Abstract
Data from animal experiments strongly suggest that ceramide is an important mediator of lipotoxicity in the heart and that accumulation of ceramide contributes to cardiomyocyte apoptosis associated with type 2 diabetes and obesity. However, it remains unknown whether a similar relationship is present also in the human heart. Therefore, we aimed to examine whether myocardial apoptosis in obese and type 2 diabetic patients is associated with elevated ceramide level. The study included 11 lean and 26 overweight or moderately obese subjects without (n = 11, OWT) or with (n = 15, T2D-OWT) a history of type 2 diabetes. Samples of the right atrial appendage were obtained from patients at the time of coronary bypass surgery. Compared with lean subjects, the extent of DNA fragmentation (a marker of apoptosis) was significantly higher in the myocardium of OWT patients and increased further in T2D-OWT subjects. However, the content of ceramide and sphingoid bases remained stable. Interestingly, the mRNA level of enzymes involved in synthesis and degradation of ceramide including serine palmitoyltransferase, sphingosine kinase 1, neutral sphingomyelinase, and ceramidases was markedly higher in the myocardium of OWT and T2D-OWT patients compared with lean subjects. Our results indicate that in the human heart, or at least in the atrium, ceramide is not a major factor in cardiomyocyte apoptosis associated with obesity and type 2 diabetes.
Keywords: atrium, diabetes, heart, human, obesity, papillary muscle, sphingolipids
Lipotoxicity is the process through which lipid overload leads to cellular dysfunction, cell death, and, eventually, organ dysfunction (1). Accumulation of neutral lipids within cardiomyocytes is a hallmark of the heart of diabetic as well as obese rodents and humans (1, 2). In recent years, a hypothesis suggesting that lipotoxicity may contribute to the development of cardiac dysfunction associated with diabetes and obesity has emerged (3, 4). Direct evidence supporting this notion comes from experiments on mice with cardiac specific overexpression of long-chain acyl CoA synthetase 1 (5), fatty acid transport protein 1 (6), and the cell membrane anchored form of LPL (7). All these transgenic animal models are characterized by augmented myocardial fatty acid uptake, lipid accumulation in the heart, and cardiomyopathy that develops in the absence of disturbances in systemic metabolism or cardiac fatty acid oxidation.
Although it is still unclear how lipid overload leads to cardiomyopathy, there is some data indicating that accumulation of ceramide is a major factor in myocardial lipotoxicity. When the balance between fatty acid uptake and oxidation is altered, excess fatty acids is directed toward synthesis of complex lipids including ceramide. In various rodent models of lipotoxic cardiomyopathy, diabetes and obesity, increased myocardial ceramide content has been observed in association with cardiac dysfunction (5, 8–11). In addition, interventions leading to reduction in ceramide level in the heart were shown to improve cardiac function in Zucker diabetic fatty (ZDF) rats and mice with cardiac specific overexpression of a cell membrane anchored form of LPL (10, 11).
Cardiomyocyte apoptosis is one of the mechanisms underlying development of diabetic cardiomyopathy and heart failure (12, 13). Ceramide was proposed to be a mediator of cardiomyocyte apoptosis induced by ischemia-reperfusion, tumor necrosis factor α, and palmitate (14–16). Accumulation of ceramide was found to be associated with myocardial apoptosis in ZDF rats as well as in mice with cardiac specific overexpression of peroxisome proliferator-activated receptor γ or long-chain acyl CoA synthetase 1 (5, 9, 10). Ceramide induces apoptosis by inhibiting a variety of pro-survival signaling kinases including Akt/protein kinase B (PKB) and protein kinase C (PKC)ζ as well as by stimulating pro-apoptosis stress-activated protein kinases like c-jun N-terminal protein kinase (JNK). These signaling pathways are involved in proapoptotic events including suppression of Bcl2 and activation of caspases (17).
The above data strongly suggest that ceramide is an important mediator of lipotoxicity in the rodent heart and that accumulation of this sphingolipid contributes to cardiomyocyte apoptosis associated with type 2 diabetes and obesity. However, it remains unknown whether a similar relationship is present also in the human heart. Moreover, cardiac ceramide metabolism in humans has not been studied yet. Therefore, the aims of this study were: 1) to determine the content of principal intermediates and expression of key enzymes of ceramide metabolism in the human heart, 2) to examine effects of type 2 diabetes and obesity on cardiac ceramide metabolism, and 3) to determine whether myocardial apoptosis in obese and type 2 diabetic patients is associated with elevated ceramide level.
MATERIALS AND METHODS
Patients
The study included 37 age-matched patients with normal valvular function (26 males and 11 females) undergoing elective coronary bypass graft surgery. The subjects were divided into three groups: 1) lean [body mass index (BMI) < 25.0, n = 11] without a history of diabetes and with normal fasting blood glucose level (<110 mg/dl), 2) overweight or moderately obese (BMI > 25.0) without a history of diabetes and with normal fasting glycemia (n = 11, OWT), and 3) overweight or moderately obese with type 2 diabetes (n = 15, T2D-OWT). Mean diabetes duration in this group was 6.6 ± 2.1 years. Ten of the diabetic patients were treated with oral hypoglycemic agents (sulfonylurea derivatives and metformin) and five were treated with insulin. Clinical characteristics of each group are given in Table 1. Samples of the right atrial appendage were retrieved at the time of the right atrial cannulation. Dissected tissues were promptly frozen in liquid nitrogen and then stored at −80°C until further processing. During the surgery, blood glucose levels were kept within the physiologic range in all patients.
TABLE 1.
Clinical characteristics of the subjects
| Lean | OWT | T2D-OWT | |
|---|---|---|---|
| Sex (males/females) | 8/3 | 7/4 | 10/5 |
| Age (years) | 63.9 ± 2.6 (51-76) | 61.4 ± 2.2 (52-76) | 67.3 ± 2.1 (52-77) |
| BMI (kg/m2) | 24.0 ± 0.4 (21.3-25.0) | 30.1 ± 0.7 (26.4-35.7)* | 28.7 ± 0.4 (26.2-31.6)* |
| History of MI | 6 | 5 | 7 |
| Blood glucose (mg%) | 104.0 ± 3.0 | 93.2 ± 1.8 | 130.7 ± 8.6*† |
| Total cholesterol (mg%) | 140.5 ± 6.6 | 171.4 ± 13.4 | 157.1 ± 9.5 |
| HDL cholesterol (mg%) | 46.6 ± 6.5 | 46.2 ± 3.6 | 37.3 ± 2.3 |
| LDL cholesterol (mg%) | 82.2 ± 6.5 | 95.3 ± 11.7 | 97.1 ± 9.4 |
| Triglyceride (mg%) | 78.0 ± 4.8 | 148.8 ± 12.6* | 120.1 ± 19.2 |
| LVEF (%) | 47.7 ± 3.5 (25-60) | 48.1 ± 3.3 (28-60) | 49.5 ± 3.3 (20-65) |
| LVEDd (mm) | 51.3 ± 1.3 | 50.9 ± 2.3 | 52.4 ± 2 |
| SWT (mm) | 11.5 ± 0.25 | 11.4 ± 0.48 | 11.8 ± 0.44 |
| LVPWT (mm) | 11.3 ± 0.3 | 11.1 ± 0.38 | 11.4 ± 0.31 |
| LVM/ht2.7 (g/m2.7) | 55.6 ± 2.2 | 55 ± 4.1 | 59.2 ± 4.1 |
| LAD (mm) | 41 ± 1.6 | 40.2 ± 1.8 | 38.8 ± 1.7 |
The results are means ± SEM. * P < 0.01 versus the lean group, † P < 0.001 versus the OWT group. OWT, overweight/obese patients, T2D-OWT, overweight/obese patients with type 2 diabetes, LVEF, left ventricular ejection fraction; LVEDd, left ventricular end-diastolic diameter; LAD, left atrial diameter; SWT, septal wall thickness; LVPWT, left ventricular posterior wall thickness; LVM/ht2.7, left ventricular mass/height to the 2.7 power.
To compare ceramide metabolism in atrial and ventricular tissue, samples of both right atrial appendage and papillary muscle of the mitral apparatus were obtained from eight additional patients (five males and three females) undergoing cardiac surgery for the mitral valve replacement. The investigation conforms with the principles outlined in the Declaration of Helsinki and was approved by the Ethical Committee for Human Studies of the Medical University of Bialystok. All patients gave their informed consent prior to their inclusion in the study.
Real-time quantitative PCR
Expression of the key genes involved in ceramide and fatty acid metabolism was measured with the use of real-time quantitative PCR. Total RNA was isolated from the samples using TriReagent (Sigma) according to the manufacturer's instructions. Following RNA purification, DNase treatment (Ambion) was performed to ensure that there was no contaminating genomic DNA. Extracted RNA was solubilized in RNase-free water and stored at −80°C until use. The quality of each RNA sample was verified by running the agarose electrophoresis with ethidium bromide. The RNA was reverse transcribed into cDNA using First Strand cDNA Synthesis Kit (Fermentas) with oligo(dT)18. Primers were designed using Beacon Designer Software (Premier Biosoft). Real-time quantitative PCR was performed with SYBR Green JumpStart Taq ReadyMix (Sigma) using a Bio-Rad Chromo4 system. PCR efficiency was examined by serially diluting the template cDNA, and a melt curve was performed at the end of each reaction to verify PCR product specificity. A sample containing no cDNA was used as a negative control to verify the absence of primer dimers. The results were normalized to β-actin expression measured in each sample. Primer sequences are available in the online supplementary data.
DNA fragmentation
DNA fragmentation was assayed by the method of Duke and Sellins (18) with minor modifications. Samples were pulverized in liquid nitrogen. Then the lysis buffer (10 mM Tris-HCl, 10 mM EDTA, and 0.5% Triton X-100, pH 8.0) was added and the samples were suspended by gentle pipetting and incubated on ice for 20 min. After incubation samples were centrifuged for 20 min at 4°C (14,000 g). The supernatant containing fragmented (soluble) DNA was transferred to a fresh tube and lysis buffer was added to the pellet containing insoluble DNA. Both fractions were treated with RNase A (0.5 mg/ml) for 1 h at 37°C and then with Proteinase K (Fermentas) for 1 h at 37°C. After adding 5 M NaCl and isopropyl alcohol, the samples were incubated overnight at −20°C, and DNA concentrations were measured. The extent of DNA fragmentation was calculated as 100% × soluble DNA/(soluble + insoluble DNA). Fragmentation of the soluble DNA fraction was confirmed by electrophoresis on 1.5% agarose gel.
Content of ceramide and sphingoid bases
The content of ceramide and free sphingoid bases including sphingosine, sphinganine, and sphingosine-1-phosphate (S1P) was determined as described previously in detail (19). Briefly, tissues were homogenized and internal standards (C17-sphingosine and C17-S1P, Avanti Polar Lipids) were added. Lipids were then extracted by addition of chloroform, 1M NaCl, and 3N NaOH and the aqueous phase containing S1P was transferred to a fresh tube. The amount of S1P was determined indirectly after dephosphorylation to sphingosine with the use of alkaline phosphatase (bovine intestinal mucosa, Fluka). Free sphingosine and sphinganine as well as sphingosine formed during dephosphorylation of S1P were converted to their o-phthalaldehyde derivatives and analyzed using HPLC system equipped with a fluorescence detector and C18 reversed-phase column (Varian Inc., OmniSpher 5, 4.6 × 150mm). For ceramide assay, a small volume of the chloroform phase containing extracted lipids was transferred to a fresh tube containing C17-sphingosine as an internal standard. The samples were then subjected to alkaline hydrolysis to deacylate ceramide. The content of free sphingosine liberated from ceramide was then analyzed by means of HPLC as described above. Because the chloroform extract used for ceramide assay contains small amounts of free sphingoid bases, the content of ceramide was corrected for the level of free sphingosine determined in the same sample.
Statistical analysis
All data are presented as means ± SEM. Statistical comparisons were made by using two-way ANOVA without interactions with one factor being overweight and the other factor being diabetes. If overall significance was demonstrated by ANOVA posthoc multiple comparisons using a Newman-Keuls test were conducted. Comparisons between atrial appendage and papillary muscle were made using Student's t-test for paired samples. P < 0.05 was considered statistically significant. One, two, or three symbols indicate a significant difference at the P < 0.05, P < 0.01, or P < 0.001 levels, respectively.
RESULTS
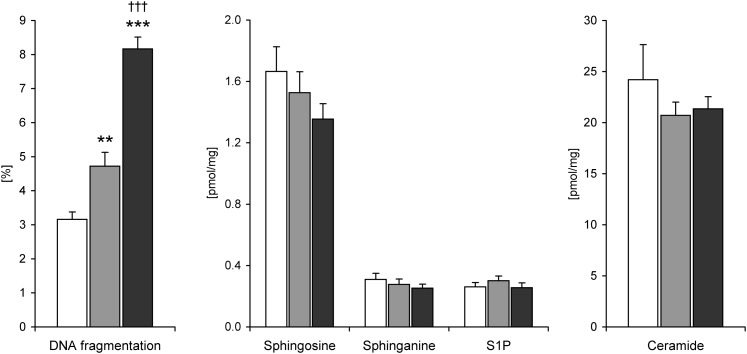
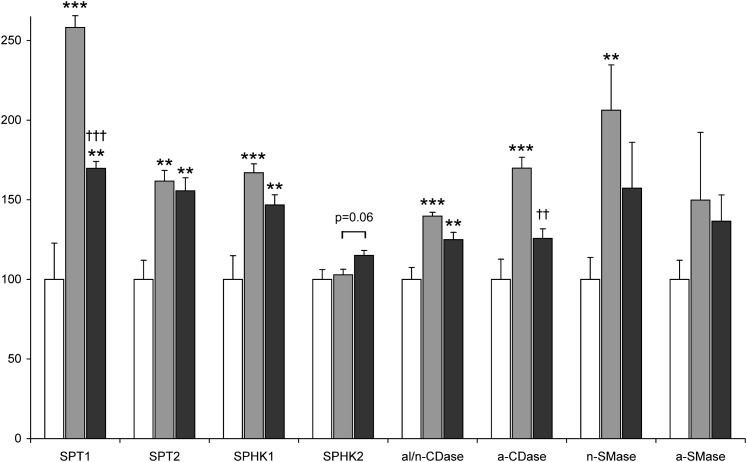
The mean content of sphingosine, sphinganine, S1P, and ceramide in the right atrial appendage of the lean patients was 1.67 ± 0.16, 0.31 ± 0.04, 0.26 ± 0.03, and 24.2 ± 3.44 pmol/mg, respectively. There were no significant sex differences in the content of these compounds. Neither obesity nor type 2 diabetes induced statistically significant changes in the level of any of the aforementioned sphingolipids (Fig. 1). Myocardial expression of serine palmitoyltransferase (SPT) subunit 1 and 2, sphingosine kinase (SPHK) 1 and alkaline/neutral ceramidase (al/n-CDase) in the OWT and T2D-OWT groups was approximately 1.5-fold higher compared with the lean patients (Fig. 2). Two-way ANOVA demonstrated a significant effect of overweight also on the mRNA level of acid ceramidase (a-CDase) and neutral sphingomyelinase (n-SMase), however, posthoc analysis showed that expression of these enzymes was increased only in the OWT patients (by 1.7 and 2.1-fold, respectively, Fig. 2). SPHK2 and acid sphingomyelinase (a-SMase) were the only investigated genes related to ceramide metabolism which expression was not affected by overweight.
Fig. 1.
Sphingolipid content and DNA fragmentation in the right atrial appendage of lean (white bars, n = 11), overweight/obese (gray bars, n = 11) and overweight/obese type 2 diabetic (black bars, n = 15) patients. Values are means ± SEM. * significant difference versus lean subjects, † significant difference versus overweight/obese patients. S1P, sphingosine-1-phosphate.
Fig. 2.
Expression of ceramide metabolism-related genes in the right atrial appendage of lean (white bars, n = 11), overweight/obese (gray bars, n = 11) and overweight/obese type 2 diabetic (black bars, n = 15) patients. mRNA levels are expressed as a % of the lean group ± SEM. * significant difference versus lean subjects, † significant difference versus overweight/obese patients. SPT, serine palmitoyltransferase; SPHK, sphingosine kinase; al/n-Cdase, alkaline/neutral ceramidase; a-Cdase, acid ceramidase; n-Smase, neutral sphingomyelinase; a-Smase, acid sphingomyelinase.
A significant effect of diabetes on the mRNA level of SPT1, SPHK2, and a-CDase was found by two-way ANOVA. Posthoc comparisons demonstrated that expression of SPT1 and a-CDase was lower in the T2D-OWT patients (by 34 and 26%, respectively) compared with the OWT group (Fig. 2). In contrast, the mRNA level of SPHK2 was slightly elevated in the myocardium of the T2D-OWT patients, however, the difference was of borderline significance (P = 0.06).
Two-way ANOVA demonstrated a significant effect of both overweight and diabetes on the extent of DNA fragmentation (a marker of apoptosis) in the myocardium. Compared with the lean patients, DNA fragmentation in the OWT group increased 1.5-fold and further elevation (1.7-fold vs. the OWT patients) was observed in the T2D-OWT subjects (Fig. 1). The extent of DNA fragmentation was significantly positively correlated with glucose level (r = 0.47, P = 0.005) and BMI (r = 0.45, P = 0.008) but not with myocardial content of ceramide or its metabolites (data not shown).
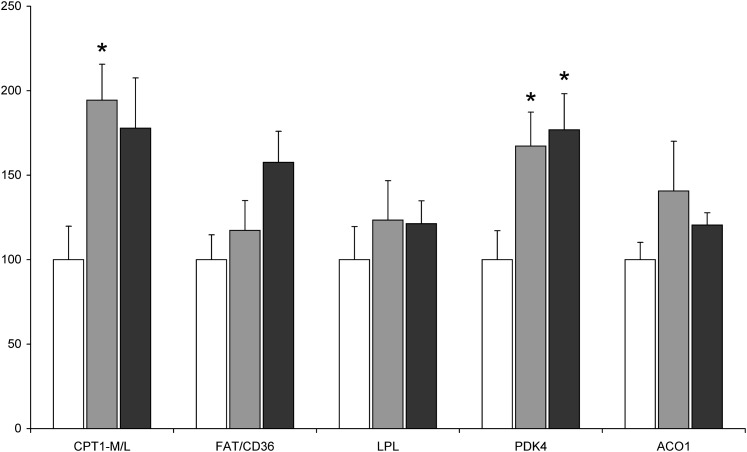
We also determined myocardial expression of the key genes involved in fatty acid metabolism (Fig. 3). A significant effect of overweight on the mRNA level of pyruvate dehydrogenase kinase (PDK) 4 and carnityne palmitoyltransferase (CPT) 1 was found by two-way ANOVA. Compared with the lean patients, expression of PDK4 and CPT1 was almost 2-fold higher in the myocardium of the OWT and T2D-OWT subjects. However, in the case of CPT1, statistically significant difference was demonstrated only for the OWT group. There was also a trend for increased mRNA level of fatty acid translocase/CD36 (FAT/CD36) in the T2D-OWT patients. However, the difference was of only borderline significance (P = 0.076 vs. the lean group).
Fig. 3.
Expression of fatty acid metabolism-related genes in the right atrial appendage of lean (white bars, n = 11), overweight/obese (gray bars, n = 11) and overweight/obese type 2 diabetic (black bars, n = 15) patients. mRNA levels are expressed as a % of the lean group ± SEM. * significant difference versus lean subjects. CPT1-M/L, carnityne palmitoyltransferase 1 muscle/liver isoform; FAT/CD36, fatty acid translocase/CD36; LPL, lipoprotein lipase, PDK4, pyruvate dehydrogenase kinase 4; ACO1, acyl-CoA oxidase 1.
To compare ceramide metabolism in atrial and ventricular tissue, samples of both atrial appendage and papillary muscle of the mitral apparatus were obtained from eight additional patients. Compared with the atrial appendage, papillary muscle was characterized by lower level of ceramide, sphinganine, and S1P (by 29, 44, and 39%, respectively, Table 2). However, in the case of ceramide, the difference was of borderline significance (P = 0.088). On the other hand, expression of all investigated enzymes involved in ceramide metabolism, with the exception of sphingomyelinases, was significantly higher in the papillary muscle (Table 2). The difference ranged from ∼2-fold (SPT subunits) to 7-fold (SPHK2).
TABLE 2.
Sphingolipid content and expression of ceramide metabolism-related genes in atrial and ventricular tissue
| Atrial Appendage | Papillary Muscle | |
|---|---|---|
| Content of sphingolipids (pmol/mg) | ||
| Sphingosine | 1.58 ± 0.15 | 1.28 ± 0.09 |
| Sphinganine | 0.26 ± 0.02 | 0.14 ± 0.01** |
| Sphingosine-1P | 0.22 ± 0.02 | 0.14 ± 0.03** |
| Ceramide | 24.8 ± 2.8 | 17.5 ± 1.7 |
| Gene expression(fold change vs. atrial appendage) | ||
| SPT1 | 1 ± 0.04 | 2.15 ± 0.04*** |
| SPT2 | 1 ± 0.08 | 1.58 ± 0.04*** |
| SPHK1 | 1 ± 0.06 | 4.12 ± 0.1*** |
| SPHK2 | 1 ± 0.05 | 6.94 ± 0.14** |
| al/n-CDase | 1 ± 0.04 | 2.89 ± 0.05*** |
| a-CDase | 1 ± 0.03 | 3.24 ± 0.05*** |
| n-SMase | 1 ± 0.19 | 1.25 ± 0.22 |
| a-SMase | 1 ± 0.11 | 1.28 ± 0.25 |
The results are means ± SEM. Samples of both right atrial appendage and papillary muscle of the mitral apparatus were obtained from patients undergoing cardiac surgery for the mitral valve replacement (n = 6–8). * significant difference versus atrial appendage.
We did not find statistically significant correlations between the measured parameters of cardiac structure and function and the level of myocardial ceramide or the extent of DNA fragmentation (data not shown). No significant differences in sphingolipid content or DNA fragmentation were found also when statistical comparisons were made between the groups of patients with impaired left ventricular ejection fraction (LVEF ≤ 45%, n = 15) and preserved (LVEF > 45%, n = 22) systolic function (data not shown). However, the concentration of plasma triacylglycerol was inversely related to the left ventricular ejection fraction (r = -0.38, P = 0.024).
DISCUSSION
To the best of our knowledge, this is the first report describing the pathways of ceramide metabolism in the human myocardium. Compared with the data from our previous paper where sphingolipid content in the rat heart was determined by the same method (19), human myocardium is characterized by similar level of ceramide but markedly lower content of sphingosine, sphinganine, and S1P. We found only one paper where ceramide content in the human cardiac muscle was measured. Sugita et al. (20) reported that the heart of a 3-year-old male patient who died of pneumonia contained 15.9 μg of ceramide per gram of wet tissue (approximately 29 pmol/mg, assuming molecular weight of C16-ceramide), which falls within the range of values observed in our study. Another difference between human and rat myocardium is relative contribution of various ceramidase isoforms to total ceramidase activity. We previously showed that in the rat heart a-CDase activity is much lower than activity of alkaline or neutral isoform of this enzyme (19). However, in the human myocardium expression of a-CDase was much higher compared with al/n-CDase, suggesting the dominance of the former isoform. Our results are in line with previous reports showing high level of a-CDase mRNA in the human but not murine heart (21, 22). Similar species difference is present also for expression of sphingosine kinase isoforms. Our data indicate that SPHK1 is the dominant subtype in the human myocardium, whereas the rodent heart expresses predominantly SPHK2 (23).
We found significant differences in ceramide metabolism between atrial and ventricular tissue. In general, the levels of ceramide and sphingoid bases were lower in the papillary muscle of the mitral apparatus compared with the right atrial appendage. However, the expression of enzymes involved in both de novo ceramide synthesis (SPT1 and SPT2) and degradation (ceramidases and sphingosine kinases) was markedly higher in the papillary muscle suggesting higher ceramide turnover in the ventricular tissue. Regional differences in the content of ceramide as well as sphingosine and sphinganine were observed also in the murine heart (24).
In our study, overweight either alone or accompanied by type 2 diabetes did not affect myocardial ceramide content. This is in contrast to animal studies showing accumulation of ceramide in the heart of ZDF rats (10) and streptozotocin-diabetic rats (M. Baranowski, unpublished observation) as well as in the neonatal rat cardiomyocytes incubated in the presence of palmitate (25). Elevated muscle ceramide level associated with obesity and diabetes is generally thought to result from increased fatty acyl-CoA availability for ceramide synthesis de novo. In addition, exogenous fatty acids can also augment ceramide synthesis by upregulating the expression of SPT (the initial and rate-limiting enzyme in the de novo synthesis of ceramide) (26). Interestingly, we observed increased mRNA level of both SPT subunits in the myocardium of overweight patients. In addition, expression of n-SMase, an enzyme producing ceramide from sphingomyelin, was also upregulated. However, these increases were accompanied by elevated mRNA level of enzymes involved in the pathway of ceramide degradation, including ceramidases (responsible for ceramide deacylation to sphingosine) and sphingosine kinase 1 (phosphorylating sphingosine to S1P, thereby preventing ceramide resynthesis). These data indicate that activation of ceramide catabolism may protect the heart of overweight humans against ceramide accumulation resulting from its augmented production de novo and increased formation from sphingomyelin.
Another explanation for the lack of change in myocardial ceramide content in spite of upregulation of enzymes responsible for its synthesis is that this upregulation represents a compensatory mechanism to maintain proper ceramide level required for normal cell function. In our study, myocardium of the overweight patients was characterized by increased expression of genes that promote fatty acid oxidation (CPT1 and PDK4), whereas the mRNA level of genes encoding proteins involved in fatty acid uptake (FAT/CD36 and LPL) remained stable. It is possible then, that in the heart of overweight humans, fatty acids are shunted toward β-oxidation rather than ceramide synthesis. This could result in upregulation of SPT expression to compensate for lower availability of substrates required for de novo ceramide synthesis. This notion is supported by the findings of Park et al. (11) who demonstrated that pharmacological inhibition of SPT markedly increased expression of both its subunits in the murine myocardium without altering ceramide level.
Type 2 diabetes did not potentiate the stimulatory effect of overweight on myocardial expression of enzymes involved in ceramide metabolism. In fact mRNA level of some genes upregulated by overweight was reduced if concomitant diabetes was present. One of these genes was a-CDase, which suggests decreased rate of ceramide degradation in the heart of overweight type 2 diabetic patients. Interestingly, the myocardial content of ceramide again remained unchanged, likely due to the fact that the expression of SPT1 was also reduced proportionally. It should be noted, however, that compared with the lean subjects, expression of almost all genes upregulated by overweight (with the exception of a-CDase and n-SMase) was still significantly higher in the heart of overweight type 2 diabetic patients. This is in line with the results of Wilson et al. (27) who reported elevated expression of ceramide metabolism-related genes in the heart of obese mice with type 2 diabetes.
We found elevated myocardial DNA fragmentation levels (a hallmark of apoptosis) in overweight subjects that were further increased in patients with concomitant type 2 diabetes. These data indicate that obesity and diabetes have additive effect on cardiac apoptosis in humans. Activation of cardiomyocyte apoptosis has been previously reported in diabetic patients as well as in ob/ob and db/db mice (genetic animal models of obesity and type 2 diabetes, respectively) (28–30). The increase in DNA fragmentation observed in our study was moderate; however, it was demonstrated that in the long term, even low albeit abnormal rates of cardiomyocyte apoptosis are sufficient to cause cardiac dysfunction (31).
As already mentioned in the introduction, ceramide was proposed to be a mediator of cardiomyocyte apoptosis resulting from lipotoxicity. It was also demonstrated that ceramide-induced apoptosis contributes to the decreased pancreatic β-cell mass in type 2 diabetes (26). Therefore, our initial hypothesis was that myocardial apoptosis in overweight and type 2 diabetic patients would be associated with accumulation of ceramide. However, despite marked differences in DNA fragmentation between the investigated groups of patients, we found no changes in ceramide content. This suggests that in the human heart or at least in the atrium, ceramide is not a major mediator of cardiomyocyte apoptosis associated with obesity and type 2 diabetes and that other factors play a dominant role in this process. It was shown that palmitate can induce cardiomyocyte apoptosis independently of ceramide via generation of reactive oxygen species resulting from increased cycling through mitochondrial β-oxidation pathway (32, 33). In addition, apoptosis of cardiomyocytes is activated by hyperglycemia through a mechanism involving excessive protein O-glycosylation (34). In the overweight type 2 diabetic patients, elevated SPHK2 expression could also contribute to increased cardiac apoptosis. This notion is also supported by the fact that the mRNA level of this enzyme was positively correlated with the extent of DNA fragmentation (r = 0.41, P = 0.018). In contrast to SPHK1 that promotes cell survival via production of S1P, SPHK2 enhances apoptosis and this effect has been attributed to its BH3 domain (35).
The toxic effects of ceramide on the heart are commonly considered to be mediated primarily by activation of apoptosis. It should be noted, however, that a recent study by Park et al. (11) suggested that cardiotoxicity of ceramide results from its influence on myocardial glucose and fatty acid catabolism rather than induction of apoptotic loss of cardiomyocytes. They used mice with cardiac-specific overexpression of the cell membrane anchored form of LPL to determine the role of ceramide in cardiac lipotoxicity. These mice were characterized by impaired systolic function, increased rate of fatty acid oxidation, and reduced glucose oxidation rate in the heart as well as by accumulation of myocardial ceramide, which, however, was not accompanied by increased cardiomyocyte apoptosis. Interestingly, pharmacological or genetic inhibition of ceramide synthesis de novo normalized fatty acid and glucose oxidation rate as well as systolic function. In addition, these interventions corrected the mismatch between myocardial glucose uptake and oxidation, which likely contributed to the development of cardiomyopathy in this model.
In summary, we found elevated DNA fragmentation level in the heart of overweight patients that was increased further in overweight type 2 diabetic subjects. However, these changes were not associated with accumulation of ceramide in the myocardium. Ceramide content remained stable despite marked effect of overweight and type 2 diabetes on the mRNA level of ceramide metabolism-related genes. This was likely due to the fact that cardiac expression of enzymes involved in synthesis and degradation of ceramide was regulated in concert. We conclude that in the human heart, or at least in the atrium, ceramide is not a major factor in cardiomyocyte apoptosis associated with obesity and type 2 diabetes.
Supplementary Material
Footnotes
Abbreviations:
- ACO1
- acyl-CoA oxidase 1
- al/n-Cdase
- alkaline/neutral ceramidase
- a-CDase
- acid ceramidase
- a-SMase
- acid sphingomyelinase
- BMI
- body mass index
- CPT1-M/L
- carnityne palmitoyltransferase 1 muscle/liver isoform
- FAT/CD36
- fatty acid translocase/CD36
- n-Smase
- neutral sphingomyelinase
- OWT
- overweight or moderately obese subjects without a history of type 2 diabetes
- PDK4
- pyruvate dehydrogenase kinase 4
- S1P
- sphingosine-1-phosphate
- SPHK
- sphingosine kinase
- SPT
- serine palmitoyltransferase
- T2D-OWT
- overweight or moderately obese subjects with a history of type 2 diabetes
- ZDF
- Zucker diabetic fatty
This work was supported by the Medical University of Bialystok grants 3-18827 and 4-18844 as well as by the Polish Ministry of Science and Higher Education (grant N N402 470937).
The online version of this article (available at http://www.jlr.org) contains supplementary data.
REFERENCES
- 1.Borradaile N. M., Schaffer J. E. 2005. Lipotoxicity in the heart. Curr. Hypertens. Rep. 7: 412–417. [DOI] [PubMed] [Google Scholar]
- 2.Harmancey R., Wilson C. R., Taegtmeyer H. 2008. Adaptation and maladaptation of the heart in obesity. Hypertension. 52: 181–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Young M. E., McNulty P., Taegtmeyer H. 2002. Adaptation and maladaptation of the heart in diabetes: Part II: potential mechanisms. Circulation. 105: 1861–1870. [DOI] [PubMed] [Google Scholar]
- 4.Park T. S., Yamashita H., Blaner W. S., Goldberg I. J. 2007. Lipids in the heart: a source of fuel and a source of toxins. Curr. Opin. Lipidol. 18: 277–282. [DOI] [PubMed] [Google Scholar]
- 5.Chiu H. C., Kovacs A., Ford D. A., Hsu F. F., Garcia R., Herrero P., Saffitz J. E., Schaffer J. E. 2001. A novel mouse model of lipotoxic cardiomyopathy. J. Clin. Invest. 107: 813–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiu H. C., Kovacs A., Blanton R. M., Han X., Courtois M., Weinheimer C. J., Yamada K. A., Brunet S., Xu H., Nerbonne J. M., et al. 2005. Transgenic expression of fatty acid transport protein 1 in the heart causes lipotoxic cardiomyopathy. Circ. Res. 96: 225–233. [DOI] [PubMed] [Google Scholar]
- 7.Yagyu H., Chen G., Yokoyama M., Hirata K., Augustus A., Kako Y., Seo T., Hu Y., Lutz E. P., Merkel M., et al. 2003. Lipoprotein lipase (LpL) on the surface of cardiomyocytes increases lipid uptake and produces a cardiomyopathy. J. Clin. Invest. 111: 419–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Finck B. N., Lehman J. J., Leone T. C., Welch M. J., Bennett M. J., Kovacs A., Han X., Gross R. W., Kozak R., Lopaschuk G. D., et al. 2002. The cardiac phenotype induced by PPARalpha overexpression mimics that caused by diabetes mellitus. J. Clin. Invest. 109: 121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Son N. H., Park T. S., Yamashita H., Yokoyama M., Huggins L. A., Okajima K., Homma S., Szabolcs M. J., Huang L. S., Goldberg I. J. 2007. Cardiomyocyte expression of PPARgamma leads to cardiac dysfunction in mice. J. Clin. Invest. 117: 2791–2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou Y. T., Grayburn P., Karim A., Shimabukuro M., Higa M., Baetens D., Orci L., Unger R. H. 2000. Lipotoxic heart disease in obese rats: implications for human obesity. Proc. Natl. Acad. Sci. USA. 97: 1784–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park T. S., Hu Y., Noh H. L., Drosatos K., Okajima K., Buchanan J., Tuinei J., Homma S., Jiang X. C., Abel E. D., et al. 2008. Ceramide is a cardiotoxin in lipotoxic cardiomyopathy. J. Lipid Res. 49: 2101–2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Foo R. S., Mani K., Kitsis R. N. 2005. Death begets failure in the heart. J. Clin. Invest. 115: 565–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boudina S., Abel E. D. 2007. Diabetic cardiomyopathy revisited. Circulation. 115: 3213–3223. [DOI] [PubMed] [Google Scholar]
- 14.Dyntar D., Eppenberger-Eberhardt M., Maedler K., Pruschy M., Eppenberger H. M., Spinas G. A., Donath M. Y. 2001. Glucose and palmitic acid induce degeneration of myofibrils and modulate apoptosis in rat adult cardiomyocytes. Diabetes. 50: 2105–2113. [DOI] [PubMed] [Google Scholar]
- 15.Bielawska A. E., Shapiro J. P., Jiang L., Melkonyan H. S., Piot C., Wolfe C. L., Tomei L. D., Hannun Y. A., Umansky S. R. 1997. Ceramide is involved in triggering of cardiomyocyte apoptosis induced by ischemia and reperfusion. Am. J. Pathol. 151: 1257–1263. [PMC free article] [PubMed] [Google Scholar]
- 16.Krown K. A., Page M. T., Nguyen C., Zechner D., Gutierrez V., Comstock K. L., Glembotski C. C., Quintana P. J., Sabbadini R. A. 1996. Tumor necrosis factor alpha-induced apoptosis in cardiac myocytes. Involvement of the sphingolipid signaling cascade in cardiac cell death. J. Clin. Invest. 98: 2854–2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morales A., Lee H., Goni F. M., Kolesnick R., Fernandez-Checa J. C. 2007. Sphingolipids and cell death. Apoptosis. 12: 923–939. [DOI] [PubMed] [Google Scholar]
- 18.Duke R. C., Sellins K. S. 1989. Target cell nuclear damage in addition to DNA fragmentation during cytotoxic T lymphocyte-mediated cytolysis. Cellular Basis of Immune Modulation. Kaplan J. G., Green D. R., Bleackley R. C., Alan R. Liss Inc., New York: 311–314. [Google Scholar]
- 19.Baranowski M., Zabielski P., Blachnio A., Gorski J. 2008. Effect of exercise duration on ceramide metabolism in the rat heart. Acta Physiol. (Oxf.). 192: 519–529. [DOI] [PubMed] [Google Scholar]
- 20.Sugita M., Iwamori M., Evans J., McCluer R. H., Dulaney J. T., Moser H. W. 1974. High performance liquid chromatography of ceramides: application to analysis in human tissues and demonstration of ceramide excess in Farber's disease. J. Lipid Res. 15: 223–226. [PubMed] [Google Scholar]
- 21.Li C. M., Hong S. B., Kopal G., He X., Linke T., Hou W. S., Koch J., Gatt S., Sandhoff K., Schuchman E. H. 1998. Cloning and characterization of the full-length cDNA and genomic sequences encoding murine acid ceramidase. Genomics. 50: 267–274. [DOI] [PubMed] [Google Scholar]
- 22.Li C. M., Park J. H., He X., Levy B., Chen F., Arai K., Adler D. A., Disteche C. M., Koch J., Sandhoff K., et al. 1999. The human acid ceramidase gene (ASAH): structure, chromosomal location, mutation analysis, and expression. Genomics. 62: 223–231. [DOI] [PubMed] [Google Scholar]
- 23.Liu H., Sugiura M., Nava V. E., Edsall L. C., Kono K., Poulton S., Milstien S., Kohama T., Spiegel S. 2000. Molecular cloning and functional characterization of a novel mammalian sphingosine kinase type 2 isoform. J. Biol. Chem. 275: 19513–19520. [DOI] [PubMed] [Google Scholar]
- 24.Noureddine L., Azzam R., Nemer G., Bielawski J., Nasser M., Bitar F., Dbaibo G. S. 2008. Modulation of total ceramide and constituent ceramide species in the acutely and chronically hypoxic mouse heart at different ages. Prostaglandins Other Lipid Mediat. 86: 49–55. [DOI] [PubMed] [Google Scholar]
- 25.Hickson-Bick D. L., Buja M. L., McMillin J. B. 2000. Palmitate-mediated alterations in the fatty acid metabolism of rat neonatal cardiac myocytes. J. Mol. Cell. Cardiol. 32: 511–519. [DOI] [PubMed] [Google Scholar]
- 26.Summers S. A. 2005. Ceramides in insulin resistance and lipotoxicity. Prog. Lipid Res. 45: 42–72. [DOI] [PubMed] [Google Scholar]
- 27.Wilson K. D., Li Z., Wagner R., Yue P., Tsao P., Nestorova G., Huang M., Hirschberg D. L., Yock P. G., Quertermous T., et al. 2008. Transcriptome alteration in the diabetic heart by rosiglitazone: implications for cardiovascular mortality. PLoS One. 3: e2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chowdhry M. F., Vohra H. A., Galinanes M. 2007. Diabetes increases apoptosis and necrosis in both ischemic and nonischemic human myocardium: role of caspases and poly-adenosine diphosphate-ribose polymerase. J. Thorac. Cardiovasc. Surg. 134: 124–131. [DOI] [PubMed] [Google Scholar]
- 29.Frustaci A., Kajstura J., Chimenti C., Jakoniuk I., Leri A., Maseri A., Nadal-Ginard B., Anversa P. 2000. Myocardial cell death in human diabetes. Circ. Res. 87: 1123–1132. [DOI] [PubMed] [Google Scholar]
- 30.Barouch L. A., Gao D., Chen L., Miller K. L., Xu W., Phan A. C., Kittleson M. M., Minhas K. M., Berkowitz D. E., Wei C., et al. 2006. Cardiac myocyte apoptosis is associated with increased DNA damage and decreased survival in murine models of obesity. Circ. Res. 98: 119–124. [DOI] [PubMed] [Google Scholar]
- 31.Wencker D., Chandra M., Nguyen K., Miao W., Garantziotis S., Factor S. M., Shirani J., Armstrong R. C., Kitsis R. N. 2003. A mechanistic role for cardiac myocyte apoptosis in heart failure. J. Clin. Invest. 111: 1497–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Listenberger L. L., Ory D. S., Schaffer J. E. 2001. Palmitate-induced apoptosis can occur through a ceramide-independent pathway. J. Biol. Chem. 276: 14890–14895. [DOI] [PubMed] [Google Scholar]
- 33.Kong J. Y., Rabkin S. W. 2002. Palmitate-induced cardiac apoptosis is mediated through CPT-1 but not influenced by glucose and insulin. Am. J. Physiol. Heart Circ. Physiol. 282: H717–H725. [DOI] [PubMed] [Google Scholar]
- 34.Fulop N., Marchase R. B., Chatham J. C. 2007. Role of protein O-linked N-acetyl-glucosamine in mediating cell function and survival in the cardiovascular system. Cardiovasc. Res. 73: 288–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alemany R., van Koppen C. J., Danneberg K., Ter Braak M., Meyer Zu Heringdorf D. 2007. Regulation and functional roles of sphingosine kinases. Naunyn Schmiedebergs Arch. Pharmacol. 374: 413–428. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.