Evidence from a variety of sources suggests that structural alterations in the brain, including neurogenesis, may play a role in both the pathogenesis of mood disorders and the mechanism of action of anti-depressants (Duman, 2004; Schmidt and Duman, 2007). Chronic antidepressant treatment has also been shown to increase TGF-b mediated phosphorylation of Smad2, suggesting that this pathway may be important in the mechanism of action of antidepressants (Dow et al., 2005). Other studies have implicated the phosphatidyl inositol-3 kinase (PI3K)- Akt pathway in the neurogenesis-promoting properties of antidepressants (reviewed in (Beech and Duman, 2005)).
FoxG1 (formerly called BF-1) is an important regulator of both of these pathways (Accili and Arden, 2004; Katoh, 2004). FoxG1 inhibits signaling through the Smad/TGF-β pathway (Dou et al., 2000) leading to a down-regulation of the growth-inhibitory protein p21 (WAF1/CIP1), and thus allows proliferation of the neuroepithelium to continue during embryogenesis (Seoane et al., 2004). FoxG1 also inhibits transcriptional activation by another forkheadbox transcription factor, FoxO1, whose activity is regulated by PI3K and its downstream activator Akt (Adesina et al., 2007; Aoki et al., 2004; Brunet et al., 1999).
FoxG1 knockout embryos die shortly before birth (E18.5)(Hanashima et al., 2004). FoxG1 heterozygous null mice (FoxG1+/−) were previously thought to have a normal developmental phenotype (Hebert and McConnell, 2000; Xuan et al., 1995). However, recent evidence shows that while FoxG1+/− mice survive and are able to breed normally, they do not produce new neurons as adults and have behavioral abnormalities including deficits in contextual fear learning (Shen et al., 2006). The role of FoxG1, if any, in the response to antidepressants is unknown.
To investigate the role of the FoxG1 gene in the response to antidepressants, we tested FoxG1+/− mice and littermate controls in two different rodent models of antidepressant action: the tail suspension test (Steru et al., 1985) and the forced swim test (Porsolt et al., 1977).
All procedures involving animals were performed in strict accordance with the NIH Guide for the Care and Use of Laboratory Animals and were approved by the Yale Animal Care and Use Committee. Founder mice with a FoxG1 heterozygous “knock-in” in which the intron-less FoxG1 coding region was replaced with the tetracycline transactivator (tTA) [FoxG1+/tTA, C57BL/6 background (Hanashima et al., 2002)] were the generous gift of Dr. Stewart Anderson, Weill Medical College of Cornell University.
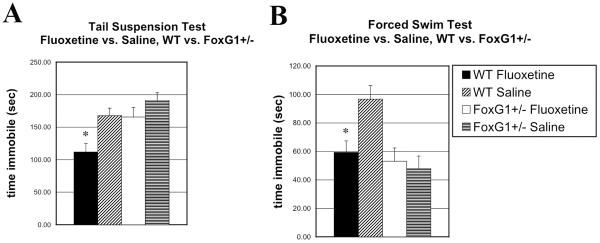
In the tail suspension test, mice received fluoxetine (10mg/kg IP) 30 minutes prior to testing. Mice were then taped by their tails (approximately 1 cm from the end) to a 36 cm piece of plastic tubing approximately 120 cm above the floor and videotaped for 6 minutes. Immobility was scored by an observer blinded to genotype and drug treatment group. As shown in figure 1A, wild-type mice treated with fluoxetine showed a significant decrease in immobility compared to saline treated controls, while FoxG1+/− mice showed no effect of antidepressant treatment. Analysis of variance showed significant main effects of both genotype [F(3,77) 10.01, p=0.002] and drug [F(3,77) 8.96, p=0.004], with post hoc tests confirming that wild type (WT) mice treated with fluoxetine spent less time immobile than WT mice that received saline injections, while FoxG1+/− mice treated with fluoxetine did not differ significantly from FoxG1+/− mice injected with saline.
figure 1.
A. Fox G1+/− mice do not respond to fluoxetine in the tail suspension test.
Wild type mice treated with fluoxetine (n=21) showed a significant (p<0.05) reduction in time immobile compared with wild-type mice treated with saline (n=21), while FoxG1+/− mice treated with fluoxetine (n=21) showed no significant change compared to FoxG1+/− mice injected with saline (n=18). Data are shown as mean (±SEM) time immobile. (*) indicates significant difference from other 3 groups (WT =wild-type).
B. Fox G1+/− mice do not respond to fluoxetine in the forced swim test.
Wild type mice treated with fluoxetine (n=20) showed a significant (p=.005) reduction in time immobile compared with wild-type mice treated with saline (n=24),. FoxG1+/− mice treated with fluoxetine (n=19) did not show a decrease in time immobile, compared to FoxG1+/− mice injected with saline (n=20).Data are shown as mean (±SEM) time immobile for first 5 minutes of test. (*) indicates significant difference from WT saline treated animals.
In the forced swim test, mice received fluoxetine (20mg/kg IP) 30 minutes prior to testing. Mice were then placed into a 4L cylinder of water at room temperature (20–22° C.), and videotaped for 15 min. Immobility, defined as absence of all movement except that required to keep the animal's head above water, was scored by two independent observers who were blinded to genotype and drug treatment group. As shown in figure 1B, wild-type mice treated with fluoxetine showed a significant reduction in immobility during the first 5 minutes of the test, while FoxG1+/− mice showed no effect of antidepressant treatment. There were no differences among groups during the last 10 minutes of the test. Analysis of variance showed significant main effects of genotype [F(3,79) 9.21, p=0.003] and a significant drug X genotype interaction [F(3,79) 5.53, p=0.021], with post hoc tests confirming that WT mice treated with fluoxetine spent less time immobile than WT mice that received saline injections, while the time spent immobile by FoxG1+/− mice treated with fluoxetine did not differ from that of FoxG1+/− mice injected with saline.
In the current study we observed no significant behavioral response in FoxG1+/− mice in two different rodent models of antidepressant action: the tail suspension test and the forced swim test. These results suggest at least two (not mutually exclusive) interpretations: (1) FoxG1 signaling may be a direct mediator of antidepressant-induced behavioral changes, or (2) immature neurons or neuronal precursors (both of which are deficient in FoxG1 heterozygous animals (Shen et al., 2006)) may have unique signaling properties that are required for behavioral responses to antidepressants. These data are consistent with previous studies indicating a role for signaling through the TGF-b/Smad pathway in both regulation of adult neurogenesis (Battista et al., 2006; Buckwalter et al., 2006; Wachs et al., 2006) and the behavioral effects of antidepressant treatments (Dow et al., 2005).
FoxG1 is a negative regulator of the FoxO/TGFbeta/Smad complex (Arden, 2004; Seoane et al., 2004). If inhibition of this complex is indeed a key step in the neurogenic and behavioral actions of antidepressants and mood stabilizers, then decreased expression of FoxG1 should be associated with decreased response to these agents. Our finding that FoxG1+/− mice do not respond behaviorally to antidepressants is consistent with that hypothesis. Similarly, we found that chronic treatment with antidepressants was not able to overcome the deficit in adult neurogenesis previously described in these mice (Shen et al., 2006), suggesting that normal levels of FoxG1 expression may be required for the pro-neurogenic properties of antidepressants.
Limitations of the current study include the fact that the deficiency in FoxG1 signaling in the FoxG1+/− mice is present throughout embryonic and post-natal development (Shen et al., 2006). Thus, differences in behavior may reflect prior developmental abnormalities (Eagleson et al., 2007), rather than a specific defect in adult neurogenesis. In addition, FoxG1+/− mice are hyperactive (Shen et al., 2006), which may be a confounding factor in the behavioral tests used here. In our experiments we found that FoxG1+/− mice were less active in tail suspension test, but more active in the forced swim test compared to wild type mice in the absence of any antidepressant treatment. The differing direction of these baseline differences makes it difficult to interpret the FoxG1+/− phenotype as either depressed-like or antidepressant-like. However, the fact that there was no change in either test when the FoxG1+/− mice were treated with fluoxetine suggests that defects in FoxG1 signaling can significantly impair antidepressant response. Future experiments using other behavioral paradigms may help to clarify more precisely the contribution of FoxG1 signaling to the behavioral and neurogenic actions of antidepressants in the adult brain.
Acknowledgements
This work was supported by NIH grants R21 MH076053-01A1, K12 DA-00167, and Culpeper Biomedical Pilot Grants (01-240 and 03-266) (RDB).
References
- Accili D, Arden KC. FoxOs at the crossroads of cellular metabolism, differentiation, and transformation. Cell. 2004;117(4):421–426. doi: 10.1016/s0092-8674(04)00452-0. [DOI] [PubMed] [Google Scholar]
- Adesina AM, Nguyen Y, Guanaratne P, Pulliam J, Lopez-Terrada D, Margolin J, Finegold M. FOXG1 is overexpressed in hepatoblastoma. Hum Pathol. 2007 doi: 10.1016/j.humpath.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Aoki M, Jiang H, Vogt PK. Proteasomal degradation of the FoxO1 transcriptional regulator in cells transformed by the P3k and Akt oncoproteins. Proc Natl Acad Sci U S A. 2004;101(37):13613–13617. doi: 10.1073/pnas.0405454101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arden KC. FoxO; Linking New Signaling Pathways. Mol Cell. 2004;14(4):416–418. doi: 10.1016/s1097-2765(04)00213-8. [DOI] [PubMed] [Google Scholar]
- Battista D, Ferrari CC, Gage FH, Pitossi FJ. Neurogenic niche modulation by activated microglia: transforming growth factor beta increases neurogenesis in the adult dentate gyrus. Eur J Neurosci. 2006;23(1):83–93. doi: 10.1111/j.1460-9568.2005.04539.x. [DOI] [PubMed] [Google Scholar]
- Beech RD, Duman RS. The role of transcription factors in the biology of depression. In: Licinio J, Wong M-L, editors. Biology of Depression: Towards a Novel Understanding and Therapeutic Strategies. Wiley-VCH; Weinheim: 2005. pp. 823–854. [Google Scholar]
- Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96(6):857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- Buckwalter MS, Yamane M, Coleman BS, Ormerod BK, Chin JT, Palmer T, Wyss-Coray T. Chronically increased transforming growth factor-beta1 strongly inhibits hippocampal neurogenesis in aged mice. Am J Pathol. 2006;169(1):154–164. doi: 10.2353/ajpath.2006.051272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou C, Lee J, Liu B, Liu F, Massague J, Xuan S, Lai E. BF-1 interferes with transforming growth factor beta signaling by associating with Smad partners. Molecular & Cellular Biology. 2000;20(17):6201–6211. doi: 10.1128/mcb.20.17.6201-6211.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dow AL, Russell DS, Duman RS. Regulation of activin mRNA and Smad2 phosphorylation by antidepressant treatment in the rat brain: effects in behavioral models. J Neurosci. 2005;25(20):4908–4916. doi: 10.1523/JNEUROSCI.5155-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS. Role of neurotrophic factors in the etiology and treatment of mood disorders. Neuromolecular Med. 2004;5(1):11–26. doi: 10.1385/NMM:5:1:011. [DOI] [PubMed] [Google Scholar]
- Eagleson KL, Schlueter McFadyen-Ketchum LJ, Ahrens ET, Mills PH, Does MD, Nickols J, Levitt P. Disruption of Foxg1 expression by knock-in of Cre recombinase: Effects on the development of the mouse telencephalon. Neuroscience. 2007;148(2):385–399. doi: 10.1016/j.neuroscience.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanashima C, Li SC, Shen L, Lai E, Fishell G. Foxg1 Suppresses Early Cortical Cell Fate. Science. 2004;303(5654):56–59. doi: 10.1126/science.1090674. [DOI] [PubMed] [Google Scholar]
- Hanashima C, Shen L, Li SC, Lai E. Brain factor-1 controls the proliferation and differentiation of neocortical progenitor cells through independent mechanisms. J Neurosci. 2002;22(15):6526–6536. doi: 10.1523/JNEUROSCI.22-15-06526.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert JM, McConnell SK. Targeting of cre to the Foxg1 (BF-1) locus mediates loxP recombination in the telencephalon and other developing head structures. Developmental Biology. 2000;222(2):296–306. doi: 10.1006/dbio.2000.9732. [DOI] [PubMed] [Google Scholar]
- Katoh M. Human FOX gene family (Review) Int J Oncol. 2004;25(5):1495–1500. [PubMed] [Google Scholar]
- Porsolt RD, Bertin A, Jalfre M. Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther. 1977;229(2):327–336. [PubMed] [Google Scholar]
- Schmidt HD, Duman RS. The role of neurotrophic factors in adult hippocampal neurogenesis, antidepressant treatments and animal models of depressive-like behavior. Behav Pharmacol. 2007;18(5-6):391–418. doi: 10.1097/FBP.0b013e3282ee2aa8. [DOI] [PubMed] [Google Scholar]
- Seoane J, Le HV, Shen L, Anderson SA, Massague J. Integration of Smad and forkhead pathways in the control of neuroepithelial and glioblastoma cell proliferation. Cell. 2004;117(2):211–223. doi: 10.1016/s0092-8674(04)00298-3. [DOI] [PubMed] [Google Scholar]
- Shen L, Nam HS, Song P, Moore H, Anderson SA. FoxG1 haploinsufficiency results in impaired neurogenesis in the postnatal hippocampus and contextual memory deficits. Hippocampus. 2006;16(10):875–890. doi: 10.1002/hipo.20218. [DOI] [PubMed] [Google Scholar]
- Steru L, Chermat R, Thierry B, Simon P. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology (Berl) 1985;85(3):367–370. doi: 10.1007/BF00428203. [DOI] [PubMed] [Google Scholar]
- Wachs FP, Winner B, Couillard-Despres S, Schiller T, Aigner R, Winkler J, Bogdahn U, Aigner L. Transforming growth factor-beta1 is a negative modulator of adult neurogenesis. J Neuropathol Exp Neurol. 2006;65(4):358–370. doi: 10.1097/01.jnen.0000218444.53405.f0. [DOI] [PubMed] [Google Scholar]
- Xuan S, Baptista CA, Balas G, Tao W, Soares VC, Lai E. Winged helix transcription factor BF- 1 is essential for the development of the cerebral hemispheres. Neuron. 1995;14(6):1141–1152. doi: 10.1016/0896-6273(95)90262-7. [DOI] [PubMed] [Google Scholar]