Summary
DNA-responsive checkpoints prevent cell cycle progression following DNA damage or replication inhibition. The mitotic activator Cdc25 is suppressed by checkpoints through inhibitory phosphorylation at Ser287 (Xenopus numbering) and docking of 14-3-3. S287 phosphorylation is a major locus of G2/M checkpoint control, though several checkpoint-independent kinases can phosphorylate this site. We reported previously that mitotic entry requires 14-3-3 removal and S287 dephosphorylation. We show here that DNA-responsive checkpoints activate PP2A/B56δ phosphatase complexes to dephosphorylate Cdc25 at a site (T138) whose phosphorylation is required for 14-3-3 release. However, phosphorylation of T138 is not sufficient for 14-3-3 release from Cdc25. Rather, our data suggest that creation of a 14-3-3 “sink”, consisting of phosphorylated 14-3-3-binding intermediate filament proteins, coupled with reduced Cdc25-14-3-3 affinity, contribute to Cdc25 activation. These observations identify PP2A/B56δ as a central checkpoint effector, and suggest a mechanism for controlling 14-3-3 interactions to promote mitosis.
Introduction
The master regulator of mitosis, Cdc2/Cyclin B, is inhibited by phosphorylation of Cdc2 on T14 and Y15, catalyzed by the Myt1 and Wee1 kinases. At the G2/M transition, these phosphorylations are reversed by the Cdc25 phosphatase, leading to Cdc2/Cyclin B activation and mitosis (Lew and Kornbluth, 1996).
During interphase, Cdc25 is inhibited by Ser287 phosphorylation (Xenopus Cdc25; Ser 216 in human Cdc25C) and this inhibitory phosphorylation is maintained by DNA-responsive checkpoints (Kumagai et al., 1998a; Peng et al., 1997). S287 is targeted by many kinases, including Chk1, Chk2, cTAK-1, PKA, p38 and MAPKAP kinase-2 suggesting that phosphorylation of this site may integrate multiple signaling inputs (Duckworth et al., 2002; Furnari et al., 1999; Manke et al., 2005; Peng et al., 1998).
Cdc25 S287 phosphorylation provides a docking site for 14-3-3 protein (Dougherty and Morrison, 2004; Peng et al., 1997). Although the role of this binding in regulating Cdc25 catalytic activity has been controversial, 14-3-3 masks a nuclear localization sequence, preventing Cdc25 nuclear translocation (Kumagai et al., 1998b; Yang et al., 1999). A Cdc25 mutant that is neither phosphorylated at S287 nor binds 14-3-3 (S287A) promotes premature mitosis even in the presence of damaged or incompletely replicated DNA (Kumagai et al., 1998a; Peng et al., 1997). At mitosis, Cdc25 activation follows S287 dephosphorylation and 14-3-3 dissociation. Although previous reports implied that 14-3-3 removal resulted from S287 dephosphorylation, we recently reported that 14-3-3 removal precedes and is required for S287 dephosphorylation by protein phosphatase 1 (PP1) (Margolis et al., 2003). Thus, we hypothesized that factors controlling 14-3-3 removal might be key targets of DNA-responsive checkpoints.
Seeking to identify factors controlling 14-3-3 removal from Cdc25, we found that phosphorylation of Cdc25 on T138 by Cdk2 was required, explaining a previously reported role for Cdk2 in regulating mitotic entry (Guadagno and Newport, 1996; Margolis et al., 2003). These data did not explain how 14-3-3 release might be checkpoint-regulated as Cdk2 activity was reportedly constitutive throughout the cell cycle in the Xenopus egg extracts used for our analyses (Guadagno and Newport, 1996; Rempel et al., 1995). This suggested that regulation of 14-3-3 release must be exerted at some other level.
Although Cdc25 is negatively regulated by S287 phosphorylation, a role for phosphorylation in activating Cdc25 has been proposed because PP2A can inhibit Cdc25. At doses that inhibit PP2A selectively, okadaic acid (OA) promotes Cdc25 activation and mitosis even when DNA-responsive checkpoints are activated (Clarke et al., 1993, Izumi et al., 1992; Kumagai et al., 1998a). Because PP2A has many substrates, the specific effects of PP2A ablation on Cdc25 have not been studied. Thus, it has not been clear how PP2A controls Cdc25 activation and whether this regulation might be under checkpoint control. In addition, the PP2A core enzyme, comprised of the catalytic subunit (C) and a structural subunit (A), can be directed to substrates by a number of different targeting subunits (B), and the targeting subunit involved in vertebrate Cdc25 regulation has not been identified.
We report here that checkpoint-regulated release of 14-3-3 from Cdc25 is controlled by PP2A bound to the B56δ targeting subunit. Binding of PP2A B56δ to Cdc25 maintains T138 dephosphorylation (and stable 14-3-3 binding) during interphase. Importantly, Chk1 phosphorylation of B56δ enhances PP2A-mediated T138 dephosphorylation, indicating that DNA-responsive checkpoints control Cdc25 through PP2A, rather than simply through S287 phosphorylation. Although T138 phosphorylation is critical for 14-3-3 release from Cdc25, it is not sufficient. Rather, T138 phosphorylation reduces the affinity of 14-3-3 for Cdc25, but efficient 14-3-3 removal involves the creation of alternative docking sites for 14-3-3 on other proteins at G2/M. We show that this 14-3-3 “sink” consists, in Xenopus egg extracts, of phosphorylated keratin and propose that other abundant mitotically phosphorylated proteins may serve this role in other cell types. These data show that PP2A is checkpoint-controlled through targeting subunit phosphorylation, suggesting that PP2A-mediated T138 dephosphorylation is the most upstream of the known regulatory events acting on Cdc25. In addition, our findings suggest a model in which the G2/M transition is driven in a concerted manner both through control of Cdc2 regulators and by phosphorylation of additional cellular proteins that serve as docking sites for 14-3-3.
Results
T138-directed phosphatase activity is cell cycle regulated
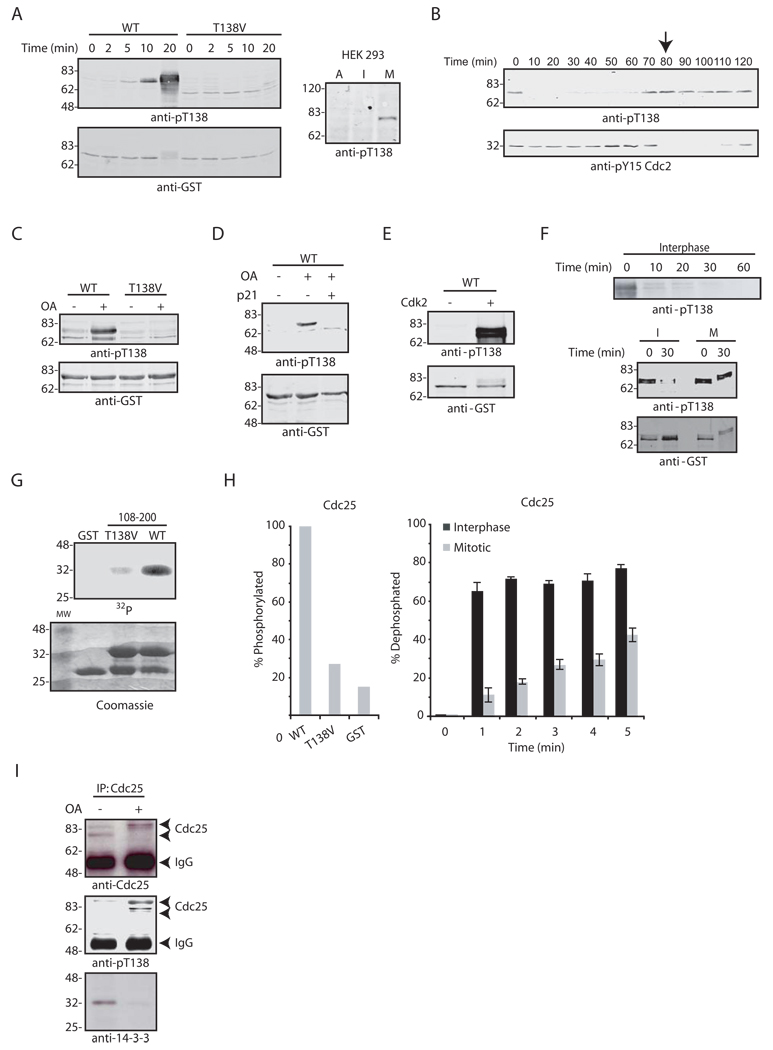
The identification of Cdk2 as the T138-directed kinase raised the issue of how this phosphorylation might be regulated, since Cdk2 kinase activity is constitutive during the early Xenopus embryonic cell cycles (Guadagno and Newport, 1996). T138 phosphorylation was absent in interphase egg extracts (prepared with cycloheximide to prevent Cyclin synthesis) and was induced by addition of recombinant Cyclin B to drive mitosis (Fig. 1A, left). Similar cell cycle variation in phosphorylation at this site was seen for endogenous Cdc25C in human HEK 293 cells (T130 in Cdc25C) (Fig. 1A, right). T138 phosphorylation appeared before Cdc2 activation in cycling egg extracts, as determined by Y15 dephosphorylation and nuclear envelope breakdown (Fig. 1B and data not shown). These data raised the possibility that a T138-directed phosphatase was cell cycle controlled. Consistent with this, addition of the phosphatase inhibitor, OA, to interphase extracts induced T138 phosphorylation (Fig. 1C). Moreover, the Cdk2 inhibitor p21 diminished T138 phosphorylation in the OA-treated interphase extracts, confirming the role of Cdk2 in regulating 14-3-3 release from Cdc25 (Fig. 1D).
Fig. 1. T138 dephosphorylation is cell cycle-regulated.
A. Wild-type (WT) and T138V GST-Cdc25 proteins were incubated in Xenopus egg interphase extract treated with Cyclin B. Samples were retrieved on glutathione-Sepharose at the indicated times, washed, and immunoblotted with anti-pT138 antibody (left). Asynchronous (A), interphase (I; aphidicolin-treated), or mitotic (M; nocodazole-treated) HEK 293 cell lysates were immunoblotted with anti-pT138 antibody (right). B. Cycling extract samples taken at the indicated times were immunoblotted for pT138 and pY15 Cdc2. The arrow indicates nuclear envelope breakdown. C. WT or T138V GST-Cdc25 was incubated in interphase extract +/− OA treatment. Samples were retrieved on glutathione-Sepharose, washed, and immunoblotted for pT138. D. N-terminal GST-Cdc25 was incubated in interphase extract +/− OA and +/− p21 and processed as in (C) for anti-pT138 blotting. E. GST-Cdc25 protein was incubated in buffer containing ATP +/− Cdk2/Cyclin E for 1 hr at 30°C, and processed as in (C). F. GST-Cdc25 was phosphorylated with Cdk2/Cyclin E, washed, and incubated in interphase extract (top) or in either interphase (I) or mitotic (M) extract (bottom). Samples were processed as in (C) for anti-pT138 immunoblotting. G. WT or T138V GST-Cdc25, amino acids 108–200, was incubated with Cdk2/Cyclin E and [γ−32P] ATP for 1 h at 30°C. Samples were resolved by SDS-PAGE and visualized by autoradiography (top) or Coomassie blue stain (bottom). H. GST, WT or T138V GST-Cdc25 108–200 proteins were phosphorylated as described in (G) and quantified by phosphorimager (left). Following in vitro phosphorylation, proteins were incubated in I or M extracts and samples were taken at the indicated times, washed and resolved by SDS-PAGE. The % dephosphorylation was quantified by phosphorimager and represents the average of 4 experiments with standard deviation (right). I. Endogenous Cdc25 was immunoprecipitated from interphase extract treated +/− OA. Samples were washed and immunoblotted with 14-3-3, Cdc25 and pT138 antibodies. Arrows indicate IgG heavy chain or hypophosphorylated/hyperphosphorylated forms of Cdc25.
To demonstrate T138 dephosphorylation during interphase, we phosphorylated GST-Cdc25 with Cdk2 in vitro (Fig. 1E), added this protein to interphase extracts, and retrieved the protein on glutathione-Sepharose at various times for anti-pT138 immunoblotting (Fig. 1F, top). As shown in Fig. 1F (bottom), T138 was rapidly dephosphorylated in interphase, but not mitotic, extracts. Similar results were obtained with a radiolabeled phosphopeptide derived from Cdc25 (aa. 108–200 containing T138 as its only phosphorylation site) (Figs. 1G–H). These data show that T138-directed phosphatase activity is cell cycle regulated and suggest that 14-3-3 release may be controlled by a T138-directed phosphatase. Indeed, OA induced release of 14-3-3 from Cdc25 in interphase extracts (Fig. 1I).
Interphase activation of T138-directed PP2A activity
OA induction of T138 phosphorylation suggested that PP1 or PP2A might regulate T138 phosphorylation. As shown in Fig. 2A, with concentrations of OA that inhibit PP2A, but not PP1, the interphase extract could not promote T138 dephosphorylation of in vitro phosphorylated GST-Cdc25 (Margolis et al., 2003). In addition, the selective PP2A inhibitor, fostriecin, prevented T138 dephosphorylation at concentrations that prevent dephosphorylation of a known PP2A substrate, phosphorylase A, arguing that PP2A dephosphorylates T138 in interphase extracts (Fig. 2B; Margolis et al., 2003).
Fig. 2. Thr138 is dephosphorylated by PP2A.
A–B. GST-Cdc25 was phosphorylated with Cdk2/Cyclin E, washed and incubated in interphase extract pre-treated with increasing concentrations of OA (A) or fostriecin (B). Samples were immunoblotted with anti-pT138 antibody. The percent of phosphorylase A dephosphorylated in the presence of fostriecin is shown. C. [γ−32P]-labeled phosphorylase A was incubated with increasing concentrations of interphase or mitotic extracts for 10 min in phosphatase buffer. [γ−32P] release from phosphorylase A was measured by scintillation counting. D. PP2A was immunoprecipitated from cycling extracts at the indicated times, washed and incubated with Threonine phosphopeptide. The pmoles of phosphate released were measured by colorimetric detection with Malachite Green Phosphate Detection Solution; results from 3 experiments were averaged with standard deviation (top). Cell cycle progression was monitored by Cdc2 Y15 immunoblotting (bottom).
PP2A activity is reportedly static during the cell cycle (Clarke et al., 1993; Ruediger et al., 1991). Consistent with this, PP2A dephosphorylated phosphorylase A similarly in interphase and mitotic extracts and no variation in PP2A activity occurred in a cycling egg extract (Figs. 2C–D). Thus, we hypothesized that cell cycle oscillation in T138 dephosphorylation resulted from a specific change in Cdc25-targeted PP2A activity.
B56δ mediates T138 dephosphorylation
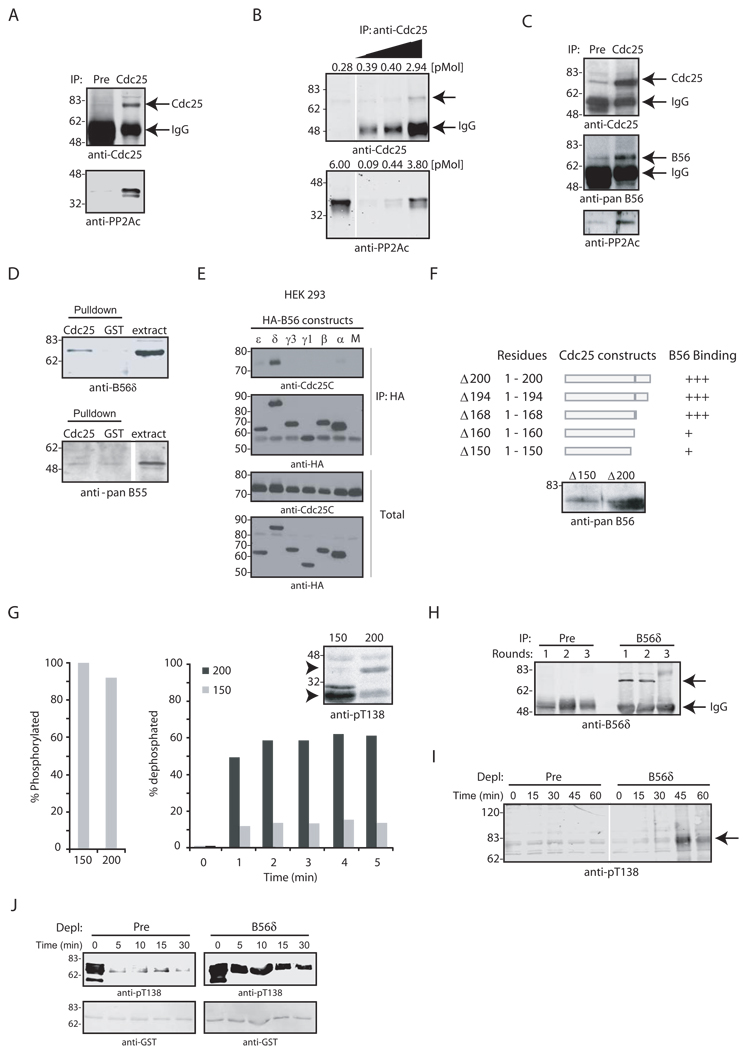
The PP2A core enzyme consists of a catalytic (C) subunit and a regulatory/scaffolding (A) subunit. A regulatory/targeting B subunit associates with the core, creating the holoenzyme (PP2A A/B/C) (Mumby and Walter, 1993). We wished to determine whether PP2A A/B/C could bind to Cdc25 and if so, which B subunit was present. As shown in Figs. 3A–B, Cdc25 co-precipitated with PP2A/C in a 1:1 stoichiometry. In S. cerevisiae, two B subunits, Cdc55 (B55 in vertebrates) and RTS1 (B56) have been suggested to regulate Cdc25 (Minshull et al., 1996; Wang and Burke, 1997; Yang et al., 2000; D. Lew, pers. communication). Though we did not detect an interaction between Xenopus Cdc25 and B55, (Fig. 3D, bottom), B56 co-precipitated with Cdc25 from interphase extracts and was retrieved from extracts with GST-Cdc25 (Fig. 3C–D, top). Human Cdc25C also co-precipitated with HA-tagged B56 (δ isoform) from transfected HEK 293 cells (Fig. 3E). Using a B56δ-specific antibody to immunoblot Xenopus extract and comparing the electrophoretic mobility of the recognized band to those in WT and B56δ knockdown HEK 293 cell lysates, we identified the Xenopus Cdc25-bound B56 as B56δ (Fig. S1 and 3D, top).
Fig. 3. The B56δ targeting subunit mediates Thr138 dephosphorylation.
A. Cdc25 was immunoprecipitated from interphase extract with CNBr sepharose-coupled preimmune or anti-Cdc25 sera and immunoblotted with anti-PP2A antibodies. B. Increasing amounts of endogenous Cdc25 were immunoprecipitated from interphase extracts, washed and immunoblotted for PP2A and Cdc25 and compared to levels of Cdc25 and PP2A present in egg extracts. C. Cdc25 was immunoprecipitated as described in (A) and immunoblotted with anti-B56 and PP2A antibodies. D. GST or GST-Cdc25 linked to glutathione-Sepharose was incubated in interphase extract, collected and immunoblotted for B56 (top) or B55 (bottom). E. HEK 293 cells were mock transfected (M) or transfected with HA-B56 isoforms (ε,δ,γ1,γ2,β,α) that were immunoprecipitated from cell lysates. Immunoprecipitates were washed and immunoblotted with anti-Cdc25C and anti-HA antibodies (top). Total Cdc25C and HA-B56 proteins were blotted for in cell lysates (bottom). F. C-terminally truncated GST-Cdc25 proteins were incubated in interphase extract, washed and immunoblotted for B56. G. GST-Cdc25 aa 108–150 and 108–200, were pre-phosphorylated with Cdk2/Cyclin E and [γ−32P] ATP (left). Fragments were incubated in interphase extract, collected at the indicated times and resolved by SDS-PAGE (bottom right). The % dephosphorylation was quantified by phosphorimager. Non-phosphorylated fragments were incubated in interphase extract, collected at the indicated times and resolved by SDS-PAGE for anti-pT138 immunoblotting (top right). Arrows indicate Cdc25 fragments. H. Cytosolic interphase extract was depleted of B56δ with three sequential incubations with 10 µg of preimmune or anti-B56δ antibodies coupled to Protein A-Sepharose. Beads were washed and immunoblotted with anti-B56δ antibody. Arrow indicates B56δ. I. Preimmune- and B56δ-depleted extracts were incubated at room temp with energy regenerating mix. Samples were withdrawn at the indicated times and immunoblotted for pT138 (arrow). J. GST-Cdc25 was phosphorylated in vitro with Cdk2/Cyclin E, washed and incubated in preimmune- or B56δ -depleted extracts. Samples were withdrawn at the indicated times, washed and immunoblotted for pT138.
To determine if PP2A B56δ-Cdc25 binding was important for T138 dephosphorylation, we created a series of Cdc25 N-terminal fragments and assessed their PP2A B56δ binding and susceptibility to T138 dephosphorylation. We found that amino acids (aa) 1–150 of Cdc25 bound weakly to PP2A B56δ, while larger N-terminal fragments interacted strongly (Fig. 3F). Cdc25 peptides spanning aa 108–200 (containing the PP2A docking site) or 108–150 (lacking the docking site) were equally phosphorylated by Cdk2, yet only the 108–200 peptide was dephosphorylated in interphase extract (Fig. 3G, left and bottom right). In addition, incubation of unphosphorylated 108–150 peptide in interphase extracts resulted in net T138 phosphorylation, while incubation of the 108–200 peptide did not (Fig. 3G, top right), most likely because the 108–150 peptide could not be dephosphorylated.
To explore the role of B56δ further, we performed multiple rounds of B56δ immunodepletion from cytosolic interphase egg extract. After 3 depletions, it was not possible to precipitate additional B56 (Fig. 3H). Following this depletion, pT138 accumulated on Cdc25 in an interphase extract (Fig. 3I). Moreover, removal of immunoprecipitable B56 resulted in a failure to dephosphorylate T138 when we added Cdk2-phosphorylated Cdc25 to the extract, consistent with a critical role for B56 in T138 dephosphorylation (Fig. 3J). These data suggest that association of B56δ with Cdc25 is required for T138 dephosphorylation during interphase and that it is the T138-directed phosphatase, rather than Cdk2, that is cell cycle regulated.
The DNA replication checkpoint controls T138 dephosphorylation
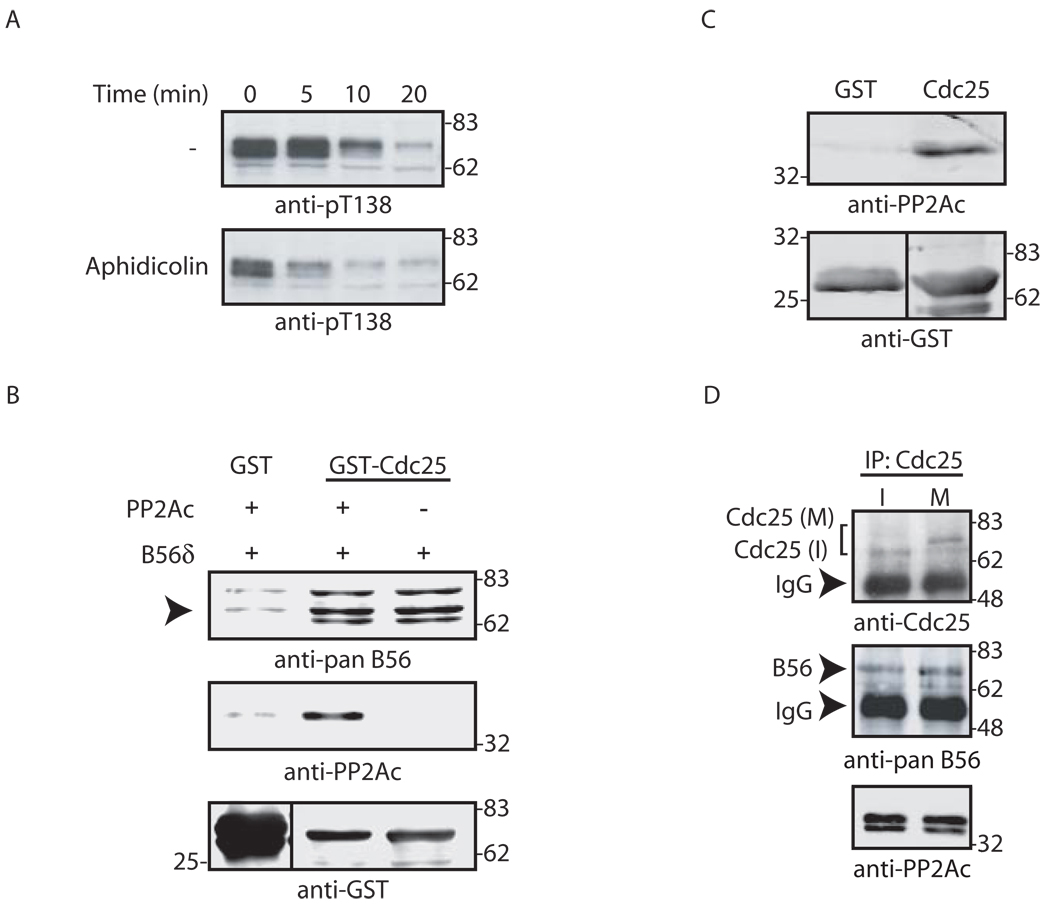
Given the importance of 14-3-3 in maintaining checkpoint suppression of Cdc25 and the role of T138 phosphorylation in 14-3-3 release, we hypothesized that T138 dephosphorylation might be checkpoint-regulated. To test this, we examined Cdc25 dephosphorylation in Xenopus egg extracts incubated with sperm chromatin and aphidicolin. As shown in Fig. 4A, we found that T138 dephosphorylation was markedly accelerated by checkpoint activation.
Fig. 4. Cell cycle regulation of Thr138 dephosphorylation and 14-3-3 release depends on B56.
A. GST-Cdc25 pre-phosphorylated by Cdk2/Cyclin E was incubated in untreated (−) or aphidicolin-treated interphase extract for the indicated times. Samples were collected, washed and immunoblotted for pT138. B. GST or GST-Cdc25 linked to glutathione-Sepharose was incubated with recombinant B56δ +/− purified PP2A A/C in buffer. Samples were washed and immunoblotted with the indicated antibodies. Arrow indicates B56δ. C. PP2A A/C was incubated in buffer with GST or GST-Cdc25 linked to glutathione-Sepharose. Samples were washed and immunoblotted for PP2A. D. Cdc25 was immunoprecipitated from interphase (I) or mitotic (M) extracts and immunoblotted for Cdc25, PP2A or B56. Arrows indicate IgG heavy chain versus B56 or Cdc25. Mitotic Cdc25 (M) migrates more slowly than interphase Cdc25 (I) due to hyperphosphorylation.
Since T138 dephosphorylation was cell cycle controlled, we speculated that alterations in PP2A B56δ-Cdc25 binding might underlie this regulation. Using recombinant proteins, we found that both PP2A A/C and B56δ could interact directly and independently with Cdc25; however, neither interaction varied with the cell cycle (Fig. 4B–D). These data suggest that cell cycle-regulated T138 dephosphorylation was not the result of altered binding of PP2A or B56δ to Cdc25.
Chk1 phosphorylates B56δ
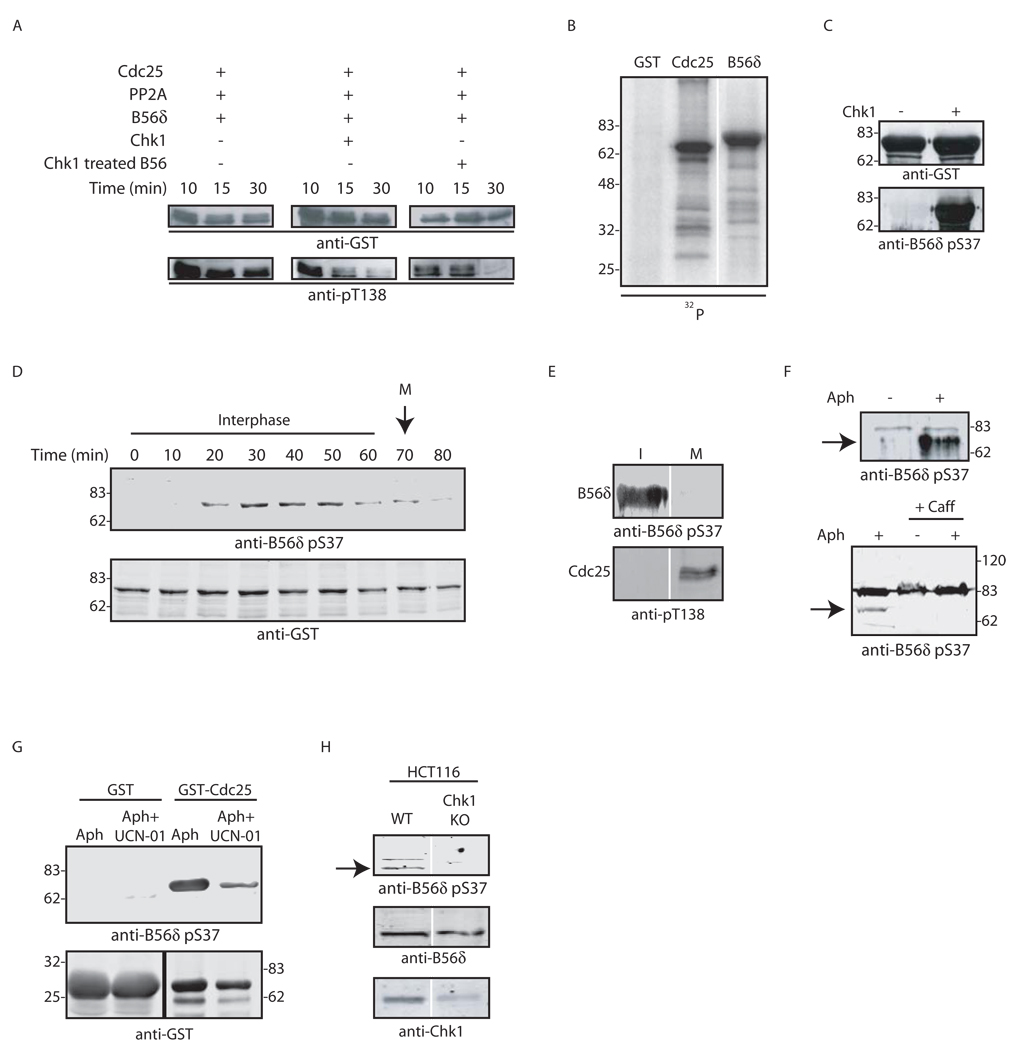
Cdc25 suppression by the replication checkpoint is controlled by Chk1 and other checkpoint kinases. Therefore, we speculated that maintenance of this suppression by T138 dephosphorylation might also be controlled by checkpoint kinases. Accordingly, we examined in vitro T138 dephosphorylation by PP2A A/C with B56δ in the presence and absence of Chk1. Interestingly, there was little T138 dephosphorylation in the absence of Chk1 and Chk1 markedly enhanced T138 dephosphorylation (Fig. 5A). Importantly, prior incubation of B56δ with Chk1 (with washes to remove Chk1), followed by incubation with Cdc25 and PP2A A/C also enhanced T138 dephosphorylation (Fig. 5A), suggesting that B56δ might be a Chk1 target. Human B56δ is a known phosphoprotein (McCright et al., 1996), and B56 phosphorylation stimulates the activity of PP2A towards specific substrates in vitro (Usui et al., 1998). Moreover, B56δ could serve as an in vitro Chk1 substrate (Fig. 5B). To identify the site(s) of phosphorylation, we used phospho-antibodies directed against four known phospho-sites on human B56δ (Usui et al., 1998; JHA and ACN, unpublished data). Although only two of these sites are conserved in Xenopus B56δ (Fig. S2), a phospho-Ser 37 antibody recognized B56δ in vitro phosphorylated by Chk1 (Fig. 5C). In addition, the enhanced T138 dephosphorylation observed upon pre-incubation of B56 with Chk1 was lost when S37A mutant B56 was used in the reaction (data not shown). Moreover, Xenopus B56δ added to interphase egg extracts was phosphorylated at S37 and dephosphorylated at mitosis, coincident with the appearance of pT138 (Figs. 5D–E). Importantly, S37 phosphorylation on endogenous Xenopus B56δ was enhanced by replication checkpoint activation and blocked by caffeine (Fig. 5F). Under checkpoint conditions, B56δ bound to Cdc25 was phosphorylated and this phosphorylation was blocked by UCN-01, which inhibits Chk1 (Fig. 5G). Consistent with this, an 85% knock-down of Chk1 protein by RNAi in HCT116 cells resulted in a marked loss of phosphorylated B56δ (Fig. 5H). Collectively, these data demonstrate that B56δ serves as a Chk1 substrate, providing the first link between DNA-responsive checkpoints and PP2A B targeting subunits.
Fig. 5. B56δ is phosphorylated in a checkpoint-dependent manner.
A. GST-Cdc25 linked to glutathione-Sepharose was incubated with Cdk2/Cyclin E in buffer containing ATP for 1 hr at 30°C. The protein was washed and incubated in fresh buffer containing ATP with PP2A A/C and B56δ +/− recombinant Chk1 (left and middle column). GST-B56δ was preincubated with Chk1 and ATP, washed extensively to remove Chk1 and then incubated in fresh buffer containing ATP, pre-phosphorylated GST-Cdc25 and PP2A A/C (right column). Samples were retrieved at the indicated times and immunoblotted with pT138 antibody. B. GST, GST-Cdc25 or GST-B56δ was incubated with Chk1 in buffer containing [γ−32P] ATP for 1 hr at 30°C. Samples were resolved by SDS-PAGE and analyzed by autoradiography. C. GST-B56δ was incubated +/− Chk1 in buffer containing ATP. Samples were immunoblotted with anti-pS37 antibody. D. GST-B56δ was incubated in interphase extract with Cyclin B. The arrow indicates mitotic entry. Samples were collected at the indicated times, washed, and immunoblotted for pS37. E. GST-Cdc25 and GST-B56δ were incubated in interphase and mitotic extracts and immunoblotted for pS37 and pT138. F. Interphase extracts supplemented with sperm chromatin were pretreated +/− 20 µg/ml aphidicolin (top) or with aphidicolin +/− 5 mM caffeine (bottom). GST-B56δ was incubated in extracts as indicated, washed and immunoblotted for pS37. Arrow indicates pS37 B56δ. G. GST or GST-Cdc25 was incubated in interphase extract pretreated with 20 µg/ml aphidicolin +/− 1 µM UCN-01, retrieved on glutathione-Sepharose and immunoblotted for pS37. H. Lysates from HCT116 cells or Chk1 knockdown HCT116 cells were immunoblotted for pS37, total B56 or Chk1. Arrow indicates pS37 B56δ.
B56δ phosphorylation enhances PP2A holoenzyme formation
To determine if B56δ S37 phosphorylation was important for interphase Cdc25 inhibition, we added S37A B56δ protein to egg extracts. As shown in Figs. 6A–B, S37A, but not WT B56δ, protein overrode a replication checkpoint. Consistent with the idea that B56δ-Cdc25 interactions were not likely to be a key point of regulation, B56δ WT and S37A mutant bound equally well to Cdc25 (Fig. 6C, top). In addition, in vitro phosphorylation of B56δ with Chk1 did not alter its ability to bind recombinant Cdc25 (Fig. 6D). Rather, B56δ phosphorylation by Chk1 enhanced its ability to bind the PP2A core, suggesting that PP2A complex formation could be increased by checkpoint function (Fig. 6E). Chk1 did not similarly enhance PP2A-S37A B56δ binding (Fig. 6F). B56δ S37A protein was also impaired in its ability to bind endogenous Xenopus PP2A (Fig. 6C). These data provided an explanation for the ability of the S37A mutant B56δ protein to impair checkpoint suppression of Cdc25. Because S37 mutation diminished recruitment of PP2A A/C without impairing Cdc25 binding, we suspected that the S37A mutant acted in a dominant negative fashion in egg extracts, docking on Cdc25 without interacting with the co-bound PP2A core enzyme. This impairment would allow inappropriate accumulation of phospho-T138 even during interphase (or checkpoint arrest), as seen in Fig. 6G. We also found that T138 dephosphorylation could be restored in extracts depleted of B56δ if recombinant WT B56δ, pretreated with Chk1, was added back to the depleted extracts. However, similarly treated S37A B56δ could not restore T138 dephosphorylation (Fig 6H). T138 dephosphorylation was somewhat slower in the depleted extracts supplemented with recombinant WT protein than in the mock depleted extract, probably reflecting the presence of some inactive protein in the recombinant B56 preparation (Fig. 3J).
Fig. 6. S37A B56 overrides a replication checkpoint.
A. Cycling extracts were incubated with GST, B56δ, or B56δ S37A +/− aphidicolin and sperm chromatin. Samples were taken at the indicated times and immunoblotted for pY15 Cdc2 and anti-PSTAIRE antibody. B. Hoechst staining of DNA was monitored by fluorescence microscopy using extracts treated as in A. C. GST-B56δ and His-Cdc25 proteins were incubated +/− Chk1 in buffer with ATP. GST-B56δ was retrieved on glutathione-Sepharose, washed, and analyzed by anti-His immunoblotting. D–E. WT or S37A GST-B56δ was incubated with PP2A A/C +/− Chk1 in ATP-containing buffer at 30°C. GST proteins were retrieved on glutathione–Sepharose, washed immunoblotted with PP2A C and GST antibodies. F. GST-B56δ WT or GST-S37A B56 was incubated in interphase extract, retrieved on glutathione-Sepharose, washed and immunoblotted with Cdc25 or PP2A antibodies. G. GST-B56δ was incubated in interphase extracts immunodepleted of Chk1, retrieved, washed, and immunoblotted for pS37, B56δ and PP2A (left). Mock or Chk1-depleted extracts were immunoblotted for Chk1 (right). Arrow indicates Chk1. H. Interphase extracts with Cyclin B were treated with aphidicolin in the presence of GST, B56δ, or B56δ S37A. Samples were taken at the indicated times and analyzed for pT138 phosphorylation. I. WT or S37A B56δ protein was preincubated with His-Chk1 in buffer containing ATP at 30°C before being added to B56δ depleted cytosolic extracts. GST-Cdc25 T138 dephosphorylation was monitored by removing samples at the indicated times, washing and immunoblotting with anti-pT138 antibody.
Consistent with a role for B56δ S37 phosphorylation in regulating PP2A subunit interactions, GST-B56δ bound PP2A A/C poorly in Chk1-depleted extracts (Fig. 6I). Note that in Fig. 6C and I, the amounts of recombinant B56δ used for the pull-downs were in excess of endogenous Cdc25 (which would bridge interactions of co-bound proteins) in order to observe alterations in the ability of free A/C subunits (which constitute ~50% of total PP2A A/C in egg extracts (Lin et al., 1998)) to bind B56 after Chk1 phosphorylation. Thus, we propose that Chk1-mediated suppression of Cdc25 acts at two distinct points: S287 phosphorylation allows 14-3-3 docking, while enhancement of B56δ-PP2A A/C association ensures that the 14-3-3 will remain docked.
Thr138 phosphorylation is required but not sufficient for 14-3-3 removal
Upon completion of DNA replication, PP2A-mediated T138 dephosphorylation ceases, allowing 14-3-3 release. However, when we treated recombinant 14-3-3-bound Cdc25 with Cdk2/Cyclin E to phosphorylate T138, we did not see any gross change in 14-3-3 binding (Fig. 7A). Nonetheless, Cdk2 phosphorylation of Cdc25 reduced the apparent affinity of 14-3-3 for Cdc25 (Fig. S3). Moreover, Cdk2/CyclinE treatment rendered 14-3-3 more susceptible to dissociation from Cdc25 by high-salt buffer (Fig. 7B). These data suggest that T138 phosphorylation, while required to induce 14-3-3 release, is not sufficient, but simply “loosens” 14-3-3 binding. Consistent with this, isolation of 14-3-3-bound Cdc25 from interphase extracts followed by OA treatment allowed phosphorylation of Cdc25 at T138 (presumably by bound Cdk2, Fig. 7D) without affecting 14-3-3 removal (Fig. 7C). These data suggest that a factor(s), present in the full extract (Fig. 1I), but not associated with the precipitated complex, was required for efficient 14-3-3 release.
Fig. 7. A keratin 14-3-3 sink promotes 14-3-3 release from Cdc25.
A. Cdc25-14-3-3 complexes isolated from interphase extract were washed and incubated +/− Cdk2/CyclinE at 30°C for 1 hr. Samples were washed with ELB + 300 mM NaCl and 0.1% Triton X-100 and immunoblotted with anti-14-3-3 sera. B. The same experiment as in (A) was performed, but samples were washed with buffer containing increasing NaCl concentrations. C. Cdc25-14-3-3 complexes were isolated as in (A) and incubated +/− OA at 30°C for 1 hr. Samples were collected, washed, and immunoblotted with anti 14-3-3 and anti-pT138 antibodies. D. GST or GST-Cdc25 linked to glutathione-Sepharose was incubated in interphase extract, washed and processed for immunoblotting with anti-Cdk2 antibody. E. GST-Cdc25-14-3-3 complexes were added to crude or cytosolic interphase egg extracts +/− OA. Samples were washed and immunoblotted with anti-14-3-3 sera. F. Extract was spun at 200,000 × g. Four reactions were generated by either mixing all fractions together (reconstituted), cytosol alone, cytosol + membrane, or cytosol + pellet. 14-3-3-bound GST-Cdc25 was incubated in each reaction + OA. Samples were collected at the indicated times, washed with ELB + 300 mM NaCl and 0.1% Triton X-100, and immunoblotted for GST and 14-3-3. G. Crude interphase extract was 33 spun at 200,000 × g. Pelleted proteins were biotinylated, added to cytosol, and incubated with DMSO or OA for 1 hr and re-pelleted at 200,000 × g. GST-14-3-3 or GST was incubated in each extract for 1 hr at 4°C. Samples were collected, washed and resolved by SDS-PAGE and probed with HRP-streptavidin. H. A similar experiment to that in (G) was done using non-biotinylated pelleted protein and samples were immunoblotted with anti-keratin sera (top). The experiment was also performed using mitotic cytosol (bottom). I. Crude extract +/− OA or Cyclin B was spun at 200,000 × g. Cytosolic extracts were treated with OA and aliquots were taken for anti-keratin and actin immunoblotting. J. Equal amounts of 14-3-3-bound GST-Cdc25 were incubated at room temp for 1h in cytosolic extract +/− OA with 71 nM or 142 nM keratin 8/18. GST-Cdc25 was retrieved on glutathione-Sepharose, washed, and immunoblotted with anti-pT138 and anti-14-3-3. K. Keratin 8/18 or GST was added to cytosolic extract in the presence of light membrane and sperm chromatin. Non-degradable Cyclin was added to extracts and samples were taken at the indicated times and immunoblotted with anti-pY15 of Cdc2. L. GST-14-3-3 was incubated in cycling egg extract, retrieved at the indicated times on glutathione-Sepharose, washed and immunoblotted with anti-keratin. Entry into mitosis was monitored by anti-Y15 Cdc2 immunoblotting.
An intermediate filament “sink” contributes to 14-3-3 removal from Cdc25
To identify the missing component(s) required for 14-3-3 release, we fractionated interphase egg extracts by ultracentrifugation at 200,000 × g into cytosolic, light membrane, heavy membrane and pelletable (glycogen, ribosomes, assembled cytoskeletal proteins, and large protein complexes) fractions. Addition of OA (or Cyclin B) to the cytosol did not induce 14-3-3 release from GST-Cdc25 (Fig. 7E, data not shown). Adding each of the other fractions back, we restored 14-3-3 removal upon addition of the pelleted fraction to OA–treated cytosol (Fig. 7F). Interestingly, pre-treatment of total extract with OA prior to pelleting, or using mitotic, rather than interphase extract, as a source of pelleted material, generated 14-3-3-releasing activity in the pure cytosol, relieving the requirement for the pellet (Fig. S4A). These data implied that the pelleted fraction contained a component required for 14-3-3 removal and that the active principle was pelletable during interphase, but cytosolic in mitosis (or in the presence of OA to promote pseudo-mitotic phosphorylations).
Interestingly, addition of 14-3-3 to extracts to double the endogenous concentration (40 µg/ml) suppressed both 14-3-3 release from Cdc25 and mitotic entry (Fig. S5). In considering this, we noted a publication by Avruch and colleagues in which treatment of Cos-7 cells with the phosphatase inhibitor calyculin A resulted in the abundant association of phosphorylated vimentin with 14-3-3 at the expense of most other 14-3-3 protein complexes (Tzivion et al., 2000). From this, they hypothesized that phospho-vimentin (and perhaps other intermediate filament proteins such as keratins) might, under specific physiological conditions, provide high affinity binding sites to sequester 14-3-3. From this, we speculated that addition of excess 14-3-3 to our extracts neutralized the 14-3-3 “sink” provided by phosphorylated intermediate filament proteins, limiting their ability to promote 14-3-3 removal from Cdc25, thereby preventing mitotic entry.
Because 14-3-3 releasing activity was soluble in mitosis (or OA-induced pseudo-mitosis), but pelletable in interphase, we hypothesized that the activity might reside in an intermediate filament protein that would be polymerized in interphase (pelletable) and monomeric (soluble) in mitosis. Therefore, relevant proteins pelleted during interphase ought to be released into the cytosol during mitosis to create a 14-3-3 sink. To evaluate this, we pelleted interphase extracts and marked all precipitated proteins by biotinylation. The biotinylated proteins were then added to cytosolic interphase extracts treated with OA. The extracts were re-fractionated by centrifugation and the soluble cytosolic proteins were bound to a GST-14-3-3 resin, resolved by SDS-PAGE and probed with HRP-streptavidin (Fig. S6). This resulted in the capture of a small number of 14-3-3 binding partners that had transitioned from a pelletable to a soluble state (Fig. 7G).
Given the hypothesis that prompted these experiments, we were struck by the presence of abundant proteins of 48 and 55 KD, the molecular weights of keratin 8/18, in the profile of 14-3-3 binding partners. Because cells from keratin knockout animals reportedly show defects in G2/M progression (in some cell types), keratins were attractive candidates for the 14-3-3-binding sink (Toivola et al., 2001). Moreover, keratin 8/18 complexes are phosphorylated and bind 14-3-3 at mitosis (Ku et al., 1998; Ku et al., 2002; Liao and Omary, 1996). Interestingly, OA treatment of egg extracts or use of mitotic extract induced keratin 18 binding to 14-3-3, suggesting that phosphatase inhibition is sufficient to allow phosphorylation of keratin at 14-3-3 binding sites to similar levels observed during mitosis (Fig. 7H). Given these data, we wished to determine whether keratin depletion/add-back affected 14-3-3-releasing activity. We found that 200,000 × g ultracentrifugation of interphase extract removed >90% of the keratin, while OA or Cyclin B pre-incubation caused an almost complete release of keratin 18 into the cytoplasm (Fig. 7I). Thus, we considered the cytosol to be a keratin-depleted extract, which was deficient in 14-3-3-releasing activity (Fig. 7J, top). We then added back nanomolar quantities of keratin 8/18 protein to the cytosolic extract and measured 14-3-3 release in the presence or absence of OA (Fig. 7J, bottom). Interestingly, keratin addition (~140nM) effectively restored 14-3-3 release. We also found that addition of keratin back to ultra-spun cytosol accelerated entry into mitosis driven by added Cyclin B, as measured by Cdc2 Y15 dephosphorylation and nuclear envelope breakdown (Fig. 7K and data not shown). Moreover, in cycling egg extracts, keratin binding to 14-3-3 occurred in advance of Cdc2 Y15 dephosphorylation, consistent with a role for keratin in Cdc25 activation (Fig. 7L). Additionally, immunofluorescent staining of synchronized HeLa cells demonstrated that keratin 18 phosphorylation at a previously characterized 14-3-3 binding site occurred prior to mitotic entry and in the presence of OA (Figs. S4B-C) (Ku et al., 1998). In aggregate, these data suggest that the reduction in affinity for 14-3-3 caused by PP2A/B56δ regulation and consequent accumulation of pT138 on Cdc25 could promote 14-3-3 removal due to the coincident mitotic phosphorylation of intermediate filaments to create a 14-3-3 sink. Together, these processes would allow efficient Cdc25 activation, dephosphorylation of Cdc2, and mitotic entry.
Discussion
Regulation of B56δ PP2A by DNA-responsive checkpoints
DNA-responsive checkpoints control mitotic entry, in part, by suppressing Cdc25 activation. While S287 phosphorylation by checkpoint kinases allows 14-3-3 docking and Cdc25 inhibition, we show here that maintaining this binding relies upon continued T138 dephosphorylation by PP2A B56δ. Thus, PP2A B56δ represents a novel checkpoint component. We have not yet determine if this regulatory pathway controls human Cdc25 proteins, though we note the conservation of T138 and 14-3-3 binding across isoforms (Fig. S7).
Because S287 is phosphorylated during an unperturbed interphase, it has been speculated that checkpoint activation simply prolongs phosphorylation of this site until DNA damage is repaired or replication is completed (Sanchez et al., 1997; Zeng et al., 1998). Indeed, checkpoint activation by DNA damage during interphase does not result in a marked increase in total S287 phosphorylation (Stanford and Ruderman, 2005). Once DNA is repaired or replication is completed, loss of checkpoint kinase activity is not sufficient to promote Cdc25 activation because removal of 14-3-3 is required to allow S287 dephosphorylation. Thus, DNA-responsive checkpoints must directly control 14-3-3 removal, which occurs independently of and prior to S287 dephosphorylation. Our data suggest that 14-3-3 removal is controlled through checkpoint regulation of T138 dephosphorylation.
Unlike S287 phosphorylation, B56δ phosphorylation is dynamically controlled by checkpoints. This phosphorylation enhances interaction of the PP2A A/C complex with B56δ, providing the first evidence of checkpoint modulation of PP2A holoenzyme composition. These data also suggest that Cdc25 serves as a scaffold for B56δ and PP2A A/C as both of these proteins bind independently to Cdc25, but are enhanced in their ability to interact with each other following checkpoint activation.
Keratin phosphorylation promotes 14-3-3 release from Cdc25
Cdc25 T138 phosphorylation reduces 14-3-3 affinity sufficiently that an increase in the availability of other 14-3-3 binding sites at mitosis promotes 14-3-3 dissociation. We were surprised to identify keratin as a potential driver of mitotic entry. These data are consistent with the prescient prediction of Avruch and colleagues that phosphorylated 14-3-3-binding intermediate filament proteins might alter signaling pathways through 14-3-3 sequestration (Tzivion et al., 2000). Interestingly, K8 null hepatocytes exhibit mis-localized 14-3-3 protein as well as elevated numbers of PCNA-positive S/G2-phase cells, with doubled DNA content, suggestive of a 14-3-3 mediated G2 arrest (Toivola et al., 2001). Although these data may indicate that the relevant “sink” in liver cells (as in egg extracts) is keratin, we propose that other abundantly phosphorylated proteins might perform this role in other cell types. A recent report implicating K17-14-3-3 interactions in the regulation of protein synthesis in epithelial cells also points to a broader role of keratins in controlling fundamental cellular processes (Kim et al., 2006).
We have not yet identified the keratin-directed kinase, though it is not likely to be Cdc2, because OA treatment of interphase extracts, which lack Cyclin B, promotes keratin-14-3-3 interactions. We observed 14-3-3 release from Cdc25 upon OA treatment of complete extracts because this led to both keratin phosphorylation and T138 phosphorylation. Whether PP2A B56δ also dephosphorylates keratin is unknown. We also note that Cdc2/Cyclin B might create novel 14-3-3 binding sites on keratin, thereby contributing to positive feedback activation of Cdc25 by Cdc2.
A model for Cdc25 activation
We suggest that the following events control Cdc25 activation (Fig. S8): During interphase, S287 is phosphorylated and 14-3-3 is bound. When replication is ongoing, checkpoint kinases phosphorylate S37 of B56δ, maintaining T138 dephosphorylation. Upon completion of replication, checkpoint kinases are turned off. We speculate, based on previously published work, that PP2A can then autodephosphorylate (Inoue et al., 1999; Tanabe et al., 1996). Once B56δ is dephosphorylated, pT138 accumulates through constitutive Cdk2 action. Once sufficient levels of phosphorylated “sink” proteins are achieved (e.g. phospho-keratin), 14-3-3 is released, allowing PP1 to dephosphorylate S287 and activate Cdc25. Cdc25 activates Cdc2/Cyclin B, which further activates Cdc25 (Margolis et al., 2006). Although S287 phosphorylation of Cdc25 must be maintained to sustain checkpoint inhibition of Cdc25, our data demonstrate that a critical component of checkpoint regulation lies with the PP2A-mediated dephosphorylation of T138 and consequent release of 14-3-3.
Experimental Procedures
Cloning and protein expression
N-terminal Cdc25 was cloned into pGEX-KG as described in Yang et al., 1999. Xenopus B56δ was cloned from a Xenopus cDNA library using 5’-CATGCCATGGGAATGCCTAATAAAAACAAGAAAGATAAAGAACCTCC-3’ and 5’-CCGCTCGAGTCAAGTAAGGGCCCATCTGTTTCC-3’. GST-B56δ S37A was generated using the QuikChange Mutagenesis Kit (Stratagene). HA-B56 proteins were cloned into pCEP4-Lerner (McCright et al., 1996). Keratin 8/18 was purchased from Prospec-Tany TechnoGene LTD. GST-14-3-3ε was expressed as described (Kumagai et al., 1998b).
Antibodies
Rabbit polyclonal anti-pT138 was raised against the peptide LPHLLCSpTPSFKKAC. B56δ antibody was raised and affinity purified using the peptide QSQPPSSNKRPSNS (amino acids 49–62 of human B56δ) Phospho-B56 antibody was raised against KRPpSNSTP. Anti-14-3-3ε (Santa Cruz), anti-PP2A (Upstate), anti-pan-B56 (Upstate), anti-phospho-Cdc2 (Y15; Cell Signaling Technology), anti-Cdc25C (Abcam), anti-cdk2 (Abcam), anti-keratin C11(Sigma) and anti-keratin-18 (Lab Vision) antibodies were purchased.
Xenopus extracts
Xenopus egg extracts and sperm chromatin were prepared as described (Murray, 1991). Interphase extracts contained cycloheximide to prevent Cyclin synthesis, while cycling extracts were prepared after egg activation without cycloheximide. Nuclei stained with Hoechst 33258 were examined using a Zeiss Axioscop with a 40× Plan-Neofluar air objective.
Cell culture and Chk1 knock-down
HeLa and HEK 293 cells were grown in Dulbecco’s Modified Eagle’s Medium (Gibco) with 10% fetal bovine serum (FBS). HCT116 cells were grown in McCoy’s 5A Medium Modified (Gibco) with 10% FBS. Cells were transfected using PLUS reagent and Lipofectamine (Invitrogen). Where noted, cells were treated with 5 µg/ml aphidicolin or 40 ng/ml nocodazole for 20 h prior to harvest. Oligonucleotides targeting human Chk1 (5’-CAGGAGAGAAGGCAATATC-3’ and its compliment) were cloned into pSUPER and HCT116 cells were transfected with Fugene 6 (Roche). Stable clones were selected with 500 µg/ml G418, grown to confluency and examined for Chk1 expression by immunoblotting.
Protein phosphorylation/dephosphorylation
To generate phosphorylated GST-Cdc25, Cdk2/Cyclin E (Upstate) and 2 µg of GST-Cdc25 were incubated in kinase buffer [10 mM Tris-HCl pH 7.5, 10 mM MgCl2, 1 mM DTT, 100 µM ATP] for 1 hr at 30°C. Xenopus B56δ was phosphorylated by incubating 2 µg GST-B56δ or GST-B56δ S37A with recombinant His-Chk1 in kinase buffer for 1 hr at 30°C
Phosphorylated GST-Cdc25 bound to glutathione-Sepharose was dephosphorylated in phosphatase buffer [50 mM Tris-HCl pH 7.5, 1 mM EDTA, 0.1% β-mercaptoethanol] supplemented with Xenopus egg extract or purified PP2A A/C (Upstate) and recombinant B56δ and Chk1. PP2A activity was measured in cycling extracts using the PP2A Immunoprecipitation Phosphatase Assay Kit (Upstate).
Co-precipitation and immunodepletions
To analyze interactions between Cdc25 and PP2A or B56δ, GST-Cdc25 proteins were incubated in interphase extract for 1 hr at 4°C, retrieved on glutathione-Sepharose, washed with egg lysis buffer [ELB: 250 mM sucrose, 2.5 mM MgCl2, 1 mM dithiothreitol (DTT), 50 mM KCl, 10 mM Hepes pH 7.7] plus 150 mM NaCl and 0.05% Triton-X 100, and immunoblotted with anti-PP2A or anti-B56δ. GST-Cdc25-14-3-3 complexes were washed with ELB + 300 mM NaCl and 0.1% Triton-X 100.
To analyze purified protein interactions, N-terminal GST-Cdc25 or GST was incubated with PP2A A/C (Upstate) in ELB + 0.1% BSA. Proteins were incubated at 4°C for 1 hr and washed with ELB + 150 mM NaCl and 0.05% Triton-X 100 and analyzed by immunoblotting. GST-B56δ or GST-B56δ S37A was incubated with PP2A A/C and His-Chk1 in kinase buffer for 1 hr at 30°C before washing and immunoblotting.
Cytosolic interphase extract was depleted of Chk1 as reported (Kumagai et al., 1998a) and was depleted of B56δ by three sequential 30 min incubations with 10 µg purified polyclonal anti-B56δ serum coupled to Protein A-Sepharose.
To analyze human protein interactions, cell lysates were prepared as described (Eide et al., 2002). HA-tagged proteins were immunoprecipitated from 300 µg total protein with anti-HA antibody (Santa Cruz) and collected with 20 µl protein A agarose (Invitrogen). Beads were washed with lysis buffer [150 mM NaCl, 30 mM HEPES pH 7.4, 0.1% Tween 20] and immunoblotted. Proteins were biotinylated using the ECL Biotinylation Module (Amersham) and quenched with the Avidin/Biotin Blocking Kit (Zymed).
Supplementary Material
Acknowledgements
We thank J. Wu, E. Gan, A. Parrish and S. Bulmer. SK was supported by R01 GM 067225. WCH was supported in part by P01 CA50661.
References
- Clarke PR, Hoffmann I, Draetta G, Karsenti E. Dephosphorylation of cdc25-C by a type-2A protein phosphatase: specific regulation during the cell cycle in Xenopus egg extracts. Mol Biol Cell. 1993;4:397–411. doi: 10.1091/mbc.4.4.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty MK, Morrison DK. Unlocking the code of 14-3-3. J Cell Sci. 2004;117:1875–1884. doi: 10.1242/jcs.01171. [DOI] [PubMed] [Google Scholar]
- Duckworth BC, Weaver JS, Ruderman JV. G2 arrest in Xenopus oocytes depends on phosphorylation of cdc25 by protein kinase A. Proc Natl Acad Sci U S A. 2002;99:16794–16799. doi: 10.1073/pnas.222661299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eide EJ, Vielhaber EL, Hinz WA, Virshup DM. The circadian regulatory proteins BMAL1 and cryptochromes are substrates of casein kinase Iepsilon. J Biol Chem. 2002;277:17248–17254. doi: 10.1074/jbc.M111466200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furnari B, Blasina A, Boddy MN, McGowan CH, Russell P. Cdc25 inhibited in vivo and in vitro by checkpoint kinases Cds1 and Chk1. Mol Biol Cell. 1999;10:833–845. doi: 10.1091/mbc.10.4.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guadagno TM, Newport JW. Cdk2 kinase is required for entry into mitosis as a positive regulator of Cdc2-cyclin B kinase activity. Cell. 1996;84:73–82. doi: 10.1016/s0092-8674(00)80994-0. [DOI] [PubMed] [Google Scholar]
- Inoue R, Usui H, Tanabe O, Nishito Y, Shimizu M, Takeda M. Studies on functions of the 63-kDa A- and 74-kDa B'(delta)-regulatory subunits in human erythrocyte protein phosphatase 2A: dissociation and reassociation of the subunits. J Biochem (Tokyo) 1999;126:1127–1135. doi: 10.1093/oxfordjournals.jbchem.a022558. [DOI] [PubMed] [Google Scholar]
- Izumi T, Walker DH, Maller JL. Periodic changes in phosphorylation of the Xenopus cdc25 phosphatase regulate its activity. Mol Biol Cell. 1992;3:927–939. doi: 10.1091/mbc.3.8.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Wong P, Coulombe PA. A keratin cytoskeletal protein regulates protein synthesis and epithelial cell growth. Nature. 2006;441:362–365. doi: 10.1038/nature04659. [DOI] [PubMed] [Google Scholar]
- Ku NO, Liao J, Omary MB. Phosphorylation of human keratin 18 serine 33 regulates binding to 14-3-3 proteins. Embo J. 1998;17:1892–1906. doi: 10.1093/emboj/17.7.1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku NO, Michie S, Resurreccion EZ, Broome RL, Omary MB. Keratin binding to 14-3-3 proteins modulates keratin filaments and hepatocyte mitotic progression. Proc Natl Acad Sci U S A. 2002;99:4373–4378. doi: 10.1073/pnas.072624299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai A, Guo Z, Emami KH, Wang SX, Dunphy WG. The Xenopus Chk1 protein kinase mediates a caffeine-sensitive pathway of checkpoint control in cell-free extracts. J Cell Biol. 1998a;142:1559–1569. doi: 10.1083/jcb.142.6.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai A, Yakowec PS, Dunphy WG. 14-3-3 proteins act as negative regulators of the mitotic inducer Cdc25 in Xenopus egg extracts. Mol Biol Cell. 1998b;9:345–354. doi: 10.1091/mbc.9.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew DJ, Kornbluth S. Regulatory roles of cyclin dependent kinase phosphorylation in cell cycle control. Curr Opin Cell Biol. 1996;8:795–804. doi: 10.1016/s0955-0674(96)80080-9. [DOI] [PubMed] [Google Scholar]
- Liao J, Omary MB. 14-3-3 proteins associate with phosphorylated simple epithelial keratins during cell cycle progression and act as a solubility cofactor. J Cell Biol. 1996;133:345–357. doi: 10.1083/jcb.133.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin XH, Walter J, Scheidtmann K, Ohst K, Newport J, Walter G. Protein phosphatase 2A is required for the initiation of chromosomal DNA replication. Proc Natl Acad Sci USA. 1998;95:14693–14698. doi: 10.1073/pnas.95.25.14693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manke IA, Nguyen A, Lim D, Stewart MQ, Elia AE, Yaffe MB. MAPKAP kinase-2 is a cell cycle checkpoint kinase that regulates the G2/M transition and S phase progression in response to UV irradiation. Mol Cell. 2005;17:37–48. doi: 10.1016/j.molcel.2004.11.021. [DOI] [PubMed] [Google Scholar]
- Margolis SS, Perry JA, Weitzel DH, Freel CD, Yoshida M, Haystead TA, Kornbluth S. A Role for PP1 in the Cdc2/Cyclin B-mediated Positive Feedback Activation of Cdc25. Mol Biol Cell. 2006;17:1779–1789. doi: 10.1091/mbc.E05-08-0751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis SS, Walsh S, Weiser DC, Yoshida M, Shenolikar S, Kornbluth S. PP1 control of M phase entry exerted through 14-3-3-regulated Cdc25 dephosphorylation. Embo J. 2003;22:5734–5745. doi: 10.1093/emboj/cdg545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCright B, Rivers AM, Audlin S, Virshup DM. The B56 family of protein phosphatase 2A (PP2A) regulatory subunits encodes differentiation-induced phosphoproteins that target PP2A to both nucleus and cytoplasm. J Biol Chem. 1996;271:22081–22089. doi: 10.1074/jbc.271.36.22081. [DOI] [PubMed] [Google Scholar]
- Minshull J, Straight A, Rudner AD, Dernburg AF, Belmont A, Murray AW. Protein phosphatase 2A regulates MPF activity and sister chromatid cohesion in budding yeast. Curr Biol. 1996;6:1609–1620. doi: 10.1016/s0960-9822(02)70784-7. [DOI] [PubMed] [Google Scholar]
- Mumby MC, Walter G. Protein serine/threonine phosphatases: structure, regulation, and functions in cell growth. Physiol Rev. 1993;73:673–699. doi: 10.1152/physrev.1993.73.4.673. [DOI] [PubMed] [Google Scholar]
- Murray AW. Cell Cycle Extracts. Methods in Cell Biology. 1991;36:581–605. [PubMed] [Google Scholar]
- Peng CY, Graves PR, Ogg S, Thoma RS, Byrnes MJ, 3rd, Wu Z, Stephenson MT, Piwnica-Worms H. C-TAK1 protein kinase phosphorylates human Cdc25C on serine 216 and promotes 14-3-3 protein binding. Cell Growth Differ. 1998;9:197–208. [PubMed] [Google Scholar]
- Peng CY, Graves PR, Thoma RS, Wu Z, Shaw AS, Piwnica-Worms H. Mitotic and G2 checkpoint control: regulation of 14-3-3 protein binding by phosphorylation of Cdc25C on serine-216. Science. 1997;277:1501–1505. doi: 10.1126/science.277.5331.1501. [DOI] [PubMed] [Google Scholar]
- Rempel RE, Sleight SB, Maller JL. Maternal Xenopus Cdk2-cyclin E complexes function during meiotic and early embryonic cell cycles that lack a G1 phase. J Biol Chem. 1995;270:6843–6855. doi: 10.1074/jbc.270.12.6843. [DOI] [PubMed] [Google Scholar]
- Ruediger R, Van Wart Hood JE, Mumby M, Walter G. Constant expression and activity of protein phosphatase 2A in synchronized cells. Mol Cell Biol. 1991;11:4282–4285. doi: 10.1128/mcb.11.8.4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez Y, Wong C, Thoma RS, Richman R, Wu Z, Piwnica-Worms H, Elledge SJ. Conservation of the Chk1 checkpoint pathway in mammals: linkage of DNA damage to Cdk regulation through Cdc25. Science. 1997;277:1497–1501. doi: 10.1126/science.277.5331.1497. [DOI] [PubMed] [Google Scholar]
- Stanford JS, Ruderman JV. Changes in regulatory phosphorylation of Cdc25C Ser287 and Wee1 Ser549 during normal cell cycle progression and checkpoint arrests. Mol Biol Cell. 2005;12:5749–5760. doi: 10.1091/mbc.E05-06-0541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe O, Nagase T, Murakami T, Nozaki H, Usui H, Nishito Y, Hayashi H, Kagamiyama H, Takeda M. Molecular cloning of a 74-kDa regulatory subunit (B" or delta) of human protein phosphatase 2A. FEBS Lett. 1996;379:107–111. doi: 10.1016/0014-5793(95)01500-0. [DOI] [PubMed] [Google Scholar]
- Toivola DM, Nieminen MI, Hesse M, He T, Baribault H, Magin TM, Omary MB, Eriksson JE. Disturbances in hepatic cell-cycle regulation in mice with assembly-deficient keratins 8/18. Hepatology. 2001;34:1174–1183. doi: 10.1053/jhep.2001.29374. [DOI] [PubMed] [Google Scholar]
- Tzivion G, Luo ZJ, Avruch J. Calyculin A-induced vimentin phosphorylation sequesters 14-3-3 and displaces other 14-3-3 partners in vivo. J Biol Chem. 2000;275:29772–29778. doi: 10.1074/jbc.M001207200. [DOI] [PubMed] [Google Scholar]
- Usui H, Inoue R, Tanabe O, Nishito Y, Shimizu M, Hayashi H, Kagamiyama H, Takeda M. Activation of protein phosphatase 2A by cAMP-dependent protein kinase-catalyzed phosphorylation of the 74-kDa B" (delta) regulatory subunit in vitro and identification of the phosphorylation sites. FEBS Lett. 1998;430:312–316. doi: 10.1016/s0014-5793(98)00684-x. [DOI] [PubMed] [Google Scholar]
- Wang Y, Burke DJ. Cdc55p, the B-type regulatory subunit of protein phosphatase 2A, has multiple functions in mitosis and is required for the kinetochore/spindle checkpoint in Saccharomyces cerevisiae. Mol Cell Biol. 1997;17:620–626. doi: 10.1128/mcb.17.2.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Jiang W, Gentry M, Hallberg RL. Loss of a protein phosphatase 2A regulatory subunit (Cdc55p) elicits improper regulation of Swe1p degradation. Mol Cell Biol. 2000;20:8143–8156. doi: 10.1128/mcb.20.21.8143-8156.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Winkler K, Yoshida M, Kornbluth S. Maintenance of G2 arrest in the Xenopus oocyte: a role for 14-3-3- mediated inhibition of Cdc25 nuclear import. Embo J. 1999;18:2174–2183. doi: 10.1093/emboj/18.8.2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Y, Forbes KC, Wu Z, Moreno S, Piwnica-Worms H, Enoch T. Replication checkpoint requires phosphorylation of the phosphatase Cdc25 by Cds1 or Chk1. Nature. 1998;395:507–510. doi: 10.1038/26766. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.