Abstract
Parkinson disease is a specific form of neurodegeneration characterized by a loss of nigra-striatal dopaminergic neurons in the midbrain of humans. The disease is also characterized by an increase in oxidative stress and a loss of glutathione in the midbrain region. A potential link between all these factors is the oxidation of dopamine to dopaminochrome (DAC). Using the murine mesencephalic cell line MN9D, we have shown that DAC [50 – 250 µM] leads to cell death in a concentration-dependent manner, whereas oxidized L-dopa, dopachrome [50–250 µM], is only toxic at the highest concentration used. Furthermore, chronic exposure of MN9D cells to low concentrations of DAC [50 – 100 µM] is cytotoxic between 48 and 96 h. DAC also increases superoxide production within MN9D cells as indicated by dihydroethidium fluorescence, that can be prevented by co-administration with the antioxidant, N-acetylcysteine [5 mM]. Moreover, the cytotoxicity induced by DAC can also be prevented by administration of N-acetylcysteine [1 – 5 mM]. Finally, depletion of reduced glutathione in MN9D cells by buthionine sulfoximine [50 – 100 µM] administration significantly enhances the cytotoxic effect of low concentrations of DAC [50 – 100 µM] and DAC [175 µM] itself reduces the proportion of oxidized glutathione in total glutathione within 30 min of administration in MN9D cells. Overall, we have shown that DAC causes MN9D cell death in an oxidatively-dependent manner that appears closely linked with a rapid loss of reduced glutathione. These findings have implications for understanding the pathogenesis of neurodegenerative pathways in Parkinson disease.
Keywords: neurodegeneration, Parkinson disease, dopamine, glutathione, oxidation
Introduction
The mechanism(s) by which neurodegeneration occurs in the midbrain of patients suffering from Parkinson disease (PD) is not fully understood, but oxidative stress appears to play an important role (Ebadi et al., 1996; Halliwell, 1992; Koutsilieri et al., 2002). This disorder becomes evident after dopaminergic neurons projecting from the substantia nigra pars compacta (SNpc) to the striatum degenerate, with up to 70% of striatal dopamine being lost before symptoms appear. A variety of genetic markers have been identified that can lead to Parkinsonian syndromes, but evidence as to their involvement in idiopathic PD cases is unclear (Foley and Riederer, 2000).
Effective PD mimetics are limited, but many of the models used in animal studies, including 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) and rotenone, are known to increase oxidative stress to a significant degree (Tipton and Singer, 1993). Indeed, when these neurotoxins are administered, they ablate dopaminergic cells of the SNpc before others throughout the midbrain, indicating that these neurons are particularly susceptible to oxidative stress (Heikkila et al., 1985; Thiffault et al., 2000).
The dopaminergic cells of the SNpc contain the pigment neuromelanin that results from polymerization of oxidized dopamine. Dopamine is readily oxidized at physiological pH first to transient quinone species, and then to the relatively stable dopaminochrome (DAC). The conversion from dopamine to DAC can be accelerated with the addition of various oxidants, including peroxynitrite and superoxide. Polymerization of DAC results in the production of neuromelanin in the SNpc (Graham, 1978; Sulzer et al., 2000).
Post-mortem studies have shown that neuromelanin is lost in the progression of PD, signifying a loss of dopaminergic neurons (Zecca et al., 2006). Along with a loss of neuromelanin in the SNpc, Parkinson patients have also been observed in post-mortem studies to lack the levels of reduced glutathione observed in normal individuals (Ebadi et al., 1996; Perry et al., 1982). Reduced glutathione (GSH) is produced in the cytosol and utilized by glutathione peroxidases located in the mitochondria and at the phospholipid membrane to reduce levels of hydroperoxides on lipids and proteins. Action by glutathione peroxidase yields oxidized glutathione (GSSG) that must be converted back to GSH in the cytosol before it can be re-used. The ratio of GSH to GSSG is important for maintaining the effectiveness of glutathione peroxidase and, thus, protection against oxidative insult within neuronal cells (Chinta et al., 2007).
DAC, the oxidized form of dopamine, is likely present in these midbrain cells as it is a necessary step in the course to neuromelanin production. DAC is potentially detrimental, therefore we evaluated the effects of this molecule on cell viability using the mesencephalic cell line, MN9D (Choi et al., 1992). We found that DAC is indeed toxic to MN9D cells, while its precursor counterpart, dopachrome, is much weaker in this capacity. Furthermore, the toxicity observed is dependent upon oxidative stress in that N-acetylcysteine is effective at improving survival, and the endogenous pool of glutathione is rapidly depleted upon DAC incubation with MN9D cells. From these data, we believe that DAC is not only toxic to MN9D cells, but it may act by depleting glutathione stores that would otherwise protect cells against oxidative stress.
Materials and Methods
Cell Culture
MN9D cells were grown in high-glucose (4500 mg/L) Dulbecco’s modified Eagle’s Medium (DMEM, Sigma-Aldrich, St. Louis, MO) with L-glutamine and 10% fetal bovine serum (Hyclone, Logan, UT) in a humidified 5% CO2 atmosphere at 37 °C. For cell viability studies, cells were differentiated for 5 days with 1 mM sodium butyrate (Sigma-Aldrich, St. Louis, MO) and seeded onto collagen-coated 96-well plates. For dihydroethidium (DHE) studies, cells were differentiated as before, but seeded to 35 mm petri dishes with a glass bottom for fluorescence visualization. For glutathione assays, cells were differentiated as before and seeded on collagen-coated 6-well plates.
Aminochrome Production
Catecholamines were oxidized by reacting NaIO4 with either dopamine or L-dopa at a 2:1 ratio in ddH2O as described previously (Graham, 1978). L-dopa was reacted with NaIO4 for 15 min. at room temperature (22 °C – 25 °C), and dopamine was reacted with NaIO4 for 45 min. at 37 °C as optimized in earlier studies by our laboratory (Ochs et al., 2005). All compounds were obtained from Sigma-Aldrich (St. Louis, MO). After the reaction was complete, all samples were tested using high pressure liquid chromatography with electrochemical detection to verify the purity of our samples, and that the conversion to dopaminochrome from dopamine yielded no remaining intermediates.
Cell Viability Assay
All cell viability measurements were carried out using the MTS assay (CellTiter96 AQ, Promega, Madison, WI). In brief, 20 µL of reagent was added to each well in the 96-well plate at the specified time points for 3 hours at 37 °C. The wells were then evaluated for colorometric absorption on a Powerwave × plate reader (Biotek instruments; Winooski, VT) and the calculation of sample value was analyzed by KC Junior Software (Biotek instruments).
Dihydroethidium Fluorescence
Dihydroethidium (DHE; Sigma-Aldrich, St. Louis, MO) is oxidized to oxyethidium in the presence of superoxide radicals, which can be visualized via fluorescence microscopy using a GFP filter (emission = 488 nm, excitation = 595 nm) (Zhao et al., 2003). In brief, cells were cultured in 35 mm petri dishes, then administered DAC. After 2 h, cells were incubated with DHE (10 µg/mL) for 20 min at 37 °C, followed by fixing with 4% paraformaldehyde for 30 min. Cells were then visualized under a fluorescent microscope and analyzed with Metamorph image analysis software.
Glutathione Assay Experiments
MN9D cells were treated with DAC for a limited time before being harvested, lysed, and treated according to the Glutathione Assay Kit (Cayman, Ann Arbor, MI). Briefly, cells were scraped from 6-well collagen-coated plates and sonicated in 50 mM 2-(N-morpholino) ethanesulphonic acid (MES) buffer. After centrifugation, the supernatant was removed and stored on ice. The assay cocktail was prepared according to the kit instructions and added to samples in a 96-well plate. The absorbance was read on a Powerwave × plate reader (Biotek instruments; Winooski, VT) at 405 nm. The absorbance values were compared with a set of standards provided by the manufacturer.
Statistical Analysis
esults are expressed as mean ± s.e.m. for (n) wells. Statistical differences between treatments were determined by Student’s t-test; by one-way analysis of variance followed by Student-Newman-Keuls test; or by two-way analysis of variance followed by Bonferroni Post-Hoc test, depending on the experimental conditions. Statistical differences were accepted when P<0.05.
Results
Dopaminochrome is toxic to MN9D cells in a concentration-dependent manner
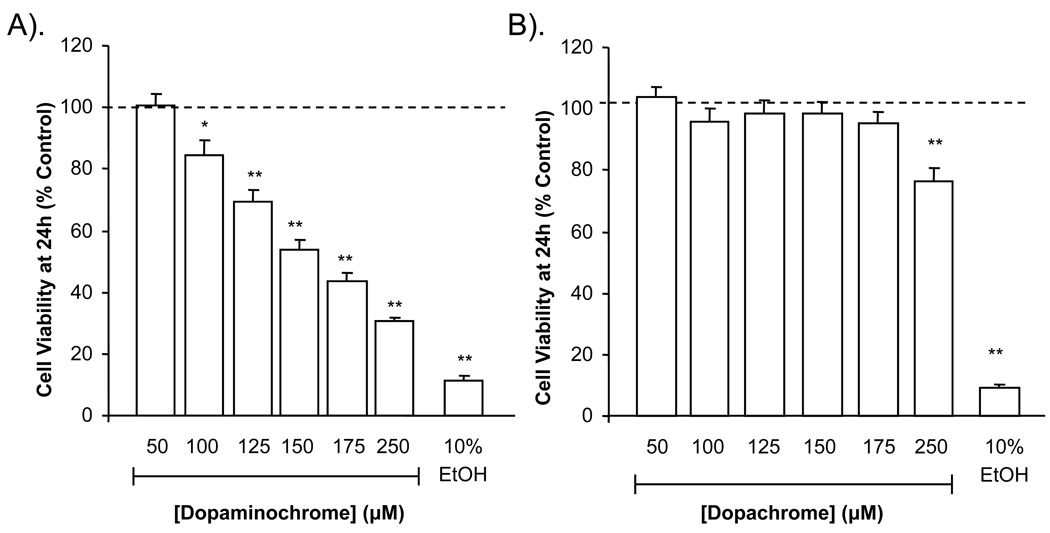
MN9D cells incubated with DAC [50–250 µM] in DMEM for 24 h exhibited a concentration-dependent decrease in cell viability when compared with untreated cells (Figure 1A). In contrast, dopachrome [DPC; 50–250 µM], the oxidation product of L–dopa, caused cytotoxicity in MN9D cells after 24 h only at the highest concentration tested (Figure 1B). In the above experiments, ethanol (10%) was used as a positive control of cell death. Production of DAC by reacting dopamine with NaIO4 results in the byproduct, NaIO3. When NaIO3 was incubated alone with MN9D cells, no detrimental effect was observed (not shown).
Figure 1. Viability of MN9D cells in presence of dopaminochrome and dopachrome.
MN9D cells were incubated with DAC or dopachrome and cell viability measured at 24 h by the MTS assay. DAC caused a concentration-dependent decrease in cell viability in MN9D cells (A). Dopachrome was only cytotoxic at the highest concentration tested (B). (n = 30 wells from 3 individual experiments) **P<0.01, ***P<0.001 compared to control.
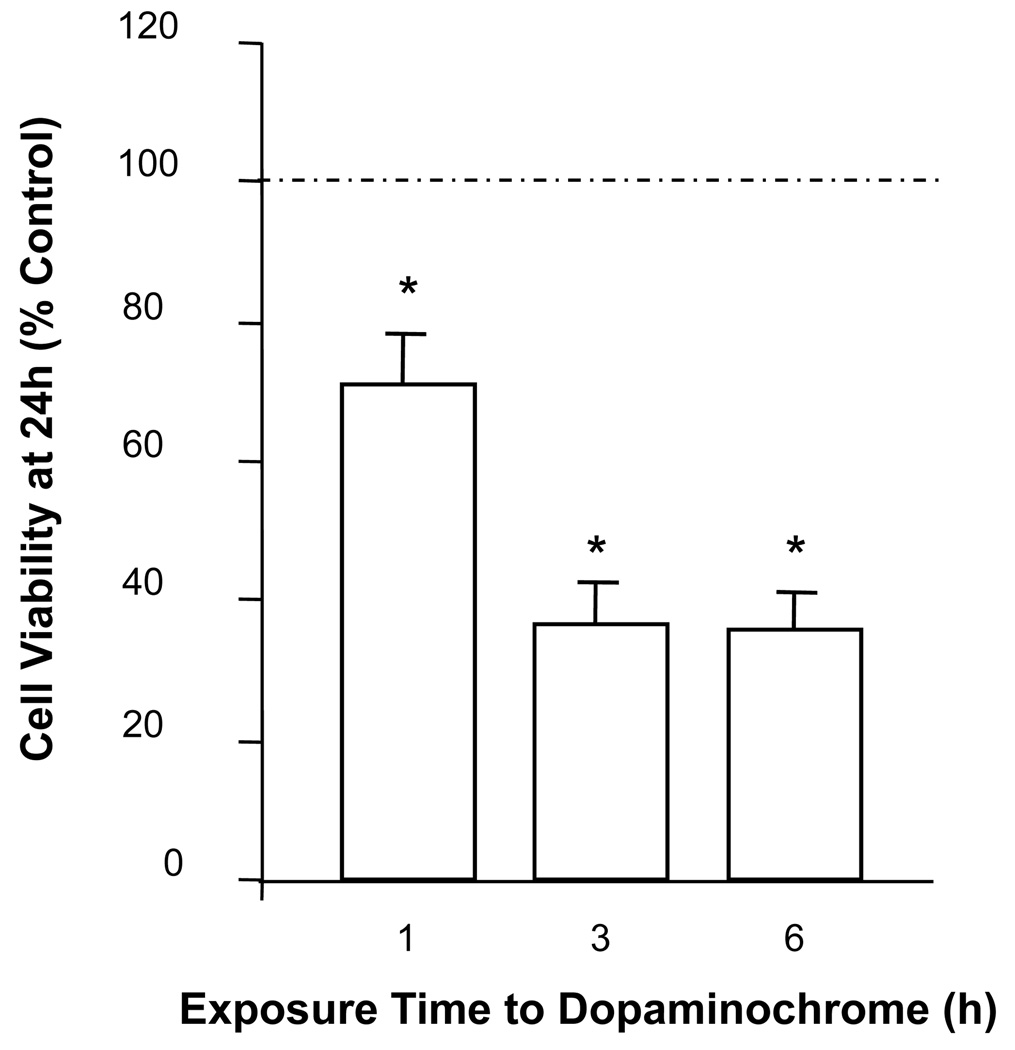
In order to establish the minimum length of cytotoxic exposure time to DAC, MN9D cells were incubated with DAC [175 µM] for 1, 3 or 6 h and, after replacement of DAC-treated media with DAC-free media, evaluated at 24 h for viability. Incubation with DAC for as little as 1 h is enough to induce significant cytotoxicity when observed at 24 h, and this toxicity is maximized by exposure for 3 h (Figure 2).
Figure 2. Time-dependent cytotoxicity of MN9D cells exposed to dopaminochrome.
Exposure of MN9D cells to DAC [175 µM] for 1, 3 or 6 h reduces cell viability at 24 h as measured by the MTS assay. (n = 40 wells from 4 individual experiments) *P<0.05 compared to control.
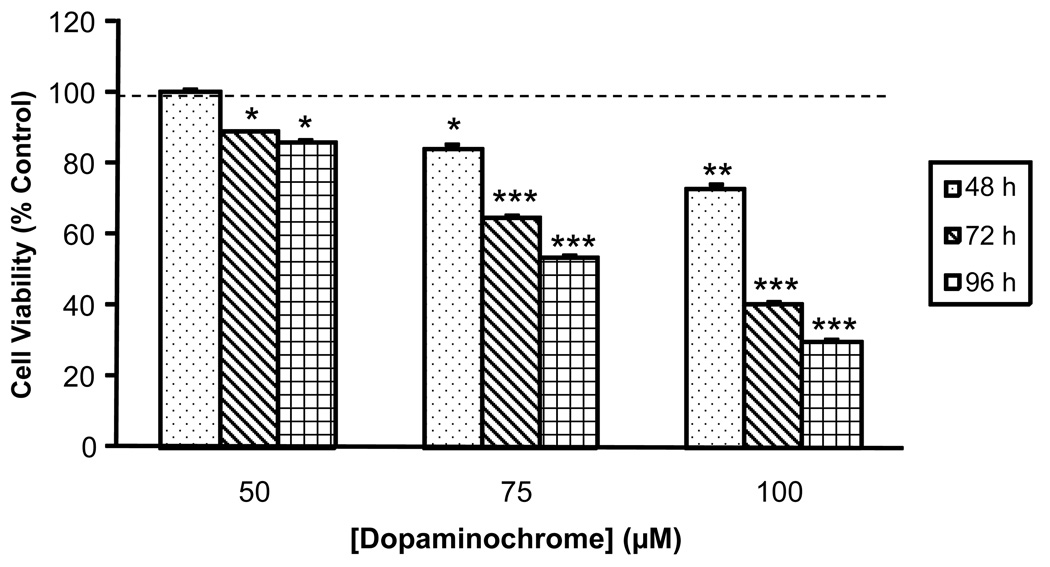
MN9D cells were incubated with concentrations of DAC [50–100 µM] that were either moderately toxic or nontoxic at 24 h, and cell viability was assessed at the end of 48, 72 or 96 h. In all experiments, MN9D cells were given fresh DMEM containing the appropriate concentration of DAC every 24 h, thereby allowing the cells to be exposed to DAC in a chronic fashion. MN9D cells exposed to 50 µM DAC exhibited no toxicity at 48 h, however by 96 h they had decreased in viability by 14%. MN9D cells given 100 µM DAC exhibited a decrease in viability of over 60% at 96 h (Figure 3).
Figure 3. Cytotoxic effects of chronic exposure of MN9D cells to low concentrations of dopaminochrome.
MN9D cells were treated for 48, 72 or 96 h (dotted, diagonal or hatched bars, respectively) with low concentrations of DAC [50, 75 or 100 µM]. Experimental media was replaced on each day with fresh media containing fresh DAC at the appropriate concentrations and evaluated at the end of the trial period for cell viability by the MTS assay. All three treatments of DAC resulted in decreasing viability of MN9D cells over 96 h. (n = 36 wells from 4 individual experiments) *P<0.05 compared with control cells; **P<0.01 compared with control cells; ***P<0.001 compared with control cells.
Taken together, these data demonstrate that exposure to DAC leads to cell death in a concentration-dependent manner in MN9D cell cultures. Furthermore, the initiation of cytotoxicity occurs rapidly since replacement of DAC-treated media with fresh media after a relatively short exposure time is ineffective at rescuing MN9D cells from progression to cell death. Finally, chronic exposure of MN9D cells to low concentrations of DAC decreases viability over a period of 96 h.
Cytotoxicity by dopaminochrome is dependent upon oxidative stress
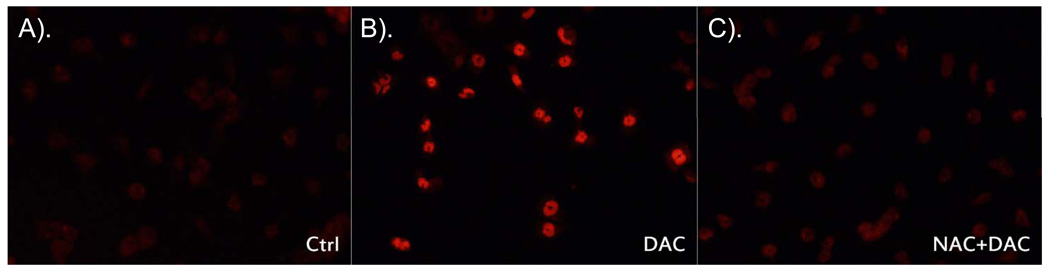
Given the correlation between neurodegeneration and oxidative stress, we wanted to evaluate whether DAC exposure increased oxidative stress in MN9D cells. To investigate this, MN9D cells were incubated for 2 h with DAC [175 µM] and then exposed to dihydroethidium (DHE; the fluorescent redox indicator for O2−) for 30 min, after which they were fixed with 4% paraformaldehyde and visualized by fluorescence microscopy. MN9D cells exposed to DAC exhibited enhanced fluorescence when compared with control cells (Figure 4). The increase in DHE fluorescence in response to DAC was prevented by co-administration of the general antioxidant, N-acetylcysteine [5 mM].
Figure 4. Dopaminochrome induces superoxide production in MN9D cells.
DAC [175 µM] caused an increase in superoxide in MN9D cells after 2 h as visualized by dihydroethidium [DHE; 10 µg/mL] fluorescence (B) compared with control (A). Images shown are representative of 3 individual experiments. The increase in DHE fluorescence can be prevented by co-administration with the antioxidant, N-acetylcysteine [5 mM] (C).
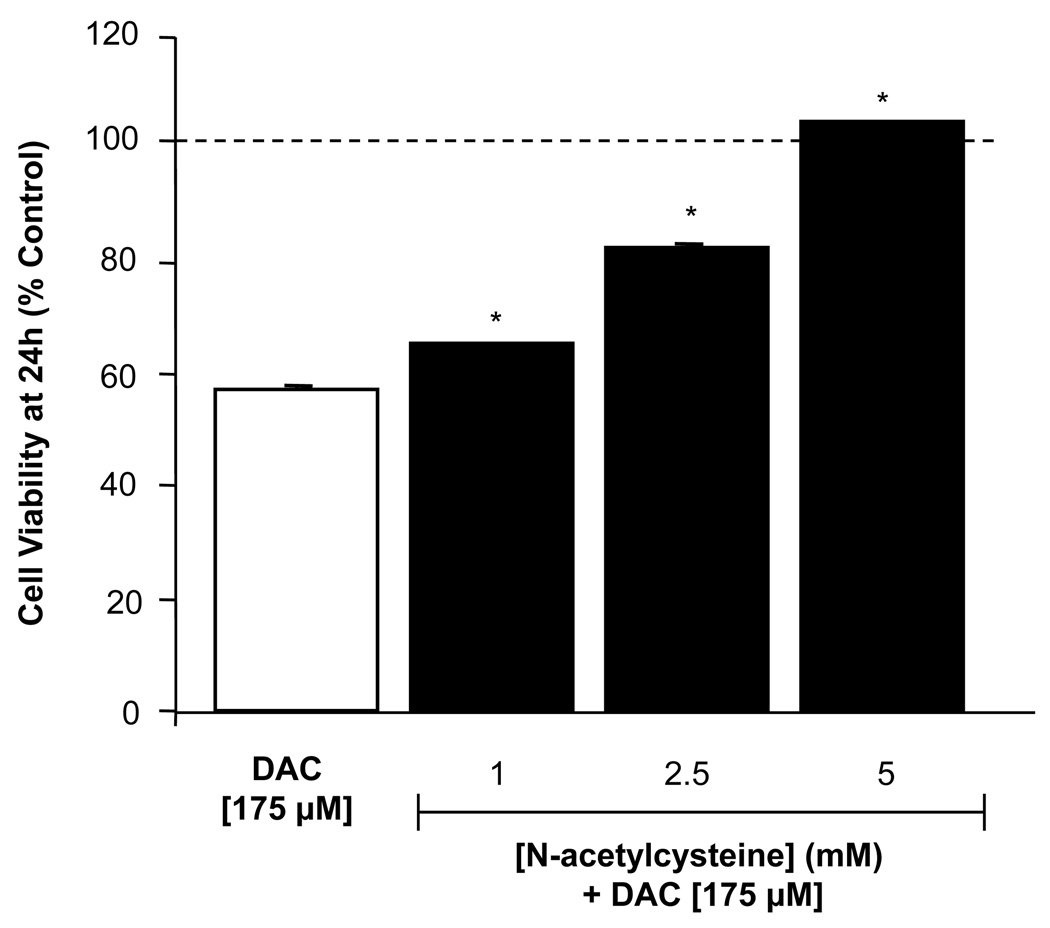
In parallel experiments, MN9D cells were pre-treated for 1 h with N-acetylcysteine [1–5 mM] and then treated for an additional 24 h with DAC [175 µM]. DAC-induced cytotoxicity in MN9D cells was attenuated in the presence of N-acetylcysteine, indicating that the observed cell death was related to an increase in oxidative stress (Figure 5). N-acetylcysteine was chosen not only because it is a potent general antioxidant, but also because it is thiol-based in its action and thus likely mimics the actions of the endogenous thiol antioxidant, glutathione. In separate experiments, we confirmed that N-acetylcysteine does not react with DAC directly, as incubation of equimolar concentrations of N-acetylcysteine and DAC does not reduce the concentration of DAC when verified by high-pressure liquid chromatography with electrochemical detection (not shown).
Figure 5. N-acetylcysteine prevents dopaminochrome cytotoxicity in MN9D cells.
N-acetylcysteine [1 – 5 mM; solid bars] was administered to MN9D cell cultures 1 h prior to DAC [175 µM] and incubated for 24 h. The presence of N-acetylcysteine prevented the decrease in cell viability observed by DAC alone [open bar]. (n=84 wells from 4 individual experiments). *P<0.05 compared with control cells.
These data indicate that DAC leads to an increase in superoxide, the appearance of which can be reduced with a thiol-based antioxidant, N-acetylcysteine. Furthermore, the addition of N-acetylcysteine to MN9D cells also prevents the toxicity that DAC would normally have, demonstrating that the death observed is linked to production of superoxide in the cells.
Cytotoxicity by dopaminochrome is dependent upon glutathione system integrity
The choice of N-acetylcysteine as an antioxidant was intended as an appropriate analog of the endogenous thiol-based antioxidant of neurons, glutathione. Reduced glutathione is necessary for protecting against damage induced by superoxide, hydrogen peroxide, and other reactive oxygen species. As N-acetylcysteine was very effective in protecting against DAC-induced cell death, we investigated the role of glutathione in the actions of DAC.
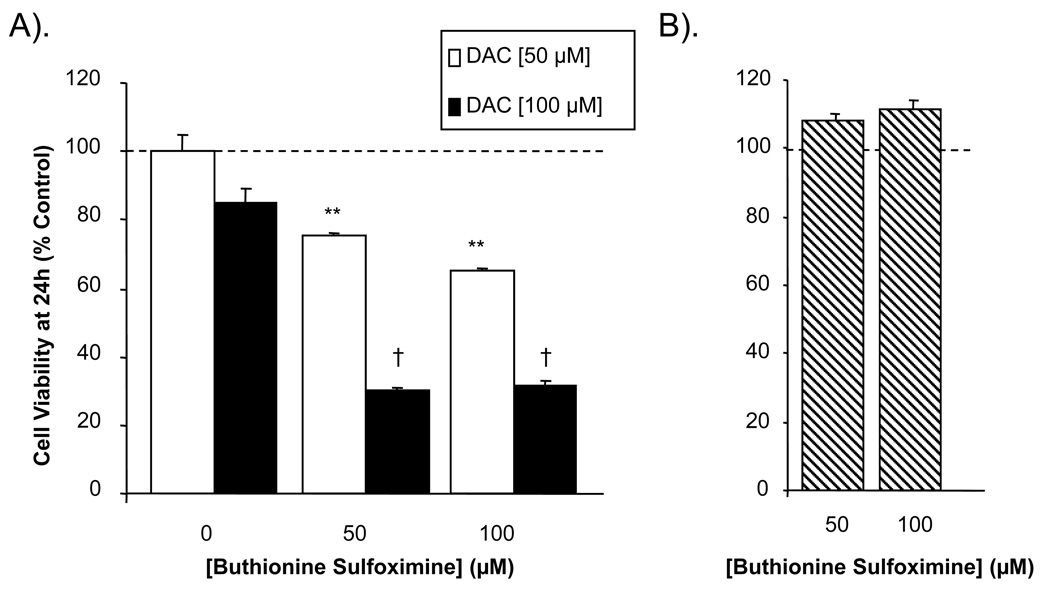
Intracellular stores of reduced glutathione (GSH) were depleted by incubating MN9D cells with buthionine sulfoximine [BSO; 50 and 100 µM] for 24 h. Thereafter, the cells were exposed to DAC [50 and 100 µM; concentrations that previously showed moderate or no toxicity at 24 h] for an additional 24 h, at which time cell viability was assessed. Pre-treatment of the MN9D cells with either concentration of BSO led to a significant decrease in cell viability in the presence of DAC compared with control cells (no DAC), or cells given DAC without BSO (Figure 6A). BSO alone yielded no significant effect on the viability of MN9D cells (Figure 6B).
Figure 6. Depletion of intracellular glutathione potentiates the cytotoxic effect of dopaminochrome on MN9D cells.
(A) Pre-treatment of MN9D cells with buthionine sulfoximine [BSO; 50 or 100 µM] potentiated the cytotoxic effects of DAC [50 or 100 µM, open or solid bars respectively] at 24 h. (B) Treatment of MN9D cells with BSO alone [50 or 100 µM, hatched bars] had no effect on cell viability. (n=28 wells from 4 individual experiments) ***P<0.001 compared with 50 µM DAC and no BSO; † P<0.001 compared with 100 µM DAC and no BSO.
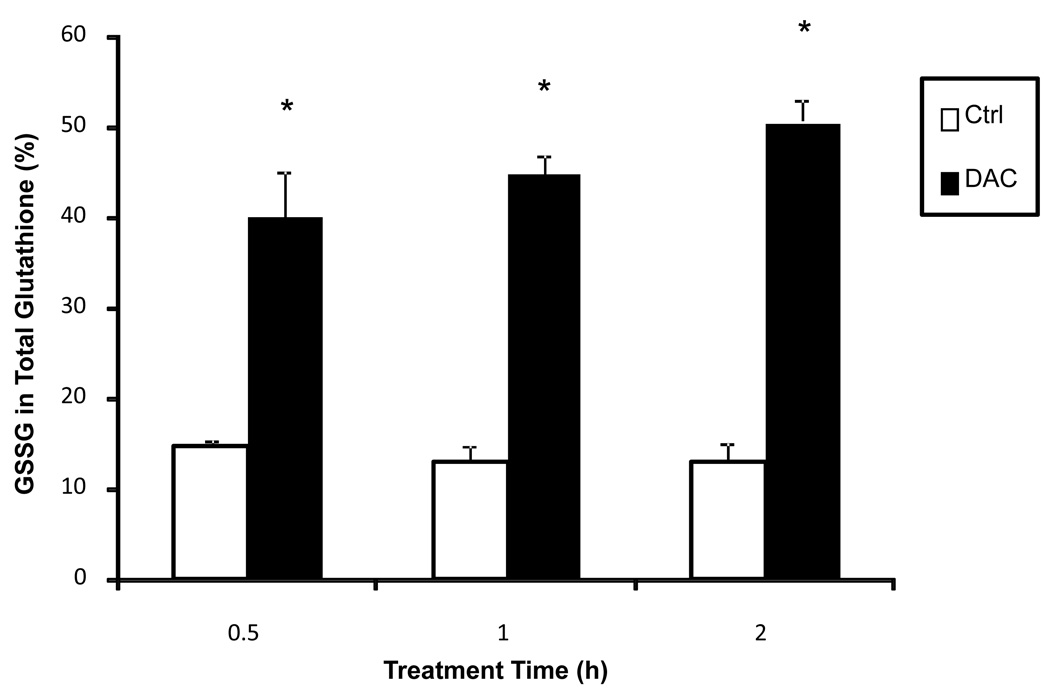
The ability to affect cellular glutathione levels was then assessed as the proportion of oxidized glutathione (GSSG) in total cellular glutathione. MN9D cells were treated with DAC [175 µM] for 0.5, 1 or 2 h and then harvested for analysis. After 0.5 h, DAC induced a decrease in total glutathione [83.82 ± 10.96 to 58.89 ± 11.42 nmol/mg protein] within MN9D cells and an increase in GSSG [14.77 ± 0.714 to 40.09 ± 5.251 nmol/mg protein]. Overall, the percentage of GSSG in total cellular glutathione increased from 13% to 50% over a period of two hours (Figure 7).
Figure 7. Dopaminochrome increases oxidized glutathione in MN9D cells.
The proportion of oxidized glutathione (GSSG) in total cellular glutathione within MN9D cells increased over control cells (open bars) after incubation with DAC [175 µM, closed bars] for 0.5, 1 or 2 h. (n=3) *P<0.05 compared to control cells.
These data implicate the glutathione system as being integral to the survival of MN9D cells when exposed to DAC. Not only are low concentrations of DAC capable of causing cell death after depletion of the reduced glutathione pool, but DAC itself causes a rapid loss of reduced glutathione. Taken together from these data, DAC administration results in the compromise of the endogenous glutathione system.
Discussion
The causes of idiopathic Parkinson disease (PD) have remained elusive, but there is strong evidence available as to the presence and potential pathogenesis of oxidized catecholamine products within the mesencephalic neurons that degenerate during the course of the disease. Although the toxicity of DAC and similar compounds (e.g. adrenochrome in myocardium) has been demonstrated in other cell types (Singal et al., 1982; Taam et al., 1986), here we present data showing that DAC reduces the levels of glutathione within a mesencephalic cell culture model, MN9D, indicating that DAC may play a role in neurodegeneration.
The presence of neuromelanin in dopaminergic neurons is evidence for DAC formation. The question remains as to whether DAC is cytotoxic if left unpolymerized. Indeed, it has been suggested that neuromelanin formation is protective to neuronal populations in the mesencephalon (Zecca et al., 2006). Our results show that DAC is indeed cytotoxic to MN9D cells, whereas dopachrome, the oxidation product of dopamine’s precursor, l-dopa, is only toxic at the highest concentration evaluated. It has been estimated that nearly 90% of dopamine is stored within the vesicles of dopaminergic neurons, protecting the neurotransmitter from oxidation (Caudle et al., 2007). The remaining dopamine, accumulating over time in the cytosol of these neurons, is mostly polymerized and stored as neuromelanin, but there is a possibility that these products could cause cellular damage in their unpolymerized form.
The initiator for oxidation of dopamine to DAC is of some debate. In our experience, DAC left in ddH2O polymerizes at room temperature to form a neuromelanin-like product; the reaction speed is increased when carried out in phosphate buffered saline. In the physiological setting, dopamine can be oxidized by a variety of mediators found within the cytosol, including superoxide, peroxynitrite, hydrogen peroxide, and heavy metal ions like iron and manganese. Indeed, it is possible that the oxidative stress resulting from exposure to MPTP or rotenone in cell culture or in vivo is not only due to disruption of mitochondrial electron transport, but may also be in part caused by DAC formation (Kweon et al., 2004; Zoccarato et al., 2005).
Our results show that MN9D cells incubated with DAC exhibit increases in the production of superoxide, as visualized by DHE fluorescence. Superoxide is a well-known oxidant that is associated with oxidative stress in most cell types (Halliwell, 1992). Co-administration of DAC with the general antioxidant, N-acetylcysteine, yielded improved viability in MN9D cells indicating that the observed cytotoxicity in presence of DAC was directly related to the increase in superoxide levels within the cell.
Parkinson disease has been associated with a decrease in glutathione levels in the SNpc, as observed in post-mortem studies (Perry et al., 1982). Our results support the idea that DAC could be a candidate for the depletion of glutathione levels within mesencephalic cells. MN9D cells were incubated with a concentration of DAC that caused 50% cell death and then tested for internal total glutathione and oxidized glutathione (GSSG) levels, the ratio of which is used as a measure of oxidative stress (Chinta et al., 2007). Typically, a neuron requires more GSH than GSSG, leaving an available pool from which GSH can be drawn to protect against oxidative insults should they arise. A rapid decrease in GSH levels must be followed by replenishment of the pool, otherwise the cell risks irreversible damage. The data presented here implicate the glutathione system in the neurodegeneration caused by DAC. First, pre-treatment of MN9D cells with buthionine sulfoximine (BSO), a glutathione synthase inhibitor, potentiates the effects of DAC where concentrations of the toxin that were previously nontoxic are rendered harmful. Treatment of MN9D cells with BSO alone was nontoxic, indicating that the depletion of GSH in of itself was not enough to cause cytotoxicity, yet co-treatment with BSO and DAC was greatly effective. Second, DAC rapidly depletes GSH in MN9D cells in as little as 30 min. The results from our dihydroethidium experiments suggest that superoxide may play a role in this depletion, as both are occurring simultaneously and GSH and other thiol-based antioxidants are important to its removal. Other toxins used in neurodegeneration studies, including rotenone, have also been shown to decrease GSH levels as a part of their toxic effects (Sherer et al., 2003). From these observations, it appears that DAC, by increasing intracellular superoxide, can deplete the available GSH pool, thus rendering the cell vulnerable against further DAC insult.
The data presented here reveal a direct relationship between DAC and glutathione depletion in vitro. Parkinson disease patients frequently exhibit depleted levels of reduced glutathione, and our results show that DAC could be a possible cause of this pathology. Glutathione has been implicated in removal of the o-quinone precursor to DAC, dopamine-o-quinone, by action of glutathione-S-transferase, effectively detoxifying the compound before it causes oxidative damage (Baez et al., 1997). Our work here clearly shows that the product of further dopamine oxidation, DAC, also depletes glutathione, but through an as yet unidentified mechanism, although superoxide production appears to be involved. While the o-quinone form readily binds to the sulfur group of glutathione, the aminochrome form is incapable (Zoccarato et al., 2005). Once cyclized, the aminochrome form is also unable to return to its precursor, the o-quinone (Graham, 1978).
The presence of DAC in MN9D cells causes an increase in oxidative stress that requires the presence of a readily available pool of GSH. If this pool is depleted, either rapidly or steadily decreased over time, the cells become less capable of handling even the low concentrations of DAC that cells would otherwise be able to counteract. Indeed, the chronic administration of DAC to MN9D cells, even at relatively low concentrations, still leads to cell death, perhaps because reduced glutathione is constantly being depleted (Figure 3). The protective benefits of co-administration of N-acetylcysteine with DAC (Figure 5) illustrate that replacement of the GSH pool with a thiol-based analog is effective at protecting against cytotoxicity.
The site of action for DAC remains unknown. The experiments outlined here utilized DAC that is applied extracellularly to MN9D cells. Experiments from our lab suggests that 3H-dopamine that has been oxidized to form 3H-dopaminochrome is able to pass through the dopamine transporter on the cell membrane, yet in separate experiments pharmacological blockade of this transporter with GBR-12935 is unable to prevent cytotoxicity (unpublished observations), suggesting an alternative site of action for DAC in this process.
The oxidation of dopamine to DAC has been well-characterized in vitro by a variety of methods (Bisaglia et al., 2007; Segura-Aguilar et al., 2001). In addition, we, in collaboration with Dr. Julie Andersen’s laboratory, have measured DAC formation in vivo (Mallajosyula et al., 2008) in an oxidatively stressed mouse model of neurodegeneration. Many groups have suggested that the oxidation products of catecholamines, in general, may be detrimental to cells in vivo, but direct analysis of the role of each oxidized species remains elusive. In this study, we sought to further establish the potential role for DAC and the mechanisms by which it may be causing neurodegeneration. Our data demonstrate that exposure of MN9D cells to DAC leads to cytotoxicity in a manner consistent with increased oxidative stress and depletion of glutathione stores raising the possibility that oxidized dopamine itself has a role in the neurodegeneration observed in Parkinsonian patients.
Acknowledgements
We wish to thank Drs. Al Heller and Lisa Won from the University of Chicago for the kind gift of MN9D cells used in these experiments.
Grant Support
This work was supported in part by NIGMS 008306.
Abbreviations
- DAC
Dopaminochrome
- SNpc
Substantia Nigra pars compacta
- PD
Parkinson disease
- GSH
Reduced Glutathione
- GSSG
Oxidized Glutathione
- DHE
dihydroethidium
- DMEM
Dulbecco’s Modified Eagle’s Medium.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
The authors declare that there are no conflicts of interest.
References
- Baez S, Segura-Aguilar J, Widersten M, Johansson AS, Mannervik B. Glutathione transferases catalyse the detoxication of oxidized metabolites (o-quinones) of catecholamines and may serve as an antioxidant system preventing degenerative cellular processes. The Biochemical journal. 1997;324(Pt 1):25–28. doi: 10.1042/bj3240025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisaglia M, Mammi S, Bubacco L. Kinetic and structural analysis of the early oxidation products of dopamine: Analysis of the interactions with alpha-synuclein. The Journal of biological chemistry. 2007;282:15597–15605. doi: 10.1074/jbc.M610893200. [DOI] [PubMed] [Google Scholar]
- Caudle WM, Richardson JR, Wang MZ, Taylor TN, Guillot TS, McCormack AL, Colebrooke RE, Di Monte DA, Emson PC, Miller GW. Reduced vesicular storage of dopamine causes progressive nigrostriatal neurodegeneration. J Neurosci. 2007;27:8138–8148. doi: 10.1523/JNEUROSCI.0319-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinta SJ, Kumar MJ, Hsu M, Rajagopalan S, Kaur D, Rane A, Nicholls DG, Choi J, Andersen JK. Inducible alterations of glutathione levels in adult dopaminergic midbrain neurons result in nigrostriatal degeneration. J Neurosci. 2007;27:13997–4006. doi: 10.1523/JNEUROSCI.3885-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi HK, Won L, Roback JD, Wainer BH, Heller A. Specific modulation of dopamine expression in neuronal hybrid cells by primary cells from different brain regions. Proc Natl Acad Sci U S A. 1992;89:8943–8947. doi: 10.1073/pnas.89.19.8943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebadi M, Srinivasan SK, Baxi MD. Oxidative stress and antioxidant therapy in parkinson's disease. Progress in neurobiology. 1996;48:1–19. doi: 10.1016/0301-0082(95)00029-1. [DOI] [PubMed] [Google Scholar]
- Foley P, Riederer P. Influence of neurotoxins and oxidative stress on the onset and progression of parkinson's disease. J Neurol. 2000;247 Suppl 2:II82–II94. doi: 10.1007/pl00007766. [DOI] [PubMed] [Google Scholar]
- Graham DG. Oxidative pathways for catecholamines in the genesis of neuromelanin and cytotoxic quinones. Mol Pharmacol. 1978;14:633–643. [PubMed] [Google Scholar]
- Halliwell B. Reactive oxygen species and the central nervous system. Journal of neurochemistry. 1992;59:1609–1623. doi: 10.1111/j.1471-4159.1992.tb10990.x. [DOI] [PubMed] [Google Scholar]
- Heikkila RE, Nicklas WJ, Vyas I, Duvoisin RC. Dopaminergic toxicity of rotenone and the 1-methyl-4-phenylpyridinium ion after their stereotaxic administration to rats: Implication for the mechanism of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine toxicity. Neuroscience letters. 1985;62:389–394. doi: 10.1016/0304-3940(85)90580-4. [DOI] [PubMed] [Google Scholar]
- Koutsilieri E, Scheller C, Grunblatt E, Nara K, Li J, Riederer P. Free radicals in parkinson's disease. J Neurol. 2002;249 Suppl 2:II1–II5. doi: 10.1007/s00415-002-1201-7. [DOI] [PubMed] [Google Scholar]
- Kweon GR, Marks JD, Krencik R, Leung EH, Schumacker PT, Hyland K, Kang UJ. Distinct mechanisms of neurodegeneration induced by chronic complex i inhibition in dopaminergic and non-dopaminergic cells. The Journal of biological chemistry. 2004;279:51783–51792. doi: 10.1074/jbc.M407336200. [DOI] [PubMed] [Google Scholar]
- Mallajosyula JK, Kaur D, Chinta SJ, Rajagopalan S, Rane A, Nicholls DG, Di Monte DA, Macarthur H, Andersen JK. Mao-b elevation in mouse brain astrocytes results in parkinson's pathology. PLoS ONE. 2008;3:e1616. doi: 10.1371/journal.pone.0001616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochs SD, Westfall TC, Macarthur H. The separation and quantification of aminochromes using high-pressure liquid chromatography with electrochemical detection. Journal of neuroscience methods. 2005;142:201–208. doi: 10.1016/j.jneumeth.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Perry TL, Godin DV, Hansen S. Parkinson's disease: A disorder due to nigral glutathione deficiency? Neuroscience letters. 1982;33:305–310. doi: 10.1016/0304-3940(82)90390-1. [DOI] [PubMed] [Google Scholar]
- Segura-Aguilar J, Metodiewa D, Baez S. The possible role of one-electron reduction of aminochrome in the neurodegenerative process of the dopaminergic system. Neurotoxicity research. 2001;3:157–165. doi: 10.1007/BF03033188. [DOI] [PubMed] [Google Scholar]
- Sherer TB, Betarbet R, Testa CM, Seo BB, Richardson JR, Kim JH, Miller GW, Yagi T, Matsuno-Yagi A, Greenamyre JT. Mechanism of toxicity in rotenone models of parkinson's disease. J Neurosci. 2003;23:10756–10764. doi: 10.1523/JNEUROSCI.23-34-10756.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singal PK, Dhillon KS, Beamish RE, Kapur N, Dhalla NS. Myocardial cell damage and cardiovascular changes due to i.V. Infusion of adrenochrome in rats. Br J Exp Pathol. 1982;63:167–176. [PMC free article] [PubMed] [Google Scholar]
- Sulzer D, Bogulavsky J, Larsen KE, Behr G, Karatekin E, Kleinman MH, Turro N, Krantz D, Edwards RH, Greene LA, Zecca L. Neuromelanin biosynthesis is driven by excess cytosolic catecholamines not accumulated by synaptic vesicles. Proc Natl Acad Sci U S A. 2000;97:11869–11874. doi: 10.1073/pnas.97.22.11869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taam GM, Takeo S, Ziegelhoffer A, Singal PK, Beamish RE, Dhalla NS. Effect of adrenochrome on adenine nucleotides and mitochondrial oxidative phosphorylation in rat heart. Can J Cardiol. 1986;6(2):88–93. [PubMed] [Google Scholar]
- Thiffault C, Langston JW, Di Monte DA. Increased striatal dopamine turnover following acute administration of rotenone to mice. Brain Res. 2000;885:283–288. doi: 10.1016/s0006-8993(00)02960-7. [DOI] [PubMed] [Google Scholar]
- Tipton KF, Singer TP. Advances in our understanding of the mechanisms of the neurotoxicity of mptp related compounds. Journal of neurochemistry. 1993;61:1191–1206. doi: 10.1111/j.1471-4159.1993.tb13610.x. [DOI] [PubMed] [Google Scholar]
- Zecca L, Zucca FA, Albertini A, Rizzio E, Fariello RG. A proposed dual role of neuromelanin in the pathogenesis of parkinson's disease. Neurology. 2006;67(6):S8–S11. doi: 10.1212/wnl.67.7_suppl_2.s8. [DOI] [PubMed] [Google Scholar]
- Zhao H, Kalivendi S, Zhang H, Joseph J, Nithipatikom K, Vasquez-Vivar J, Kalyanaraman B. Superoxide reacts with hydroethidine but forms a fluorescent product that is distinctly different from ethidium: Potential implications in intracellular fluorescence detection of superoxide. Free radical biology & medicine. 2003;34:1359–1368. doi: 10.1016/s0891-5849(03)00142-4. [DOI] [PubMed] [Google Scholar]
- Zoccarato F, Toscano P, Alexandre A. Dopamine-derived dopaminochrome promotes H(2)O(2) release at mitochondrial complex i: Stimulation by rotenone, control by ca(2+), and relevance to parkinson disease. The Journal of biological chemistry. 2005;280:15587–15594. doi: 10.1074/jbc.M500657200. [DOI] [PubMed] [Google Scholar]