Abstract
Objective:
Both phosphorylated (p) mTOR and p70S6K are known to regulate protein synthesis and are affected during intrauterine growth restriction (IUGR). We studied the mTOR pathway during hyperthermia (HT) induced IUGR in the sheep.
Study Design:
Beginning at 40 dGA, four ewes were exposed to HT for 55 days and four were exposed for 80 to induce IUGR. Western blot analyses were performed for mTOR, p70S6K, 4EBP1, ERK, and AKT.
Results:
HT animals had: smaller fetuses and placentas near-term, reduced placental weight at mid-gestation, increased p-mTOR, p-ERK and p-AKT and decreased p70S6K in the near-term cotyledons, and decreased p- p70S6K and increased p-ERK in the caruncles (maternal) near-term.
Conclusion:
Near-term IUGR ovine cotyledons showed upregulation of p-mTOR, while p70S6K was decreased. This suggest that the changes in placental mTOR signaling proteins could be driven by the fetal stress observed near-term in this model of IUGR.
Introduction
The mammalian target of rapamycin (mTOR) protein is a phosphatidylinositol kinase-regulated protein kinase that regulates cell growth in response to nutrients and growth factors. 1-3 Downstream effectors in the mTOR pathway include the 70-kDa ribosomal protein S6 kinase 1 (p70S6K) and the eukaryotic translation initiation factor 4E-binding protein 1 (4EBP1). 4;5 Activation-phosphorylation of these proteins by mTOR is known to regulate translation initiation and protein synthesis. 3;6 Regulation of mTOR and p70S6K activity is mediated by PI-3 Kinase/AKT signaling. 7-9 Under physiological conditions, activation of p70S6K is also mediated by the activation of the ERK pathway. 10-12 In the human placenta, mTOR has been localized to the syncytiotrophoblast cells suggesting a role for this protein in nutrient sensing during pregnancy. 4 This is further supported by studies showing a migratory and proliferative role for mTOR in trophoblast function in the placenta. Furthermore, experiments in HTR8/SVneo trophoblast cells have shown that a decrease in mTOR protein resulted in a decrease of trophoblast cells migration when stimulated by epidermal growth factor (EGF). 12 Experiments in the same cells also showed a correlation between mTOR and the induction of proliferation of trophoblast cells by angiopoietin-2. 9 Other studies have shown that placental total mTOR protein is increased in human intrauterine growth restriction (IUGR) while there was a down-regulation in the placental phospho (p)-p70S6K protein during this disease. This suggests a different mechanism for regulation of these proteins in the IUGR condition, which have yet not been further defined. 4
IUGR is an obstetric complication known to increase the risk for fetal and infant morbidity and mortality. 13-17 Abnormal placentation with placental insufficiency is the most common cause for the development of this disease. 18;19 Our laboratory utilizes an ovine model of IUGR with numerous similarities to the human IUGR condition including asymmetric growth, umbilical artery Doppler abnormalities, abnormal trophoblast apoptosis and limited placental nutrient transport. 20-22 We chose to study this model to further define the molecular mechanisms related to mTOR and its effector proteins not previously defined in IUGR. In addition, using this model enabled us to define these mechanisms earlier in pregnancy in a controlled fashion that is not achievable in humans.
The primary hypothesis of this study was that mTOR and its downstream effector proteins would be decreased in the placentae of ovine IUGR pregnancies, and that these findings would be true both at term and midgestation. Our specific objectives were to asses the following at mid-gestation (95 days of gestational age; dGA) and near-term (130 dGA) in the fetal (cotyledon) and maternal (caruncle) components of the placenta: 1) phospho (p)-mTOR protein concentration, 2) activation of p70S6K and 4EBP1, and 3) activation of ERK and AKT.
Materials and Methods
Animal care
A total of 16 mixed-breed Columbia-Rambouillet ewes with time-dated singleton pregnancies were used for this study, which was approved by the University of Colorado at Denver and Health Sciences Center Animal Care and Use Committee. To induce placental insufficiency and intrauterine growth restriction (PI-IUGR), ewes were exposed to environmental conditions as previously described. 23;24 Briefly, the environmental conditions consisted of: (1) temperature maintained at 40°C for 12 hours during the day and decreased to 35°C at night; (2) humidity kept between 35% and 40%. Eight ewes were placed in the environmental chamber beginning at 40 days of gestation (dGA; term=147 days) and then separated into two groups based on gestational age at necropsy. In the first group, four ewes were housed in the environmental chamber for 55 days and four ewes were housed at ambient temperature (20 ± 2°C) to serve as controls. IUGR fetuses were diagnosed as having IUGR if their placental weights were reduced compared to controls and if the umbilical artery Doppler velocimetry studies showed elevated systolic/diastolic ratios. These animals underwent necropsy at 95dGA (mid-gestation). In the second group, four ewes were exposed to HT conditions for 80 days and were removed to control conditions at approximately 120 days gestation (dGA) together with four ewes kept at ambient temperature as controls. The latter animal group was euthanized at 130 dGA (near-term). All ewes were pair-fed and offered water ad libitum. No fetal loss was found in these studies. At the time of necropsy, fetal and placentome weights were recorded. The placentomes were divided into cotyledon (fetal) and caruncle (maternal) components, and frozen in liquid nitrogen for western blot analysis. Whole placentomes were sectioned, fixed in 4% paraformaldehyde and sent for paraffin embedding for histochemical studies.
Western blot analysis
Cotyledon and caruncle tissues were homogenized in protein lysis buffer containing: 10mM of PMSF, 10mM of Na3VO4, 1× triton X-100, 150mM NaCl, 20mM Tris Base, 5μM of AEBSF, 5μM of EDTA, 10nM of E-64, 10nm of Leupeptin and 10ng/ml of Aprotinin. Tissue lysates containing 50μg of total protein were separated on 2-14% Bis-TRIS gel and transferred to a nitrocellulose membrane. Membranes were incubated in rabbit antibodies against phospho-AKT (Thr308), total AKT, phospho-p44/42 MAPK (Thr202/Tyr204), total p44/42 MAPK, phospho-mTOR (Ser2448), total mTOR, phospho-p70 S6 Kinase (Thr389), total p70 S6K, phospho-4E-BP1 (Thr37/46) or total 4E-BP1 (all 1:500 in TBST with 5% BSA; Cell Signaling Technology, Dancers, MA). A secondary anti-rabbit Ig-HRP antibody (dilution 1:5,000) (Cell signalling, Dancers, MA) was incubated for 1 hour at room temperature. After rinsing, the membranes were incubated with ECL substrate (Amersham, Priceton, NJ) for 5 min. The emission of light was detected using x-ray film. Each membrane was stripped of antibodies and reprobed utilizing antibody against mouse beta-actin (dilution 1:4,000; MP Biomedicals, Aurora, OH) to confirm loading consistencies in each lane. Presence of these proteins was quantified by densitometry.
Immunohistochemistry
Immunohistochemistry (IHC) was performed on paraffin-embedded whole placentome sections. Slides were de-waxed with 100% xylene. Slide preparation was done using the Mach 2 double staining reagents from Biocare Medical (Concord, CA). Protocol was followed as suggested by manufacturer. Briefly, slides were incubated with Peroxidase for 10 min followed by PBS rinses. Diva was used for antigen retrieval for 20 min. Slides were blocked with Sniper for 15 min followed by rinses with TBS. Slides were incubated for one hour with a mouse monoclonal primary antibody against Pan-Cytokeratin (dilution of 1:500; Sigma, Saint Louis, MO) for trophoblast localization, rabbit anti-mTOR (dilution of 1:500; Cell signalling, Dancers, MA) antibody or with an universal IgG negative control (dilution of 1:500; Biocare Medical; Concord, CA). Sections were washed in 1X TBS. Slides were incubated with Mach 2 double stain polymer for 30 min. Color development was done by incubating the sides for 5 min with diaminobenzidine (DAB; brown color) substrate for the rabbit antibody (mTOR) for 5 min and Vulcan fast red (red color) for the mouse antibody (cytokeratin) for 12 min. Hematoxylin was used for nuclear counterstaining. Slides were mounted using Permount mounting media.
Statistical analysis
Comparisons of the following end-points were made between control and IUGR pregnancies: fetal and placental weights, phosphorylated p-ERK p-AKT, p-mTOR, p-p70S6K and p-4EBP1. All data were assessed for normality and treatment effects were determined using Mann-Whitney test with p<0.05 considered significant.
Results
There was no difference in fetal weights between PI-IUGR and control pregnancies at 95dGA (682±205g vs. 715±11g; p≤0.79). However, PI-IUGR pregnancies demonstrated significant reductions in fetal weight compared to controls near-term (1.8-fold; 1718±433g vs. 2914±201g; p≤0.008). At 95 dGA, PI-IUGR pregnancies exhibited a statistically significant decrease (2.4-fold; 186±18g vs. 440±50g; p≤0.003) in placental weight. The difference in placental weights persisted near-term (2.0-fold; 169±43g vs. 349±21g; p≤0.004).
Cotyledon tissues (fetal side of placenta)
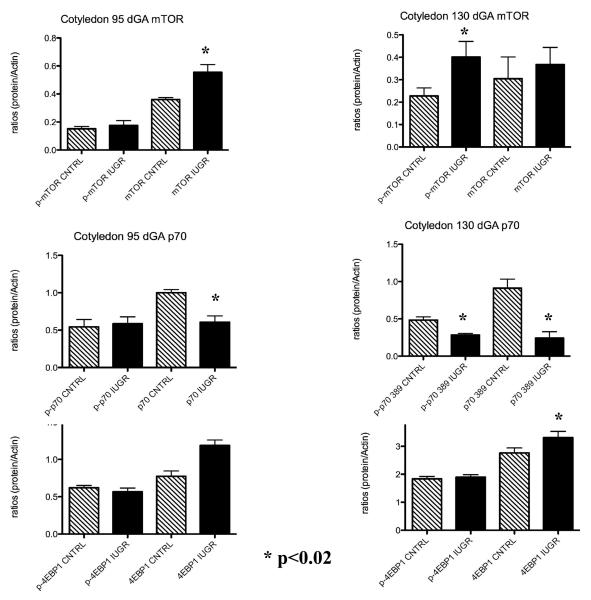
A characteristic western for near-term cotyledon mTOR, p70S6K, and 4EBP1 is shown in Figure 1. No other western blot pictures will be shown in interest of space. A significant increase in cotyledon p-mTOR was observed at 130 dGA (1.8-fold). In contrast, cotyledon total mTOR was increase at 95 dGA while p-mTOR was unchanged in treated animals as compared to controls (Figure 2). p-p70S6K and p70S6K were decreased (p<0.02) in the cotyledon of treated animals near-term while p70S6K only was decreased in this tissue at 95 dGA (Figure 2). There was no significant difference observed for 4EBP1 at 95 dGA during PI-IUGR in the sheep while a significant increase in the 4EBP1 (1.5-fold; p< 0.02) protein was seen in the cotyledon near-term (Figure 2).
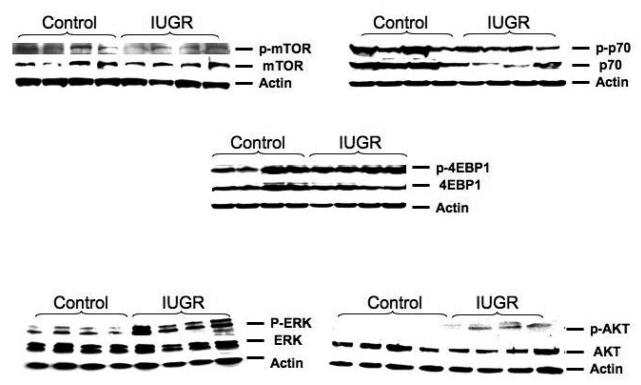
Figure 1.
Characteristic cotyledon western blot pictures.. A characteristic western blot for near-term cotyledon mTOR, p70 and 4EBP1, ERK and AKT proteins are shown in this figure.
Figure 2.
Increase cotyledon mTOr and decrease in p70S6K during IUGR. A significant increase of cotyledon mTOR and a significant decrease in p- p70S6K was observed in treated animals near-term.
Caruncle tissues (maternal side of the placenta)
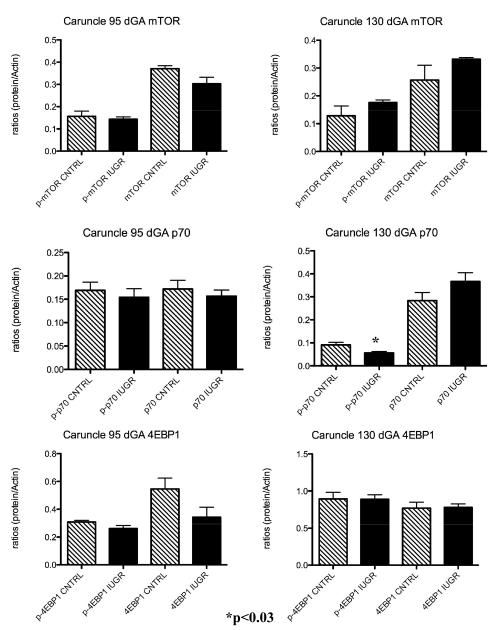
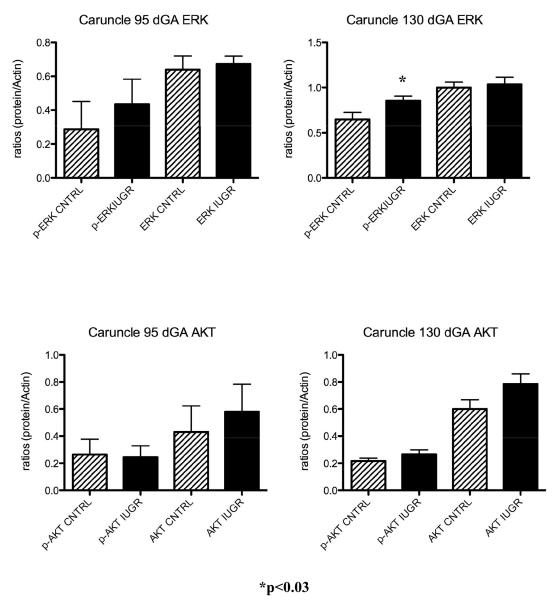
p-mTOR protein was not impacted in the PI-IUGR caruncle at either 95 or 130 dGA (Figure 3). Similarly, treatment did not result in differences at either gestational period for the mTOR downstream effector p-4EBP1 protein. However, there was a significant decrease in p- p70S6K in the caruncle tissues near-term between treated animals and controls (Figure 3).
Figure 3.
Caruncle mTOR pathway proteins . A significant decrease in p-p70 was observed in the caruncle of treated animals vs. controls near term. No differences were present for these proteins at mid-gestation.
ERK and AKT
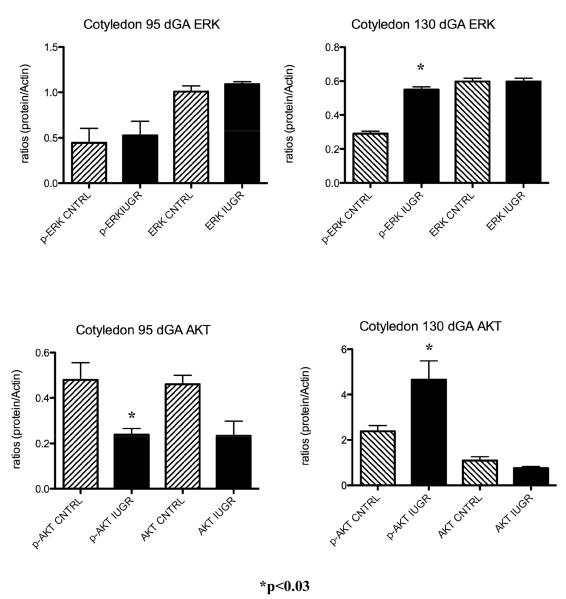
A characteristic western for near-term cotyledon ERK and AKT is shown in Figure 1. When assessing the ERK and AKT proteins in the sheep placenta during IUGR, there was a significant increase for both p-ERK (1.4-fold; p<0.02) and p-AKT (2.6-fold; p<0.03) associated with HT treatment in the cotyledon of the sheep placenta near-term (Figure 4). In the caruncular tissues, only p-ERK was increase at this gestational point (Figure 5). A decrease in p-AKT was found in the cotyledon at mid-gestation (Figure 4) while no changes were observed for either ERK and AKT proteins in the caruncle of treated animal as compared to controls (Figure 5). These findings are shown in Figure 3.
Figure 4.
Cotyledon ERK and AKT. ERK and AKT are increased in the cotyledon of IUGR animal near-term.
Figure 5.
Caruncle ERK and AKT. ERK is increased in the caruncle of IUGR animal near-term.
Immunohistochemistry
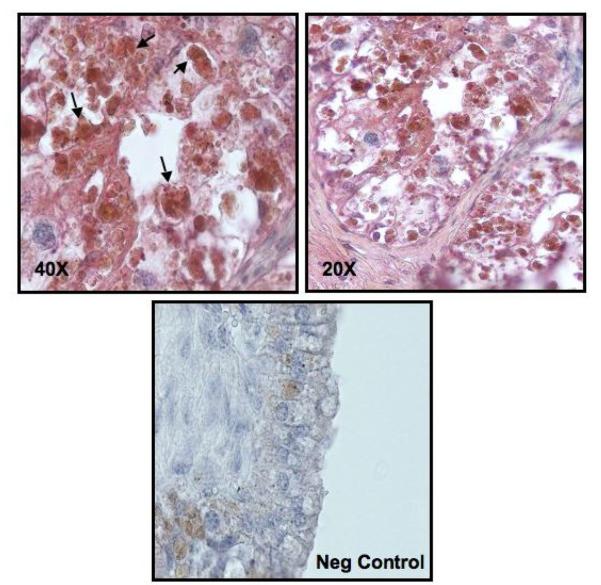
Tissues samples were confirmed to contain trophoblast cells with cytokeratin positive staining. mTOR protein colocalized to the cytokeratin positive trophoblast cells (black arrows) in the villi of the sheep placentome (Figure 6).
Figure 6.
Localization of mTOR protein in the ovine placentome. mTOR (brown) co-localize to the trophoblast cells (red) in the cotyledon of the human placentome. Black Arrows show co-localization of mTOR to trophoblast cells in the villi of the ovine placentome.
Discussion
In the present study, mTOR and its downstream effectors were not phosphorylated-activated under conditions of PI-IUGR at mid-gestation. At near-term, however, we found that the phosphorylation of AKT, mTOR and ERK proteins were significantly increased while total and phosphorylated p70S6K were decreased in the cotyledon of PI-IUGR animals. Previous human studies have shown an increase in total mTOR protein during IUGR in the human placenta at term. In the same study they found a downregulation of the phosphorylated downstream effector protein p70S6K while 4EBP1 was unchanged. 4 Our results parallel the human IUGR studies suggesting that a similar mechanism exist for regulating the mTOR pathway proteins in the ovine placenta in this model of IUGR. Previous studies have shown that p70S6K activation is mediated by the activation of AKT and ERK in fibroblast cells, our results showed that although ERK and AKT were upregulated, activated p70S6K was decrease suggesting a different mechanism of regulation for this protein in this IUGR model. 7-12
mTOR activation is usually associated with the activation of the effector proteins p70S6K and for 4EBP1, this PI-IUGR ovine model showed an increase in phosphorylated mTOR with a decrease in total and phosphorylated p70S6K and no changes in 4EBP1 near-term. While this was an unexpected finding, previous reports have shown changes in mTOR and p70S6K, but not 4EBP1 in other cells, suggesting a different mechanism of regulation for the 4EBP1 protein in the mTOR pathway. 2;25 Furthermore, in vitro studies have demonstrated that the regulation of 4EBP1 occurs via five phosphorylation sites that have been identified (Thr 37, Thr 46, Thr 70, Ser 64 and Ser 82). 26 We only evaluated the Thr 37/46 phosphorylation site of the protein, but it may be that other sites are affected differently under conditions of PI-IUGR.
Interestingly, we showed that it is in the period of maximal placental size in the final 1/3 of pregnancy and near term where mTOR pathway changes occur. This also coincides with the period of maximal slope of growth of the fetus. More specifically, we found that the differences in the regulation of the cotyledon mTOR pathway between mid and near-term gestation during PI-IUGR could be secondary to protein production required during the peak of fetal growth observed in the latter one third of pregnancy (130 dGA) in sheep pregnancy. 20;21;27;28 These results suggest that the increase in mTOR may be a compensatory mechanism attempting to increase protein synthesis and thus fetal growth, near-term in this model of PI-IUGR. There could be a different factor or mechanism that decreases p70S6K which could be affecting placental protein synthesis at this point. This is speculative, as mTOR signaling has not been evaluated at mid-gestation in human IUGR.
When examining the mTOR signaling pathway in the maternal section (caruncle) under conditions of PI-IUGR, we only found a significant effect in total p70S6K expression near-term. This is an interesting result, which will require further evaluation. In contrast, mTOR was not changed in this tissue at any other gestational points studied. This is consistent with the IHC experiments which showed that in this model of hyperthermia-induced placental insufficiency and IUGR, mTOR is primarily localized to the cotyledon tissues, suggesting that hyperthermia effects on mTOR pathway endpoints occur primarily in the cotyledon or on the fetal side of the placenta. Although little staining occurred in the carcuncular tissues, it is likely that there are still other mechanisms involved in the mTOR regulation in the maternal oriented, caruncular tissues.
IUGR is a common clinical problem with several abnormal placental characteristics, which also includes an alteration in the expression and activity of placental nutrient transporters that are important for fetal development. 29-33 Amino acids are known to regulate signal pathways including the activation of the mTOR pathway. 6 Activation of this pathway regulates cell growth in response to nutrient stimuli. 34 More specifically, this signaling pathway regulates protein synthesis and modulates insulin signals both of which are important mechanisms that control cell growth. 6;35;36 mTOR has been shown to regulate trophoblast proliferation and differentiation suggesting an important role for mTOR in trophoblast function within the placenta. 9;37 Roos et al. (2007) has further shown mTOR to be expressed in the syncytiotrophoblast which are the cells responsible for exchange of nutrients between maternal and fetal blood. 4 This suggests that mTOR is actively involved in the uptake of amino acids for fetal development during pregnancy. In this study, phospho-mTOR was increased near-term in the cotyledon of treated at animals during IUGR, which was associated with an increase phospho-AKT and phospho-ERK. A reduction in phospho and total p70S6K confirms that although mTOR was activated, this was not enough to increase p70S6K for the purpose of increasing protein synthesis in IUGR. Trophoblast cell activation of these proteins in the mTOR pathway regulates protein translation in a synergistic fashion. 38 Our model is characterized by a smaller placenta with placental insufficiency and a decrease in amino acid uptake. 39-41 Although placental mTOR expression is increased, this is not sufficient to maintain normal fetal weight in the latter third of gestation in our ovine model of PI-IUGR and may simply represent a compensatory effort by the placenta to increase protein synthesis for fetal benefit. 39 Perhaps this can be explained in part by an increase apoptosis in the cotyledon of HT IUGR animals near-term resulting in decreased amino acid transport because of reduced trophoblast volume even though mTOR protein is increase. 42 These findings provide further insight into the molecular mechanisms of placental dysfunction at mid-gestation and near-term in our ovine model of IUGR.
Acknowledgments
This study was supported by the NIH grant HL071990.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Blume-Jensen P, Hunter T. Oncogenic kinase signalling. Nature. 2001;411:355–65. doi: 10.1038/35077225. [DOI] [PubMed] [Google Scholar]
- 2.Jansson N, Pettersson J, Haafiz A, Ericsson A, Palmberg I, Tranberg M, et al. Down-regulation of placental transport of amino acids precedes the development of intrauterine growth restriction in rats fed a low protein diet. J.Physiol. 2006;576:935–46. doi: 10.1113/jphysiol.2006.116509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–84. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 4.Roos S, Jansson N, Palmberg I, Saljo K, Powell TL, Jansson T. Mammalian target of rapamycin in the human placenta regulates leucine transport and is down-regulated in restricted fetal growth. J.Physiol. 2007;582:449–59. doi: 10.1113/jphysiol.2007.129676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Volarevic S, Thomas G. Role of S6 phosphorylation and S6 kinase in cell growth. Prog.Nucleic Acid Res.Mol.Biol. 2001;65:101–27. doi: 10.1016/s0079-6603(00)65003-1. [DOI] [PubMed] [Google Scholar]
- 6.Patti ME, Brambilla E, Luzi L, Landaker EJ, Kahn CR. Bidirectional modulation of insulin action by amino acids. J.Clin.Invest. 1998;101:1519–29. doi: 10.1172/JCI1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chung T, Crilly KS, Anderson WH, Mukherjee JJ, Kiss Z. ATP-dependent choline phosphate-induced mitogenesis in fibroblasts involves activation of pp70 S6 kinase and phosphatidylinositol 3′-kinase through an extracellular site. Synergistic mitogenic effects of choline phosphate and sphingosine 1-phosphate. J.Biol.Chem. 1997;272:3064–72. doi: 10.1074/jbc.272.5.3064. [DOI] [PubMed] [Google Scholar]
- 8.Ming XF, Burgering BM, Wennstrom S, Claesson-Welsh L, Heldin CH, Bos JL, et al. Activation of p70/p85 S6 kinase by a pathway independent of p21ras. Nature. 1994;371:426–29. doi: 10.1038/371426a0. [DOI] [PubMed] [Google Scholar]
- 9.Wen HY, Abbasi S, Kellems RE, Xia Y. mTOR: a placental growth signaling sensor. Placenta. 2005;26(Suppl A):S63–S69. doi: 10.1016/j.placenta.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 10.Eguchi S, Iwasaki H, Ueno H, Frank GD, Motley ED, Eguchi K, et al. Intracellular signaling of angiotensin II-induced p70 S6 kinase phosphorylation at Ser(411) in vascular smooth muscle cells. Possible requirement of epidermal growth factor receptor, Ras, extracellular signal-regulated kinase, and Akt. J.Biol.Chem. 1999;274:36843–51. doi: 10.1074/jbc.274.52.36843. [DOI] [PubMed] [Google Scholar]
- 11.Iijima Y, Laser M, Shiraishi H, Willey CD, Sundaravadivel B, Xu L, et al. c-Raf/MEK/ERK pathway controls protein kinase C-mediated p70S6K activation in adult cardiac muscle cells. J.Biol.Chem. 2002;277:23065–75. doi: 10.1074/jbc.M200328200. [DOI] [PubMed] [Google Scholar]
- 12.Qiu Q, Yang M, Tsang BK, Gruslin A. Both mitogen-activated protein kinase and phosphatidylinositol 3-kinase signalling are required in epidermal growth factor-induced human trophoblast migration. Mol.Hum.Reprod. 2004;10:677–84. doi: 10.1093/molehr/gah088. [DOI] [PubMed] [Google Scholar]
- 13.Barker DJ. The intrauterine origins of cardiovascular disease. Acta Paediatr Suppl. 1993;82(Suppl 391):93–99. doi: 10.1111/j.1651-2227.1993.tb12938.x. [DOI] [PubMed] [Google Scholar]
- 14.Barker DJ, Gluckman PD, Godfrey KM, Harding JE, Owens JA, Robinson JS. Fetal nutrition and cardiovascular disease in adult life. Lancet. 1993;341:938–41. doi: 10.1016/0140-6736(93)91224-a. [DOI] [PubMed] [Google Scholar]
- 15.Barker DJ. The developmental origins of adult disease. J.Am.Coll.Nutr. 2004;23:588S–95S. doi: 10.1080/07315724.2004.10719428. [DOI] [PubMed] [Google Scholar]
- 16.Bernstein IM, Horbar JD, Badger GJ, Ohlsson A, Golan A. Morbidity and mortality among very-low-birth-weight neonates with intrauterine growth restriction. The Vermont Oxford Network. Am.J.Obstet.Gynecol. 2000;182:198–206. doi: 10.1016/s0002-9378(00)70513-8. [DOI] [PubMed] [Google Scholar]
- 17.Seeds JW, Peng T. Impaired growth and risk of fetal death: is the tenth percentile the appropriate standard? Am.J.Obstet.Gynecol. 1998;178:658–69. doi: 10.1016/s0002-9378(98)70475-2. [DOI] [PubMed] [Google Scholar]
- 18.Kingdom J, Huppertz B, Seaward G, Kaufmann P. Development of the placental villous tree and its consequences for fetal growth. Eur J Obstet Gynecol Reprod Biol. 2000;92:35–43. doi: 10.1016/s0301-2115(00)00423-1. [DOI] [PubMed] [Google Scholar]
- 19.Rutland CS, Mukhopadhyay M, Underwood S, Clyde N, Mayhew TM, Mitchell CA. Induction of Intrauterine Growth Restriction by Reducing Placental Vascular Growth with the Angioinhibin TNP-470. Biol Reprod. 2005 doi: 10.1095/biolreprod.105.043893. [DOI] [PubMed] [Google Scholar]
- 20.Barry JS, Anthony RV. The pregnant sheep as a model for human pregnancy. Theriogenology. 2008;69:55–67. doi: 10.1016/j.theriogenology.2007.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Galan HL, Hussey MJ, Barbera A, Ferrazzi E, Chung M, Hobbins JC, et al. Relationship of fetal growth to duration of heat stress in an ovine model of placental insufficiency. Am J Obstet Gynecol. 1999;180:1278–82. doi: 10.1016/s0002-9378(99)70629-0. [DOI] [PubMed] [Google Scholar]
- 22.Galan HL, Regnault TR, Le Cras TD, Tyson RW, Anthony RV, Wilkening RB, et al. Cotyledon and binucleate cell nitric oxide synthase expression in an ovine model of fetal growth restriction. J Appl Physiol. 2001;90:2420–26. doi: 10.1152/jappl.2001.90.6.2420. [DOI] [PubMed] [Google Scholar]
- 23.Bell AW, Wilkening RB, Meschia G. Some aspects of placental function in chronically heat-stressed ewes. J Dev Physiol. 1987;9:17–29. [PubMed] [Google Scholar]
- 24.Regnault TR, Orbus RJ, Battaglia FC, Wilkening RB, Anthony RV. Altered arterial concentrations of placental hormones during maximal placental growth in a model of placental insufficiency. J Endocrinol. 1999;162:433–42. doi: 10.1677/joe.0.1620433. [DOI] [PubMed] [Google Scholar]
- 25.Dreyer HC, Glynn EL, Lujan HL, Fry CS, Dicarlo SE, Rasmussen BB. Chronic paraplegia-induced muscle atrophy downregulates the mTOR/S6K1 signaling pathway. J.Appl.Physiol. 2007 doi: 10.1152/japplphysiol.00736.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mothe-Satney I, Yang D, Fadden P, Haystead TA, Lawrence JC., Jr. Multiple mechanisms control phosphorylation of PHAS-I in five (S/T)P sites that govern translational repression. Mol.Cell Biol. 2000;20:3558–67. doi: 10.1128/mcb.20.10.3558-3567.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Galan HL, Hussey MJ, Barbera A, Ferrazzi E, Chung M, Hobbins JC, et al. Relationship of fetal growth to duration of heat stress in an ovine model of placental insufficiency. Am J Obstet Gynecol. 1999;180:1278–82. doi: 10.1016/s0002-9378(99)70629-0. [DOI] [PubMed] [Google Scholar]
- 28.Thureen PJ, Trembler KA, Meschia G, Makowski EL, Wilkening RB. Placental glucose transport in heat-induced fetal growth retardation. Am.J.Physiol. 1992;263:R578–R585. doi: 10.1152/ajpregu.1992.263.3.R578. [DOI] [PubMed] [Google Scholar]
- 29.Brar HS, Rutherford SE. Classification of intrauterine growth retardation. Semin.Perinatol. 1988;12:2–10. [PubMed] [Google Scholar]
- 30.Glazier JD, Cetin I, Perugino G, Ronzoni S, Grey AM, Mahendran D, et al. Association between the activity of the system A amino acid transporter in the microvillous plasma membrane of the human placenta and severity of fetal compromise in intrauterine growth restriction. Pediatr.Res. 1997;42:514–19. doi: 10.1203/00006450-199710000-00016. [DOI] [PubMed] [Google Scholar]
- 31.Jansson T, Powell TL. Placental nutrient transfer and fetal growth. Nutrition. 2000;16:500–02. doi: 10.1016/s0899-9007(00)00323-3. [DOI] [PubMed] [Google Scholar]
- 32.Norberg S, Powell TL, Jansson T. Intrauterine growth restriction is associated with a reduced activity of placental taurine transporters. Pediatr.Res. 1998;44:233–38. doi: 10.1203/00006450-199808000-00016. [DOI] [PubMed] [Google Scholar]
- 33.Pollack RN, Divon MY. Intrauterine growth retardation: definition, classification, and etiology. Clin Obstet Gynecol. 1992;35:99–107. doi: 10.1097/00003081-199203000-00015. [DOI] [PubMed] [Google Scholar]
- 34.Kim DH, Sarbassov DD, Ali SM, King JE, Latek RR, Erdjument-Bromage H, et al. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell. 2002;110:163–75. doi: 10.1016/s0092-8674(02)00808-5. [DOI] [PubMed] [Google Scholar]
- 35.Wang X, Proud CG. The mTOR pathway in the control of protein synthesis. Physiology.(Bethesda.) 2006;21:362–69. doi: 10.1152/physiol.00024.2006. [DOI] [PubMed] [Google Scholar]
- 36.Xu G, Kwon G, Marshall CA, Lin TA, Lawrence JC, Jr., McDaniel ML. Branched-chain amino acids are essential in the regulation of PHAS-I and p70 S6 kinase by pancreatic beta-cells. A possible role in protein translation and mitogenic signaling. J.Biol.Chem. 1998;273:28178–84. doi: 10.1074/jbc.273.43.28178. [DOI] [PubMed] [Google Scholar]
- 37.Pollheimer J, Knofler M. Signalling pathways regulating the invasive differentiation of human trophoblasts: a review. Placenta. 2005;26(Suppl A):S21–S30. doi: 10.1016/j.placenta.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 38.Chambard JC, Lefloch R, Pouyssegur J, Lenormand P. ERK implication in cell cycle regulation. Biochim.Biophys.Acta. 2007;1773:1299–310. doi: 10.1016/j.bbamcr.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 39.Arroyo JA, Anthony RV, Parker TA, Galan HL. Differential expression of placental and vascular endothelial nitric oxide synthase in an ovine model of fetal growth restriction. Am.J.Obstet.Gynecol. 2006;195:771–77. doi: 10.1016/j.ajog.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 40.Regnault TR, Friedman JE, Wilkening RB, Anthony RV, Hay WW., Jr. Fetoplacental transport and utilization of amino acids in IUGR--a review. Placenta. 2005;26(Suppl A):S52–S62. doi: 10.1016/j.placenta.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 41.Ziebell BT, Galan HL, Anthony RV, Regnault TR, Parker TA, Arroyo JA. Ontogeny of endothelial nitric oxide synthase mRNA in an ovine model of fetal and placental growth restriction. Am.J.Obstet.Gynecol. 2007;197:420–25. doi: 10.1016/j.ajog.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 42.Arroyo JA, Anthony RV, Galan HL. Decreased placental X-linked inhibitor of apoptosis protein in an ovine model of intrauterine growth restriction. Am.J.Obstet.Gynecol. 2008;199:80–88. doi: 10.1016/j.ajog.2007.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]