Abstract
The formation of bile acids/bile alcohols is of major importance for the maintenance of cholesterol homeostasis. Besides their functions in lipid absorption, bile acids/bile alcohols are regulatory molecules for a number of metabolic processes. Their effects are structure-dependent, and numerous metabolic conversions result in a complex mixture of biologically active and inactive forms. Advanced methods are required to characterize and quantify individual bile acids in these mixtures. A combination of such analyses with analyses of the proteome will be required for a better understanding of mechanisms of action and nature of endogenous ligands. Mass spectrometry is the basic detection technique for effluents from chromatographic columns. Capillary liquid chromatography-mass spectrometry with electrospray ionization provides the highest sensitivity in metabolome analysis. Classical gas chromatography-mass spectrometry is less sensitive but offers extensive structure-dependent fragmentation increasing the specificity in analyses of isobaric isomers of unconjugated bile acids. Depending on the nature of the bile acid/bile alcohol mixture and the range of concentration of individuals, different sample preparation sequences, from simple extractions to group separations and derivatizations, are applicable. We review the methods currently available for the analysis of bile acids in biological fluids and tissues, with emphasis on the combination of liquid and gas phase chromatography with mass spectrometry.
Keywords: liquid chromatography-mass spectrometry, electrospray ionization, gas-liquid chromatography-mass spectrometry, sample preparation
Bile acids constitute a large family of steroids carrying a carboxyl group in the side chain (Scheme 1). Bile alcohols have similar structures and are formed as intermediates or side products in bile acid biosynthesis or as end products in lower vertebrates. Metabolism of cholesterol to bile acids/bile alcohols is of major importance for the maintenance and regulation of cholesterol homeostasis. Depending on their structure, bile acids/bile alcohols can be ligands to nuclear receptors with different regulatory functions in animals, including even Caenorhabditis elegans (1). Bile acids can be formed in some marine microorganisms (2). These findings have increased interest in methods for specific and sensitive analysis of bile acids.
Scheme. 1.
Structures of some human bile acids and precursors. (I) cholesterol, (II) R1=OH, R2=H in 7α-hydroxycholesterol; R1=H, R2=OH in 27-hydroxycholesterol; R1=OH, R2=OH in 7α,27-dihydroxycholesterol (some confusion may arise concerning the nomenclature of 27-hydroxycholesterol and related compounds. According to rules of priority of numbering, the correct description of 27-hydroxycholesterol is 25R,26-hydroxycholesterol. However, the common name is 27-hydroxycholesterol, which will be used here). (III) R1=H in 3-oxocholest-4-enoic acid; R1=OH in 7α-hydroxy-3-oxocholest-4-enoic acid. (IV) R1=H, R2=H, in lithocholic acid (LCA); R1=OH, R2=H in chenodeoxycholic acid (CDCA); R1=H, R2=OH in deoxycholic acid (DCA); R1=OH, R2=OH in cholic acid (CA). (V) glycochenodeoxycholic acid (GCDCA). (VI) taurochenodeoxycholic acid (TCDCA). (VII) taurochenodeoxycholic acid 3α-sulfate. (VIII) taurochenodeoxycholic acid 3α-glucuronide. (IX) tauroursodeoxycholic acid 7β-N-acetylglucosaminide. (X) deoxycholic acid 24-glucuronide.
Conjugated forms of bile acids/bile alcohols in bile (bile salts) are essential participants in the absorption of lipids and lipid soluble compounds. Studies of this function, bile secretion, enterohepatic circulation, levels in blood, urine, and feces required methods for analysis of bile acids. In 1844, M. von Pettenkofer described his classical color reaction between cholic acid(s) and sugar in conc. sulfuric acid. During the subsequent 100 years, the reaction was modified in numerous ways. This period is described by Sobotka (3, 4), who pointed out the discrepancies between the poor quality of the analytical methods of his days and the better understanding of bile acid physiology and pathology. He made the following remarks, well worth considering today:
“The value of a method lies not only in its specificity but also in the ease with which interfering factors may be specifically removed. To test the usefulness of a method, it is insufficient to demonstrate that solutions of the pure substance yield correct results and that known amounts of the substance, when added to biological material, may be completely recovered. It is absolutely necessary to prove that the analytical reaction given by the biological material or extract is due to the substance for which one tests. The unequivocal identification of this substance, in amounts consistent with the analytical data, should always precede the introduction of a new analytical method; thus many mistakes in the interpretation of laborious analytical work may be avoided.” (ref. 4, p. 152)
The bile acid literature contains many examples where this has not been appreciated.
The situation changed with the introduction of chromatographic methods that could be used for quantification of individual bile acids in bile and revealed, for example, that chenodeoxycholic, assumed to be a minor bile acid, was in fact a major bile acid in humans (see refs.5, 6). The power of methods providing profile analysis of bile acids became obvious. Combined with the use of radioactively labeled bile acids, they provided the basic knowledge about hepatic and bacterial bile acid metabolism and their kinetics (see ref. 5).
A major step forward occurred when Horning's group (see refs. 6, 7) described methods for preparation of gas chromatographic (GLC) columns for separation and quantification of molecules as large as steroids and bile acids. By using the molecule separator designed by Ryhage (8), the column effluent could be directed into a mass spectrometer (MS), and GLC-MS provided new means of identification and specific quantification. The sensitivity was sufficient for estimation of bile acids in normal human plasma. Complex mixtures, such as those in feces, could be analyzed following appropriate sample preparation (9). An important drawback with the method was the need for cleavage of conjugated bile acids and derivatization to provide the necessary volatility. This was circumvented by the introduction, a few years later, of enzymatic methods based on the use of dehydrogenases acting on hydroxyl groups in the bile acid structures [see the review by Murphy, Maghsoudloo, and Qureshi (10)]. However, only so-called "total bile acids" were estimated and the method was based on the assumption that the chemical nature of the bile acid(s) to be measured was known. Development of immunoassays provided another biological approach to bile acid analysis (11)
The GLC requirement for volatility was eliminated when high-performance liquid chromatography (HPLC) was introduced (12). Separation efficiencies of GLC and HPLC methods have been greatly improved since the time of their introduction. Problems with HPLC have been limitations in detector sensitivity and specificity. These problems were greatly reduced by the introduction of soft ionization methods, particularly electrospray ionization (ESI), permitting the use of mass spectrometers as detectors in HPLC also (13). Recent advances in bile acid analysis have been made primarily in HPLC-MS and this is the field in which much of the future improvements in speed, sensitivity, and simplicity in bile acid analysis can be expected. However, GLC-MS will continue to be important in analysis of complex mixtures of unconjugated bile acids, particularly because of the extensive structural information given by electron impact ionization and fragmentation and the large number of published reference spectra. The importance of NMR in structure analysis is obvious, but the use of NMR in quantitative, high-speed ultra-micro analysis of bile acids remains to be seen and NMR is not included in this review.
NATURALLY OCCURRING BILE ACIDS
The choice of analytical method is determined by the question to be answered. The nature of the bile acids present in the sample is a major determinant. Simpler methods often have limitations, which may not be realized or are not mentioned. It is important to be aware of the potential complexity and to qualitatively characterize the bile acid mixture to be analyzed. Complex mixtures usually require use of time-consuming methods. This situation is changing with the advances in LC-MS. Examples of biological materials containing complex and variable mixtures of bile acids are feces, meconium, amniotic fluid, and urine, but blood also contains many bile acids besides the major biliary bile acids.
For biosynthetic reasons (14–16), the predominant bile acids and alcohols possess a 7α-hydroxy group (in most species) and, in modern species, have a 3α-hydroxy-5β(H) configuration. Bile acid biosynthesis proceeds via a number of intermediates that may have biological functions and are present in blood. Secondary metabolism of intermediates by hydroxylations, oxidoreductions, and other reactions may occur, particularly in genetic diseases of bile acid biosynthesis. Thus, bile acids occur with differences in the length of the side chain (1–10 carbon atoms), the number, positions, and stereochemistry of hydroxyl groups [1α/β, 2β, 3α/β, 4α/β, 5β, 7α/β, 12α/β, 15α/β, 16α, 19, 21, 22R, 23R/S, 24R/S, 25, 26/27 (25R/S) (not all found in humans)], the presence of carbonyl groups, and double bonds [e.g., Δ1, Δ2, Δ3, Δ4, Δ5, Δ6, Δ22, and the A/B-ring stereochemistry (5β(H) (normal), 5α(H) (allo)]. Species differences, which are sometimes/often neglected in the choice of method, can be large, e.g., between humans and rodents. Sex differences are seen in some species. Bile acid composition can differ between neonatal and adult periods of life, e.g., in humans. Bile acid patterns in healthy adults, infants, and patients with liver disease differ not only with respect to concentrations of common bile acids but also by introduction of hydroxyl groups at different positions, e.g., at carbons 1, 2, 4, 5, or 6. Thus, conventional methods described for analysis of the three common bile acids (in humans), cholic, chenodeoxycholic, and deoxycholic acids, and ursodeoxycholic (used as a drug and metabolized into a host of hydroxylated metabolites not included in the conventional analyses) and lithocholic acid (also present as metabolites that are not analyzed) are of limited value when comparisons are to be made between populations in which patterns of metabolites differ due to differences in metabolism.
Bile acids are secreted into bile after conjugation of the carboxyl group with glycine or taurine and, in exceptional cases, other amino acids. They undergo an enterohepatic circulation during which the bile acid composition is modified through bacterial metabolism in the intestine. Important reactions are deconjugation, 7-dehydroxylation, and oxidoreductions resulting in epimerization of hydroxyl groups. Free bile acids are reconjugated upon return to the liver, in some species hydroxylated, and ketones are fully or partly reduced. Both free and conjugated bile acids may become hydroxylated (and by different hydroxylases), so that different forms of the same free bile acid may have different metabolic origins. Depending on the questions asked, this may be of importance in the choice of analytical method.
Bile alcohols are secreted into bile, usually as sulfates or glucuronides. Bile acids can also be found as sulfates, glucuronides, glucosides, galactosides, and N-acetylglucosaminides, particularly in urine but also in blood and, to a less known extent, in bile and other fluids. Glutathione conjugates and their analysis in rat bile have recently been described (17). The positions of conjugation differ depending on the structures both of the bile acid and the conjugating moiety. For example, in humans, glucuronidation occurs at C-6 or C-3 and at the carboxyl group, glucosidation at C-3, N-acetylglucosaminidation at C-7β, and galactosidation at the carboxyl group. The modes of conjugation influence the biological activity of a bile acid. For example, sulfation decreases the toxicity of lithocholic acid, whereas sulfation increases the pheromone activity of petromyzonol in the sea lamprey (18, 19). Species differences are important. For example, cholic acid sulfate carries the sulfate group at C-3 in humans and at C-7 in mice (20, 21). Such differences should influence the choice of analytical methods. Esterification of bile acids with fatty acids at C-3 occurs in the colon (22, 23), affecting the methodology for analysis of fecal bile acids (24). Polydeoxycholic acid is an ester that is rarely considered in analyses of fecal bile acids (25).
Bile acids and bile alcohols can be simultaneously conjugated at different positions, resulting in double and triple conjugates. Aminoacyl amidation is often combined with sulfation or, to a lesser extent, different types of glycosylation [e.g., Alvelius et al. (26); Iida et al. (27)]. Different conjugated forms may indicate multiple metabolic origins of a bile acid that may require separate analysis. Most published methods do not include these types of sometimes major conjugates.
DIRECT INFUSION ESI-MS
Whenever possible, the analysis of a biological fluid or tissue extract should start with a characterization by direct soft ionization MS. Today, this may be achieved by exploiting direct infusion ESI-MS or nanospray ESI-MS (or in the past by fast atom bombardment-MS), often following a simple solid phase extraction step utilizing a C18 cartridge (see below). This strategy has been extensively utilized by Setchell and colleagues in Cincinnati (28–30), Clayton and coworkers in London (31–34) and in the Karolinska laboratory (26, 35–37).
Whereas the levels of bile acids in bile are sufficiently high (∼10 mg/ml) to allow direct analysis following dilution with water or aqueous methanol, this may not be true for analysis of other media. Although the levels of bile acids in both plasma (1–4 μg/ml) and urine (<10 μg/ml) from healthy humans are appreciable, and well in excess of modern instrument detection limits (pg–ng injected), the complexity of the biological matrix and the competitive nature of the ESI event can make their detection difficult. The situation is different when analyzing urine or blood from patients suffering from cholestatic liver disease or synthetic defects in bile acid synthesis, in which case, bile acids/alcohols are readily detected in urine using negative-ion ESI-MS and previously with negative-ion FAB-MS (26, 28–30, 35–38) (Fig. 1). When bile acid synthetic pathways are intact, ESI-MS spectra reveal ions corresponding to the conjugates of normal (primary) bile acids chenodeoxycholic acid and cholic acid (m/z 448, 464, 498, 514). However, if the liver disease is caused by a mutation in a gene encoding one of the enzymes involved in bile acid biosynthesis, the ESI-MS spectrum reveals a series of ions that characterize the particular genetic defect.
Fig. 1.
Negative-ion FAB-MS (upper panel) and ESI-MS (lower panel) spectra of a urine extract from an infant with cholestatic liver disease. Major peaks correspond to di-, tri-, and tetrahydroxycholanoylglycine (m/z 448, 464, and 480, respectively), di-, tri-, and tetrahydroxycholanoyltaurine (m/z 498, 514, and 530, respectively), sulfated dihydroxycholanoylglycine (m/z 528), and -taurine (m/z 578 in FAB spectrum and doubly charged at m/z 288.6 in ESI spectrum), glycine- and taurine-conjugated 7-hydroxy- (m/z 444 and 494, respectively), and 7,12-dihydroxy-3-oxochol-4-enoic acids (m/z 460 and 510, respectively). Glucuronides of tri- and tetrahydroxycholest-4-en-3-ones are seen at m/z 607 and 623, respectively, and m/z 657 corresponds to the 3-sulfate, 24-glucuronide of 24-hydroxycholesterol (see Tables 1 and 2 in ref. 38). Reproduced with permission from (38).
The major advantages of the direct infusion or nanospray approaches are simplicity, in that sample preparation is minimal, and the "gentle" nature of the procedure, allowing the analysis of labile bile acids that may be abundant in inherited or acquired deficiencies of bile acid biosynthesis or metabolism, e.g., 7-hydroxy-5-enes, 7-hydroxy-4-en-3-ones. Further, the absence of a derivatization step excludes the possible generation of chemical artifacts. High-throughput analysis is now made simple with the use of chip-based ESI, such as the NanoMate from Advion Biosystems, which, when coupled to a modern instrument such as the LTQ-Orbitap from Thermo Fisher that gives high resolution (100,000, FWHM, full width at half maximum) and exact mass (<2 ppm) spectra in a matter of seconds, successive samples can be analyzed approximately every two minutes. A significant advantage of this system over regular direct infusion methods is the absence of any carryover as a new ESI tip and sample loader is used for each sample.
Direct infusion ESI methods suffer from the problem of competitive ionization, which limits the dynamic range of analysis. To overcome this problem, it is possible to include an ion-exchange step in the sample preparation protocol (see below) (26, 38, 39). However, a more popular route is to exploit LC separation and perform LC-MS (see below).
In the absence of HPLC separation, isomeric bile acids are indistinguishable by simple ESI-MS, although the application of exact mass measurement at high resolution allows the differentiation of compounds of similar nominal mass, e.g., disulfates of trihydroxycholanoyltaurine ([M-H]− m/z 674.1980, C26H44NO13S3−) and monoglucuronides of dihydroxycholanoyltaurine ([M-H]− m/z 674.3216, C32H52NO12S−) (Fig. 2). Further information can also be obtained by utilization of collision-induced dissociation (CID). This may be performed either "in-source", when utilizing ESI-MS or in a collision cell, when tandem mass spectrometry (MS/MS or MSn) is employed. For example, the [M-H]− ion of a disulfate of trihydroxycholanoyltaurine will fragment with the loss of the one and two SO3 groups, giving ions at m/z 594 ([M-H-80]−) and 514 ([M-H-2 × 80]−), respectively(Fig. 2), whereas the [M-H]− ion of a glucuronide of a dihydroxycholanoyltaurine will lose the glucuronic acid moiety and 176 Da giving a fragment at m/z 498 ([M-H-176]−). Of course, if both the glucuronide and disulfate are present as a mixture, a composite spectrum will result. This is a recurrent problem for direct infusion measurements whether MS/MS is used or not.
Fig. 2.
Theoretical mass and isotopic pattern for [M-H]− ions of a disulfate of a trihydroxycholanoyltaurine ([M-H]− m/z 674.1980) (upper panel), and (center panel) a glucuronide of a dihydroxycholanoyltaurine ([M-H]− m/z 674.3216). Resolution is 30,000 FWHM. Negative-ion ESI-MS spectrum utilizing "in-source" CID of a mixture of a disulfate of a trihydroxycholanoyltaurine ([M-H]− m/z 674, [M-H-80]− m/z 594, [M-H-2 × 80]− m/z 514) and a monoglucuronide of a dihydroxycholanoyltaurine ([M-H]− m/z 674, [M-H-176]− m/z 498] (lower panel). The compounds were isolated from urine by solid phase extraction from an infant with cholestatic liver disease (as in Fig. 1).
When ESI-MS/MS is performed, fragmentation is achieved in a collision cell that separates the two mass analyzers, MS1 and MS2, either in space as on beam instruments or in time as on ion-traps. Today, most MS/MS experiments are performed at low collision-energy (<100 eV), in which case product-ion spectra are dominated by simple neutral losses and/or low-mass fragment ions. For conjugated bile acids, specific neutral losses or low-mass fragment ions are observed that define the type of conjugation (see Tables 2 and 3 in ref. 40). In the negative-ion mode, taurine conjugated bile acids give characteristic fragment ions at m/z 124 (H2NC2H4SO3−), 107 (CH2CHSO3−), and 80 (SO3−), glycine conjugates give an ion at m/z 74 (H2NCH2CO2−), whereas sulfated bile acids give a characteristic fragment ion at m/z 97 (HSO4−) and a 80 Da (SO3) neutral loss (Fig. 3). These ions are readily observed on beam instruments but are lost in most ion-trap applications when the bottom third of the spectrum is lost (41). In such cases, significant ions are observed that result from the loss of the taurine residue and water molecule(s) to give ions, e.g., at m/z 353 ([M-H-125-36]−) and 355 ([M-H-125-18]−) when [M-H]− ions of trihydroxycholanoyltaurine and dihydroxycholanoyltaurine are fragmented, respectively (42). With trihydroxy- and dihydroxycholanoylglycine, major fragment ions are observed at m/z 402 ([M-H-44-18]−) and 404 ([M-H-44]−), resulting from the loss of CO2 with and without a molecule of water, respectively. The intensity of these ions is dependent on the location of the ring hydroxyl groups, and their pattern may offer some degree of isomer differentiation. Similar ions are also formed in experiments performed on beam instruments but can be swamped by abundant low-mass fragment ions. Bile acids conjugated with sugars, such as glucose, N-acetylglucosamine, or glucuronic acid, give neutral losses of 162, 203, and 176 Da, respectively (Fig. 3). As mentioned above, bile acids are conjugated with sugars via the anomeric carbon of the sugar and an alcohol or carboxyl group of the bile acid. In the case of ester conjugates, loss of the sugar group is particularly facile and allows the differentiation of the two types of conjugation (43).
Fig. 3.
Low-energy MS/MS spectra of ESI-generated [M-H]− ions of (a) cholic acid m/z 407, glycocholic acid m/z 464, taurocholic acid m/z 514: (b) taurolithocholic acid m/z 482, lithocholic acid 3-sulfate m/z 455, lithocholic acid 3-glucuronide m/z 551: (c) hyodeoxycholic acid 3-glucoside m/z 553, ursodeoxycholic acid 7β-N-acetylglucosaminide m/z 594. Spectra were recorded on beam instruments.
The observation of distinct fragment ions and characteristic neutral-losses upon CID allows the application of precursor-ion scans and neutral-loss scans, which pinpoint the precursor-ions generating a specific fragment ion or undergoing the defined neutral loss. This is nicely exemplified by Libert et al. (44), who performed neutral-loss scans for 80 Da to identify [M-H]− ions of sulfated glycine (m/z 528, 544, 560) and taurine (m/z 578, 594, 610) conjugates of di-,tri-, and tetrahydroxycholanoic acids in a urine sample from a patient suffering from biliary atresia, and precursor-ion scans for m/z 124 to identify [M-H]− ions of taurine conjugated bile acids (m/z 498, 514, 556, 572) in serum from a patient with neonatal adrenoleukodystrophy. Precursor ions at m/z 556 and 572 were suggested to correspond to [M-H]− ions of taurine conjugated tri- and tetrahydroxycholestanoic acids. To gain maximum sensitivity and still operate the mass spectrometer in the MS/MS mode, selected reaction monitoring (SRM), (also called multiple reaction monitoring, MRM), can be performed on both beam and ion-trap instruments. In this case, sample is injected, and throughout the time of the infusion, only specific fragmentation channels are monitored, e.g., on beam instruments [M-H]−→80 for taurine-conjugated bile acids and [M-H]−→74 for glycine conjugates. Using this procedure, Mushtaq et al. (45) have measured the levels of di- and trihydroxycholanoylglycine and taurine in blood spots on Guthrie cards, and Yousef et al. (46) have quantified bile acids in urine.
Negative-ion ESI-MS/MS spectra of isomers of conjugated bile acids differing in the position of ring hydroxyl groups are similar when recorded at low collision-energy. This is because of the dominance of a few abundant ions that are characteristic of the conjugating group. This is less evident in spectra recorded on ion-trap instruments where high abundance low-mass fragment ions are lost, and in fact, taurochenodeoxycholic acid and taurodeoxycholic acid can be differentiated from their MS2 spectra on account of differences in ion abundance. Similar results are also obtained with FAB-MS/MS (47). With respect to unconjugated bile acids, the major fragment-ions result from the neutral loss of CO2 (44 Da) or H2CO2 (46 Da) and with accompanying water molecules (44 or 46 + n × 18 Da).
Although seldom used today, high-energy CID (>400 eV) offers advantages for the differentiation of bile acid isomers. When bile acid [M-H]− ions undergo high-energy CID, carbon-carbon bonds are cleaved within the ring system and side-chain, allowing the location of substituents. This has been well documented in studies by Tomer and Gross (48) and the authors of this review (40, 49). Today, high-energy CID is still performed in a few laboratories on magnetic sector instruments interfaced with either fast-atom bombardment or ESI ion-sources but more commonly on tandem time-of-flight (TOF-TOF) instruments using matrix-assisted laser desorption/ionization (MALDI) (50). Bile acids give [M-H]− ions in MALDI (51, 52) so should also give structurally informative MALDI-MS/MS spectra.
Whether MS/MS is performed with high- or low-energy CID isomer differentiation from biological mixtures can be difficult as it is probable that the [M-H]− ion itself is a composite peak comprising not only chemical isomers of the target molecule but also of other compounds with the same nominal mass. The only satisfactory way to overcome this problem is to incorporate a prior chromatographic step, preferably on-line (see below).
In this section, we have concentrated on negative-ion ESI-MS and ESI-MS/MS; however, with this form of ionization, unconjugated bile acids tend to give a lower response than either aminoacyl amidated or sulfuric acid conjugated bile acids (13, 38). This is also true of unconjugated bile alcohols, and these molecules may be better analyzed by GLC-MS following appropriate derivatization (see below).
SAMPLE PREPARATION PROCEDURES
A detailed discussion of various techniques for extraction and group separation of bile acids has been published (53) and only the most common ones are discussed here. The selection also reflects the authors' personal preferences.
Extraction of bile acids
The need for sample preparation is determined by the ratio between levels of bile acids and levels of interfering substances in the sample matrix. When the bile acid concentration is high (e.g., in bile), dilution may suffice. In other cases, extraction with solvent or sorbent is the start of an analysis. The purpose is to remove proteins and desalt and/or delipidate the sample. HPLC and LC-MS methods using a precolumn incorporate the extraction step on-line.
Bile acids can usually be extracted from fluids and tissues with ethanol or acetonitrile. Ultrasonication or heating is used, and some authors recommend addition of ammonium or sodium hydroxide to decrease protein binding (see refs. 53, 54). However, base can catalyze hydrolysis of labile conjugates, e.g., ester glycosides, or elimination of hydroxyl groups in labile positions (e.g., 7-hydroxy-Δ5 or -Δ4-3-one). A solvent/sample ratio of at least 10 is recommended. The extract from plasma can be directly analyzed by LC-MS (e.g., ref. 55) and other HPLC methods.
In extractions of tissues and feces, an ethanol extraction may be followed by extraction with a less polar solvent, e.g., chloroform (or methylene chloride)/methanol (1:1 or 2:1) or 2-propanol, (in some studies by Soxhlet extraction) to recover less polar bile acid derivatives (e.g., the fatty acid esters) and bile acids remaining in a lipid residue (24, 56–58). Early studies using feces from individuals given radioactively labeled bile acids gave evidence for quantitative extraction (59–61). A relatively recent comparison of different methods to extract fecal bile acids and sterols was made by Batta et al. (62). Although not directly comparable, the results support an extraction sequence as above. However, the simplified method described by Batta et al. (and many other methods) is based on the assumption that the fecal bile acids are unconjugated, which is not a valid assumption for infants, many clinical conditions, and other experimental conditions in animals. An important aspect of the analysis of fecal bile acids is the irregularity of bile acid excretion and the inhomogeneity of fecal samples as carefully studied by Setchell et al. (63). Many studies of fecal bile acid excretion have neglected this problem and the importance of analyzing aliquots of thoroughly homogenized 4–5 day collections of feces. Together with poorly designed methods for analysis of fecal bile acids, this has affected the evaluation of a potential involvement of bile acids in the causation of large bowel cancer on the basis of correlations between bile acid levels and profiles in feces and the occurrence of cancer (see refs. 57, 64).
Recoveries of bile acids in any extraction procedure are difficult to evaluate. Recovery of added reference compounds is not sufficient proof of efficiency because protein-bound bile acids may not become equilibrated with the added bile acids. Radioactive labeling of the endogenous bile acids to be extracted is usually not possible. An example: Mano et al. (65) found that ethanol alone could not extract chenodeoxycholic acid from a brain cytosolic fraction unless 7.3 M guanidine hydrochloride in Tris buffer pH 8.6 was first added. Obviously, addition of external chenodeoxycholic acid would not necessarily have revealed the loss of endogenous bile acid in the absence of guanidine hydrochloride.
The alternative to solvent extraction of fluids is sorbent (solid phase) extraction. Since the early 1980s, this has been done predominantly with octadecylsilane substituted (ODS) silica. The primary aim is to obtain a solution of bile acids without proteins, inorganic salts, and polar organic compounds from the intermediary metabolism. There are many brands of ODS silica with unknown differences in performance and the literature has many examples of difficulties in reproducing published procedures (see ref. 66). Several factors contribute to these difficulties, e.g., ion exchange, protein binding, and experimental conditions (67). In many procedures, sodium hydroxide is added to the sample, which may damage the ODS silica structure. Extraction problems can arise not only with plasma but also with urine, and recoveries vary with structure of bile acid and type of conjugation (68). Rodrigues and Setchell (69) compared several brands of ODS silica and found large variations in recoveries of common bile acids in plasma. High and reproducible extraction efficiencies are obtained if the sample is diluted with one volume of 0.5 M triethylamine sulfate, pH 7, and heated at 60–64°C before being passed through the cartridge of ODS silica (68). After a wash, the bile acids are eluted from the cartridge and can be analyzed by ESI-MS and LC-MS.
Another factor whose importance often is not appreciated is the poor solubility of compounds to be extracted. When tissue or feces is extracted with solvents, the dried extract is frequently "dissolved" in water or aqueous methanol and passed through an ODS silica cartridge. Depending on the structure of the bile acids/sterols, the extraction can be very inefficient because many of the compounds are not soluble in water/dilute methanol and pass unextracted through the sorbent bed (see ref. 56). Instead, the extract should be dissolved in methanol or ethanol, and the effluent cycled repeatedly through the bed, each time with reduction of the alcohol content of the effluent (56, 70). Nonpolar sterols and polar bile acid conjugates will all be retained by the sorbent (see also ref. 71). An alternative to the recycling is to pass the sample through a bed of hydrophobic Lipidex 5000 followed by ODS silica (58).
An important advantage of solid phase extraction compared with solvent extraction is that inorganic ions are removed. A solid phase extract of plasma or urine can be directly analyzed by negative ion ESI-MS without the presence of sodium adduct ions of double conjugates carrying two acidic groups (see Figs. 1 and 2). The removal of polar organic acids also diminishes a potential suppression of the bile acid ions.
Group separation of bile acids
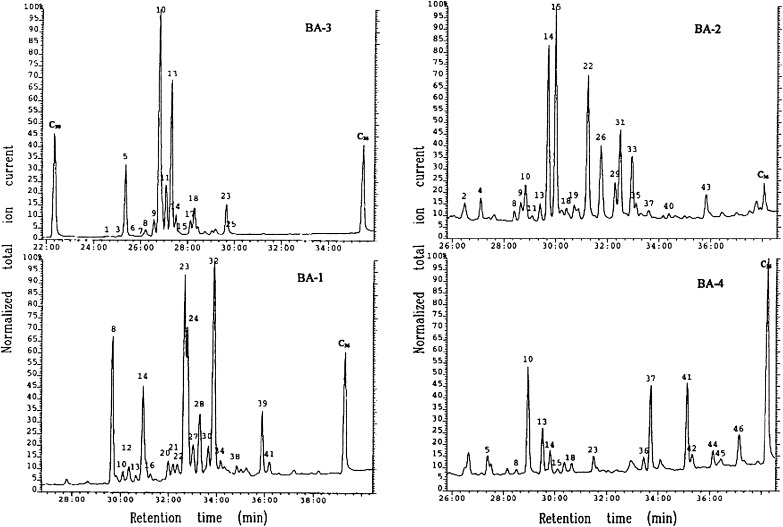
When the bile acid mixtures are complex, many types of conjugates are present, or the range of concentrations of individuals is wide, a group separation may be needed. This is preferably based on the charge differences of conjugates and/or the differences in the polarity of the bile acid nucleus (the latter is discussed in ref. 53). Groups of conjugates are most conveniently separated by ion exchange chromatography. The best documented methods use ion exchangers prepared by attachment of ionic groups to Sephadex LH-20. In contrast to many other cross-linked polymers, the Sephadex matrix gives little or no nonspecific or irreversible adsorption in ethanol/(methanol)/water/chloroform mixtures. Thus, separations depend entirely on ion exchange and not on interactions with uncharged polar groups. The anion exchanging groups most commonly used in separations of bile acids/bile alcohols and their conjugates are diethylaminohydroxypropyl- [DEAP-LH-20 or Lipidex-DEAP, weak anion exchanger (72)], piperidinohydroxypropyl- [(PHP-LH-20, strong anion exchanger (73)], and triethylaminohydroxypropyl- [(TEAP-LH-20, strong anion exchanger (74–77)]. Unfortunately, only PHP-LH-20 is commercially available today (Shimadzu). Literature for the simple and rapid synthesis of the other ion exchangers is cited in ref. 74. The ion exchangers are preferably used in their acetate or bicarbonate forms to avoid chemical reactions catalyzed by the base form of strong anion exchangers. Solvent is chosen depending on the solubility of the extract to be separated. Aqueous methanol or ethanol (70–90%) (with addition of methylene chloride or propan-2-ol if needed) are suitable. Elution of conjugate groups, from the weak free bile acids to the strongly acidic taurine conjugates and disulfates, is effected by addition of acetic acid, formic acid, ammonium acetate buffers at different concentrations and increasing pH, finished by ammonia or ammonium bicarbonate at high pH to recover the disulfates (these eluants are volatile and compatible with ESI-MS analyses). The ion exchangers can be reused and are stable for years in their acetate form in methanol or ethanol in a refrigerator. Details on column sizes, solvent systems, and buffers for elution of different conjugate groups are found in a large number of papers concerned with the analysis of bile acid profiles in biological material. Examples from the Japanese and Swedish laboratories using ion exchange as a sample preparation prior to GLC-MS or HPLC include refs. 39 and 75–93. Some of these references contain complete descriptions of methods for analysis of bile acid profiles in different biological materials. The large differences in bile acid profiles between different groups of conjugates separated by ion exchange chromatography are exemplified for human urine in Fig. 4.
Fig. 4.
Total-ion current chromatograms obtained in the GLC-MS analyses of bile acid profiles of different (selected) groups of conjugates isolated by ion exchange chromatography of a solid phase extract of urine from a healthy pregnant woman in the 38th week of gestation. Samples equivalent to 60 μl, 100 μl, 120 μl, and 200 μl of urine were injected from fractions BA-3 (sulfates with or without aminoacyl amidation), BA-1 (aminoacyl amidated only), BA-2 (glucuronidated only), and BA-4 (doubly conjugated with sulfate and glucuronide) following removal of the conjugating groups and derivatisation [from Meng and Sjövall (77), with permission]. Further subfractionation of conjugate groups is possible if needed. However, the figure clearly illustrates the differences in bile acid profiles between the groups. The numbers above the peaks refer to identified or partially characterized bile acids listed in Table 5 of the cited paper. C36 is the peak of hexatriacontane added before injection. The figure (and Table 5 in the cited paper) also illustrates the complexity of bile acid profiles in urine which would be difficult to elucidate by direct LC-MS analysis of the intact conjugates.
Other ion exchangers [DEAE-Sephadex (24); Bond-Elut SAX (94)] have been used for group separation of free, aminoacyl amidated, and sulfated bile acids. Other conjugates were not studied and further work is needed to establish mobilities of different conjugates, potential adsorptive losses, and compatibility with less polar solvent mixtures. The large number of different ion exchangers now available motivates a systematic study of their possible use in bile acid analysis. Because of the great improvements in mass spectrometric sensitivity and resolution, improved sample preparation procedures could be developed based on automated on-line capillary systems.
The fractions from the ion exchange separation can be analyzed by ESI-MS. The weaker acids can be detected with higher sensitivity after separation from the stronger acids, which may cause ion suppression. Also, cation adducts of doubly charged anions are eliminated by the elution with acids or ammonium acetate buffers.
Ion exchange is a quick and simple method for separation of bile alcohols and bile acids. Mass spectra of TMS ethers of bile acid methyl esters and bile alcohols can be very similar, because the side chain of a C27 bile alcohol is 2 Da lighter than the side chain of a C24 bile acid methyl ester and the nuclear substituents may be the same. Thus, it can be difficult to distinguish a bile alcohol from a bile acid. The physical separation of bile acids and bile alcohols prior to GLC-MS eliminates the problem. Another method, using derivatization, is described in the section on GLC-MS.
HPLC AND LC-MS
LC-MS
When a mixture is to be analyzed, LC-MS is the first method of choice, depending on the levels of bile acids and the biological matrix. Currently, most LC-MS (and LC-MS/MS) methods for bile acid analysis rely on reverse-phase chromatography and atmospheric pressure ionization. A wide range of flow rates and column dimensions can be used, extending from 1 ml/min on 4.6 mm (inner diameter) columns down to ∼1 μl/min on 180 μm (i.d) columns (13, 38, 83, 95). Bile acids are acidic molecules with pKa values of ∼1.5 for taurine-conjugated, ∼4.5 for glycine-conjugated, and ∼6 for unconjugated bile acids (96), thus, to achieve satisfactory chromatographic performance, it is preferable to use a buffered mobile phase. Some groups prefer work at neutral pH, e.g., 2–30 mM ammonium acetate in aqueous methanol or acetonitrile, in which case bile acids are predominantly in their deprotonated form (38, 97) whereas others prefer acidic conditions; e.g., ∼10 mM ammonium acetate in aqueous acetonitrile and/or methanol adjusted to pH ∼4 with acetic or formic acid (55, 98) where taurine conjugates are deprotonated, and glycine and unconjugated bile acids exist predominantly in the unionized form. When using ESI, it is important to avoid the use of nonvolatile additives in the LC solvents that may have a detrimental effect on the spray-ionization process.
LC-MS (and MS/MS) has been exploited for bile acid analysis of human and rodent urine (43, 55, 83, 86, 97, 99–102), plasma/serum (13, 55, 97, 98, 103–110), bile (13, 55, 111, 112), cecal content (113, 114), liver (55, 97, 114), feces (114), and more surprisingly, brain (65, 115). However, care must be taken in interpreting LC-MS data, and to postulate a structure based on the mass of an [M-H]− ion alone can lead to mistaken identifications. In quantitative studies, several groups use plasma stripped with charcoal to remove all bile acids and then show that this "plasma" does not quench signals of added bile acids. They also use this as "proof" for an absence of interference from a "plasma" background. However, it is likely that stripping removes not only bile acids but also related material that can both give peaks and cause suppression of bile acid signals. So the fact that stripped plasma does not influence the analyses does really not prove anything.
High sensitivity can be achieved by utilizing MRM, e.g., [M-H]−→124 or 80 (54, 55, 98, 103, 104), [M-H]−→74 (54, 98, 103, 104), and [M-H]−→ 97 (101) for taurine, glycine, and sulfuric acid conjugated bile acids, respectively.
Quantification is best performed by addition of isotope-labeled internal standards, when available, and then including the necessary transition in the MRM inclusion list. Internal standards should be added as early as possible in the analytical process so as to account for analyte loss during sample preparation. Isotope labeled bile acids are commercially available from, e.g., CDN isotopes and Steraloids, and many have been prepared in the analytical laboratories. For the quantification of unconjugated bile acids, many authors have opted to use the residual precursor-ion following MS/MS (55, 104). This practice is not recommended as the specificity of MRM is lost.
The advent of high resolution (100,000 FWHM) mass spectrometers that are able to perform full mass scans on a chromatographic time scale has opened the possibility of performing bile acid analysis using full-scan LC-MS on samples that have undergone a minimum of sample preparation, e.g., protein precipitation. Using this procedure, Bobeldijk et al. (97) were able to identify 13 different bile acids from samples of mouse serum and plasma (50 μl) in a 40 min chromatographic run (C18 2.5 μm, 2.1 × 50 mm column) utilizing exact mass and retention time with reference to commercially available authentic standards. Semi-quantitative data were also provided based on comparison of ion-current to an isotope labeled internal standard. Modern mass spectrometers provide impressive detection limits (e.g., 10–50 pg on-column utilizing LC-ESI-MS on an LTQ-FT from Thermo Fisher), meaning that as little as 10 μl of plasma was required for injection on-column by Bobeldijk et al. (97). The reader is reminded once more that it is unsatisfactory to postulate a bile acid structure from a mass value alone in the absence of confirmatory data such as retention time or MS/MS fragmentation and reference to an authentic standard. Similar sensitivity to that achieved by LC-high resolution MS can be achieved in MRM experiments on modern tandem quadrupole instruments where limits of detection are of the order of 20–50 pg on-column (55). Using ultra performance/pressure liquid chromatography (UPLC) on a 1.7 μm particle C18 column (100 × 2.1 mm) in a 25 min run, Alnouti et al. (55) were able to identify 15 bile acids in mouse bile but only five in mouse plasma and none in mouse urine. Quantitative estimates were made based on the addition of two isotope labeled standards. When utilizing MRM in LC-MS/MS experiments on tandem quadrupole instruments, dynamic range may be of the order of 2,000.
Although most LC-MS (and MS/MS) studies are targeted toward the analysis of C24 bile acids, longer alkyl chain bile acids are also present in body fluids and tissues (38, 103, 115–117). Johnson et al. (118) developed an ESI-MS/MS method for analysis of C27 bile acids in plasma of patients with peroxisomal disorders. Unconjugated C27 bile acids, which are precursors of the C24 bile acids, are abundant in plasma/serum of healthy humans (e.g., 7α-hydroxy-3-oxocholest-4-enoic acid 80 ng/ml, cf. cholic acid 110 ng/ml) (75, 115) but tend to be ignored in most targeted approaches to bile acid analysis. However, specific LC-MS/MS methods have been developed for the analysis of oxo- and hydroxycholestenoic acids and their bile alcohol precursors (95, 119–121).
The recent introduction of UPLC using columns packed with smaller particles (<2 μm) has provided an improvement in LC performance over HPLC, allowing analysis with increased peak capacity or, alternatively, at greater speed. UPLC tends to be performed at higher flow rates (600–200 μl/min) (55, 105, 114), although it has also been performed utilizing microbore columns (100 μl/min) (122). As discussed above, Alnouti et al. (55) have exploited UPLC in combination with MRM and were able to separate 17 standard bile acids in a 20 min gradient, and Hagio et al. (114), using selected ion recording (SIR), have separated 22 bile acid standards in a 24 min gradient. It should be noted that in both of these studies, the bile acid standards analyzed were unconjugated, glycine-, or taurine-conjugated forms of cholic, muricholic, hyodeoxycholic, chenodeoxycholic, deoxycholic, ursodeoxycholic, or lithocholic acid. Other forms of conjugation or modified steroid skeleton were not considered. The introduction of UPLC, where peaks can now elute in a few seconds, has shifted analytical challenges back to the manufacturers of MS instruments to provide ever increasing scan rates. This is particularly challenging for the recording of product-ion scans that are inherently less sensitive than MRM and MS scans.
Many LC-MS and LC-MS/MS studies of bile acids are either focused on a few predefined targets (<20) when using MRM (55, 98, 101, 103, 104, 111) or SIR (86, 99, 114) or are performed as part of an "omic" study where attention is focused on maximizing the number of observable features rather than specifically identifying the bile acid species themselves (105, 122). By their very nature, both these types of study tend to ignore many of the known bile acids present in the matrix of interest. In this regard, it is important to consider the value of bile acid profiling studies where samples have been prefractionated according to functional groups prior to analysis by either GLC-MS or LC-MS/MS. Using this methodology, Yang et al. (38) were able to partially characterize over 150 bile acids and conjugated bile alcohols in urine from a child with cholestatic liver disease.
HPLC
HPLC without mass spectrometric detection is still being used for simultaneous analysis of free bile acids and intact conjugates. An advantage is that solvent systems containing nonvolatile components can be chosen. A large number of systems have been described mostly based on methanol, acetonitrile, and phosphate or acetate buffers. Alternative systems using ion-pairing or complexing with cyclodextrins have been described. The subject has been reviewed (see, e.g., refs. 96, 123–125). Examples of the excellent ability of HPLC (isocratic or gradient systems) to separate bile acids in biological materials are found, e.g., in refs. 126 (human bile), 80 (3-sulfates of aminoacyl amidated bile acids in human bile), 127 (rat bile and serum), 124 (30 bile acids in rat liver tissue using only one extractive delipidation and one solid phase extraction prior to HPLC), 128 (human bile and stool), 129 (mouse bile), 130 (bird bile), and 131 (reference bile acid acyl 24-glycosides). Useful tables of retention times of bile acids and different conjugates can be found in refs. 80, 127, 131, and 132.
Tables and figures presented in the papers mentioned above suggest that analysis of bile acid mixtures as complex as those in feces or urine will be difficult or completely unsatisfactory using direct HPLC without mass spectrometric detection. For most samples except bile, UV detection also lacks the sensitivity required (particularly in the absence of peptide bond or oxo group). Other detection methods include an immobilized 3α-hydroxysteroid dehydrogenase (HSD) system capable of detecting 10–20 pmol bile acid, [(133) used e.g., by Sakakura et al. (124, 127), see above, in their studies of bile acids in rat bile, liver, and plasma] and evaporative light-scattering mass detector (detection limit 80 pmol, preventing use of nonvolatile buffer) (128, 129). For comparison, the concentrations of individual bile acids in serum from healthy subjects are in the 0.1–1 μM range (134, 135) and modern mass spectrometers can detect bile acid quantities of just a few fmol, thus, requiring greatly reduced sampling volumes. It should be emphasized that the HSD detection system only measures bile acids with a free 3α-hydroxy group and that the influence of potentially hindering groups (e.g., 1-, 2-, 4-, or 5-hydroxy or 7-sulfoxy groups) is not known.
Precolumn derivatization with UV-absorbing or fluorescent groups has been described to improve sensitivity of detection [listed in the review by Roda, Piazza, and Baraldini (123)]. The 3α-hydroxyl (or 3-oxo) or the carboxyl group are sites of derivatization. Many important bile acids will become excluded from the analysis when a 3α-hydroxyl group is chosen for derivatization (cf. the HSD detection method) and taurine conjugates will not become derivatized when the carboxyl group is selected. The sensitivity with fluorescent derivatives is high [e.g., 1–2 pmol when the carboxyl group is derivatized with 2-bromoacetyl-6-methoxynaphthalene (136) and 15 fmol with 3-(4-bromomethylphenyl)-7-diethylaminocoumarin (137)] and may be chosen for specific analytical needs, e.g., analysis of bile acid 3-sulfates (138) or low levels of 24-hydroxycholestanoic acids (137). 3-(1-Anthroyl) derivatives have been used with a detection limit of 20 fmol in quantifications of 7- and 12- sulfates in human urine (139). However, the derivatization methods are not the choice for analysis of comprehensive bile acid profiles in feces or urine.
GLC AND GLC-MS
Historically, GLC and GLC-MS have been the most commonly used methods for analysis of bile acid profiles. Unless complemented with MS detection, the specificity of GLC (e.g., with flame ionization detection) is only partial, in analogy with the case for HPLC without MS. Following the introduction of LC-MS, the importance of GLC-MS has decreased, particularly in routine applications. This is mainly because of the need for removal of charged groups that make the conjugated bile acids "non-volatile" and partly because of the need for prior separation of groups of conjugates to obtain information on the state of conjugation of the individual bile acids. However, GLC-MS also has important advantages. Complex mixtures of unconjugated bile acids are best analyzed by this method. The separations on capillary GLC columns are still superior to those obtained in liquid-phase separations, and the electron impact mass spectra of suitable derivatives provide more structural information than can be obtained in LC-MS/MS analyses. Published retention indices (RIs) and mass spectra aid in the identification of bile acids by GLC-MS. Thus, LC-MS/MS and GLC-MS are complementary methods that provide an abundance of information in analyses of bile acid profiles and permit quantification based on monitoring of specific fragment ions and use of internal standards, preferably analogs labeled with stable isotopes.
Cleavage of conjugates
Cleavage reactions may be incomplete or give side reactions. If the samples can be analyzed by ESI-MS before and after the reaction, comparisons of the spectra and the GLC-MS results may reveal such problems. In our experience, the efficiencies are increased when the cleavage reactions are applied to samples after ion exchange subfractionation. Glycine and taurine conjugates are usually hydrolyzed with cholylglycine hydrolase (140, 141). The reaction rates are influenced by the number and positions of hydroxyl and oxo groups (decrease with an increasing number) and by the structure of the side chain. Conjugates of C27 bile acids are not hydrolyzed by the commercial enzyme from Clostridium perfringens (142, 143), but hydrolysis can be achieved by anaerobic incubation with a rat fecal suspension (142). In a method for analysis of fecal bile acids, the fecal homogenate was incubated in water for an hour to hydrolyze glycine and taurine conjugates prior to solvent extraction (54). However, in such a procedure, other microbial reactions may occur that will change the composition of the bile acid mixture. The applicability of the method to samples containing other conjugates, e.g., sulfates in mouse (20) and infant (144) feces needs to be evaluated. Glycine and taurine conjugates of 3- or 7-sulfated bile acids are cleaved by cholylglycine hydrolase, leaving the sulfates intact (145). Amidated bile acids that are not hydrolyzed by enzyme may be split by vigorous alkaline hydrolysis, but this destroys sulfates, glycosides, and bile acids with oxo or allylic hydroxyl groups. A new method using brief exposure to alkali in propylene glycol in a microwave oven may offer an alternative (146).
Sulfates are best cleaved by solvolysis [available sulfatases are too structure-selective and fail to hydrolyze many sulfates (147)]. Many conditions have been described, often producing changes of bile acid structure. Methods involving use of dimethoxypropane or acetone cause formation of acetonides from vicinal cis glycol structures (e.g., 6,7-diols in rodents) and in other methods, ethyl acetate causes transesterification. We have found the method described by Hirano et al. (148), with a modification to prevent destruction of 7-hydroxy-5-ene structures (76), to be reliable. Another method, based on the use of HCl in aq. dioxane, was developed after comprehensive studies by Princen, Meijer, and Kuipers (149). It should be pointed out that the rates of solvolysis differ depending on the site of sulfation (150).
Ester glycosides are hydrolyzed by brief exposure to 0.3 M sodium hydroxide (100) for analysis by LC-MS and GLC-MS. Ether glycosides require use of enzymes, the β-glucuronidase from Helix pomatia being generally used for hydrolysis of 3- and 6-glucuronides (151). Less is known about hydrolysis of glucosides and N-acetylglucosaminides, and there are no ideal enzymes for analytical use (152).
Derivatization
Analysis by GLC and GLC-MS requires derivatization of polar groups. Side reactions and partial derivatizations are potential problems. Methylation (Me) with fresh diazomethane continues to be the best method for derivatization of the carboxyl group. Trimethylsilyldiazomethane in toluene is a potential alternative reagent (26). Butyl instead of methyl esters have been prepared in order to increase the retention times and to separate bile acid derivatives from derivatives of sterols and bile alcohols in analyses of serum and fecal bile acids (62, 153–155). However, the acidic conditions and heating during butylation are harmful to labile bile acids found, e.g., as normal and pathological intermediates in bile acid biosynthesis, and reactions with sulfates and glycosidic conjugates are not well defined.
Hydroxyl groups are most commonly protected by alkylsilylation or acetylation. The former is preferred both from chemical and mass spectrometric points of view. Acetylation was used early (see ref. 6), but groups using this method have changed their derivatization to trimethylsilylation (e.g., refs 62, 154–156). TMS and dimethylethylsilyl (DMES) ethers are the most common derivatives (see refs. 157–159). The DMES ethers are preferred by the Japanese groups and have advantages both from the GLC separation and mass spectrometric points of view. Retention times of Me-DMES derivatives increase more with an increasing number of hydroxyl groups than those of the TMS derivatives, thus, increasing separation factors between isomeric mono-, di-, tri-, and tetrahydroxylated acids. Mass spectra of DMES ethers give a more intense M-29 ion than the M-15 ion given by TMS ethers, thus, giving better information about the molecular weight. The spectra are otherwise analogous.
Alkylsilyl ethers can be prepared in many different ways (160). Methods using volatile reagents are most convenient. Care must be taken to avoid hydrolysis of the acid-labile ethers during the workup of the reaction mixture. Reaction rates depend on the position, configuration, and steric hindrance of the hydroxyl groups. This can be helpful in establishing the identity of unknown GLC peaks. Forceful reaction conditions can result in enolization and derivatization of oxo groups. To prevent this reaction, the oxo groups can be converted into oximes (56) or methyloximes (56, 161, 162). This is also a convenient method to distinguish between an unsaturated hydroxy and a saturated oxo bile acid by ESI-MS. Syn (Z) and anti (E) isomers are formed, which may or may not separate upon GLC or HPLC.
Bile acid profiles are usually analyzed using a nonselective nonpolar stationary phase with low bleeding. Retention times on such phases are primarily dependent on molecular size and shape. For comparison with literature data, retention times are best expressed as Kovats RIs or methylene units (MUs; RI × 0.01). Functional groups give additive (sometimes negative) contributions to RI depending on their position, orientation (α or β, equatorial or axial), interactions between neighboring groups, and the configuration of the steroid skeleton. Absolute RI or MU values differ between studies, but differences induced by a particular functional group (or grouping) are usually similar. Typical examples for comparison are found, e.g., in ref. 163. Extensive tables of RI or MU values can be found in the literature both for different derivatives and different stationary phases, e.g., refs. 77 and 90, 156, 164, 165–171. RI/MU values for derivatives of bile alcohols and oxysterols (172–174) are also valuable when evaluating contributions of different functional groups to the RI (MU) values in analyses of bile acids.
GLC-MS
Mass spectral analysis of the GLC peaks is required for identification and establishment of analytical specificity. This is also evident from the chromatograms in Fig. 4 and the list of bile acids in Table 5 in ref. 77 giving RIs for the 46 peaks seen in Fig. 4. It is outside the scope of this review to discuss mass spectra of bile acids and the reader is referred to reviews, e.g., by Sjövall, Lawson, and Setchell (157); Lawson and Setchell (175); Setchell and Lawson (158); and Sjövall et al. (176). Mass spectra of Me-TMS and Me-DMES derivatives provide information about the number and nature of oxygen substituents. Molecular ions are usually lacking in the case of tri- and tetrahydroxy bile acids, but [M-15]+ (-OTMS) and particularly [M-29]+ (-ODMES) may help to determine molecular weights. Fragmentation can be decreased/optimized by lowering the ionizing energy from the common 70 eV. The general structure of a bile acid may be deduced from typical losses of nxTMSOH (90 Da) [nxDMESOH (104 Da)], side chain (115 Da for a saturated side chain in a C24 methyl ester, varying with side chain length, substituents, and unsaturation), side chain plus D-ring (157 Da except for 12-oxo bile acids when a McLafferty rearrangement results in loss of 155 Da). The combined loss of TMSOH:s (DMESOH:s) and side chain yields an ABCD-ring ion whose mass is increased by 14 Da when an oxo group is present. Addition of 16 Da to an ion indicates an underivatized hydroxyl group, which can be confirmed by more vigorous silylation conditions (e.g., 5-hydroxy). Positional isomers of hydroxycholanoates can usually be distinguished by the formation of fragment ions characteristic of the substituted position(s) (exemplified by m/z 129 from a 3-OTMS-Δ5 or -Δ6 structure, m/z 217 from a 1,3-OTMS structure (and 22-OTMS-C27-OMe), m/z 142 from a 2,3-OTMS structure, m/z 181 from a 3α,4β-OTMS structure, m/z 195 from a 3,6,7-OTMS structure, m/z 243 from 2,3,7-OTMS or 15-OTMS structures, m/z 285 from 3,6,7β-OTMS structures, m/z 208 from a 12-OTMS structure, m/z 249 from 6- or 7-OTMS or Δ5 or Δ6 structures, m/z 175 from 22-OTMS-C24-OMe structures. Typical losses are also seen, e.g., 145 Da from 3,6-OTMS structures, 103 Da from OTMS derivatives of primary hydroxyls, 89 Da for loss of the last OTMS from bile acids with vicinal OTMS groups. Stereoisomers give spectra with essentially the same peaks, but relative intensities can be markedly different. Together with RI values, this helps to suggest a tentative structure.
GLC-MS is used for quantification by construction of selected ion chromatograms from full range scans. Higher sensitivity is obtained by SIR. Common ions to be monitored are [M-nx90]+ (nx104 for DMES derivatives) or structure-specific ions. A major problem is the lack of authentic bile acids for determination of responses relative to the response of a suitable internal standard and the lack of isotopically labeled standards for accurate analysis of targeted bile acids. Semiquantitative results can be obtained by converting fragment ion current to total ion current (from the full-scan spectrum) and making the approximating assumption that the compounds analyzed give the same total ion currents. A less separated semiquantitative profile analysis may be obtained using the flame ionization detector, which gives essentially the same response for all the components (except for some, e.g., with oxo groups, being lost to a minor extent by adsorption in the GLC column or injector). However, in all cases it is essential to characterize the peaks assumed to represent bile acids by preceding GLC-MS analyses of representative samples. It is important to realize that bile acid profiles can differ markedly depending on experimental conditions, so that proofs of specificity must be obtained for each experimental condition studied.
Artifacts and contaminants may confuse the analyses. For example, ethyl esters (+14 Da) may be formed by transesterification in the presence of ethanol and –OTMS (+58 Da) (-ODMES, +72 Da) esters if methylation is incomplete. Degradation products from ODS silica and HPLC phases, plant sterols from rubber materials, compounds from plastics, silicones, etc. may contaminate samples and promote a careful choice and handling of materials used in the analytical procedure.
ENZYMATIC ASSAYS AND IMMUNOASSAYS
Enzymatic assays
Enzymatic methods are used because they are simple. An NAD+ dependent steroid dehydrogenase oxidizes hydroxycholanoates with formation of NADH, which is measured by UV or fluorimetric methods. Because this procedure often lacks the necessary sensitivity, the reaction can be coupled with a reaction using the NADH to reduce a substrate that can be detected with higher sensitivity by fluorimetry. 3α-Hydroxysteroid dehydrogenase is used in clinical chemistry for analysis of plasma bile acids to monitor changes in liver disease. Obviously, the method is highly dependent on the purity of the enzyme, because the bile acids are only indirectly determined from the production of NADH. It is difficult to evaluate the specificity for individual samples, and major abnormalities in bile acid profiles may escape detection. For example, bile acids with a 3β-hydroxy group in patients with HSD3B7-deficiency or 3-oxo bile acids in patients with 3-oxo-Δ4-steroid 5β-reductase deficiency will not be detected, and the analysis will indicate normal or low bile acid levels. The reaction is inhibited by structural features of the bile acid, e.g., neighboring hydroxyl groups, glucuronidation of a 6-hydroxyl group (177), and sulfation of a 7-hydroxyl group. Other dehydrogenases (3β-, 7α-, 7β- and 12α-hydroxysteroid dehydrogenases) have been used for analysis of groups of bile acids containing hydroxyl groups at specific positions. Methods based on oxidation with 3α-hydroxysteroid dehydrogenase are often considered to measure total bile acids, e.g., when used in analyses of plasma bile acids or for determination of fecal bile acids (see references in ref. 178). Although the enzymatic method may be of use in clinical practice, this is clearly a questionable assumption, as the fecal bile acid mixture includes considerable, sometimes major, amounts of 3β-hydroxy and 3-oxo components (56, 179), as well as sulfated (144) and fatty acylated (22, 24) derivatives that are not oxidized by the enzyme. Alkaline hydrolysis, dependent on the conditions, produces unsaturated bile acids from sulfates that may not be determined (21). Comparisons of enzymatic and GLC-MS methods have been made with variable results (see ref. 57). In a recent study with good agreement between the methods (178), no 3β-hydroxy bile acids were detected by GLC. This is a very unusual finding considering the results of many studies published in the past 50 years (see refs. 57–59, 179). It is possible that populations exist [e.g., patients with malabsorption studied by Porter et al. (178)] in whom bacterial transformation of bile acids is minimal and the two methods both measure total bile acids. An experimentally induced change of a value obtained by an enzymatic method reflects either a change of total bile acids or a change of bile acid composition, i.e., is ambiguous. The enzymatic method has to be validated for the particular sample type and experimental conditions studied using LC-MS or GLC-MS methods providing separation of isomeric bile acids and their conjugates.
Immunoassays
Many radioimmunoassays and enzyme immunoassays for simple and rapid targeted analysis of common bile acids in plasma and in in vitro studies were developed 20–25 years ago. An important drawback of immunoassays is their questionable specificity, particularly at low concentrations of bile acid. Specificity is claimed if a series of bile acids are found not to interfere with the measurement of the targeted bile acid. This does not prove an absence of nontargeted unknown material in the sample reacting with the antibody. A reason for use of immunoassays would be the need for a simple method to determine a particular bile acid found by LC-MS and/or GLC-MS profiling to be of diagnostic or prognostic value or for monitoring of treatments with bile acid drugs. This is exemplified by recent development of enzyme-linked immunosorbent assay systems for ursodeoxycholic acid 7-N-acetylglucosaminides (180), ursodeoxycholic acid 3-sulfate (181), and glycolithocholic acid 3-sulfate (182).
CONCLUSIONS AND PERSPECTIVES
The introduction of soft ionization methods and LC-MS/MS has made the analysis of conjugated bile acids possible with high sensitivity, small sample requirements, and simplified sample preparation procedures. Although LC-MS/MS may provide sufficient specificity in analyses of common conjugated bile acids, e.g., in bile or plasma, mixtures such as those found in urine and feces require a combination of LC-MS/MS and GLC-MS to provide sufficient information about the structure of the isomeric bile acids and bile alcohols present in unconjugated, singly, doubly, or triply conjugated forms. Metabolomic studies of native unconjugated bile acids with potential functions as ligands to nuclear receptors are probably still best carried out using GLC-MS. Improved methods for fragmentation of the bile acid skeleton in ions produced by ESI may change this situation. Non mass spectrometric methods, particularly the non chromatographic ones, rely on assumptions regarding the bile acid composition of the material analyzed, and their application should be restricted only to defined samples (defined by mass spectrometric methods) and particular experimental conditions in vitro.
The next generation of methods for bile acid analysis is most likely based on mass spectrometric detection. Instruments and software are available and being developed providing exceptional resolution and sensitivity for high specificity analyses, calculation of elemental composition, and multi-labeling isotope experiments. Considering the close structural similarity of isobaric bile acid isomers in biological materials, coupling to chromatographic separation will usually be required. Sample preparation and separation are the rate limiting steps in high-throughput analyses, and on-line automated sample preparation procedures will be developed. Equipment is already commercially available. The high sensitivity of mass spectrometers permit work in the nanoscale range, and microfluidic systems are available. This development is accelerating because it is needed in all fields of proteomics, peptidomics, lipidomics, metabolomics, etc., and high-sensitivity analysis.
Footnotes
Abbreviations:
- CID
- collision-induced dissociation
- DMES
- dimethylethylsilyl
- HSD
- 3α-hydroxysteroid dehydrogenase
- Me
- methylation
- MRM
- multiple reaction monitoring
- MU
- methylene unit
- ODS
- octadecylsilane substituted
- RI
- retention index
- SIR
- selected ion recording
- SRM
- selected reaction monitoring
- UPLC
- ultra performance/pressure liquid chromatography
This work was supported by Karolinska Institutet and the UK Research Council (BBSRC grant no. BBC5157712, BBC5113561).
REFERENCES
- 1.Motola D. L., Cummins C. L., Rottiers V., Sharma K. K., Li T., Li Y., Suino-Powell K., Xu H. E., Auchus R. J., Antebi A., et al. 2006. Identification of ligands for DAF-12 that govern dauer formation and reproduction in C. elegans. Cell. 124: 1209–1223. [DOI] [PubMed] [Google Scholar]
- 2.Maneerat S., Nitoda T., Kanzaki H., Kawai F. 2005. Bile acids are new products of a marine bacterium, Myroides sp. strain SM1. Appl. Microbiol. Biotechnol. 67: 679–683. [DOI] [PubMed] [Google Scholar]
- 3.Sobotka H. (1937) Physiological chemistry of the bile. Ballière, Tyndall & Cox, London. [Google Scholar]
- 4.Sobotka H. (1938) Chemistry of the sterids. Ballière, Tyndall & Cox, London. [Google Scholar]
- 5.Bergström S., Danielsson H., Samuelsson B. (1960) Formation and metabolism of bile acids. Lipide Metabolism. Block K., editor John Wiley & Sons, New York: 291–366. [Google Scholar]
- 6.Eneroth P., Sjövall J. (1971) Extraction, purification and chromatographic analysis of bile acids in biological materials. The Bile Acids, Volume 1. Kritchevsky D., Nair P. P., Plenum Press, New York: 209–248. [Google Scholar]
- 7.Horning E. C., Brooks C. J., Vanden Heuvel W. J. 1968. Gas phase analytical methods for the study of steroids. Adv. Lipid Res. 6: 273–392. [DOI] [PubMed] [Google Scholar]
- 8.Ryhage R. 1964. Use of a mass spectrometer as a detector and analyzer for effluents emerging from high temperature gas liquid chromatography columns. Anal. Chem. 36: 759–764. [Google Scholar]
- 9.Sjövall J., Eneroth P., Ryhage R. (1971) Mass spectra of bile acids. The Bile Acids; Chemistry, Physiology and Metabolism. Nair P., Kritchevsky D., Plenum Press, New York. [Google Scholar]
- 10.Murphy G. M., Maghsoudloo M., Qureshi M. Y. (1995) Assay of bile acids in biological samples. Steroid Analysis. Makin H. L. J., Gower D. B., Kirk D. B., Blackie Academic & Professional, Glasgow: 527–561. [Google Scholar]
- 11.Simmonds W. J., Korman M. G., Go V. L., Hofmann A. F. 1973. Radioimmunoassay of conjugated cholyl bile acids in serum. Gastroenterology. 65: 705–711. [PubMed] [Google Scholar]
- 12.Shaw R., Elliott W. H. 1976. Bile acids. XLVIII. Separation of conjugated bile acids by high-pressure liquid chromatography. Anal. Biochem. 74: 273–281. [DOI] [PubMed] [Google Scholar]
- 13.Roda A., Gioacchini A. M., Cerre C., Baraldini M. 1995. High-performance liquid chromatographic-electrospray mass spectrometric analysis of bile acids in biological fluids. J. Chromatogr. B Biomed. Appl. 665: 281–294. [DOI] [PubMed] [Google Scholar]
- 14.Russell D. W., Setchell K. D. 1992. Bile acid biosynthesis. Biochemistry. 31: 4737–4749. [DOI] [PubMed] [Google Scholar]
- 15.Une M., Hoshita T. 1994. Natural occurrence and chemical synthesis of bile alcohols, higher bile acids, and short side chain bile acids. Hiroshima J. Med. Sci. 43: 37–67. [PubMed] [Google Scholar]
- 16.Russell D. W. 2003. The enzymes, regulation, and genetics of bile acid synthesis. Annu. Rev. Biochem. 72: 137–174. [DOI] [PubMed] [Google Scholar]
- 17.Mitamura K., Watanabe S., Mitsumoto Y., Sakai T., Sogabe M., Wakamiya T., Ikegawa S. 2009. Formation and biliary excretion of glutathione conjugates of bile acids in the rat as shown by liquid chromatography/electrospray ionization-linear ion trap mass spectrometry. Anal. Biochem. 384: 224–230. [DOI] [PubMed] [Google Scholar]
- 18.Sorensen P. W., Fine J. M., Dvornikovs V., Jeffrey C. S., Shao F., Wang J., Vrieze L. A., Anderson K. R., Hoye T. R. 2005. Mixture of new sulfated steroids functions as a migratory pheromone in the sea lamprey. Nat. Chem. Biol. 1: 324–328. [DOI] [PubMed] [Google Scholar]
- 19.Fine J. M., Sorensen P. W. 2005. Biologically relevant concentrations of petromyzonol sulfate, a component of the sea lamprey migratory pheromone, measured in stream water. J. Chem. Ecol. 31: 2205–2210. [DOI] [PubMed] [Google Scholar]
- 20.Parmentier G., Mertens J., Eyssen H. (1975) Cholic acid-7-sulfate, a major bile acid in the large intestine of mouse. Advances in Bile Acid Research. Matern S., Hackenschmidt J., Back P., Schattauer Verlag, Stuttgart: 39–144. [Google Scholar]
- 21.Eyssen H. J., Parmentier G. G., Mertens J. A. 1976. Sulfate bile acids in germ-free and conventional mice. Eur. J. Biochem. 66: 507–514. [DOI] [PubMed] [Google Scholar]
- 22.Norman A., Palmer R. H. 1964. Metabolites of lithocholic acid-24-C-14 in human bile and feces. J. Lab. Clin. Med. 63: 986–1001. [PubMed] [Google Scholar]
- 23.Kelsey M. I., Molina J. E., Huang S. K., Hwang K. K. 1980. The identification of microbial metabolites of sulfolithocholic acid. J. Lipid Res. 21: 751–759. [PubMed] [Google Scholar]
- 24.Korpela J. T., Fotsis T., Adlercreutz H. 1986. Multicomponent analysis of bile acids in faeces by anion exchange and capillary column gas-liquid chromatography: application in oxytetracycline treated subjects. J. Steroid Biochem. 25: 277–284. [DOI] [PubMed] [Google Scholar]
- 25.Benson G. M., Haskins N. J., Eckers C., Moore P. J., Reid D. G., Mitchell R. C., Waghmare S., Suckling K. E. 1993. Polydeoxycholate in human and hamster feces: a major product of cholate metabolism. J. Lipid Res. 34: 2121–2134. [PubMed] [Google Scholar]
- 26.Alvelius G., Hjalmarson O., Griffiths W. J., Björkhem I., Sjövall J. 2001. Identification of unusual 7-oxygenated bile acid sulfates in a patient with Niemann-Pick disease, type C. J. Lipid Res. 42: 1571–1577. [PubMed] [Google Scholar]
- 27.Iida T., Kakiyama G., Hibiya Y., Miyata S., Inoue T., Ohno K., Goto T., Mano N., Goto J., Nambara T., et al. 2006. Chemical synthesis of the 3-sulfooxy-7-N-acetylglucosaminyl-24-amidated conjugates of 3beta,7beta-dihydroxy-5-cholen-24-oic acid, and related compounds: unusual, major metabolites of bile acid in a patient with Niemann-Pick disease type C1. Steroids. 71: 18–29. [DOI] [PubMed] [Google Scholar]
- 28.Setchell K. D., Schwarz M., O'Connell N. C., Lund E. G., Davis D. L., Lathe R., Thompson H. R., Weslie T. R., Sokol R. J., Russell D. W. 1998. Identification of a new inborn error in bile acid synthesis: mutation of the oxysterol 7alpha-hydroxylase gene causes severe neonatal liver disease. J. Clin. Invest. 102: 1690–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Setchell K. D., Heubi J. E., Bove K. E., O'Connell N. C., Brewsaugh T., Steinberg S. J., Moser A., Squires R. H., Jr 2003. Liver disease caused by failure to racemize trihydroxycholestanoic acid: gene mutation and effect of bile acid therapy. Gastroenterology. 124: 217–232. [DOI] [PubMed] [Google Scholar]
- 30.Setchell K. D., Heubi J. E. 2006. Defects in bile acid biosynthesis–diagnosis and treatment. J. Pediatr. Gastroenterol. Nutr. 43(Suppl 1): S17–S22. [DOI] [PubMed] [Google Scholar]
- 31.Lemonde H. A., Johnson A. W., Clayton P. T. 1999. The identification of unusual bile acid metabolites by tandem mass spectrometry: use of low-energy collision-induced dissociation to produce informative spectra. Rapid Commun. Mass Spectrom. 13: 1159–1164. [DOI] [PubMed] [Google Scholar]
- 32.Lemonde H. A., Custard E. J., Bouquet J., Duran M., Overmars H., Scambler P. J., Clayton P. T. 2003. Mutations in SRD5B1 (AKR1D1), the gene encoding delta(4)-3-oxosteroid 5beta-reductase, in hepatitis and liver failure in infancy. Gut. 52: 1494–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clayton P. T., Verrips A., Sistermans E., Mann A., Mieli-Vergani G., Wevers R. 2002. Mutations in the sterol 27-hydroxylase gene (CYP27A) cause hepatitis of infancy as well as cerebrotendinous xanthomatosis. J. Inherit. Metab. Dis. 25: 501–513. [DOI] [PubMed] [Google Scholar]
- 34.Clayton P. T. 2003. Diagnosis of inherited disorders of liver metabolism. J. Inherit. Metab. Dis. 26: 135–146. [DOI] [PubMed] [Google Scholar]
- 35.Egestad B., Pettersson P., Skrede S., Sjövall J. 1985. Fast atom bombardment mass spectrometry in the diagnosis of cerebrotendinous xanthomatosis. Scand. J. Clin. Lab. Invest. 45: 443–446. [DOI] [PubMed] [Google Scholar]
- 36.Clayton P. T., Leonard J. V., Lawson A. M., Setchell K. D., Andersson S., Egestad B., Sjövall J. 1987. Familial giant cell hepatitis associated with synthesis of 3 beta, 7 alpha-dihydroxy-and 3 beta,7 alpha, 12 alpha-trihydroxy-5-cholenoic acids. J. Clin. Invest. 79: 1031–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fischler B., Bodin K., Stjernman H., Olin M., Hansson M., Sjövall J., Björkhem I. 2007. Cholestatic liver disease in adults may be due to an inherited defect in bile acid biosynthesis. J. Intern. Med. 262: 254–262. [DOI] [PubMed] [Google Scholar]
- 38.Yang Y., Griffiths W. J., Nazer H., Sjövall J. 1997. Analysis of bile acids and bile alcohols in urine by capillary column liquid chromatography-mass spectrometry using fast atom bombardment or electrospray ionization and collision-induced dissociation. Biomed. Chromatogr. 11: 240–255. [DOI] [PubMed] [Google Scholar]
- 39.Meng L. J., Griffiths W. J., Nazer H., Yang Y., Sjövall J. 1997. High levels of (24S)-24-hydroxycholesterol 3-sulfate, 24-glucuronide in the serum and urine of children with severe cholestatic liver disease. J. Lipid Res. 38: 926–934. [PubMed] [Google Scholar]
- 40.Griffiths W. J. 2003. Tandem mass spectrometry in the study of fatty acids, bile acids, and steroids. Mass Spectrom. Rev. 22: 81–152. [DOI] [PubMed] [Google Scholar]
- 41.Wang Y., Griffiths W. J. (2008) Mass spectrometry for metabolite identification. Metabolomics, Metabonomics and Metabolite Profiling. Griffiths W. J., editor The Royal Society of Chemistry, Cambridge: 1–31. [Google Scholar]
- 42.Hager J. W., Yves Le Blanc J. C. 2003. Product ion scanning using a Q-q-Q linear ion trap (Q TRAP) mass spectrometer. Rapid Commun. Mass Spectrom. 17: 1056–1064. [DOI] [PubMed] [Google Scholar]
- 43.Ikegawa S., Okuyama H., Oohashi J., Murao N., Goto J. 1999. Separation and detection of bile acid 24-glucuronides in human urine by liquid chromatography combined with electrospray ionization mass spectrometry. Anal. Sci. 15: 625–631. [Google Scholar]
- 44.Libert R., Hermans D., Draye J. P., Van Hoof F., Sokal E., de Hoffmann E. 1991. Bile acids and conjugates identified in metabolic disorders by fast atom bombardment and tandem mass spectrometry. Clin. Chem. 37: 2102–2110. [PubMed] [Google Scholar]
- 45.Mushtaq I., Logan S., Morris M., Johnson A. W., Wade A. M., Kelly D., Clayton P. T. 1999. Screening of newborn infants for cholestatic hepatobiliary disease with tandem mass spectrometry. BMJ. 319: 471–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yousef I. M., Perwaiz S., Lamireau T., Tuchweber B. 2003. Urinary bile acid profile in children with inborn errors of bile acid metabolism and chronic cholestasis; screening technique using electrospray tandem mass-spectrometry (ES/MS/MS). Med. Sci. Monit. 9: MT21–MT31. [PubMed] [Google Scholar]
- 47.Stroobant V., de Hoffmann E., Libert R., Van Hoof F. 1995. Fast-atom bombardment mass spectrometry and low energy collision-induced tandem mass spectrometry of tauroconjugated bile acid anions. J. Am. Soc. Mass Spectrom. 6: 588–596. [DOI] [PubMed] [Google Scholar]
- 48.Tomer K. B., Gross M. L. 1988. Fast atom bombardment and tandem mass spectrometry for structure determination: remote site fragmentation of steroid conjugates and bile salts. Biomed. Environ. Mass Spectrom. 15: 89–98. [DOI] [PubMed] [Google Scholar]
- 49.Zhang J., Griffiths W. J., Bergman T., Sjövall J. 1993. Derivatization of bile acids with taurine for analysis by fast atom bombardment mass spectrometry with collision-induced fragmentation. J. Lipid Res. 34: 1895–1900. [PubMed] [Google Scholar]
- 50.Pittenauer E., Allmaier G. 2009. High-energy collision induced dissociation of biomolecules: MALDI-TOF/RTOF mass spectrometry in comparison to tandem sector mass spectrometry. Comb. Chem. High Throughput Screen. 12: 137–155. [DOI] [PubMed] [Google Scholar]
- 51.Mims D., Hercules D. 2003. Quantification of bile acids directly from urine by MALDI-TOF-MS. Anal. Bioanal. Chem. 375: 609–616. [DOI] [PubMed] [Google Scholar]
- 52.Mims D., Hercules D. 2004. Quantification of bile acids directly from plasma by MALDI-TOF-MS. Anal. Bioanal. Chem. 378: 1322–1326. [DOI] [PubMed] [Google Scholar]
- 53.Sjövall J., Setchell K. D. (1988) Techniques for extraction and group separation of bile acids. The Bile Acids. Vol. 4. Setchell K. D., Kritchevsky D., Nair P. P., Plenum Press, New York: 1–42. [Google Scholar]
- 54.Perwaiz S., Mignault D., Tuchweber B., Yousef I. M. 2002. Rapid and improved method for the determination of bile acids in human feces using MS. Lipids. 37: 1093–1100. [DOI] [PubMed] [Google Scholar]
- 55.Alnouti Y., Csanaky I. L., Klaassen C. D. 2008. Quantitative-profiling of bile acids and their conjugates in mouse liver, bile, plasma, and urine using LC-MS/MS. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 873: 209–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Axelson M., Sjövall J. (1985) Studies on the selective analysis of ketonic bile acids and steroids in faeces. Enterohepatic Circulation of Bile Acids and Sterol Metabolism. Paumgartner G., Stiehl A., Gerok W., MTP Press Ltd, Lancaster, UK: 249–258 [Google Scholar]
- 57.Setchell K. D., Street J. M., Sjövall J. (1988) Fecal bile acids. The Bile Acids: Methods and Applications. Setchell K. D., Kritchevsky D., Nair P. P., Plenum Press, New York: 441–570. [Google Scholar]
- 58.Setchell K. D., Lawson A. M., Tanida N., Sjövall J. 1983. General methods for the analysis of metabolic profiles of bile acids and related compounds in feces. J. Lipid Res. 24: 1085–1100. [PubMed] [Google Scholar]
- 59.Eneroth P., Hellström K., Sjövall J. 1968. A method for quantitative determination of bile acids in human feces. Bile acids and steroids 195. Acta Chem. Scand. 22: 1729–1744. [DOI] [PubMed] [Google Scholar]
- 60.Gustafsson B. E., Norman A. 1969. Influence of the diet on the turnover of bile acids in germ-free and conventional rats. Br. J. Nutr. 23: 429–442. [DOI] [PubMed] [Google Scholar]
- 61.Hedenborg G., Norlander A., Norman A. 1986. Bile acid conjugates present in tissues during extrahepatic cholestasis. Scand. J. Clin. Lab. Invest. 46: 539–544. [DOI] [PubMed] [Google Scholar]
- 62.Batta A. K., Salen G., Rapole K. R., Batta M., Batta P., Alberts D., Earnest D. 1999. Highly simplified method for gas-liquid chromatographic quantitation of bile acids and sterols in human stool. J. Lipid Res. 40: 1148–1154. [PubMed] [Google Scholar]
- 63.Setchell K. D., Ives J. A., Cashmore G. C., Lawson A. M. 1987. On the homogeneity of stools with respect to bile acid composition and normal day-to-day variations: a detailed qualitative and quantitative study using capillary column gas chromatography-mass spectrometry. Clin. Chim. Acta. 162: 257–275. [DOI] [PubMed] [Google Scholar]
- 64.Haines A., Hill M. J., Thompson M. H., Owen R. W., Williams R. E., Meade T. W., Wilkes H., Griffin M. 2000. A prospective study of faecal bile acids and colorectal cancer. Eur. J. Cancer Prev. 9: 317–323. [DOI] [PubMed] [Google Scholar]
- 65.Mano N., Goto T., Uchida M., Nishimura K., Ando M., Kobayashi N., Goto J. 2004. Presence of protein-bound unconjugated bile acids in the cytoplasmic fraction of rat brain. J. Lipid Res. 45: 295–300. [DOI] [PubMed] [Google Scholar]
- 66.Nuber R., Maucher H., Stange E. F. 1990. Size exclusion chromatography for extraction of serum bile acids. J. Lipid Res. 31: 1517–1522. [PubMed] [Google Scholar]
- 67.Axelson M., Sahlberg B-L. 1981. On solid extraction of steroids and steroid conjugates in biological fluids. Anal. Lett. 14: 771–782. [Google Scholar]
- 68.Wahlén E., Tamasawa N., Ichimiya H., Egestad B., Sjövall J. 1994. Conditions for solid-phase extraction of urinary bile acids with octadecylsilane-bonded silica. J. Lipid Res. 35: 1902–1906. [PubMed] [Google Scholar]
- 69.Rodrigues C. M., Setchell K. D. 1996. Performance characteristics of reversed-phase bonded silica cartridges for serum bile acid extraction. Biomed. Chromatogr. 10: 1–5. [DOI] [PubMed] [Google Scholar]
- 70.Björkhem I., Andersson U., Ellis E., Alvelius G., Ellegård L., Diczfalusy U., Sjövall J., Einarsson C. 2001. From brain to bile. Evidence that conjugation and omega-hydroxylation are important for elimination of 24S-hydroxycholesterol (cerebrosterol) in humans. J. Biol. Chem. 276: 37004–37010. [DOI] [PubMed] [Google Scholar]
- 71.Persson E., Löfgren L., Hansson G., Abrahamsson B., Lennernäs H., Nilsson R. 2007. Simultaneous assessment of lipid classes and bile acids in human intestinal fluid by solid-phase extraction and HPLC methods. J. Lipid Res. 48: 242–251. [DOI] [PubMed] [Google Scholar]
- 72.Almé B., Bremmelgaard A., Sjövall J., Thomassen P. 1977. Analysis of metabolic profiles of bile acids in urine using a lipophilic anion exchanger and computerized gas-liquid chromatography-mass spectrometry. J. Lipid Res. 18: 339–362. [PubMed] [Google Scholar]
- 73.Goto J., Hasegawa M., Kato H., Nambara T. 1978. A new method for simultaneous determination of bile acids in human bile without hydrolysis. Clin. Chim. Acta. 87: 141–147. [DOI] [PubMed] [Google Scholar]
- 74.Sjövall J., Axelson M. 1984. Sample work-up by column techniques. J. Pharm. Biomed. Anal. 2: 265–280. [DOI] [PubMed] [Google Scholar]
- 75.Axelson M., Mörk B., Sjövall J. 1988. Occurrence of 3 beta-hydroxy-5-cholestenoic acid, 3 beta,7 alpha-dihydroxy-5-cholestenoic acid, and 7 alpha-hydroxy-3-oxo-4-cholestenoic acid as normal constituents in human blood. J. Lipid Res. 29: 629–641. [PubMed] [Google Scholar]
- 76.Ichimiya H., Egestad B., Nazer H., Baginski E. S., Clayton P. T., Sjövall J. 1991. Bile acids and bile alcohols in a child with hepatic 3 beta-hydroxy-delta 5–C27-steroid dehydrogenase deficiency: effects of chenodeoxycholic acid treatment. J. Lipid Res. 32: 829–841. [PubMed] [Google Scholar]
- 77.Meng L. J., Sjövall J. 1997. Method for combined analysis of profiles of conjugated progesterone metabolites and bile acids in serum and urine of pregnant women. J. Chromatogr. B Biomed. Sci. Appl. 688: 11–26. [DOI] [PubMed] [Google Scholar]
- 78.Axelson M., Mörk B., Everson G. T. 1991. Bile acid synthesis in cultured human hepatoblastoma cells. J. Biol. Chem. 266: 17770–17777. [PubMed] [Google Scholar]
- 79.Axelson M., Ellis E., Mörk B., Garmark K., Abrahamsson A., Björkhem I., Ericzon B. G., Einarsson C. 2000. Bile acid synthesis in cultured human hepatocytes: support for an alternative biosynthetic pathway to cholic acid. Hepatology. 31: 1305–1312. [DOI] [PubMed] [Google Scholar]
- 80.Goto J., Kato H., Saruta Y., Nambara T. 1981. Studies on steroids. CLXX. Separation and determination of bile acid 3-sulfates in human bile by high-performance liquid chromatography. J. Chromatogr. A. 226: 13–24. [PubMed] [Google Scholar]
- 81.Goto J., Miura H., Inada M., Nambara T., Nagakura T., Suzuki H. 1988. Studies on steroids. CCXXXVIII. Determination of bile acids in liver tissue by gas chromatography-mass spectrometry with negative ion chemical ionization detection. J. Chromatogr. A. 452: 119–129. [DOI] [PubMed] [Google Scholar]
- 82.Goto J., Murao N., Nakada C., Motoyama T., Oohashi J., Yanagihara T., Niwa T., Ikegawa S. 1998. Separation and characterization of carboxyl-linked glucuronides of bile acids in incubation mixture of rat liver microsomes. Steroids. 63: 186–192. [DOI] [PubMed] [Google Scholar]
- 83.Goto T. 2007. Development of a highly sensitive methods for determination of activated carboxylic acids in biological specimens. Chromatography. 28: 9–17. [Google Scholar]
- 84.Ikegawa S., Hirabayashi N., Yoshimura T., Tohma M., Maeda M., Tsuji A. 1992. Determination of conjugated bile acids in human urine by high-performance liquid chromatography with chemiluminescence detection. J. Chromatogr. A. 577: 229–238. [DOI] [PubMed] [Google Scholar]
- 85.Ikegawa S., Yoshimura T., Ito K., Kurosawa T., Tohma M. 1995. Simultaneous flurometric determination of conjugated fetal bile acids in newborns by high-performance liquid chromatography. Anal. Sci. 11: 91–96. [Google Scholar]
- 86.Ikegawa S., Murao N., Motoyama T., Yanagihara T., Niwa T., Goto J. 1996. Separation and detection of bile acid 3-glucuronides in human urine by liquid chromatography/electrospray ionization-mass spectrometry. Biomed. Chromatogr. 10: 313–317. [DOI] [PubMed] [Google Scholar]
- 87.Marschall H. U., Green G., Egestad B., Sjövall J. 1988. Isolation of bile acid glucosides and N-acetylglucosaminides from human urine by ion-exchange chromatography and reversed-phase high-performance liquid chromatography. J. Chromatogr. A. 452: 459–468. [DOI] [PubMed] [Google Scholar]
- 88.Marschall H. U., Griffiths W. J., Gotze U., Zhang J., Wietholtz H., Busch N., Sjövall J., Matern S. 1994. The major metabolites of ursodeoxycholic acid in human urine are conjugated with N-acetylglucosamine. Hepatology. 20: 845–853. [DOI] [PubMed] [Google Scholar]
- 89.Matoba N., Une M., Hoshita T. 1986. Identification of unconjugated bile acids in human bile. J. Lipid Res. 27: 1154–1162. [PubMed] [Google Scholar]
- 90.Meng L. J., Reyes H., Palma J., Hernandez I., Ribalta J., Sjövall J. 1997. Effects of ursodeoxycholic acid on conjugated bile acids and progesterone metabolites in serum and urine of patients with intrahepatic cholestasis of pregnancy. J. Hepatol. 27: 1029–1040. [DOI] [PubMed] [Google Scholar]
- 91.Niwa T., Fujita K., Goto J., Nambara T. 1993. Separation and characterization of ursodeoxycholate 7-N-acetylglucosaminides in human urine by high performance liquid chromatography with fluorescence detection. J. Liq. Chromatogr. 16: 2531–2544. [Google Scholar]
- 92.Une M., Tsujimura K., Kihira K., Hoshita T. 1989. Identification of (22R)-3 alpha,7 alpha,12 alpha,22- and (23R)-3 alpha,7 alpha,12 alpha,23-tetrahydroxy-5 beta-cholestanoic acids in urine from a patient with Zellweger's syndrome. J. Lipid Res. 30: 541–547. [PubMed] [Google Scholar]
- 93.Wahlén E., Egestad B., Strandvik B., Sjövall J. 1989. Ketonic bile acids in urine of infants during the neonatal period. J. Lipid Res. 30: 1847–1857. [PubMed] [Google Scholar]
- 94.Scalia S. 1990. Group separation of free and conjugated bile acids by pre-packed anion-exchange cartridges. J. Pharm. Biomed. Anal. 8: 235–241. [DOI] [PubMed] [Google Scholar]
- 95.Griffiths W. J., Hornshaw M., Woffendin G., Baker S. F., Lockhart A., Heidelberger S., Gustafsson M., Sjövall J., Wang Y. 2008. Discovering oxysterols in plasma: a window on the metabolome. J. Proteome Res. 7: 3602–3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Scalia S. 1995. Bile acid separation. J. Chromatogr. B Biomed. Appl. 671: 299–317. [DOI] [PubMed] [Google Scholar]
- 97.Bobeldijk I., Hekman M., de Vries-van der Weij J., Coulier L., Ramaker R., Kleemann R., Kooistra T., Rubingh C., Freidig A., Verheij E. 2008. Quantitative profiling of bile acids in biofluids and tissues based on accurate mass high resolution LC-FT-MS: compound class targeting in a metabolomics workflow. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 871: 306–313. [DOI] [PubMed] [Google Scholar]
- 98.Burkard I., von Eckardstein A., Rentsch K. M. 2005. Differentiated quantification of human bile acids in serum by high-performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 826: 147–159. [DOI] [PubMed] [Google Scholar]
- 99.Ikegawa S., Yanagihara T., Murao N., Watanabe H., Goto J., Niwa T. 1997. Separatory determination of bile acid 3-sulfates by liquid chromatography/electrospray ionization mass spectrometry. J. Mass Spectrom. 32: 401–407. [Google Scholar]
- 100.Goto T., Shibata A., Sasaki D., Suzuki N., Hishinuma T., Kakiyama G., Iida T., Mano N., Goto J. 2005. Identification of a novel conjugate in human urine: bile acid acyl galactosides. Steroids. 70: 185–192. [DOI] [PubMed] [Google Scholar]
- 101.Goto T., Myint K. T., Sato K., Wada O., Kakiyama G., Iida T., Hishinuma T., Mano N., Goto J. 2007. LC/ESI-tandem mass spectrometric determination of bile acid 3-sulfates in human urine. 3beta-Sulfooxy-12alpha-hydroxy-5beta-cholanoic acid is an abundant nonamidated sulfate. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 846: 69–77. [DOI] [PubMed] [Google Scholar]
- 102.Shinka T., Inoue Y., Ohse M., Kuhara T. 2007. Simple and quantitative analysis of urinary sulfated tauro- and glycodihydroxycholic acids in infant with cholestasis by electrospray ionization mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 855: 104–108. [DOI] [PubMed] [Google Scholar]
- 103.Bootsma A. H., Overmars H., van Rooij A., van Lint A. E., Wanders R. J., van Gennip A. H., Vreken P. 1999. Rapid analysis of conjugated bile acids in plasma using electrospray tandem mass spectrometry: application for selective screening of peroxisomal disorders. J. Inherit. Metab. Dis. 22: 307–310. [DOI] [PubMed] [Google Scholar]
- 104.Ando M., Kaneko T., Watanabe R., Kikuchi S., Goto T., Iida T., Hishinuma T., Mano N., Goto J. 2006. High sensitive analysis of rat serum bile acids by liquid chromatography/electrospray ionization tandem mass spectrometry. J. Pharm. Biomed. Anal. 40: 1179–1186. [DOI] [PubMed] [Google Scholar]
- 105.Williams R., Lenz E. M., Wilson A. J., Granger J., Wilson I. D., Major H., Stumpf C., Plumb R. 2006. A multi-analytical platform approach to the metabonomic analysis of plasma from normal and Zucker (fa/fa) obese rats. Mol. Biosyst. 2: 174–183. [DOI] [PubMed] [Google Scholar]
- 106.Tessier E., Neirinck L., Zhu Z. 2003. High-performance liquid chromatographic mass spectrometric method for the determination of ursodeoxycholic acid and its glycine and taurine conjugates in human plasma. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 798: 295–302. [DOI] [PubMed] [Google Scholar]
- 107.Ye L., Liu S., Wang M., Shao Y., Ding M. 2007. High-performance liquid chromatography-tandem mass spectrometry for the analysis of bile acid profiles in serum of women with intrahepatic cholestasis of pregnancy. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 860: 10–17. [DOI] [PubMed] [Google Scholar]
- 108.Yang L., Xiong A., He Y., Wang Z., Wang C., Wang Z., Li W., Yang L., Hu Z. 2008. Bile acids metabonomic study on the CCl4- and alpha-naphthylisothiocyanate-induced animal models: quantitative analysis of 22 bile acids by ultraperformance liquid chromatography-mass spectrometry. Chem. Res. Toxicol. 21: 2280–2288. [DOI] [PubMed] [Google Scholar]
- 109.Bentayeb K., Batlle R., Sanchez C., Nerin C., Domeno C. 2008. Determination of bile acids in human serum by on-line restricted access material-ultra high-performance liquid chromatography-mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 869: 1–8. [DOI] [PubMed] [Google Scholar]
- 110.Xiang X., Han Y., Neuvonen M., Pasanen M. K., Kalliokoski A., Backman J. T., Laitila J., Neuvonen P. J., Niemi M. 2009. Effect of SLCO1B1 polymorphism on the plasma concentrations of bile acids and bile acid synthesis marker in humans. Pharmacogenet. Genomics. 19: 447–457. [DOI] [PubMed] [Google Scholar]
- 111.Perwaiz S., Tuchweber B., Mignault D., Gilat T., Yousef I. M. 2001. Determination of bile acids in biological fluids by liquid chromatography-electrospray tandem mass spectrometry. J. Lipid Res. 42: 114–119. [PubMed] [Google Scholar]
- 112.Plumb R. S., Rainville P. D., Potts W. B., III, Johnson K. A., Gika E., Wilson I. D. 2009. Application of ultra performance liquid chromatography-mass spectrometry to profiling rat and dog bile. J. Proteome Res. 8: 2495–2500. [DOI] [PubMed] [Google Scholar]
- 113.Hamilton J. P., Xie G., Raufman J. P., Hogan S., Griffin T. L., Packard C. A., Chatfield D. A., Hagey L. R., Steinbach J. H., Hofmann A. F. 2007. Human cecal bile acids: concentration and spectrum. Am. J. Physiol. Gastrointest. Liver Physiol. 293: G256–G263. [DOI] [PubMed] [Google Scholar]
- 114.Hagio M., Matsumoto M., Fukushima M., Hara H., Ishizuka S. 2009. Improved analysis of bile acids in tissues and intestinal contents of rats using LC/ESI-MS. J. Lipid Res. 50: 173–180. [DOI] [PubMed] [Google Scholar]
- 115.Ferdinandusse S., Denis S., Faust P. L., Wanders R. J. 2009. Bile acids: role of peroxisomes. J. Lipid Res. 50: 2139–2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Parmentier G. G., Janssen G. A., Eggermont E. A., Eyssen H. J. 1979. C27 bile acids in infants with coprostanic acidemia and occurrence of a 3 alpha,7 alpha,12 alpha-trihydroxy-5 beta-C29 dicarboxylic bile acid as a major component in their serum. Eur. J. Biochem. 102: 173–183. [DOI] [PubMed] [Google Scholar]
- 117.Van Eldere J. R., Parmentier G. G., Eyssen H. J., Wanders R. J., Schutgens R. B., Vamecq J., Van Hoof F., Poll-The B. T., Saudubray J. M. 1987. Bile acids in peroxisomal disorders. Eur. J. Clin. Invest. 17: 386–390. [DOI] [PubMed] [Google Scholar]
- 118.Johnson D. W., ten Brink H. J., Schuit R. C., Jakobs C. 2001. Rapid and quantitative analysis of unconjugated C(27) bile acids in plasma and blood samples by tandem mass spectrometry. J. Lipid Res. 42: 9–16. [PubMed] [Google Scholar]
- 119.Lövgren-Sandblom A., Heverin M., Larsson H., Lundström E., Wahren J., Diczfalusy U., Björkhem I. 2007. Novel LC-MS/MS method for assay of 7alpha-hydroxy-4-cholesten-3-one in human plasma. Evidence for a significant extrahepatic metabolism. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 856: 15–19. [DOI] [PubMed] [Google Scholar]
- 120.Honda A., Yamashita K., Numazawa M., Ikegami T., Doy M., Matsuzaki Y., Miyazaki H. 2007. Highly sensitive quantification of 7alpha-hydroxy-4-cholesten-3-one in human serum by LC-ESI-MS/MS. J. Lipid Res. 48: 458–464. [DOI] [PubMed] [Google Scholar]
- 121.Honda A., Yamashita K., Hara T., Ikegami T., Miyazaki T., Shirai M., Xu G., Numazawa M., Matsuzaki Y. 2009. Highly sensitive quantification of key regulatory oxysterols in biological samples by LC-ESI-MS/MS. J. Lipid Res. 50: 350–357. [DOI] [PubMed] [Google Scholar]
- 122.Lenz E. M., Williams R. E., Sidaway J., Smith B. W., Plumb R. S., Johnson K. A., Rainville P., Shockcor J., Stumpf C. L., Granger J. H., et al. 2007. The application of microbore UPLC/oa-TOF-MS and 1H NMR spectroscopy to the metabonomic analysis of rat urine following the intravenous administration of pravastatin. J. Pharm. Biomed. Anal. 44: 845–852. [DOI] [PubMed] [Google Scholar]
- 123.Roda A., Piazza F., Baraldini M. 1998. Separation techniques for bile salts analysis. J. Chromatogr. B Biomed. Sci. Appl. 717: 263–278. [DOI] [PubMed] [Google Scholar]
- 124.Sakakura H., Kimura N., Takeda H., Komatsu H., Ishizaki K., Nagata S. 1998. Simultaneous determination of bile acids in rat liver tissue by high-performance liquid chromatography. J. Chromatogr. B Biomed. Sci. Appl. 718: 33–40. [DOI] [PubMed] [Google Scholar]
- 125.Shimada K., Mitamura K., Higashi T. 2001. Gas chromatography and high-performance liquid chromatography of natural steroids. J. Chromatogr. A. 935: 141–172. [DOI] [PubMed] [Google Scholar]
- 126.Rossi S. S., Converse J. L., Hofmann A. F. 1987. High pressure liquid chromatographic analysis of conjugated bile acids in human bile: simultaneous resolution of sulfated and unsulfated lithocholyl amidates and the common conjugated bile acids. J. Lipid Res. 28: 589–595. [PubMed] [Google Scholar]
- 127.Sakakura H., Suzuki M., Kimura N., Takeda H., Nagata S., Maeda M. 1993. Simultaneous determination of bile acids in rat bile and serum by high-performance liquid chromatography. J. Chromatogr. A. 621: 123–131. [DOI] [PubMed] [Google Scholar]
- 128.Roda A., Cerre C., Simoni P., Polimeni C., Vaccari C., Pistillo A. 1992. Determination of free and amidated bile acids by high-performance liquid chromatography with evaporative light-scattering mass detection. J. Lipid Res. 33: 1393–1402. [PubMed] [Google Scholar]
- 129.Torchia E. C., Labonte E. D., Agellon L. B. 2001. Separation and quantitation of bile acids using an isocratic solvent system for high performance liquid chromatography coupled to an evaporative light scattering detector. Anal. Biochem. 298: 293–298. [DOI] [PubMed] [Google Scholar]
- 130.Kakiyama G., Iida T., Goto T., Mano N., Goto J., Nambara T., Hagey L. R., Schteingart C. D., Hofmann A. F. 2006. Identification of a novel bile acid in swans, tree ducks, and geese: 3alpha,7alpha,15alpha-trihydroxy-5beta-cholan-24-oic acid. J. Lipid Res. 47: 1551–1558. [DOI] [PubMed] [Google Scholar]
- 131.Kakiyama G., Hosoda A., Iida T., Fujimoto Y., Goto T., Mano N., Goto J., Nambara T. 2006. A direct method for the separation and quantification of bile acid acyl glycosides by high-performance liquid chromatography with an evaporative light scattering detector. J. Chromatogr. A. 1125: 112–116. [DOI] [PubMed] [Google Scholar]
- 132.Moschetta A., Xu F., Hagey L. R., van Berge-Henegouwen G. P., van Erpecum K. J., Brouwers J. F., Cohen J. C., Bierman M., Hobbs H. H., Steinbach J. H., et al. 2005. A phylogenetic survey of biliary lipids in vertebrates. J. Lipid Res. 46: 2221–2232. [DOI] [PubMed] [Google Scholar]
- 133.Okuyama S., Kokubunn N., Higashidate S., Uemura D., Hirata Y. 1979. A new analytical method of individual bile acids using high-performance liquid chromatography and immobilized 3α-hydroxysteroid dehydrogenase in column form. Chem. Lett. 8: 1443–1446. [Google Scholar]
- 134.Björkhem I., Falk O. 1983. Assay of the major bile acids in serum by isotope dilution-mass spectrometry. Scand. J. Clin. Lab. Invest. 43: 163–170. [DOI] [PubMed] [Google Scholar]
- 135.Stellaard F., Paumgartner G. 1987. Quantitation of serum bile acids by isotope dilution with 13C-labelled homologs. Clin. Chim. Acta. 162: 45–51. [DOI] [PubMed] [Google Scholar]
- 136.Gatti R., Roda A., Cerre C., Bonazzi D., Cavrini V. 1997. HPLC-fluorescence determination of individual free and conjugated bile acids in human serum. Biomed. Chromatogr. 11: 11–15. [DOI] [PubMed] [Google Scholar]
- 137.Kurosawa T., Sato H., Sato M., Takechi H., Machida M., Tohma M. 1997. Analysis of stereoisomeric C27-bile acids by high performance liquid chromatography with fluorescence detection. J. Pharm. Biomed. Anal. 15: 1375–1382. [DOI] [PubMed] [Google Scholar]
- 138.Budai K., Javitt N. B. 1997. Bile acid analysis in biological fluids: a novel approach. J. Lipid Res. 38: 1906–1912. [PubMed] [Google Scholar]
- 139.Goto J., Chikai T., Nambara T. 1987. Studies on steroids. CCXXVII. Separation and determination of bile acid 7- and 12-sulphates in urine by high-performance liquid chromatography with fluorescence labelling. J. Chromatogr. A. 415: 45–52. [PubMed] [Google Scholar]
- 140.Karlaganis G., Paumgartner G. 1979. Determination of bile acids in serum by capillary gas-liquid chromatography. Clin. Chim. Acta. 92: 19–26. [DOI] [PubMed] [Google Scholar]
- 141.Huijghebaert S. M., Hofmann A. F. 1986. Influence of the amino acid moiety on deconjugation of bile acid amidates by cholylglycine hydrolase or human fecal cultures. J. Lipid Res. 27: 742–752. [PubMed] [Google Scholar]
- 142.Batta A. K., Salen G., Cheng F. W., Shefer S. 1979. Cleavage of the taurine conjugate of 3 alpha,7 alpha,12 alpha-trihydroxy-5 beta-cholestan-26-oic acid by rat fecal bacteria. J. Biol. Chem. 254: 11907–11909. [PubMed] [Google Scholar]
- 143.Batta A. K., Salen G., Shefer S. 1984. Substrate specificity of cholylglycine hydrolase for the hydrolysis of bile acid conjugates. J. Biol. Chem. 259: 15035–15039. [PubMed] [Google Scholar]
- 144.Hofmann A. F., Loening-Baucke V., Lavine J. E., Hagey L. R., Steinbach J. H., Packard C. A., Griffin T. L., Chatfield D. A. 2008. Altered bile acid metabolism in childhood functional constipation: inactivation of secretory bile acids by sulfation in a subset of patients. J. Pediatr. Gastroenterol. Nutr. 47: 598–606. [DOI] [PubMed] [Google Scholar]
- 145.Lepage G., Roy C. C., Weber A. M. 1981. Separation of sulfated from non-sulfated serum bile acids without the use of Sephadex columns. J. Lipid Res. 22: 705–711. [PubMed] [Google Scholar]
- 146.Dayal B., Ertel N. H. 1998. Rapid hydrolysis of bile acid conjugates using microwaves: retention of absolute stereochemistry in the hydrolysis of (25R) 3 alpha,7 alpha,12 alpha-trihydroxy-5 beta-cholestan-26-oyltaurine. Lipids. 33: 333–338. [DOI] [PubMed] [Google Scholar]
- 147.Huijghebaert S., Parmentier G., Eyssen H. 1984. Specificity of bile salt sulfatase activity in man, mouse and rat intestinal microflora. J. Steroid Biochem. 20: 907–912. [DOI] [PubMed] [Google Scholar]
- 148.Hirano Y., Miyazaki H., Higashidate S., Nakayama F. 1987. Analysis of 3-sulfated and nonsulfated bile acids by one-step solvolysis and high performance liquid chromatography. J. Lipid Res. 28: 1524–1529. [PubMed] [Google Scholar]
- 149.Princen H. M., Meijer P., Kuipers F. 1990. One-step solvolysis of 3-, 7- and 12-sulfated free and conjugated bile acids. Clin. Chim. Acta. 192: 77–83. [DOI] [PubMed] [Google Scholar]
- 150.Parmentier G., Eyssen H. 1975. Synthesis of the specific monosulfates of cholic acid. Steroids. 26: 721–729. [DOI] [PubMed] [Google Scholar]
- 151.Almé B., Sjövall J. 1980. Analysis of bile acid glucuronides in urine. Identification of 3 alpha, 6 alpha, 12 alpha-trihydroxy-5 beta-cholanoic acid. J. Steroid Biochem. 13: 907–916. [DOI] [PubMed] [Google Scholar]
- 152.Momose T., Maruyama J., Iida T., Goto J., Nambara T. 1997. Comparative abilities and optimal conditions for beta-glycosidase enzymes to hydrolyse the glucuronide, glucoside, and N-acetylglucosaminide conjugates of bile acids. Biol. Pharm. Bull. 20: 828–833. [DOI] [PubMed] [Google Scholar]
- 153.Child P., Aloe M., Mee D. 1987. Separation and quantitation of fatty acids, sterols and bile acids in feces by gas chromatography as the butyl ester-acetate derivatives. J. Chromatogr. A. 415: 13–26. [DOI] [PubMed] [Google Scholar]
- 154.Batta A. K., Salen G., Rapole K. R., Batta M., Earnest D., Alberts D. 1998. Capillary gas chromatographic analysis of serum bile acids as the n-butyl ester-trimethylsilyl ether derivatives. J. Chromatogr. B Biomed. Sci. Appl. 706: 337–341. [DOI] [PubMed] [Google Scholar]
- 155.Batta A. K., Salen G., Batta P., Tint G. S., Alberts D. S., Earnest D. L. 2002. Simultaneous quantitation of fatty acids, sterols and bile acids in human stool by capillary gas-liquid chromatography. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 775: 153–161. [DOI] [PubMed] [Google Scholar]
- 156.Batta A. K., Salen G., Batta M., Earnest D., Alberts D. 1997. Capillary gas-liquid chromatography of acetate-methyl esters of bile acids. J. Chromatogr. A. 766: 286–291. [DOI] [PubMed] [Google Scholar]
- 157.Sjövall J., Lawson A. M., Setchell K. D. (1985) Mass spectrometry of bile acids. Methods in Enzymology. Law J. H., Rilling H. C., Academic Press, London: 63–113. [DOI] [PubMed] [Google Scholar]
- 158.Setchell K. D., Lawson A. M. (1989) Bile acids. Mass Spectrometry. Clinical Biochemistry-Principles, Methods, Applications. Lawson A. M., editor Walter de Gruyter, Berlin, New York: 54–125. [Google Scholar]
- 159.Miyazaki H., Ishibashi M., Yamashita K. 1978. Use of a new silylating agent for separation of bile acids and cholesterol by selected ion monitoring with the computer controlled intensity matching technique. Biomed. Mass Spectrom. 5: 469–476. [DOI] [PubMed] [Google Scholar]
- 160.Evershed R. P. (1993) Advances in silylation. Handbook of Derivatives for Chromatography. Blau K., Halket J. M., Wiley, London: 52–108. [Google Scholar]
- 161.Kimura A., Suzuki M., Murai T., Inoue T., Kato H., Hori D., Nomura Y., Yoshimura T., Kurosawa T., Tohma M. 1997. Perinatal bile acid metabolism: analysis of urinary bile acids in pregnant women and newborns. J. Lipid Res. 38: 1954–1962. [PubMed] [Google Scholar]
- 162.Thenot J-P., Horning E. C. 1972. MO-TMS derivatives of human urinary steroids for GC and GC-MS studies. Anal. Lett. 5: 21–33. [Google Scholar]
- 163.Shoda J., Axelson M., Sjövall J. 1993. Synthesis of potential C27-intermediates in bile acid biosynthesis and their deuterium-labeled analogs. Steroids. 58: 119–125. [DOI] [PubMed] [Google Scholar]
- 164.Batta A. K., Aggarwal S. K., Tint G. S., Batta M., Salen G. 1995. Capillary gas-liquid chromatography of 6-hydroxylated bile acids. J. Chromatogr. A. 704: 228–233. [DOI] [PubMed] [Google Scholar]
- 165.Batta A. K., Salen G. 1999. Gas chromatography of bile acids. J. Chromatogr. B Biomed. Sci. Appl. 723: 1–16. [DOI] [PubMed] [Google Scholar]
- 166.Dumaswala R., Setchell K. D., Zimmer-Nechemias L., Iida T., Goto J., Nambara T. 1989. Identification of 3 alpha,4 beta,7 alpha-trihydroxy-5 beta-cholanoic acid in human bile: reflection of a new pathway in bile acid metabolism in humans. J. Lipid Res. 30: 847–856. [PubMed] [Google Scholar]
- 167.Iida T., Chang F. C., Matsumoto T., Tamura T. 1983. Separation of mono-, di-, and trihydroxy stereoisomers of bile acids by capillary gas-liquid chromatography. J. Lipid Res. 24: 211–215. [PubMed] [Google Scholar]
- 168.Iida T., Komatsubara I., Chang F. C., Goto J., Nambara T. 1990. Capillary gas liquid chromatographic separation of oxo and oxo-hydroxy bile acid isomers of allo and normal series. Lipids. 25: 753–755. [Google Scholar]
- 169.Iida T., Komatsubara I., Chang F. C., Goto J., Nambara T. 1991. Capillary gas chromatographic separation of stereoisomeric bile acids with vicinal glycol structure by "mixed" alkylboronate derivatives. J. Chromatogr. A. 537: 345–356. [Google Scholar]
- 170.Lawson A. M., Setchell K. D. (1988) Appendix. The Bile Acids. Volume 4: Methods and Applications. Setchell K. D., Kritchevsky D., Nair P. P., Plenum Press, New York: 571–575. [Google Scholar]
- 171.Shoda J., Osuga T., Mahara R., Tohma M., Matsuura K., Tanaka N., Matsuzaki Y., Miyazaki H. 1989. Altered metabolism of bile acids in cholestasis: determination of 1 beta- and 6 alpha-hydroxylated metabolites. J. Chromatogr. A. 488: 315–328. [DOI] [PubMed] [Google Scholar]
- 172.Batta A. K., Aggarwal S. K., Mirchandani R., Shefer S., Salen G. 1992. Capillary gas-liquid chromatographic separation of bile alcohols. J. Lipid Res. 33: 1403–1407. [PubMed] [Google Scholar]
- 173.Iida T., Hikosaka M., Goto J., Nambara T. 2001. Capillary gas chromatographic behaviour of tert.-hydroxylated steroids by trialkylsilylation. J. Chromatogr. A. 937: 97–105. [DOI] [PubMed] [Google Scholar]
- 174.Shan H., Pang J., Li S., Chiang T. B., Wilson W. K., Schroepfer G. J., Jr 2003. Chromatographic behavior of oxygenated derivatives of cholesterol. Steroids. 68: 221–233. [DOI] [PubMed] [Google Scholar]
- 175.Lawson A. M., Setchell K. D. (1988) Mass spectrometry of bile acids. The Bile Acids. Volume 4: Methods and Applications. Setchell K. D., Kritchevsky D., Nair P. P., Plenum Press, New York: 167–268. [Google Scholar]
- 176.Sjövall J., Griffiths W. J., Setchell K. D., Mano N., Goto J. (2009) Analysis of bile acids, Steroid Analysis, 2nd edition Makin H. L. J., Gower D. B., Springer. In press. [Google Scholar]
- 177.Little J. M., Zimniak P., Radominska A., Lester R. 1987. Hyodeoxycholate-6-O-glucuronide cannot be quantitated with 3 alpha-hydroxysteroid dehydrogenase. J. Lipid Res. 28: 1370–1372. [PubMed] [Google Scholar]
- 178.Porter J. L., Fordtran J. S., Santa Ana C. A., Emmett M., Hagey L. R., Macdonald E. A., Hofmann A. F. 2003. Accurate enzymatic measurement of fecal bile acids in patients with malabsorption. J. Lab. Clin. Med. 141: 411–418. [DOI] [PubMed] [Google Scholar]
- 179.Danielsson H., Eneroth P., Hellström K., Lindstedt S., Sjövall J. 1963. On the turnover and excretory products of cholic and chenodeoxycholic acid in man. J. Biol. Chem. 238: 2299–2304. [PubMed] [Google Scholar]
- 180.Kobayashi N., Oiwa H., Goto J. 1998. Production and characterization of group-specific monoclonal antibodies recognizing nonamidated, glycine- and taurine-amidated ursodeoxycholic acid 7-N-acetylglucosaminides. J. Steroid Biochem. Mol. Biol. 64: 171–177. [DOI] [PubMed] [Google Scholar]
- 181.Kobayashi N., Katsumata H., Katayama H., Oiwa H., Goto J., Takeuchi Y. 2000. A monoclonal antibody-based enzyme-linked immunosorbent assay of ursodeoxycholic acid 3-sulfates in human urine. J. Steroid Biochem. Mol. Biol. 72: 265–272. [DOI] [PubMed] [Google Scholar]
- 182.Kobayashi N., Katsumata H., Uto Y., Goto J., Niwa T., Kobayashi K., Mizuuchi Y. 2002. A monoclonal antibody-based enzyme-linked immunosorbent assay of glycolithocholic acid sulfate in human urine for liver function test. Steroids. 67: 827–833. [DOI] [PubMed] [Google Scholar]