Abstract
Objective
To determine whether multivitamin supplements modify the relationship between alcohol consumption during pregnancy and the risk of miscarriage.
Study Design
We utilized data from a population-based cohort study of pregnant women (n=1061; response rate=39%). Participants were asked about their alcohol consumption and vitamin intake during pregnancy.
Results
Among multivitamin nonusers, women who drank alcohol during their pregnancy were more likely to have a miscarriage compared to women who abstained (adjusted Hazard Ratio (aHR): 1.67, 95%CI: 1.04, 2.69). However among multivitamin users, there was no difference in the risk of miscarriage between alcohol consumers and abstainers. Results suggest the volume of alcohol as well as the timing of multivitamin supplementation may also be important.
Conclusions
Our findings suggest that a woman of child-bearing years might decrease her risk of miscarriage associated with alcohol intake by taking multivitamin supplements. However, our findings should be interpreted with caution and future research replicating these findings is necessary.
Keywords: alcohol consumption during pregnancy, miscarriage, multivitamin supplementation, reproductive outcomes
Introduction
A majority of reproductive-age women in the US consume alcohol to some extent1–4. In fact, general population studies suggest that rates of fetal alcohol exposure may be as high as 50%, and that fetal exposure to large quantities of alcohol may be as high as 12%.
Research suggests a relationship exists between alcohol consumption during pregnancy and miscarriage5–11, however the mechanism through which alcohol causes damage to the fetus resulting in miscarriage remains unknown. It is possible that alcohol's toxicity occurs partly through its disruption of maternal and/or fetal nutrition. Research on the deleterious effects of alcohol on nutrition has shown that alcohol consumption can lead to various micronutrient deficiencies (such as vitamins C and E, zinc, iron and potassium)12–16. Deficiencies of these nutrients during early pregnancy may result in adverse events ranging from fetal growth restriction to mental retardation and developmental delay17–20. Based on this evidence, multivitamin supplements may potentially provide protection.
We are not aware of any studies that have evaluated whether multivitamin supplements modify the relationship between alcohol consumption during pregnancy and miscarriage. To examine this potential relationship, we utilized data from a prospective cohort study to evaluate whether 1) taking multivitamin supplements modifies the effect of any alcohol consumption during pregnancy and miscarriage, 2) taking multivitamin supplements modifies the relationship between the average number of drinks a week during pregnancy (4+ drinks/week, < 4 drinks/week, and no alcohol intake) and miscarriage and 3) the timing of multivitamin supplement exposure modifies the relationship between any alcohol consumption during pregnancy and miscarriage.
Materials and Methods
The current secondary data analysis was conducted utilizing data from a population-based prospective cohort study conducted in the Kaiser Permanente Medical Care Program (KPMCP) in Northern California. All KPMCP women members who lived in San Francisco County and parts of San Mateo County who had a positive pregnancy test at one of two San Francisco KPMCP facilities from October 1996 to October 1998 were identified through the computerized laboratory database as potential eligible subjects. A woman's second pregnancy, if any, during the study period was not eligible for the study. All women submitting a urine sample for a pregnancy test were given a flyer describing the purpose and procedures of the study and a postage-paid and self-addressed return refusal card. Women with positive pregnancy tests who did not return a refusal postcard were contacted by a trained female interviewer to determine their eligibility for the study. English-speaking women who intended to carry their pregnancy to term and whose gestational age at the pregnancy test was less than or equal to 10 complete weeks were eligible for the study. The median gestational age at study entry was 40 days. The original study recruited women to evaluate the relationship between electromagnetic field exposure during pregnancy and miscarriage21.
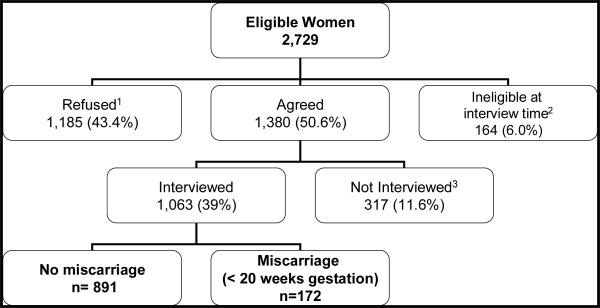
A total of 2,729 pregnant women were identified as eligible for the original study (Figure 1). Of the eligible women, 1,380 (50.6%) initially agreed to participate in the study, of whom 1,061 (39%) completed an in-person interview. In-person interviews were conducted by a trained interviewer to obtain detailed information about alcohol consumption, previous pregnancy history, demographic characteristics and other possible confounders. The present analysis includes 1061 women who completed an in-person interview.
Figure 1.
Recruitment Process
1The main reasons for refusing participation were 1) too busy/not interested/too stressful to participate (47.9%), 2) husband's objection (11.1%), 3) had miscarried already and would rather not talk about it (7.3%), 4) unwilling to wear the meter (required for the original study) (6.2%), 5) other miscellaneous reasons (8.3%), and 6) no specific reasons given (19%).
2Participants were not interviewed because they were too far along in their pregnancy (>15 weeks gestation) when they were reached by the interviewers
3Participants were never able to schedule an interview.
Institutional Review Board approval for this study was obtained from Kaiser Permanente's Human Subjects Committee.
Measures
Miscarriage
Miscarriage was defined as a fetal loss occurring prior to 20 complete weeks of gestation and was based on the date of the woman's last menstrual period (LMP). It was ascertained for all participants through one of the following methods: electronic KPMCP databases, reviewing medical charts, and telephoning women whose outcomes could not be identified through either of the previous methods.
Alcohol Use
Participants were asked if they drank any alcoholic beverages “since becoming pregnant or since LMP.” Women who drank any alcohol were then asked the number of beers (one beer was equal to 12 ounces), the number of glasses of wine or champagne (one glass was equal to 4 ounces), and the number of mixed drinks (one drink was equivalent to 1 ounce of hard liquor) consumed since becoming pregnant. A variable approximating the average number of alcoholic drinks consumed per week was calculated by adding the total number of alcoholic beverages consumed since the beginning of pregnancy, and dividing by the gestational age in weeks at the time of the interview. Based on the distribution of the average number of drinks per week, our sample size and previous research, alcohol consumption was further categorized into three mutually exclusive categories: 1) no alcohol intake (n=626), 2) drank < 4 drinks per week (n=403), 3) drank 4+ drinks per week (n=32).
Multivitamin Use
Participants were asked if they had taken any vitamins, including multiple, prenatal and single vitamins or any other type of supplements “since becoming pregnant or their LMP”. Women were asked about the type and brand of supplement for each of the supplements they reported taking. Additionally, they were asked when they started taking the vitamin supplement.
All women who reported taking either a multivitamin or prenatal vitamin during their pregnancy were considered multivitamin users (n=730). Multivitamins and prenatal vitamins are similar in the content of vitamins and other micronutrients. We also considered the timing of the multivitamin exposure window further categorizing women into three categories: 1) periconceptional user (began taking multivitamins prior to pregnancy and continued in pregnancy, n=475), 2) prenatal user (began taking multivitamins during pregnancy, n=252) and, 3) non-user (n=331). Three women who reported taking multivitamins did not report when they began taking them.
Covariates
Participants were asked about various behaviors during their pregnancy. These included whether they used any illicit drugs, engaged in regular exercise (physical activity for 30 minutes or more at least three times a week), smoked at all, or drank any caffeine during pregnancy. Pre-pregnancy Body Mass Index (BMI) was calculated (kg/m2) and categorized into 2 categories 1) underweight/normal <= 24.9, 2) overweight/obese 25.0 + 22. Other demographic characteristics considered were race (White, Black, Hispanic, Asian, and Native American), education (≤ high school, some college/technical training, graduated from college, attended graduate school) and marital status (married, have a partner but not married, and single). Women were also asked about their income, but 59 (6%) did not respond and therefore were categorized separately (<$35k, $35k-$59k, $60k+, non-responders). Age was dichotomized as less than or equal to 35 and 36 or older, because pregnancies occurring among women aged 36 or older are considered high risk. Finally, we considered whether this pregnancy was intended and previous miscarriage history.
Data Analysis
Stata version 9 was used for all analyses. Pearson chi-square tests were conducted to test differences between categorical variables.
Cox Proportional Hazards analysis was used to examine whether multivitamin supplements modify the relationship between alcohol exposure and miscarriage at any given gestational age while controlling for other possible confounders. This analysis considered all covariates significantly associated with both alcohol and miscarriage. The period for which a woman was considered at risk began at the gestational age when she had a positive pregnancy test (study entry) and continued until she had a miscarriage, ectopic pregnancy or induced abortion (3.6%), or was censored at 20 weeks gestation (80%) because by definition, a miscarriage occurs through 20 weeks of gestation. The time variable used in the proportional hazards model (gestational age in days) was left-truncated at their positive pregnancy test to reflect participants' actual contribution of their person-time23.
Three separate sets of Cox Proportional Hazards Models were conducted. The first set of analyses evaluated any multivitamin use (any use during pregnancy versus no use), any alcohol consumption (any alcohol intake versus no intake), and miscarriage. The second set of analyses evaluated any multivitamin use, the average number of drinks a week (drank 4+ drinks/week, drank <4 drinks/week, no intake) and miscarriage. The final set of analyses evaluated the timing of multivitamin exposure (nonuse, prenatal, periconceptional), any alcohol consumption during pregnancy and miscarriage.
Each set of analyses began with a likelihood ratio test comparing the Cox Proportional Hazards Model which included alcohol use, multivitamin use, all covariates, and an interaction term for alcohol use and multivitamin use, to the same model with the exclusion of the interaction term. The interaction term differed for each set of analyses as described above. Upon a p-value of less than 0.10 for the likelihood ratio test, additional Cox Proportional Hazards Models were conducted stratified by 1) any multivitamin use (first and second sets of analyses) or 2) timing of multivitamin exposure (third set of analyses) to assess the relationship between alcohol consumption during pregnancy and miscarriage. As tests for interaction generally have less power to test for statistical significance24, prior to any data analysis, it was determined that a corresponding p-value of less than 0.10 for the likelihood ratio tests would be the cut-off for conducting the stratified analyses.
Results
Sixteen percent (n=172) of the women in our study had a miscarriage. Forty-one percent of the women reported drinking any alcohol, with 3% drinking 4 or more drinks a week and 38% drinking less than 4 drinks a week (Table 1). The mean number of drinks per week among women who drank alcohol was 1.24 (SD: 2.5). Most women (69%; n=730) took multivitamin supplements; 475 (65%) began taking them during pregnancy and 252 (35%) began taking them prior to pregnancy.
Table 1.
Demographic Characteristics, Pregnancy Behaviors, Previous Miscarriage History, and Alcohol Consumption by Miscarriage
| Total Sample n=1061 n (%) | Miscarriage n=172 n (%) | p-value1 | Any Alcohol Intake n=435 n (%) | p-value2 | |
|---|---|---|---|---|---|
| Demographic Characteristics | |||||
| Maternal age 36 + | 570 (54) | 104 (18) | 0.053 | 246 (43) | 0.123 |
| Maternal age < 36 | 491 (46) | 68 (14) | 189 (39) | ||
| Marital Status | |||||
| Single | 97 (9) | 24 (25) | 0.028 | 51 (53) | 0.01 |
| Married | 849 (80) | 134 (16) | 329 (39) | ||
| Partner | 113 (11) | 13 (12) | 54 (48) | ||
| Race | |||||
| White | 449 (43) | 74 (16) | 0.76 | 267 (59) | <0.001 |
| Black | 82 (8) | 15 (18) | 29 (35) | ||
| Hispanic | 219 (21) | 38 (17) | 80 (37) | ||
| Native American | 13 (1) | 1 (8) | 4 (31) | ||
| Asian | 291 (28) | 42 (14) | 53 (18) | ||
| Income | |||||
| <$35k | 289 (27) | 52 (18) | 0.56 | 94 (33) | <0.001 |
| $35K–$59K | 326 (31) | 46 (14) | 118 (36) | ||
| $60k+ | 388 (37 | 63 (16) | 200 (52) | ||
| non-responders | 58 (5) | 11 (19) | 23 (40) | ||
| Education | |||||
| <=HS | 250 (24) | 43 (17) | 0.756 | 95 (38) | <0.001 |
| some college/tech | 339 (32) | 51 (15) | 112 (33) | ||
| graduated college | 307 (29) | 47 (15) | 135 (44) | ||
| grad school | 164 (15) | 30 (18) | 91 (55) | ||
| Pregnancy Behaviors | |||||
| Illicit Drug Use | |||||
| Used drugs | 60 (6) | 10 (17) | 0.921 | 45 (75) | <0.001 |
| Did not use drugs | 1001 (94) | 162 (16) | 390 (39) | ||
| Smoking Status | |||||
| Smoked | 129 (12) | 23 (18) | 0.595 | 72 (56) | <0.001 |
| Did not smoke | 932 (88) | 149 (16) | 363 (39) | ||
| Exercise Status | |||||
| Exercised regularly | 319 (30) | 56 (18) | 0.424 | 159 (50) | <0.001 |
| Did not exercise regularly | 738 (70) | 115 (16) | 274 (37) | ||
| Caffeine Consumption | |||||
| Drank any caffeinated beverages | 797 (75) | 139 (17) | 0.059 | 359 (45) | <0.001 |
| Did not drink caffeinated beverages | 264 (25) | 33 (13) | 76 (29) | ||
| Pregnancy Intention | |||||
| Did not plan this pregnancy | 376 (35) | 72 (19) | 0.048 | 173 (46) | 0.015 |
| Planned this pregnancy | 684 (65) | 99 (14) | 262 (38) | ||
| Body Mass Index | |||||
| Underweight/normal | 709 (69) | 112 (16) | 0.822 | 295 (42) | 0.55 |
| Overweight/obese | 318 (31) | 52 (16) | 126 (40) | ||
| Previous Miscarriage History | |||||
| No miscarriage | 843 (79) | 134 (16) | 0.583 | 352 (42) | 0.324 |
| 1 + miscarriage | 218 (21) | 38 (17) | 83 (38) | ||
| Timing of Current Miscarriage | |||||
| Early (< 10 weeks gestation) | 12 (59) | NA | 47 (46) | 0.676 | |
| Late (10–20 weeks gestation) | 70 (41) | NA | 30 (43) | ||
| Alcohol Consumption | |||||
| Drank any alcohol | 435 (41) | 77 (18) | 0.272 | NA | |
| Did not drink any alcohol | 626 (59) | 95 (15) | NA | ||
| Frequency of Alcohol Consumption | |||||
| High (4+ drinks/week) | 32 (3) | 11 (34) | 0.016 | NA | |
| Low (<4 drinks/week) | 403 (38) | 66 (16) | NA | ||
| Abstainer | 626 (59) | 95 (15) | NA | ||
| Type of Alcohol | |||||
| Spirits | 56 (5) | 18 (32) | 0.013 | NA | |
| Wine | 160 (15) | 22 (14) | NA | ||
| Beer | 47 (4) | 10 (21) | NA | ||
| Combination | 172 (16) | 27 (16) | NA | ||
| Abstainer | 626 (59) | 95 (15) | NA | ||
| Multivitamin Supplements | |||||
| Did not take multivitamin supplements | 331 (31) | 74 (22) | 114 (34) | 0.003 | |
| Took multivitamin supplements | 730 (69) | 98 (13) | <0.001 | 321 (44) | |
| Timing of Multivitamin Supplement Commencement | |||||
| Non Multivitamin User | 331 (31) | 74 (22) | <0.001 | 114 (34) | 0.001 |
| Prenatal User | 475 (45) | 53 (11) | 196 (41) | ||
| Periconceptional User | 252 (24) | 45 (18) | 125 (50) |
p-value comparison for miscarriage versus no miscarriage
p-value comparison for alcohol intake versus no alcohol intake
We found few possible covariates significantly associated with miscarriage in the bivariate analyses (Table 1). While not statistically significant at p<0.05, the data suggest that women who were over 35 years of age and women who drank any caffeinated beverage during pregnancy were more likely to have a miscarriage. These (maternal age and caffeine intake) and two other significant covariates, marital status and unintended pregnancy, were all also significantly associated with alcohol consumption and were included as possible confounders in all multivariate analyses.
In the first set of multivariable models, the results for the likelihood ratio test (p=0.07) conducted for the analyses evaluating any multivitamin use and any alcohol consumption indicate multivitamin use modified the relationship between alcohol and miscarriage. Therefore we conducted additional analyses stratified by multivitamin use. Among women who did not take multivitamins, women who drank alcohol were 1.67 times more likely to have a miscarriage compared to women who abstained, after adjusting for possible confounders (adjusted Hazard Ratio (aHR): 1.67, 95%CI: 1.04, 2.69) (Table 2). However, among multivitamin users, there was no difference in the risk of miscarriage between women who drank alcohol and women who abstained (aHR: 0.98, 95%CI 0.65, 1.48).
Table 2.
Any Alcohol Consumption during Pregnancy and the Hazard Ratio (HR) for Miscarriage by Multivitamin Status
| Multivitamin Non-user (n=331) | |||||
|---|---|---|---|---|---|
| Alcohol Consumption | Miscarriage n=74 n (%) | HRcrude | 95% CI | HR1 | 95% CI |
| Any alcohol intake (n=114) | 33 (29) | 1.84 | 1.16, 2.91 | 1.67 | 1.04, 2.69 |
| No alcohol intake (n=217) | 41 (19) | 1.0 | 1.0 | ||
| Multivitamin User (n=730) | |||||
|---|---|---|---|---|---|
| Alcohol Consumption | Miscarriage n=98 n (%) | HRcrude | 95% CI | HR1 | 95% CI |
| Any alcohol intake (n=321) | 44 (14) | 1.07 | 0.72, 1.60 | 0.98 | 0.65, 1.48 |
| No alcohol intake (n=409) | 54 (13) | 1.0 | 1.0 | ||
adjusting for maternal age, caffeine, unintended pregnancy and marital status
The p-value for the likelihood ratio test assessing the interaction between any multivitamin use and the average number of drinks per week was 0.098. Table 3 shows the Hazard Ratios for the relationship between three categories of the average number of drinks per week during pregnancy and miscarriage stratified by multivitamin use. Among multivitamin nonusers, women who drank at least four drinks a week were over 6 times as likely to have a miscarriage compared to women who abstained (aHR: 6.30, 95%CI: 2.32, 17.05) and those who drank fewer than four drinks a week had a non-significant increased risk of miscarriage, compared to women who abstained (aHR: 1.51, 95%CI: 0.92, 2.47) (Table 3). However, among multivitamin users, the risk of miscarriage associated with alcohol intake during pregnancy significantly diminished for women who drank four or more drinks a week (aHR:1.87, 95% CI: 0.79, 4.45) and there was no increased risk of miscarriage for women who drank fewer than four drinks a week (aHR: 0.92, 95%CI: 0.60, 1.41).
Table 3.
Frequency of Alcohol Consumption during Pregnancy and the Hazard Ratio (HR) for Miscarriage Stratified by Multivitamin Exposure
| Multivitamin Non-user (n=331) | |||||
|---|---|---|---|---|---|
| Alcohol Consumption | Miscarriage n=74 n (%) | HRcrude | 95% CI | HR1 | 95% CI |
| 4+ drinks/week (n=10) | 5 (50) | 5.34 | 2.10, 13.55 | 6.30 | 2.32, 17.05 |
| < 4 drinks/week (n=104) | 28 (27) | 1.65 | 1.02, 2.66 | 1.51 | 0.92, 2.47 |
| No alcohol intake (n=217) | 41 (19) | 1 | 1.0 | ||
| Multivitamin User (n=730) | |||||
|---|---|---|---|---|---|
| Alcohol Consumption | Miscarriage n=98 n (%) | HRcrude | 95% CI | HR1 | 95% CI |
| 4 + drinks/week (n=22) | 6 (27) | 2.19 | 0.94, 5.10 | 1.87 | 0.79, 4.45 |
| < 4 drinks/week (n=299) | 38 (13) | 0.99 | 0.65, 1.50 | 0.92 | 0.60, 1.41 |
| No alcohol intake (n=409) | 54 (13) | 1 | 1.00 | ||
adjusting for maternal age, caffeine, unintended pregnancy and marital status
The p-value for the likelihood ratio test assessing the interaction between the timing (none, prenatal, periconceptional) of multivitamin exposure and any alcohol consumption was 0.008, therefore, we stratified by the timing of multivitamin use. Table 4 displays the Hazard Ratios for the relationship between alcohol consumption and miscarriage disaggregated by the timing of multivitamin exposure. A trend emerged with the timing of multivitamin use in which the timing of multivitamin use appeared to have a differential but diminished effect on alcohol consumption and the risk of miscarriage, compared to multivitamin non-users.
Table 4.
Any Alcohol Consumption during Pregnancy and Hazard Ratio (HR) for Miscarriage Stratified by Timing of Multivitamin Exposure
| Multivitamin Non-user (n=331) | |||||
|---|---|---|---|---|---|
| Miscarriage n=74 n (%) | HRcrude | 95%CI | HR1 | 95%CI | |
| Any alcohol intake (n= 114) | 33 (29) | 1.84 | 1.16, 2.91 | 1.67 | 1.04, 2.69 |
| No alcohol intake (n=217) | 41 (19) | 1.00 | 1.00 | ||
| Prenatal Multivitamin User2 (n= 475) | |||||
|---|---|---|---|---|---|
| Miscarriage n=53 n (%) | HRcrude | 95% CI | HR1 | 95% CI | |
| Any alcohol intake (n=196) | 27 (14) | 1.51 | 0.89, 2.60 | 1.47 | 0.85, 2.55 |
| No alcohol intake (n=279) | 26 (9) | 1.00 | 1.00 | ||
| Periconceptional Multivitamin User2 (n=252) | |||||
|---|---|---|---|---|---|
| Miscarriage n=45 n (%) | HRcrude | 95%CI | HR1 | 95% CI | |
| Any alcohol intake (n=125) | 17 (14) | 0.64 | 0.35, 1.17 | 0.57 | 0.30, 1.10 |
| No alcohol intake (n=127) | 28 (22) | 1.00 | 1.00 | ||
adjusting for maternal age, caffeine, unintended pregnancy and marital status
Three women did not report when they began taking multivitamins and were not included in this analysis
Additional analyses were conducted to evaluate the relationship between the timing of multivitamin exposure and the average number of drinks per week and miscarriage. Results followed a similar pattern, regardless of the amount of alcohol consumed (results not shown).
To ensure the robustness of our results, additional analyses were conducted which included other demographic characteristic and behavior variables (regular exercise, white versus other race, illicit drug use, and education) significantly associated with alcohol intake and multivitamin use, but not considered traditional confounders. Similar trends and results emerged for all analyses.
Comment
Our results indicate multivitamin status as an important modifier in the relationship between pregnancy drinking and miscarriage that should be considered when evaluating the relationship between alcohol consumption and miscarriage. We found the risk of miscarriage was greatest for women who drank alcohol and reported no multivitamin supplementation. Commencement of multivitamin supplementation prior to pregnancy appeared to have the greatest impact on the relationship between alcohol consumption during pregnancy and miscarriage. Yet, our findings also suggest that multivitamin supplementation started during pregnancy may provide benefits as well.
These findings should be interpreted in light of certain limitations. First, we note that miscarriage was based on a clinical diagnosis and therefore miscarriages which occurred prior to pregnancy awareness were not included. Second, 105 (61%) of the women were interviewed after they had a miscarriage. However, the proportion of women who reported alcohol use during pregnancy was the same for women interviewed either pre- or post- miscarriage (44% for both), with no indication of differential reporting due to the timing of interview in relation to miscarriage.
Third, the generalizeability of the findings may be limited due to the study's low response rate. We can not rule out with certainty that participation was not associated with factors related to both alcohol consumption and multivitamin use; however the requirements of participation in the original study resulted in many refusals. We were able to obtain the percent of miscarriage among non-participants which was similar to that of participants (17.2% versus 16.4%, respectively) somewhat reducing this concern. In addition, other papers published from these data have reported findings consistent with previous research21, 25, 26. Nevertheless, low participation could potentially impact the interpretation of the findings.
The accuracy of self-reported alcohol consumption during pregnancy is a concern in all studies evaluating alcohol-related reproductive and birth outcomes. Comparisons of self-reported pregnancy drinking with the use of vessels (varying sizes of beer, wine and other glasses) to visualize the amount of alcohol consumed have found an underestimation of alcohol consumption resulting from self-report27. Further, studies comparing pregnancy drinking as measured by antenatal self-report and retrospective self-report have documented an under-reporting for antenatal assessment, especially among heavy drinkers28, 29. However, self-report antenatal assessment has been found to be a more accurate and valid measure30.
Finally, we are unable to establish which micronutrients are most important in modifying the relationship between prenatal alcohol exposure and miscarriage. Although information was collected on the brand and type of vitamin supplements consumed, the small number of women taking any particular individual vitamin or micronutrient supplement made analyses of these individual micronutrients unfeasible. Of the 776 women who reported taking vitamins, 78% reported taking only multivitamins, and only 5% reported not taking multivitamins.
Previous research has found an increased risk of miscarriage when alcohol consumption is measured in discrete levels5–11. To an extent, our findings support previous research regarding this relationship. For example, our findings suggest an increased risk of miscarriage for women who drank four or more drinks a week compared to abstainers, among both multivitamin users and non-users. However, the magnitude of the risk of miscarriage associated with alcohol consumption was smaller for multivitamin users. We note that our results are based on a small sample which may affect the precision of our results. Contrary to the literature which has not found an increased risk of miscarriage at lower levels of alcohol intake, our findings suggest an increased risk of miscarriage for women who drank less than four drinks a week, but only among multivitamin non-users.
Research has shown that optimal nutrition at the start of pregnancy is important for healthy pregnancy outcomes. For example, studies evaluating multivitamin supplementation suggest the timing of supplementation may be important in affecting preterm birth and small for gestational age births31, 32. In addition, sufficient levels of folic acid within the first few months of pregnancy are important to protect against birth defects such as neural tube defects33. The results from our study appear to support previous research suggesting the timing of optimal nutrition during pregnancy is important. Our findings suggest that starting multivitamin supplementation during pregnancy may reduce the risk of miscarriage for women who drank alcohol during pregnancy compared to abstainers. Further, although based on a small sample, a non-significant, but protective trend was found for periconceptional users. While further research is needed to replicate this finding, this association is consistent with previous reports that perinatal vitamin use is beneficial to a healthy pregnancy31–33.
The epidemiologic literature lacks research assessing the potential role of nutrition as a modifier in the relationship between alcohol consumption and adverse pregnancy outcomes. However nutrition has been found to be an important modifier between alcohol consumption and several chronic diseases such as cancer34, 35. In addition, interactions between alcohol and select nutrients can affect fetal development, as demonstrated in animal studies36–45. Thus it is plausible that multivitamins may mitigate the risk of adverse pregnancy outcomes including miscarriage, associated with alcohol use during pregnancy.
Conclusions
Our study has important implications for women of child-bearing age in the US. Nearly half of the pregnancies in the US are unintended and surveys have shown that binge-drinking is prevalent among women of child-bearing age3, 4. Our findings suggest that a woman of child-bearing years might decrease her risk of miscarriage by taking multivitamin supplements as part of her daily nutrition. However, we acknowledge that our findings should be interpreted with caution and future research replicating these findings is necessary.
Acknowledgements
The authors would like to thank Drs. Barbara Abrams and Sylvia Guendelman, for their thoughtful comments and suggestions on earlier versions of this manuscript. In addition, we would like to thank Dr. Mark Hudes for his statistical consult. The original data collection of this study was supported by the California Public Health Foundation. This manuscript was a chapter of Lyndsay Ammon's dissertation which was supported by the NIAAA Training Grant: T32-AA007240-29.
This manuscript was a chapter of Lyndsay Ammon Avalos's dissertation which was supported by the NIAAA Training Grant: T32-AA007240-29.
Footnotes
Condensation: Our findings suggest that a woman of child-bearing years might decrease her risk of miscarriage associated with alcohol use by taking multivitamin supplements daily.
Clinical Implications • Women of child-bearing years may decrease her risk of miscarriage from early pregnancy alcohol consumption by taking multivitamin supplements
References
- 1.Ebrahim SH, Gfroerer J. Pregnancy-related substance use in the United States during 1996-1998. Obstet Gynecol. 2003;101:374–379. doi: 10.1016/s0029-7844(02)02588-7. [DOI] [PubMed] [Google Scholar]
- 2.Ebrahim SH, Diekman ST, Floyd RL, Decoufle P. Comparison of binge drinking among pregnant and nonpregnant women, United States, 1991-1995. Am J Obstet Gynecol. 1999 Jan;180(1 Pt 1):1–7. doi: 10.1016/s0002-9378(99)70139-0. [DOI] [PubMed] [Google Scholar]
- 3.Alcohol consumption among women who are pregnant or who might become pregnant-- United States, 2002. MMWR Morb Mortal Wkly Rep. 2004 Dec 24;53(50):1178–1181. [PubMed] [Google Scholar]
- 4.Nayak MB, Kaskutas LA. Risky drinking and alcohol use patterns in a national sample of women of childbearing age. Addiction. 2004;99(11):1393–1402. doi: 10.1111/j.1360-0443.2004.00840.x. [DOI] [PubMed] [Google Scholar]
- 5.Kesmodel U, Wisborg K, Olsen SF, Henriksen TB, Secher NJ. Moderate alcohol intake in pregnancy and the risk of spontaneous abortion. Alcohol Alcohol. 2002 Jan-Feb;37(1):87–92. doi: 10.1093/alcalc/37.1.87. [DOI] [PubMed] [Google Scholar]
- 6.Windham GC, Von Behren J, Fenster L, Schaefer C, Swan SH. Moderate maternal alcohol consumption and risk of spontaneous abortion. Epidemiology. 1997 Sep;8(5):509–514. doi: 10.1097/00001648-199709000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Rasch V. Cigarette, alcohol, and caffeine consumption: risk factors for spontaneous abortion. Acta Obstet Gynecol Scand. 2003 Feb;82(2):182–188. doi: 10.1034/j.1600-0412.2003.00078.x. [DOI] [PubMed] [Google Scholar]
- 8.Henriksen TB, Hjollund NH, Jensen TK, et al. Alcohol consumption at the time of conception and spontaneous abortion. Am J Epidemiol. 2004 Oct 1;160(7):661–667. doi: 10.1093/aje/kwh259. [DOI] [PubMed] [Google Scholar]
- 9.Harlap S, Shiono PH. Alcohol, smoking, and incidence of spontaneous abortions in the first and second trimester. Lancet. 1980 Jul 26;2(8187):173–176. doi: 10.1016/s0140-6736(80)90061-6. [DOI] [PubMed] [Google Scholar]
- 10.McDonald AD, Armstrong BG, Sloan M. Cigarette, alcohol, and coffee consumption and prematurity. Am J Public Health. 1992 Jan;82(1):87–90. doi: 10.2105/ajph.82.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kline J, Shrout P, Stein Z, Susser M, Warburton D. Drinking during pregnancy and spontaneous abortion. Lancet. 1980 Jul 26;2(8187):176–180. doi: 10.1016/s0140-6736(80)90062-8. [DOI] [PubMed] [Google Scholar]
- 12.Kay HH, Grindle KM, Magness RR. Ethanol exposure induces oxidative stress and impairs nitric oxide availability in the human placental villi: a possible mechanism of toxicity. Am J Obstet Gynecol. 2000 Mar;182(3):682–688. doi: 10.1067/mob.2000.104201. [DOI] [PubMed] [Google Scholar]
- 13.Henderson GI, Devi BG, Perez A, Schenker S. In utero ethanol exposure elicits oxidative stress in the rat fetus. Alcohol Clin Exp Res. 1995 Jun;19(3):714–720. doi: 10.1111/j.1530-0277.1995.tb01572.x. [DOI] [PubMed] [Google Scholar]
- 14.Lieber CS. Metabolism of alcohol. Clin Liver Dis. 2005 Feb;9(1):1–35. doi: 10.1016/j.cld.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 15.Goodlett CR, Horn KH. Mechanisms of alcohol-induced damage to the developing nervous system. Alcohol Res Health. 2001;25(3):175–184. [PMC free article] [PubMed] [Google Scholar]
- 16.Church MW, Jen KL, Pellizzon MA, Holmes PA. Prenatal cocaine, alcohol, and undernutrition differentially alter mineral and protein content in fetal rats. Pharmacol Biochem Behav. 1998 Mar;59(3):577–584. doi: 10.1016/s0091-3057(97)00478-4. [DOI] [PubMed] [Google Scholar]
- 17.Chaftez MD. Nutrition and Neurotransmitters: The nutrient bases of behavior. Prentice Hall; Englewood Cliffs, NJ: 1990. [Google Scholar]
- 18.Meadows NJ, Ruse W, Smith MF, et al. Zinc anc small babies. Lancet. 1981 November 21;2(8256):1135–1137. doi: 10.1016/s0140-6736(81)90587-0. [DOI] [PubMed] [Google Scholar]
- 19.Neggers YH, Cutter GR, Alvarez JO, et al. The relationship between maternal serum zinc levels during pregnancy and birthweight. Early Human Development. 1991 May;25(2):75–85. doi: 10.1016/0378-3782(91)90186-7. [DOI] [PubMed] [Google Scholar]
- 20.Shaw GM, Liberman RF, Todoroff K, Wasserman CR. Low birth weight, preterm delivery, and periconceptional vitamin use. J Pediatr. 1997 Jun;130(6):1013–1014. doi: 10.1016/s0022-3476(97)70303-2. [DOI] [PubMed] [Google Scholar]
- 21.Li DK, Odouli R, Wi S, et al. A population-based prospective cohort study of personal exposure to magnetic fields during pregnancy and the risk of miscarriage. Epidemiology. 2002 Jan;13(1):9–20. doi: 10.1097/00001648-200201000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Institute of Medicine (United States) Nutrition During Pregnancy. 1-23. National Academy Press; Washington DC: 1990. Subcommittee on nutritional status and weight gain during pregnancy; pp. 96–120. [Google Scholar]
- 23.Howards PP, Hertz-Picciotto I, Poole C. Conditions for bias from differential left truncation. Am J Epidemiol. 2007 Feb 15;165(4):444–452. doi: 10.1093/aje/kwk027. [DOI] [PubMed] [Google Scholar]
- 24.Frazier PA, Tix AP, Barron KE. Testing moderator and mediator effects in counseling psychology research. Journal of Counseling Psychology. 2004;51(1):115–134. [Google Scholar]
- 25.Weng X, Odouli R. Maternal caffeine consumption during pregnancy and the risk of miscarriage: a prospective cohort study. Am J Obstet Gynecol. 2008 doi: 10.1016/j.ajog.2007.10.803. [DOI] [PubMed] [Google Scholar]
- 26.Li DK, Janevic T, Odouli R, Liu L. Hot tub use during pregnancy and the risk of miscarriage. Am J Epidemiol. 2003 Nov 15;158(10):931–937. doi: 10.1093/aje/kwg243. [DOI] [PubMed] [Google Scholar]
- 27.Armstrong MA, Kaskutas LA, Witbrodt J, et al. Using drink size to talk about drinking during pregnancy: a randomized clinical trial of early start plus. Social Work in Health Care. 2008 doi: 10.1080/00981380802451210. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alvik A, Haldorsen T, Groholt B, Lindemann R. Alcohol consumption before and during pregnancy comparing concurrent and retrospective reports. Alcohol Clin Exp Res. 2006 Mar;30(3):510–515. doi: 10.1111/j.1530-0277.2006.00055.x. [DOI] [PubMed] [Google Scholar]
- 29.Ernhart CB, Morrow-Tlucak M, Sokol RJ, Martier S. Underreporting of alcohol use in pregnancy. Alcohol Clin Exp Res. 1988;12:506–511. doi: 10.1111/j.1530-0277.1988.tb00233.x. [DOI] [PubMed] [Google Scholar]
- 30.Jacobson SW, Chiodo LM, Sokol RJ, Jacobson JL. Validity of maternal report of prenatal alcohol, cocaine, and smoking in relation to neurobehavioral outcome. Pediatrics. 2002 May;109(5):815–825. doi: 10.1542/peds.109.5.815. [DOI] [PubMed] [Google Scholar]
- 31.Vahratian A, Siega-Riz AM, Savitz DA, Thorp JM., Jr. Multivitamin use and the risk of preterm birth. Am J Epidemiol. 2004 Nov 1;160(9):886–892. doi: 10.1093/aje/kwh305. [DOI] [PubMed] [Google Scholar]
- 32.Scholl TO, Hediger ML, Bendich A, Schall JI, Smith WK, Krueger PM. Use of multivitamin/mineral prenatal supplements: influence on the outcome of pregnancy. Am J Epidemiol. 1997 Jul 15;146(2):134–141. doi: 10.1093/oxfordjournals.aje.a009244. [DOI] [PubMed] [Google Scholar]
- 33.Czeizel AE, Dudas I. Prevention of the first occurrence of neural-tube defects by periconceptional vitamin supplementation. N Engl J Med. 1992 Dec 24;327(26):1832–1835. doi: 10.1056/NEJM199212243272602. [DOI] [PubMed] [Google Scholar]
- 34.Jiang R, Hu FB, Giovannucci EL, et al. Joint association of alcohol and folate intake with risk of major chronic disease in women. Am J Epidemiol. 2003 Oct 15;158(8):760–771. doi: 10.1093/aje/kwg221. [DOI] [PubMed] [Google Scholar]
- 35.Corrao G, Torchio P, Zambon A, D'Amicis A, Lepore AR, di Orio F. Alcohol consumption and micronutrient intake as risk factors for liver cirrhosis: a case-control study. The Provincial Group for the study of Chronic Liver Disease. Ann Epidemiol. 1998 Apr;8(3):154–159. doi: 10.1016/s1047-2797(97)00193-2. [DOI] [PubMed] [Google Scholar]
- 36.Thomas JD, LaFiette MH, Quinn VRE, Riley EP. Neonatal choline supplementation ameliorates the effects of prenatal alcohol exposure on a discrimination learning task in rats. Neurotoxicology and Teratology. 2000 January;22(1):703–711. doi: 10.1016/s0892-0362(00)00097-0. [DOI] [PubMed] [Google Scholar]
- 37.Thomas JD, Biane JS, O'Bryan KA, O'Neill TM, Dominguez HD. Choline supplementation following third-trimester-equivalent alcohol exposure attenuates behavioral alterations in rats. Behav Neurosci. 2007;121(1):120–130. doi: 10.1037/0735-7044.121.1.120. [DOI] [PubMed] [Google Scholar]
- 38.Wentzel P, Rydberg U, Eriksson UJ. Antioxidative treatment diminishes ethanol-induced congenital malformations in the rat. Alcohol Clin Exp Res. 2006 Oct;30(10):1752–1760. doi: 10.1111/j.1530-0277.2006.00208.x. [DOI] [PubMed] [Google Scholar]
- 39.Heaton MB, Mitchell JJ, Paiva M. Amelioration of ethanol-induced neurotoxicity in the neonatal rat central nervous system by antioxidant therapy. Alcohol Clin Exp Res. 2000 Apr;24(4):512–518. [PubMed] [Google Scholar]
- 40.Tanaka H, Nasu F, Inomata K. Fetal alcohol effects: decreased synaptic formations in the field CA3 of fetal hippocampus. Int J Dev Neurosci. 1991;9(5):509–517. doi: 10.1016/0736-5748(91)90037-m. [DOI] [PubMed] [Google Scholar]
- 41.Xu Y, Li Y, Tang Y, et al. The maternal combined supplementation of folic acid and Vitamin B(12) suppresses ethanol-induced developmental toxicity in mouse fetuses. Reprod Toxicol. 2006 Jul;22(1):56–61. doi: 10.1016/j.reprotox.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 42.Cano MJ, Ayala A, Murillo ML, Carreras O. Protective effect of folic acid against oxidative stress produced in 21-day postpartum rats by maternal-ethanol chronic consumption during pregnancy and lactation period. Free Radic Res. 2001 Jan;34(1):1–8. doi: 10.1080/10715760100300011. [DOI] [PubMed] [Google Scholar]
- 43.Ba A, Seri BV, Aka KJ, Glin L, Tako A. Comparative effects of developmental thiamine deficiencies and ethanol exposure on the morphometry of the CA3 pyramidal cells. Neurotoxicol Teratol. 1999 Sep-Oct;21(5):579–586. doi: 10.1016/s0892-0362(99)00014-8. [DOI] [PubMed] [Google Scholar]
- 44.Yeh LC, Cerklewski FL. Interaction between ethanol and low dietary zinc during gestation and lactation in the rat. J Nutr. 1984 Nov;114(11):2027–2033. doi: 10.1093/jn/114.11.2027. [DOI] [PubMed] [Google Scholar]
- 45.Zidenberg-Cherr S, Benak PA, Hurley LS, Keen CL. Altered mineral metabolism: a mechanism underlying the fetal alcohol syndrome in rats. Drug Nutr Interact. 1988;5(4):257–274. [PubMed] [Google Scholar]