Abstract
Amyloid-β (Aβ) accumulation in the brain extracellular space is a hallmark of Alzheimer’s disease (AD). The factors regulating this process are only partly understood. Aβ aggregation is a concentration-dependent process that is likely to be dependent on changes in brain interstitial fluid (ISF) levels of Aβ. Using in vivo microdialysis, we found that ISF Aβ levels correlated with wakefulness. ISF Aβ levels also significantly increased during acute sleep deprivation and during orexin infusion, whereas they decreased with infusion of a dual orexin receptor antagonist. Importantly, chronic sleep restriction significantly increased and a dual orexin receptor antagonist decreased Aβ plaque formation in amyloid precursor protein transgenic mice. Thus, the sleep-wake cycle and orexin may play a role in the pathogenesis of AD.
Alzheimer’s disease (AD) is the most common cause of dementia. The accumulation of the amyloid-β (Aβ) peptide in the brain extracellular space is a critical event in the pathogenesis of AD. Aβ is produced by neurons and secreted into the brain interstitial fluid (ISF). An initiating factor in AD pathogenesis occurs when soluble, monomeric Aβ undergoes a conformational change and converts into forms such as oligomers, protofibrils, and fibrils. The accumulation of these forms of Aβ is concentration-dependent and confers toxicity (1). Elucidating factors that regulate soluble Aβ levels is important for understanding AD pathogenesis. Synaptic activity regulates the release of Aβ from neurons into the ISF (2, 3). How ISF Aβ is regulated by normal physiology is poorly understood.
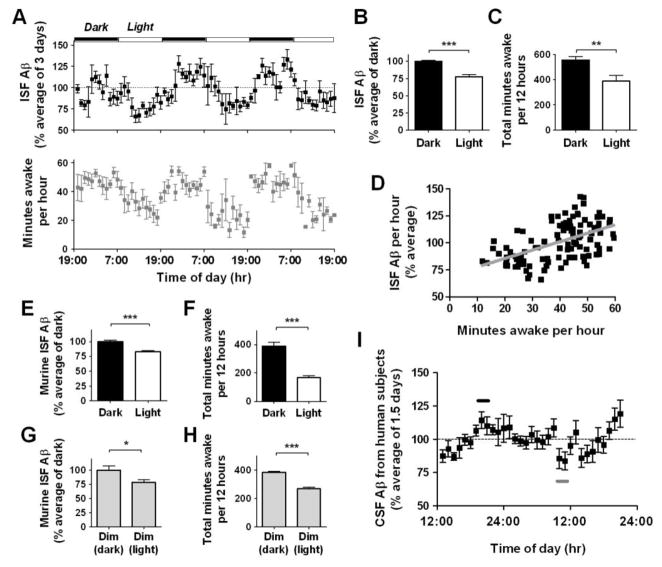
To investigate ISF Aβ metabolism, we monitored hippocampal Aβ levels using in vivo microdialysis in both wild-type mice and human APP transgenic (Tg2576) mice, which express a mutated form of human amyloid precursor protein (APP) (4). ISF Aβ was assessed in Tg2576 mice at 3 months of age, several months earlier than Aβ deposition begins. We found diurnal variation of ISF Aβ levels. Aβ levels were significantly increased during the dark period compared to the light period (Fig. 1A). ISF Aβ levels fluctuated over a 24-hour period with mean levels during the light period being ~75% of mean Aβ levels during the dark period (Fig. 1B). ISF Aβ levels were significantly correlated with the amount of time spent awake (Fig. 1, C–D). Conversely, ISF Aβ levels were negatively correlated with the amount of time spent asleep. This negative correlation was even stronger with non-REM sleep (Fig. S1). Despite fluctuations in ISF Aβ levels, full-length APP, APP C-terminal fragments, and Aβ1–40 and Aβ1–42 were not significantly different in total tissue homogenates of hippocampus between dark and light periods (Fig. S2). Thus, the pool of ISF Aβ is likely to be independently regulated from total intracellular and membrane-associated Aβ.
Figure 1.
Diurnal rhythm of ISF Aβ levels in the hippocampus of mice and CSF Aβ levels in human subjects. (A) ISF human Aβ levels expressed as a percentage of basal ISF Aβ levels over 6 light-dark periods in Tg2576 mice (n = 8). Total number of minutes spent awake per hour in the same mice. (B, C) Mean ISF Aβ levels were 24.4% higher (***P < 0.0001, n = 8) and the number of minutes awake were 167 minutes higher (**P = 0.007, n = 7) during dark vs. light periods. (D) ISF Aβ levels correlate with the number of minutes awake per hour (r = 0.53, ***P < 0.0001, n = 7). (E, F) Mean ISF murine Aβ levels and minutes awake over 2 days in C57BL6 mice. Under 12 hr dark/12 hr light conditions, ISF murine Aβ levels were 18.5% higher (***P < 0.0001, n = 10) and the number of minutes awake was 223 minutes higher (***P =0.0001, n = 5) during the dark periods. (G, H) Under constant dim light conditions, ISF Aβ levels were 22.7% higher (*P = 0.05, n = 10) and the number of minutes awake was 114 minutes higher (***P =0.0003, n = 5) during the typical hours for the dark phase. (I) CSF Aβ1–40 levels from human subjects expressed as a percentage of basal CSF Aβ1–40 levels over 33 hours (n = 10). Mean peak CSF Aβ1–40 levels (black bar) at 8–10 PM were 27.6% higher than mean trough CSF Aβ levels (gray bar) at 9–10 AM (112.3 ± 6% vs. 84.7 ± 6% respectively, P = 0.004). Data shown are mean ± SEM.
Next, we asked if diurnal Aβ fluctuation was also present in C57BL6, wild-type mice. Similar to Tg2576 mice, C57BL6 mice also showed a significant difference in ISF Aβ levels between dark and light phases, when samples were pooled over longer periods of time (Fig. 1, E and F). Thus, the diurnal variation in Aβ is intrinsic to normal cellular physiology.
To determine the underlying mechanism of the diurnal variation in ISF Aβ levels, we tested whether the light stimulus itself could affect ISF Aβ levels. Using C57BL6 mice, we measured ISF Aβ levels over 2 days under constant dim light conditions. Diurnal fluctuations of ISF Aβ still occurred, as did normal sleep-wake behavior (Fig. 1, G and H). Thus, ISF Aβ fluctuations are linked to the sleep-wake cycle and not to light or dark exposure.
To see whether the diurnal fluctuation of ISF Aβ is present in humans, we assessed cerebrospinal fluid (CSF) levels of Aβ in N=10 young healthy male volunteers via lumbar catheters over a 33 hour period and found clear evidence of diurnal fluctuation of Aβ in the CSF. Aβ levels increased throughout the first day with a peak in the evening, then decreased overnight, and again increased throughout the second day (Fig. 1I).
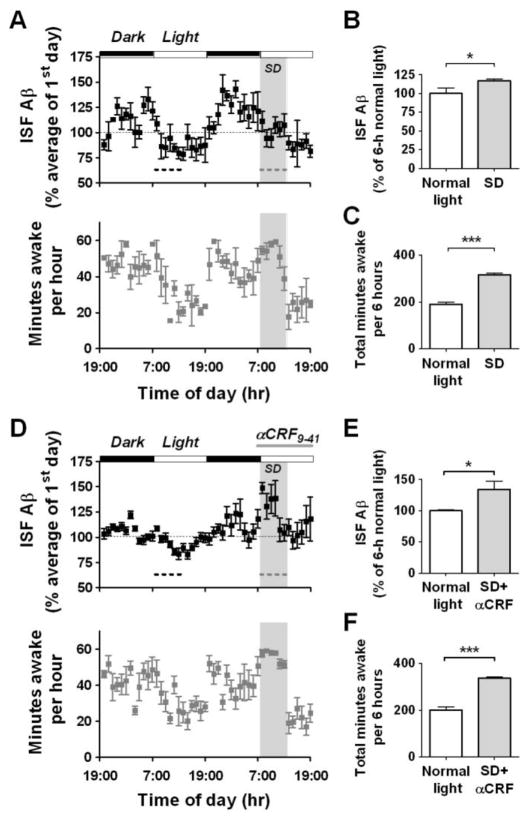
Because Aβ levels correlated with wakefulness, we asked whether manipulating sleep behavior would alter ISF Aβ levels. Mice were forced into wakefulness for 6 hours at the beginning of the second 12 hour light period when they would naturally be asleep. During sleep deprivation (SD), ISF Aβ levels were significantly higher compared to ISF Aβ levels during the normal light period 24 hours previously (Fig. 2, A–C). Following SD, mice spent more time sleeping and had an immediate reduction in ISF Aβ levels. Thus, the state of wakefulness, and not time of day, is associated with increased ISF Aβ levels.
Figure 2.
Acute sleep deprivation alters ISF Aβ diurnal rhythm independently of CRF receptor signaling in Tg2576 mice. (A) Mice underwent acute sleep deprivation (SD – grey dashed line) for 6 hours at the beginning of the light period. This prevented the normal decrease in ISF Aβ levels that occurs during this period (n = 8). (B) Mean ISF Aβ levels during SD were 16.8% higher compared to those during the light period 24 hours earlier (black dashed line, P = 0.05, n = 8). (C) Animals spent 126 more minutes awake during SD (***P < 0.0001, n = 5). (D) Mice underwent acute SD (grey dashed line) at the beginning of the light period. 860 pmoles of αCRF9–41 was infused into the hippocampus from 30 min before SD until the end of the light period (n = 8). (E) Mean ISF Aβ levels during SD with αCRF9–41 infusion were 33.7% higher compared to those during the light period 24 hours earlier (black dashed line, P = 0.01, n = 8). (F) Mice spent 136 more minutes awake during SD with αCRF9–41 infusion (***P < 0.0001, n = 5). Data represent mean ± SEM. SD = sleep deprivation.
Restraint stress in Tg2576 mice can acutely increase ISF Aβ mediated by corticotropin releasing factor (CRF) (5). αCRF9–41, an antagonist of CRF receptors, was administered by reverse microdialysis at the beginning of SD. In the presence of the CRF receptor antagonist, ISF Aβ levels were still significantly higher compared to ISF Aβ levels during the normal light period 24 hours previously (Fig. 2, D–F). The SD-induced increase in ISF Aβ did not significantly differ in the presence or absence of the CRF antagonist, thereby excluding the CRF stress pathway as mechanism of action for SD to increase Aβ levels.
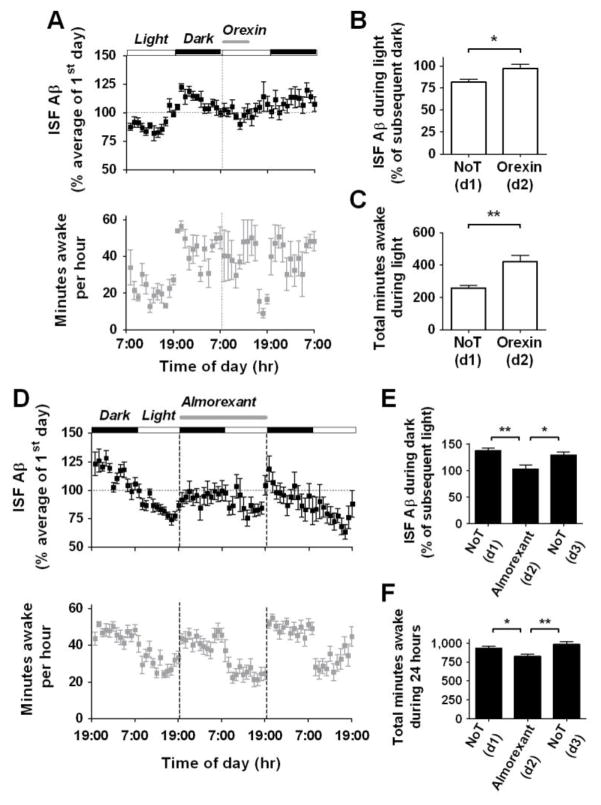
We next asked what molecular mechanism might mediate the diurnal fluctuation of Aβ levels. Orexin is a molecule that regulates wakefulness and other physiological functions, and is strongly implicated in narcolepsy/cataplexy and disorders of sleep and arousal (6). Orexin release from hypothalamic neurons shows a diurnal fluctuation similar to that of ISF Aβ (7). Orexin neurons project to the hippocampus where orexin receptors are expressed and is the location where we monitored ISF Aβ (8). We asked if orexin administration would modulate ISF Aβ levels. Intracerebroventricular (icv) infusion of orexin-A (1.5 pmole/hr) was given for 6 hours at the beginning of the light period. This dose induces wakefulness in rodents (9). During orexin infusion, ISF Aβ levels were significantly increased compared to ISF Aβ levels measured during the light period of the preceding day (Fig. 3, A–B). Infusion of vehicle did not significantly affect ISF Aβ (Fig. S3, A–B).
Figure 3.
Effects of orexin and a dual orexin receptor antagonist on ISF Aβ levels in Tg2576 mice. (A) After 24 hr of baseline measurement, 1.5 pmole/hr of orexin was infused icv for 6 hours at the beginning of the light period. This sustained ISF Aβ levels from the dark period, and kept the mice awake longer. (B, C) Infusion with orexin increased Aβ levels duirng the light period and thereby abolished the normal 20% difference between the dark and light period (*P = 0.01, n = 7). Orexin increased the amount of minutes awake by 163 minutes (**P = 0.009, n = 5), compared to that during the light period 24 hours previously. (D) After 24 hr of baseline measurement, 13.9 nmole/hr of almorexant was infused icv for 24 hr from the beginning of the dark period (n = 8). This continued to suppress ISF Aβ levels from the light period. (E) Mean ISF Aβ levels differed by 29% between the dark and light period during the control days, whereas there was no difference between the dark and light period during the 24 hour infusion of almorexant (**P = 0.001, n = 8). (F) During almorexant treatment, the number of minutes spent awake was decreased by 108 minutes over the 24 hour period compared to control days (**P = 0.005, n = 13). Data represent mean ± SEM. NoT = no treatment; d = day.
The orexin family (orexin-A and orexin-B) has two receptor subtypes: orexin receptor 1 (OXR1) and orexin receptor 2 (OXR2). We asked whether endogenous orexin signaling via orexin receptors is involved in the diurnal variation of Aβ levels. We infused a dual orexin receptor antagonist, almorexant, during in vivo microdialysis for ISF Aβ. Icv administration of almorexant for 24 hours suppressed ISF Aβ levels and abolished the natural diurnal variation of Aβ (Fig. 3, D–E). Removal of almorexant immediately restored the diurnal rhythm in ISF Aβ levels during the next 24 h period. Control icv infusions of vehicle did not affect ISF Aβ levels (Fig. S3, C–D). Almorexant decreased the total amount of time spent awake by approximately 10% (Fig. 3F). Thus, endogenous orexin signaling via orexin receptors is required for the diurnal rhythm of ISF Aβ levels.
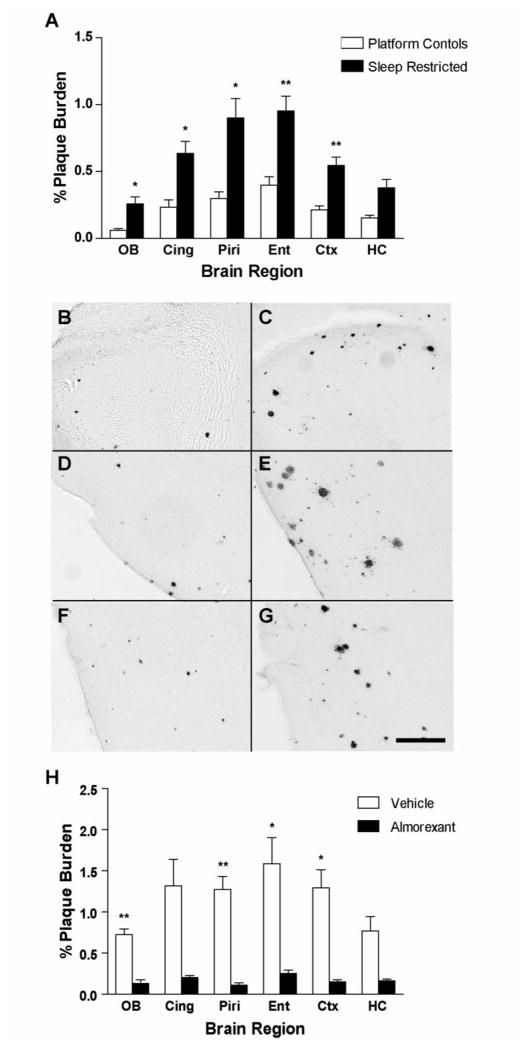
Because sleep-wake behavior modulates ISF Aβ levels, we asked whether chronic sleep deprivation could ultimately affect Aβ plaque deposition in the brain. APP transgenic mice of the APPswe/PS1dE9 genotype were subjected to chronic sleep restriction for 20 hours daily for 21 days. Sleep-restricted animals showed markedly greater Aβ plaque deposition compared to their age-matched littermate controls (Fig. 4A–G). We also observed significantly greater Aβ plaque burden using Tg2576 mice (Fig. S4). We next asked whether chronic orexin receptor blockade could decrease Aβ plaque deposition in APPswe/PS1dE9 mice at an age when plaques are just forming. Systemic treatment with almorexant once daily for 8 weeks significantly decreased Aβ plaque formation in several brain regions compared to vehicle-treated age-matched control mice (Fig. 4H).
Figure 4.
Aβ plaque deposition after chronic sleep restriction and chronic orexin receptor blockade in APPswe/PS1dE9 transgenic mice (A) Mice that underwent chronic sleep restriction for 21 days showed significantly greater Aβ plaque deposition in multiple subregions of the cortex compared to age-matched control mice (**P < 0.0008, *P < 0.008, n = 9–11 per group, using Bonferroni-adjusted P<0.0083 for multiple t-tests. For hippocampus, P<0.009). Representative photomicrographs of Aβ plaques are shown in (B) control and (C) sleep restricted olfactory bulb (D) control and (E) sleep restricted piriform cortex, (F) control and (G) sleep restricted entorhinal cortex. (H) Mice treated with daily i.p. injections of almorexant for 8 weeks showed significantly less Aβ plaque deposition in multiple subregions of the cortex compared to age-matched vehicle controls (**P < 0.0008, *P < 0.008 n = 5 per group, using Bonferroni-adjusted P<0.0083 for multiple t-tests. For cingulate cortex and hippocampus, P<0.009). Scale bar = 200 μm. OB = olfactory bulb, Cing = cingulate cortex, Piri = piriform cortex, Ent = entorhinal cortex, Ctx = cortex (immediately dorsal to dorsal hippocampus), and HC = hippocampus.
Herein, we demonstrated diurnal variation in Aβ levels in the brain of awake and behaving animals. Perturbations in both orexin signaling and the sleep-wake cycle had acute effects upon Aβ dynamics. Furthermore, chronic sleep restriction accelerates Aβ plaque burden, while enhancing sleep via orexin receptor blockade markedly inhibits Aβ plaque accumulation.
One factor that influences Aβ levels is synaptic activity. Periods of wakefulness are associated with a net increase in synaptic strength, and periods of sleep are associated with a net decrease in synaptic strength (10–12). Differences in synaptic activity between sleep and wake states, specifically via orexin signaling, may underlie the dynamic fluctuations in ISF Aβ levels.
How might changes in hourly ISF Aβ levels contribute to eventual Aβ plaque deposition? Recent work with a gamma secretase inhibitor has shown that changes in ISF Aβ levels as little as 20% blocks plaque formation and growth over weeks (13). Thus, behavioral and pharmacological manipulations of wakefulness that resulted in changes in ISF Aβ of 20–25% likely caused the observed changes in Aβ accumulation.
Sleep is a complex behavioral state whose ultimate functions remain poorly understood. Sleep disturbances, in addition to being prominent in neurodegenerative diseases (14), could exacerbate a fundamental process leading to neurodegeneration, and optimization of sleep time could potentially inhibit aggregation of toxic proteins and slow the progression of AD.
Supplementary Material
References and Notes
- 1.Selkoe DJ. Nat Cell Biol. 2004 Nov;6:1054. doi: 10.1038/ncb1104-1054. [DOI] [PubMed] [Google Scholar]
- 2.Kamenetz F, et al. Neuron. 2003 Mar 27;37:925. doi: 10.1016/s0896-6273(03)00124-7. [DOI] [PubMed] [Google Scholar]
- 3.Cirrito JR, et al. Neuron. 2005 Dec 22;48:913. doi: 10.1016/j.neuron.2005.10.028. [DOI] [PubMed] [Google Scholar]
- 4.Hsiao K, et al. Science. 1996;274:99. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- 5.Kang JE, Cirrito JR, Dong H, Csernansky JG, Holtzman DM. Proc Natl Acad Sci U S A. 2007 Jun 19;104:10673. doi: 10.1073/pnas.0700148104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kilduff TS, et al. J Neurosci. 2008 Nov 12;28:11814. doi: 10.1523/JNEUROSCI.3768-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoshida Y, et al. Eur J Neurosci. 2001 Oct;14:1075. doi: 10.1046/j.0953-816x.2001.01725.x. [DOI] [PubMed] [Google Scholar]
- 8.Peyron C, et al. J Neurosci. 1998 Dec 1;18:9996. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang ZL, et al. Proc Natl Acad Sci U S A. 2001 Aug 14;98:9965. doi: 10.1073/pnas.181330998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vyazovskiy VV, Cirelli C, Pfister-Genskow M, Faraguna U, Tononi G. Nat Neurosci. 2008 Feb;11:200. doi: 10.1038/nn2035. [DOI] [PubMed] [Google Scholar]
- 11.Gilestro GF, Tononi G, Cirelli C. Science. 2009 Apr 3;324:109. doi: 10.1126/science.1166673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donlea JM, Ramanan N, Shaw PJ. Science. 2009 Apr 3;324:105. doi: 10.1126/science.1166657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yan P, et al. J Neurosci. 2009 Aug 26;29:10706. doi: 10.1523/JNEUROSCI.2637-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gagnon JF, Petit D, Latreille V, Montplaisir J. Curr Pharm Des. 2008;14:3430. doi: 10.2174/138161208786549353. [DOI] [PubMed] [Google Scholar]
- 15.We thank E. D. Herzog and G.M. Freeman Jr. for assistance and P. J. Shaw for discussion. This work was supported by NIH grants AG025824, AG030946, NS065667, AG029524, MH072525, Neuroscience Blueprint Center Core Grant P30 NS057105, Cure Alzheimer’s Fund, Alzheimer’s Association Zenith Award, and Eli Lilly.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.