Abstract
Curcumin, the active compound of the rhizome of Curcuma longa has anti-inflammatory, antioxidant and antiproliferative activities. This agent has been shown to regulate numerous transcription factors, cytokines, protein kinases, adhesion molecules, redox status and enzymes that have been linked to inflammation. While curcumin has been identified as an activator of apopotosis in several cell lines, the mechanism by which it initiates apoptosis, however, remains poorly understood. We considered curcumin from the point of view of its ability to protect against oxidative stress, the latter being one factor strongly implicated in the development of Parkinson’s disease. Althougth the etiology of Parkinson’s disease remains unknown, epidemiological studies have linked exposure to pesticides such paraquat to an increased risk of developing the condition. Analysis of the neurotoxic properties of these pesticide compounds has been focused on their ability to induce oxidative stress in neural cells. Given curcumin’s capacity to protect against oxidative stress, it has been considered as a potential therapeutic agent for neurodegenerative diseases such as Parkinson’s disease that involve an oxidative stress component. In the present report we describe the effect of curcumin in paraquat-mediated apoptosis of N27 mesencepahlic cells. We show that subtoxic concentrations of curcumin sensitize N27 mesencephalic cells to paraquat-mediated apoptosis.
Keywords: paraquat, curcumin, Parkinson’s disease, oxidative stress, apoptosis
Introduction
Curcuma longa L. (turmeric) has been used for hundreds of years as a flavoring, coloring, and preservative. Curcumin, an important constituent of turmeric, is a nutriceutical compound reported to possess therapeutic properties against a variety of diseases ranging from cancer to cystic fibrosis (Liu et al., 1996). Curcumin has been shown to exhibit a variety of biological activities including antioxidative activity (Wei et al., 2006), scavenging of superoxide anions and nitric oxide radicals (Sreejayan and Rao, 1997), an inhibition of lipid peroxidation (Sreejayan and Rao, 1996) and lipoxygenase/cycloxygenase activity (Huang et al., 1991). The strong antioxidant activity of curcumin makes it an interesting candidate for use in counteracting oxidative stress-induced damage. Given our interest in Parkinson’s disease (PD), curcumin could be potentially useful in combating the oxidative damage triggered by neurotoxicants such as paraquat, which has been implicated in causing PD. To this extent, curcumin has been proposed as a neuroprotective agent in neurodegenerative disorders such Alzheimer’s disease (Aggarwal and Harikumar, 2008;Baum et al., 2008;Begum et al., 2008;Cole et al., 2007a) and Parkinson’s disease (Cole et al., 2007;Jagatha et al., 2008;Rajeswari and Sabesan, 2008).
Recent studies have highlighted how curcumin is able to enhace cell apoptosis via a mechanism which is dependent on the generation of reactive oxygen species (ROS) (Thayyullathil et al., 2008;Su et al., 2006). However, other reports have indicated how curcumin is able to inhibit ROS, and consequently apoptosis, through its well known antioxidant propierties (Chen et al., 2006;Chan et al., 2005).
Paraquat (1,1-dimethyl-4,42-bipyridinium dichloride), a widely used herbicide, has been suggested as a potential etiologic factor for the development of PD, a neurodegenerative disorder characterized by the selective loss of dopaminergic neurons in the substantia nigra (SN) (Burke, 1998). This is accompanied by typical clinical manifestations, such as rest tremors, bradykinesia, rigidity, and postural instability. However, the causes of PD remain unknown, and over the years the mechanism of genetic inheritance versus environmental factors has been debated. Exposure to environmental agents such paraquat have been linked repeatedly with the development of PD (Tanner et al., 1989; Tanner, 1989; Gorell et al., 1998). PQ enhances cell death through a mechanism that is dependent on ROS production and subsequent oxidative stress damage (Thrash et al., 2007;Drechsel and Patel, 2008;Chen et al., 2008;Yang and Tiffany-Castiglioni, 2005). In addition, PQ has been identified as a trigger for neural cell apoptosis (Moran et al., 2008;Gonzalez-Polo et al., 2004;Gonzalez-Polo et al., 2007a,b).
Due to its well known anti-apoptic and antioxidant properties, and the fact that it has been proposed as a putative agent in the treatment of some neurodegenerative disorders, such PD, we hypothesized that curcumin would be able to prevent the PQ-mediated apoptosis of rat mesencephalic N27 cells. The results that we present here differ from previous hypotheses, and show how curcumin is not able to protect against PQ-mediated neurotoxicity but enhances the PQ-mediated apoptosis of N27 cells.
Materials and methods
Cell culture
Rat mesencephalon-derived cell line (1RB3AN27; hereafter referred to as N27 cells), represents a homogenous population of tyrosine hydroxylase-positive cells with functional characteristics resembling dopaminergic neurons (Prasad et al., 1998), as an in vitro model for studies of dopaminergic neurodegeneration (Kitazawa et al., 2003). Diverse studies have established that N27 cells are an adequate cell culture model for studying dopaminergic neurodegeneration, compared to PC12 and SH-SY5Y cells, as N27 cells are derived from the mesencephalon, a brain region directly affected in some neurodegeneratives diseases like PD (Anantharam et al., 2002; Kaul et al., 2003; Yang et al., 2004; Kaul et al., 2005; Kanthasamy et al., 2006; Sun et al., 2006) MPP+ treatment in N27 cells induces acute generation of ROS in a time- and dose-dependent manner (Kaul et al., 2003; Kaul et al., 2005), and that ROS generation precedes changes in mitochondrial membrane potential or cytochrome C release. Immortalized N27 cells were grown in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS, Hyclone, Brevieres, France), 1% L-glutamine, penicillin (100 U/ml), and streptomycin (100 U/ml). Cells were seeded and maintained at a density of 1×106/cm2 in 75-cm2 tissue culture flasks (Corning, New York, NY) and incubated at 37°C under saturating humidity in 5% CO2/95% air.
Reagents, treatment and induction of cell death
Confluent cells (≃80%) in 75-cm2 tissue culture flasks were trypsinized and seeded in 24-well cell culture plates to viability and for flow cytometry assay or 6 well to western blot analysis at a concentration of 2.5–3.5 ×104 cells/ml. The N27 cells were preincubated with curcumin-concentrations for 24 hours prior to the addition of PQ, and then incubated for 24 hours at 37°C. When carried out, preincubation with antioxidants N-Acetyl-Cysteine (NAC) (10 mM) and Vitamin E (VitE) (200 μM), was carried out 30 mins prior to the addition of PQ or curcumin.
MTT reduction assay
The MTT assay for measuring cytotoxicity and cell growth developed by Mosman (1983) was performed following the modifications described by Hansen (1989). Viable cells with active mitochondria reduce the colourless tetrazolium salt MTT (Sigma, St. Louis, MO), producing dark blue water-insoluble formazan crystals. To perform the assay, MTT was dissolved at a concentration of 5 mg/ml in PBS. Two hours before the end of the experiment, the MTT solution was added to 24-well plates (50 μl per well) and the plates were returned to the incubator. Following the 2 h incubation period, the medium was decanted, and the formazan precipitates were solubilized with acidic isopropanol (0.04–0.1 N HCl in absolute isopropanol). Optical density (OD) was measured at 570 nm (reference wavelength 630 nm), using the extraction solution as a blank. The absorbance of the converted dye was measured at a wavelength of 570 nm with background subtraction at 630–690 nm. The absorbance of untreated cultures was set at 100%.
Flow cytometry
production, superoxide anion generation and apoptotic cell death were monitored using cytofluorometry. We used 4-amino-5-methylamino-2′,7′-difluorofluorescein diacetate (DAF-FM diacetate; 5μM) for the measurement of NO production, and hydroethidine (HE; 10μM) for the determination of superoxide anion generation. Apoptotic cell death was determined by assessment of phosphatidylserine exposure, we used Annexin-V labeled with fluorescein isothiocyanate. Propidium iodide (PI; 1 μg/ml) was used to determine cell viability. All of these reagents were obtained from Molecular Probes, Eugene, OR. After exposure to different experimental conditions, cells were trypsinized and labelled with the fluorochromes at 37°C, followed by cytofluorometric analysis with a FACS scanner (Becton Dickinson, New York, NY). A total of 10000 events were analyzed for each condition.
Immunofluorescence microscopy
Cells were cultured on coverslips pretreated with poly-L-lysine. After the experimental period, the cells were first fixed with paraformaldehyde (4% w:v) and then permeabilized with Triton X-100 solution (Triton 0.2% in PBS) for 10 minutes. After blocking for 20 minutes with bovine serum albumin (BSA) solution (1 mg/ml in PBS), cells were incubated with primary antibodies for 1.5 hours at RT with antibody directed against cleaved Caspase-3 (Cell Signaling Technology), cytochrome c (Santa Cruz Biotechnology) and 3-nitrotyrosine (3NT; Sigma-Aldrich) developed with an anti-mouse or anti-rabbit immunoglobulin Alexa®fluor conjugate (Molecular Probes, Eugene, OR), and finally counterstained with Hoechst 33342 (2 μM; Sigma) before mounting. Fluorescence was analysed using an Olympus IX51 microscope equipped with a DC300F camera. The quantitative measurement of the fluorescence signal was performed as described by Kirkeby and Thomsen (2005).
Western blot analysis
Following experimental treatments, the cells were rinsed twice with cold PBS and removed by scraping. Cells were lysed in buffer containing 50 mM Tris-HCl pH 6.8, 10% glycerol, and 2% SDS, heated at 95 °C for 10 minutes and then stored at −80°C until required for analysis by western blot. Protein concentration was measured based on the BCA method, using a Bicinchoninic Acid Kit (Sigma, St. Louis, MO) according to the manufacturer’s instructions, with BSA used as a standard. Equal amounts of protein (40μg/condition) were resolved by 8–12% SDS-gel electrophoresis and transferred to polyvinylidene fluoride (PVDF) membranes according to a partially modified conventional method (Fuentes et al., 2000). Briefly, proteins were transferred (250 mA for 60 min) to PVDF membranes using a Mini Trans-Blot Cell apparatus (Bio-Rad, Hercules, CA). The procedure for immunodetection included the transfer and blocking of the membrane (60 min at room temperature) with TTBS (10 mM Tris/HCl pH 7.5, 150 mM NaCl and 0.2 % Tween-20) containing 5% BSA (Sigma). Membranes were then incubated overnight at 4°C with primary anti-IκBα antibody (diluted 1:500; Santa Cruz Biotechnology) or anti-nitrotyrosine antibody (diluted 1:1000; Sigma-Aldrich). After washing (for two 5-min periods with TTBS), membranes were incubated (60 min at RT) with peroxidase-conjugated secondary antibodies (1:5000 in TTBS with 10% non-fat dry milk). After washing (for two 5-min periods and one 10-min period), the detection of bound antibodies was visualized by chemiluminescence using the ECL-plus reagent (GE Healthcare, Bucks, UK). Actin content was analysed as a control using a rabbit polyclonal antibody (Santa Cruz Biotechnology).
RNA extraction and relative quantification of gene expression
Total RNA was extracted from N27 cells with TRI Reagent® (Ambion, Austin, Texas, USA) according to manufacturer’s instructions. RNA was quantified using a Biowave II® spectrophotometer (Biochrom Ltd, Cambridge, UK), and its integrity was checked electrophoretically. Reverse transcription and amplification were performed in one step with the RNA-to-Ct® kit (Applied Biosystems, Madrid, Spain), according to the manufacturer’s instructions. For relative quantification of gene expression, the 5′ exonuclease (TaqMan) assay, which produces a directly proportional readout with the progression of PCR, was used. Amplification of cDNA derived from 50 ng total RNA was performed in a 20 μl reaction volume according to standard conditions provided by the manufacturer. Briefly, amplified cDNA of studied samples was quantified in 20 μL reactions containing 10 μl TaqMan Gene Expression Master Mix, 0.5 μl TaqMan RT Enzyme, 1 μL of the individual TaqMan Gene Expression Assay (all from Applied Biosystems), 8 μl nuclease free-H2O and 0.5 μl total RNA. Thermal cycler conditions were 15 min at 48 °C, 10 min at 95 °C followed by 40 cycles of 30 s at 95 °C to denature the DNA and 30 s at 60 °C to anneal and extend the template. All reactions were performed in duplicate (endogenous control GAPDH was measured in triplicate) in a Model 7500 thermocycler (Applied Biosystems). The values obtained for the target gene expression were normalized to GAPDH and quantified relative to the expression in control samples. For the calculation of relative quantification, the 2−ΔΔCT formula was used, where −ΔΔCT = (CT,target−CT,GAPDH) experimental sample − (CT,target−CT,GAPDH) control sample.
Statistical analysis
Each experiment was repeated at least three times, with satisfactory correlation between the results of individual experiments. The data shown are those of representative experiments; each result being the average of three to four culture dishes. In each experiment, differences between groups were assessed by appropriate statistical methods. Statistical significance was evaluated using the Chi-square test, and all comparisons giving a p value less than 0.05 (p<0.05) were considered statistically significant. Data are expressed as the mean ± SEM. All data were analyzed with SPSS 12.0 software (Chicago, IL) for Windows
Results
Cell viability after exposure to PQ and curcumin
The viability of N27 mesencephalic cells was evaluated after 24 hr of exposure to PQ (Fig. 1A). Cell viability was assayed by the MTT method and significant effects were found for PQ concentrations from 100 to 500 μM. Cells were exposed for 24 hr to curcumin concentrations ranging from 0.001 nM to 50 μM, with significant toxicity observed for concentrations in excess of 10 μM (data not shown) (Fig. 1B).
Fig. 1.
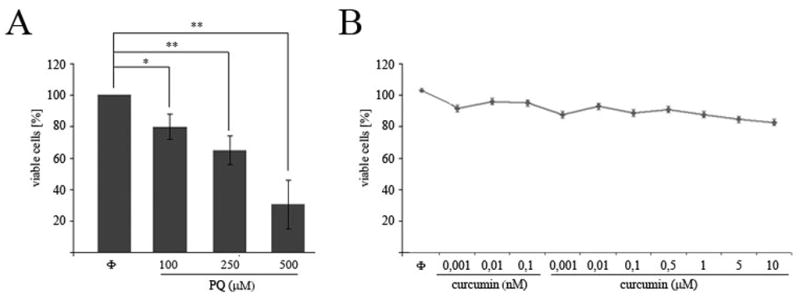
Cell viability measurement after exposure to PQ or curcumin. N27 cells were exposed for 24 h to the indicated concentrations of curcumin or PQ. Cell viability measured by MTT assay after PQ (A) or curcumin (B) exposure in N27 cells. Data are expressed as the mean ± SEM. **p<0.01.
Curcumin increases cell death in PQ-exposed N27 cells
N27 cells were co-incubated with PQ concentrations ranging from 100 μM to 500 μM and 10 nM curcumin for 24 hr (Fig. 2). As shown in Fig. 2A, compared to control, the presence of curcumin exacerbated the extent of cell death induced at each concentration of PQ. This effect, however, was independent of the concentration of curcumin used (Fig. 2B).
Fig. 2.
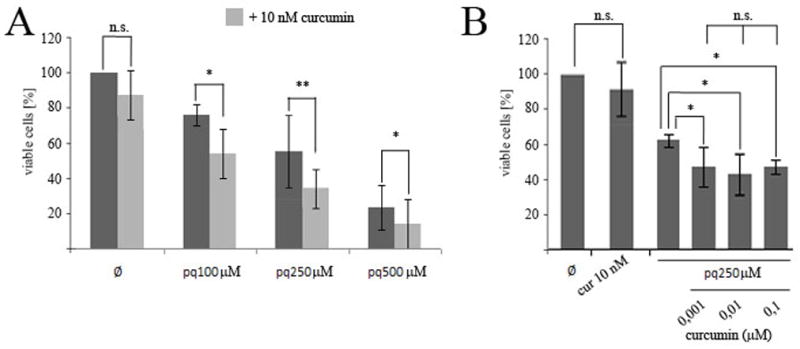
Effect of curcumin exposure on the viability of PQ-exposed N27 cells. Cells were preincubated for 24h with the indicated amounts of curcumin before exposure to the indicated amounts of PQ for 24h. Cell viability was measured by the MTT assay (A,B). Data are expressed as the mean ± SEM. n.s. not significant,*p<0.05, **p<0.01.
Curcumin enhances the PQ-mediated induction of apoptosis in N27 cells
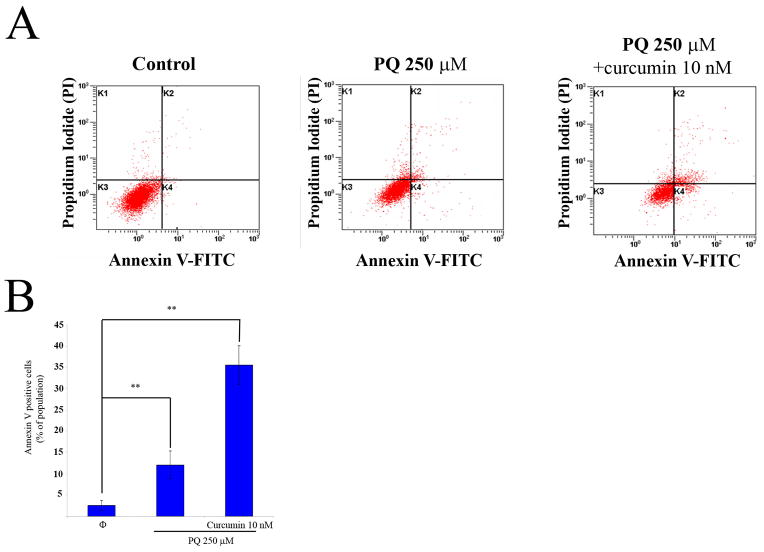
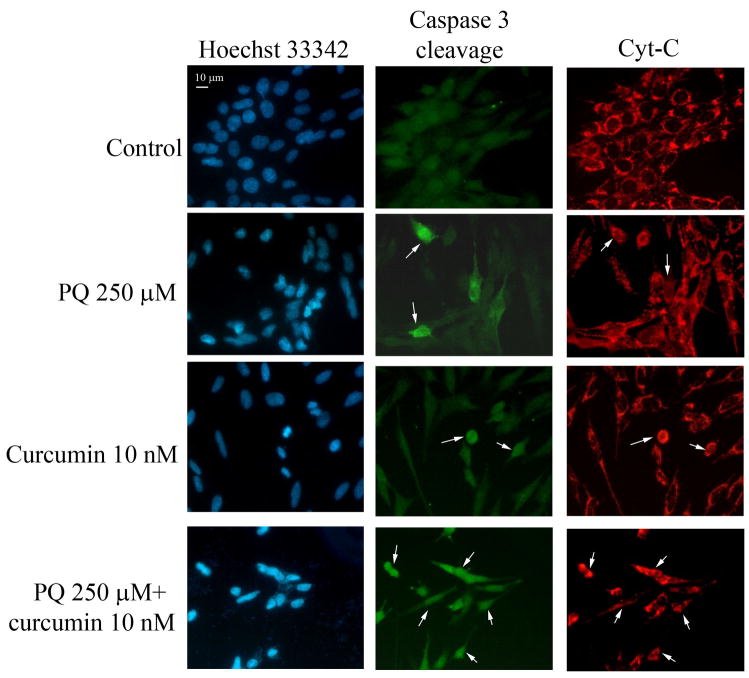
We wished to characterize the type of cell death involved in the experiments outlined above. To examine whether PQ causes apoptosis of N27 cells, we performed flow cytometry experiments with Annexin V/PI on cells treated with 250 μM PQ for 24 hours (Fig. 3A). N27 cells treated with 10 nM curcumin alone did not show any detectable evidence of apoptosis (data not shown); however, the co-incubation of N27 cells with PQ and curcumin resulted in a 34 % increase in the number of apoptotic cells detected (Fig 3B). Using fluorescence microscopy we also found that PQ induces chromatin condensation, caspase 3 cleavage, and triggers the release of cytochrome c into the cytosol (Fig 4).
Fig. 3.
Curcumin increases phosphatidylserine outsourcing induced by PQ in N27 cells. Apoptotic cells detected by flow cytometry with Annexin V/IP. (A) Representative plots of flow cytometry with Annexin V/IP of N27 cells after exposure to PQ 250 μM or PQ 250μM plus curcumin 10nM. (B) Measurement of phosphatidylserine outsourcing after treatment with or without exposed to PQ 250μM for 24h. Data are expressed as the mean ± SEM. **p<0.01.
Fig. 4.
Curcumin increases PQ-induced apoptotic cell death in N27 cells. Inmunocytochemical analysis of active Caspase-3 (Casp-3 cleavage), released cytochrome C (Cyt-C) and chromatin condensation (Hoecthst 3342) in N27 cells treated with PQ, curcumin or both.
Curcumin-enhanced PQ-induced apoptosis of N27 cells is a ROS-dependent mechanism
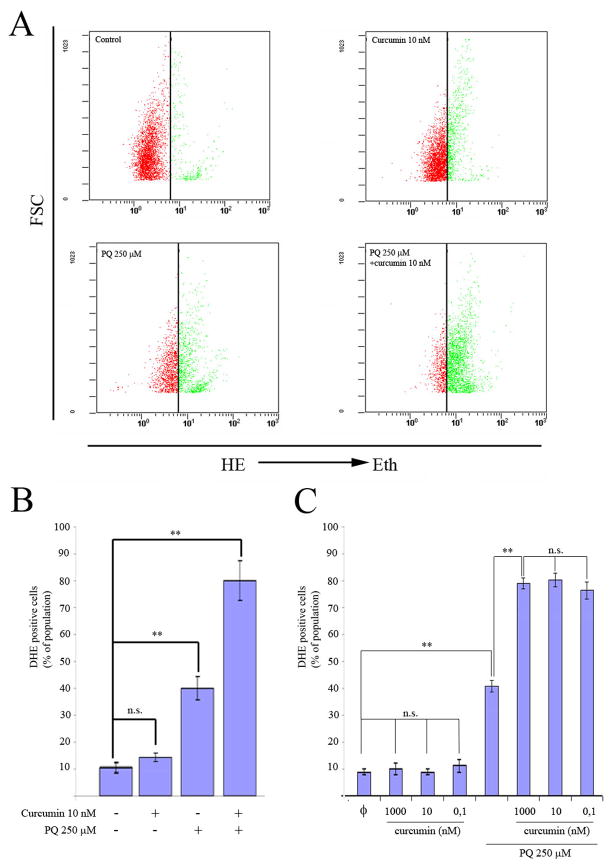
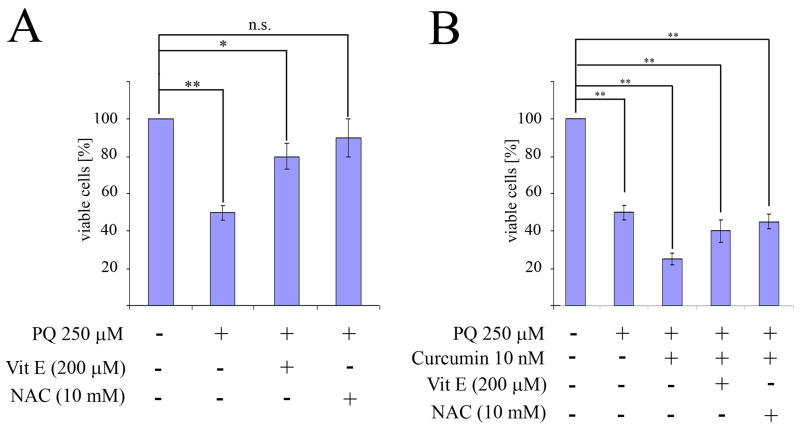
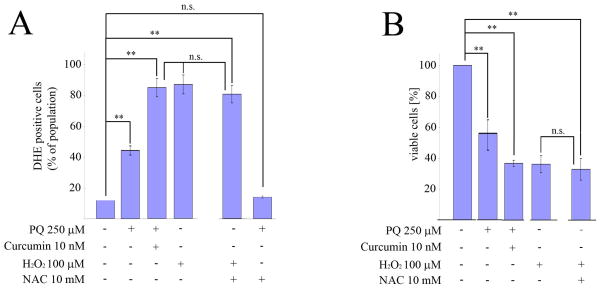
Given that an important mechanism by which many stimuli induce apoptosis is via the generation of ROS, we explored whether this was also the case in relation to PQ+curcumin-exposed cells. To assess intracellular ROS production, we used flow cytometry to measure the oxidation of HE. As anticipated, the exposure of N27 cells to 250 μM PQ for 24 hr resulted in a significant increase in ROS production, while control cells and cells exposed to curcumin alone had relatively modest levels of ROS production compared to PQ-exposed cells (Fig 5A). Surprisingly, cells co-incubated with curcumin and PQ 250 μM exhibited a heightened production of ROS (Fig 5B). This effect was not dependent on the concentration of curcumin used (0.1 nM to 1 μM) (Fig. 5C). In the presence of the potent antioxidants NAC (10 mM) and VitE (200 μM), cell viability measured using the MTT test increased significantly after PQ exposure (250 μM) compared to cells not treated with antioxidants (Fig. 6A). However when N27 cells were co-incubated with PQ (250μM) plus curcumin (10 nM), in addition to the indicated antioxidants, cell viability did not increase (Fig 6B). These data suggest that cell susceptibility to PQ+curcumin stimulation might be mediated by ROS, but they also indicate that rescue of N27 cells by NAC or VitE antioxidant therapy is not a viable approach.
Fig. 5.
Exposure to PQ plus curcumin enhances ROS generation. (A) Generation of DHE oxidation analyzed by flow cytometry in N27 cells exposed to the indicated amounts of PQ, curcumin, or both. (B) Quantification of oxidation of DHE analyzed by flow cytometry in N27 cells exposed to the indicated amounts of PQ, curcumin, or both. (C) Measurement of DHE-positive cells by flow cytometry in N27 cells treated with different curcumin-concentrations with or without exposure to PQ 250μM for 24h. Data are expressed as the mean ± SEM. n.s. not significant,*p<0.05, **p<0.01.
Fig. 6.
Antioxidants protect N27 cells from PQ-mediated toxicity but not from toxicity induced by treatment with PQ+curcumin. Cell viability was measured by MTT assay. (A) N27 cells were preincubated for 30 min prior to PQ exposure with vitamin E (Vit E) and N-acetyl-cysteine (NAC) at indicated concentrations. (B) N27 cells were preincubated for 30 min prior to treatment with PQ or PQ plus curcumin with vitamin E (Vit E) and N-acetyl-cysteine (NAC) at indicated concentrations. Data are expressed as the mean ± SEM. n.s. not significant,*p<0.05, **p<0.01.
To expand on this result, we incubated N27 cells for 5 hr with the well known ROS generating molecule H2O2 (0.1 μM to 500 μM) and then measured ROS production by flow cytometry with DHE. As observed in Fig. 7A, exposure of cells to 100 μM H2O2 increased ROS levels in a similar manner to that obtained with 250 μM PQ +10 nM curcumin, with similar cell viability levels as measured with the MTT test (Fig. 7B). This level of oxidative stress could not be rescued by co-incubation with NAC (Fig 7B), indicating that the burst of ROS generated by the co-incubation with PQ (250 μM) and curcumin (10 nM) irreversibly initiates cell death cascades.
Fig. 7.
Effect of oxidative stress generated after treatment with PQ plus curcumin cannot be rescued by co-incubation with antioxidants. (A) Flow cytometry analysis of ROS generation by N27 cells co-incubated with 10 mM NAC for 30 min prior to treatment with H2O2. (B) The MTT assay was used to measure the viability of N27 cells co-incubated with 10 mM NAC for 30 min prior to treatment with H2O2 at the indicated concentrations. Data are expressed as the mean ± SEM. n.s. not significant, **p<0.01.
A NOS-dependent mechanism of curcumin-enhanced PQ-mediated cell death
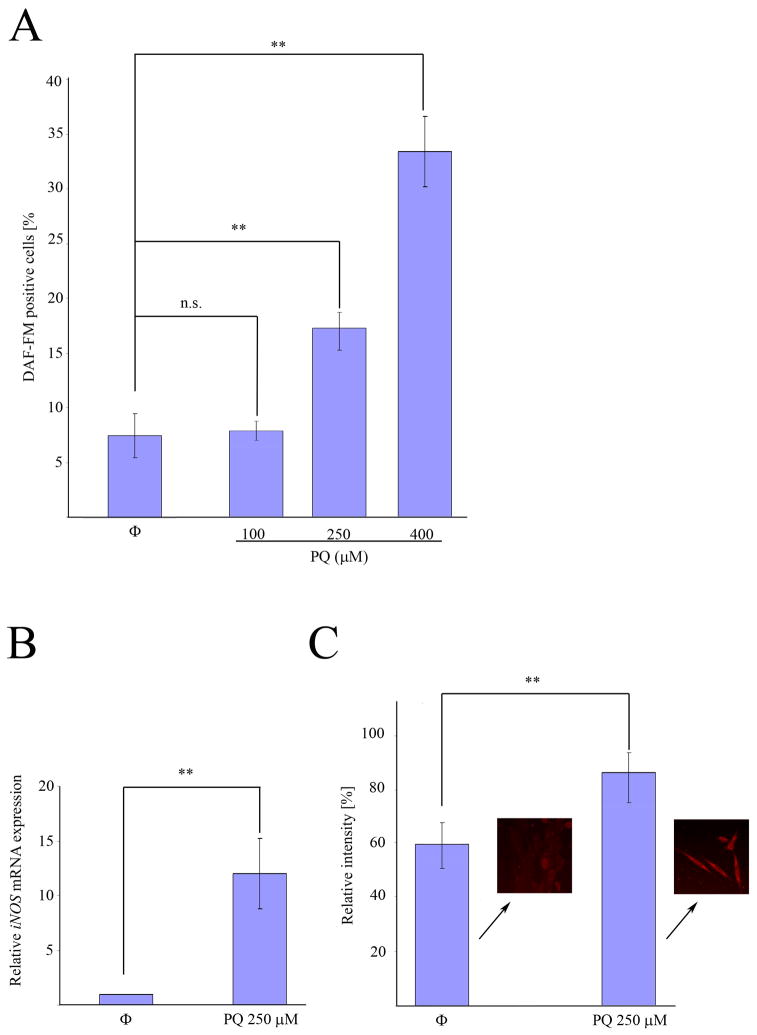
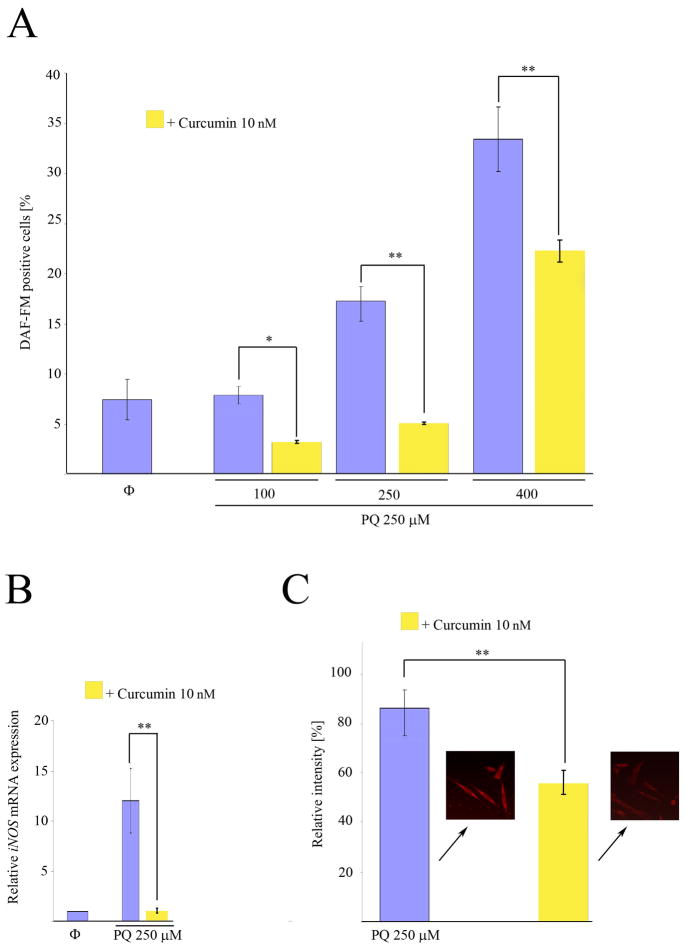
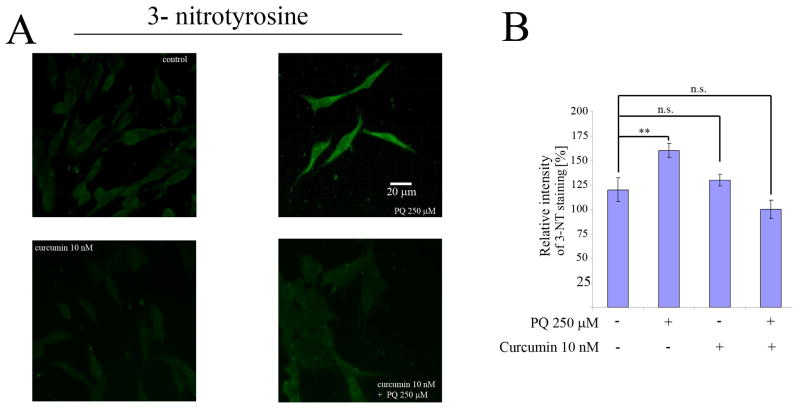
Once we determined that the enhacement of cell death in PQ+curcumin-exposed cells was ROS-dependent, we considered other means by which curcumin could be able to trigger this effect. Although the molecular mechanisms underlying curcumin activity are poorly understood, one process, related to the inhibition of expression of the inducible isoform of nitrogen oxide synthase (iNOS), has been widely reported. On this basis we first assessed if indeed PQ-exposed N27 cells produce NO. As shown in Fig. 8A, flow cytometry experiments using the dye DAF-FM demonstrated that N27 cells produce NO in a manner dependent on the PQ concentration to which they are exposed. Real time PCR experiments showed that this production was accompanied by an increase in the level of iNOS mRNA expression after 24 hr exposure to 250 μM PQ (Fig. 8B), and an increase in iNOS protein expression as determined by immunofluorescence (Fig. 8C). When cells were co-incubated with 250 μM PQ and 10 nM curcumin, the nitric oxide production was partially inhibited (Fig. 9A), and iNOS mRNA expression (Fig. 9B) and protein production (Fig. 9C) after 24 hr were reduced. As the mechanism of inhibition of iNOS expression by curcumin is Iκκ dependent, (Singh and Khar, 2006) we evaluated the integrity of IκB in PQ− and PQ+curcumin-exposed cells. As shown in Fig. 10, curcumin effectively inhibited IκB degradation, thus abrogating NF-κB translocation and therefore iNOS expression. Overall, the data demonstrate that curcumin inhibits NO production in N27-exposed cells. Superoxide scavenging by NO to generate peroxynitrite has been proposed as a defensive mechanism in PQ-mediated cell death, thereby protecting the cell in part against oxidative stress generated by superoxide. This process does, however, also generate the toxic compound peroxynitrite. We thus assessed the production of peroxynitrite in terms of the generation of 3-NT in PQ−, curcumin- and PQ+curcumin-exposed cells. As can be seen in Fig. 11, 3-NT production is increased in PQ-exposed cells (250 μM for 24 hr) and decreased in N27 cells co-incubated with 250 μM PQ and 10 nM curcumin.
Fig. 8.
PQ exposure induces an increase of iNOS mRNA and protein expression in N27 cells. (A) Analysis of DAF-FM-positive cells by flow cytometry after PQ treatment at indicated concentrations. (B) Quantification of iNOS mRNA expression by real-time RT-PCR after incubation with 250μM PQ for 24h.(C) Relative quantification of iNOS fluorescence signal by immunocytochemistry in N27 cells exposed to 250 μM PQ. Data are expressed as the mean ± SEM. n.s. not significant, **p<0.01.
Fig. 9.
Inhibition by curcumin of NO production, and expression of iNOS mRNA and protein in N27 cells after treatment with PQ. (A) Analysis of DAF-FM positive N27 cells by flow cytometry after exposure to PQ or PQ plus curcumin at the indicated concentrations. (B) Quantification of iNOS mRNA expression by real-time RT-PCR after incubation with 250 μM PQ or 250 μM PQ plus 10 nM curcumin for 24h.(C) Relative quantification of iNOS fluorescence signal by immunocytochemistry in N27 cells exposed to 250 μM PQ or 250 μM PQ plus curcumin 10 nM for 24h. Data are expressed as the mean ± SEM. n.s. not significant, **p<0.01.
Fig. 10.
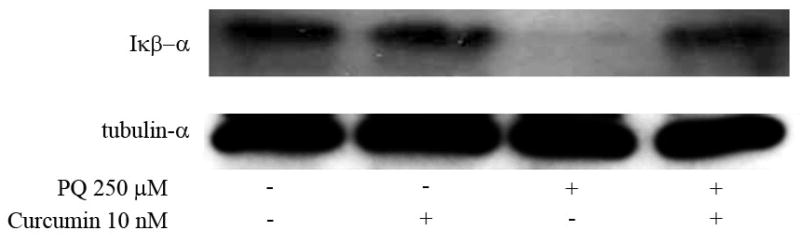
Curcumin inhibits the PQ-induced degradation of IκBα. Western blot analysis of IκBα expression in N27 cells treated with PQ 250μM, 10nM curcumin or both. α-tubulin was used for equal loading.
Fig. 11.
Inhibition by curcumin of PQ-induced 3-Nitrotyrosine (3-NT) formation. (A) Immunocytochemical analysis of 3-NT formation in rat N27 cells after exposure to 250 μM PQ, 10 nM curcumin, or both. (B) Relative quantification by immunocytochemistry of 3-NT fluorescence signal after exposure of N27 cells to 250 μM PQ, 10 nM curcumin, or both. Data are expressed as the mean ± SEM. n.s. not significant, **p<0.01.
Discussion
Curcumin, a polyphenol derived from the plant Curcuma longa, is known to be a potent antioxidant (Menon and Sudheer, 2007;Bengmark, 2006) and could therefore have desirable preventive or putative therapeutic properties in medicine Curcumin has thus been proposed as a potential candidate to treat neurodegenerative diseases involving oxidative stress such as PD (Jagatha et al., 2008;Zbarsky et al., 2005), Alzheimer’s Disease (Ringman et al., 2005;Ono et al., 2004;Lim et al., 2001) and other neurodegenerative disorders (Calabrese et al., 2008;Cole et al., 2007;Bengmark, 2006).
PQ has been proposed as a putative etiologic factor in the development of PD (Thrash et al., 2007;Jones and Miller, 2008;Barlow et al., 2007;Dick, 2006;Dinis-Oliveira et al., 2006). The redox cycling of the pesticide PQ is characterized by the generation of ROS (Bonneh-Barkay et al., 2005) an event that has been described as a key functional element in the relation of the pesticide with PD (Miller et al., 2009;Drechsel and Patel, 2008;Chen et al., 2008;Castello et al., 2007;Yang and Tiffany-Castiglioni, 2005;Przedborski and Ischiropoulos, 2005;Thiruchelvam et al., 2005).
Antioxidant treatments have thus been proposed as potential strategies in the prevention of neuronal injury after exposure to PQ (Chen et al., 2008;Thiruchelvam et al., 2005;Reiter et al., 1997). As curcumin exhibits potent antioxidant properties, we considered that it could serve as a potential protective agent against the PQ-mediated toxicity of N27 mesencephalic cells. Surprisingly, curcumin was not able to protect N27 cells against PQ-mediated toxicity, but rather it exacerbated the cell death and dramatically decreased cell viability. This effect was clearly synergistic in nature, because curcumin itself at the concentrations used was not toxic to N27 cells. Combined use of curcumin with PQ, however, increased cell death significantly compared to levels reached with the pesticide alone.
Our aim was to characterize the nature of cell death that occurred in response to the co-incubation of cells with curcumin and PQ. As has been previously reported, PQ exposure was able to initiate apoptosis in SH-SY5Y cells (Moran et al., 2008;Yang and Tiffany-Castiglioni, 2008;Gonzalez-Polo et al., 2007a,b; Gonzalez-Polo et al., 2004a ). Although curcumin has been widely described as a inhibitor of apoptosis in several cell models (Reuter et al., 2008;Shishodia et al., 2007;Kuttan et al., 2007;Karunagaran et al., 2005), we observed here that co-incubation with PQ led to an increase of apoptosis in N27 mesencephalic cells compared to that observed in cells exposed to PQ alone. As we and other groups have previously described that the increase of apoptosis in PQ-exposed neural cells is mainly a ROS-dependent event, (Gonzalez-Polo et al., 2007a;Chen et al., 2008) we decided to evaluate ROS production after co-incubation of cells with curcumin and PQ. The results obtained indicate that the co-incubation leads to an increase in the ROS production which we characterized as a synergistic process given that curcumin itself was not able to increase ROS production in cells. This result is consistent with previously described findings that curcumin, although a potent antioxidant with ROS scavenging properties, is able to increase ROS production in different models (Javvadi et al., 2008;Cui et al., 2007;Nakamura et al., 1998). Moreover, several in vitro studies have shown that curcumin-induced apoptosis is associated with ROS production (Thayyullathil et al., 2008;Das et al., 2008;Jung et al., 2005;Woo et al., 2003;Bhaumik et al., 1999).
As the cell death that was observed after the PQ+curcumin exposure was found to be a ROS-dependent event, we sought to reverse this effect by using NAC or VitE, two well known antioxidants. Although both antioxidants were able to significantly protect against the cell death generated by the exposure of cells to PQ alone, we were no able to prevent cell death when the N27 cells were co-incubated with PQ+curcumin. As we have previously observed that the co-incubation generated a burst of ROS we evaluated if this effect sent the cell into a “no-way-back” cascade leading to cell death that could not be retrieved by using antioxidants. To this extent, when ROS levels exceed the cells’ defense or antioxidant capacities, injury occurs; growing evidence indicates that this burst leads to modification of proteins, lipids and DNA, altering their functions, and activating related signaling pathways, eventually leading to apoptosis of these cells. To address this question, N27 mesencephalic cells were exposed to hydrogen peroxide, a major oxidant generated when oxidative stress occurs. In experiments with the ROS-sensitive dye HE, we have observed that when ROS production in H2O2-exposed cells reached levels similar to those observed following the exposure of cells to PQ+curcumin, the recovery of cell viability with the antioxidants NAC or VitE could not be achieved.
To further explore the molecular mechanism that mediated the enhancement of cell death in PQ+curcumin-exposed cells, we analyzed the expression of NO and its regulation by curcumin. As superoxide has been described as the major ROS produced by the redox cycling of the pesticide PQ, (Hassan, 1984) and given that previous reports have shown that NO could act as a superoxide scavenger both in vitro (Day et al., 1999) and in vivo, (Cho et al., 2005) we analyzed the expression of NO after PQ exposure in N27 cells. We found that PQ exposure increases the production of NO in N27 cells and that this exposure leads to an upregulation of the expression of the inducible isoform of NOS in a time-dependent manner. As previously reported (Day et al., 1999), nitric oxide interacts with superoxide to generate the strong oxidant and nitrating intermediate peroxynitrite. As curcumin is an extremely specific inhibitor of iNOS expression (Lin, 2007), we hypothesized that the heightened ROS production observed after the exposure of cells to PQ+curcumin could be due to a failure in the nitric oxide production system leading to an increase in superoxide. We observed that curcumin effectively inhibits the expression of iNOS mRNA and that this effect is due to the inhibition of IκB degradation leading to an inhibition of the translocation of NF-κB, the nuclear factor that initiates the expression of the inducible isoform of NOS. As this inhibition attenuates the levels of NO produced after exposure of N27 cells to PQ, this effect leads to an overall increase in ROS levels due to a failure in the scavenging of superoxide by NO. This effect was seen in the attenuation of the increase of peroxynitrite formation (as evidenced by the formation of 3-NT) after exposure of cells to PQ in the presence of curcumin.
Although a definitive mechanism for the toxicity of PQ is yet to be elucidated, a cyclic single electron reduction/oxidation of the parent molecule is a critical mechanistic event. The redox cycling of paraquat has two potentially important consequences relevant to the development of toxicity: generation of “activated oxygen” (e.g., superoxide anions, hydrogen peroxide, hydroxyl radicals) which is highly reactive to cellular macromolecules; and/or oxidation of reducing equivalents (e.g., NADPH, reduced glutathione) necessary for normal cell function. The action of superoxide is normally limited by its low lipid solubility, its limited membrane transport, and also by its removal by SOD, the rate constant of which is 2 × 109 M−1s−1 (Huie and Padmaja, 1993). The production of nitric oxide after PQ exposure has been described for both in vitro (Yeh et al., 2006) and in vivo models (Berisha et al., 1994). Although the toxicity of NO is modest, it is greatly enhanced by reacting with superoxide to form peroxynitrite. The rate constant for this reaction is 6.7 × 109 M−1s−1 (Beckman, 1994). This suggests that as superoxide production increases after PQ exposure, peroxynitrite formation will be favored over superoxide removal by SOD. To this extent, NO is the only known biological molecule that is produced in high enough concentrations and reacts rapidly enough to outcompete SOD for superoxide, which in fact leads to an attenuation of the superoxide levels due to a scavenging effect. The peroxynitrite formed reacts relatively slowly with most biological molecules, making itself a selective oxidant. Peroxynitrite modifies tyrosine in proteins to create nitrotyrosines, leaving a detectable footprint both in vivo and in vitro. Because in PQ mediated toxicity the generation of superoxide is the acute event due to redox cycling of the herbicide the inhibition of nitric oxide production by the iNOS expression curcumin leads to an exacerbation of the ROS due to an attenuation of the generation of peroxynitrites, which in fact enhances cell death after PQ exposure.
Curcumin has pleiotropic effects on cell death and survival. It can modulate the expression and activation of cellular regulatory systems such as NF-κB, leading to the inhibition of iNOS expression. Although curcumin has been proposed as a putative agent to protect against the oxidative stress generated after PQ injury, (Peiro et al., 2007;Soudamini et al., 1992) our data with N27 cells do not support this hypothesis and suggest that the use of curcumin as a therapeutic agent in the case of neurodegenerative disorders must be reevaluated.
Acknowledgments
This research was supported by grants PRI08A016, PR06B124 and GRU09017 from the Junta de Extremadura, Spain, and PI070400 (Fondo de Investigación Sanitaria, Ministerio de Sanidad y Consumo, Spain) and National Institute of Health (NIH) grants ES10586 and NS038644. J.M.M. was supported by a postdoctoral fellowship from CIBERNED; M.A.O. and J.M.B were supported by Junta of Extremadura fellowships; R.A.G.P. was supported by a Miguel Servet (Fondo de Investigación Sanitaria, Ministerio de Sanidad y Consumo, Spain) contract and M.N.S. was supported by a fellowship from CIBERNED.
Footnotes
Conflict of Interest statement
The authors declare that there are no conflicts of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aggarwal BB, Harikumar KB. Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int J Biochem Cell Biol. 2008;41(1):40–59. doi: 10.1016/j.biocel.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anantharam V, Kitazawa M, Wagner J, Kaul S, Kanthasamy AG. Caspase-3-dependent proteolytic cleavage of protein kinase Cdelta is essential for oxidative stress-mediated dopaminergic cell death after exposure to methylcyclopentadienyl manganese tricarbonyl. J Neurosci. 2002;22 (5):1738–1751. doi: 10.1523/JNEUROSCI.22-05-01738.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow BK, Cory-Slechta DA, Richfield EK, Thiruchelvam M. The gestational environment and Parkinson’s disease: evidence for neurodevelopmental origins of a neurodegenerative disorder. Reprod Toxicol. 2007;23:457–470. doi: 10.1016/j.reprotox.2007.01.007. [DOI] [PubMed] [Google Scholar]
- Baum L, Lam CW, Cheung SK, Kwok T, Lui V, Tsoh J, Lam L, Leung V, Hui E, Ng C, Woo J, Chiu HF, Goggins WB, Zee BC, Cheng KF, Fong CY, Wong A, Mok H, Chow MS, Ho PC, Ip SP, Ho CS, Yu XW, Lai CY, Chan MH, Szeto S, Chan IH, Mok V. Six-month randomized, placebo-controlled, double-blind, pilot clinical trial of curcumin in patients with Alzheimer disease. J Clin Psychopharmacol. 2008;28:110–113. doi: 10.1097/jcp.0b013e318160862c. [DOI] [PubMed] [Google Scholar]
- Beckman JS. Peroxynitrite versus hydroxyl radical: the role of nitric oxide in superoxide-dependent cerebral injury. Ann N Y Acad Sci. 1994;738:69–75. doi: 10.1111/j.1749-6632.1994.tb21791.x. [DOI] [PubMed] [Google Scholar]
- Begum AN, Jones MR, Lim GP, Morihara T, Kim P, Heath DD, Rock CL, Pruitt MA, Yang F, Hudspeth B, Hu S, Faull KF, Teter B, Cole GM, Frautschy SA. Curcumin structure-function, bioavailability, and efficacy in models of neuroinflammation and Alzheimer’s disease. J Pharmacol Exp Ther. 2008;326:196–208. doi: 10.1124/jpet.108.137455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengmark S. Curcumin, an atoxic antioxidant and natural NFkappaB, cyclooxygenase-2, lipooxygenase, and inducible nitric oxide synthase inhibitor: a shield against acute and chronic diseases. JPEN J Parenter Enteral Nutr. 2006;30:45–51. doi: 10.1177/014860710603000145. [DOI] [PubMed] [Google Scholar]
- Berisha HI, Pakbaz H, Absood A, Said SI. Nitric oxide as a mediator of oxidant lung injury due to paraquat. Proc Natl Acad Sci USA. 1994;91:7445–7449. doi: 10.1073/pnas.91.16.7445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaumik S, Anjum R, Rangaraj N, Pardhasaradhi BV, Khar A. Curcumin mediated apoptosis in AK-5 tumor cells involves the production of reactive oxygen intermediates. FEBS Lett. 1999;456:311–314. doi: 10.1016/s0014-5793(99)00969-2. [DOI] [PubMed] [Google Scholar]
- Bonneh-Barkay D, Langston WJ, Di Monte DA. Toxicity of redox cycling pesticides in primary mesencephalic cultures. Antioxid Redox Signal. 2005;7:649–653. doi: 10.1089/ars.2005.7.649. [DOI] [PubMed] [Google Scholar]
- Burke RE. Programmed cell death and Parkinson’s disease. Mov Disord. 1998;13 (Suppl 1):17–23. [PubMed] [Google Scholar]
- Calabrese V, Bates TE, Mancuso C, Cornelius C, Ventimiglia B, Cambria MT, Di Renzo L, De Lorenzo A, Dinkova-Kostova AT. Curcumin and the cellular stress response in free radical-related diseases. Mol Nutr Food Res. 2008;52:1062–1073. doi: 10.1002/mnfr.200700316. [DOI] [PubMed] [Google Scholar]
- Castello PR, Drechsel DA, Patel M. Mitochondria are a major source of paraquat-induced reactive oxygen species production in the brain. J Biol Chem. 2007;282:14186–14193. doi: 10.1074/jbc.M700827200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan WH, Wu HJ, Hsuuw YD. Curcumin inhibits ROS formation and apoptosis in methylglyoxal-treated human hepatoma G2 cells. Ann N Y Acad Sci. 2005;1042:372–378. doi: 10.1196/annals.1338.057. [DOI] [PubMed] [Google Scholar]
- Chen J, Tang XQ, Zhi JL, Cui Y, Yu HM, Tang EH, Sun SN, Feng JQ, Chen PX. Curcumin protects PC12 cells against 1-methyl-4-phenylpyridinium ion-induced apoptosis by bcl-2-mitochondria-ROS-iNOS pathway. Apoptosis. 2006;11:943–953. doi: 10.1007/s10495-006-6715-5. [DOI] [PubMed] [Google Scholar]
- Chen P, Chen Z, Li A, Lou XC, Wu XK, Zhao CJ, Wang SL, Liang LP. Catalytic metalloporphyrin protects against paraquat neurotoxicity in vivo. Biomed Environ Sci. 2008;21:233–238. doi: 10.1016/S0895-3988(08)60035-5. [DOI] [PubMed] [Google Scholar]
- Cho JH, Yang DK, Kim L, Ryu JS, Lee HL, Lim CM, Koh YS. Inhaled nitric oxide improves the survival of the paraquat-injured rats. Vascul Pharmacol. 2005;42:171–178. doi: 10.1016/j.vph.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Cole GM, Teter B, Frautschy SA. Neuroprotective effects of curcumin. Adv Exp Med Biol. 2007;595:197–212. doi: 10.1007/978-0-387-46401-5_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui L, Miao J, Cui L. Cytotoxic effect of curcumin on malaria parasite Plasmodium falciparum: inhibition of histone acetylation and generation of reactive oxygen species. Antimicrob Agents Chemother. 2007;51:488–494. doi: 10.1128/AAC.01238-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das R, Roy A, Dutta N, Majumder HK. Reactive oxygen species and imbalance of calcium homeostasis contributes to curcumin induced programmed cell death in Leishmania donovani. Apoptosis. 2008;13:867–882. doi: 10.1007/s10495-008-0224-7. [DOI] [PubMed] [Google Scholar]
- Day BJ, Patel M, Calavetta L, Chang LY, Stamler JS. A mechanism of paraquat toxicity involving nitric oxide synthase. Proc Natl Acad Sci USA. 1999;96:12760–12765. doi: 10.1073/pnas.96.22.12760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick FD. Parkinson’s disease and pesticide exposures. Br Med Bull. 2006;79–80:219–231. doi: 10.1093/bmb/ldl018. [DOI] [PubMed] [Google Scholar]
- Dinis-Oliveira RJ, Remiao F, Carmo H, Duarte JA, Navarro AS, Bastos ML, Carvalho F. Paraquat exposure as an etiological factor of Parkinson’s disease. Neurotoxicology. 2006;27:1110–1122. doi: 10.1016/j.neuro.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Drechsel DA, Patel M. Role of reactive oxygen species in the neurotoxicity of environmental agents implicated in Parkinson’s disease. Free Radic Biol Med. 2008;44:1873–1886. doi: 10.1016/j.freeradbiomed.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentes JM, Lompre AM, Moller JV, Falson P, le Maire M. Clean Western blots of membrane proteins after yeast heterologous expression following a shortened version of the method of Perini et al. Anal Biochem. 2000;285:276–278. doi: 10.1006/abio.2000.4784. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Polo RA, Niso-Santano M, Ortiz-Ortiz MA, Gomez-Martin A, Moran JM, Garcia-Rubio L, Francisco-Morcillo J, Zaragoza C, Soler G, Fuentes JM. Inhibition of paraquat-induced autophagy accelerates the apoptotic cell death in neuroblastoma SH-SY5Y cells. Toxicol Sci. 2007a;97:448–458. doi: 10.1093/toxsci/kfm040. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Polo RA, Niso-Santano M, Ortiz-Ortiz MA, Gomez-Martin A, Moran JM, Garcia-Rubio L, Francisco-Morcillo J, Zaragoza C, Soler G, Fuentes JM. Relationship between autophagy and apoptotic cell death in human neuroblastoma cells treated with paraquat: could autophagy be a “brake” in paraquat-induced apoptotic death? Autophagy. 2007b;3:366–367. doi: 10.4161/auto.4194. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Polo RA, Rodriguez-Martin A, Moran JM, Niso M, Soler G, Fuentes JM. Paraquat-induced apoptotic cell death in cerebellar granule cells. Brain Res. 2004;1011:170–176. doi: 10.1016/j.brainres.2004.02.078. [DOI] [PubMed] [Google Scholar]
- Gorell JM, Johnson CC, Rybicki BA, Peterson EL, Richardson RJ. The risk of Parkinson’s disease with exposure to pesticides, farming, well water, and rural living. Neurology. 1998;50:1346–1350. doi: 10.1212/wnl.50.5.1346. [DOI] [PubMed] [Google Scholar]
- Hansen MB, Nielsen SE, Berg K. Re-examination and further development of a precise and rapid dye method for measuring cell growth/cell kill. J Immunol Methods. 1989;119:203–210. doi: 10.1016/0022-1759(89)90397-9. [DOI] [PubMed] [Google Scholar]
- Hassan HM. Exacerbation of superoxide radical formation by paraquat. Methods Enzymol. 1984;105:523–532. doi: 10.1016/s0076-6879(84)05072-2. [DOI] [PubMed] [Google Scholar]
- Huang MT, Lysz T, Ferraro T, Abidi TF, Laskin JD, Conney AH. Inhibitory effects of curcumin on in vitro lipoxygenase and cyclooxygenase activities in mouse epidermis. Cancer Res. 1991;51:813–819. [PubMed] [Google Scholar]
- Huie RE, Padmaja S. The reaction of no with superoxide. Free Radic Res Commun. 1993;18:195–199. doi: 10.3109/10715769309145868. [DOI] [PubMed] [Google Scholar]
- Jagatha B, Mythri RB, Vali S, Bharath MM. Curcumin treatment alleviates the effects of glutathione depletion in vitro and in vivo: therapeutic implications for Parkinson’s disease explained via in silico studies. Free Radic Biol Med. 2008;44:907–917. doi: 10.1016/j.freeradbiomed.2007.11.011. [DOI] [PubMed] [Google Scholar]
- Javvadi P, Segan AT, Tuttle SW, Koumenis C. The chemopreventive agent curcumin is a potent radiosensitizer of human cervical tumor cells via increased reactive oxygen species production and overactivation of the mitogen-activated protein kinase pathway. Mol Pharmacol. 2008;73:1491–1501. doi: 10.1124/mol.107.043554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DC, Miller GW. The effects of environmental neurotoxicants on the dopaminergic system: A possible role in drug addiction. Biochem Pharmacol. 2008;76:569–581. doi: 10.1016/j.bcp.2008.05.010. [DOI] [PubMed] [Google Scholar]
- Jung EM, Lim JH, Lee TJ, Park JW, Choi KS, Kwon TK. Curcumin sensitizes tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis through reactive oxygen species-mediated upregulation of death receptor 5 (DR5) Carcinogenesis. 2005;26:1905–1913. doi: 10.1093/carcin/bgi167. [DOI] [PubMed] [Google Scholar]
- Karunagaran D, Rashmi R, Kumar TR. Induction of apoptosis by curcumin and its implications for cancer therapy. Curr Cancer Drug Targets. 2005;5:117–129. doi: 10.2174/1568009053202081. [DOI] [PubMed] [Google Scholar]
- Kaul S, Kanthasamy A, Kitazawa M, Anantharam V, Kanthasamy AG. Caspase-3 dependent proteolytic activation of protein kinase C delta mediates and regulates 1-methyl-4-phenylpyridinium (MPP+)-induced apoptotic cell death in dopaminergic cells: relevance to oxidative stress in dopaminergic degeneration. Eur J Neurosci. 2003;18 (6):1387–1401. doi: 10.1046/j.1460-9568.2003.02864.x. [DOI] [PubMed] [Google Scholar]
- Kaul S, Anantharam V, Yang Y, Choi CJ, Kanthasamy A, Kanthasamy AG. Tyrosine phosphorylation regulates the proteolytic activation of protein kinase Cdelta in dopaminergic neuronal cells. J Biol Chem. 2005;280:28721–30. doi: 10.1074/jbc.M501092200. [DOI] [PubMed] [Google Scholar]
- Kanthasamy AG, Anantharam V, Zhang D, Latchoumycandane C, Jin H, Kaul S, Kanthasamy A. A novel peptide inhibitor targeted to caspase-3 cleavage site of a proapoptotic kinase protein kinase C delta (PKCdelta) protects against dopaminergic neuronal degeneration in Parkinson’s disease models. Free Radic Biol Med. 2006;41:1578–89. doi: 10.1016/j.freeradbiomed.2006.08.016. [DOI] [PubMed] [Google Scholar]
- Kirkeby S, Thomsen CE. Quantitative immunohistochemistry of fluorescence labelled probes using low-cost software. J Immunol Methods. 2005;301:102–113. doi: 10.1016/j.jim.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Kitazawa M, Anantharam V, Kanthasamy AG. Dieldrin induces apoptosis by promoting caspase-3-dependent proteolytic cleavage of protein kinase Cdelta in dopaminergic cells: relevance to oxidative stress and dopaminergic degeneration. Neuroscience. 2003;119 (4):945–964. doi: 10.1016/s0306-4522(03)00226-4. [DOI] [PubMed] [Google Scholar]
- Kuttan G, Kumar KB, Guruvayoorappan C, Kuttan R. Antitumor, anti-invasion, and antimetastatic effects of curcumin. Adv Exp Med Biol. 2007;595:173–184. doi: 10.1007/978-0-387-46401-5_6. [DOI] [PubMed] [Google Scholar]
- Lim GP, Chu T, Yang F, Beech W, Frautschy SA, Cole GM. The curry spice curcumin reduces oxidative damage and amyloid pathology in an Alzheimer transgenic mouse. J Neurosci. 2001;21:8370–8377. doi: 10.1523/JNEUROSCI.21-21-08370.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JK. Molecular targets of curcumin. Adv Exp Med Biol. 2007;595:227–243. doi: 10.1007/978-0-387-46401-5_10. [DOI] [PubMed] [Google Scholar]
- Liu F, Fung MC, Ooi VE, Chang ST. Induction in the mouse of gene expression of immunomodulating cytokines by mushroom polysaccharide-protein complexes. Life Sci. 1996;58:1795–1803. doi: 10.1016/0024-3205(96)00163-4. [DOI] [PubMed] [Google Scholar]
- Menon VP, Sudheer AR. Antioxidant and anti-inflammatory properties of curcumin. Adv Exp Med Biol. 2007;595:105–125. doi: 10.1007/978-0-387-46401-5_3. [DOI] [PubMed] [Google Scholar]
- Miller RL, James-Kracke M, Sun GY, Sun AY. Oxidative and inflammatory pathways in Parkinson’s disease. Neurochem Res. 2009;34:55–65. doi: 10.1007/s11064-008-9656-2. [DOI] [PubMed] [Google Scholar]
- Moran JM, Gonzalez-Polo RA, Ortiz-Ortiz MA, Niso-Santano M, Soler G, Fuentes JM. Identification of genes associated with paraquat-induced toxicity in SH-SY5Y cells by PCR array focused on apoptotic pathways. J Toxicol Environ Health A. 2008;71:1457–1467. doi: 10.1080/15287390802329364. [DOI] [PubMed] [Google Scholar]
- Mosman T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assay. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Ohto Y, Murakami A, Osawa T, Ohigashi H. Inhibitory effects of curcumin and tetrahydrocurcuminoids on the tumor promoter-induced reactive oxygen species generation in leukocytes in vitro and in vivo. Jpn J Cancer Res. 1998;89:361–370. doi: 10.1111/j.1349-7006.1998.tb00572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono K, Hasegawa K, Naiki H, Yamada M. Curcumin has potent anti-amyloidogenic effects for Alzheimer’s beta-amyloid fibrils in vitro. J Neurosci Res. 2004;75:742–750. doi: 10.1002/jnr.20025. [DOI] [PubMed] [Google Scholar]
- Prasad KN, Clarkson ED, La Rosa FG, Edwards-Prasad J, Freed CR. Efficacy of grafted immortalized dopamine neurons in an animal model of parkinsonism: a review. Mol Genet Metab. 1998;65 (1):1–9. doi: 10.1006/mgme.1998.2726. [DOI] [PubMed] [Google Scholar]
- Peiro AM, Zapater P, Alenda C, Ramirez A, Gutierrez A, Perez-Mateo M, Such J. Hepatotoxicity related to paraquat and diquat absorption through intact skin. Dig Dis Sci. 2007;52:3282–3284. doi: 10.1007/s10620-005-9056-2. [DOI] [PubMed] [Google Scholar]
- Przedborski S, Ischiropoulos H. Reactive oxygen and nitrogen species: weapons of neuronal destruction in models of Parkinson’s disease. Antioxid Redox Signal. 2005;7:685–693. doi: 10.1089/ars.2005.7.685. [DOI] [PubMed] [Google Scholar]
- Rajeswari A, Sabesan M. Inhibition of monoamine oxidase-B by the polyphenolic compound, curcumin and its metabolite tetrahydrocurcumin, in a model of Parkinson’s disease induced by MPTP neurodegeneration in mice. Inflammopharmacology. 2008;16:96–99. doi: 10.1007/s10787-007-1614-0. [DOI] [PubMed] [Google Scholar]
- Reiter RJ, Carneiro RC, Oh CS. Melatonin in relation to cellular antioxidative defense mechanisms. Horm Metab Res. 1997;29:363–372. doi: 10.1055/s-2007-979057. [DOI] [PubMed] [Google Scholar]
- Reuter S, Eifes S, Dicato M, Aggarwal BB, Diederich M. Modulation of anti-apoptotic and survival pathways by curcumin as a strategy to induce apoptosis in cancer cells. Biochem Pharmacol. 2008;76:1340–1351. doi: 10.1016/j.bcp.2008.07.031. [DOI] [PubMed] [Google Scholar]
- Ringman JM, Frautschy SA, Cole GM, Masterman DL, Cummings JL. A potential role of the curry spice curcumin in Alzheimer’s disease. Curr Alzheimer Res. 2005;2:131–136. doi: 10.2174/1567205053585882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shishodia S, Chaturvedi MM, Aggarwal BB. Role of curcumin in cancer therapy. Curr Probl Cancer. 2007;31:243–305. doi: 10.1016/j.currproblcancer.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Singh S, Khar A. Biological effects of curcumin and its role in cancer chemoprevention and therapy. Anticancer Agents Med Chem. 2006;6:259–270. doi: 10.2174/187152006776930918. [DOI] [PubMed] [Google Scholar]
- Soudamini KK, Unnikrishnan MC, Soni KB, Kuttan R. Inhibition of lipid peroxidation and cholesterol levels in mice by curcumin. Indian J Physiol Pharmacol. 1992;36:239–243. [PubMed] [Google Scholar]
- Sreejayan N, Rao MN. Free radical scavenging activity of curcuminoids. Arzneimittelforschung. 1996;46:169–171. [PubMed] [Google Scholar]
- Sreejayan, Rao MN. Nitric oxide scavenging by curcuminoids. J Pharm Pharmacol. 1997;49:105–107. doi: 10.1111/j.2042-7158.1997.tb06761.x. [DOI] [PubMed] [Google Scholar]
- Su CC, Lin JG, Li TM, Chung JG, Yang JS, Ip SW, Lin WC, Chen GW. Curcumin-induced apoptosis of human colon cancer colo 205 cells through the production of ROS, Ca2+ and the activation of caspase-3. Anticancer Res. 2006;26:4379–4389. [PubMed] [Google Scholar]
- Sun F, Anantharam V, Zhang D, Latchoumycandane C, Kanthasamy A, Kanthasamy AG. Proteasome inhibitor MG-132 induces dopaminergic degeneration in cell culture and animal models. Neurotoxicology. 2006;27:807–15. doi: 10.1016/j.neuro.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Tanner CM. The role of environmental toxins in the etiology of Parkinson’s disease. Trends Neurosci. 1989;12:49–54. doi: 10.1016/0166-2236(89)90135-5. [DOI] [PubMed] [Google Scholar]
- Tanner CM, Chen B, Wang W, Peng M, Liu Z, Liang X, Kao LC, Gilley DW, Goetz CG, Schoenberg BS. Environmental factors and Parkinson’s disease: a case-control study in China. Neurology. 1989;39:660–664. doi: 10.1212/wnl.39.5.660. [DOI] [PubMed] [Google Scholar]
- Thayyullathil F, Chathoth S, Hago A, Patel M, Galadari S. Rapid reactive oxygen species (ROS) generation induced by curcumin leads to caspase-dependent and -independent apoptosis in L929 cells. Free Radic Biol Med. 2008 doi: 10.1016/j.freeradbiomed.2008.08.014. [DOI] [PubMed] [Google Scholar]
- Thiruchelvam M, Prokopenko O, Cory-Slechta DA, Buckley B, Mirochnitchenko O. Overexpression of superoxide dismutase or glutathione peroxidase protects against the paraquat + maneb-induced Parkinson disease phenotype. J Biol Chem. 2005;280:22530–22539. doi: 10.1074/jbc.M500417200. [DOI] [PubMed] [Google Scholar]
- Thrash B, Uthayathas S, Karuppagounder SS, Suppiramaniam V, Dhanasekaran M. Paraquat and maneb induced neurotoxicity. Proc West Pharmacol Soc. 2007;50:31–42. [PubMed] [Google Scholar]
- Wei QY, Chen WF, Zhou B, Yang L, Liu ZL. Inhibition of lipid peroxidation and protein oxidation in rat liver mitochondria by curcumin and its analogues. Biochim Biophys Acta. 2006;1760:70–77. doi: 10.1016/j.bbagen.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Woo JH, Kim YH, Choi YJ, Kim DG, Lee KS, Bae JH, Min DS, Chang JS, Jeong YJ, Lee YH, Park JW, Kwon TK. Molecular mechanisms of curcumin-induced cytotoxicity: induction of apoptosis through generation of reactive oxygen species, down-regulation of Bcl-XL and IAP, the release of cytochrome c and inhibition of Akt. Carcinogenesis. 2003;24:1199–1208. doi: 10.1093/carcin/bgg082. [DOI] [PubMed] [Google Scholar]
- Yang Y, Kaul S, Zhang D, Anantharam V, Kanthasamy AG. Suppression of caspase-3-dependent proteolytic activation of protein kinase C delta by small interfering RNA prevents MPP+-induced dopaminergic degeneration. Mol Cell Neurosci. 2004;25 (3):406–421. doi: 10.1016/j.mcn.2003.11.011. [DOI] [PubMed] [Google Scholar]
- Yang W, Tiffany-Castiglioni E. The bipyridyl herbicide paraquat produces oxidative stress-mediated toxicity in human neuroblastoma SH-SY5Y cells: relevance to the dopaminergic pathogenesis. J Toxicol Environ Health A. 2005;68:1939–1961. doi: 10.1080/15287390500226987. [DOI] [PubMed] [Google Scholar]
- Yang W, Tiffany-Castiglioni E. Paraquat-induced apoptosis in human neuroblastoma SH-SY5Y cells: involvement of p53 and mitochondria. J Toxicol Environ Health A. 2008;71:289–299. doi: 10.1080/15287390701738467. [DOI] [PubMed] [Google Scholar]
- Yeh ST, Guo HR, Su YS, Lin HJ, Hou CC, Chen HM, Chang MC, Wang YJ. Protective effects of N-acetylcysteine treatment post acute paraquat intoxication in rats and in human lung epithelial cells. Toxicology. 2006;223:181–190. doi: 10.1016/j.tox.2006.03.019. [DOI] [PubMed] [Google Scholar]
- Zbarsky V, Datla KP, Parkar S, Rai DK, Aruoma OI, Dexter DT. Neuroprotective properties of the natural phenolic antioxidants curcumin and naringenin but not quercetin and fisetin in a 6-OHDA model of Parkinson’s disease. Free Radic Res. 2005;39:1119–1125. doi: 10.1080/10715760500233113. [DOI] [PubMed] [Google Scholar]