment between the two groups is unclear. It is well established that HDL-cholesterol concentrations are inversely associated with triglyceride concentrations due to their interrelated metabolic fates. In addition to the role of HDL in promoting cholesterol efflux, HDL composition and structure are also emerging as being important for this process (22). In one small study, patients with CHD or CHD risk factors had proinflammatory HDL relative to matched controls at baseline, and about one-half continued to have proinflammatory HDL after statin therapy despite a profound decrease in plasma lipids. Proinflammatory HDL has been shown to have diminished ability to promote cholesterol efflux (23). These data suggest that statin therapy may have modified HDL function in some but not all of our subjects, and this variable may have accounted for differences in clinical outcome despite similar plasma lipoprotein profiles.
In addition to the lipid-related effects, statins have also been shown to have beneficial effects on several nonlipid-related CVD risk factors such as decreasing smooth muscle cell proliferation, endothelial activation, and reductions in C-reactive protein, antioxidant, and antithrombotic properties. Furthermore, genetic polymorphisms have been documented that influence response to statin treatment. Pravastatin has been shown to be less effective in lowering cholesterol synthesis in carriers of the SLCO1B1*17 haplotype compared with noncarriers (24). Genetic variation at the LDL receptor locus as well as the ATP-binding cassette (ABC) transporters ABCG5 and ABCG8 have also been shown to affect response to pravastatin and CVD risk, respectively (25, 26). While plasma CRP concentrations (mean ± SD) were similar between cases (3.58 ± 3.21 and 3.01 ± 2.84) and controls (3.33 ± 3.29 and 3.88 ± 3.01, males and females, respectively), we cannot rule out the possibility that the magnitude of other nonlipid-related effects of statin therapy as well as frequency of genetic polymorphisms known to influence cholesterol homeostasis differed between cases and controls and contributed to the reduction in coronary events.
In conclusion, our results suggest that in an elderly population with a high CVD prevalence, the decrease in cholesterol synthesis in response to pravastatin therapy is accompanied by a modest compensatory increase in markers of fractional cholesterol absorption. The magnitude of these alterations was similar between cases and controls and does not explain differences in outcomes with pravastatin treatment.
APPENDIX
The PROSPER study group
Executive Committee: (Glasgow) J. Shepherd, University Department of Pathological Biochemistry, North Glasgow University NHS Trust, Glasgow, Scotland, UK (Chairman and Principal Investigator); S. M. Cobbe, Division of Cardiovascular and Medical Sciences, University of Glasgow, Glasgow, Scotland; I. Ford, Robertson Centre for Biostatistics, University of Glasgow, Glasgow, Scotland, UK; A. Gaw, University Department of Pathological Biochemistry, North Glasgow University NHS Trust, Glasgow, Scotland, UK; P. W. Macfarlane, Division of Cardiovascular and Medical Sciences, University of Glasgow, Glasgow, Scotland, UK; C. J. Packard, University Department of Pathological Biochemistry, North Glasgow University NHS Trust, Glasgow, Scotland, UK; D. J. Stott, Division of Cardiovascular and Medical Sciences, University of Glasgow, Glasgow, Scotland, UK; (Leiden) G. J. Blauw, Section of Gerontology and Geriatrics, Leiden University Medical Centre, Leiden, The Netherlands (Principal Investigator); E. L. E. M. Bollen, Department of Neurology, Leiden University Medical Centre, Leiden, The Netherlands; A. M. Kamper, Section of Gerontology and Geriatrics, Leiden University Medical Centre, Leiden, The Netherlands; R. G. J. Westendorp, Section of Gerontology and Geriatrics, Leiden University Medical Centre, Leiden, The Netherlands; (Cork) M. B. Murphy, Department of Pharmacology and Therapeutics, Cork University Hospital, Wilton, Cork, Ireland (Principal Investigator); B. M. Buckley, Department of Pharmacology and Therapeutics, Cork University Hospital, Wilton, Cork, Ireland; M. Hyland, University College Cork, and Department of Geriatric Medicine, Cork University Hospital, Wilton, Cork, Ireland; I. J. Perry, Department of Epidemiology and Public Health, Cork University Hospital, Wilton, Cork, Ireland.
Endpoint Committee: S. M. Cobbe (Chairman); J. W. Jukema, Department of Cardiology, Leiden University Medical Centre, Leiden, The Netherlands; P W Macfarlane; A. E. Meinders, Department of General Internal Medicine, Leiden University Medical Centre, Leiden, The Netherlands; D J Stott; B. J. Sweeney, Department of Neurology, Cork University Hospital, Wilton, Cork, Ireland; C. Twomey, University College Cork, and Department of Geriatric Medicine, Cork University Hospital, Wilton, Cork, Ireland.
Acknowledgments
The authors acknowledge the cooperation of the study subjects, without whom this investigation would not be possible.
Footnotes
Abbreviations:
- ABC
- ATP-binding cassette
- CHD
- coronary heart disease
- MI
- myocardial infarct
- PROSPER
- Prospective Study of Pravastatin in the Elderly at Risk
This work is supported by a grant from the National Institutes of Health (R01 HL-74753) and an investigator initial grant from Bristol Myers Squibb Inc., Princeton, NJ.
REFERENCES
- 1.AHA. 2007. Heart disease and stroke statistics–2007 update. Accessed January 2009, at http://www.americanheart.org/presenter.jhtml?identifier=1928.
- 2.National Cholesterol Education Program (NCEP) Expert Panel on Detection Evaluation and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). 2002. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 106: 3143–3421. [PubMed] [Google Scholar]
- 3.Baigent C., Keech A., Kearney P. M., Blackwell L., Buck G., Pollicino C., Kirby A., Sourjina T., Peto R., Collins R., et al. for the Cholesterol Treatment Trialists' (CTT) Collaborators. 2005. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 366: 1267–1278. [DOI] [PubMed] [Google Scholar]
- 4.Heart Protection Study Collaborative Group. 2002. MRC/BHF Heart Protection Study of antioxidant vitamin supplementation in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 360: 23–33.12114037 [Google Scholar]
- 5.Long-term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. 1998. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N. Engl. J. Med. 339: 1349–1357. [DOI] [PubMed] [Google Scholar]
- 6.Nakamura H., and MEGA Study Group. 2007. Primary prevention of cardiovascular diseases among hypercholesterolemic Japanese with a low dose of pravastatin. Atheroscler. Suppl. 8: 13–17. [DOI] [PubMed] [Google Scholar]
- 7.Sacks F. M., Pfeffer M. A., Moye L. A., Rouleau J. L., Rutherford J. D., Cole T. G., Brown L., Warnica J. W., Arnold J. M., Wun C. C., et al. 1996. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N. Engl. J. Med. 335: 1001–1009. [DOI] [PubMed] [Google Scholar]
- 8.The Scandinavian Simvastatin Survival Study. 1994. Randomized trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet. 344: 1386–1389. [PubMed] [Google Scholar]
- 9.Ward S., Lloyd Jones M., Pandor A., Holmes M., Ara R., Ryan A., Yeo W., Payne N. 2007. A systematic review and economic evaluation of statins for the prevention of coronary events. Health Technol. Assess. 11: 1–160. [DOI] [PubMed] [Google Scholar]
- 10.West of Scotland Coronary Prevention Study Group. 1995. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. N. Engl. J. Med. 333: 1301–1307. [DOI] [PubMed] [Google Scholar]
- 11.Shepherd J., Blauw G. J., Murphy M. B., Bollen E. L., Buckley B. M., Cobbe S. M., Ford I., Gaw A., Hyland M., Jukema J. W., et al. , on behalf of the PROSPER study group. 2002. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet. 360: 1623–1630. [DOI] [PubMed] [Google Scholar]
- 12.Knopp R. H., Paramsothy P., Atkinson B., Dowdy A. 2008. Comprehensive lipid management versus aggressive low-density lipoprotein lowering to reduce cardiovascular risk. Am. J. Cardiol. 101: 48B–75B. [DOI] [PubMed] [Google Scholar]
- 13.Miettinen T. A., Gylling H. 2002. Ineffective decrease of serum cholesterol by simvastatin in a subgroup of hypercholesterolemic coronary patients. Atherosclerosis. 164: 147–152. [DOI] [PubMed] [Google Scholar]
- 14.van Himbergen T. M., Matthan N. R., Resteghini N. A., Otokozawa S., Ai M., Stein E. A., Jones P. H., Schaefer E. J. 2009. Comparison of the effects of maximal dose atorvastatin and rosuvastatin therapy on cholesterol synthesis and absorption markers. J. Lipid Res. 50: 730–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Friedewald W. T., Levy R. I., Fredrickson D. S. 1972. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 18: 499–502. [PubMed] [Google Scholar]
- 16.Matthan N. R., Giovanni A., Schaefer E. J., Brown B. G., Lichtenstein A. H. 2003. Impact of simvastatin, niacin, and/or antioxidants on cholesterol metabolism in CAD patients with low HDL. J. Lipid Res. 44: 800–806. [DOI] [PubMed] [Google Scholar]
- 17.Matthan N. R., LaRocque J. M., Pencina M., D'Agostino R. B., Schaefer E. J., Lichtenstein A. H. 2009. Alterations in cholesterol absorption and synthesis characterize Framingham Offspring Study participants with coronary heart disease. J. Lipid Res. 50: 1927–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reihner E., Rudling M., Stahlberg D., Berglund L., Ewerth S., Bjorkhem I., Einarsson K., Angelin B. 1990. Influence of pravastatin, a specific inhibitor of HMG-CoA reductase, on hepatic metabolism of cholesterol. N. Engl. J. Med. 323: 224–228. [DOI] [PubMed] [Google Scholar]
- 19.Miettinen T. A., Gylling H., Strandberg T., Sarna S. 1998. Baseline serum cholestanol as predictor of recurrent coronary events in subgroup of Scandinavian simvastatin survival study. Finnish 4S investigators. BMJ. 316: 1127–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miettinen T. A., Strandberg T. E., Gylling H. 2000. Noncholesterol sterols and cholesterol lowering by long-term simvastatin treatment in coronary patients: relation to basal serum cholestanol. Arterioscler. Thromb. Vasc. Biol. 20: 1340–1346. [DOI] [PubMed] [Google Scholar]
- 21.Gylling H., Miettinen T. A. 2002. Baseline intestinal absorption and synthesis of cholesterol regulate its response to hypolipidaemic treatments in coronary patients. Atherosclerosis 160: 477–481. [DOI] [PubMed] [Google Scholar]
- 22.Navab M., Yu R., Gharavi N., Huang W., Ezra N., Lotfizadeh A., Anantharamaiah G. M., Alipour N., Van Lenten B. J., Reddy S. T., et al. 2007. High-density lipoprotein: antioxidant and anti-inflammatory properties. Curr. Atheroscler. Rep. 9: 244–248. [DOI] [PubMed] [Google Scholar]
- 23.Ansell B. J., Navab M., Hama S., Kamranpour N., Fonarow G. C., Hough G., Rahmani S., Mottahedeh R., Dave R., Reddy S. T., et al. 2003. Inflammatory/antiinflammatory properties of high-density lipoprotein distinguish patients from cholesterol subjects better than high-density lipoprotein cholesterol levels and are favorably affected by simvastatin treatment. Circulation. 108: 2751–2756. [DOI] [PubMed] [Google Scholar]
- 24.Niemi M., Neuvonen P. J., Hofman U., Backman J. T., Schwab M., Lutjohann D., Von Bergmann K., Eichelbaum M., Kivisto K. T. 2005. Acute effects of pravastatin on cholesterol synthesis are associated with SLCO1B1 (encoding OATP1B1) haplotype*17. Pharmacogenet. Genomics. 15: 303–309. [DOI] [PubMed] [Google Scholar]
- 25.Plösch T., Kosters A., Groen A. K., Kuipers F. T. 2005. The ABC of hepatic and intestinal cholesterol transport. Handb. Exp. Pharmacol. 170: 465–482. [DOI] [PubMed] [Google Scholar]
- 26.Polisecki E., Muallem H., Maeda N., Peter I., Robertson M., McMahon A. D., Ford I., Packard C., Shepherd J., Jukema J. W., et al. on behalf of the Prospective Study of Pravastatin in the Elderly at Risk (PROSPER) Investigators. 2008. Genetic variation at the LDL receptor and HMG-CoA reductase gene loci, lipid levels, statin response, and cardiovascular disease incidence in PROSPER. Atherosclerosis. 200: 109–114. [DOI] [PMC free article] [PubMed] [Google Scholar]