Abstract
Background
Change in high sensitivity C-reactive protein (CRP) from low-fat diet (diet) and physical activity (PA) interventions is relatively unknown for adults with metabolic syndrome.
Objective
To assess CRP change (ΔCRP) with diet and/or PA in men and women with and without metabolic syndrome.
Design
Men (n=149) and postmenopausal women (n=125) with elevated LDL-C and low HDL-C were recruited into a one-year randomized controlled trial. Treatment groups were: control, diet (reduced total fat, saturated fat and cholesterol intake), PA (45-60 minutes at 60-85% maximum heart rate) or diet plus physical activity (diet+PA). Weight loss was not an intervention focus. Metabolic syndrome was defined using the American Heart Association/National Heart, Lung and Blood Institute/ criteria. Stored plasma samples were analyzed for CRP. ΔCRP was compared between treatments, within gender and metabolic syndrome status, using ANCOVA, including covariates for baseline CRP and body fat change.
Results
For women with metabolic syndrome (n=39) ΔCRP was greater in diet vs. control (-1.2 ± 0.4, p = 0.009), diet+PA vs. control (-1.3 ± 0.4,p = 0.006), and diet+PA vs. PA (-1.1 ± 0.4, p = 0.02). Women with metabolic syndrome receiving the diet component (diet or diet+PA) had greater ΔCRP compared with those who did not (control or PA)(p = 0.001). ΔCRP was not significantly different between intervention groups in men overall, women overall, men with (n=47) or without metabolic syndrome (n=102), or women without metabolic syndrome (n=86).
Conclusion
Low-fat diet may be the most effective treatment for reducing CRP in women with metabolic syndrome.
Introduction
The process of atherosclerosis leading to cardiovascular disease is hypothesized to be controlled through inflammation1. Chronic inflammation is characterized by elevated levels of C-reactive protein (CRP), an acute phase reactant released from the liver in response to various cytokines, such as interleukin-6 (IL-6) and tumor necrosis factor (TNF-α). It is also hypothesized that CRP is a feature of metabolic syndrome, a constellation of metabolic and/or lipid abnormalities which predispose one to cardiovascular disease 2.
Approximately 36% of Americans have metabolic syndrome3 and the majority of individuals with metabolic syndrome also have elevated CRP.4 While CRP and metabolic syndrome are each independent predictors of cardiovascular events, 5;6 the combination adds predictive and prognostic value to the estimation of cardiovascular disease events6;7.
Positive lifestyle habits such as incorporation of a low-fat diet and increasing physical activity improve cardiovascular risk and mortality.8;9 Diet- or physical activity-induced weight loss is associated with improvements of the individual components of metabolic syndrome (waist circumference, high density lipoprotein cholesterol (HDL-C), triglycerides, blood pressure, fasting glucose)9 and CRP levels10. The release of CRP is controlled, in part, by cytokines that can be stored and released from adipose tissue.11 Therefore, reduction in body fat10 is associated with a reduction in the level of circulating CRP. Studies that combined dietary change and physical activity show reductions in CRP for individuals with metabolic syndrome12-14, even when weight loss was not an intervention goal12. However, few studies have compared the independent and combined effects of low-fat diet and physical activity, without the influence of intentional weight loss, on CRP levels in individuals with metabolic syndrome.
Although inconclusive, some research suggests that women have higher CRP levels than their age-matched male counterparts.15 Some diet and/or physical activity studies that examine CRP as an outcome are either in a single gender 16;17 or combine genders in the analysis12;13;18 without statistical adjustment 12;13. Higher CRP values at baseline can result in larger decreases in CRP with both low-fat diet 19;20 and exercise 21-23. Thus, analyses of CRP from lifestyle treatment should test for possible differing gender responses. Gender-stratified analyses in insulin-resistant individuals revealed similar magnitudes of CRP change from diet plus physical activity 24, however these results may not be generalizable to individuals who have multiple lipid and metabolic disorders.
Thus, the purpose of this study was to assess CRP change from a low-fat diet and /or physical activity treatment, relative to controls, in men and women with and without metabolic syndrome.
Methods
The Diet and Exercise for Elevated Risk Trial (DEER) was a one year-long, single-center randomized controlled clinical trial initiated in 1992 at Stanford Medical School’s Prevention Research Center25. The primary and secondary outcomes were the effects on HDL-C and low density lipoprotein cholesterol (LDL-C), respectively, of a low-fat diet and/or increased physical activity intervention in individuals with elevated cardiovascular disease risk (defined as having low HDL-C and elevated LDL-C). Results of the original study have been published.25 This project utilizes the existing data set and stored baseline and one year plasma blood samples to examine, retrospectively, the effect of the diet and physical activity interventions on hs-CRP.
Specific eligibility criteria were: for men, age 30-64 years, and HDL-C < 45 mg/dL combined with LDL-C 126-189 mg/dL; for women, age 45-64 years, postmenopausal, HDL-C < 60 mg/dL combined with LDL-C 126-209 mg/dL. Exclusion criteria included body mass index (BMI) ≥ 34 kg/m2 for men and ≥ 32 kg/m2 for woman, and for both genders: blood pressure ≥ 160/85 mmHg; fasting triglycerides ≥ 500 mg/dL; fasting glucose ≥ 140 mg/dL; abnormal baseline maximal exercise treadmill test; history of heart disease, stroke, insulin-dependent diabetes mellitus, recent cancer, or diagnosis of a life-threatening disease; neuromuscular or orthopaedic disability which would preclude brisk walking; use of lipid lowering medication or antihypertensive medications; non-euthyroid; low hematocrit; smoking > 9 cigarettes per day; > 4 alcoholic drinks per day; inability to attend sessions; by judgment of a physician; or, unwillingness to accept random assignment to a diet or exercise intervention.
Participant eligibility and baseline information was assessed by telephone and clinic screening prior to randomization. All clinic staff performing measurement were blinded to the participants’ group assignment.
Venous blood was collected in the morning at two separate visits at baseline and at one-year, after participants had fasted, i.e. no food or drink (except water), for 12 hours, no alcohol consumption or vigorous physical activity for 24 hours, and abstained from smoking for one hour. Blood collected for plasma was mixed with 1.5 mg/mL of EDTA, placed on ice before and after refrigerated centrifugation, and alliquoted samples were stored and kept frozen at -80° C.
Plasma high-sensitivity CRP (hs-CRP) concentrations were determined on stored plasma samples randomly chosen from two available samples available at each time point. Immunoturbidimetric assay was performed on the Hitachi 917 analyzer (Roche Diagnostics - Indianapolis, IN), using reagents and calibrators from DiaSorin (Stillwater, MN). This assay has a sensitivity of 0.03 mg/L. The day-to-day variability of the assay at concentrations of 0.91, 3.07 and 13.38 mg/L are 2.81, 1.61 and 1.1%, respectively.
Total cholesterol and triglycerides were measured using enzymatic procedures. 26;27 HDL-C was measured using dextran sulfate—magnesium precipitation28 as well as enzymatic measurement of non-precipitated cholesterol.26 Very low density lipoprotein (VLDL) was calculated as triglycerides divided by five29, unless triglyceride levels exceeded 400 mg/dL, in which case enzymatic methods were used 26 after ultracentrifugation for 18 hours30. LDL-C was calculated as total cholesterol minus the sum of HDL-C + VLDL 29. Lipoprotein values were averaged between the two baseline fasting values.
Blood pressure was measured from the brachial artery utilizing a mercury sphygmomanometer and stethoscope. Averages for two readings of the first and fifth Korotkoff phase were recorded as systolic and diastolic blood pressure.31
Body weight was measured with a standard medical beam balance scale. Height was measured using a Harpenden stadiometer. BMI was calculated as body weight, in kilograms, divided by height, in meters squared (kg/m2). BMI categories were determined using National Institutes of Health guidelines (NIH): 1) normal weight (18.5-24.9 kg/m2), 2) overweight (25.0 – 29.9 kg/m2), and obese (>30 kg/m2)32. Waist circumference was taken at the narrowest circumference of the torso when viewed from the front. Skinfold measures were made in triplicate on the right side of the body and averaged. For males, the locations of the skinfolds were chest, abdomen, thigh. For women, the locations were triceps, suprailiac and thigh. Body density was estimated using generalized equations 33;34 and percent body fat was calculated using the Siri equation 35.
The presence of metabolic syndrome was determined using the joint American Heart Association/National Heart, Lung and Blood Institute’s guidelines.36 Clinical identification of the metabolic syndrome includes at least three of the following 1) waist circumference > 102 cm in men; > 88 cm in women, 2) triglycerides ≥150 mg/dL, or on drug treatment, 3) HDL-C < 40 mg/dL for men; < 50 mg/dL for women, or on drug treatment, 4) blood pressure ≥130/85 mmHg, or on drug treatment, and 5) fasting glucose ≥ 100 mg/dL, or on drug treatment.36
Eligible participants were recruited and then computer randomized within cohort into treatment groups, using a modified Efron procedure which weighted the probability of assignment in order to balance groups for sample size, HDL-C and LDL-C measures.37 The four treatment groups were: 1) control, 2) diet, 3) physical activity (PA) and 4) diet plus physical activity (diet+PA).
The control group was instructed to maintain their usual lifestyle habits for the duration of the trial.
The dietary goals for the diet group were based on the National Cholesterol Education Program (NCEP) Step II Guidelines 38: 1) reduce total fat to less than 30% of total calories, 2) reduce saturated fat to less 7% of total calories and 3) reduce dietary cholesterol to less than 200 mg/day. Each participant met with a dietician to individualize dietary recommendations and attended eight group sessions about the NCEP Step II Guidelines.
Participants in the PA group had an individualized physical activity prescription based on the results of a breath-by-breath maximal treadmill exercise test. All PA participants began with six weeks of aerobics sessions three days a week for one hour. After the adoption phase, PA participants were instructed to perform 20 minutes three times a week at 60-85% maximum heart rate, increasing duration over the course of the year to 45-60 total minutes. Progression of the PA program was negotiated between the exercise leader and the participant. PA individuals who were already active prior to randomization were asked to add at least 20 minutes three times a week to their existing programs, in order to elicit physiological changes from an increase in PA. Participants either opted to continue supervised training or adopt a home program for the remaining eight months. The typical PA program involved a minimum of ten miles per week of brisk walking, jogging, or running.
Participants in the diet+PA group received both the diet and PA treatments as individual treatments. To prevent contamination, diet+PA had separate diet and PA sessions from the other groups, and project staff leading the individual sessions made no reference to the other treatment groups.
The diet, PA and diet+PA groups did not emphasize weight loss as an intervention goal.
All intervention methods and laboratory analyses were approved by the Institutional Review Board of Stanford University. CRP secondary data analyses were approved by the Institutional Review Board of the University of Maryland.
Statistical Analysis
All statistical analyses were performed by using SAS software version 9.1 (SAS Institute, Cary, NC). Participants with hs-CRP levels at baseline or follow-up over 10 mg/L were removed from the analysis to eliminate the acute effects of infection (n=9).39 Because the original trial was designed with sex-specific eligibility criteria to identify high risk individuals, it was powered for gender-stratified analyses.25 Because of this, and our a priori hypotheses, all analyses were stratified by gender
Wilcoxon rank sum tests were used to compare hs-CRP baseline level between metabolic syndrome status groups. Analysis of covariance (ANCOVA) was used to test the effects of diet and PA on hs-CRP change (ΔCRP) between treatment groups for: men overall, women overall, men with metabolic syndrome, women with metabolic syndrome, men without metabolic syndrome and women without metabolic syndrome. Older age is a known influence for both metabolic syndrome3 and hs-CRP15. Furthermore, hs-CRP also increases with metabolic syndrome status4, thus it was necessary to account for these differences in the analysis. The analyses in men and women with metabolic syndrome are exploratory due to the limited sample size in each intervention group.
ΔCRP was calculated as a difference between the follow-up and baseline value and was a normally distributed variable, thus a transformation was not necessary. Differences between treatment groups for ΔCRP were compared for: 1) control versus diet, 2) control versus PA, 3) control versus diet+PA, 4) diet versus diet+PA and 5) PA versus diet+PA. An α level of 0.01 was adopted to control for type I error and to account for the multiple statistical comparisons. ANCOVA analyses controlled for baseline hs-CRP, cohort, baseline body fat (%), change in body fat (%), cigarettes per day, alcoholic drinks per day, age and for women, menopausal hormonal therapy (MHT) status. hs-CRP was significantly higher at baseline and follow-up for women on MHT, thus it was included as a covariate. However, change in CRP, was not significantly different between women with or without MHT, thus MHT-stratified analyses were not warranted. Despite weight loss not being a focus of the current intervention, preliminary analysis found significantly greater weight loss, BMI reductions, and percent body fat loss in the diet and diet+PA groups relative to control in both men and women. To control for group differences and to represent changes in body composition resulting from weight loss, percent body fat and baseline body fat were chosen as covariates for the models. Analyses were also run without the covariates for body fat.. In the case of significant differences in ΔCRP between intervention groups, a two-way ANCOVA was analyzed in which diet (yes/no), PA (yes/no), and its interaction were used to distinguish the most important lifestyle component(s).
Results
Of the total 377 DEER participants who were randomized for the study, 278 participants (73%, 149 men and 125 women) were analyzed for ΔCRP. Participants were not included in the analysis due to incomplete data, which was assumed to be missing completely at random. hs-CRP baseline levels were not different between participants included in this analysis and participants with incomplete data (data not shown).
Men and women had an average age of 49.0 ± 8.8 and 57.6 ± 5.0 years, respectively. Participants were mostly Caucasian (∼85%), non-smokers (∼ 98%) and consumed less than one alcoholic drink per day (∼ 95%). Men and women were highly educated: 61% of men and 43% of women had a college degree or greater. The mean BMI for men was approximately 26 kg/m2, with 26% normal weight, 58% overweight and 16% obese. For women, mean BMI was approximately 26 kg/m2, with 38% normal weight, 48% overweight and 14% obese. Approximately 43% of women were on MHT. Mean (± standard deviation) baseline values for hs-CRP were 1.3 ± 1.3 mg/L (median: 0.9, interquartile range: 0.5-1.4) for men and 2.0 ± 1.8 mg/L (median: 1.5, interquartile range: 0.6-2.6) in women. There were no between-intervention group differences at baseline for hs-CRP in either men or women. Table 1 shows the baseline study variables by treatment groups for men and women.
Table 1.
Baseline Characteristics for Men and Women According to Intervention Group1.
| Men (n=149) | Control | Diet | Physical Activity | Diet plus Physical Activity |
|---|---|---|---|---|
| N | 33 | 39 | 35 | 42 |
| Age | 48.8 ± 9.8 | 49.3 ± 9.2 | 49.5 ± 8.7 | 48.5 ± 8.1 |
| Caucasian (%) | 91 | 79.5 | 91 | 81 |
| Non-Smokers (%) | 97 | 100 | 100 | 100 |
| ≤1 Alcoholic Drink per day (%) | 88 | 92 | 91 | 95 |
| HDL-C(mg/dL) | 33.6 ± 5.7 | 35.1 ± 5.3 | 34.7 ± 4.2 | 34.5 ± 4.8 |
| LDL-C (mg/dL) | 158.6 ± 16.8 | 156.2 ± 17.9 | 156.1 ± 16.2 | 156.5 ± 16.4 |
| Waist Circumference (cm) | 95.3 ± 9.4 | 95.9 ± 10.2 | 95.4 ± 7.9 | 94.8 ± 8.5 |
| Body Fat (%) | 21.1 ± 3.6 | 21.3 ± 4.5 | 22.3 ± 5.0 | 21.6 ± 4.1 |
| BMI (kg/m2) | 26.7 ± 3.2 | 26.9 ± 3.1 | 26.9 ± 2.6 | 26.6 ± 2.6 |
| Metabolic Syndrome n (%) | 9 (28) | 13(33) | 11(31) | 14(33) |
| hs-CRP (mg/L) | ||||
| Mean ± SD | 1.4 ± 1.5 | 1.0 ± 1.2 | 1.3 ± 1.3 | 1.4 ± 1.3 |
| Median2 (Interquartile range) | 0.9 (0.3-1.4) | 0.7 (0.4-1.0) | 0.8 (0.4-1.3) | 1.0 (0.4-1.7) |
| High risk CRP (>3mg/L) n (%) | 3 (9) | 2 (5) | 5 (14) | 5 (12) |
| Women (n=125) | Control | Diet | Physical Activity | Diet plus Physical Activity |
|---|---|---|---|---|
| N | 34 | 32 | 28 | 31 |
| Age | 58.4 ± 4.8 | 57.9 ± 5.4 | 57.5 ± 5.4 | 56.6± 4.7 |
| Caucasian (%) | 85 | 91 | 89 | 90 |
| Non-Smokers (%) | 100 | 100 | 100 | 90 |
| ≤1 Alcoholic Drink per day (%) | 94 | 97 | 93 | 100 |
| HDL-C (mg/dL) | 45.1 ± 7.3 | 44.7 ± 7.3 | 44.3 ± 7.8 | 45.0± 7.2 |
| LDL-C (mg/dL) | 166.1 ± 19.1 | 163.1 ± 24.3 | 166.1 ± 23.3 | 165.7± 20.6 |
| Waist Circumference (cm) | 85.4 ± 11.6 | 85.3 ± 7.8 | 83.7 ± 7.0 | 83.8± 9.8 |
| Body Fat (%) | 31.7 ± 5.7 | 31.8 ± 4.8 | 31.9 ± 4.8 | 32.7± 5.3 |
| BMI (kg/m2) | 26.0 ± 3.9 | 26.6 ± 2.8 | 25.9 ± 2.4 | 26.4± 3.5 |
| Metabolic Syndrome n(%) | 9(26) | 9(28) | 9(32) | 12(39) |
| hs-CRP (mg/L) | ||||
| Mean ± SD | 2.2 ± 2.2 | 1.9 ± 1.6 | 1.8 ± 1.6 | 2.0 ±1.9 |
| Median2 (Interquartile range) | 1.4 (0.3-2.6) | 1.3 (0.4-2.2) | 1.5(0.6-2.2) | 1.5 (0.6-2.5) |
| High risk CRP (>3mg/L) n (%) | 9 (25) | 6 (21) | 4 (14) | 5 (19) |
Key: hs-CRP: high-sensitivity C-reactive protein; BMI: body mass index, HDL-C: high density lipoprotein cholesterol, LDL-C: low density lipoprotein cholesterol; MHT: menopausal hormonal therapy; SD: standard deviation.
Mean ± SD is presented. No significant differences any variables presented between groups in either men or women at baseline.
hs-CRP is skewed variable, thus both mean and median values are provided.
Metabolic syndrome prevalence was present in 30% of men and 32% of women at baseline. In men with metabolic syndrome, baseline hs-CRP (mean ± standard deviation) was 1.5 ± 1.4 mg/L (median: 0.9, interquartile range: 0.3-1.5), compared with 1.2 ± 1.2 mg/L (median: 0.9, interquartile range: 0.4-1.4) in men without metabolic syndrome (p = 0.24). Baseline hs-CRP was 2.4 ± 1.7 mg/L (median: 1.9, interquartile range: 0.4-3.4) in women with metabolic syndrome and 1.8 ± 1.9 mg/L (median: 1.3, interquartile range: 0.6-2.1) in women without metabolic syndrome (p = 0.008).
There was little loss to follow-up. Retention was 98% and 96% in men and women, respectively.25 Data from the original DEER study indicate adherence to the assigned treatment groups: the change in fitness (ml/kg/min from VO2max) from baseline was significantly greater in the PA group (men: +1.9 ± 4.3; women: +2.4± 3.3 ml/kg/min) and diet+PA groups (men: 4.7 ± 4.8; women: 3.7 ± 3.8 ml/kg/min) relative to controls25. Changes in total fat, saturated fat and cholesterol were significantly greater within the diet group and diet+PA groups relative to the control group for both men and women. For example, for women, reductions in total fat intake (%) were greater in the diet (- 5.7 % ± 7.4) and diet+PA (-8.0 % ± 5.8 ) relative to controls25 The main findings from DEER with respect to the lipid parameters showed similar changes in men and women: no change in for HDL-C for any group, and a significant decrease in LDL-C in the diet+PA versus control.25
ΔCRP for Men and Women Overall
For men, 15 (10%) were classified as high CRP risk (> 3mg/L)39 at baseline, and at follow-up, 9 (6%) remained in the high risk category. For women at baseline, 24 (19%) were identified at high risk CRP, and 21 (17%) women remained in the high risk category at follow-up. There were no differences for ΔCRP between control, diet, PA and diet+PA for men overall (p = 0.99) and women overall (p=0.06) (Figure 1).
Figure 1.
Adjusted change in CRP (mg/L) (ΔCRP) are presented (mean ± SE) for men and woman overall. ANCOVA statistical comparisons between treatment groups adjusted for the following: baseline hs-CRP, baseline body fat percentage, Δ body fat, cohort, cigarettes per day, alcoholic drinks per day, age and MHT (as appropriate).
A. No differences for ΔCRP were found in men (n = 149) between control, diet, physical activity or diet plus physical activity groups.
B. No differences for ΔCRP were found in women (n = 125) between control, diet, physical activity or diet plus physical activity groups.
ΔCRP for Men and Women with Metabolic Syndrome
For men with metabolic syndrome, no differences were found for ΔCRP between treatments (p = 0.77) (Figure 2). For women with metabolic syndrome, ΔCRP differed between treatment groups (p < 0.01): Women with metabolic syndrome had greater ΔCRP when comparing the diet versus controls (-1.2 ± 0.4 mg/L; p = 0.009), diet+PA versus controls (-1.3 ± 0.4 mg/L; p = 0.006), and diet+PA versus PA (-1.1 ± 0.4 mg/L; p = 0.02) (Figure 2). The two-way ANCOVA analysis found that woman with metabolic syndrome who received the diet component (diet and diet+PA) had greater ΔCRP than those who did not receive the diet (control and PA) (1.2 ± 0.3 mg/L p = 0.001).
Figure 2.
Adjusted change in CRP (mg/L) (ΔCRP) are shown (mean ± SE) for men and woman with metabolic syndrome. ANCOVA comparisons between treatment groups adjusted for the following: baseline hs-CRP, baseline body fat percentage, cohort, Δ body fat, cigarettes per day, alcoholic drinks per day, age and MHT (as appropriate).
A. ΔCRP for men with metabolic syndrome (n = 47) was not different between control, low-fat diet, physical activity or diet plus physical activity in men with metabolic syndrome.
B. ΔCRP for women with metabolic syndrome (n = 39) was different between the control and diet groups (p = 0.009), control and diet plus physical activity groups (p = 0.006) and physical activity and diet plus physical activity groups (p = 0.02).
ΔCRP for Men and Women without Metabolic Syndrome
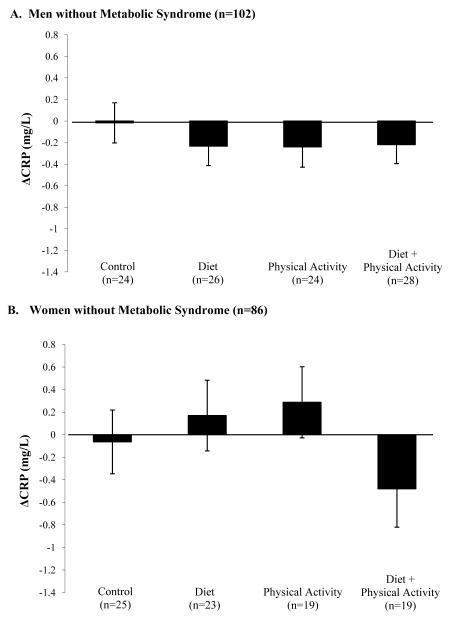
No differences were found for ΔCRP between treatments for men without metabolic syndrome (p = 0.79) or for women without metabolic syndrome (p = 0.31) (Figure 3). No change in results were noted when ΔCRP was analyzed as a percent change or when removing the covariates for baseline and changes in percent body fat for men and women overall, and with or without the metabolic syndrome. Change in percent body fat was not a significant covariate for hs-CRP change for men or women overall, or men or women with or without metabolic syndrome.
Figure 3.
Adjusted change in CRP (mg/L) (ΔCRP) are shown for men and woman without metabolic syndrome (means ± SE). ANCOVA statistical comparisons between treatment groups adjusted for the following: baseline hs-CRP, baseline body fat percentage, cohort, Δ body fat, cigarettes per day, alcoholic drinks per day, age and MHT (as appropriate).
A. ΔCRP for men without metabolic syndrome (n = 102) did not show significant differences between treatment groups.
B. ΔCRP in women without metabolic syndrome (n = 86) did not show differences between treatment groups.
Discussion
To our knowledge, this is the first paper to explore lifestyle changes on hs-CRP stratified by metabolic syndrome separately in men and women.. Women with metabolic syndrome randomized to diet or diet plus physical activity had greater reductions in hs-CRP compared with both the control group and physical activity group. These results suggest that the diet component was an effective treatment for women with metabolic syndrome, though it is difficult to know whether the reduction was due to a decrease in total fat, saturated fat, cholesterol intake, change in macronutrient content, or a combination of these factors Results were not observed for men.
In the present study, women with metabolic syndrome had significantly higher hs-CRP levels than women without metabolic syndrome at baseline. Some low-fat diet and physical activity interventions found decreased hs-CRP levels only in individuals who initially had elevated levels of hs-CRP. 19;21;22 As expected, we found baseline hs-CRP to be a significant predictor of change in CRP (men: r2= 0.19; women r2 = 0.16), such that the higher the baseline hs-CRP, the greater the magnitude of change in CRP. hs-CRP levels for men with metabolic syndrome were not significantly elevated above their healthy counterparts, which may have weakened the hs-CRP response to diet and/or physical activity. Future studies are needed to clarify whether the significant hs-CRP reductions were an influence of gender or a result of higher baseline values. Our results found that women with metabolic syndrome reduced their hs-CRP levels with diet and diet plus physical activity, however, no differences were found between these two groups. Further analysis revealed that the subjects who received the diet component resulted in a significantly different hs-CRP than those who did not. Taken together, these results suggest that low-fat diet may be the most important component for reducing hs-CRP levels for women with metabolic syndrome. Other comparisons between low-fat diet and diet plus exercise have shown that the combination results in larger changes in CRP than diet alone, although none of these studies included a control condition. 13;17;18
Several diet plus physical activity interventions incorporated purposeful weight loss, 13;17;18;24 which may be related to the change in CRP 24. Thus, comparisons between the independent and combined effects of diet and exercise on CRP are difficult to distinguish from the influence of weight loss, or more likely, fat loss. Our study specifically did not promote weight loss and adjusted for changes in body composition using baseline body fat percentage and change in body fat. Only a few studies also accounted for the change in body fat in their analysis13 or allowed ad-libitum dietary consumption16. All of these studies found CRP reductions significant within the diet plus physical activity group, which is consistent with our work.
Several lifestyle interventions explored the effects of low-fat diet and/or physical activity treatments in other high cardiovascular risk adults. Jenkins et al., also found that a low-fat diet lowered CRP in dyslipidemic women, but not men, compared to a control group.20 Exercise training has shown equivocal results for the changes in CRP in dyslipidemic adults22;23. The combination of diet and physical activity has shown decreases in CRP for insulin-resistant adults 24, adults with multiple cardiovascular risk factors12;16. Dyslipidemia and hypertension are only part of the metabolic syndrome, and the clustering of multiple risk factors could alter changes in CRP in response to diet or increased physical activity. A few studies have examined changes in CRP in adults with metabolic syndrome13;14, however, these study designs cannot account for differences in gender or nor do they include a control group for comparison. Though previous studies in high cardiovascular risk individuals likely include participants with metabolic syndrome, our study exclusively examined the results based on the men and women’s metabolic syndrome status.
Several physiological mechanisms exist to explain the changes in CRP from low-fat diet and/or physical activity. Low-fat foods may simultaneously change macronutrient intake and quality20 which can increase intake of naturally anti-inflammatory foods, such as fruits and vegetables, which may ultimately lower CRP 40. Another proposed mechanism involves low-fat foods limiting the postprandial glucose response, thereby inhibiting the cytokine release into the bloodstream41 and subsequent CRP release from the endothelium 42. Physical activity releases IL-6 from muscle tissue initiating the secretion of anti-inflammatory markers (interleukin-10 and interleukin-1 receptor antagonist) which downregulate the pro-inflammatory effects of TNF-α.11 This negative feedback loop suggests that chronic physical activity decreases CRP levels. While the mechanisms that describe low-fat diet and physical activity and their relationship to CRP reduction appear to be independent, one could theorize that physiological effects could be additive. However, the exact physiological mechanism relating the decrease in CRP from diet plus physical activity is poorly understood.
We did not directly compare men and women for hs-CRP response to lifestyle treatment. Due to eligibility criteria for the enrollment, women were approximately ten years older than the men. As previously mentioned, hs-CRP levels may be higher in women,15 but also increase with aging15. Therefore, we performed gender-stratified analyses. Also, since metabolic syndrome status increases with age3, and hs-CRP increases with metabolic syndrome status4, we also gender-stratified the metabolic syndrome status analyses. However, these analyses had a small sample size, which may have reduced power. Although significant findings were found for women, these analyses were exploratory and further study is warranted. Since our participants had elevated cardiovascular disease risk (elevated LDL-C and low HDL-C), women were postmenopausal, and most were Caucasian, highly educated, and highly motivated, our results may be limited to persons with similar characteristics. Despite these limitations, our study included a randomized controlled design that examined both the independent and combined effects of diet and physical activity. Our analysis included individuals free of existing disease, excluded those on lipid lowering medications, and adjusted for baseline body fat and changes in body fat, all of which have known influences on the level of circulating CRP.
In conclusion, women with metabolic syndrome reduced hs-CRP levels with low-fat diet and diet plus physical activity compared to controls, suggesting that lifestyle approaches may have benefit. Furthermore, low-fat diet appears to be the most effective component.
Acknowledgments
Supported by funds from the National Institute of Health grant R21 HL086651. The authors responsibilities were as follows: Sarah M. Camhi- CRP data collection, statistical analysis, results interpretation, manuscript drafting. Deborah R. Young- analysis, results interpretation, manuscript drafting. Marcia L. Stefanick- study design, data collection supervision, results interpretation, manuscript revisions. Paul M. Ridker - results interpretation, manuscript revisions, advice and consultation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 2.Festa A, D’Agostino R, Jr., Howard G, et al. Chronic subclinical inflammation as part of the insulin resistance syndrome: the Insulin Resistance Atherosclerosis Study (IRAS) Circulation. 2000;102:42–47. doi: 10.1161/01.cir.102.1.42. [DOI] [PubMed] [Google Scholar]
- 3.Churilla JR, Fitzhugh EC, Thompson DL. The Metabolic Syndrome: How Definition Impacts the Prevalence and Risk in U.S. Adults: 1999-2004 NHANES. Metab Syndr.Relat Disord. 2007;5:331–342. doi: 10.1089/met.2007.0010. [DOI] [PubMed] [Google Scholar]
- 4.Ford ES. The metabolic syndrome and C-reactive protein, fibrinogen, and leukocyte count: findings from the Third National Health and Nutrition Examination Survey. Atherosclerosis. 2003;168:351–358. doi: 10.1016/s0021-9150(03)00134-5. [DOI] [PubMed] [Google Scholar]
- 5.Rutter MK, Meigs JB, Sullivan LM, et al. C-reactive protein, the metabolic syndrome, and prediction of cardiovascular events in the Framingham Offspring Study. Circulation. 2004;110:380–385. doi: 10.1161/01.CIR.0000136581.59584.0E. [DOI] [PubMed] [Google Scholar]
- 6.Sattar N, Gaw A, Scherbakova O, et al. Metabolic syndrome with and without C-reactive protein as a predictor of coronary heart disease and diabetes in the West of Scotland Coronary Prevention Study. Circulation. 2003;108:414–419. doi: 10.1161/01.CIR.0000080897.52664.94. [DOI] [PubMed] [Google Scholar]
- 7.Ridker PM, Buring JE, Cook NR, et al. C-reactive protein, the metabolic syndrome, and risk of incident cardiovascular events: an 8-year follow-up of 14 719 initially healthy American women. Circulation. 2003;107:391–397. doi: 10.1161/01.cir.0000055014.62083.05. [DOI] [PubMed] [Google Scholar]
- 8.Paffenbarger RS, Jr., Hyde RT, Wing AL, et al. The association of changes in physical-activity level and other lifestyle characteristics with mortality among men. N.Engl.J.Med. 1993;328:538–545. doi: 10.1056/NEJM199302253280804. [DOI] [PubMed] [Google Scholar]
- 9.Yu-Poth S, Zhao G, Etherton T, et al. Effects of the National Cholesterol Education Program’s Step I and Step II dietary intervention programs on cardiovascular disease risk factors: a meta-analysis. Am.J.Clin.Nutr. 1999;69:632–646. doi: 10.1093/ajcn/69.4.632. [DOI] [PubMed] [Google Scholar]
- 10.Selvin E, Paynter NP, Erlinger TP. The effect of weight loss on C-reactive protein: a systematic review. Arch.Intern.Med. 2007;167:31–39. doi: 10.1001/archinte.167.1.31. [DOI] [PubMed] [Google Scholar]
- 11.Petersen AM, Pedersen BK. The anti-inflammatory effect of exercise. J.Appl.Physiol. 2005;98:1154–1162. doi: 10.1152/japplphysiol.00164.2004. [DOI] [PubMed] [Google Scholar]
- 12.Bo S, Ciccone G, Baldi C, et al. Effectiveness of a lifestyle intervention on metabolic syndrome. A randomized controlled trial. J.Gen.Intern.Med. 2007;22:1695–1703. doi: 10.1007/s11606-007-0399-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Esposito K, Marfella R, Ciotola M, et al. Effect of a mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: a randomized trial. JAMA. 2004;292:1440–1446. doi: 10.1001/jama.292.12.1440. [DOI] [PubMed] [Google Scholar]
- 14.Roberts CK, Won D, Pruthi S, et al. Effect of a short-term diet and exercise intervention on oxidative stress, inflammation, MMP-9, and monocyte chemotactic activity in men with metabolic syndrome factors. J.Appl.Physiol. 2006;100:1657–1665. doi: 10.1152/japplphysiol.01292.2005. [DOI] [PubMed] [Google Scholar]
- 15.Ford ES, Giles WH, Mokdad AH, et al. Distribution and correlates of C-reactive protein concentrations among adult US women. Clin.Chem. 2004;50:574–581. doi: 10.1373/clinchem.2003.027359. [DOI] [PubMed] [Google Scholar]
- 16.Wegge JK, Roberts CK, Ngo TH, et al. Effect of diet and exercise intervention on inflammatory and adhesion molecules in postmenopausal women on hormone replacement therapy and at risk for coronary artery disease. Metabolism. 2004;53:377–381. doi: 10.1016/j.metabol.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 17.You T, Berman DM, Ryan AS, et al. Effects of hypocaloric diet and exercise training on inflammation and adipocyte lipolysis in obese postmenopausal women. J.Clin.Endocrinol.Metab. 2004;89:1739–1746. doi: 10.1210/jc.2003-031310. [DOI] [PubMed] [Google Scholar]
- 18.Nicklas BJ, Ambrosius W, Messier SP, et al. Diet-induced weight loss, exercise, and chronic inflammation in older, obese adults: a randomized controlled clinical trial. Am.J.Clin.Nutr. 2004;79:544–551. doi: 10.1093/ajcn/79.4.544. [DOI] [PubMed] [Google Scholar]
- 19.Seshadri P, Iqbal N, Stern L, et al. A randomized study comparing the effects of a low-carbohydrate diet and a conventional diet on lipoprotein subfractions and C-reactive protein levels in patients with severe obesity. Am.J.Med. 2004;117:398–405. doi: 10.1016/j.amjmed.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 20.Jenkins DJ, Kendall CW, Marchie A, et al. Direct comparison of dietary portfolio vs statin on C-reactive protein. Eur.J.Clin.Nutr. 2005;59:851–860. doi: 10.1038/sj.ejcn.1602152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goldhammer E, Tanchilevitch A, Maor I, et al. Exercise training modulates cytokines activity in coronary heart disease patients. Int.J.Cardiol. 2005;100:93–99. doi: 10.1016/j.ijcard.2004.08.073. [DOI] [PubMed] [Google Scholar]
- 22.Lakka TA, Lakka HM, Rankinen T, et al. Effect of exercise training on plasma levels of C-reactive protein in healthy adults: the HERITAGE Family Study. Eur.Heart J. 2005;26:2018–2025. doi: 10.1093/eurheartj/ehi394. [DOI] [PubMed] [Google Scholar]
- 23.Huffman KM, Samsa GP, Slentz CA, et al. Response of high-sensitivity C-reactive protein to exercise training in an at-risk population. Am.Heart J. 2006;152:793–800. doi: 10.1016/j.ahj.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 24.Haffner S, Temprosa M, Crandall J, et al. Intensive lifestyle intervention or metformin on inflammation and coagulation in participants with impaired glucose tolerance. Diabetes. 2005;54:1566–1572. doi: 10.2337/diabetes.54.5.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stefanick ML, Mackey S, Sheehan M, et al. Effects of diet and exercise in men and postmenopausal women with low levels of HDL cholesterol and high levels of LDL cholesterol. N.Engl.J.Med. 1998;339:12–20. doi: 10.1056/NEJM199807023390103. [DOI] [PubMed] [Google Scholar]
- 26.Allain CC, Poon LS, Chan CS, et al. Enzymatic determination of total serum cholesterol. Clin.Chem. 1974;20:470–475. [PubMed] [Google Scholar]
- 27.Sampson EJ, Demers LM, Krieg AF. Faster enzymatic procedure for serum triglycerides. Clin.Chem. 1975;21:1983–1985. [PubMed] [Google Scholar]
- 28.Warnick GR, Benderson J, Albers JJ. Dextran sulfate-Mg2+ precipitation procedure for quantitation of high-density-lipoprotein cholesterol. Clin.Chem. 1982;28:1379–1388. [PubMed] [Google Scholar]
- 29.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin.Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 30.Government Printing Office; Washington DC: AnonymousManual of laboratory operations: lipid and lipoprotein anlaysis. 1982
- 31.Fortmann SP, Haskell WL, Wood PD. Effects of weight loss on clinic and ambulatory blood pressure in normotensive men. Am.J.Cardiol. 1988;62:89–93. doi: 10.1016/0002-9149(88)91370-7. [DOI] [PubMed] [Google Scholar]
- 32.Expert Panel on the Identification, Evaluation and Treatment of Overweight in Adults Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: executive summary. Expert Panel on the Identification, Evaluation, and Treatment of Overweight in Adults. Am.J.Clin.Nutr. 1998;68(4):899–917. doi: 10.1093/ajcn/68.4.899. (GENERIC) Ref Type: Report. [DOI] [PubMed] [Google Scholar]
- 33.Jackson AS, Pollock ML. Generalized equations for predicting body density of men. Br.J.Nutr. 1978;40:497–504. doi: 10.1079/bjn19780152. [DOI] [PubMed] [Google Scholar]
- 34.Jackson AS, Pollock ML, Ward A. Generalized equations for predicting body density of women. Med.Sci.Sports Exerc. 1980;12:175–181. [PubMed] [Google Scholar]
- 35.Siri WE. Body composition from fluid spaces and density - analysis of methods. In: Brozek J, Henschel A, editors. Techniques for measuring body composition. National Academy of Sciences; Washington, DC: 1961. pp. 223–244. [Google Scholar]
- 36.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 37.Efron B. Forcing a sequential experiment to be balanced. Biometrika. 1971;58:403–417. [Google Scholar]
- 38.Expert Panel on Detection, Evaluation and Treatment of High Blood Pressure Summary of the second report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel II) JAMA. 1993 Jun 16;269(23):3015–3023. (GENERIC) Ref Type: Report. [PubMed] [Google Scholar]
- 39.Myers GL, Rifai N, Tracy RP, et al. CDC/AHA Workshop on Markers of Inflammation and Cardiovascular Disease: Application to Clinical and Public Health Practice: report from the laboratory science discussion group. Circulation. 2004;110:e545–e549. doi: 10.1161/01.CIR.0000148980.87579.5E. [DOI] [PubMed] [Google Scholar]
- 40.Middleton E., Jr Effect of plant flavonoids on immune and inflammatory cell function. Adv.Exp.Med.Biol. 1998;439:175–182. doi: 10.1007/978-1-4615-5335-9_13. [DOI] [PubMed] [Google Scholar]
- 41.McCarty MF. Low-insulin-response diets may decrease plasma C-reactive protein by influencing adipocyte function. Med.Hypotheses. 2005;64:385–387. doi: 10.1016/j.mehy.2004.03.039. [DOI] [PubMed] [Google Scholar]
- 42.De CR, Liao JK, Libby P. Fatty acid modulation of endothelial activation. Am.J.Clin.Nutr. 2000;71:213S–223S. doi: 10.1093/ajcn/71.1.213S. [DOI] [PubMed] [Google Scholar]