Introduction
The prevalence of type 2 diabetes mellitus (DM2) is increasing precipitously as a consequence of the aging population and obesity epidemic. Type 2 diabetes mellitus is characterized by both pancreatic beta cell dysfunction and insulin resistance in multiple tissues, abnormalities which precede and predict the development of DM2 [1, 2]. Because of its large mass, skeletal muscle accounts for the majority of insulin mediated glucose uptake in peripheral tissue [3, 4]. Thus, understanding the mechanism by which insulin resistance develops in skeletal muscle may offer insight into potential therapies for the treatment or prevention of DM2.
Abnormalities in skeletal muscle lipid metabolism and mitochondrial dysfunction have been proposed as possible mechanisms for skeletal muscle insulin resistance [5]. In multiple studies of untrained subjects, elevation of intramyocellular lipid (IMCL) correlates with insulin resistance [6-8]. Lipid infusion induces insulin resistance several hours after FFA elevation [9, 10]. In humans, insulin resistance has been associated with diminished mitochondrial function [8, 11, 12], reduced expression of oxidative metabolism genes [13, 14], decreased mitochondrial size [15], and reduced mitochondrial density [15].
The purpose of this review is to explore the complex relationship between local lipid exposure, mitochondrial dysfunction, and insulin resistance at the level of human skeletal muscle. The focus will be on human studies although selected animal and cell-based studies will be presented for more detailed insights into pathophysiology. We begin with a brief overview of the normal physiology of insulin mediated glucose disposal and the associated abnormalities observed in insulin resistance. We will then discuss the evidence for local lipid excess contributing to skeletal muscle insulin resistance. Next, we will briefly review the relationship between skeletal muscle mitochondrial function and skeletal muscle insulin resistance with a focus on local lipid excess as a cause and/or consequence of mitochondrial dysfunction. After reviewing the role of lipid metabolism in skeletal muscle insulin resistance, we will present the concept that multiple abnormalities of non-muscle tissues and “cross talk” between tissues also contributes to the insulin insensitivity of skeletal muscle. Our major goal is to illustrate the conflicting nature of the evidence with regard to the biochemical pathogenesis of DM2 and, thereby, to highlight the difficulties in determining a causal relationships between lipid excess, insulin resistance and mitochondrial function in skeletal muscle.
Mechanism of Skeletal Muscle Insulin Resistance
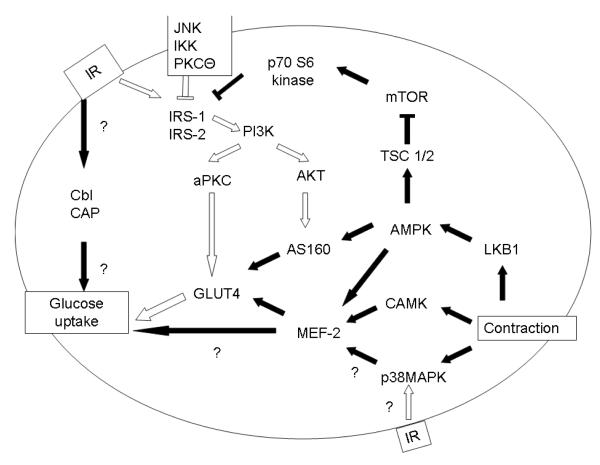
Skeletal muscle is the largest site of insulin mediated glucose disposal in the human body [3, 4]. The physiology of skeletal muscle glucose uptake is shown in Figure 1. Normally, the binding of insulin to the insulin receptor stimulates autophosphorylation of tyrosine residues [16, 17] and subsequent activation of a receptor tyrosine kinase. This tyrosine kinase phosphorylates multiple intracellular substrates, including insulin receptor substrate (IRS) 1 [18] and 2 [19], which play significant roles in the insulin response [20]. The insulin response is mediated by IRS activation of phosphatidylinositol 3-kinase (PI 3-Kinase), a critical player in insulin signaling [18, 21-23] particularly with regards to glucose homeostasis [21, 24]. PI 3-Kinase facilitates the translocation of the insulin responsive glucose transporter (GLUT4) to the plasma membrane [25-27] through a mechanism likely mediated by phosphorylation of protein kinase B (AKT) [28], and/or an atypical protein kinase C (aPKC) [29, 30]. The translocation of GLUT 4 from the intracellular pool to the plasma membrane plays a crucial role in insulin mediated glucose entry into the skeletal muscle [31]. Current evidence suggests a minimal role for insulin stimulating the ecotropic retroviral transforming sequence homolog (Cbl) and Cbl-associated pathway in mediating skeletal muscle glucose uptake [32, 33]
Figure 1.
Glucose uptake in Skeletal Muscle
The insulin receptor is phosphorylated at a tyrosine residue, which then activates tyrosine phosphorylation of IRS-1 and IRS-2. This allows the activation of PI 3-Kinase, which proceeds through either an atypical PKC or AKT mechanism to activate GLUT4 translocation. In insulin resistance (white arrows), decreased tyrosine phosphorylation of the insulin receptor, increased serine/threonine phosphorylation of IRS-1/IRS2, and decreased PI3K activity leads to decreased GLUT4 translocation. IKK, JNK, mTOR and PKCΘ are serine/threonine kinases which act on IRS-1 to inhibit IRS-1 activity. Insulin resistance may also impair p38MAPK response to insulin without affecting p38MAPK response to contraction
Contraction mediates skeletal muscle glucose uptake through activation of p38MAPK, CAMK, and AMPK. Intersection of contraction mediated glucose uptake and insulin mediated glucose uptake include effects at IRS-1, AS160 and p38MAPK.
Contraction is another critical mediator of skeletal glucose uptake. Contraction stimulates glucose uptake through activation of p38 mitogen activated protein kinase (p38MAPK) [34] [35], calmodulin dependent kinase (CAMK) [36, 37], and AMP activated protein kinase (AMPK) [38] via a protein kinase (LKB1) [39]. Enhanced glucose uptake from contraction appears to be due to increased GLUT-4 translocation due to several mediators: activation of myocyte enhancer factor-2 (MEF-2) [36, 37, 40], activation of a Rab GTPase-activating protein (AS160) [41-43] and improved insulin signaling [44], possibly through the tuberous sclerosis complex (TSC) [45] inhibitory effects on the mammalian target of rapamycin (mTOR) [46] and p70 S6 kinase [46, 47].
The convergence of contraction mediated glucose uptake and insulin mediated glucose uptake has been investigated. Insulin has been shown to stimulate p38MAPK activation [34]. AMPK activation has been shown to improve insulin signaling [44]. There is significant evidence that AS160 activation regulates both contraction mediated glucose uptake and insulin mediated glucose uptake [42, 43]
Skeletal muscle insulin resistance is characterized by impaired glucose uptake [4] resulting from impaired insulin receptor signaling [48-50]. Subjects with obesity [49] and subjects with DM2 [50] exhibit reduced IRS-1 tyrosine phosphorylation and reduced PI 3-Kinase activity compared with their respective controls. The reduction in tyrosine phosphorylation of IRS-1 and IRS-2 has been related to increased serine/threonine phosphorylation of IRS-1 and IRS-2 [51]. Proposed IRS serine/theronine kinases include inhibitor kappa B kinase (IKK) [52], c-Jun amino-terminal kinases (JNK) [53, 54], mTOR [55, 56], and protein kinase C isoforms [57, 58]. As a result of IRS serine/threonine phosphorylation, PI 3-Kinase levels are reduced which subsequently alters downstream effectors, i.e., decreased activity of aPKC [59-61] and possibly AKT [62-65] that decreases glucose uptake, presumably by reduced GLUT4 activity/translocation [5, 66, 67] A mouse model of insulin resistance (ob/ob mice) has demonstrated insulin resistance associated with impaired p38MAPK response to insulin but preserved p38MAPK response to contraction [68] (Figure 1).
Is Lipotoxicity a Mediator of Skeletal Muscle Insulin Resistance?
Lipotoxicity, defined as the elevation of lipids and/or lipid metabolites within blood or tissues with subsequent metabolic derangement, is a postulated mechanism for skeletal muscle insulin resistance. In humans, the normal plasma fasting free fatty acid (FFA) concentration ranges from 350 to 550 umol/L [69, 70] and increases with prolonged fasting [69, 71], obesity [69, 72] insulin resistance [72] and DM2 [73]. As the level of insulin resistance in untrained subjects correlates strongly with IMCL content as measured by muscle biopsy [74] or magnetic resonance imaging (MRI) [6], prolonged FFA exposure was initially hypothesized to increase skeletal muscle insulin resistance by increasing IMCL content.
This hypothesis was supported by findings from human studies involving lipid infusion. Lipid infusion has been shown to increase IMCL content [75, 76] and inhibit insulin stimulated glucose uptake in subjects who are healthy [77] and in subjects with DM2 [10]. The inhibition of insulin stimulated glucose uptake inversely correlates with the increased FFA levels produced by the lipid infusion [78]. An increase of plasma FFA levels to approximately 700 umol/l has been shown to disrupt insulin signaling by inhibiting insulin receptor tyrosine phosphorylation, IRS-1 tyrosine phosphorylation, PI 3-Kinase activity, and AKT serine phosphorylation [78]. In healthy human subjects, the onset of lipid infusion induced insulin resistance (steady state FFA of approximately 1200 umol/l) was associated with a concurrent increase in activity of protein kinase C, an increase in the level of diacylglycerol (DAG) and a decrease in the level of IkappaB-α, an inhibitor of the nuclear factor—kappa B pathway [79]. Animal studies have reported that lipid infusion induced insulin resistance is associated with activation of serine kinases such as protein kinase Θ [58, 80] and inhibitor kappa B kinase—beta (IKKB) , an activator of nuclear factor—kappa B pathway [81]. Thus, short term exposure to a lipid substrate-rich environment (i.e. lipid infusion) stimulates skeletal muscle to shift substrate use towards fatty acids, with the consequence of inducing skeletal muscle insulin resistance and reduction of glucose uptake.
Although lipid infusion is a well documented method of producing skeletal muscle insulin resistance [10, 77], the evidence is less conclusive for high fat diets producing insulin resistance. In theory, a high fat diet should elevate blood triglyceride and FFA levels to increase skeletal muscle fatty acid accumulation and increase insulin resistance. Although animal studies have shown that chronic high fat feeding increases insulin resistance [82, 83], the data from human studies are contradictory [76 , 84, 85, 86 , 87, 88]. A portion of the uncertainty in human studies likely relates to the metabolic effects of dietary fat composition and quantity. When compared with subjects on a control diet, subjects given a Mediterranean-style diet (low in saturated fat and rich in monounsaturated and polyunsaturated fats) for 2 years reduced their weight (−5%), decreased insulin resistance (as measured by homeostasis model assessment), improved endothelial function (as measured by the L-arginine test) and lowered systemic inflammatory markers (as measured by high sensitivity-C reactive protein, Interleukin 6, Interleukin 7, Interleukin 18) [89]. A prospective cohort study (4.4 years duration) of 13380 subjects found that participants who were the most adherent to a Mediterranean diet had a lower incidence of diabetes (0.41, 95% CI of 0.19 to 0.87) compared to participants who were least adherent to a Mediterranean diet [90]. Unfortunately, the effects of this diet on IMCL levels and muscle lipid metabolites were not measured in these studies.
However, even when focusing on a high fat diet comprised of saturated fatty acids, the evidence for high fat intake increasing insulin resistance in humans remains unclear. In one longitudinal observational cohort study, high total and saturated fat intake was associated with higher fasting insulin levels, suggesting that a high saturated fat diet is associated with insulin resistance [91]. However, several interventional studies in healthy humans have not documented a clear correlation between a high saturated fat diet and increased insulin resistance as measured by the hyperinsulinemic, euglycemic glucose clamp [76, 84, 86, 87] or by the frequently sampled intravenous glucose tolerance test [85, 88].
There is growing interest that increased levels of fatty acid metabolites, rather than IMCL alone, may be the mechanism responsible for lipid induced insulin resistance. The possibility that IMCL may not be harmful has been raised by the observations in endurance athletes who have levels of IMCL comparable to those of subjects with diabetes but do not have insulin resistance [92]. In addition, elevated IMCL levels in transgenic mice do not also obligatorily correlate with insulin resistance. Transgenic mice overexpressing lipoprotein lipase in skeletal muscle have increased IMCL, fatty acyl CoA, diacylglycerol and ceramide levels in the setting of reduced muscle glucose uptake [93]. In contrast, transgenic mice overexpressing diacylglycerol acyltransferase in skeletal muscle increased skeletal muscle IMCL in the setting of reduced diacyglycerol (DAG) and ceramide levels; these mice were protected from high fat diet induced insulin resistance [94]. Proposed fatty acid metabolites responsible for lipid induced insulin resistance include 4-hydroxynonenal (4-HNE) [95] DAG [58, 79] ceramide,[94, 96] and long chain fatty acyl CoA (LC-CoA) [97] .
4-hydroxynonenal (4-HNE) is a by product of lipid peroxidation and an important mediator of free radical damage [98]. In humans, 4-HNE elevation has been associated with obesity [95] but not with DM2 [99] or reduced skeletal muscle mitochondrial respiration [99].
Diacylglycerol (DAG) is generated by lipid hydrolysis or by de novo synthesis. DAG is elevated in obese subjects compared to controls [100]. Much interest has been on DAG activation of protein kinase C (PKC) as a mechanism of lipid induced insulin resistance and the activation of the novel PKCs (nPKC δ, Θ,ε,ŋ) in particular. In humans, lipid infusion induced insulin resistance has been associated with elevated skeletal muscle DAG level, elevated protein kinase C activity (elevated PKC δ and PKC ßII but not PKC Θ or ε) and decreased IkappaB-α protein, an inhibitor of NF-kappa beta [79]. In rats, lipid infusion induced insulin resistance occurs in the context of increased skeletal muscle levels of DAG, PKC Θ activation, IRS-1 serine phosphorylation and decreased PI 3-Kinase activity, suggesting that lipid infusion alters insulin signaling in skeletal muscle [58]. Due to the evidence linking DAG accumulation to abnormal insulin signaling, much effort has been made to modify DAG levels and examine the subsequent effects on insulin resistance. Reduction of DAG effects through increased fatty acid oxidation [101, 102] and increased IMCL synthesis [94, 101] have been associated with improvements in insulin sensitivity.
Ceramide is derived from long chain saturated fatty acids and serves as a precursor for complex sphingolipids. Ceramides may be produced by sphingomyelin hydrolysis or de novo synthesis from palmitoyl CoA[103]. High levels of ceramides have been observed in obese insulin resistant humans [100, 104 ]. Exercise training is associated with improved insulin resistance and reduced ceramide levels [101, 102]. In healthy humans, total ceramide levels in skeletal muscle inversely correlate with insulin resistance, as measured by a hyperinsulinemic euglycemic clamp [105]. Lipid induced insulin resistance (goal FFA at 1750 umol/l) has been associated with elevated skeletal muscle total ceramide levels [105]. Lower rates of lipid infusion (goal FFA at ~ 800 umol/l) does not appear to affect intramuscular ceramide levels in humans; however, this particular study did not measure the effect of the lower rate of lipid infusion on insulin resistance or DAG levels[106]. The mechanism of ceramide producing insulin resistance may be disruption of the insulin signaling cascade, since ceramide related inhibition of AKT [104, 107] and tyrosine phosphorylation of IRS-1[108] have been observed. An extensive discussion of ceramide effects on insulin resistance is presented in a review by Summers et al [96].
Evidence also suggests that fatty acid composition, specifically fatty acid chain length and saturation, may influence skeletal muscle insulin resistance. In humans, insulin resistance has been shown to correlate with increased saturated fatty acids in IMCL [109] and LC-CoA content in skeletal muscle [110]. Animal studies have shown high fat feeding associated with increased LC-CoA and insulin resistance [82, 111]. Myotubes (C2C12) exposed to oleate or short saturated fatty acids (laurate and myristate) generate less DAG and ceramide and have less inhibition of AKT than myotubes exposed to palmitate (16:0) and other long chain saturated chain fatty acids (stearate, arachidate, and lignocerate) [112]. In subjects with DM2, acipimox treatment for seven days reduced plasma FFA levels (−49%), improved insulin sensitivity (+33%), and decreased total intramuscular LC-CoA levels (−22%) [113].
Are Mitochondrial Abnormalities Involved in Skeletal Muscle Insulin Resistance?
Because the mitochondria are the primary cellular site for fatty acid oxidation and utilization, there is much interest in the role of reduced mitochondrial function contributing to “toxic” lipid metabolite accumulation and consequent insulin resistance. The mitochondria generate ATP through oxidative phosphorylation, which couples oxidation of reducing equivalents (ie NADH, FADH2) to the phosphorylation of ADP. Reducing equivalents generated from glycolysis (NADH), the tricarboxylic acid (TCA) cycle (NADH, FADH2) and beta-oxidation of fatty acids (NADH, FADH2) are oxidized by the electron transport chain (ETC) to produce the inner mitochondrial membrane proton gradient that drives ATP synthesis.
In humans and animals, numerous measures of mitochondrial capacity have been used, ranging from quantitation of mitochondrial density and enzyme content to functional evaluation of isolated mitochondria and in situ mitochondria. In vivo mitochondrial function has also been evaluated through the use of magnetic resonance spectrometry (MRS). Table 1 provides a selected listing of mitochondrial measurements, particularly those pertinent to this review.
Table 1.
Measures of Mitochondrial Function
| Mitochondrial Measurements |
Examples | Description |
|---|---|---|
| Mitochondrial structure | ||
| 1) Histology | Measurement of mitochondrial size and morphology on electron microscopy |
|
| 2) mt DNA copy number | Measurement of mitochondrial DNA content | |
| 3)cardiolipin | Measurement of mitochondrial inner membrane area | |
| Markers of Mitochondrial function | ||
| Fatty acid oxidation | ||
| 1) Carnitine palmitoyltransferase I |
Enzyme located in outer mitochondrial membrane and facilitates transport of long chain fatty acids across the membrane |
|
| 2) beta-hydroxyacyl dehydrogenase |
Enzyme involved in formation of acetyl coA from fatty acid oxidation |
|
| 3) fatty acid translocase | Long chain transporter of fatty acids found in plasma and mitochondrial membranes |
|
| Oxidative phosphorylation | ||
| 1) pyruvate dehydrogenase | Enzyme involved in the conversion of pyruvate to Acetyl CoA | |
| 2) citrate synthase | Enzyme in the 1st step of the TCA cycle (encoded from nuclear DNA) |
|
| 3) succinate dehydrogenase | Enzyme in TCA cycle and ETC (complex 2 - encoded from nuclear DNA) |
|
| 4) NADH dehydrogenase | Enzyme in electron transport chain (complex 1 - encoded from nuclear and mitochondrial DNA) |
|
| 5) cytochrome c oxidase | Enzyme in electron transport chain (complex 4-encoded from nuclear and mitochondrial DNA) |
|
| Uncoupling proteins | ||
| UCP1-3 | Dissipates proton gradient across inner mitochondrial membrane to separate electron transport from oxidative phosphorylation |
|
| Measures of mitochondrial function | ||
| 1) O2 consumption | Measurement of O2 consumption under various scenarios of substate/ ADP/ ATP inhibitor availability |
|
| 2) fatty acid oxidation | ||
| a) lipid utilization | Extraction of mitochondrial and measurement of mitochondrial CO2 production after exposure to lipid |
|
| b) fatty acid transport | Extraction of mitochondria and measurement of lipid uptake | |
| 3) In vitro ATP production | Extraction of mitochondria and measurement of ATP production with various substrates |
|
| 4) In vivo ATP production | Magnetic resonance spectroscopy based measurement of TCA cycle rate using 13C |
|
| Magnetic resonance spectroscopy based measurement of ATP synthesis or phosphocreatinine resynthesis using 31P |
||
| Mitochondrial biogenesis markers | ||
| 1) Peroxisome proliferator- activated receptor gamma coactivator-1 alpha (PGC-1a) |
Master regulator of mitochondrial function | |
| 2) AMP-activated protein kinase (AMPK) |
Enhances oxidative (instead of glycolytic) energy production, objects of activation include PGC-1a and TFAM |
|
| 3) Mitochondrial transcription factor A (TFAM) |
Activator of mitochondrial transcription and mitochondrial genome replication |
|
Techniques for quantifying mitochondria range from electron microscopy based morphometric measures of size, morphology and tissue density [15], to measurements of mitochondrial DNA copy number (mtDNA)[114], to quantification of inner mitochondrial membrane area [115]. Measures of mitochondrial function include measures of fatty acid oxidation and oxidative phosphorylation. Indicators of fatty acid oxidation potential include measurements of enzymes associated with fatty acid transport protein into the cell [fatty acid transport protein (FAT/CD36)[116] ], fatty acid transport into the mitochondria [carnitine palmitoyltransferase I (CPT1) [117]], and fatty acid beta oxidation [beta hydroxyacyl-Coenzyme A dehydrogenase (HADH)] [118] .Estimates of oxidative phosphorylation potential include measurement of enzymes associated with the TCA cycle (pyruvate dehydrogenase, citrate synthase, succinate dehydrogenase) and electron transport chain ( NADH dehydrogenase, succinate dehydrogenase, cytochrome c oxidase) [119]. Active measures of mitochondria function include extraction of mitochondria to measure O2 consumption capacity with varying substrates [99, 120], CO2 production with lipid substrates [121, 122], and maximal ATP production with various substrates supporting oxidative phosphorylation [12]. Recently, magnetic resonance spectroscopy has been employed to measure mitochondrial function in vivo by measuring the rate of ATP phosphorylation [123] , the rate of post ischemic phosphocreatinine recovery [124, 125] and TCA cycle flux [126, 127]. Markers of mitochondrial biogenesis include peroxisome proliferator-activated receptor gamma coactivator-1 alpha (PGC-1a) [128, 129], mitochondrial transcription factor A (TFAM) [130] and AMPK [131, 132].
Uncoupling proteins (UCPs) play a key role in mitochondrial function. Uncoupling proteins ameliorate the generation of reactive oxygen radicals by allowing protons to leak across the inner mitochondrial membrane, reducing mitochondrial inner membrane potential [133]. A side effect of UCP action is reduction of the efficiency of ATP synthesis in relation to the mitochondrial respiratory rate. UCP1 appears to be limited to the brown adipose tissue [134], UCP2 is expressed in a wide variety of tissues (including adipose tissue, skeletal muscle) [135], and UCP3 in humans appears to be restricted to skeletal muscle [136].
Does Mitochondrial Dysfunction Correlate with Skeletal Muscle Insulin Resistance?
Evidence for reduced mitochondrial function has been seen both in the context of DM2 [11-15, 99, 124, 137] and in insulin resistant subjects without DM2 [8, 14, 127]. Specific measures of reduced mitochondrial function in such subjects include smaller mitochondrial size [15], decreased expression of oxidative phosphorylation genes [13, 14], lower levels of mitochondrial enzyme activity [11, 12] lower mitochondrial ATP production [11, 12], lower ATP synthetic flux rates [123, 137], slower TCA cycle flux rates [127], and slower post-ischemic phosphocreatinine recovery [124].
Reduced mitochondrial function can also take the form of “metabolic inflexibility,” a reduced ability to switch from predominant fat utilization during fasting to predominant glucose utilization during fed (insulin-stimulated) conditions [138]. The severity of metabolic inflexibility (assessed by in vivo and in vitro measures) has been associated with the degree of insulin insensitivity in healthy subjects [122] and in subjects with a family history of DM2 [139].
The existence of multiple types of mitochondrial functional capacity measurements, however, has lead to discrepant and often contradictory observations between individual studies examining mitochondrial function and insulin resistance. When insulin resistant subjects were compared with control subjects, reductions in insulin signaling , mitochondrial density and cytochrome C oxidase activity were observed but the anticipated concordant changes in levels of succinate dehydrogenase (protein), pyruvate dehydrogenase (protein), mtDNA copy number, PGC-1alpha (mRNA and protein), and PGC-1beta (mRNA and protein) and TFAM (mRNA and protein) were not observed [140]. In subjects with DM2, a reduction in mitochondrial respiration in the absence of expected changes in citrate synthase activity and fatty acid oxidation (HADH levels) were observed [99]. In another study in subjects with DM2, reductions in skeletal muscle mitochondrial respiration were not observed when mitochondria respiration was normalized to mitochondrial DNA content or citrate synthase activity [120].
In vivo 31P MRS estimates of mitochondrial capacity using post exercise measures of phosphocreatinine and ADP recovery times have showed no differences between subjects with long standing insulin treated DM2 (>5 years), subjects with prediabetes, subjects with recently diagnosed DM2 (<1 month) and sedentary normal controls [125]. In further support of a possible disconnect between mitochondrial ATP synthetic capacity and insulin resistance, comparison between Northern European subjects with DM2 and Asian Indian subjects with DM2 found that Asian Indians with DM2 had higher levels of insulin resistance despite the presence of higher skeletal muscle mitochondrial capacity, as measured by mitochondrial DNA copy number, mRNA of oxidative phosphorylation genes, citrate synthase enzyme activity, and maximal ATP production rate [141]. Thus in humans, the relationship between mitochondrial function and insulin resistance remains indeterminate, with varying degrees of correlation observed depending on mitochondrial function measurement and patient selection. Given the recent findings in Asian Indians with DM2 [141], ethnicity may play a significant role in the etiology of insulin resistance and the mitochondrial abnormalities reported in non-Asian subjects [11-15, 99, 124, 137] are likely less relevant to the development of insulin resistance in Asian Indians.
Data from animal studies have also questioned the role of mitochondrial dysfunction in causing insulin resistance. A recent longitudinal study comparing Zucker diabetic fatty (ZDF) rats versus lean heterozygote littermates showed ZDF rats developing diabetes in association with increasing IMCL content, while skeletal muscle mitochondrial function (measured by 31P MRS, succinate dehydrogenase activity, citrate synthase activity) remained comparable to the lean littermates and mitochondrial fatty acid oxidation (measured by very long chain Acyl-CoA dehydrogenase activity) was increased compared with lean littermates [142]. Several animal models of mitochondrial dysfunction including whole body LC-CoA dehydrogenase knockout mice [143], skeletal muscle specific PGC-1a knockout mice [144] and mice with targeted dysfunction of oxidative phosphorylation [145] have not demonstrated skeletal muscle insulin resistance.
Does Lipid Exposure Affect Skeletal Muscle Mitochondrial Function?
Not surprisingly, the aforementioned effects of lipid excess on insulin sensitivity have generated interest in defining the role of excess lipid exposure on the development of skeletal muscle mitochondrial dysfunction. Although IMCL levels have been shown to be inversely correlate with MRS based measurement of in vivo mitochondrial function [123] this finding has not been consistently observed in other in vivo MRS studies [124, 137]. Exposure of isolated human and mice mitochondria to doses of free fatty acid metabolites (palmitoyl-L-carnitine, palmitoyl CoA, oleoyl CoA) similar to intramyocellular concentrations (0.5 to 2 umol/L) have been shown to stimulate ATP synthesis, whereas exposure to high levels of FFA metabolites (> 5 umol/L) showed a dose dependent inhibition of ATP synthesis [146]. This evidence raises the possibility that exposure to high levels of FFA may cause mitochondrial dysfunction and lead to insulin resistance.
What is the role of lipid infusion on mitochondrial function?
In 1963, Randle et al. proposed that excess lipid exposure inhibited glucose oxidation by increasing intramitochondrial CoA levels with subsequent inhibition of pyruvate dehydrogenase, and phosphofructokinase, two critical enzymes in the glycolytic pathway [147]. As lipid infusion is a well established model for lipid insulin resistance in humans [10, 75-77], there is much interest in studying the effects of lipid infusion on skeletal muscle mitochondrial function. In humans, lipid infusion (6 hours, plateau FFA 2300±300 umol/L) has been shown to decrease activity of pyruvate dehydrogenase and increase mRNA levels of pyruvate dehydrogenase kinase isoform 4 ,an inhibitor of pyruvate dehydrogenase, suggesting that skeletal muscle preferentially oxidizes lipid in the setting of lipid infusion [148]. In humans, lipid infusion (6 hour, plateau FFA 1037 ±29 umol/l) decreased mitochondrial function in vivo as measured by 31P MRS, with a decline in insulin sensitivity that correlated with declines in intramyocellular glucose-6-phosphate and insulin stimulated Pi →ATP flux [149]. This decline in glucose-6-phosphate suggests that a major effect of lipid infusion is to decrease glucose entry into the cell, thus limiting glycolysis and glucose oxidation.
Lipid infusion effects on mitochondrial parameters have been measured. The effects of short term (6 hours or less) lipid infusion on mitochondrial gene expression have been mixed. A crossover study of lipid (6 hours, plateau FFA 1475±88 umol/l) versus glycerol (plateau FFA 129±14 umol/l) infusion in healthy humans demonstrated lipid infusion increasing IMCL, insulin resistance, and UCP3 mRNA levels while decreasing PGC-1a mRNA and PGC-1b mRNA levels [150]. In contrast, another crossover study comparing lipid (5 hours, plateau FFA 1670 ±130 umol/l) versus saline (plateau FFA 490 ±87 umol/l ) versus saline+heparin (plateau FFA 670±90 umol/l) infusion showed no changes in PGC-1a or UCP3 mRNA levels [151]. The discrepancy between these two studies, particularly with regards to PGC-1 and UCP3 measurements, may be related to several factors, including use of different controls (glycerol [150]vs saline [151] ), shorter duration of infusion (6 [150] vs 5 hours [151] ), use of heparin (no use [150] vs use [151]), concurrent hyperinsulinemic-euglycemic clamp (present [150] or absent [151]) and reference for reporting mRNA changes ( reference gene 36B4 [150], reference gene ß-actin [151], fold change from baseline levels [151] ) In particular, the effects of the hyperinsulinemic-euglycemic clamp on mitochondrial parameters must be considered, as the hyperinsulinemic euglycemic clamp has been shown to increase PGC-1a mRNA levels [152].This issue was clarified by a cross over study of prolonged lipid infusion (48 hours, plateau FFA 1730 ±430 umol/l) vs saline (48 hours, plateau FFA of 480 ±20 umol/l) in healthy subjects which demonstrated that lipid infusion increased insulin resistance (+24%) and decreased mRNA expression of numerous nuclear encoded mitochondrial genes including PGC-1 [153]. In comparison with the studies described earlier, this study did not have a concurrent heparin infusion [151] and the muscle biopsy for evaluation of mitochondrial gene expression was performed after the lipid/saline infusion and prior to the hyperinsulinemic-euglycemic clamp [150]. In contrast to the earlier studies [150, 151], microarray analysis of muscle gene expression was used, with specific confirmation of mRNA changes (in particular PGC-1) by PCR analysis (reference gene 18S ribosomal RNA) [153].
What is the role of high fat diet on mitochondrial function?
Although lipid infusion appears to reduce measures of mitochondrial function, the consequences of a high fat diet on mitochondrial function have been mixed. Based on the aforementioned lipid infusion data, a high fat diet would be predicted to increase FFA availability and to decrease glucose uptake by skeletal muscle. Therefore the accumulation of IMCL and lipid metabolites expected to occur with the ingestion of a high fat diet could promote mitochondrial damage. Conversely, intrinsic mitochondrial dysfunction might facilitate the development of insulin resistance as ingestion of a high fat diet in the setting of mitochondrial dysfunction may facilitate accumulation of IMCL and lipid metabolites. However, there are conflicting animal and human reports showing that a high fat diet may decrease [154, 155] not affect, [118, 156, 157], or increase [158-160] measures of skeletal muscle mitochondrial performance.
Human studies of high fat feeding on mitochondrial function
A short term high fat diet has been shown to reduce markers of skeletal muscle mitochondrial function. Compared to insulin sensitive subjects without a family history of DM2, insulin sensitive subjects with a family history of DM2 exhibited a decline in mRNA expression of PGC-1aand FAT/CD36 three hours after ingesting a single high fat meal (76% fat), suggesting that impaired ability to oxidize fat upon exposure to a high fat meal precedes the development of insulin resistance in subjects with a family history of DM2 [154]. In another study, healthy young men who completed a three-day, high fat diet (50% fat) experienced reductions in the gene expression of PGC-1a(decline of 20%, p<0.01), PGC-1β (decline of 25%, p<0.05), and six genes involved in oxidative phosphorylation [155].
Compared to the short term (3 hours to 3 days) high fat diets in which skeletal muscle mitochondrial function was found to be reduced, longer duration of high fat feeding (3 to 7 weeks) in humans have had equivocal effects on measures of mitochondrial function, with alterations of beta oxidative capacity but not oxidative phosphorylation capacity. Comparing athletes feed a high fat (69% fat) versus usual diet (30% fat) for 15 days , skeletal muscle CPT1 activity increased without alterations of citrate synthase activity or HADH activity , suggesting that high fat diet in this study increased capacity for transfer of fatty acids into mitochondria but did not increase mitochondrial oxidative capacity [156]. Similarly, athletes fed a high (53%) versus low fat (17%) fat diet for 5 weeks increased skeletal muscle IMCL levels but did not alter mitochondrial density [157]. In untrained humans receiving a high fat (62%) diet for seven weeks, increased HADH activity but no change in citrate synthase enzyme activity was observed [118].
Animal studies of high fat feeding on mitochondrial function
Short term high fat diets in animals have been shown to downregulate measures of mitochondrial function. A three day high fat diet (33% fat) in rats (age not stated: 320 grams average weight) downregulates gene expression of several oxidative phosphorylation enzymes (malate dehydrogenase, ATP synthase, NADH dehydrogenase, cytochrome c oxidase) in skeletal muscle [161]. A high fat diet in mice (age 5 weeks, 45% high fat diet for 3 weeks) was associated with declines in skeletal muscle mRNA expression of numerous mitochondrial genes compared with control mice (10% fat diet for 3 weeks) as well as PGC-1a (decline of 90%, p<0.01), PGC-1β (decline of 90%, p<0.05), yet the activity of citrate synthase, cytochrome C oxidase, and HADH were not significantly changed [155]. A high fat, high sucrose diet (36% fat, 35% carbohydrate , 50% sucrose) in mice (age 4 weeks) produced glucose intolerance at 4 weeks and diabetes at 16 weeks [162]. Yet, these mice did not have evidence of mitochondrial dysfunction at 4 weeks as determined by measures of mitochondrial function such as mitochondrial density (mtDNA and electron microscopy), PGC-1a, and oxygen consumption of isolated muscle fibers; however, there was evidence of mitochondrial dysfunction by 16 weeks, as demonstrated by reduced mitochondrial density (mtDNA and electron microscopy), reduced mitochondrial gene expression (Cox 1, Cox 3, PGC-1a), and reduced oxygen consumption of isolated fibers[162]. Due to the lack of normalization relative to mitochondrial content, mitochondrial dysfunction at the level of the individual mitochondria could not be assessed; nevertheless, the authors concluded that mitochondrial dysfunction at the level of skeletal muscle does not precede the development of insulin resistance [162].
Although prolonged high fat feeding in animals has been shown to reduce skeletal muscle mitochondrial function [155, 162], other studies have shown prolonged high fat feeding in animals to have no effect [163 , 164] or may even increase measures of mitochondrial function [158-160, 165, 166]. Rats (age not stated, weight 245 to 300 grams) fed a 5 week high fat diet (78% fat) as compared to a control diet (11% fat) had higher citrate synthase activity in skeletal muscle after 1 week of feeding, which was not maintained after five weeks of feeding, while HADH rose after 1 week of a high fat diet and continued to remain high after 5 weeks of a high fat diet [167]. In a study of more prolonged feeding, rats (starting age 10 weeks) fed a high fat (60% fat) diet compared with rats fed a control diet (25% fat) for 36 weeks had no change in mitochondrial ATP production, citrate synthase activity, or cytochrome c oxidase mRNA levels [164]. Insulin resistance in these rats was not specifically reported, however the high fat diet fed rats were heavier (~ 730 -770 grams) compared with the control diet fed rats (626 grams) [164].
Increases in mitochondrial function measures have also been observed in high fat feeding studies. Compared with a 15 day low fat diet (10.6%), recently weaned rats (age 25 days) subjected to a 15 day high fat diet (50%) had comparable succinate dehydrogenase and citrate synthase activity in skeletal muscle, yet the isolated skeletal muscle mitochondria from the high fat diet rats had higher rates of mitochondrial respiration and had higher rates of uncoupling with palmitate exposure [158]. Mice (8 week old) fed a high fat diet (45% fat) versus a control diet (8% from fat) had higher rates of palmitate oxidation to CO2 production in muscle homogenates (at 5 and 20 weeks), increased oxygen consumption in isolated mitochondria (at 20 weeks) given sufficient substrate, and increased activity (at 5 and 20 weeks) of citrate synthase, HADH, medium chain acyl coA dehydrogenase, and CPT-1 [159]. Similar to the previous study [158], a high fat diet may increase mitochondrial uncoupling [159] as evidenced by elevated protein expression of UCP3 (at 5 and 20 weeks) in the high fat diet mice compared with low fat diet mice. In another study, rats (age 6 weeks, personal communication with Dr. Holloszy) fed a high fat diet specifically designed to raise plasma FFAs (4 week diet, 60% fat+daily heparin injection for activation of lipoprotein lipase) demonstrated increased mtDNA copy number, increased palmitate oxidation to CO2 from muscle homogenate, increased enzyme levels of citrate synthase , increased oxidative phosphorylation enzyme levels (COX1, COX4, ATP synthase subunit α, and increased fatty acid oxidation enzyme levels (medium chain acyl CoA dehydrogenase, LC-CoA dehydrogenase, very long chain Acyl CoA dehydrogenase) [166]. In contrast with previous studies, older rats (3 month old) subjected to a high fat diet (45% fat vs 13.8% fat for 40 days) initially increased in vitro muscle fiber mitochondrial ATP synthesis (day 14 to day 20) and mitochondrial respiration (day 14) which was not sustained and actually declined (compared with the low fat group) with continued feeding (day 40) [165] Mechanistically, this decline in in vitro muscle fiber muscle ATP synthesis and mitochondrial respiration observed at the conclusion of the high fat diet (day 40) was associated with increased fractional synthesis rate of mitochondrial and mixed muscle proteins, suggesting possible effects from protein turnover rates or disassociation of in vitro muscle fiber muscle ATP synthesis and mitochondrial respiration measurements from muscle/mitochondrial protein synthesis [160].
The inconsistency between high fat feeding and markers of mitochondrial function is likely related to inconsistency between the individual studies. A high fat diet appears to increase mitochondrial measures of beta oxidation. Whether it may affect mitochondrial oxygen consumption or ATP generation capacity may be related to contributing factors such as differences in active versus passive measures mitochondrial function, species, age at initiation of diet, diet composition, diet duration, diet effects on plasma fatty acid levels, and sampling timing during the dietary program. Most notably, the age of the animal at the time of exposure to the high fat diet may influence the effects of the high fat diet on mitochondrial function. Because enhanced mitochondrial function with high fat feeding has generally be observed in younger animals, this suggests an adaptive mechanism to high fat feeding that is more prominent in younger animals [158, 159, 166].
Does mitochondrial dysfunction facilitate accumulation of toxic lipid species?
The available evidence demonstrates that lipid exposure may cause mitochondrial dysfunction; however, it has also been theorized that mitochondrial dysfunction may lead to accumulation of toxic lipid species with subsequent effects on insulin resistance [168]. Mitochondria from untrained rats had higher rates of incomplete fatty acid oxidation compared with mitochondria from trained (4 weeks treadmill running) rats [169]. In rats, prolonged blockage (4 weeks) of fatty acid beta oxidation by inhibition of CPT1 increased IMCL content and increased high fat diet induced insulin resistance [170]. However, it appears that the effect of mitochondrial dysfunction on lipid metabolite accumulation and insulin resistance may be particularly dependent on the mechanism used to cause mitochondrial dysfunction and the extent of metabolite accumulation. Mice with blockage of fatty acid beta oxidation by a whole body knockout of LC-CoA dehydrogenase had increased skeletal muscle long chain Co A levels compared with control mice, yet maintained similar muscle glucose uptake and insulin signaling; this observation was attributed to comparable skeletal muscle diacylglycerol levels between the knockout and the control mice [143]. Mice with partial CPT1 inhibition through a whole body knock out of malonyl-CoA decarboxylase, an enzyme which degrades malonyl-CoA — a natural inhibitor of CPT1, had lower rates of incomplete fatty acid catabolism and were protected from developing insulin resistance after a 12 week high fat diet [171]. It has been hypothesized that increased beta oxidation in the setting of reduced TCA cycling or oxidative phosphorylation may lead to increased rates of incomplete fatty acid catabolism and reduced insulin signaling [171].
There is also interest in skeletal muscle insulin resistance arising from mitochondrial generation of ROS [172]. In the process of generating ATP by oxidative phosphorylation, the mitochondrial electron transport chain produces ROS [173]. Consequently, mitochondria have evolved several mechanisms to mitigate the effects of ROS including oxygen radical scavengers (ie mitochondrial superoxide dismutase - SOD2) [174] and UCPs [175].
Studies have examined the physiological effects of ROS by disrupting the protective mechanisms found in mitochondria. Compared with wild type mice, homozygous mutant mice lacking SOD2 have reduced succinate dehydrogenase activity and normal cytochrome c oxidase activity in the skeletal muscle and heart. These mice die approximately 10 days after birth from a dilated cardiomyopathy [176]. Follow up preliminary studies of heterozygote mice with reduced levels of SOD2 (50%) demonstrated no difference in skeletal muscle insulin resistance (as measured by hyperinsulinemic clamp or in vitro glucose uptake at level of vastus lateralis muscle) compared with wild type mice [177].
In mitochondria, UCPs antagonize the generation of ROS by allowing protons to leak across the inner mitochondrial membrane and reduce the mitochondrial inner membrane potential [133]. This process decreases the efficiency of oxidative phosphorylation by partially uncoupling respiration from ATP synthesis [133]. Because generation of mitochondrial ROS depends on the mitochondrial inner membrane potential, reduction of the inner membrane potential through uncoupling reduces the rate of generation of ROS . Several types of uncoupling proteins (UCP 1 - 5) have been described [178] of which UCP3 has generated particular interest due to its expression in skeletal muscle [136, 179] and its possible role in exporting fatty acids out of mitochondria [180, 181] or transporting fatty acid peroxide anions across the inner mitochondrial membrane [182]
UCP3 was discovered in 1997 by screening a human skeletal muscle cDNA library [183]. Increased function [133] and expression [162] of UCP3 has been observed with exposure to 4HNE [184] and ROS [133 , 162]. In humans, skeletal muscle UCP3 mRNA levels rise with fasting [185], lipid infusion [151], and saline infusion [151]. Unlike adipose tissue UCP2 mRNA levels, skeletal muscle UCP2 and UCP3 mRNA levels do not correlate with BMI [185]. In humans, the correlation between UCP3 expression and insulin resistance has been inconsistent. Increased skeletal muscle UCP3 mRNA levels has been observed in obesity [186] and DM2 [186 , 187]. Yet, reduced skeletal muscle UCP3 mRNA levels in DM2 [188] and reduced UCP3 protein levels in DM2 [189]have been observed as well. Although more studies are necessary to reconcile the differences, the discrepancy between UCP3 mRNA expression level in DM2 may be influenced by differences in withholding diabetes treatment prior to the biopsy (held overnight [188] vs more than 1 week [186, 187]) as well as use of a prebiopsy standardized diet (not used [188] vs 2 days [186, 187]). Thus, the human studies relating alterations in UCP3 to DM2 have remained inconclusive.
Of interest, several UCP3 knockout mouse models have been created. One mouse knockout model showed increased respiratory coupling in skeletal muscle mitochondria associated with increased generation of ROS; however these mice did not display any differences in weight, exercise tolerance, fatty acid oxidation, fasting glucose, or glucose oxidation compared with wild type mice [190]. Another mouse UCP3 knockout model displayed fasting glucose levels comparable to wild type mice [191]. Magnetic resonance spectroscopy has been used to measure skeletal muscle mitochondrial function in UCP3 knock out mice with contradictory results. Increased Pi → ATP flux observed with 31P NMR suggested elevated ATP synthesis, yet 13C NMR measurements from 13C acetate infusion demonstrated no change in TCA cycle flux rate[192]. Thus, although UCP3 underexpression is associated with increased oxidative damage [193], the effects of the UCP3 underexpression on mitochondrial performance and insulin resistance remain uncertain.
Several mouse models of UCP3 overexpression have also been created. In one model, UCP3 was overexpressed using the rat myosin light chain 2 promoter [194]. Compared with wild type mice, the transgenic mice had higher skeletal muscle oxygen consumption, lower fasting glucose levels, improved glucose tolerance and were resistant to diet induced obesity and diet induced insulin resistance [194]. In another mouse model of UCP3 overexpression in skeletal muscle, the human α-skeletal actin promoter was used [195]. These mice were hyperphagic, yet were leaner and more insulin sensitive than wild type controls [195]. Further studies of these mice demonstrated maintenance of insulin signaling in the setting of a high fat diet. This appeared to result from lower DAG levels, reduced PKCΘ activity and preserved PI 3-Kinase activity [196]. The effect of UCP3 overexpression on ROS remains unclear. Overexpression of UCP3 in L6 myotubes decreased the production of mitochondrial ROS and increased fatty acid oxidation [197]. However, when L6 myotubes overexpressing UCP3 were deliberately exposed to palmitate, an increase in intracellular ROS was observed compared with control L6 myotubes [198]. This discrepancy highlights the complex role of UCP3. Although UCP3 overexpression may reduce coupling and, thereby, reduce ROS production, perhaps the increased fatty acid oxidation arising from UCP3 overexpression may overwhelm the uncoupling effects. Thus, although UCP3 is the primary uncoupling protein in skeletal muscle, UCP3 overexpression appears to have a more significant physiological impact than its absence in diet induced obesity and insulin resistance. The mechanism of this protection remains unclear, but may be related to decreased generation of ROS or increased ability to oxidize lipids.
A Broader View of the Etiology of Skeletal Muscle Insulin Resistance
Although the skeletal muscle is the largest site of insulin mediated glucose disposal [4], skeletal muscle specific alterations may not necessarily improve overall insulin resistance. A mouse with skeletal muscle specific deletion of lipoprotein lipase exhibited improved insulin signaling and glucose uptake in skeletal muscle yet exhibited increased insulin resistance in the heart, white adipose tissue, and liver [199]. As plasma FFA, TG and cytokine (Interleukin-1ß and Interleukin-6) were not significantly different between the knockout and wild type mice, the authors proposed that the observed effects may be due to the heart and liver shifting to lipid oxidation in compensation for the reduced skeletal muscle lipid oxidation [199]. Mice with skeletal muscle specific knockout of PGC-1a demonstrated impaired glucose tolerance, in the surprising context of reduced beta cell function and normal skeletal muscle insulin sensitivity [144]. Further investigation demonstrated increased expression of proinflammatory genes in the skeletal muscle (ie SOCS1, SOCS3, TNF-α, Interleukin-6, CD68) as well as increased serum Interleukin-6 levels [144]. Exposure of beta cells to Interleukin-6 in vitro suppressed insulin secretion in response to hyperglycemia, providing evidence for “crosstalk” between skeletal muscle and beta cells [144].
Likewise, changes at the level of the liver and adipose tissue may influence skeletal muscle insulin resistance. In mice with adipose specific knock out of GLUT-4, muscle glucose uptake was impaired in vivo but preserved in vitro [200], suggesting the presence of circulatory factor (such as serum retinol binding protein 4, cytokines, etc) affecting skeletal muscle insulin sensitivity [201]. Stress at the level of the endoplasmic reticulum in liver and adipose tissue (but not skeletal muscle) is associated with obesity and insulin resistance [202]. Yet in mice, treatment of endoplasmic reticulum stress with orally administered ursodeoxycholic acid improved peripheral insulin sensitivity and muscle glucose uptake [203]. These observations suggest skeletal muscle specific changes must be interpreted in the context of “cross talk” with other organs involved in glucose homeostasis. These observations suggest that the causes of alterations of skeletal muscle mitochondrial function and insulin sensitivity may be related to exogenous and endogenous factors.
Summary
This review explores the complex relationship between excess lipid exposure, mitochondrial dysfunction, and insulin resistance at the level of human skeletal muscle. Lipotoxicity — i.e., the elevation of lipids and/or associated lipid metabolites within blood and tissues with subsequent metabolic derangement — has been proposed as a possible mechanism of skeletal muscle insulin resistance. Intravenous lipid infusion is a well-documented method of inducing insulin resistance. Although IMCL content has been correlated with insulin resistance, there is increasing evidence that lipid metabolites such as 4-HNE, DAG, ceramide, and LC-CoA may play a more significant role than triglycerides in producing skeletal muscle insulin resistance.
The association between mitochondrial dysfunction and insulin resistance is unclear, particularly due to the varied options for measuring mitochondrial function. The effect of acute lipid exposure producing skeletal muscle insulin resistance in humans is well documented. The effects of chronic lipid exposure from dietary ingestion on skeletal muscle insulin resistance and skeletal muscle mitochondrial function remain disputed. The effects of skeletal muscle mitochondrial dysfunction on accumulation of lipotoxic species and skeletal muscle insulin resistance also remain uncertain. Certainly, pursuit of the role of lipid metabolites and their roles in the generation of skeletal muscle insulin resistance remain an exciting area for future research.
Moreover, alteration in skeletal muscle insulin resistance does not occur in isolation as functional perturbations of any component of the glucose homeostatic system may initiate development of insulin resistance in skeletal muscle through “cross talk” between tissues. Nevertheless, understanding pathophysiology of skeletal muscle insulin resistance remains critically important due to its role in preceding and facilitating the development of Type 2 diabetes.
Acknowledgments
Funding support:
Lisa Chow MD: Dr. Chow gratefully acknowledges support from NIH 5K12RR023247-02 grant.
Arthur From MD: Dr. From gratefully acknowledges support from NIH NCRR P41 RR008079 grant.
Elizabeth Seaquist MD:
Glossary
- (IR)
Insulin receptor
- (IRS1)
Insulin receptor substrate 1
- (IRS2)
Insulin receptor substrate 2
- (PI 3-Kinase)
phosphatidylinositol 3 kinase
- AKT
- aPKC
(atypical protein kinase C)
- (GLUT4)
glucose transporter 4
- (p38 MAPK)
p38 mitogen activated protein kinase
- (CAMK)
calmodulin dependent kinase
- (AMPK)
AMP activated protein kinase
- (MEF-2)
myocyte enhancer factor-2
- (TSC)
tuberous sclerosis complex
- (mTOR)
mammalian target of rapamycin
- (PKCΘ )
protein kinase c Θ
- (IKK)
inhibitor kappa B kinase
- (JNK)
c-Jun amino-terminal kinases
- (aPKC)
atypical protein kinase C
- (Cbl)
ecotropic retroviral transforming sequence homolog
- (CAP)
Cbl associated protein
- (AS160)
Rab GTPase-activating protein
Footnotes
Conflicts of interest:
Lisa Chow MD: none stated
Arthur From MD: none stated
Elizabeth Seaquist MD: none stated
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
BIBILIOGRAPHY
- [1].Lillioja S, Mott DM, Spraul M, Ferraro R, Foley JE, Ravussin E, et al. Insulin-Resistance and Insulin Secretory Dysfunction as Precursors of Non-Insulin-Dependent Diabetes-Mellitus - Prospective Studies of Pima-Indians. New England Journal of Medicine. 1993 Dec;329(27):1988–92. doi: 10.1056/NEJM199312303292703. [DOI] [PubMed] [Google Scholar]
- [2].Warram JH, Martin BC, Krolewski AS, Soeldner JS, Kahn CR. Slow Glucose Removal Rate and Hyperinsulinemia Precede the Development of Type-II Diabetes in the Offspring of Diabetic Parents. Annals of Internal Medicine. 1990 Dec;113(12):909–15. doi: 10.7326/0003-4819-113-12-909. [DOI] [PubMed] [Google Scholar]
- [3].Shulman GI, Rothman DL, Jue T, Stein P, Defronzo RA, Shulman RG. Quantitation of Muscle Glycogen-Synthesis in Normal Subjects and Subjects with Non-Insulin-Dependent Diabetes by C-13 Nuclear Magnetic-Resonance Spectroscopy. New England Journal of Medicine. 1990 Jan;322(4):223–8. doi: 10.1056/NEJM199001253220403. [DOI] [PubMed] [Google Scholar]
- [4].Defronzo RA, Jacot E, Jequier E, Maeder E, Wahren J, Felber JP. The Effect of Insulin on the Disposal of Intravenous Glucose - Results from Indirect Calorimetry and Hepatic and Femoral Venous Catheterization. Diabetes. 1981;30(12):1000–7. doi: 10.2337/diab.30.12.1000. [DOI] [PubMed] [Google Scholar]
- [5].Shulman GI. Cellular mechanisms of insulin resistance. Journal of Clinical Investigation. 2000 Jul;106(2):171–6. doi: 10.1172/JCI10583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Perseghin G, Scifo P, De Cobelli F, Pagliato E, Battezzati A, Arcelloni C, et al. Intramyocellular triglyceride content is a determinant of in vivo insulin resistance in humans: a 1H-13C nuclear magnetic resonance spectroscopy assessment in offspring of type 2 diabetic parents. Diabetes. 1999 Aug;48(8):1600–6. doi: 10.2337/diabetes.48.8.1600. [DOI] [PubMed] [Google Scholar]
- [7].Thamer C, Machann J, Bachmann O, Haap M, Dahl D, Wietek B, et al. Intramyocellular lipids: Anthropometric determinants and relationships with maximal aerobic capacity and insulin sensitivity. Journal of Clinical Endocrinology and Metabolism. 2003 Apr;88(4):1785–91. doi: 10.1210/jc.2002-021674. [DOI] [PubMed] [Google Scholar]
- [8].Petersen KF, Dufour S, Befroy D, Garcia R, Shulman GI. Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. New England Journal of Medicine. 2004 Feb;350(7):664–71. doi: 10.1056/NEJMoa031314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Boden G, Jadali F, White J, Liang Y, Mozzoli M, Chen X, et al. Effects of fat on insulin-stimulated carbohydrate metabolism in normal men. Journal of Clinical Investigation. 1991 Sep;88(3):960–6. doi: 10.1172/JCI115399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Boden G, Chen XH. Effects of Fat on Glucose-Uptake and Utilization in Patients with Non-Insulin-Dependent Diabetes. Journal of Clinical Investigation. 1995 Sep;96(3):1261–8. doi: 10.1172/JCI118160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Asmann YW, Stump CS, Short KR, Coenen-Schimke JM, Guo Z, Bigelow ML, et al. Skeletal muscle mitochondrial functions, mitochondrial DNA copy numbers, and gene transcript profiles in type 2 diabetic and nondiabetic subjects at equal levels of low or high insulin and euglycemia. Diabetes. 2006 Dec;55(12):3309–19. doi: 10.2337/db05-1230. [DOI] [PubMed] [Google Scholar]
- [12].Stump CS, Short KR, Bigelow ML, Schimke JM, Nair KS. Effect of insulin on human skeletal muscle mitochondrial ATP production, protein synthesis, and mRNA transcripts. Proceedings of the National Academy of Sciences of the United States of America. 2003 Jun;100(13):7996–8001. doi: 10.1073/pnas.1332551100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Patti ME, Butte AJ, Crunkhorn S, Cusi K, Berria R, Kashyap S, et al. Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: Potential role of PGC1 and NRF1. Proceedings of the National Academy of Sciences of the United States of America. 2003 Jul 8;100(14):8466–71. doi: 10.1073/pnas.1032913100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Mootha VK, Lindgren CM, Eriksson KF, Subramanian A, Sihag S, Lehar J, et al. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nature Genetics. 2003 Jul;34(3):267–73. doi: 10.1038/ng1180. [see comment] [DOI] [PubMed] [Google Scholar]
- [15].Kelley DE, He J, Menshikova EV, Ritov VB. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes. 2002 Oct;51(10):2944–50. doi: 10.2337/diabetes.51.10.2944. [DOI] [PubMed] [Google Scholar]
- [16].Kasuga M, Karlsson FA, Kahn CR. Insulin Stimulates the Phosphorylation of the 95,000-Dalton Subunit of Its Own Receptor. Science. 1982;215(4529):185–7. doi: 10.1126/science.7031900. [DOI] [PubMed] [Google Scholar]
- [17].Kasuga M, Zick Y, Blithe DL, Crettaz M, Kahn CR. Insulin Stimulates Tyrosine Phosphorylation of the Insulin-Receptor in a Cell-Free System. Nature. 1982;298(5875):667–9. doi: 10.1038/298667a0. [DOI] [PubMed] [Google Scholar]
- [18].Sun XJ, Rothenberg P, Kahn CR, Backer JM, Araki E, Wilden PA, et al. Structure of the Insulin-Receptor Substrate Irs-1 Defines a Unique Signal Transduction Protein. Nature. 1991 Jul;352(6330):73–7. doi: 10.1038/352073a0. [DOI] [PubMed] [Google Scholar]
- [19].Araki E, Lipes MA, Patti ME, Bruning JC, Haag B, Johnson RS, et al. Alternative Pathway of Insulin Signaling in Mice with Targeted Disruption of the Irs-1 Gene. Nature. 1994 Nov;372(6502):186–90. doi: 10.1038/372186a0. [DOI] [PubMed] [Google Scholar]
- [20].White MF. IRS proteins and the common path to diabetes. American Journal of Physiology-Endocrinology and Metabolism. 2002 Sep;283(3):E413–E22. doi: 10.1152/ajpendo.00514.2001. [DOI] [PubMed] [Google Scholar]
- [21].Okada T, Kawano Y, Sakakibara T, Hazeki O, Ui M. Essential Role of Phosphatidylinositol 3-Kinase in Insulin-Induced Glucose-Transport and Antilipolysis in Rat Adipocytes - Studies with a Selective Inhibitor Wortmannin. Journal of Biological Chemistry. 1994 Feb;269(5):3568–73. [PubMed] [Google Scholar]
- [22].Ruderman NB, Kapeller R, White MF, Cantley LC. Activation of Phosphatidylinositol 3-Kinase by Insulin. Proceedings of the National Academy of Sciences of the United States of America. 1990 Feb;87(4):1411–5. doi: 10.1073/pnas.87.4.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Backer JM, Myers MG, Shoelson SE, Chin DJ, Sun XJ, Miralpeix M, et al. Phosphatidylinositol 3′-Kinase Is Activated by Association with Irs-1 During Insulin Stimulation. Embo Journal. 1992 Sep;11(9):3469–79. doi: 10.1002/j.1460-2075.1992.tb05426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Cheatham B, Vlahos CJ, Cheatham L, Wang L, Blenis J, Kahn CR. Phosphatidylinositol 3-Kinase Activation Is Required for Insulin Stimulation of Pp70 S6 Kinase, DNA-Synthesis, and Glucose-Transporter Translocation. Molecular and Cellular Biology. 1994 Jul;14(7):4902–11. doi: 10.1128/mcb.14.7.4902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Frevert EU, Kahn BB. Differential effects of constitutively active phosphatidylinositol 3-kinase on glucose transport, glycogen synthase activity, and DNA synthesis in 3T3-L1 adipocytes. Molecular and Cellular Biology. 1997 Jan;17(1):190–8. doi: 10.1128/mcb.17.1.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Katagiri H, Asano T, Ishihara H, Inukai K, Shibasaki Y, Kikuchi M, et al. Overexpression of catalytic subunit p110 alpha of phosphatidylinositol 3-kinase increases glucose transport activity with translocation of glucose transporters in 3T3-L1 adipocytes. Journal of Biological Chemistry. 1996 Jul;271(29):16987–90. doi: 10.1074/jbc.271.29.16987. [DOI] [PubMed] [Google Scholar]
- [27].Tanti JF, Gremeaux T, Grillo S, Calleja V, Klippel A, Williams LT, et al. Overexpression of a constitutively active form of phosphatidylinositol 3-kinase is sufficient to promote glut 4 translocation in adipocytes. Journal of Biological Chemistry. 1996 Oct;271(41):25227–32. doi: 10.1074/jbc.271.41.25227. [DOI] [PubMed] [Google Scholar]
- [28].Kohn AD, Summers SA, Birnbaum MJ, Roth RA. Expression of a constitutively active Akt Ser/Thr kinase in 3T3-L1 adipocytes stimulates glucose uptake and glucose transporter 4 translocation. Journal of Biological Chemistry. 1996 Dec;271(49):31372–8. doi: 10.1074/jbc.271.49.31372. [DOI] [PubMed] [Google Scholar]
- [29].Standaert ML, Galloway L, Karnam P, Bandyopadhyay G, Moscat J, Farese RV. Protein kinase C-zeta as a downstream effector of phosphatidylinositol 3-kinase during insulin stimulation in rat adipocytes - Potential role in glucose transport. Journal of Biological Chemistry. 1997 Nov;272(48):30075–82. doi: 10.1074/jbc.272.48.30075. [DOI] [PubMed] [Google Scholar]
- [30].Kotani K, Ogawa W, Matsumoto M, Kitamura T, Sakaue H, Hino Y, et al. Requirement of atypical protein kinase C lambda for insulin stimulation of glucose uptake but not for Akt activation in 3T3-L1 adipocytes. Molecular and Cellular Biology. 1998 Dec;18(12):6971–82. doi: 10.1128/mcb.18.12.6971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Zisman A, Peroni OD, Abel ED, Michael MD, Mauvais-Jarvis F, Lowell BB, et al. Targeted disruption of the glucose transporter 4 selectively in muscle causes insulin resistance and glucose intolerance. Nature Medicine. 2000 Aug;6(8):924–8. doi: 10.1038/78693. [DOI] [PubMed] [Google Scholar]
- [32].Thirone ACP, Carvalheira JBC, Hirata AE, Velloso LA, Saad MJA. Regulation of Cbl-associated protein/Cbl pathway in muscle and adipose tissues of two animal models of insulin resistance. Endocrinology. 2004 Jan;145(1):281–93. doi: 10.1210/en.2003-0575. [DOI] [PubMed] [Google Scholar]
- [33].Wadley GD, Bruce CR, Konstantopoulos N, Macaulay SL, Howlett KF, Hawley JA, et al. The effect of insulin and exercise on c-Cbl protein abundance and phosphorylation in insulin-resistant skeletal muscle in lean and obese Zucker rats. Diabetologia. 2004 Mar;47(3):412–9. doi: 10.1007/s00125-003-1322-2. [DOI] [PubMed] [Google Scholar]
- [34].Somwar R, Perreault M, Kapur S, Taha C, Sweeney G, Ramlal T, et al. Activation of p38 mitogen-activated protein kinase alpha and beta by insulin and contraction in rat skeletal muscle - Potential role in the stimulation of glucose transport. Diabetes. 2000 Nov;49(11):1794–800. doi: 10.2337/diabetes.49.11.1794. [DOI] [PubMed] [Google Scholar]
- [35].Goodyear LJ, Chang PY, Sherwood DJ, Dufresne SD, Moller DE. Effects of exercise and insulin on mitogen-activated protein kinase signaling pathways in rat skeletal muscle. American Journal of Physiology-Endocrinology and Metabolism. 1996 Aug;271(2):E403–E8. doi: 10.1152/ajpendo.1996.271.2.E403. [DOI] [PubMed] [Google Scholar]
- [36].Ojuka EO, Jones TE, Nolte LA, Chen M, Wamhoff BR, Sturek M, et al. Regulation of GLUT4 biogenesis in muscle: evidence for involvement of AMPK and Ca2+ American Journal of Physiology-Endocrinology and Metabolism. 2002 May;282(5):E1008–E13. doi: 10.1152/ajpendo.00512.2001. [DOI] [PubMed] [Google Scholar]
- [37].Wright DC, Hucker KA, Holloszy JO, Han DH. Ca2+ and AMPK both mediate stimulation of glucose transport by muscle contractions. Diabetes. 2004 Feb;53(2):330–5. doi: 10.2337/diabetes.53.2.330. [DOI] [PubMed] [Google Scholar]
- [38].Hayashi T, Hirshman MF, Kurth EJ, Winder WW, Goodyear LJ. Evidence for 5 ′ AMP-activated protein kinase mediation of the effect of muscle contraction on glucose transport. Diabetes. 1998 Aug;47(8):1369–73. doi: 10.2337/diab.47.8.1369. [DOI] [PubMed] [Google Scholar]
- [39].Sakamoto K, McCarthy A, Smith D, Green KA, Hardie DG, Ashworth A, et al. Deficiency of LKB1 in skeletal muscle prevents AMPK activation and glucose uptake during contraction. Embo Journal. 2005 May;24(10):1810–20. doi: 10.1038/sj.emboj.7600667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].McGee SL, Hargreaves M. Exercise and myocyte enhancer factor 2 regulation in human skeletal muscle. Diabetes. 2004 May;53(5):1208–14. doi: 10.2337/diabetes.53.5.1208. [DOI] [PubMed] [Google Scholar]
- [41].Treebak JT, Glund S, Deshmukh A, Klein DK, Long YC, Jensen TE, et al. AMPK-mediated AS160 phosphorylation in skeletal muscle is dependent on AMPK catalytic and regulatory subunits. Diabetes. 2006 Jul;55(7):2051–8. doi: 10.2337/db06-0175. [DOI] [PubMed] [Google Scholar]
- [42].Kramer HF, Witczak CA, Taylor EB, Fujii N, Hirshman MF, Goodyear LJ. AS160 regulates insulin- and contraction-stimulated glucose uptake in mouse skeletal muscle. Journal of Biological Chemistry. 2006 Oct;281(42):31478–85. doi: 10.1074/jbc.M605461200. [DOI] [PubMed] [Google Scholar]
- [43].Bruss MD, Arias EB, Lienhard GE, Cartee GD. Increased phosphorylation of Akt substrate of 160 kDa (AS160) in rat skeletal muscle in response to insulin or contractile activity. Diabetes. 2005 Jan;54(1):41–50. doi: 10.2337/diabetes.54.1.41. [DOI] [PubMed] [Google Scholar]
- [44].Jakobsen SN, Hardie DG, Morrice N, Tornqvist HE. 5 ′-AMP-activated protein kinase phosphorylates IRS-1 on Ser-789 in mouse C2C12 myotubes in response to 5-aminoimidazole-4carboxamide riboside. Journal of Biological Chemistry. 2001 Dec;276(50):46912–6. doi: 10.1074/jbc.C100483200. [DOI] [PubMed] [Google Scholar]
- [45].Inoki K, Zhu TQ, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003 Nov;115(5):577–90. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- [46].Shah OJ, Wang ZY, Hunter T. Inappropriate activation of the TSC/Rheb/mTOR/S6K cassette induces IRS1/2 depletion, insulin resistance, and cell survival deficiencies. Curr Biol. 2004 Sep;14(18):1650–6. doi: 10.1016/j.cub.2004.08.026. [DOI] [PubMed] [Google Scholar]
- [47].Harrington LS, Findlay GM, Gray A, Tolkacheva T, Wigfield S, Rebholz H, et al. The TSC1-2 tumor suppressor controls insulin-PI3K signaling via regulation of IRS proteins. Journal of Cell Biology. 2004 Jul;166(2):213–23. doi: 10.1083/jcb.200403069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Cusi K, Maezono K, Osman A, Pendergrass M, Patti ME, Pratipanawatr T, et al. Insulin resistance differentially affects the PI3-kinase- and MAP kinase-mediated signaling in human muscle. Journal of Clinical Investigation. 2000 Feb;105(3):311–20. doi: 10.1172/JCI7535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Goodyear LJ, Giorgino F, Sherman LA, Carey J, Smith RJ, Dohm GL. Insulin-Receptor Phosphorylation, Insulin-Receptor Substrate-1 Phosphorylation, and Phosphatidylinositol 3-Kinase Activity Are Decreased in Intact Skeletal-Muscle Strips from Obese Subjects. Journal of Clinical Investigation. 1995 May;95(5):2195–204. doi: 10.1172/JCI117909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Bjornholm M, Kawano Y, Lehtihet M, Zierath JR. Insulin receptor substrate-1 phosphorylation and phosphatidylinositol 3-kinase activity in skeletal muscle from NIDDM subjects after in vivo insulin stimulation. Diabetes. 1997 Mar;46(3):524–7. doi: 10.2337/diab.46.3.524. [DOI] [PubMed] [Google Scholar]
- [51].Paz K, Hemi R, LeRoith D, Karasik A, Elhanany E, Kanety H, et al. Elevated serine/threonine phosphorylation of IRS-1 and IRS-2 inhibits their binding to the juxtamembrane region of the insulin receptor and impairs their ability to undergo insulin-induced tyrosine phosphorylation. Journal of Biological Chemistry. 1997 Nov;272(47):29911–8. doi: 10.1074/jbc.272.47.29911. [DOI] [PubMed] [Google Scholar]
- [52].Gao ZG, Hwang D, Bataille F, Lefevre M, York D, Quon M, et al. Serine phosphorylation of insulin receptor substrate 1 by inhibitor kappa B kinase complex. Journal of Biological Chemistry. 2002 Dec;277(50):48115–21. doi: 10.1074/jbc.M209459200. [DOI] [PubMed] [Google Scholar]
- [53].Hirosumi J, Tuncman G, Chang LF, Gorgun CZ, Uysal KT, Maeda K, et al. A central role for JNK in obesity and insulin resistance. Nature. 2002 Nov;420(6913):333–6. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- [54].Aguirre V, Uchida T, Yenush L, Davis R, White MF. The c-Jun NH2-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of Ser(307) Journal of Biological Chemistry. 2000 Mar;275(12):9047–54. doi: 10.1074/jbc.275.12.9047. [DOI] [PubMed] [Google Scholar]
- [55].Li JP, DeFea K, Roth RA. Modulation of insulin receptor substrate-1 tyrosine phosphorylation by an Akt/phosphatidylinositol 3-kinase pathway. Journal of Biological Chemistry. 1999 Apr;274(14):9351–6. doi: 10.1074/jbc.274.14.9351. [DOI] [PubMed] [Google Scholar]
- [56].Ozes ON, Akca H, Mayo LD, Gustin JA, Maehama T, Dixon JE, et al. A phosphatidylinositol 3-kinase/Akt/mTOR pathway mediates and PTEN antagonizes tumor necrosis factor inhibition of insulin signaling through insulin receptor substrate-1. Proceedings of the National Academy of Sciences of the United States of America. 2001 Apr;98(8):4640–5. doi: 10.1073/pnas.051042298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Ravichandran LV, Esposito DL, Chen J, Quon MJ. Protein kinase C-zeta phosphorylates insulin receptor substrate-1 and impairs its ability to activate phosphatidylinositol 3-kinase in response to insulin. Journal of Biological Chemistry. 2001 Feb;276(5):3543–9. doi: 10.1074/jbc.M007231200. [DOI] [PubMed] [Google Scholar]
- [58].Yu C, Chen Y, Cline GW, Zhang D, Zong H, Wang Y, et al. Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. Journal of Biological Chemistry. 2002 Dec 27;277(52):50230–6. doi: 10.1074/jbc.M200958200. [DOI] [PubMed] [Google Scholar]
- [59].Kim YB, Kotani K, Ciaraldi TP, Henry RR, Kahn BB. Insulin-stimulated protein kinase C lambda/zeta activity is reduced in skeletal muscle of humans with obesity and type 2 diabetes - Reversal with weight reduction. Diabetes. 2003 Aug;52(8):1935–42. doi: 10.2337/diabetes.52.8.1935. [DOI] [PubMed] [Google Scholar]
- [60].Vollenweider P, Menard B, Nicod P. Insulin resistance, defective insulin receptor substrate 2-associated phosphatidylinositol-3 ′ kinase activation, and impaired atypical protein kinase C (zeta/lambda) activation in myotubes from obese patients with impaired glucose tolerance. Diabetes. 2002 Apr;51(4):1052–9. doi: 10.2337/diabetes.51.4.1052. [DOI] [PubMed] [Google Scholar]
- [61].Beeson M, Sajan MP, Dizon M, Grebenev D, Gomez-Daspet J, Miura A, et al. Activation of protein kinase C-zeta by insulin and phosphatidylinositol-3,4,5-(PO4)(3) is defective in muscle in type 2 diabetes and impaired glucose tolerance - Amelioration by rosiglitazone and exercise. Diabetes. 2003 Aug;52(8):1926–34. doi: 10.2337/diabetes.52.8.1926. [DOI] [PubMed] [Google Scholar]
- [62].Kim YB, Nikoulina SE, Ciaraldi TP, Henry RR, Kahn BB. Normal insulin-dependent activation of Akt/protein kinase B, with diminished activation of phosphoinositide 3-kinase, in muscle in type 2 diabetes. Journal of Clinical Investigation. 1999 Sep;104(6):733–41. doi: 10.1172/JCI6928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Kruszynska YT, Worrall DS, Ofrecio J, Frias JP, Macaraeg G, Olefsky JM. Fatty acid-induced insulin resistance: Decreased muscle PI3K activation but unchanged Akt phosphorylation. Journal of Clinical Endocrinology and Metabolism. 2002 Jan;87(1):226–34. doi: 10.1210/jcem.87.1.8187. [DOI] [PubMed] [Google Scholar]
- [64].Krook A, Roth RA, Jiang XJ, Zierath JR, Wallberg-Henriksson H. Insulin-stimulated Akt kinase activity is reduced in skeletal muscle from NIDDM subjects. Diabetes. 1998 Aug;47(8):1281–6. doi: 10.2337/diab.47.8.1281. [DOI] [PubMed] [Google Scholar]
- [65].Burgering BMT, Coffer PJ. Protein-Kinase-B (C-Akt) in Phosphatidylinositol-3-Oh Inase Signal-Transduction. Nature. 1995 Aug;376(6541):599–602. doi: 10.1038/376599a0. [DOI] [PubMed] [Google Scholar]
- [66].Farese RV. Function and dysfunction of aPKC isoforms for glucose transport in insulin-sensitive and insulin-resistant states. American Journal of Physiology-Endocrinology and Metabolism. 2002 Jul;283(1):E1–E11. doi: 10.1152/ajpendo.00045.2002. [DOI] [PubMed] [Google Scholar]
- [67].Shepherd PR, Kahn BB. Mechanisms of disease - Glucose transporters and insulin action - Implications for insulin resistance and diabetes mellitus. New England Journal of Medicine. 1999 Jul;341(4):248–57. doi: 10.1056/NEJM199907223410406. [DOI] [PubMed] [Google Scholar]
- [68].Leng Y, Steiler TL, Zierath JR. Effects of insulin, contraction, and phorbol esters on mitogen-activated protein kinase signaling in skeletal muscle from lean and ob/ob mice. Diabetes. 2004 Jun;53(6):1436–44. doi: 10.2337/diabetes.53.6.1436. [DOI] [PubMed] [Google Scholar]
- [69].Wolfe RR, Peters EJ, Klein S, Holland OB, Rosenblatt J, Gary H. Effect of Short-Term Fasting on Lipolytic Responsiveness in Normal and Obese Human-Subjects. American Journal of Physiology. 1987 Feb;252(2):E189–E96. doi: 10.1152/ajpendo.1987.252.2.E189. [DOI] [PubMed] [Google Scholar]
- [70].Jensen MD, Haymond MW, Rizza RA, Cryer PE, Miles JM. Influence of Body-Fat Distribution on Free Fatty-Acid Metabolism in Obesity. Journal of Clinical Investigation. 1989 Apr;83(4):1168–73. doi: 10.1172/JCI113997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Dobbins RL, Chester MM, Daniels MB, McGarry JD, Stein DT. Circulating fatty acids are essential for efficient glucose-stimulated insulin secretion after prolonged fasting in humans. Diabetes. 1998 Oct;47(10):1613–8. doi: 10.2337/diabetes.47.10.1613. [DOI] [PubMed] [Google Scholar]
- [72].Baldeweg SE, Golay A, Natali A, Balkau B, Del Prato S, Coppack SW. Insulin resistance, lipid and fatty acid concentrations in 867 healthy Europeans. European Journal of Clinical Investigation. 2000 Jan;30(1):45–52. doi: 10.1046/j.1365-2362.2000.00597.x. [DOI] [PubMed] [Google Scholar]
- [73].Reaven GM, Hollenbeck C, Jeng CY, Wu MS, Chen YDI. Measurement of Plasma-Glucose, Free Fatty-Acid, Lactate, and Insulin for 24-H in Patients with NIDDM. Diabetes. 1988 Aug;37(8):1020–4. doi: 10.2337/diab.37.8.1020. [DOI] [PubMed] [Google Scholar]
- [74].Pan DA, Lillioja S, Kriketos AD, Milner MR, Baur LA, Bogardus C, et al. Skeletal muscle triglyceride levels are inversely related to insulin action. Diabetes. 1997 June 1;46(6):983–8. doi: 10.2337/diab.46.6.983. 1997. [DOI] [PubMed] [Google Scholar]
- [75].Brechtel K, Dahl DB, Machann J, Bachmann OP, Wenzel I, Maier T, et al. Fast elevation of the intramyocellular lipid content in the presence of circulating free fatty acids and hyperinsulinemia: A dynamic H-1-MRS study. Magnetic Resonance in Medicine. 2001 Feb;45(2):179–83. doi: 10.1002/1522-2594(200102)45:2<179::aid-mrm1023>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- [76].Bachmann OP, Dahl DB, Brechtel K, Machann J, Haap M, Maier T, et al. Effects of intravenous and dietary lipid challenge on intramyocellular lipid content and the relation with insulin sensitivity in humans. Diabetes. 2001 Nov;50(11):2579–84. doi: 10.2337/diabetes.50.11.2579. [DOI] [PubMed] [Google Scholar]
- [77].Boden G, Jadali F, White J, Liang Y, Mozzoli M, Chen X, et al. Effects of Fat on Insulin-Stimulated Carbohydrate-Metabolism in Normal Men. Journal of Clinical Investigation. 1991 Sep;88(3):960–6. doi: 10.1172/JCI115399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Belfort R, Mandarino L, Kashyap S, Wirfel K, Pratipanawatr T, Berria R, et al. Dose-response effect of elevated plasma free fatty acid on insulin signaling. Diabetes. 2005 Jun;54(6):1640–8. doi: 10.2337/diabetes.54.6.1640. [DOI] [PubMed] [Google Scholar]
- [79].Itani SI, Ruderman NB, Schmieder F, Boden G. Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IkappaB-alpha. Diabetes. 2002 Jul;51(7):2005–11. doi: 10.2337/diabetes.51.7.2005. [DOI] [PubMed] [Google Scholar]
- [80].Griffin ME, Marcucci MJ, Cline GW, Bell K, Barucci N, Lee D, et al. Free fatty acid-induced insulin resistance is associated with activation of protein kinase C theta and alterations in the insulin signaling cascade. Diabetes. 1999 Jun;48(6):1270–4. doi: 10.2337/diabetes.48.6.1270. [DOI] [PubMed] [Google Scholar]
- [81].Kim JK, Kim YJ, Fillmore JJ, Chen Y, Moore I, Lee JS, et al. Prevention of fat-induced insulin resistance by salicylate. Journal of Clinical Investigation. 2001 Aug;108(3):437–46. doi: 10.1172/JCI11559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Oakes ND, Cooney GJ, Camilleri S, Chisholm DJ, Kraegen EW. Mechanisms of liver and muscle insulin resistance induced by chronic high-fat feeding. Diabetes. 1997 Nov;46(11):1768–74. doi: 10.2337/diab.46.11.1768. [DOI] [PubMed] [Google Scholar]
- [83].Storlien LH, James DE, Burleigh KM, Chisholm DJ, Kraegen EW. Fat Feeding Causes Widespread Invivo Insulin Resistance, Decreased Energy-Expenditure, and Obesity in Rats. American Journal of Physiology. 1986 Nov;251(5):E576–E83. doi: 10.1152/ajpendo.1986.251.5.E576. [DOI] [PubMed] [Google Scholar]
- [84].Fasching P, Ratheiser K, Schneeweiss B, Rohac M, Nowotny P, Waldhausl W. No effect of short-term dietary supplementation of saturated and poly- and monounsaturated fatty acids on insulin secretion and sensitivity in healthy men. Annals of Nutrition and Metabolism. 1996 Mar-Apr;40(2):116–22. doi: 10.1159/000177904. [DOI] [PubMed] [Google Scholar]
- [85].Schwab US, Niskanen LK, Maliranta HM, Savolainen MJ, Kesaniemi YA, Uusitupa MIJ. Lauric and Palmitic Acid-Enriched Diets Have Minimal Impact on Serum-Lipid and Lipoprotein Concentrations and Glucose-Metabolism in Healthy-Young Women. Journal of Nutrition. 1995 Mar;125(3):466–73. doi: 10.1093/jn/125.3.466. [DOI] [PubMed] [Google Scholar]
- [86].Borkman M, Campbell LV, Chisholm DJ, Storlien LH. Comparison of the Effects on Insulin Sensitivity of High-Carbohydrate and High-Fat Diets in Normal Subjects. Journal of Clinical Endocrinology and Metabolism. 1991 Feb;72(2):432–7. doi: 10.1210/jcem-72-2-432. [DOI] [PubMed] [Google Scholar]
- [87].Bisschop PH, de Metz J, Ackermans MT, Endert E, Pijl H, Kuipers F, et al. Dietary fat content alters insulin-mediated glucose metabolism in healthy men. American Journal of Clinical Nutrition. 2001 Mar;73(3):554–9. doi: 10.1093/ajcn/73.3.554. [DOI] [PubMed] [Google Scholar]
- [88].Vessby B, Uusitupa M, Hermansen K, Riccardi G, Rivellese AA, Tapsell LC, et al. Substituting dietary saturated for monounsaturated fat impairs insulin sensitivity in healthy men and women: The KANWU study. Diabetologia. 2001 Mar;44(3):312–9. doi: 10.1007/s001250051620. [DOI] [PubMed] [Google Scholar]
- [89].Esposito K, Marfella R, Ciotola M, Di Palo C, Giugliano F, Giugliano G, et al. Effect of a Mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome - A randomized trial. Jama-Journal of the American Medical Association. 2004 Sep;292(12):1440–6. doi: 10.1001/jama.292.12.1440. [DOI] [PubMed] [Google Scholar]
- [90].Martinez-Gonzalez MA, de la Fuente-Arrillaga C, Nunez-Cordoba JM, Basterra-Gortari FJ, Beunza JJ, Vazquez Z, et al. Adherence to Mediterranean diet and risk of developing diabetes: prospective cohort study. British Medical Journal. 2008 Jun;336(7657):1348–51. doi: 10.1136/bmj.39561.501007.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Marshall JA, Bessesen DH, Hamman RF. High saturated fat and low starch and fibre are associated with hyperinsulinaemia in a non-diabetic population: The San Luis Valley Diabetes Study. Diabetologia. 1997 Apr;40(4):430–8. doi: 10.1007/s001250050697. [DOI] [PubMed] [Google Scholar]
- [92].Goodpaster BH, He J, Watkins S, Kelley DE. Skeletal muscle lipid content and insulin resistance: evidence for a paradox in endurance-trained athletes. Journal of Clinical Endocrinology & Metabolism. 2001 Dec;86(12):5755–61. doi: 10.1210/jcem.86.12.8075. [DOI] [PubMed] [Google Scholar]
- [93].Kim JK, Fillmore JJ, Chen Y, Yu CL, Moore IK, Pypaert M, et al. Tissue-specific overexpression of lipoprotein lipase causes tissue-specific insulin resistance. Proceedings of the National Academy of Sciences of the United States of America. 2001 Jun;98(13):7522–7. doi: 10.1073/pnas.121164498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Liu L, Zhang Y, Chen N, Shi X, Tsang B, Yu Y-H. Upregulation of myocellular DGAT1 augments triglyceride synthesis in skeletal muscle and protects against fat-induced insulin resistance. Journal of Clinical Investigation. 2007 June 1;117(6):1679–89. doi: 10.1172/JCI30565. 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Russell AP, Gastaldi G, Bobbioni-Harsch E, Arboit P, Gobelet C, Deriaz O, et al. Lipid peroxidation in skeletal muscle of obese as compared to endurance-trained humans: a case of good vs. bad lipids? Febs Letters. 2003 Sep;551(13):104–6. doi: 10.1016/s0014-5793(03)00875-5. [DOI] [PubMed] [Google Scholar]
- [96].Summers SA. Ceramides in insulin resistance and lipotoxicity. Progress in Lipid Research. 2006;45(1):42–72. doi: 10.1016/j.plipres.2005.11.002. [DOI] [PubMed] [Google Scholar]
- [97].Laybutt DR, Schmitz-Peiffer C, Saha AK, Ruderman NB, Biden TJ, Kraegen EW. Muscle lipid accumulation and protein kinase C activation in the insulin-resistant chronically glucose-infused rat. American Journal of Physiology-Endocrinology and Metabolism. 1999 Dec;277(6):E1070–E6. doi: 10.1152/ajpendo.1999.277.6.E1070. [DOI] [PubMed] [Google Scholar]
- [98].Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radical Biology & Medicine. 1991;11(1):81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- [99].Mogensen M, Sahlin K, Fernstrom M, Glintborg D, Vind BF, Beck-Nielsen H, et al. Mitochondrial respiration is decreased in skeletal muscle of patients with type 2 diabetes. Diabetes. 2007 Jun;56(6):1592–9. doi: 10.2337/db06-0981. [DOI] [PubMed] [Google Scholar]
- [100].Straczkowski M, Kowalska I, Baranowski M, Nikolajuk A, Otziomek E, Zabielski P, et al. Increased skeletal muscle ceramide level in men at risk of developing type 2 diabetes. Diabetologia. 2007 Nov;50(11):2366–73. doi: 10.1007/s00125-007-0781-2. [DOI] [PubMed] [Google Scholar]
- [101].Dube JJ, Amati F, Stefanovic-Racic M, Toledo FGS, Sauers SE, Goodpaster BH. Exercise-induced alterations in intramyocellular lipids and insulin resistance: the athlete’s paradox revisited. American Journal of Physiology-Endocrinology and Metabolism. 2008 May;294(5):E882–E8. doi: 10.1152/ajpendo.00769.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Bruce CR, Thrush AB, Mertz VA, Bezaire V, Chabowski A, Heigenhauser GJF, et al. Endurance training in obese humans improves glucose tolerance and mitochondrial fatty acid oxidation and alters muscle lipid content. American Journal of Physiology-Endocrinology and Metabolism. 2006 Jul;291(1):E99–E107. doi: 10.1152/ajpendo.00587.2005. [DOI] [PubMed] [Google Scholar]
- [103].Merrill AH. De novo sphingolipid biosynthesis: A necessary, but dangerous, pathway. Journal of Biological Chemistry. 2002 Jul;277(29):25843–6. doi: 10.1074/jbc.R200009200. [DOI] [PubMed] [Google Scholar]
- [104].Adams JM, 2nd, Pratipanawatr T, Berria R, Wang E, DeFronzo RA, Sullards MC, et al. Ceramide content is increased in skeletal muscle from obese insulin-resistant humans. Diabetes. 2004 Jan;53(1):25–31. doi: 10.2337/diabetes.53.1.25. [DOI] [PubMed] [Google Scholar]
- [105].Straczkowski M, Kowalska I, Nikolajuk A, Dzienis-Straczkowska S, Kinalska I, Baranowski M, et al. Relationship between insulin sensitivity and sphingomyelin signaling pathway in human skeletal muscle. Diabetes. 2004 May;53(5):1215–21. doi: 10.2337/diabetes.53.5.1215. [DOI] [PubMed] [Google Scholar]
- [106].Serlie MJ, Meijer AJ, Groener JE, Duran M, Endert E, Fliers E, et al. Short-term manipulation of plasma free fatty acids does not change skeletal muscle concentrations of ceramide and glucosylceramide in lean and overweight subjects. Journal of Clinical Endocrinology and Metabolism. 2007 Apr;92(4):1524–9. doi: 10.1210/jc.2006-2347. [DOI] [PubMed] [Google Scholar]
- [107].Schmitz-Peiffer C, Craig DL, Biden TJ. Ceramide generation is sufficient to account for the inhibition of the insulin-stimulated PKB pathway in C2C12 skeletal muscle cells pretreated with palmitate. The Journal of biological chemistry. 1999 Aug 20;274(34):24202–10. doi: 10.1074/jbc.274.34.24202. [DOI] [PubMed] [Google Scholar]
- [108].Kanety H, Hemi P, Papa MZ, Karasik A. Sphingomyelinase and ceramide suppress insulin-induced tyrosine phosphorylation of the insulin receptor substrate-1. Journal of Biological Chemistry. 1996 Apr;271(17):9895–7. doi: 10.1074/jbc.271.17.9895. [DOI] [PubMed] [Google Scholar]
- [109].Manco M, Mingrone G, Greco AV, Capristo E, Gniuli D, De Gaetano A, et al. Insulin resistance directly correlates with increased saturated fatty acids in skeletal muscle triglycerides. Metabolism: Clinical & Experimental. 2000 Feb;49(2):220–4. doi: 10.1016/s0026-0495(00)91377-5. [DOI] [PubMed] [Google Scholar]
- [110].Ellis BA, Poynten A, Lowy AJ, Furler SM, Chisholm DJ, Kraegen EW, et al. Long-chain acyl-CoA esters as indicators of lipid metabolism and insulin sensitivity in rat and human muscle. American Journal of Physiology-Endocrinology and Metabolism. 2000 Sep;279(3):E554–E60. doi: 10.1152/ajpendo.2000.279.3.E554. [DOI] [PubMed] [Google Scholar]
- [111].Chen MT, Kaufman LN, Spennetta T, Shrago E. Effects of High Fat-Feeding to Rats on the Interrelationship of Body-Weight, Plasma-Insulin, and Fatty Acyl-Coenzyme-a Esters in Liver and Skeletal-Muscle. Metabolism-Clinical and Experimental. 1992 May;41(5):564–9. doi: 10.1016/0026-0495(92)90221-u. [DOI] [PubMed] [Google Scholar]
- [112].Chavez JA, Summers SA. Characterizing the effects of saturated fatty acids on insulin signaling and ceramide and diacylglycerol accumulation in 3T3-L1 adipocytes and C2Cl2 myotubes. Archives of Biochemistry and Biophysics. 2003 Nov;419(2):101–9. doi: 10.1016/j.abb.2003.08.020. [DOI] [PubMed] [Google Scholar]
- [113].Bajaj M, Suraamornkul S, Romanelli A, Cline GW, Mandarino LJ, Shulman GI, et al. Effect of a sustained reduction in plasma free fatty acid concentration on intramuscular long-chain fatty acyl-CoAs and insulin action in type 2 diabetic patients. Diabetes. 2005 Nov;54(11):3148–53. doi: 10.2337/diabetes.54.11.3148. [DOI] [PubMed] [Google Scholar]
- [114].Williams RS. Mitochondrial Gene-Expression in Mammalian Striated-Muscle - Evidence That Variation in Gene Dosage Is the Major Regulatory Event. Journal of Biological Chemistry. 1986 Sep;261(26):2390–4. [PubMed] [Google Scholar]
- [115].Menshikova E, Ritov V, Ferrell R, Azuma K, Goodpaster B, Kelley D. Characteristics of skeletal muscle mitochondrial biogenesis induced by moderate-intensity exercise and weight loss in obesity. Journal of Applied Physiology. 2007 Jul;103(1):21–7. doi: 10.1152/japplphysiol.01228.2006. [DOI] [PubMed] [Google Scholar]
- [116].Schaffer JE, Lodish HF. Expression Cloning and Characterization of a Novel Adipocyte Long-Chain Fatty-Acid Transport Protein. Cell. 1994 Nov;79(3):427–36. doi: 10.1016/0092-8674(94)90252-6. [DOI] [PubMed] [Google Scholar]
- [117].McGarry JD, Brown NF. The mitochondrial carnitine palmitoyltransferase system - From concept to molecular analysis. Eur J Biochem. 1997 Feb;244(1):1–14. doi: 10.1111/j.1432-1033.1997.00001.x. [DOI] [PubMed] [Google Scholar]
- [118].Helge JW, Kiens B. Muscle enzyme activity in humans: Role of substrate availability and training. American Journal of Physiology-Regulatory Integrative and Comparative Physiology. 1997 May;41(5):R1620–R4. doi: 10.1152/ajpregu.1997.272.5.R1620. [DOI] [PubMed] [Google Scholar]
- [119].Holloszy JO, Coyle EF. Adaptations of skeletal muscle to endurance exercise and their metabolic consequences. Journal of Applied Physiology. 1984 April 1;56(4):831–8. doi: 10.1152/jappl.1984.56.4.831. 1984. [DOI] [PubMed] [Google Scholar]
- [120].Boushel R, Gnaiger E, Schjerling P, Skovbro M, Kraunsoe R, Dela F. Patients with type 2 diabetes have normal mitochondrial function in skeletal muscle. Diabetologia. 2007 Apr;50(4):790–6. doi: 10.1007/s00125-007-0594-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Kim JY, Hickner RC, Cortright RL, Dohm GL, Houmard JA. Lipid oxidation is reduced in obese human skeletal muscle. American Journal of Physiology-Endocrinology and Metabolism. 2000 Nov;279(5):E1039–E44. doi: 10.1152/ajpendo.2000.279.5.E1039. [DOI] [PubMed] [Google Scholar]
- [122].Ukropcova B, McNeil M, Sereda O, de Jonge L, Xie H, Bray GA, et al. Dynamic changes in fat oxidation in human primary myocytes mirror metabolic characteristics of the donor. Journal of Clinical Investigation. 2005 Jul;115(7):1934–41. doi: 10.1172/JCI24332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Petersen KF, Befroy D, Dufour S, Dziura J, Ariyan C, Rothman DL, et al. Mitochondrial dysfunction in the elderly: Possible role in insulin resistance. Science. 2003 May;300(5622):1140–2. doi: 10.1126/science.1082889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Schrauwen-Hinderling VB, Kooi ME, Hesselink MKC, Jeneson JAL, Backes WH, van Echteld CJA, et al. Impaired in vivo mitochondrial function but similar intramyocellular lipid content in patients with type 2 diabetes mellitus and BMI-matched control subjects. Diabetologia. 2007 Jan;50(1):113–20. doi: 10.1007/s00125-006-0475-1. [DOI] [PubMed] [Google Scholar]
- [125].De Feyter HM, van den Broek NMA, Praet SFE, Nicolay K, van Loon LJC, Prompers JJ. Early or advanced stage type 2 diabetes is not accompanied by in vivo skeletal muscle mitochondrial dysfunction. Eur J Endocrinol. 2008 May;158(5):643–53. doi: 10.1530/EJE-07-0756. [DOI] [PubMed] [Google Scholar]
- [126].Lebon V, Dufour S, Petersen KF, Ren JM, Jucker BM, Slezak LA, et al. Effect of triiodothyronine on mitochondrial energy coupling in human skeletal muscle. Journal of Clinical Investigation. 2001 Sep;108(5):733–7. doi: 10.1172/JCI11775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Befroy DE, Petersen KF, Dufour S, Mason GF, de Graaf RA, Rothman DL, et al. Impaired mitochondrial substrate oxidation in muscle of insulin-resistant offspring of type 2 diabetic patients. Diabetes. 2007 May;56(5):1376–81. doi: 10.2337/db06-0783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Puigserver P, Wu ZD, Park CW, Graves R, Wright M, Spiegelman BM. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998 Mar;92(6):829–39. doi: 10.1016/s0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]
- [129].Wu ZD, Puigserver P, Andersson U, Zhang CY, Adelmant G, Mootha V, et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999 Jul;98(1):115–24. doi: 10.1016/S0092-8674(00)80611-X. [DOI] [PubMed] [Google Scholar]
- [130].Larsson NG, Wang JM, Wilhelmsson H, Oldfors A, Rustin P, Lewandoski M, et al. Mitochondrial transcription factor A is necessary for mtDNA maintenance and embryogenesis in mice. Nature Genetics. 1998 Mar;18(3):231–6. doi: 10.1038/ng0398-231. [DOI] [PubMed] [Google Scholar]
- [131].Zong HH, Ren JM, Young LH, Pypaert M, Mu J, Birnbaum MJ, et al. AMP kinase is required for mitochondrial biogenesis in skeletal muscle in response to chronic energy deprivation. Proceedings of the National Academy of Sciences of the United States of America. 2002 Dec;99(25):15983–7. doi: 10.1073/pnas.252625599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Winder WW, Holmes BF, Rubink DS, Jensen EB, Chen M, Holloszy JO. Activation of AMP-activated protein kinase increases mitochondrial enzymes in skeletal muscle. Journal of Applied Physiology. 2000 Jun;88(6):2219–26. doi: 10.1152/jappl.2000.88.6.2219. [DOI] [PubMed] [Google Scholar]
- [133].Echtay KS, Roussel D, St-Pierre J, Jekabsons MB, Cadenas S, Stuart JA, et al. Superoxide activates mitochondrial uncoupling proteins. Nature. 2002 Jan;415(6867):96–9. doi: 10.1038/415096a. [DOI] [PubMed] [Google Scholar]
- [134].Heaton GM, Wagenvoord RJ, Kemp A, Nicholls DG. Brown-Adipose-Tissue Mitochondria - Photoaffinity Labeling of Regulatory Site of Energy-Dissipation. Eur J Biochem. 1978;82(2):515–21. doi: 10.1111/j.1432-1033.1978.tb12045.x. [DOI] [PubMed] [Google Scholar]
- [135].Fleury C, Neverova M, Collins S, Raimbault S, Champigny O, LeviMeyrueis C, et al. Uncoupling protein-2: A novel gene linked to obesity and hyperinsulinemia. Nature Genetics. 1997 Mar;15(3):269–72. doi: 10.1038/ng0397-269. [DOI] [PubMed] [Google Scholar]
- [136].Boss O, Samec S, Paoloni-Giacobino A, Rossier C, Dulloo A, Seydoux J, et al. Uncoupling protein-3: A new member of the mitochondrial carrier family with tissue-specific expression. Febs Letters. 1997 May;408(1):39–42. doi: 10.1016/s0014-5793(97)00384-0. [DOI] [PubMed] [Google Scholar]
- [137].Szendroedi J, Schmid AI, Chmelik M, Toth C, Brehm A, Krssak M, et al. Muscle mitochondrial ATP synthesis and glucose transport/phosphorylation in type 2 diabetes. Plos Medicine. 2007 May;4(5):858–67. doi: 10.1371/journal.pmed.0040154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Kelley DE, Mandarino LJ. Fuel selection in human skeletal muscle in insulin resistance - A reexamination. Diabetes. 2000 May;49(5):677–83. doi: 10.2337/diabetes.49.5.677. [DOI] [PubMed] [Google Scholar]
- [139].Ukropcova B, Sereda O, de Jonge L, Bogacka I, Nguyen T, Xie H, et al. Family history of diabetes links impaired substrate switching and reduced mitochondrial content in skeletal muscle. Diabetes. 2007 Mar;56(3):720–7. doi: 10.2337/db06-0521. [DOI] [PubMed] [Google Scholar]
- [140].Morino K, Petersen KF, Dufour S, Befroy D, Frattini J, Shatzkes N, et al. Reduced mitochondrial density and increased IRS-1 serine phosphorylation in muscle of insulin-resistant offspring of type 2 diabetic parents. Journal of Clinical Investigation. 2005 Dec;115(12):3587–93. doi: 10.1172/JCI25151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Nair KS, Bigelow ML, Asmann YW, Chow LS, Coenen-Schimke JM, Klaus KA, et al. Asian Indians have enhanced skeletal muscle mitochondrial capacity to produce ATP in association with severe insulin resistance. Diabetes. 2008 May;57(5):1166–75. doi: 10.2337/db07-1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].De Feyter HM, Lenaers E, Houten SM, Schrauwen P, Hesselink MK, Wanders RJA, et al. Increased intramyocellular lipid content but normal skeletal muscle mitochondrial oxidative capacity throughout the pathogenesis of type 2 diabetes. Faseb Journal. 2008 November 1;22(11):3947–55. doi: 10.1096/fj.08-112318. 2008. [DOI] [PubMed] [Google Scholar]
- [143].Zhang DY, Liu ZX, Choi CS, Tian LQ, Kibbey R, Dong JY, et al. Mitochondrial dysfunction due to long-chain Acyl-CoA dehydrogenase deficiency causes hepatic steatosis and hepatic insulin resistance. Proceedings of the National Academy of Sciences of the United States of America. 2007 Oct;104(43):17075–80. doi: 10.1073/pnas.0707060104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Handschin C, Choi CS, Chin S, Kim S, Kawamori D, Kurpad AJ, et al. Abnormal glucose homeostasis in skeletal muscle-specific PGC-1 alpha knockout mice reveals skeletal muscle-pancreatic beta cell crosstalk. Journal of Clinical Investigation. 2007 Nov;117(11):3463–74. doi: 10.1172/JCI31785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Pospisilik JA, Knauf C, Joza N, Benit P, Orthofer M, Cani PD, et al. Targeted Deletion of AIF Decreases Mitochondrial Oxidative Phosphorylation and Protects from Obesity and Diabetes. Cell. 2007;131(3):476–91. doi: 10.1016/j.cell.2007.08.047. [DOI] [PubMed] [Google Scholar]
- [146].Abdul-Ghani MA, Muller FL, Liu YH, Chavez AO, Balas B, Zuo PG, et al. Deleterious action of FA metabolites on ATP synthesis: possible link between lipotoxicity, mitochondrial dysfunction, and insulin resistance. American Journal of Physiology-Endocrinology and Metabolism. 2008 Sep;295(3):E678–E85. doi: 10.1152/ajpendo.90287.2008. [DOI] [PubMed] [Google Scholar]
- [147].Randle PJ, Garland PB, Hales CN, Newsholme EA, Randle PJ, Garland PB, et al. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963 Apr 13;1(7285):785–9. doi: 10.1016/s0140-6736(63)91500-9. [DOI] [PubMed] [Google Scholar]
- [148].Tsintzas K, Chokkalingam K, Jewell K, Norton L, Macdonald IA, Constantin-Teodosiu D. Elevated free fatty acids attenuate the insulin-induced suppression of PDK4 gene expression in human skeletal muscle: Potential role of intramuscular long-chain acyl-coenzyme a. Journal of Clinical Endocrinology and Metabolism. 2007 Oct;92(10):3967–72. doi: 10.1210/jc.2007-1104. [DOI] [PubMed] [Google Scholar]
- [149].Brehm A, Krssak M, Schmid AI, Nowotny P, Waldhausl W, Roden M. Increased lipid availability impairs insulin-stimulated ATP synthesis in human skeletal muscle. Diabetes. 2006 Jan;55(1):136–40. [PubMed] [Google Scholar]
- [150].Hoeks J, Hesselink MKC, Russell AP, Mensink M, Saris WHM, Mensink RP, et al. Peroxisome proliferator-activated receptor-gamma coactivator-1 and insulin resistance: acute effect of fatty acids. Diabetologia. 2006 Oct;49(10):2419–26. doi: 10.1007/s00125-006-0369-2. [DOI] [PubMed] [Google Scholar]
- [151].Tunstall RJ, Cameron-Smith D. Effect of elevated lipid concentrations on human skeletal muscle gene expression. Metabolism-Clinical and Experimental. 2005 Jul;54(7):952–9. doi: 10.1016/j.metabol.2005.02.012. [DOI] [PubMed] [Google Scholar]
- [152].Ling C, Poulsen P, Carlsson E, Ridderstrale M, Almgren P, Wojtaszewski J, et al. Multiple environmental and genetic factors influence skeletal muscle PGC-1 alpha and PGC-1 beta gene expression in twins. Journal of Clinical Investigation. 2004 Nov;114(10):1518–26. doi: 10.1172/JCI21889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Richardson DK, Kashyap S, Bajaj M, Cusi K, Mandarino SJ, Finlayson J, et al. Lipid infusion decreases the expression of nuclear encoded mitochondrial genes and increases the expression of extracellular matrix genes in human skeletal muscle. Journal of Biological Chemistry. 2005 Mar;280(11):10290–7. doi: 10.1074/jbc.M408985200. [DOI] [PubMed] [Google Scholar]
- [154].Heilbronn LK, Gregersen S, Shirkhedkar D, Hu D, Campbell LV. Impaired fat oxidation after a single high-fat meal in insulin-sensitive nondiabetic individuals with a family history of type 2 diabetes. Diabetes. 2007 Aug;56(8):2046–53. doi: 10.2337/db06-1687. [DOI] [PubMed] [Google Scholar]
- [155].Sparks LM, Xie H, Koza RA, Mynatt R, Hulver MW, Bray GA, et al. A high-fat diet coordinately downregulates genes required for mitochondrial oxidative phosphorylation in skeletal muscle. Diabetes. 2005 Jul;54(7):1926–33. doi: 10.2337/diabetes.54.7.1926. [DOI] [PubMed] [Google Scholar]
- [156].Goedecke JH, Christie C, Wilson G, Dennis SC, Noakes TD, Hopkins WG, et al. Metabolic adaptations to a high-fat diet in endurance cyclists. Metabolism: Clinical & Experimental. 1999 Dec;48(12):1509–17. doi: 10.1016/s0026-0495(99)90238-x. [DOI] [PubMed] [Google Scholar]
- [157].Vogt M, Puntschart A, Howald H, Mueller B, Mannhart C, Gfeller-Tuescher L, et al. Effects of dietary fat on muscle substrates, metabolism, and performance in athletes. Medicine and Science in Sports and Exercise. 2003 Jun;35(6):952–60. doi: 10.1249/01.MSS.0000069336.30649.BD. [DOI] [PubMed] [Google Scholar]
- [158].Iossa S, Mollica MP, Lionetti L, Crescenzo R, Botta M, Liverini G. Skeletal muscle oxidative capacity in rats fed high-fat diet. International Journal of Obesity. 2002 Jan;26(1):65–72. doi: 10.1038/sj.ijo.0801844. [DOI] [PubMed] [Google Scholar]
- [159].Turner N, Bruce CR, Beale SM, Hoehn KL, So T, Rolph MS, et al. Excess lipid availability increases mitochondrial fatty acid oxidative capacity in muscle - Evidence against a role for reduced fatty acid oxidation in lipid-induced insulin resistance in rodents. Diabetes. 2007 Aug;56(8):2085–92. doi: 10.2337/db07-0093. [DOI] [PubMed] [Google Scholar]
- [160].Chanseaume E, Giraudet C, Gryson C, Walrand S, Rousset P, Boirie Y, et al. Enhanced muscle mixed and mitochondrial protein synthesis rates after a high-fat or high-sucrose diet. Obesity. 2007 Apr;15(4):853–9. doi: 10.1038/oby.2007.582. [DOI] [PubMed] [Google Scholar]
- [161].Obici S, Wang JL, Chowdury R, Feng ZH, Siddhanta U, Morgan K, et al. Identification of a biochemical link between energy intake and energy expenditure. Journal of Clinical Investigation. 2002 Jun;109(12):1599–605. doi: 10.1172/JCI15258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Bonnard C, Durand A, Peyrol S, Chanseaume E, Chauvin MA, Morio B, et al. Mitochondrial dysfunction results from oxidative stress in the skeletal muscle of diet-induced insulin-resistant mice. Journal of Clinical Investigation. 2008 Feb;118(2):789–800. doi: 10.1172/JCI32601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Miller WC, Bryce GR, Conlee RK. Adaptations to a High-Fat Diet That Increase Exercise Endurance in Male-Rats. Journal of Applied Physiology. 1984;56(1):78–83. doi: 10.1152/jappl.1984.56.1.78. [DOI] [PubMed] [Google Scholar]
- [164].Sreekumar R, Unnikrishnan J, Fu A, Nygren J, Short KR, Schimke J, et al. Impact of high-fat diet and antioxidant supplement on mitochondrial functions and gene transcripts in rat muscle. American Journal of Physiology-Endocrinology and Metabolism. 2002 May;282(5):E1055–E61. doi: 10.1152/ajpendo.00554.2001. [DOI] [PubMed] [Google Scholar]
- [165].Chanseaume E, Tardy AL, Salles J, Giraudet C, Rousset P, Tissandier A, et al. Chronological approach of diet-induced alterations in muscle mitochondrial functions in rats. Obesity. 2007 Jan;15(1):50–9. doi: 10.1038/oby.2007.511. [DOI] [PubMed] [Google Scholar]
- [166].Garcia-Roves P, Huss JM, Han DH, Hancock CR, Iglesias-Gutierrez E, Chen M, et al. Raising plasma fatty acid concentration induces increased biogenesis of mitochondria in skeletal muscle. Proceedings of the National Academy of Sciences of the United States of America. 2007 Jun;104(25):10709–13. doi: 10.1073/pnas.0704024104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Miller WC, Bryce GR, Conlee RK. Adaptations to a high-fat diet that increase exercise endurance in male rats. 1984:78–83. doi: 10.1152/jappl.1984.56.1.78. [DOI] [PubMed] [Google Scholar]
- [168].Ruderman NB, Saha AK, Vavvas D, Witters LA. Malonyl-CoA, fuel sensing, and insulin resistance. American Journal of Physiology-Endocrinology and Metabolism. 1999 Jan;276(1):E1–E18. doi: 10.1152/ajpendo.1999.276.1.E1. [DOI] [PubMed] [Google Scholar]
- [169].Koves TR, Li P, An J, Akimoto T, Slentz D, Ilkayeva O, et al. Peroxisome proliferator-activated receptor-gamma co-activator 1 alpha-mediated metabolic remodeling of skeletal myocytes mimics exercise training and reverses lipid-induced mitochondrial inefficiency. Journal of Biological Chemistry. 2005 Sep;280(39):33588–98. doi: 10.1074/jbc.M507621200. [DOI] [PubMed] [Google Scholar]
- [170].Dobbins RL, Szczepaniak LS, Bentley B, Esser V, Myhill J, McGarry JD. Prolonged inhibition of muscle carnitine palmitoyltransferase-1 promotes intramyocellular lipid accumulation and insulin resistance in rats. Diabetes. 2001 Jan;50(1):123–30. doi: 10.2337/diabetes.50.1.123. [DOI] [PubMed] [Google Scholar]
- [171].Koves TR, Ussher JR, Noland RC, Slentz D, Mosedale M, Ilkayeva O, et al. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metabolism. 2008 Jan;7(1):45–56. doi: 10.1016/j.cmet.2007.10.013. [DOI] [PubMed] [Google Scholar]
- [172].Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature. 2006 Apr;440(7086):944–8. doi: 10.1038/nature04634. [DOI] [PubMed] [Google Scholar]
- [173].Turrens JF. Superoxide production by the mitochondrial respiratory chain. Bioscience Reports. 1997 Feb;17(1):3–8. doi: 10.1023/a:1027374931887. [DOI] [PubMed] [Google Scholar]
- [174].Esposito LA, Melov S, Panov A, Cottrell BA, Wallace DC. Mitochondrial disease in mouse results in increased oxidative stress. Proceedings of the National Academy of Sciences of the United States of America. 1999 Apr;96(9):4820–5. doi: 10.1073/pnas.96.9.4820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000 Nov;408(6809):239–47. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- [176].Li YB, Huang TT, Carlson EJ, Melov S, Ursell PC, Olson TL, et al. Dilated Cardiomyopathy and Neonatal Lethality in Mutant Mice Lacking Manganese Superoxide-Dismutase. Nature Genetics. 1995 Dec;11(4):376–81. doi: 10.1038/ng1295-376. [DOI] [PubMed] [Google Scholar]
- [177].Conniff ME, James FD, Huang T-T, Wasserman DH. Reduction in Mn-superoxide dismutase (SOD2) corrects insulin resistance due to high fat feeding in oxidative tissues of mice. Faseb Journal. 2008;22:1226.44. 2008. [Google Scholar]
- [178].Echtay KS. Mitochondrial uncoupling proteins - What is their physiological role? Free Radical Biology and Medicine. 2007 Nov;43(10):1351–71. doi: 10.1016/j.freeradbiomed.2007.08.011. [DOI] [PubMed] [Google Scholar]
- [179].VidalPuig A, Solanes G, Grujic D, Flier JS, Lowell BB. UCP3: An uncoupling protein homologue expressed preferentially and abundantly in skeletal muscle and brown adipose tissue. Biochemical and Biophysical Research Communications. 1997 Jun;235(1):79–82. doi: 10.1006/bbrc.1997.6740. [DOI] [PubMed] [Google Scholar]
- [180].Schrauwen P, Hoeks J, Schaart G, Kornips E, Binas B, van de Vusse GJ, et al. Uncoupling protein 3 as a mitochondrial fatty acid anion exporter. Faseb Journal. 2003 Oct;17(13):2272–4. doi: 10.1096/fj.03-0515fje. [DOI] [PubMed] [Google Scholar]
- [181].Himms-Hagen J, Harper ME. Physiological role of UCP3 may be export of fatty acids from mitochondria when fatty acid oxidation predominates: An hypothesis. Experimental Biology and Medicine. 2001 Feb;226(2):78–84. doi: 10.1177/153537020122600204. [DOI] [PubMed] [Google Scholar]
- [182].Goglia F, Skulachev VP. A function for novel uncoupling proteins: antioxidant defense of mitochondrial matrix by translocating fatty acid peroxides from the inner to the outer membrane leaflet. Faseb Journal. 2003 Sep;17(12):1585–91. doi: 10.1096/fj.03-0159hyp. [DOI] [PubMed] [Google Scholar]
- [183].Boss O, Samec S, PaoloniGiacobino A, Rossier C, Dulloo A, Seydoux J, et al. Uncoupling protein-3: A new member of the mitochondrial carrier family with tissue-specific expression. Febs Letters. 1997 May;408(1):39–42. doi: 10.1016/s0014-5793(97)00384-0. [DOI] [PubMed] [Google Scholar]
- [184].Echtay KS, Esteves TC, Pakay JL, Jekabsons MB, Lambert AJ, Portero-Otin M, et al. A signalling role for 4-hydroxy-2-nonenal in regulation of mitochondrial uncoupling. Embo Journal. 2003 Aug;22(16):4103–10. doi: 10.1093/emboj/cdg412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Millet L, Vidal H, Andreelli F, Larrouy D, Riou JP, Ricquier D, et al. Increased uncoupling protein-2 and -3 mRNA expression during fasting in obese and lean humans. Journal of Clinical Investigation. 1997 Dec;100(11):2665–70. doi: 10.1172/JCI119811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Bao S, Kennedy A, Wojciechowski B, Wallace P, Ganaway E, Garvey WT. Expression of mRNAs encoding uncoupling proteins in human skeletal muscle - Effects of obesity and diabetes. Diabetes. 1998 Dec;47(12):1935–40. doi: 10.2337/diabetes.47.12.1935. [DOI] [PubMed] [Google Scholar]
- [187].Vidal H, Langin D, Andreelli F, Millet L, Larrouy D, Laville M. Lack of skeletal muscle uncoupling protein 2 and 3 mRNA induction during fasting in type-2 diabetic subjects. American Journal of Physiology-Endocrinology and Metabolism. 1999 Nov;277(5):E830–E7. doi: 10.1152/ajpendo.1999.277.5.E830. [DOI] [PubMed] [Google Scholar]
- [188].Krook A, Digby J, O’Rahilly S, Zierath JR, Wallberg-Henriksson H. Uncoupling protein 3 is reduced in skeletal muscle of NIDDM patients. Diabetes. 1998 Sep;47(9):1528–31. doi: 10.2337/diabetes.47.9.1528. [DOI] [PubMed] [Google Scholar]
- [189].Schrauwen P, Mensink M, Schaart G, Moonen-Kornips E, Sels J-P, Blaak EE, et al. Reduced Skeletal Muscle Uncoupling Protein-3 Content in Prediabetic Subjects and Type 2 Diabetic Patients: Restoration by Rosiglitazone Treatment. Journal of Clinical Endocrinology & Metabolism. 2006 April 1;91(4):1520–5. doi: 10.1210/jc.2005-1572. 2006. [DOI] [PubMed] [Google Scholar]
- [190].Vidal-Puig AJ, Grujic D, Zhang CY, Hagen T, Boss O, Ido Y, et al. Energy metabolism in uncoupling protein 3 gene knockout mice. Journal of Biological Chemistry. 2000 May;275(21):16258–66. doi: 10.1074/jbc.M910179199. [DOI] [PubMed] [Google Scholar]
- [191].Gong DW, Monemdjou S, Gavrilova O, Leon LR, Marcus-Samuels B, Chou CJ, et al. Lack of obesity and normal response to fasting and thyroid hormone in mice lacking uncoupling protein-3. Journal of Biological Chemistry. 2000 May;275(21):16251–7. doi: 10.1074/jbc.M910177199. [DOI] [PubMed] [Google Scholar]
- [192].Cline GW, Vidal-Puig AJ, Dufour S, Cadman KS, Lowell BB, Shulman GI. In vivo effects of uncoupling protein-3 gene disruption on mitochondrial energy metabolism. Journal of Biological Chemistry. 2001 Jun;276(23):20240–4. doi: 10.1074/jbc.M102540200. [DOI] [PubMed] [Google Scholar]
- [193].Brand MD, Pamplona R, Portero-Otin M, Requena JR, Roebuck SJ, Buckingham JA, et al. Oxidative damage and phospholipid fatty acyl composition in skeletal muscle mitochondria from mice underexpressing or overexpressing uncoupling protein 3. Biochemical Journal. 2002 Dec;368:597–603. doi: 10.1042/BJ20021077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Li B, Nolte LA, Ju JS, Han DH, Coleman T, Holloszy JO, et al. Skeletal muscle respiratory uncoupling prevents diet-induced obesity and insulin resistance in mice. Nature Medicine. 2000 Oct;6(10):1115–20. doi: 10.1038/80450. [DOI] [PubMed] [Google Scholar]
- [195].Clapham JC, Arch JRS, Chapman H, Haynes A, Lister C, Moore GBT, et al. Mice overexpressing human uncoupling protein-3 in skeletal muscle are hyperphagic and lean. Nature. 2000 Jul;406(6794):415–8. doi: 10.1038/35019082. [DOI] [PubMed] [Google Scholar]
- [196].Choi CS, Fillmore JJ, Kim JK, Liu Z-X, Kim S, Collier EF, et al. Overexpression of uncoupling protein 3 in skeletal muscle protects against fat-induced insulin resistance. Journal of Clinical Investigation. 2007 July 2;117(7):1995–2003. doi: 10.1172/JCI13579. 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].MacLellan JD, Gerrits MF, Gowing A, Smith PJS, Wheeler MB, Harper ME. Physiological increases in uncoupling protein 3 augment fatty acid oxidation and decrease reactive oxygen species production without uncoupling respiration in muscle cells. Diabetes. 2005 Aug;54(8):2343–50. doi: 10.2337/diabetes.54.8.2343. [DOI] [PubMed] [Google Scholar]
- [198].Duval C, Camara Y, Hondares E, Sibille B, Villarroya F. Overexpression of mitochondrial uncoupling protein-3 does not decrease production of the reactive oxygen species, elevated by palmitate in skeletal muscle cells. Febs Letters. 2007 Mar;581(5):955–61. doi: 10.1016/j.febslet.2007.01.085. [DOI] [PubMed] [Google Scholar]
- [199].Wang H, Knaub LA, Jensen DR, Jung D Young, Hong E-G, Ko H-J, et al. Skeletal Muscle-Specific Deletion of Lipoprotein Lipase Enhances Insulin Signaling in Skeletal Muscle but Causes Insulin Resistance in Liver and Other Tissues. Diabetes. 2009 January 1;58(1):116–24. doi: 10.2337/db07-1839. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Abel ED, Peroni O, Kim JK, Kim YB, Boss O, Hadro E, et al. Adipose-selective targeting of the GLUT4 gene impairs insulin action in muscle and liver. Nature. 2001 Feb;409(6821):729–33. doi: 10.1038/35055575. [DOI] [PubMed] [Google Scholar]
- [201].Yang Q, Graham TE, Mody N, Preitner F, Peroni OD, Zabolotny JM, et al. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature. 2005 Jul;436(7049):356–62. doi: 10.1038/nature03711. [DOI] [PubMed] [Google Scholar]
- [202].Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004 Oct;306(5695):457–61. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- [203].Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, et al. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006 Aug;313(5790):1137–40. doi: 10.1126/science.1128294. [DOI] [PMC free article] [PubMed] [Google Scholar]