Abstract
The neurodegenerative disorder Huntington disease (HD) is caused by an expanded CAG repeat in the huntingtin gene, resulting in loss of striatal and cortical neurons. Although, the gene product is widely expressed, it remains unclear why neurons are selectively targeted. Here, we demonstrate the relationship between synaptic and extrasynaptic activity, inclusion formation of mutant huntingtin protein (mtHtt), and neuronal survival. Synaptic NMDA receptor (NMDAR) activity induces mtHtt inclusions via a TCP1 ring complex (TRiC)-dependent mechanism, rendering neurons more resistant to mtHtt-mediated cell death. In contrast, stimulation of extrasynaptic NMDARs increases vulnerability of mtHtt-neurons to cell death by impairing a neuroprotective CREB—PGC-1α cascade and increasing the small guanine nucleotide-binding protein Rhes, which is known to sumoylate and disaggregate mtHtt. Treatment of transgenic YAC128 HD mice with low-dose memantine blocks extrasynaptic (but not synaptic) NMDARs and ameliorates neuropathological and behavioral manifestations. By contrast, high-dose memantine also blocks synaptic NMDAR activity, decreases neuronal inclusions, and worsens these outcomes. Our findings offer a rational therapeutic approach for protecting susceptible neurons in HD.
Huntington disease (HD) is an inherited neurodegenerative disorder caused by an expansion of a polyglutamine repeat in the N-terminal region of huntingtin1. Aggregation of mutant huntingtin (mtHtt) into insoluble macro inclusions, and early selective cell death of striatal and cortical neurons are features of the disease2. Interestingly, most if not all neurodegenerative disorders, whether or not genetically inherited like HD, manifest abnormal misfolded proteins3. Although it has been known for many years that excessive NMDAR activity can mimic HD4 and that such activity might contribute to disease pathogenesis5-7, it remains unknown if normal synaptic activity influences inclusion formation and neuronal survival. Additionally, mechanistic details relating electrophysiological activity and molecular pathways to protein misfolding and aggregation in disorders such as HD have been lacking. Moreover, a mechanism-based treatment has not yet proven successful in HD patients.
RESULTS
NMDA receptor-mediated synaptic activity is necessary for inclusion formation by mtHtt
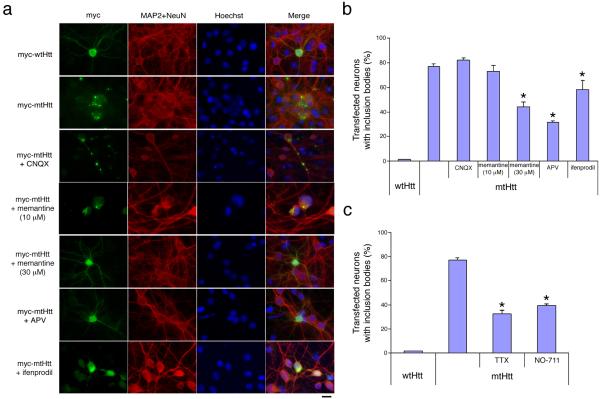
To investigate the relationship between synaptic activity, inclusion formation and neurotoxicity induced by mtHtt, we initially used a neuronal cell culture model of HD in which primary striatal or cortical neurons were transiently transfected with constructs of either full-length or N-terminal Htt encoding wild-type or expanded polyglutamine repeats. Neurons transfected with wtHtt displayed diffuse cytoplasmic expression of huntingtin by immunocytochemistry, while both intranuclear and cytoplasmic/neuropil macroscopic inclusions were present in neurons transfected with mtHtt (Fig. 1a, b for cortical neurons and Supplementary Fig. 1a, b online for striatal neurons). Strikingly, when endogenous NMDAR activity was curtailed in these cultures with NMDAR antagonists d-(-)-2 amino-5-phosphonovaleric acid (d-APV), memantine or ifenprodil, we observed a significant decrease in the number of mtHtt-containing inclusions. In contrast, the AMPA (α-amino-3-hydroxy-5-methyl-4-isooxazole propionic acid)-sensitive glutamate receptor antagonist, 6-cyano-7-nitroquinoxaline-2, 3-dione (CNQX), did not affect inclusion formation. Moreover, neither CNQX nor NMDAR antagonists had any effect on normal huntingtin expression in transfected neurons (Supplementary Fig. 2 online). Hence, the suppressive effect of NMDAR antagonists on mtHtt inclusion formation could not be attributed to a global suppression of protein expression. Importantly, memantine decreased mtHtt-containing inclusions only at high (30 μM) concentrations, but not at lower (≤10 μM) concentrations (Fig. 1a, b; Supplementary Fig. 1a, b). To mimic more closely the pathophysiological situation, we next utilized neurons transfected with full-length mtHtt bearing a 44Q expansion, or neurons from the striatum of the transgenic YAC128 HD mouse, which has a yeast artificial chromosome (YAC) encoding the entire human HD gene containing 128 CAG repeats8. These models produced similar findings (Supplementary Figs. 3 and 4 online).
Figure 1.
Suppression of excitatory NMDAR synaptic transmission ameliorates inclusion formation in mtHtt-expressing neurons. (a, b) Immunostaining and quantification of inclusion bodies in rat primary cortical neurons transfected with wtHtt (Myc-wtHtt-N63-18Q) or mtHtt (Myc-mtHtt-N63-148Q) after treatment with AMPA receptor antagonist CNQX (10 μM) or with NMDAR antagonists memantine (10 μM or 30 μM), d-APV (150 μM), or ifenprodil (10 μM), a relatively selective inhibitor of NR2B subunits of the NMDAR. Values are mean ± s.e.m. (n ≥ 1,200). Scale bar, 10 μm. *, P < 0.01 by ANOVA. (c) Inclusion formation was quantified in rat primary neurons transfected with wtHtt or mtHtt and treated with synaptic activity suppressors (0.2 μM TTX or 100 μM NO-711). Values are mean ± s.e.m. *, P < 0.01 by ANOVA.
Unlike other NMDAR antagonists, such as ifenprodil and d-APV, we have shown that memantine is an open-channel blocker, acting via an uncompetitive/fast ‘off-rate’ mechanism whereby low micromolar concentrations of the drug preferentially inhibit excessive, primarily extrasynaptic, stimulation of NMDARs but relatively preserve physiological synaptic transmission9,10. In contrast, higher concentrations of memantine lose this selectivity and also inhibit normal NMDAR-mediated synaptic activity like other NMDAR antagonists. Thus, blockade of inclusion formation by memantine at higher concentrations, but not lower, most likely results from interrupting endogenous synaptic activity in these cultures. To further characterize the role of synaptic activity in mtHtt aggregation, we used two additional pharmacological tools to suppress normal excitatory synaptic transmission. Both tetrodotoxin (TTX, to block Na+ channels and thus the propagation of action potentials that lead to endogenous synaptic activity) and NO-711 (to block GABA uptake and hence augment inhibitory neurotransmission) decreased mtHtt-containing inclusions (Fig. 1c). Taken together, our data suggest that interruption of normal excitatory synaptic activity (mediated by NMDARs) ameliorates macro inclusion formation.
Electrophysiological validation of pharmacological modifiers of synaptic activity
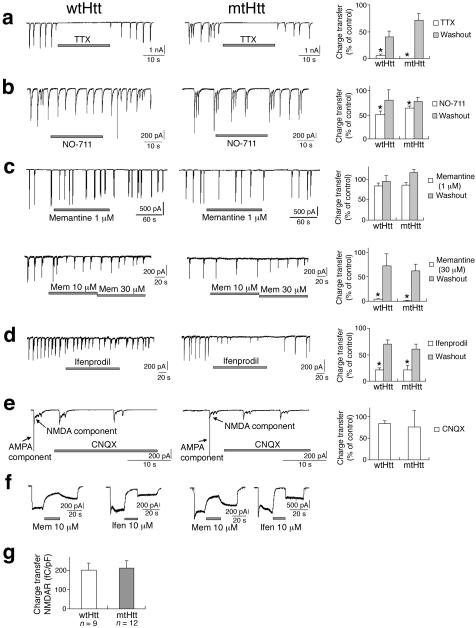
To confirm the notion that our pharmacological manipulations modified synaptic activity, we used whole-cell recording with patch electrodes to study spontaneous excitatory postsynaptic currents (sEPSCs). These experiments were designed to analyze the effect of short-term additions of drugs to determine their mechanism of action. The same drugs were used to prevent sEPSCs from triggering the intracellular signaling cascades involved in inclusion formation or neuronal cell death (Fig. 1; Supplementary Fig. 1). We found that total charge transfer during the NMDAR-mediated component of sEPSCs was significantly reduced by TTX or NO-711, in both wtHtt and mtHtt transfected cortical neurons (Fig. 2a, b). Similarly, high (30 μM) memantine significantly inhibited the NMDAR-mediated component of sEPSCs, while low (1-10 μM) memantine relatively spared this synaptic activity (Fig. 2c). In contrast, ifenprodil (10 μM) blocked this synaptic activity (Fig. 2d). However, in the presence of 10 μM CNQX, NMDAR-mediated synaptic activity was not significantly attenuated (Fig. 2e).
Figure 2.
Pharmacology of NMDAR-mediated sEPSCs and whole-cell currents recorded from wtHtt- and mtHtt-transfected neurons. (a-e) sEPSCs (left-hand panels) and charge transfer (right-hand panels), normalized for each cell (see Supplementary Methods). Both wtHtt- and mtHtt-transfected neurons were treated with TTX (a), NO-711 (b), memantine (c), or ifenprodil (d). Recording solutions in (a-e) contained 20 μM glycine; for (c. d), CNQX and bicuculline (10 μM each) were also added. Values are mean ± s.e.m. (n ≥ 4 cells in each case); *, P < 0.03 (paired t-test on raw data). (f) Effects of memantine and ifenprodil on extrasynaptic NMDAR-mediated currents. Incomplete reversal was observed because of slow washout in this system. Recordings were performed in the presence of 0.1-1 μM TTX. NMDA currents were evoked by co-application of 100 μM NMDA and 20 μM glycine. (g) Charge transfer (fC/pF) for NMDAR-sEPSCs in mtHtt- and wtHtt-transfected neurons.
Additionally, both memantine (even at low concentrations), and ifenprodil, attenuated extrasynaptic NMDAR-mediated currents (Fig. 2f). The effect of low-dose memantine on extrasynaptic NMDAR-mediated currents was further confirmed by isolating extrasynaptic NMDAR activity using the previously published MK-801 protocol11 (Supplementary Fig. 5 online). These electrophysiological findings validate the specificity of our pharmacological tools for NMDARs when studying mtHtt aggregation and neuronal cell death. Next, we examined whether NMDAR-sEPSCs were affected by mtHtt under our conditions. We found that total charge transfer during sEPSCs was not significantly different in mtHtt- vs. wtHtt-transfected neurons (Fig. 2g). Similar findings were observed in striatal slice recordings from the YAC72 mouse model of HD during low-frequency cortical afferent stimulation12, simulating the spontaneous activity level in our cultures. At higher levels of stimulation, mtHtt itself is known to enhance NMDAR activity6.
Chaperonin TRiC facilitates synaptic activity-dependent inclusion formation
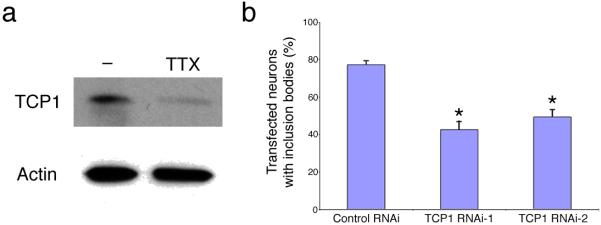
Next, we investigated the molecular mechanism of mtHtt-induced inclusion formation. Recently, three groups reported in yeast and cell lines that the chaperonin TRiC (TCP1 ring complex, also known as CCT) detoxifies mtHtt, at least in part by forming multiple mtHtt inclusions, while decreasing toxic soluble microaggregates of mtHtt and thereby decreasing toxicity13. Augmentation of TRiC activity increased the appearance of multiple, small mtHtt-containing inclusions (Fig. 3c and 5c in Tam et al.14), reminiscent of our findings in neurons with preserved synaptic activity (our Fig. 1a; Supplementary Figs. 1a and 3). Hence, we examined if synaptic activity regulates the expression of TCP1, the subunit of TRiC that preferentially interacts with mtHtt14. We added TTX to our neuronal cultures to block synaptic activity and found that it significantly reduced the expression of TCP1 (Fig. 3a). While low-dose memantine (10 μM) did not affect the TCP1 expression, both high-dose memantine (30 μM) and ifenprodil also decreased TCP1 levels (Supplementary Fig. 6 online), supporting the notion that synaptic NMDAR activity is necessary for TCP1 expression.
Figure 3.
Involvement of the chaperonin TRiC TCP1 subunit in synaptic activity-mediated inclusion formation. (a) Immunoblotting demonstrating the level of TCP1 protein expression in neurons exposed to TTX (0.2 μM) or control conditions for 24 h. Actin served as a loading control. (b) Inclusion formation was quantified in neurons transfected with mtHtt plus two different small hairpin vectors for TCP1 or control vector. Values are mean ± s.e.m. for n ≥ 300. *, P ≤ 0.001 by ANOVA.
To test if TCP1 is involved in synaptic activity-induced inclusion formation, we generated two small hairpin RNA interference constructs (shRNAs designated TCP1 RNAi-1 and RNAi-2) to knockdown TCP1 expression. TCP1 RNAi-1 and RNAi-2 reduced TCP1 levels to 70% and 60%, respectively (Supplementary Fig. 7 online). Both small hairpin TCP1 RNAi vectors significantly decreased inclusion formation from mtHtt (Fig. 3b). Additionally, knockdown of TCP1 by RNAi occluded the inhibitory effect on inclusion formation exerted by suppression of synaptic activity with high-dose (30 μM) memantine (Supplementary Fig. 8 online). Taken together, our results indicate that synaptic activity induces expression of TCP1, which in turn mediates inclusion formation in neurons. Moreover, TRiC has been reported to cooperate with the Hsp70 chaperone system15-17; Hsp70 attaches to mtHtt inclusions to protect cells from death17,18. Indeed, here we observed co-localization of inclusions and Hsp70 (Supplementary Fig. 9 online), suggesting that the TRiC-Hsp70 system might ameliorate mtHtt toxicity.
mtHtt renders neurons more vulnerable to excitotoxic insult via extrasynaptic NMDA receptors
We have thus shown that physiological synaptic NMDAR activity is necessary for macro inclusion formation in mtHtt-expressing neurons. However, the relationship of mtHtt-induced inclusion formation and neurotoxicity in HD pathogenesis has remained contentious19,20. Large aggregates of abnormally folded proteins have been hypothesized to contribute to synaptic damage in neurodegenerative disorders21, but a recent report suggests that inclusion formation protects neurons from cell death22, possibly by decreasing the level of toxic soluble forms of mtHtt23. Neurodegeneration in HD has also been proposed to represent excitotoxic-mediated apoptotic cell death triggered by excessive activation of NMDARs24. Increased sensitivity to NMDAR-mediated excitotoxicity has been observed in the YAC128 mouse model of HD6. On the other hand, recent studies have indicated that physiological levels of synaptic NMDAR activity (predominantly composed of NR2A subunits in mature neurons) promote survival in the face of various forms of stress11. The exact role of normal neuronal electrical activity in the pathogenesis of HD has not previously been studied.
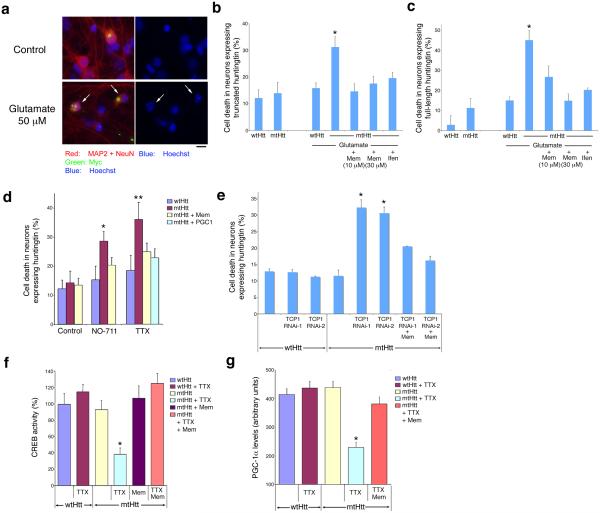
Our observation that endogenous synaptic NMDAR activity promotes formation of mtHtt-containing inclusions prompted our investigation of whether this normal activity could protect neurons. Conversely, we hypothesized that excessive activation of extrasynaptic NMDARs may increase neuronal vulnerability induced by mtHtt. To test this notion, we initially applied a relatively low concentration of exogenous glutamate (50 μM), which by itself was not excitotoxic to untransfected or wtHtt-transfected neurons under these conditions, to stimulate both extrasynaptic and synaptic receptors. While transfection of mtHtt by itself did not result in neuronal death, as shown previously6, we found that mtHtt neurons became more vulnerable in the presence of 50 μM glutamate (Fig. 4a-c). When mtHtt neurons were treated with memantine or ifenprodil, neuronal cell death induced by exogenous glutamate was significantly reduced. Of note, we found that low concentrations of memantine (1-10 μM), which selectively block excessive extrasynaptic NMDAR activity while sparing physiological synaptic NMDARs (Fig. 2; Supplementary Figs. 5 and 10 online)9,10, were sufficient to protect mtHtt-transfected neurons from glutamate challenge. Additionally, extrasynaptic NMDARs preferentially contain NR2B subunits25, and ifenprodil, a relatively selective inhibitor of NR2B, blocked this neurotoxicity6,11. Taken together, these results imply that mtHtt renders neurons more vulnerable to acute excitotoxic insults and that blockade of extrasynaptic NMDARs efficiently ameliorates this excitotoxic cell death. In this regard, we observed similar results for both truncated (N-terminal fragment) (Fig. 4b) and full-length Htt constructs (Fig. 4c).
Figure 4.
Excitatory synaptic versus extrasynaptic activity in HD-related neuronal cell death. (a) Excessive glutamate insult (50 μM for 40 h) led to neuronal cell death in mtHtt-transfected cortical neurons in the presence of inclusion formation (scale bar, 10 μm). Neurons transfected with mtHtt contained inclusions and displayed healthy nuclei. (b, c) Cell death analysis after transfection with the N-terminal fragment of mtHtt (b) and full-length mtHtt (c). Values represent mean ± s.e.m. for n ≥ 1,200. *, P < 0.01 by ANOVA. (d) Memantine attenuated neuronal cell death induced by blockade of physiological excitatory synaptic activity with NO-711 or TTX in mtHtt-transfected neurons. Co-transfection of PGC-1α mitigated neuronal cell death induced by blockade of synaptic activity with TTX. Values represent mean ± s.e.m. for n ≥ 1,200. *, P < 0.05; **, P < 0.01 by ANOVA. (e) Cell death was quantified in neurons transfected with wtHtt or mtHtt plus two different small hairpin vectors for TCP1 or a control vector. Values are mean ± s.e.m. for n ≥ 300. *, P ≤ 0.01 by ANOVA. (f) Low concentration (5 μM) memantine abrogated the decrease in CREB activity induced by blockade of physiological excitatory synaptic activity with TTX in mtHtt-transfected neurons. Neurons were transfected with a CRE-luciferase reporter construct and wtHtt or mtHtt. Ordinate axis represents relative luciferase activity. Values represent mean ± s.e.m. *, P < 0.01 by ANOVA. (g) Low concentration memantine ameliorated the decrease in PGC-1α levels induced by blockade of synaptic activity with TTX in mtHtt-transfected neurons. PGC-1α levels were quantified by immunofluorescence under deconvolution microscopy in wtHtt- or mtHtt-transfected neurons. Values represent mean ± s.e.m. for n ≥ 300. *, P < 0.01 by ANOVA.
Physiological synaptic activity protects mtHtt expressing-neurons from cell death
Next, we examined the effect of normal synaptic activity on survival of neurons expressing mtHtt. We found that TTX, which suppresses normal excitatory synaptic transmission, or NO-711, which enhances inhibitory neurotransmission, triggered significant cell death in mtHtt- but not wtHtt-expressing neurons (Fig. 4d; Supplementary Fig. 11 online). We then asked if extrasynaptic NMDAR activity causes cell death in mtHtt neurons when protective (physiological) synaptic NMDAR activity is inhibited. Accordingly, we investigated if an NMDAR antagonist that preferentially blocks extrasynaptic activity could protect mtHtt neurons from toxicity induced by TTX or NO-711. Indeed, we found that treating mtHtt cultures with low concentrations (~5 μM) of memantine significantly reduced neuronal death induced by TTX or NO-711 (Fig. 4d). The question arises where the extrasynaptic glutamate originates in our culture system in the presence of neuronal activity blockade with TTX because synaptic release is inhibited. Since the intracellular pool of glutamate, used for metabolic purposes, may be significant, up to 10 mM per cell26, mtHtt-insulted neurons may potentially leak glutamate from nonsynaptic sites. Additionally, similar to the intact brain, our culture system contains nonneuronal cells, predominantly astrocytes. Even in the absence of synaptic activity, glutamate released from astrocytes can activate extrasynaptic NMDARs in neurodegenerative conditions27,28.
Effect of electrical activity on the molecular pathways involved in inclusion formation and cell survival in mtHtt-expressing neurons
We next wanted to investigate the relationship of macro inclusion formation, electrical activity, and neuronal survival pathways. Since we had demonstrated earlier that TCP1 mediates inclusion formation (Fig. 3b), we examined if TCP1 is involved in the survival of neurons expressing mtHtt. We found that knockdown of TCP1 not only decreased inclusion formation but also significantly increased neuronal cell death in mtHtt-expressing neurons (Fig. 4e). These RNAi experiments imply causality between TCP1 levels, inclusion formation, and neuronal cell death. Importantly, this form of cell death was significantly ameliorated by blockade of extrasynaptic NMDARs using low-dose memantine (Fig. 4e). Taken together, our results suggest that synaptic activity induces expression of TCP1, which in turn contributes to protective inclusion formation and decreased neurotoxicity. Conversely, blockade of synaptic activity reduces TCP1 levels, which in turn decreases mtHtt inclusion formation, and contributes to neuronal cell death in conjunction with excessive extrasynaptic NMDARs activity.
Additionally, using low-dose memantine, we investigated if the level of Rhes, a small guanine nucleotide-binding protein recently reported to mediate mtHtt sumoylation, disaggregation and cytotoxicity29, is affected by blockade of extrasynaptic NMDARs. We found that low-dose memantine (5-10 μM) reduced Rhes levels (Supplementary Fig. 12 online), consistent with the notion that extrasynaptic NMDAR activity controls Rhes expression. Thus, the detrimental effects of excessive extrasynaptic NMDAR activity in the context of mtHtt may be ascribed, at least in part, to a relative increase in Rhes.
NMDAR activity modulates the neuroprotective CREB—PGC-1α pathway in neurons expressing mtHtt
CREB/CBP transcriptional activity, which triggers the neuroprotective PGC-1α pathway, has been shown to be decreased by extrasynaptic NMDAR activity11 as well as by binding to soluble mtHtt30. We therefore hypothesized that impairment of the CREB—PGC-1α cascade may contribute to neuronal cell death in HD31. Hence, we determined if CREB function was compromised in mtHtt-expressing neurons when synaptic activity was blocked. In mtHtt- but not wtHtt-transfected neurons we found a significant decrease in CREB activity in the presence of TTX, which suppresses excitatory synaptic activity (Fig. 4f). Additionally, blockade of extrasynaptic NMDARs with low concentrations of memantine restored CREB function, suggesting that mtHtt and activation of extrasynaptic NMDARs are necessary for CREB inactivation.
We next determined the level of PGC-1α under these same conditions. We observed a significant decrease in PGC-1α levels in mtHtt-transfected neurons in the presence of TTX, but preservation of PGC-1α levels in the presence of low concentrations of memantine (Fig. 4g; Supplementary Fig. 13 online). Additionally, we found that RNAi knockdown of TCP1 decreased CREB activity and PGC-1α levels in mtHtt-expressing neurons (Supplementary Fig. 14 online). These findings support the notion that mtHtt in the absence of TCP1, in conjunction with excessive extrasynaptic NMDAR activity, interferes with the neuroprotective CREB—PGC-1α cascade. Importantly, decreased levels of PGC-1α likely contribute to cell death in mtHtt-transfected neurons exposed to TTX (and thus in neurons with blocked synaptic activity and decreased mtHtt inclusions). As would be predicted, cell death under these conditions was diminished by co-transfection with PGC-1α (Fig. 4d).
NMDAR activity modulates toxicity and behavior in HD transgenic mice
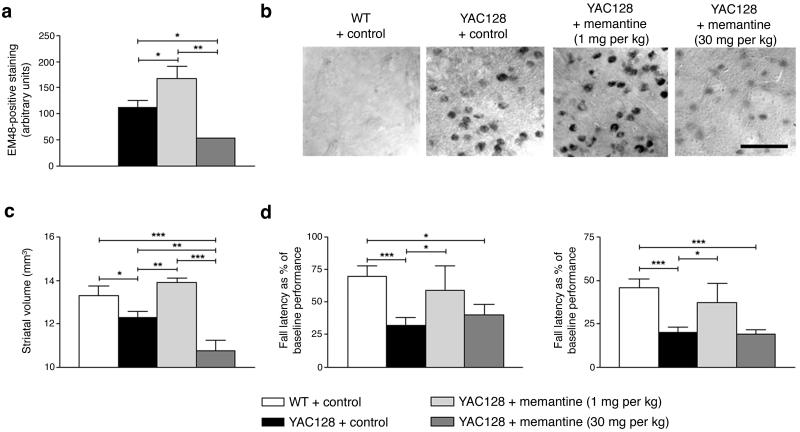
These findings raise the possibility that long-term blockade of extrasynaptic activity would be advantageous while simultaneous blockade of synaptic activity might prove deleterious in vivo, for example, in the transgenic YAC128 HD mouse8 (Supplementary Fig. 4b). We hypothesized that the balance between synaptic and extrasynaptic activity in the face of mtHtt would determine neuronal survival. Therefore, while short-term blockade of both synaptic and extrasynaptic activity might cause transient improvement, eventually blockade of synaptic activity would lead to neuronal loss. In contrast, maintenance of synaptic activity with abrogation of excessive extrasynaptic activity would be most beneficial to combat the deleterious effects of mtHtt. To test this hypothesis in vivo, YAC128 mice were treated using drinking water with low-dose memantine (1 mg kg-1) to block extrasynaptic NMDAR activity or high-dose memantine (30 mg kg-1) to additionally block synaptic NMDAR activity since we knew that these doses would produce low and high concentrations of memantine approaching those used in the culture experiments9,10,32-34. Treatment commenced at 2 months of age and continued for 10 months until 12 months of age. We first analyzed mtHtt inclusions using immunostaining with antibody EM48, which recognizes N-terminal huntingtin and is highly specific for aggregates35. Treatment with low-dose memantine increased inclusion formation (Fig. 5a, b), as confirmed using a filter trap assay (Supplementary Fig. 15 online), while high-dose memantine significantly decreased inclusion formation in 12-month-old YAC128 mice (Fig. 5a, b). Accordingly, high-dose memantine also increased soluble mtHtt (Supplementary Fig. 16 online). These effects of memantine treatment on inclusion formation were not due to alterations in mtHtt protein expression since low and high doses of memantine did not affect mtHtt levels (Supplementary Fig. 17 online). On the other hand, TCP1 levels increased with low-dose memantine and decreased with high-dose memantine (Supplementary Fig. 18 online), supporting the notion that normal synaptic NMDAR activity increases TCP1 and promotes inclusion formation.
Figure 5.
Long-term treatment with memantine affects neuropathology and motor function in a dose-specific manner in transgenic YAC128 HD animals. (a) The extent of inclusion formation was assessed using unbiased densitometry of striatal neurons stained with EM48. (b) Representative immunohistological photographs of EM48-stained striata from WT, untreated YAC128 animals, and YAC128 animals treated with 1 or 30 mg kg-1 memantine. The photomicrographs were taken using an 100x objective (scale bar, 50 μm). (c) Striatal volumes were determined by tracing the perimeter of the striatum in serial sections spanning the striatum. (d) Effects of low-dose or high-dose memantine on the fixed-speed (left) or accelerating rotarod task (right) in YAC128 animals. Values represent the mean change from baseline latency to fall. Data represent mean + s.e.m. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Next, we measured loss in striatal volume in YAC128 mice, a cardinal neuropathological feature of HD36. Low-dose memantine improved striatal volume, while high-dose memantine worsened this parameter (Fig. 5c). These findings support our hypothesis that maintenance of synaptic activity, while inhibiting excessive extrasynaptic activity, is protective, and that long-term blockade of synaptic activity is detrimental. We also monitored motor function by rotatrod testing at 12 months of age. YAC128 HD mice treated with low-dose memantine demonstrated improvements on these motor tests, while high-dose-treated mice showed no improvement (Fig. 5d). Taken together, these results support the premise that maintenance of synaptic activity with abrogation of extrasynaptic activity is beneficial in YAC128 HD mice.
DISCUSSION
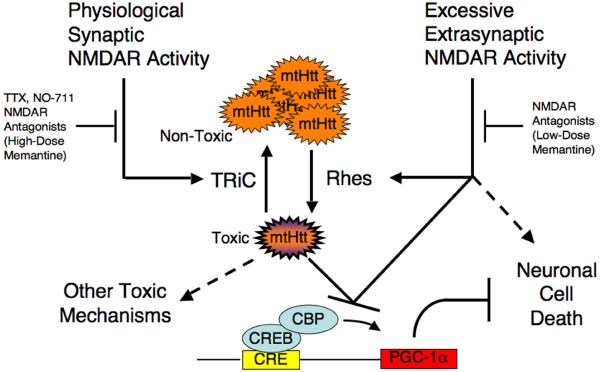
Our results provide a mechanistic framework to help us understand the selective vulnerability of striatal and cortical neurons in HD. We show here that synaptic activity controls expression of the chaperonin TRiC, which in turn modulates inclusion formation and toxicity of mtHtt. Moreover, the electrical properties of these neurons coupled with the predominant effect of the small guanine nucleotide-binding protein Rhes and the CREB—PGC-1α pathway in this cell type29,37 renders them particularly sensitive to the toxic effects of mtHtt. Specifically, excessive extrasynaptic activity increases the relative Rhes level and, in conjunction with mtHtt, decreases CREB—PGC-1α activity to promote neuronal cell death. Coupled with a decrease in synaptic transmission, which also contributes to a decrease in CREB activity in the presence of mtHtt, neurons become increasingly vulnerable to injury and death (Fig. 6). We speculate that these findings imply that therapies for HD that minimize excessive extrasynaptic activity while maintaining or enhancing normal synaptic activity will yield considerable benefit. Notably, it has been contentious whether mtHtt inclusions are cytotoxic. The presence of inclusions may reflect either successful sequestration of toxic soluble oligomers of mtHtt and thus contribute to neuroprotection, or insufficient sequestration of toxic soluble mtHtt, which can no longer be accommodated by the inclusion formation process, and is thus toxic. Here, we report that synaptic activity-driven inclusions are neuroprotective, supporting the former premise.
Figure 6.
Schematic model showing the role of physiological synaptic vs. excessive extrasynaptic NMDAR activity in the neurodegeneration of Huntington’s disease. Physiological synaptic NMDAR activity promotes neuroprotection, in part by facilitating non-toxic aggregation of mtHtt via the chaperonin TRiC. Otherwise toxic mtHtt would interfere with the neuroprotective CREB-PGC-1α pathway. In contrast, extrasynaptic NMDAR activity promotes neuronal cell injury and death, in part by increasing the relative level of Rhes, and, in conjunction with soluble/toxic mtHtt, by contributing to transcriptional deregulation of the CREB-PGC-1α cascade. Drugs inhibiting extrasynaptic or synaptic activity are indicated. Note that most NMDAR antagonists, as well as high concentrations of memantine, block both synaptic and extrasynaptic NMDAR-mediated currents, while low concentrations of memantine block predominantly the extrasynaptic component, thus relatively sparing synaptic activity and promoting neuroprotection.
Nonetheless, we also demonstrate that cultured neurons containing mtHtt inclusions become increasingly vulnerable to excitotoxic insults, consistent with the notion that over time, accumulation of excitotoxic challenges may eventually contribute to the death of mtHtt-expressing neurons in HD6. We show not only that mtHtt increases the vulnerability of neurons to relatively low concentrations of exogenous glutamate, but also that suppressing spontaneous excitatory synaptic activity can mimic this phenomenon via the excitotoxic effect of endogenous glutamate apparently acting on extrasynaptic receptors.
Our data using primary neurons manifesting synaptic activity may also explain why others had reported direct mtHtt toxicity in the absence of excitotoxic insult in yeast, various cell lines, and synaptically-immature neurons. That is, the presence of normal synaptic activity in our mature neuronal cultures leads to the formation of inclusions, thereby avoiding toxic oligomers of mtHtt, whereas these other cell types lack synaptic activity and therefore are exposed to soluble mtHtt oligomers.
Our findings further suggest that the balance between synaptic and extrasynaptic NMDAR activity may be critical in determining neuronal cell survival in HD. We show that low concentrations of the NMDAR antagonist, memantine, afford the advantage of restoring excitatory balance by maintaining physiological synaptic activity while blocking excessive extrasynaptic NMDAR stimulation9,10. Inhibiting excessive extrasynaptic NMDAR activity decreased Rhes levels, thus preserving mtHtt inclusions and lessening mtHtt cytotoxicity29. To the contrary, blocking synaptic activity with high-dose memantine decreased inclusion formation and increased soluble mtHtt. Soluble mtHtt interferes with the neuroprotective CREB—PGC-1α pathway, thus accelerating neuronal cell death. Indeed, prolonged administration of high-dose memantine may contribute to toxicity for several reasons, including interference with other neuroprotective pathways that are dependent on synaptic activity, such as the phosphatidylinositol 3 kinase (PI3K)-Akt cascade38,39. Thus, we posit that restoring excitatory balance, which is disrupted by mtHtt protein, can affect protein misfolding and protect neurons in HD, as also suggested in a small, open-label human clinical trial of memantine in HD patients40. This novel concept of balancing synaptic and extrasynaptic neuronal NMDAR activity may also lead to strategies to combat cell injury and death associated with other neurodegenerative disorders.
Supplementary Material
ACKOWLEDGMENTS
We thank C. A. Ross (Johns Hopkins University School of Medicine) and L. M. Ellerby (Buck Institute for Age Research) for N-terminal and full-length constructs of huntingtin, T. F. Newmeyer and H. Fang for providing primary neuronal cultures, X.-J. Li (Emory University School of Medicine) for providing the EM48 antibody, and E. Bossy-Wetzel, Y.S. Choo, W. Zago, and T. Nakamura for assistance or discussion. This work was supported in part by NIH grants P01 HD29587, R01 EY09024, R01 EY05477 and R01 NS41207, and a Senior Scholar Award in Aging Research from the Ellison Medical Foundation (S.A.L.). Additional support was provided by the NIH Blueprint Grant for La Jolla Interdisciplinary Neuroscience Center Cores P30 NS057096. M.A.P. was supported by the Canadian Institute of Health Research and the Michael Smith Foundation for Health Research. M.R.H. was supported by grants from the Canadian Institutes of Health Research, the Huntington Society of Canada, the Hereditary Disease Foundation, the Huntington’s Disease Society of America, CHDI Inc., and the HighQ Foundation.
Appendix
ONLINE METHODS
Cell culture and transfection
Primary cerebrocortical or striatal cultures, containing both neurons and glia, were made from E16 rat pups and grown on 12-mm glass coverslips, as we have described41. Cells were transfected via LipofectAMINE 2000 (Invitrogen) on the 15-20th DIV with either N-terminal fragments of Htt consisting of exon 1 (Myc-wtHtt-N63-18Q or Myc-mtHtt-N63-148Q), or with full-length Htt (wtHtt with 15Q or mtHtt with 138Q). In a number of experiments, cells were co-transfected with enhanced green fluorescence protein (EGFP) to facilitate identification of transduced cells. Labeled cells could be subsequently stained with anti-NeuN or anti-MAP-2 antibody, verifying their neuronal identity. In a series of experiments, a PGC-1α expression vector (ATCC) was also co-transfected. Transfection efficiency was 10-15% for cortical neurons, and 5-10% for striatal neurons. In experiments manipulating synaptic activity, drugs were added to cultures five hours post transfection. Extracellular Mg2+ was set at ≤ 0.4 mM to produce spontaneous NMDAR activity in the absence of excessive AMPAR stimulation, as assessed by patch-clamp recordings42. CREB transcriptional activity was analyzed using a CRE luciferase reporter (Stratagene) and renilla luciferase internal control vector (Promega), as previously described41. siSTRIKE plasmids expressing shRNAs targeting TCP1 and a non-targeted sequence were constructed according to the manufacturer’s protocol (Promega). TCP1 shRNA targeting the sequences were designed using Promega’s siRNA Target Designer.
Assessment of inclusion formation and neuronal cell death
For assessment of inclusions, Htt-transfected cortical or striatal neurons were stained with anti-myc (for truncated N-terminal Htt constructs, Santa Cruz), or anti-huntingtin (for full-length Htt constructs, Chemicon) to visualize huntingtin expression under deconvolution microscopy. The expression level of transfected full-length Htt was sufficiently above baseline to allow us to easily distinguish transfected from endogenous Htt (Supplementary Fig. 3). Images were captured with a Zeiss Axiovert 100M microscope equipped with deconvolution software (see Supplementary Methods).
Inclusion formation was assessed 15-17 h after drug application. Immunocytochemistry for MAP2 (Sigma) and NeuN (Chemicon) were used to identify neurons. The total number of transfected neurons with Htt-positive inclusions and the total number of transfected neurons on each coverslip were counted. In a series of experiments combining inclusion and neuronal cell death analysis, wtHtt or mtHtt was co-transfected with EGFP, the latter to facilitate identification of transduced cells; in this manner, we could normalize the number of viable, dead, or inclusion-containing neurons to the total number of transfected neurons for each treatment paradigm. In the cell death experiments, wtHtt- or mtHtt-expressing neurons were incubated in media with or without 50 μM glutamate for 40 h. In another series of experiments, cultures were incubated for 20 h with TTX or NO-711. In some experiments, PGC-1α was also expressed. Cells were then stained with anti-MAP2/anti-NeuN, anti-myc antibodies, anti-PGC-1α antibody (Santa Cruz), anti-TCP1 antibody (Santa Cruz), and Hoechst dye 33342. Neuronal cell death was assessed on the basis of condensed nuclear morphology (with Hoechst dye) and shrunken neurites. Neurons were scored as viable if they possessed normal nuclei and neuritic processes30. In the assessment of both inclusion formation and neuronal cell death, for each experimental condition, ≥100 neurons were counted on triplicate coverslips, and the experiments were repeated at least four times; thus, n ≥ 1200 in each case. Data are expressed as mean ± s.e.m., and statistical significance was determined using an ANOVA followed by Dunnett’s post-hoc test.
Electrophysiological recordings
Whole-cell recordings were performed with an Axopatch 200B amplifier (Axon Instruments) from EGFP-positive neurons 48 to 96 h after transfection42. The extracellular solution contained (in mM): NaCl 135, KCl 2.5, CaCl2 2, MgCl2 0-0.01, NaHCO3 1, Na2HPO4 0.34, KH2PO4 0.44, glucose 20, Hepes 10, pH 7.4. Patch electrodes with a final tip resistance of 4-7 MΩ, were filled with a solution containing (in mM): CsCl 120, tetraethylammonium chloride (TEA-Cl) 20, Hepes 10, EGTA 2.25, CaCl2 1, MgCl2 2, and phalloidin 0.001, pH 7.4. To examine NMDAR-mediated sEPSCs, we used “charge transfer.” Details of this analysis are given in the Analysis of NMDAR-mediated sEPSCs section in the Supplementary Methods and in Supplementary Fig. 10. pCLAMP 8-9.2 software (Axon Instruments) was used for data acquisition and analysis. All recordings were made at room temperature with a holding potential of -60 mV. Currents were digitally sampled at 10-20 kHz and filtered at 2-5 kHz. Data are expressed as mean ± s.e.m., and statistical significance was determined using a Student’s t-test for pairwise comparisons.
Transgenic YAC128 HD mice
Male and female YAC128 mice expressing expanded human huntingtin with 128 CAG repeats and WT littermates maintained on the FVB/N strain (Charles River) were used for these experiments8,43. Mice were housed singly or in pairs in duplex cages with littermates of mixed genotype and maintained under a 12 L:12 D light cycle (lights on at 2300) in a clean facility and given free access to food and water. Experimenters were blind to the genotype of the mice. All experiments were performed with the approval of the animal care committee at the University of British Columbia.
Drug treatment
For administration of drugs in the drinking water, water consumption of individual cages was monitored on a biweekly-basis along with animal body weights. The concentrations of drug solutions for each cage were then adjusted accordingly. The drug solutions were replaced twice/week and provided ad libitum. Animals were treated starting at 2 months of age with 1 mg kg-1 day-1 to 30 mg kg-1 day-1 of memantine for 10 months, and their neuropathology and motor function was assessed at 12 months of age. The apparent effective concentration of memantine at the NMDAR-associated channel in vivo under these conditions after administration of low-dose memantine was estimated to be in the range of 5-10 μM based on our current observations (see Supplementary Methods).
Assessment of motor function
Training and baseline testing for motor function tasks were carried out prior to memantine treatment initiation at 2 months of age during the dark cycle. Assessment of the effect of long-term (10-month) treatment with memantine on motor function was carried out by testing at 12 months of age. Motor coordination and balance were assessed using accelerating and fixed-speed rotarod tasks (UGO Basile, Comerio, Italy). In the accelerating task, the rotarod sped up from 5 revolutions per minute (RPM) to 40 RPM over 4 minutes. In the fixed task, the rotarod revolved at 24 RPM. Performance in the rotarod tasks was assessed by the amount of time that a mouse could remain running on the rotarod. During training, mice were given three trials per day for three consecutive days.
Neuropathological analysis
A series of 25 μm-thick coronal sections spaced 200 μm apart spanning the striatum was stained with NeuN antibody (Chemicon) overnight at room temperature, followed by incubation with biotinylated anti-mouse antibody (Vector Laboratories, Burlingame). The signal was amplified with an ABC Elite kit (Vector) and detected with diaminobenzidine (DAB; Pierce). Striatal volumes were determined from a series of mounted sections using StereoInvestigator software (Microbrightfield) by tracing the perimeter of the striatum in serial sections spanning the striatum.
Assessment of huntingtin inclusions in vivo
Perfused brain sections of 25 μm thickness from WT, untreated YAC128, 1 mg kg-1- and 30 mg kg-1-treated YAC128 animals were immunoassayed with the antibody EM48 to assess the presence of mtHtt inclusions, as described previously35. Polyclonal EM48 antibody was used at 1:500 and DAB was used as the chromogen (Vector). Htt inclusions were defined as EM48-positive staining at the light microscope level. Photographs of mounted sections were taken on a light microscope (Zeiss) under a 100X objective using a MetaMorph Imaging System (Molecular Devices), and the intensity of the staining of striatal neurons was measured.
Statistical analysis
Data are expressed as mean ± s.e.m. When suitable, results were interpreted using a one-way ANOVA with a Student-Newman-Keuls (SNK) post-hoc test. Pairwise comparisons between genotypes/treatments were assessed with a Student’s t-test. Differences were considered statistically significant at P < 0.05.
Additional methods
Supporting methodology is described in the Supplementary Methods.
- 41.Okamoto S.-i., et al. Dominant-interfering forms of MEF2 generated by caspase cleavage contribute to NMDA-induced neuronal apoptosis. Proc. Natl. Acad. Sci. USA. 2002;99:3974–3979. doi: 10.1073/pnas.022036399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lei SZ, et al. Effect of nitric oxide production on the redox modulatory site of the NMDA receptor-channel complex. Neuron. 1992;8:1087–1099. doi: 10.1016/0896-6273(92)90130-6. [DOI] [PubMed] [Google Scholar]
- 43.Van Raamsdonk JM, et al. Cognitive dysfunction precedes neuropathology and motor abnormalities in the YAC128 mouse model of Huntington’s disease. J. Neurosci. 2005;25:4169–4180. doi: 10.1523/JNEUROSCI.0590-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Footnotes
COMPETING INTERESTS STATEMENT
The authors declare competing financial interests: details accompany the full-text HTML version of the paper at http://www.nature.com/naturemedicine/.
References
- 1.The Huntington’s Disease Collaborative Research Group A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. Cell. 1993;72:971–983. doi: 10.1016/0092-8674(93)90585-e. [DOI] [PubMed] [Google Scholar]
- 2.DiFiglia M, et al. Aggregation of huntingtin in neuronal intranuclear inclusions and dystrophic neurites in brain. Science. 1997;277:1990–3. doi: 10.1126/science.277.5334.1990. [DOI] [PubMed] [Google Scholar]
- 3.Ciechanover A, Brundin P. The ubiquitin proteasome system in neurodegenerative diseases: sometimes the chicken, sometimes the egg. Neuron. 2003;40:427–446. doi: 10.1016/s0896-6273(03)00606-8. [DOI] [PubMed] [Google Scholar]
- 4.Beal MF, et al. Replication of the neurochemical characteristics of Huntington’s disease by quinolinic acid. Nature. 1986;321:168–171. doi: 10.1038/321168a0. [DOI] [PubMed] [Google Scholar]
- 5.Ferrante RJ, et al. Therapeutic effects of coenzyme Q10 and remacemide in transgenic mouse models of Huntington’s disease. J. Neurosci. 2002;22:1592–1599. doi: 10.1523/JNEUROSCI.22-05-01592.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zeron MM, et al. Increased sensitivity to N-methyl-d-aspartate receptor-mediated excitotoxicity in a mouse model of Huntington’s disease. Neuron. 2002;33:849–860. doi: 10.1016/s0896-6273(02)00615-3. [DOI] [PubMed] [Google Scholar]
- 7.Heng MY, Detloff PJ, Wang PL, Tsien JZ, Albin RL. In vivo evidence for NMDA receptor-mediated excitotoxicity in a murine genetic model of Huntington disease. J. Neurosci. 2009;29:3200–3205. doi: 10.1523/JNEUROSCI.5599-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Slow EJ, et al. Selective striatal neuronal loss in a YAC128 mouse model of Huntington disease. Human Molec. Genet. 2003;12:1555–1567. doi: 10.1093/hmg/ddg169. [DOI] [PubMed] [Google Scholar]
- 9.Lipton SA. Paradigm shift in neuroprotection by NMDA receptor blockade: Memantine and beyond. Nat. Rev. Drug Disc. 2006;5:160–170. doi: 10.1038/nrd1958. [DOI] [PubMed] [Google Scholar]
- 10.Lipton SA. Pathologically activated therapeutics for neuroprotection. Nat. Rev. Neurosci. 2007;8:803–808. doi: 10.1038/nrn2229. [DOI] [PubMed] [Google Scholar]
- 11.Hardingham GE, Fukunaga Y, Bading H. Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat. Neurosci. 2002;5:405–414. doi: 10.1038/nn835. [DOI] [PubMed] [Google Scholar]
- 12.Li L, Murphy TH, Hayden MR, Raymond LA. Enhanced striatal NR2B-containing N-methyl-d-aspartate receptor-mediated synaptic currents in a mouse model of Huntington disease. J. Neurophysiol. 2004;92:2738–2746. doi: 10.1152/jn.00308.2004. [DOI] [PubMed] [Google Scholar]
- 13.Pickett J. Folding away the bad guys. Nat. Rev. Neurosci. 2006;7:832–833. [Google Scholar]
- 14.Tam S, Geller R, Spiess C, Frydman J. The chaperonin TRiC controls polyglutamine aggregation and toxicity through subunit-specific interactions. Nat. Cell Biol. 2006;8:1155–1162. doi: 10.1038/ncb1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Melville MW, McClellan AJ, Meyer AS, Darveau A, Frydman J. The Hsp70 and TRiC/CCT chaperone systems cooperate in vivo to assemble the von Hippel-Lindau tumor suppressor complex. Mol. Cell. Biol. 2003;23:3141–3151. doi: 10.1128/MCB.23.9.3141-3151.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Behrends C, et al. TRiC promotes the assembly of polyQ expansion proteins into nontoxic oligomers. Mol. Cell. 2006;23:887–897. doi: 10.1016/j.molcel.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 17.Kitamura A, et al. Cytosolic chaperonin prevents polyglutamine toxicity with altering the aggregation state. Nat. Cell Biol. 2006;8:1163–1170. doi: 10.1038/ncb1478. [DOI] [PubMed] [Google Scholar]
- 18.King MA, et al. Cytoplasmic inclusions of Htt exon1 containing an expanded polyglutamine tract suppress execution of apoptosis in sympathetic neurons. J. Neurosci. 2008;31:14401–14415. doi: 10.1523/JNEUROSCI.4751-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davies SW, et al. Formation of neuronal intranuclear inclusions underlies the neurological dysfunction in mice transgenic for the HD mutation. Cell. 1997;90:537–548. doi: 10.1016/s0092-8674(00)80513-9. [DOI] [PubMed] [Google Scholar]
- 20.Saudou F, Finkbeiner S, Devys D, Greenberg ME. Huntingtin acts in the nucleus to induce apoptosis but death does not correlate with the formation of intranuclear inclusions. Cell. 1998;95:55–66. doi: 10.1016/s0092-8674(00)81782-1. [DOI] [PubMed] [Google Scholar]
- 21.Tsai J, Grutzendler J, Duff K, Gan WB. Fibrillar amyloid deposition leads to local synaptic abnormalities and breakage of neuronal branches. Nat. Neurosci. 2004;7:1181–1183. doi: 10.1038/nn1335. [DOI] [PubMed] [Google Scholar]
- 22.Arrasate M, Mitra S, Schweitzer ES, Segal MR, Finkbeiner S. Inclusion body formation reduces levels of mutant huntingtin and the risk of neuronal death. Nature. 2004;431:805–810. doi: 10.1038/nature02998. [DOI] [PubMed] [Google Scholar]
- 23.Sanchez I, Mahlke C, Yuan J. Pivotal role of oligomerization in expanded polyglutamine neurodegenerative disorders. Nature. 2003;421:373–379. doi: 10.1038/nature01301. [DOI] [PubMed] [Google Scholar]
- 24.Friedlander RM. Apoptosis and caspases in neurodegenerative diseases. N. Engl. J. Med. 2003;348:1365–1375. doi: 10.1056/NEJMra022366. [DOI] [PubMed] [Google Scholar]
- 25.Tovar KR, Westbrook GL. The incorporation of NMDA receptors with a distinct subunit composition at nascent hippocampal synapses in vitro. J. Neurosci. 1999;19:4180–4188. doi: 10.1523/JNEUROSCI.19-10-04180.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lipton SA, Rosenberg PA. Excitatory amino acids as a final common pathway for neurologic disorders. N. Engl. J. Med. 1994;330:613–622. doi: 10.1056/NEJM199403033300907. [DOI] [PubMed] [Google Scholar]
- 27.Fellin T, et al. Neuronal synchrony mediated by astrocytic glutamate through activation of extrasynaptic NMDA receptors. Neuron. 2004;43:729–743. doi: 10.1016/j.neuron.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 28.Tian GF, et al. An astrocytic basis of epilepsy. Nat. Med. 2005;11:973–981. doi: 10.1038/nm1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Subramaniam S, Sixt KM, Barrow R, Snyder SH. Rhes, a striatal specific protein, mediates mutant-huntingtin cytotoxicity. Science. 2009;324:1327–1330. doi: 10.1126/science.1172871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nucifora FC, Jr., et al. Interference by huntingtin and atrophin-1 with cbp-mediated transcription leading to cellular toxicity. Science. 2001;291:2423–2428. doi: 10.1126/science.1056784. [DOI] [PubMed] [Google Scholar]
- 31.McGill JK, Beal MF. PGC-1α, a new therapeutic target in Huntington’s disease? Cell. 2006;127:465–468. doi: 10.1016/j.cell.2006.10.023. [DOI] [PubMed] [Google Scholar]
- 32.Chen HS, et al. Open-channel block of N-methyl-d-aspartate (NMDA) responses by memantine: therapeutic advantage against NMDA receptor-mediated neurotoxicity. J Neurosci. 1992;12:4427–4436. doi: 10.1523/JNEUROSCI.12-11-04427.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hesselink MB, De Boer BG, Breimer DD, Danysz W. Brain penetration and in vivo recovery of NMDA receptor antagonists amantadine and memantine: a quantitative microdialysis study. Pharm. Res. 1999;16:637–642. doi: 10.1023/a:1018856020583. [DOI] [PubMed] [Google Scholar]
- 34.Parsons CG, Stöffler A, Danysz W. Memantine: a NMDA receptor antagonist that improves memory by restoration of homeostasis in the glutamatergic system--too little activation is bad, too much is even worse. Neuropharmacology. 2007;53:699–723. doi: 10.1016/j.neuropharm.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 35.Gutekunst CA, et al. Nuclear and neuropil aggregates in Huntington’s disease: relationship to neuropathology. J. Neurosci. 1999;19:2522–2534. doi: 10.1523/JNEUROSCI.19-07-02522.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vonsattel JP, et al. Neuropathological classification of Huntington’s disease. J. Neuropath. Exp. Neurol. 1985;44:559–577. doi: 10.1097/00005072-198511000-00003. [DOI] [PubMed] [Google Scholar]
- 37.Cui L, et al. Transcriptional repression of PGC-1α by mutant huntingtin leads to mitochondrial dysfunction and neurodegeneration. Cell. 2006;127:59–69. doi: 10.1016/j.cell.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 38.Papadia S, Stevenson P, Hardingham NR, Bading H, Hardingham GE. Nuclear Ca2+ and the cAMP response element-binding protein family mediate a late phase of activity-dependent neuroprotection. J. Neurosci. 2005;25:4279–4287. doi: 10.1523/JNEUROSCI.5019-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Soriano FX, et al. Preconditioning doses of NMDA promote neuroprotection by enhancing neuronal excitability. J. Neurosci. 2006;26:4509–4518. doi: 10.1523/JNEUROSCI.0455-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beister A, et al. The N-methyl-d-aspartate antagonist memantine retards progression of Huntington’s disease. J. Neural Transm. Suppl. 2004;68:117–122. doi: 10.1007/978-3-7091-0579-5_14. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.