Abstract
Objective
To determine whether SED1, a protein secreted by the mouse epididymis that coats sperm and participates in sperm adhesion to the zona pellucida, is present on human sperm and in human epididymal tissue.
Design
SED1 expression was analyzed by immunoblot and indirect immunofluorescence assays.
Setting
Academic clinical and research laboratories.
Patient(s)
Human breast milk was donated. Unused semen was donated by men presenting for semen analysis or in vitro fertilization (IVF). Cadaveric epididymal tissue was obtained from the institutional body donor program.
Intervention(s)
Human milk fat globule membranes and human seminal plasma proteins were analyzed by immunoblot. Human sperm and epididymis were analyzed by indirect immunofluorescence microscopy. Acrosomal status was determined by staining with fluorescein isothiocyanate-Pisum sativum agglutinin.
Main Outcome Measure(s)
Immunoblot and indirect immunofluorescence assays.
Result(s)
Human SED1 is recognized by two different polyclonal anti-SED1 antisera. SED1 is localized to the plasma membrane of human sperm overlying the intact acrosome. In acrosome-reacted sperm, SED1 is localized to the equatorial segment. SED1 is expressed by the epithelium of the anterior caput epididymis.
Conclusion(s)
SED1 is expressed on the surface of acrosome-intact human sperm and in the anterior caput of the human epididymis, similar to that seen in mouse.
Keywords: SED1, sperm adhesion, acrosome reaction
SED1 is a sperm-associated protein that facilitates mouse sperm adhesion to the egg zona pellucida (1). SED1 was initially identified as P47, a 47-kDa protein isolated by affinity chromatography of solubilized boar sperm membranes applied to immobilized porcine zona pellucida glycoproteins (2). Sequence analysis demonstrates that P47 is homologous to the short isoform of MFG-E8, also known as lactadherin, a predominant milk fat globule membrane protein found in several species, including pig, cow, and human; P47 is also expressed in pig, cow, mouse, and human testis. The localization and intensity of P47 expression changes during posttes-ticular maturation and capacitation of porcine sperm (3).
To gain more insight into P47 function, the mouse homologue was cloned and renamed SED1 to indicate a Secreted protein containing two NH2-terminal notch-like epidermal growth factor (EGF) repeats and two COOH-terminal Discoi-din/F5/8 complement domains. Some SED1 is derived from the Golgi complex of spermatogenic cells, but the majority of SED1 is secreted from the initial segment of the epididymis, where it binds to the sperm plasma membrane overlying the acrosome (4). Anti-SED1 antibodies block mouse sperm–egg binding, as does recombinant SED1, which binds to the zona pellucida of unfertilized but not fertilized oocytes. SED1 knockout male mice are subfertile in that they produce smaller litters than control males. Furthermore, sperm from SED1 knockout mice are incapable of binding the egg zona pellucida in vitro, despite apparently normal sperm motility and morphology. Thus, SED1 plays an important role during sperm–egg binding in mouse, as assayed by both in vitro and in vivo models (4). In addition to its role during sperm–egg binding, mouse SED1/MFG-E8 has also been shown to play critical roles during mammary gland development (5), clearance of apoptotic cells during mammary gland involution (6), repair of the intestinal epithelium (7), and phagocytosis of photoreceptor outer segments (8).
Human SED1, also known as human breast carcinoma antigen BA46, has been isolated from the milk of healthy donors as a 50-kDa full-length form and a 30-kDa truncated form containing the COOH-terminal discoidin domains (9). Human and mouse SED1 proteins are highly homologous, except that human SED1 lacks the first EGF-like domain. Although SED1 function in human has not been explored, it has been suggested that SED1/MFG-E8 present in milk may prevent rotavirus infection in breast-fed infants (10, 11). SED1 is also found in the sera of women with breast cancer and has therefore been evaluated for a role in breast cancer diagnosis and treatment (12–14). Irregardless, as a first assessment of SED1’s potential role in human sperm–egg binding, we determined in this study whether SED1 is expressed on human sperm and in human epididymal tissue.
MATERIALS AND METHODS
Development of Recombinant SED1 and Anti-SED1 Antibody
The cloning of mouse SED1, the production of affinity-purified recombinant SED1, and characterization of rabbit antibodies raised against recombinant SED1 have been described previously (4).
Human Milk Preparation
SED1 was isolated from donated human milk using a modification of a previously published method (9). Briefly, the cream was isolated from fresh human milk by centrifugation at 4,000 × g for 20 minutes and washed with 10 mM Na2PO4, phosphate-buffered saline (PBS), pH 7.2. After centrifugation at 4,000 × g for 20 minutes, the isolated washed cream was resuspended in five volumes of PBS and sonicated to disrupt the milk fat globule membranes (MFGMs). The resultant MFGMs were collected by centrifugation at 40,000 × g for 1 hour. The membrane pellet was resuspended in PBS with 2.5% Triton (vol/vol), sonicated, and mixed overnight at 4°C. Phase separation was achieved by heating the solution in a 37°C water bath for 1 hour, followed by centrifugation at 4,000 × g for 20 minutes. The lower detergent phase was collected and the protein precipitated overnight in 10 volumes of acetone at −20°C. The resultant SED1-containing fraction was collected by centrifugation at 4,000 × g for 20 minutes. The acetone was removed and the pellet stored at −20°C.
Human Sperm Acquisition
After informed consent, excess semen from 105 men aged 18–50 years presenting for semen analysis or in vitro fertilization (IVF) was donated to the study. Sperm used in this study were from men with normal standard semen analysis parameters, according to World Health Organization 1999 criteria for count and motility (15) and Kruger strict criteria for morphology >4% (16).
Participants were instructed to abstain from ejaculation for 2–5 days before sample collection. Semen samples were collected by masturbation and allowed to liquefy for 30–60 minutes before being centrifuged over an 80% single-layer density gradient (PureCeption; SAGE In-Vitro Fertilization, Inc., a CooperSurgical Company, Trumbull, CT) for 20 minutes. The resultant pellet was suspended in synthetic human tubular fluid (HTF) (Quinn’s; SAGE In-Vitro Fertilization, Inc., a CooperSurgical Company) with 6% serum substitute supplement (SSS) (Irvine Scientific, Santa Ana, CA) and centrifuged for 5 minutes. The supernatant was discarded; fresh HTF with 6% SSS was applied over the pellet and incubated at 5% CO2, 37°C for 30 minutes for capacitation and “swim-up” separation. Sperm from the top portion of the fluid column, the “swim-up” sperm, were used for immunohisto-chemical analysis.
Western Blot of Human Milk and Seminal Plasma
The MFGM isolated from human milk and the soluble proteins from human seminal plasma were resolved by both one- and two-dimensional electrophoresis. Proteins were transferred to polyvinylidene fluoride, which was subsequently blocked overnight in 5% normal goat serum (NGS) at 4°C. The polyvinylidene fluoride membrane was incubated in rabbit anti-SED1 antiserum at a concentration of 1:2,000 in 5% NGS phosphate buffered saline plus 1% Tween-20 (PBST) for 1 hour. The membrane was washed with PBST three times before incubation with 1:30,000 horseradish peroxidase–conjugated goat antirabbit IgG (Santa Cruz Biotechnology, Santa Cruz, CA) in 5% NGS PBST for 1 hour. The membrane was washed three times in PBST before application of electrochemical luminescence Western detection reagent, film exposure, and development. Preimmune antiserum was used at the same concentration to determine background levels of immunoreactivity. Two-dimensional electrophoresis provided the best protein separation to compare SED1 profiles between samples and species. Both sources of anti-SED1 antisera (anti-SED1, anti-MFG-E8) produced similar results.
Induction of the Sperm Acrosome Reaction
A portion of the swim-up sperm was incubated with the calcium ionophore A23187 (10 μM) in 5% CO2 at 37°C for 30 minutes to induce the acrosome reaction. The sperm were then washed in HTF with 6% SSS for 5 minutes before being dried on slides.
Immunofluorescence of Human Sperm
Sperm were evaluated for SED1 expression using indirect immunofluorescence noting presence or absence of staining on the sperm membrane. A portion of the swim-up sperm was dried on slides, which were subsequently blocked in 5% NGS for 1 hour. Rabbit anti-SED1 IgG (60 μg/mL) was applied to slides for 1 hour. After three washes in PBS, slides were incubated in biotinylated goat antirabbit IgG (1:1,000) (Vector Laboratories, Burlingame, CA) for 1 hour. After three washes in PBS, fluorescent streptavidin (1:1,000) was applied for 1 hour. Slides were rinsed in PBS and water before mounting. Preimmune IgG at the same concentration was used to determine background levels of immunoreactivity. Parallel assays using anti-SED1 antiserum produced similar results as anti-SED1 IgG. To confirm that SED1 was expressed on the intact sperm plasma membrane, live sperm were blocked and exposed to primary antibodies as above before being dried on slides and exposed to secondary antibodies.
Determination of Acrosomal Status
Acrosomal status was determined by staining with fluorescein isothiocyanate–Pisum sativum agglutinin (FITC-PSA) (Sigma, St. Louis, MO) (17). Briefly, slides were dried before permeabilization and fixation with methanol for 30 seconds at room temperature. After a distilled water wash, slides were dried and then incubated with 100 μg/mL FITC-PSA for 10 minutes in the dark. After a distilled water wash, slides were covered with fluorescent mounting media, cover-slipped, and viewed on an Olympus fluorescence microscope (Tokyo, Japan).
Immunohistochemistry of Human Epididymal Tissue
Cadaveric human testes and epididymal tissue from a single cadaver of unknown prior fertility were obtained from the institutional body donor program. Tissue was fixed in Bouin’s solution for 48 hours and embedded in paraffin. Ten-micrometer sections of the anterior caput epididymis were subjected to antigen retrieval by boiling in 10 mM sodium citrate (pH 6) for 6 minutes in the microwave oven. Thinner sections did not withstand the rigors of subsequent staining owing to the fragile and porous nature of the tissue. Sections were cooled and blocked overnight with 5% NGS in PBS. Rabbit anti-SED1 antiserum (1:200) was applied in 5% NGS PBS for 1 hour. After three PBS washes, sections were incubated with FITC goat antirabbit Fab fragment (Invitrogen, Carlsbad, CA) at 1:1,000 for 1 hour. After a brief wash in PBS, slides were mounted and examined. Preimmune antiserum was used at the same concentration to determine background levels of immunoreactivity.
This protocol was conducted with the approval of the Emory University Institutional Review Board.
RESULTS
Antisera Raised Against Recombinant Murine SED1 Recognizes Human SED1
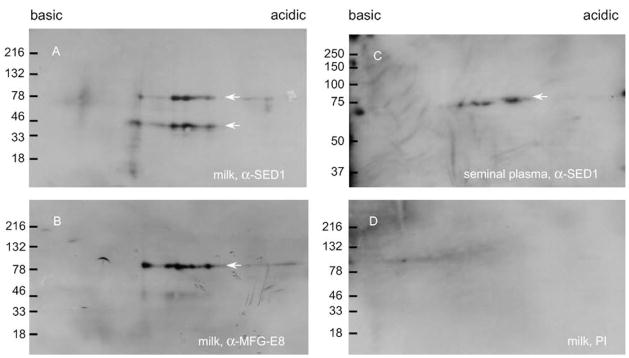
Rabbit antisera raised against recombinant murine SED1 cross-reacts with the two SED1 isoforms known to be present in human milk (9) (Fig. 1). Furthermore, both anti-SED1 antibodies produced similar patterns of immunoreactivity (Fig. 1), indicating that these antisera are useful reagents to probe SED1 expression in human. The cross-reactivity between mouse and human SED1 proteins is not unexpected, given their high homology (76% identity complementary DNA; 57% protein sequence; 68% conservative substitutions) (18).
FIGURE 1.
Antibodies against recombinant murine SED1 recognize human SED1. (A) Rabbit antiserum raised against bacterially expressed recombinant murine SED1 (α-SED1) recognizes the two SED1 isoforms present in human milk (9). (B) A similar pattern of immunoreactivity is seen with an independent antibody raised against murine MFG-E8 (α-MFG-E8). (C) α-SED1 identifies only one isoform in human seminal plasma. (D) Preimmune (PI) serum fails to show any specific immunoreactivity. Arrows indicate SED1 isoforms.
SED1 Localization on Human Sperm
Motile sperm were isolated from semen and subjected to immunofluorescence detection of SED1. SED1 was found to be localized on the plasma membrane overlying the intact acrosome (Fig. 2). Similar patterns of reactivity were found using both live unfixed sperm and fixed sperm, consistent with SED1 expression on the intact plasma membrane. Furthermore, SED1 was found to localize to the equatorial segment on acrosome-reacted sperm (Fig. 2). Although the relative intensity of SED1 staining varied between patients and among methods, this pattern has been replicated in 15 separate samples. In all instances, substitution of anti-SED1 antiserum or IgG with preimmune reagents produced background reactivity.
FIGURE 2.
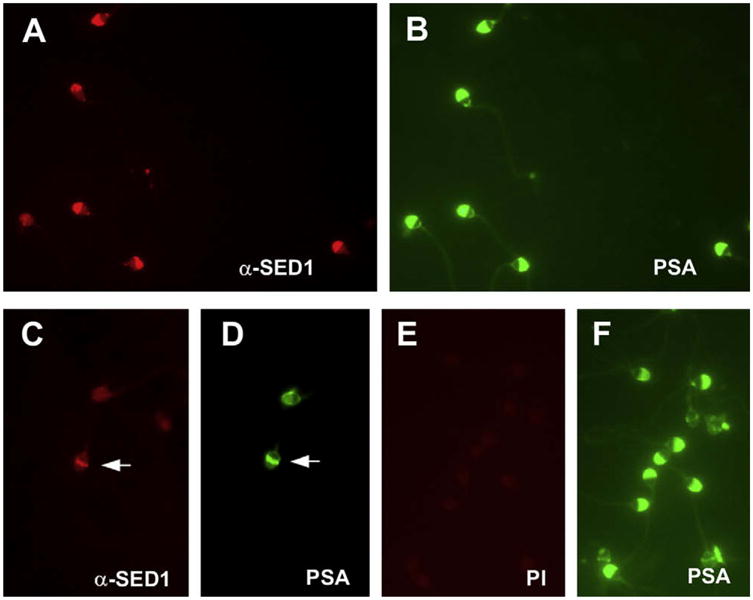
SED1 is localized on the sperm plasma membrane overlying the intact acrosome and is expressed on the equatorial segment after acrosomal exocytosis. (A, C) Anti-SED1 IgG; (E) preimmune (PI) IgG; (B, D, F) FITC-PSA staining of the acrosomal contents. (A, B) In acrosome-intact human sperm, SED1 is localized on the anterior aspect of the intact plasma membrane. (C, D) In acrosome-reacted sperm, SED1 is localized to the equatorial segment (arrows). (E, F) Preimmune IgG produces background levels of immunoreactivity.
SED1 Expression in Human Epididymal Tissue
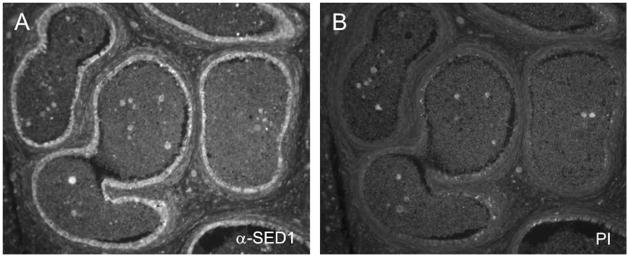
In mouse, SED1 is secreted by the initial segment of the epididymis, where it coats sperm as they progress through the epididymal lumen (4). We therefore examined whether SED1 shows a similar expression profile in human. Despite suboptimal architecture of the cadaveric tissue, SED1-specific staining was evident in the epithelium of the anterior caput of the human epididymis (Fig. 3). Preimmune antiserum produced background reactivity.
FIGURE 3.
SED1 is expressed in the epithelium of the human anterior caput epididymis. (A) Anti-SED1 antiserum localizes SED1 to the epithelium of the human epididymis. (B) Preimmune (PI) serum produces background levels of immunoreactivity.
DISCUSSION
This study provides evidence that SED1 is expressed on the surface of human sperm overlying the intact acrosome and that it is expressed in the anterior caput of the human epididymis, where it is likely secreted during sperm passage. These results are similar to that seen in mouse, in which SED1 has been shown to have a critical role in mediating sperm binding to the egg zona pellucida (4). It is of interest that unlike that seen in mouse, human sperm retain SED1 after acrosomal exocytosis, at which time it localizes to the equatorial segment, where it may stabilize adhesion of acrosome-reacted sperm to the zona pellucida before penetration; however, this possibility awaits direct testing.
SED1 contributes to many cellular interactions via its two binding motifs: the RGD sequence in the second EGF domain binds to αVβ5/3 integrins, whereas the COOH-terminal discoidin domains associate with phospholipid bilayers and/or extracellular matrices (19, 20). In this regard, mouse macrophages secrete SED1, which binds anionic phospholipids on apoptotic lymphocytes and initiates phagocytosis by binding and activating αVβ5/3 integrins on macrophages (21). Similarly, mouse SED1 participates in intestinal epithelial cell migration and response to intestinal mucosal damage (7), as well as facilitating retinal pigment epithelium interactions with photoreceptor outer segments (8). SED1 also plays critical roles during mouse mammary gland development by signaling between luminal epithelial and myoepithelial cells, as well as during involution after lactation by promoting engulfment of apoptotic cells by phagocytic mammary epithelium and macrophages (5, 6).
Unlike these cellular interactions that require both SED1 binding motifs, SED1 function during sperm–egg interactions does not seem to require the RGD motif, given that all of its biologic activity can be attributed to its COOH-terminal discoidin domains. Nevertheless, SED1 is just one of many components thought to participate in sperm–egg interactions. Studies in mice suggest that SED1 facilitates the initial adhesion of sperm to the zona pellucida, which enables the binding of zona pellucida glycoprotein 3 (ZP3) to β1,4-galactosyl-transferase-1 (GalT) on the sperm surface; subsequent GalT aggregation by ZP3 glycan chains initiates the acrosome reaction enabling sperm penetration through the zona matrix. Consequently, sperm from SED1-null males do not bind the zona pellucida in vitro, and SED1-null males are subfertile in vivo. In contrast, sperm from GalT-null mice bind the zona pellucida normally but fail to undergo zona-induced ac-rosomal exocytosis (22, 23).
The demonstration that SED1 is expressed on the surface of human sperm is consistent with the possibility that SED1 facilitates sperm–egg binding in human as it does in mouse; however, this awaits more direct analysis. Furthermore, future studies must include men with proven fertility as well as those with unexplained infertility to determine whether SED1 expression correlates with clinical prognosis. By continuing to clarify the role of SED1 in human sperm–egg binding, we will gain insight into the mechanism of fertilization, which will lead to advances in evaluation and treatment of infertility and potential contraceptive applications. In this regard, because SED1 is secreted onto the surface of mature sperm, SED1 could be added vaginally before intercourse to increase the fertilizing potential of infertile males. Contraceptive applications would also be plausible by developing suppositories containing antibodies or site-specific inhibitors of SED1 that would coat the sperm, decrease sperm–egg binding, and prevent fertilization. Further study is necessary to explore the significance and feasibility of SED1-based approaches for controlling reproduction.
Acknowledgments
The authors thank the scientific advisers Adam Raymond, B.S., Michael Ensslin, Ph.D., Weirong Shang, Ph.D., and Sharon Wiker for technical assistance. The authors would also like to thank Tsukasa Matsuda (Nagoya University) for kindly providing a second source of rabbit anti-SED1/MFG-E8 antisera.
This work was supported by National Institutes of Health grant HD23479 to B.D.S.
Footnotes
S.D.C. has nothing to disclose. A.A.M. has nothing to disclose. B.D.S. has nothing to disclose.
References
- 1.Shur BD, Ensslin MA, Rodeheffer C. SED1 function during mammalian sperm-egg adhesion. Curr Opin Cell Biol. 2004;16:477–85. doi: 10.1016/j.ceb.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 2.Ensslin M, Vogel T, Calvete JJ, Thole HH, Schmidtke J, Matsuda T, et al. Molecular cloning and characterization of p47, a novel boar sperm-associated zona pellucida-binding protein homologous to a family of mammalian secretory proteins. Biol Reprod. 1998;58:1057–64. doi: 10.1095/biolreprod58.4.1057. [DOI] [PubMed] [Google Scholar]
- 3.Petrunkina AM, Lakamp A, Gentzel M, Ekhlasi-Hundrieser M, Topfer-Petersen E. Fate of lactadherin P47 during post-testicular maturation and capacitation of boar spermatozoa. Reproduction. 2003;125:377–87. [PubMed] [Google Scholar]
- 4.Ensslin MA, Shur BD. Identification of mouse sperm SED1, a bimotif EGF repeat and discoidin-domain protein involved in sperm-egg binding. Cell. 2003;114:405–17. doi: 10.1016/s0092-8674(03)00643-3. [DOI] [PubMed] [Google Scholar]
- 5.Ensslin MA, Shur BD. The EGF repeat and discoidin domain protein, SED1/MFG-E8, is required for mammary gland branching morphogenesis. Proc Natl Acad Sci U S A. 2007;104:2715–20. doi: 10.1073/pnas.0610296104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hanayama R, Nagata S. Impaired involution of mammary glands in the absence of milk fat globule EGF factor 8. Proc Natl Acad Sci U S A. 2005;102:16886–91. doi: 10.1073/pnas.0508599102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bu HF, Zuo XL, Wang X, Ensslin MA, Koti V, Hsueh W, et al. Milk fat globule-EGF factor 8/lactadherin plays a crucial role in maintenance and repair of murine intestinal epithelium. J Clin Invest. 2007;117:3673–83. doi: 10.1172/JCI31841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nandrot EF, Anand M, Almeida D, Atabai K, Sheppard D, Finnemann SC. Essential role for MFG-E8 as ligand for alphavbeta5 integrin in diurnal retinal phagocytosis. Proc Natl Acad Sci U S A. 2007;104:12005–10. doi: 10.1073/pnas.0704756104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giuffrida MG, Cavaletto M, Giunta C, Conti A, Godovac-Zimmermann J. Isolation and characterization of full and truncated forms of human breast carcinoma protein BA46 from human milk fat globule membranes. J Protein Chem. 1998;17:143–8. doi: 10.1023/a:1022531500370. [DOI] [PubMed] [Google Scholar]
- 10.Kvistgaard AS, Pallesen LT, Arias CF, Lopez S, Petersen TE, Heegaard CW, et al. Inhibitory effects of human and bovine milk constituents on rotavirus infections. J Dairy Sci. 2004;87:4088–96. doi: 10.3168/jds.S0022-0302(04)73551-1. [DOI] [PubMed] [Google Scholar]
- 11.Newburg DS, Peterson JA, Ruiz-Palacios GM, Matson DO, Morrow AL, Shults J, et al. Role of human-milk lactadherin in protection against symptomatic rotavirus infection. Lancet. 1998;351:1815–6. doi: 10.1016/s0140-6736(97)10322-1. [DOI] [PubMed] [Google Scholar]
- 12.Ceriani RL, Sasaki M, Sussman H, Wara WM, Blank EW. Circulating human mammary epithelial antigens in breast cancer. Proc Natl Acad Sci USA. 1982;79:5420–4. doi: 10.1073/pnas.79.17.5420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Larocca D, Peterson JA, Urrea R, Kuniyoshi J, Bistrain AM, Ceriani RL. A Mr 46,000 human milk fat globule protein that is highly expressed in human breast tumors contains factor VIII-like domains. Cancer Res. 1991;51:4994–8. [PubMed] [Google Scholar]
- 14.Couto JR, Blank EW, Peterson JA, Ceriani RL. Anti-BA46 monoclonal antibody Mc3: humanization using a novel positional consensus and in vivo and in vitro characterization. Cancer Res. 1995;55:1717–22. [PubMed] [Google Scholar]
- 15.World Health Organization. Laboratory manual for human semen and sperm cervical mucus interaction. 4. New York: Cambridge University Press; 1999. [Google Scholar]
- 16.Kruger TF, Acosta AA, Simmons KF, Swanson RJ, Matta JF, Oehninger S. Predictive value of abnormal sperm morphology in in vitro fertilization. Fertil Steril. 1988;49:112–7. doi: 10.1016/s0015-0282(16)59660-5. [DOI] [PubMed] [Google Scholar]
- 17.Jaiswal BS, Eisenbach M, Tur-Kaspa I. Detection of partial and complete acrosome reaction in human spermatozoa: which inducers and probes to use? Mol Hum Reprod. 1999;5:214–9. doi: 10.1093/molehr/5.3.214. [DOI] [PubMed] [Google Scholar]
- 18.Carmon L, Bobilev-Priel I, Brenner B, Bobilev D, Paz A, Bar-Haim E, et al. Characterization of novel breast carcinoma-associated BA46-derived peptides in HLA-A2.1/D(b)-beta2m transgenic mice. J Clin Invest. 2002;110:453–62. doi: 10.1172/JCI14071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anderson MH, Berglund L, Rasmussen JT, Petersen TE. Bovine PAS-6/7 binds αVβ5 integrin and anionic phospholipids through two domains. Biochemistry. 1997;36:5441–6. doi: 10.1021/bi963119m. [DOI] [PubMed] [Google Scholar]
- 20.Anderson MH, Graversen H, Fedosov SN, Petersen TE, Rasmussen JT. Functional analyses of two cellular binding domains of bovine lactadherin. Biochemistry. 2000;39:6200–6. doi: 10.1021/bi992221r. [DOI] [PubMed] [Google Scholar]
- 21.Hanayama R, Tanaka M, Miwa K, Shinohara A, Iwamatsu A, Nagata S. Identification of a factor that links apoptotic cells to phagocytes. Nature. 2002;417:182–7. doi: 10.1038/417182a. [DOI] [PubMed] [Google Scholar]
- 22.Lyng R, Shur BD. Sperm-egg binding requires a multiplicity of receptor-ligand interactions: new insights into the nature of gamete receptors derived from reproductive tract secretions. Soc Reprod Fertil Suppl. 2007;65:335–51. [PubMed] [Google Scholar]
- 23.Ensslin MA, Lyng R, Raymond A, Copland S, Shur BD. Novel gamete receptors that facilitate sperm adhesion to the egg coat. Soc Reprod Fertil Suppl. 2007;63:367–83. [PubMed] [Google Scholar]