Abstract
Background
The purpose of the present investigation was to more clearly define blood-alcohol parameters associated with alcohol dependence produced by alcohol vapor inhalation and alcohol-containing liquid diet.
Methods
Alcohol levels in blood and brain were compared during and after four hours of acute alcohol vapor exposure; also, brain-alcohol levels were assessed in alcohol-exposed (14d alcohol vapor) and alcohol-naïve rats during and after four hours of acute alcohol vapor exposure. A separate group of rats were implanted with i.v. catheters, made dependent on alcohol via vapor inhalation, and tested for operant alcohol responding; blood-alcohol levels (BALs) were measured throughout operant alcohol drinking sessions during alcohol withdrawal. A final group of rats consumed alcohol-liquid diet until they were dependent, and those rats were then tested for operant behavior at various withdrawal time points; BALs were measured at different withdrawal time points and after operant sessions.
Results
Blood-and brain-alcohol levels responded similarly to vapor, but brain-alcohol levels peaked at a higher point and more slowly returned to zero in alcohol-naïve rats relative to alcohol-exposed rats. Alcohol vapor exposure also produced an upward shift in subsequent operant alcohol responding and resultant BALs. Rats consumed large quantities of alcohol-liquid diet, most of it during the dark cycle, sufficient to produce high blood-alcohol levels and elevated operant alcohol responding when tested during withdrawal from liquid diet.
Conclusions
These results emphasize that the key determinants of excessive alcohol drinking behavior are the BAL range and pattern of chronic high-dose alcohol exposure.
Keywords: Alcohol dependence, blood-alcohol levels, alcohol-liquid diet, alcohol vapor
INTRODUCTION
Animal models of alcohol dependence are important for understanding the behavioral and biological components of alcoholism (Lieber & DeCarli, 1973; Majchrowicz et al., 1975; Rogers et al., 1979). These models have traditionally been used to model the somatic symptoms associated with withdrawal in alcohol dependence in humans (e.g., Isbell et al., 1955; Victor & Adams, 1953). Over time, refinements have been made to facilitate the study of motivational symptoms associated with withdrawal in alcohol dependence (e.g., O’Dell et al., 2004; Overstreet et al., 2004; Roberts et al., 1996, 2000). Perhaps the two most frequently used methods of producing alcohol dependence are alcohol vapor inhalation and alcohol liquid diet.
In a now classic study, alcohol was added in high concentrations to a liquid diet that represented the sole source of nutrition for rats (Lieber & DeCarli, 1973). The diet was composed such that its nutritional value overcame the aversive gustatory properties of alcohol and produced intakes of up to 14–16 g alcohol/kg per day. Shortly thereafter, an alcohol vapor inhalation model was developed in an attempt to address some of the limitations of the alcohol-liquid diet model of dependence induction (Rogers et al., 1979). Each of these techniques has limitations as an ideal animal model for induction of alcohol dependence, specifically route of administration (vapor inhalation), experimenter control of alcohol dose and exposure pattern (liquid diet), and forced alcohol administration (both techniques). However, the “success” of liquid diet and vapor inhalation models in producing dependence was historically measured by their ability to produce a specific constellation of somatic withdrawal symptoms in otherwise healthy animals (Frye et al., 1981; Majchrowicz, 1975; Rogers et al., 1979).
Currently, alcohol dependence in rats can be defined by the presence of physical and affective disturbances in the absence of alcohol, as well as changes in the motivational properties of alcohol. Physical symptoms of alcohol dependence manifest during acute withdrawal, subside within 24–48 hours following termination of alcohol exposure, and include convulsions, motor abnormalities and autonomic disturbances (Gilpin et al., 2008c; Majchrowicz, 1975). Following termination of chronic exposure to high doses of alcohol, rats exhibit increases in anxiety-like behavior, increased alcohol drinking, and increased willingness to work for alcohol that are present during acute withdrawal, even when animals still have alcohol in blood from vapor exposure (Funk et al., 2007; Gehlert et al., 2007; Gilpin et al., 2008a; O’Dell et al., 2004; Rimondini et al., 2002; Roberts et al., 1996; Valdez et al., 2002; Walker et al., 2007; Zhao et al., 2007). These withdrawal-induced behaviors are reversed by GABAergic agonists (Roberto et al., 2008; Roberts et al., 1996) and corticotropin releasing factor (CRF) receptor antagonists (Funk et al., 2007; Gehlert et al., 2007; Richardson et al., 2008a; Valdez et al., 2002).
However, little work has explored the dynamics of blood and brain alcohol changes during the alcohol vapor inhalation and liquid diet procedures. The overall objective of the present study was to better define the parameters associated with the liquid diet and vapor inhalation models of alcohol dependence. The purpose of Experiment 1 was to track the rise and fall of blood- and brain-alcohol levels during alcohol vapor exposure withdrawal in naïve and alcohol-experienced animals, and also to assess metabolic tolerance in chronic alcohol-exposed animals. The purpose of Experiment 2 was to examine BAL time course during operant alcohol sessions that followed induction of alcohol dependence via vapor inhalation. The purpose of Experiment 3 was to examine BAL time course during alcohol-liquid diet access, during withdrawal from liquid diet, and following post-dependence operant alcohol sessions. Finally, Experiments 2 and 3 provide a direct comparison of post-dependence operant alcohol response data following two different methods of dependence induction.
METHODS
Animals
Adult male Wistar rats (n=51) obtained from Charles River (Kingston, NY) were used for all experiments. Rats were housed in standard plastic cages with wood chip bedding under a 12 hr light/12 hr dark cycle. Animals were given ad libitum access to food and water throughout except during experimental drinking sessions. All procedures were conducted in the dark cycle and met the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Experimental design
Experiment 1
The purpose of this experiment was to track alcohol levels in blood and brain of previously alcohol-naïve versus alcohol-experienced rats during alcohol vapor exposure and subsequent withdrawal. Subjects were 15 male Wistar rats that weighed 300–350 g at the start of the experiment.
Stereotaxic Surgery
Surgical implantation of cannulae was conducted using aseptic procedures. Rats were anesthetized via inhalation of a mixture of halothane/CO2/O2 and implanted with a single cannula (20 gauge, Plastics One Inc., Roanoke, VA) aimed at the nucleus accumbens; the stereotaxic coordinates (AP +1.7, ML ±1.5, DV -6.1) were taken from Paxinos and Watson (1998). A dummy cannula (28 gauge) that was placed in the guide cannula at all times except during dialysate sample collection. The dialysate probe extended 1.0 mm beyond the tip of the guide cannula when inserted. At the completion of all experimental manipulations, animals were sacrificed via overdose of halothane and the brains were removed and stored in 10% formalin. To verify microdialysis probe placements, the brain was frozen on a cryostat and sliced into 30 µm coronal sections that were stained with cresyl violet. Slices were examined under a microscope to verify placement using the atlas of Paxinos and Watson (1998).
Microdialysis Probes
Microdialysis probes were constructed as described previously (Smith and Justice, 1993). Briefly, two lengths of fused silica (100 µm o.d. 40 µm i.d.) were inserted into a 5-mm section of cellulose dialysis tubing (200 µm; 6000 molecular weight cutoff, Spectrum Medical Industries, Houston, TX). Polyimide resin (Alltech Associates, Waukegon, IL) was used to seal both ends of the dialysis membrane. The inlet silica line extended beyond the outlet silica line by 2 mm inside the dialysis membrane to define the active area of the probe. The remaining portions of the dialysis membrane were coated with polyimide resin.
Alcohol Vapor Inhalation & Blood/Dialysate Sample Collection
Acute Alcohol Vapor Exposure
Following recovery from stereotaxic and i.v. catheterization surgeries (see General Methods for details of i.v. catheterization surgery), animals (n=5) were transferred to vapor inhalation chambers and the dialysate probe was inserted into the guide cannula. The probe was connected to a 500 µl syringe mounted on a CMA 100 syringe pump (Carnegie Medicine) and perfused with artificial cerebrospinal fluid (aCSF) containing 149 mM NaCl, 2.8 mM KCl, 1.2 mM CaCl2, 1.2 mM MgCl2, 0.25 mM ascorbic acid, and 5.4 mM D-glucose, pH 7.2 to 7.4, at a rate of 0.5 µl/min overnight while rats were exposed to air vapor. This aCSF perfusion subsequently persisted throughout the experiment. Approximately 12 hours following the start of aCSF perfusion, the i.v. connection tube was attached to catheters. Two baseline samples of dialysate (from cannula probe in brain) and blood (from catheter in jugular vein) were collected from all rats at 30-min intervals during air vapor exposure. Alcohol vapor was then introduced into the chambers and animals were exposed to alcohol vapor for 4 hours. Dialysate and blood sample collection continued throughout this period of alcohol vapor exposure on 30-min intervals, and also 8 hrs following termination of alcohol vapor exposure.
Chronic Alcohol Vapor Exposure
Following recovery from stereotaxic surgery, animals were separated into 2 groups that would be exposed to either chronic intermittent alcohol vapor (alcohol-exposed group; n=5) or air vapor (alcohol-naïve group; n = 5) for a period of 14 days. Animals in the alcohol-exposed group were group-housed in inhalation chambers and exposed daily to 12 hrs (6pm – 6 am) of alcohol vapor. Control animals were treated similarly but exposed to chronic air vapor that did not contain alcohol. Tail blood samples were collected from all rats every third day just prior to the termination of alcohol vapor exposure (6 a.m.); evaporated ethanol values (ml/h) were adjusted as necessary to maintain BALs in the 125–250 mg% (mg/dL) range. On the eve of the 14th day of exposure (approximately 12 hours following the start of aCSF perfusion), dialysate probes were inserted into guide cannulae and perfused with aCSF at 0.5 µl/min overnight while rats were exposed to air vapor. Three baseline samples of dialysate were collected from all rats at 30-min intervals during the final 90 minutes of ambient air exposure. Alcohol vapor was then introduced into the chambers and animals were exposed to alcohol vapor for 4 hours. Dialysate sample collection continued throughout this period of alcohol vapor exposure on 30-min intervals, and also throughout the 8 hrs following termination of alcohol vapor exposure on 30-min intervals.
Experiment 2
The purpose of this experiment was to characterize the time course of operant alcohol self-administration behavior and resultant BALs in animals made dependent using chronic intermittent exposure to alcohol vapor. Subjects were 14 male Wistar rats that weighed 150–225 g at the start of the experiment.
Alcohol Vapor Inhalation
Animals were exposed to chronic intermittent alcohol vapor to model the human condition in which alcohol exposure occurs in a series of extended exposures followed by periods of withdrawal. Vapor was delivered on an intermittent schedule (on at 18:00 h, off at 08:00 h) for a period of 4 weeks before post-vapor testing began, and on the same schedule during the behavioral testing phase of the experiment. Chronic exposure to intermittent vapor elicits higher alcohol self-administration than continuous vapor exposure (O'Dell et al., 2004). Blood-alcohol levels were assessed via tail vein sampling, and evaporated ethanol values (ml/h) were adjusted as necessary to maintain BALs in the 125–250 mg% (mg/dL) range.
Operant Alcohol Self-administration
Animals were trained to orally self-administer alcohol or water on a continuous reinforcement (fixed ratio-1, FR1) schedule in a concurrent, two-lever, free-choice contingency (see General Methods for procedural details). Following 19 operant sessions and stabilization of responding for 10% w/v ethanol, rats were divided into 2 groups and exposed to either chronic intermittent alcohol vapor (dependent group; n=6) or control ambient air (non-dependent group; n=8). Following 4 weeks of vapor exposure, rats were tested twice per week (total of 19 operant sessions) at 6–8 hrs withdrawal for dependence-induced changes in operant alcohol responding. During pre-vapor and post-vapor operant testing rats were attached to tethers via i.v. catheters for acclimation purposes.
BAL Determinations
On the day of jugular vein blood sampling (see General Methods for procedural details), catheters were connected to tubing attached to a syringe containing heparinzed saline. Rats were placed in operant boxes where they remained awake and freely moving, which allowed for collection of blood without disturbing the animals. Animals rested for 2–3 h after connection to ensure that they were not stressed during the session, which causes drastic changes in operant behavior. BALs were accessed at four time points: 0, 15, 30, and 45 min. The first blood sample was then taken just prior to presentation of the levers (0 min). During self-administration, a blood sample was taken at 15 min and another at 30 min (just prior to retracting the levers. Following the operant session, the animals remained in the operant boxes for 15 min and a final blood sample was taken (45 min).
Experiment 3
The goals of this experiment were to: [a] track alcohol-liquid diet intake and resultant BALs across the circadian cycle, [b] assess the ability of alcohol-liquid diet to mimic dependence-induced operant responding produced by chronic alcohol vapor exposure, and [c] determine BALs produced by operant responding at various withdrawal time points from chronic alcohol-liquid diet. Subjects were 22 rats that weighed between 209–252 g at the start of the experiment.
Operant Alcohol Self-administration
Animals were trained to orally self-administer alcohol or water on a continuous reinforcement (fixed ratio-1, FR1) schedule in a concurrent, two-lever, free-choice contingency (see General Methods for procedural details). Following 20 operant sessions and stabilization of responding for 10% w/v ethanol, rats were divided into 2 groups (n=11/group) and given either alcohol-containing liquid diet (dependent group) or isocaloric control diet (non-dependent group).
Alcohol-Liquid Diet Exposure
Immediately prior to the start of alcohol-liquid diet exposure, lab chow was removed from cages. From that point forward, the sole source of nutrition available to rats in the home cage was alcohol- or control-liquid diet. One liter of alcohol-liquid diet contained 3 g vitamins (MP Biomedicals, LLC, Solon, OH), 5 g salt (ICN Biomedicals, Inc., Aurora, OH), 92 ml 95% v/v ethanol, 711 ml Boost (High-protein chocolate-flavored nutritional energy drink, Columbus, IN), and 197 ml water; one liter of control-liquid diet was similar except that it contained 126 g sucrose (isocalorcially matched to alcohol-liquid diet; Sigma-Aldrich, St. Louis, MO) instead of 95% v/v ethanol. Early in diet exposure, two parameters were altered every few days in an attempt to maximize alcohol intake: [1] the concentration of ethanol and [2] the percentage of calories derived from ethanol (EDC). Eventually, the optimal parameters were determined and from that point forward, rats were allowed ad libitum access to 9.2% (v/v) ethanol-liquid diet in which 41% of diet calories were derived from ethanol. Seven days later, rats were tested for operant alcohol responding during withdrawal from diet and these tests continued for several weeks at a frequency of twice per week. Tail blood samples were collected from rats following operant test sessions, and also at various time points during liquid-diet access and withdrawal on days that rats were not tested.
BAL Determinations
Tail blood was sampled (see General Methods for procedural details) during the dark cycle and light cycle when rats were drinking alcohol-liquid diet in the home cage. Tail blood was also sampled at various withdrawal time points following the removal of diet. Finally, tail bloods were sampled immediately following operant drinking sessions that were conducted at the same withdrawal time points.
General Methods
Alcohol Vapor Inhalation (Experiments 1 & 2 only)
Alcohol vapor inhalation was employed to gradually increase and maintain blood-alcohol levels (BALs) between 150 and 200 mg% (details of the time course are described for each experiment above). Rats were exposed to alcohol/air vapor in a rodent alcohol inhalation system (La Jolla Alcohol Research, Inc., San Diego, CA) that has been described in detail elsewhere (Gilpin et al., 2008b; Lee et al., 2000). During vapor exposure, rats were dually housed in standard plastic tub style cages with opaque walls. Rats were monitored daily and weighed twice per week, food and water were replenished as necessary, room temperature and humidity settings were monitored daily, and vapor settings were adjusted according to alcohol concentrations within the cages as well as observed BALs and behavior of rats.
Experiment 1 utilized 2 weeks of chronic vapor exposure for practical reasons (e.g., financial cost), and because longer vapor exposure would have been extraneous for the purposes of that experiment. We know from past studies in our lab that vapor exposure parameters must be intensified even in the first 2–3 days of vapor exposure to maintain rats in the same BAL range (Gilpin et al., Curr Protocol Neurosci 2008). In Experiment 1, two weeks of alcohol vapor exposure are sufficient to produce brain metabolic tolerance in rats (Figure 2). For behavioral end points (e.g., operant alcohol self-administration), 4 weeks of intermittent vapor exposure is the standard protocol used by our laboratory before operant testing begins. It is important to note that although rats experience 4 weeks of vapor exposure prior to the first operant test, rats are eventually exposed to much more than 4 weeks of alcohol vapor. That is, rats are typically tested for operant self-administration twice per week over a period of several weeks (e.g., 19 tests in Experiment 2) to establish stable baseline withdrawal-induced responding, and then experimental manipulations (e.g., surgeries, pharmacological manipulations, or post-operant BAL assessment) are introduced. These procedural details precluded matching the time course of vapor exposure in Experiments 1 and 2.
Fig. 2.
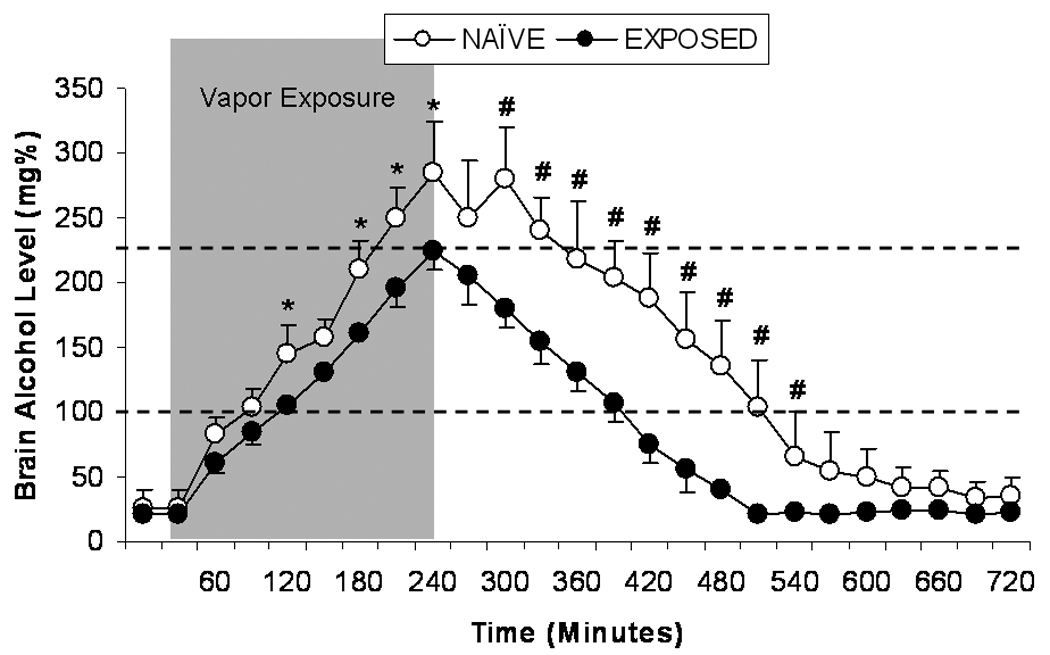
Mean ± SEM brain-alcohol levels (mg%) in previously alcohol-naïve (n=5; open circles) or alcohol-exposed (14 days of intermittent vapor; n=5; closed circles) rats 30 minutes prior to alcohol vapor exposure, during 4 hours of alcohol vapor exposure (gray area), and 8 hours following termination of alcohol vapor exposure. * p<0.05, # p<0.01 significant difference from alcohol-naïve group.
Intravenous (i.v.) Catherization (Experiments 1 & 2 only)
Animals were anesthetized by inhalation of either a mixture of halothane/CO2/O2 (Experiment 1) or isoflurane (Experiment 2), and intravenous catheters were aseptically inserted in the right jugular vein using a modified version of a procedure previously described (Caine et al., 1993). The vein was punctured with a 27 gauge needle and the tubing was inserted and secured inside the vein by tying the vein with suture thread. The catheter assembly consisted of a 14 cm length of silastic tubing (0.025 inch inner diameter, 0.047 inch outer diameter, Dow Corning, Midland, MI) that was attached to a guide cannula (Plastics One, Roanoke, VA) bent at a near right angle and embedded in dental acrylic anchored with a 2 ×2 cm square of mesh. The catheter was fed subcutaneously from where it was secured in the jugular vein to the animal’s back. It exited through a small incision on the back, and the base was sealed with a small plastic cap and metal cover cap. This design helps keep the catheter base sterile and protected from cage mates (animals were housed in groups of 2–3). Catheters were flushed daily with heparinized saline (10 units/ml of heparin sodium, American Pharmaceutical Partners, Schaumburg, IL, in 0.9% bacteriostatic sodium chloride, Hospira, Lake Forest, IL) containing antibiotic (20 mg/0.2 ml, Timetin, GlaxoSmithKline, once daily for 1 week, then once every 7–14 days) followed by heparinized saline.
Operant Alcohol Self-Administration (Experiments 2 & 3 only)
Operant alcohol self-administration was conducted in standard operant chambers (Coulbourn Instruments, Allentown, PA) housed in sound-attenuated ventilated cubicles. Animals were trained to orally self-administer alcohol or water in a concurrent, two-lever, free-choice contingency during daily 30-min daily sessions that occurred at the start of the dark cycle. Rats were trained to respond for ethanol using a saccharin fading procedure in which rats were initially allowed to respond for supersaccharin (SS; 3.0% glucose + 0.125% saccharin), then SS + 10% w/v ethanol, then 0.125% saccharin + 10% w/v ethanol, and eventually 10% w/v ethanol alone. Syringe pumps (Razel Scientific Instruments, Stamford, CT) dispensed alcohol or water into two stainless steel drinking cups mounted 4.0 cm above the grid floor in the middle of one side panel. Two retractable levers were located 4.5 cm to either side of the drinking cups. Fluid delivery and recording of operant self-administration were controlled by a standard PC computer. Lever presses were not recorded during the 0.5 s in which the pumps were active. A continuous reinforcement [fixed ratio-1 (FR1)] schedule was used such that each response resulted in delivery of 0.1 ml of fluid. Fluid delivery and recording of operant responding were controlled by a microcomputer. All rats were weighed prior to drinking sessions twice per week.
Alcohol Level Determinations
Tail vein blood sampling
Blood samples were collected by the tail-snip method (0.1–0.2 ml) from all animals (both alcohol vapor-exposed dependent and control air-exposed nondependent groups) just after the vapors turned off and 5 µl of plasma was used for measurement of BALs, as described below. As expected, BALs were always undetectable (< 20 mg%) in nondependent animals, but tail bleeding was performed to control for any stress experienced during this procedure.
Jugular vein blood sampling
In Experiment 1, blood samples were collected via jugular catheters throughout acute alcohol vapor exposure (see above for time course). In Experiment 2, blood samples were collected via jugular catheters before, during, and following operant self-administration (see above for time course). Jugular catheters were attached to PE50 tubing that exited the operant chamber. A 1cc syringe was attached to the other end of the tubing, and blood samples were collected by pulling back on the plunger, thus leaving the animal undisturbed.
Brain dialysate sampling
In Experiment 1, brain dialysate samples were collected throughout acute and chronic alcohol vapor exposure (see above for time course). The dialysate probe was connected to a 500 µl syringe mounted on a CMA 100 syringe pump (Carnegie Medicine). Probes were perfused with artificial cerebrospinal fluid (aCSF) at 0.5 µl/min overnight while rats were exposed to air vapor, and this aCSF perfusion continued throughout the experiment. Dialysate samples were assayed for ethanol content using the NAD-NADH spectrophotometric method (Sigma Chemicals).
Measurement of BALs
Blood (0.2 ml) was collected and centrifuged, the plasma extracted and injected into an oxygen-rate alcohol analyzer (Analox Instruments) for BAL determination. The reaction is based on the oxidation of alcohol by alcohol oxidase in the presence of molecular oxygen (alcohol + O2 ➔ acetaldehyde + H2O2). The rate of oxygen consumption is directly proportional to the alcohol concentration. Single point calibrations are done for each set of samples with reagents provided by Analox Instruments (25–400 mg%).
Statistical Analysis
All data are expressed as mean ±SEM. Alcohol consumption from operant sessions is normalized for body weight [i.e. (operant responses × 0.1 ml/response × 0.1 g ethanol/ml)/kg body weight = g/kg]. In Experiment 1, brain- and blood-alcohol levels during and following acute vapor exposure were analyzed using one-way repeated-measures analysis of variance (RM ANOVA) with a single factor (time). Following chronic vapor exposure, brain-alcohol levels were assessed relative to controls using two-way (exposure history × time) RM ANOVA, and peak brain-alcohol levels were compared in chronic and naïve using a two-samples t-test. In Experiments 2 and 3, data for operant responding, alcohol intake (g/kg), and alcohol preference (i.e. alcohol consumed/total fluid consumed) at the various intoxication, withdrawal, and operant test time points were separately analyzed using a series of two-way RM ANOVA and independent-samples t-tests. In Experiment 2, operant intake data were analyzed using two-way RM ANOVAs where day (pre-vapor baseline vs post-vapor test) was the within-subjects factor and vapor history (dependent vs non-dependent) was the between-subjects factor. Also in Experiment 2, BALs data were analyzed using two-way RM ANOVAs where time (0, 15, 30, 45 min post-vapor) was the within-subjects factor and vapor history (dependent vs non-dependent) was the between-subjects factor. In Experiment 3, two-way RM ANOVAs were used where day (baseline vs test time point) was the within-subjects factor and alcohol history (dependent vs non-dependent) was the between-subjects factor. Dependence history was the between-subjects factor for Experiment 3 t-tests of operant behavior and BALs. Also in Experiment 3, Pearson correlations were used to determine whether liquid-diet consumption was correlated with BALs at various time points, whether operant alcohol responding was correlated with post-operant BALs, and whether BALs achieved during liquid-diet consumption were correlated with operant alcohol responding at the various withdrawal time points. Post-hoc comparisons were made using the Student Newman-Keuls test. Statistical significance was set at p<0.05. There were no differences in the body weights of dependent and non-dependent animals during behavioral testing in Experiments 2 and 3.
RESULTS
Experiment 1
Acute Alcohol Vapor Exposure
Alcohol absorption and elimination profiles were similar in blood and brain during and following acute 4-hr exposure to alcohol vapor (Figure 1). The maximum levels of alcohol attained in blood (208 ± 15 mg%) and brain (215 ± 25 mg%) during vapor exposure were not different. Eight hours following the termination of alcohol vapor exposure, blood- and brain-alcohol levels returned to pre-vapor baseline. Linear regression analysis of the descending limb of the time course curve indicated no differences in the rate of elimination of alcohol from brain and blood following termination of alcohol vapor exposure.
Fig. 1.
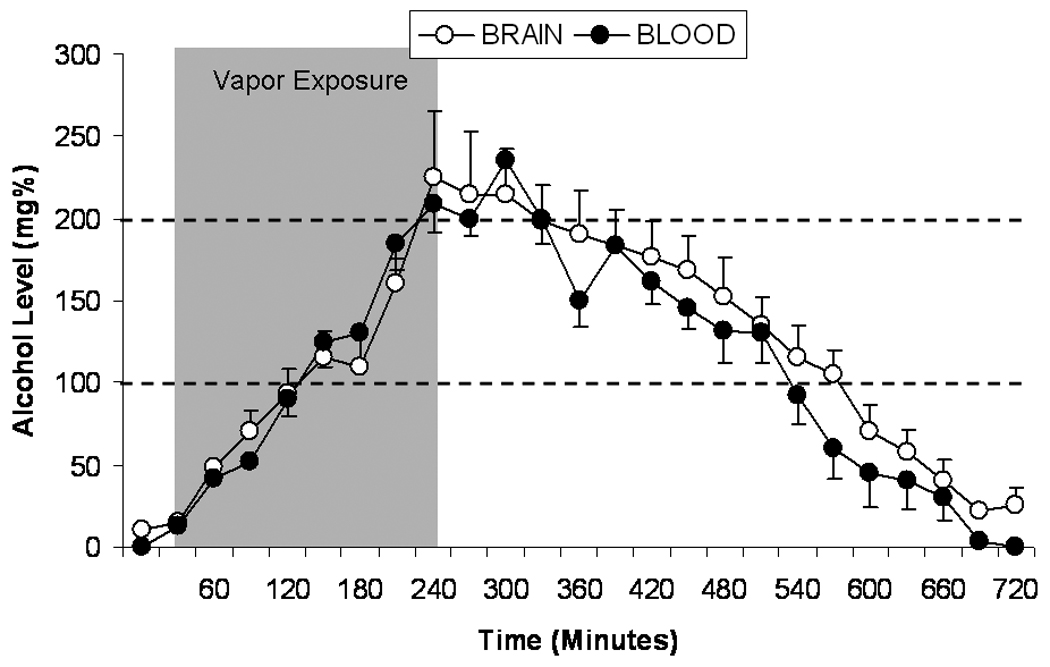
Mean ± SEM alcohol levels (mg%) in brain (open circles) and blood (closed circles) of previously alcohol-naïve rats (n=5) 30 minutes prior to alcohol vapor exposure, during 4 hours of alcohol vapor exposure (gray area), and 8 hours following termination of alcohol vapor exposure.
Chronic Alcohol Vapor Exposure
A two-way (time ×alcohol history) RM ANOVA revealed that brain-alcohol levels changed significantly over time in alcohol-naïve and alcohol-exposed rats, F(24,192)=173.94, p<0.001. Across time, alcohol-naïve rats exhibited significantly higher brain-alcohol levels than alcohol-exposed rats F(1,8)=25.67, p=0.001, suggesting brain metabolic tolerance in rats previously exposed to alcohol. Finally, there was a significant time × history interaction effect on brain-alcohol levels, F(24,192)=7.24, p<0.001. An independent-samples t-test indicated that the maximum brain-alcohol level observed during 4 hrs of alcohol vapor exposure was significantly higher in alcohol-naive (283.3 ± 43.1 mg%) versus alcohol-exposed (226 ± 11.5 mg%) animals (p < 0.05; Figure 2). On the descending limb of the brain-alcohol concentration curve, alcohol-naive animals maintained significantly higher brain-alcohol levels for the first 6 hrs following termination of alcohol vapor exposure (p<0.01). However, there was no difference in the elimination rate between the two groups (−0.75 mg%/min and −0.76 mg%/min for the control and ethanol group, respectively) as measured by regression analysis of the linear portion of the descending limb of the time course curve (Figure 2).
Experiment 2
Vapor-Induced Operant Responding
Figure 3A illustrates operant ethanol responding by animals exposed to chronic intermittent alcohol vapor during the days (3-day baseline) immediately preceding the start of vapor exposure and also at 6–8 hrs withdrawal during representative post-vapor test days (2-day mean). A two-way RM ANOVA revealed a significant main effect of vapor history, F(1,12)=17.08, p=0.001, a significant main effect of day, F(1,12)=52.66, p<0.001, and a significant history × day interaction effect, F(1,12)=19.79, p<0.001, on operant ethanol responding. Chronic intermittent alcohol vapor exposure produced increases in operant ethanol responding relative to baseline (p<0.001), and also relative to non-dependent controls (p<0.001). A separate two-way RM ANOVA revealed a significant main effect of vapor history, F(1,12)=14.55, p=0.002, a significant main effect of day, F(1,12)=48.29, p<0.001, and a significant history × day interaction effect, F(1,12)=21.98, p<0.001, on ethanol intake (g/kg). Post-hoc analysis indicated that chronic intermittent alcohol vapor exposure produced increases in ethanol intake (g/kg) relative to baseline (p<0.001), and also relative to non-dependent controls (p<0.001). A separate two-way RM ANOVA revealed no significant effects on operant responding for water.
Fig. 3.
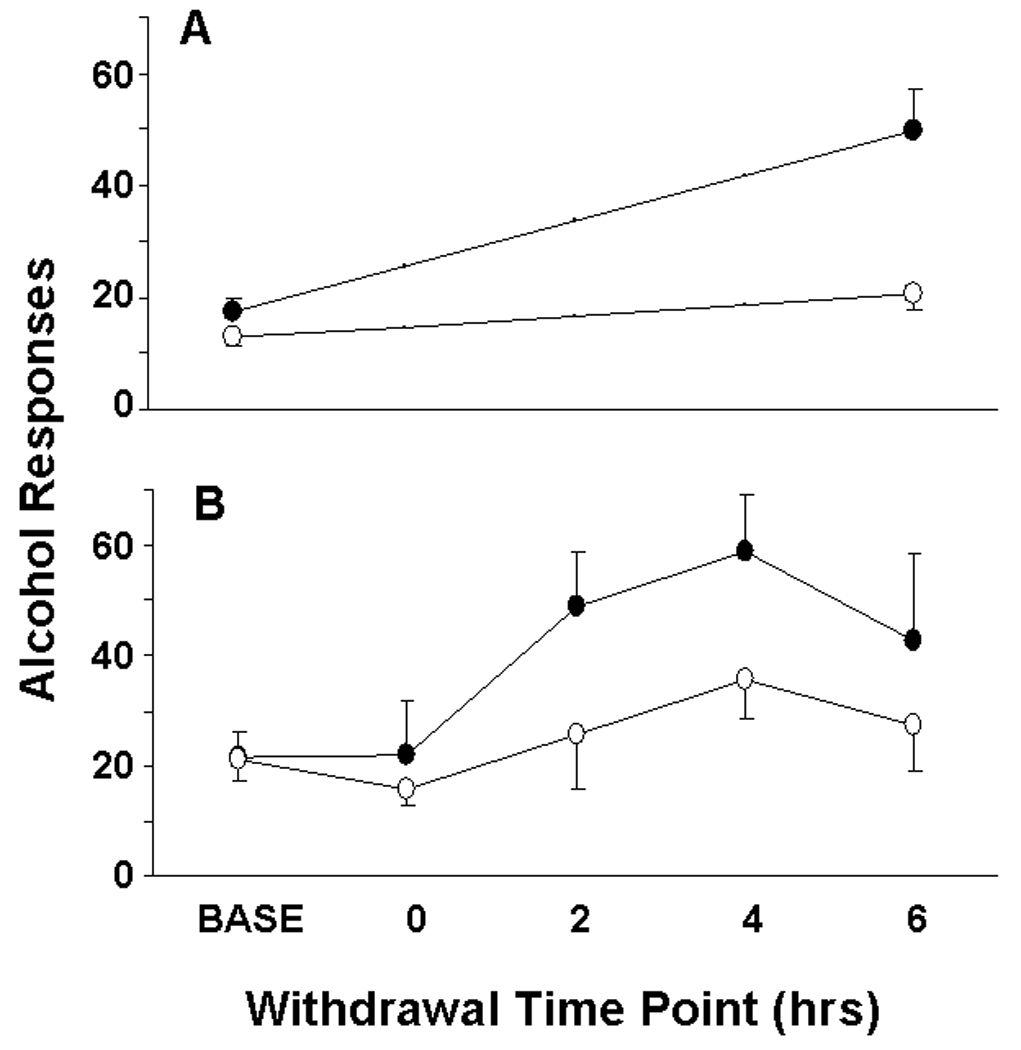
(A) Mean ± SEM operant responses for 10% w/v alcohol by dependent (n=6; black bars) and non-dependent (n=8; white bars) rats during pre-vapor baseline (average of final three pre-vapor sessions) and following chronic alcohol vapor exposure (average of first two post-vapor sessions). Post-vapor operant test sessions always occurred ~6 hrs following the termination of vapor exposure. (B) Mean ± SEM operant responses for 10% w/v alcohol by high alcohol responders (>12 responses/30 min during baseline period) in 30-min test sessions during the baseline period and also 0, 2, 4, and 6 hrs into withdrawal from liquid diet. Alcohol-dependent rats consumed alcohol-liquid diet (n=5; closed circles) and non-dependent rats consumed control diet (n=5; open circles). Liquid diet bottles were removed from the cage lids of all rats at various time points preceding operant tests.
BALs During and Following Dependence-Induced Operant Responding
Figure 4 illustrates BALs in rats at 15-minute increments from the start of the operant test session until 15 minutes following the end of the operant test session. A one-way RM ANOVA revealed that dependent rats exhibited higher BALs across the operant test session than non-dependent controls, F(1,36)=5.36, p=0.039, and also a main effect of time, F(3,36)=38.68, p<0.001. Collapsed across vapor history, rats exhibited elevated BALs 15 minutes, 30 minutes, and 45 minutes (p<0.001 in all cases) following the start of the operant test session relative to the 0-minute time point.
Fig. 4.
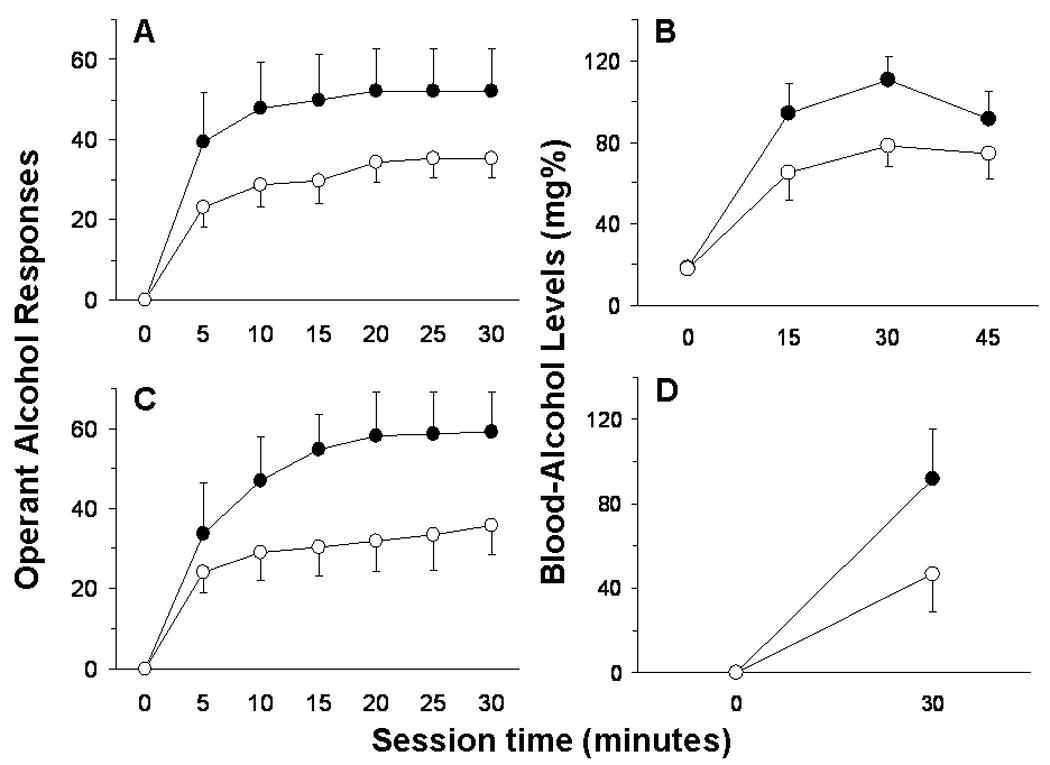
(A) Mean ± SEM cumulative operant responses for 10% w/v alcohol by dependent (n=6; black circles) and non-dependent (n=8; white circles) rats in 5-minute bins across the 30-min test session that occurred 6–8 hrs into withdrawal from alcohol vapor, and (B) mean ± SEM resultant blood-alcohol levels (mg%) in those rats 0, 15, 30, and 45 minutes following the start of the operant test session. (C) Mean ± SEM cumulative operant responses for 10% w/v alcohol by dependent (n=5; black circles) and non-dependent (n=5; white circles) rats in 5-minute bins across the 30-min test session that occurred 4 hrs into withdrawal from alcohol liquid-diet, and (D) mean ± SEM resultant blood-alcohol levels (mg%) in those rats 0 and 30 minutes following the start of the operant test session.
Experiment 3
Liquid Diet Intake and Resultant BALs
Average daily intake of the 9.2% (v/v) alcohol-liquid diet by alcohol-dependent rats was 79.04 (± 3.64) ml across all days of the experiment, which was equivalent to 9.52 (± 0.27) g ethanol/kg body weight/day. Amounts of control-liquid diet available daily to non-dependent rats was yoked to liquid diet intake levels for dependent rats on the previous day and adjusted for body weight (i.e. ml diet/kg body weight). This yoking procedure allowed for matched caloric intake and equivalent body weights for rats in the dependent (597.71 ± 15.35 grams) and non-dependent (599.86 ± 10.28 grams) groups at the end of the experiment.
Alcohol-dependent rats maintained higher BALs during the dark cycle than during the light cycle, and alcohol-liquid diet intake during the first two hours of the dark cycle by those rats was highly correlated with BALs measured at the same time point (Figure 5A). Still, alcohol-dependent rats consumed enough alcohol-liquid diet during the light cycle to maintain pharmacologically relevant BALs that were significantly different from BALs of control rats (t = 5.50, p<0.001), and these BALs were presumably high enough to avoid the manifestation of alcohol withdrawal symptoms (Figure 5B). When liquid diet bottles were removed 4 hrs prior to the start of the dark cycle (the time point at which operant tests occurred), BALs returned to negligible levels that were not different from BALs of control rats (t = 0.98, p>0.05) by the start of the dark cycle (Figure 5C). Therefore, animals [1] consume intoxicating doses of ethanol in the liquid-diet procedure, [2] achieve pharmacologically relevant BALs, [3] maintain BALs even during the inactive phase, and BALs returned to zero by the time behavioral testing occurred.
Fig. 5.
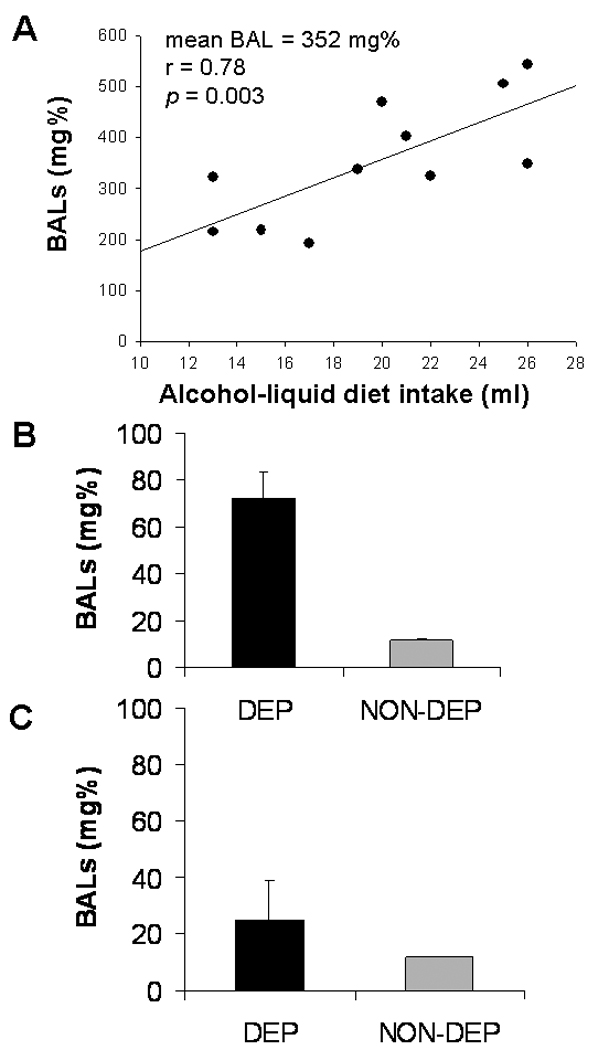
(A) Scatter plot of alcohol-liquid diet intake by alcohol-dependent rats during the first two hrs of the dark cycle and resultant BALs measured two hrs into the dark cycle; (B) mean BALs in alcohol-dependent and –non-dependent rats measured 8 hrs into the light cycle; and (C) mean BALs in alcohol-dependent and –non-dependent rats measured at the start of the dark cycle, following 4 hrs with no liquid diet (i.e. the time point when 4-hr withdrawal operant sessions occurred on behavioral test days).
Dependence-Induced Operant Responding and Resultant BALs
Dependent and non-dependent rats were divided into high and low responders based on number of alcohol presses during each 30-min session of the 6-day baseline period that preceded the start of liquid diet exposure (high responder > 12 responses/30-min session > low responder). High responders and low responders were separated for two reasons: [1] to show that the alcohol liquid-diet procedure is capable of elevating operant alcohol responding in animals that are already “high alcohol responders” prior to diet availability and also in animals that are “low alcohol responders” based on pre-diet response levels, and [2] to compare the relevant high responder group in Experiment 3 to the vapor-exposed animals in Experiment 2 (low responders were removed from the experiment prior to vapor exposure per standard protocol in our lab). Table 1 presents operant response data and resultant BALs for all rats (divided into high and low responders) at various withdrawal time points following removal of liquid diet. Figure 3B illustrates operant alcohol responding by high-responder rats at various time points of withdrawal from alcohol-liquid diet.
Table 1.
Operant responses for 10% w/v ethanol (±SEM) and resultant blood-alcohol levels (± SEM) by dependent (DEP) and non-dependent (NON) rats 2 hrs, 4 hrs, and 6hrs into withdrawal from liquid diet. were designated as either high (> 12) or low (< 12) operant alcohol responders based on pre-diet baseline alcohol responding (see Results).
| Operant Alcohol Responses | Blood-Alcohol Levels (mg%) | |
|---|---|---|
| Pre-Diet Median Split | ||
| DEP – High Responder (n=5) | 21.57 (4.68) | N/A |
| DEP – Low Responder (n=6) | 7.42 (2.02) | N/A |
| NON – High Responder (n=5) | 21.17 (3.78) | N/A |
| NON – Low Responder (n=6) | 7.19 (1.75) | N/A |
| 2 hrs Withdrawal | ||
| DEP – High Responder (n=5) | 49.00 (9.67) | 98.56 (22.54) |
| DEP – Low Responder (n=6) | 23.00 (9.27) | 67.37 (23.17) |
| NON – High Responder (n=5) | 25.60 (9.94) | 43.72 (11.19) |
| NON – Low Responder (n=6) | 12.00 (2.45) | 13.27 (0.73) |
| 4 hrs Withdrawal | ||
| DEP – High Responder (n=5) | 59.00 (10.08) | 91.58 (23.84) |
| DEP – Low Responder (n=6) | 23.67 (6.54) | 46.80 (20.95) |
| NON – High Responder (n=5) | 35.80 (7.28) | 46.64 (18.00) |
| NON – Low Responder (n=6) | 15.83 (2.09) | 17.33 (3.36) |
| 6 hrs Withdrawal | ||
| DEP – High Responder (n=5) | 42.60 (15.67) | 76.14 (18.69) |
| DEP – Low Responder (n=6) | 20.17 (8.33) | 47.12 (18.34) |
| NON – High Responder (n=5) | 27.20 (8.24) | 45.22 (13.74) |
| NON – Low Responder (n=6) | 7.50 (3.06) | 11.08 (0.57) |
A two-way RM ANOVA of alcohol response data from all rats revealed no significant effects of dependence history or day on operant alcohol responding 0 hours into “withdrawal.” A separate two-way RM ANOVA revealed a significant history × day interaction effect on operant alcohol responding 2 hours into withdrawal from liquid diet, F(1,20)=5.16, p=0.034. Post-hoc analyses indicated that alcohol-dependent rats responded more for alcohol relative to control rats (p=0.016) and also relative to their own baseline (p<0.001). There was also a significant history × day interaction effect on operant alcohol responding 4 hours into withdrawal from liquid diet, F(1,20)=4.76, p=0.041. Post-hoc analyses indicated that alcohol-dependent rats responded more for alcohol relative to control rats (p=0.033) and also relative to their own baseline (p<0.001). There was a tendency toward a history × day interaction effect on operant alcohol responding 6 hours into withdrawal from liquid diet (p=0.063). There was a main effect of day such that all rats responded more for alcohol 6 hrs into withdrawal relative to baseline, F(1,20)=7.90, p=0.011. Analyses of alcohol intake (g/kg) at these time points yielded similar results.
When rats were tested for operant behavior immediately following the removal of liquid diet from the home cage (i.e. 0 hrs “withdrawal”), a two-way RM ANOVA revealed that rats responded less for water on test day relative to baseline, F(1,20)=12.91, p=0.002. There were no effects of day or dependence history on operant water responding at any other withdrawal test time point. A separate series of two-way RM ANOVAs revealed that, relative to baseline, rats exhibited a higher preference for alcohol 0 hrs, F(1,20)=5.07, p=0.036, 2 hrs, F(1,20)=22.52, p<0.001, 4 hrs, F(1,20)=16.60, p<0.001, and 6 hrs, F(1,20)=8.06, p=0.01, into withdrawal from liquid diet (data not shown).
Blood-alcohol levels were assessed in alcohol-dependent rats directly following 30-min operant test sessions that took place 2 hrs, 4 hrs, and 6 hrs into withdrawal from alcohol-liquid diet. Figure 6 illustrates a representative scatter plot of operant alcohol responses and resultant BALs at 4 hrs withdrawal. A series of analyses indicated significant Pearson correlations between operant alcohol responses and resultant BALs at two hrs, r(22)=0.885, p<0.001, four hrs, r(22)=0.958, p<0.001, and six hrs, r(22)=0.883, p<0.001, into withdrawal from alcohol-liquid diet. There were no significant correlations between peak BALs achieved during liquid-diet consumption and operant alcohol responding at any of the withdrawal time points tested (p>0.05 in all cases).
Fig. 6.
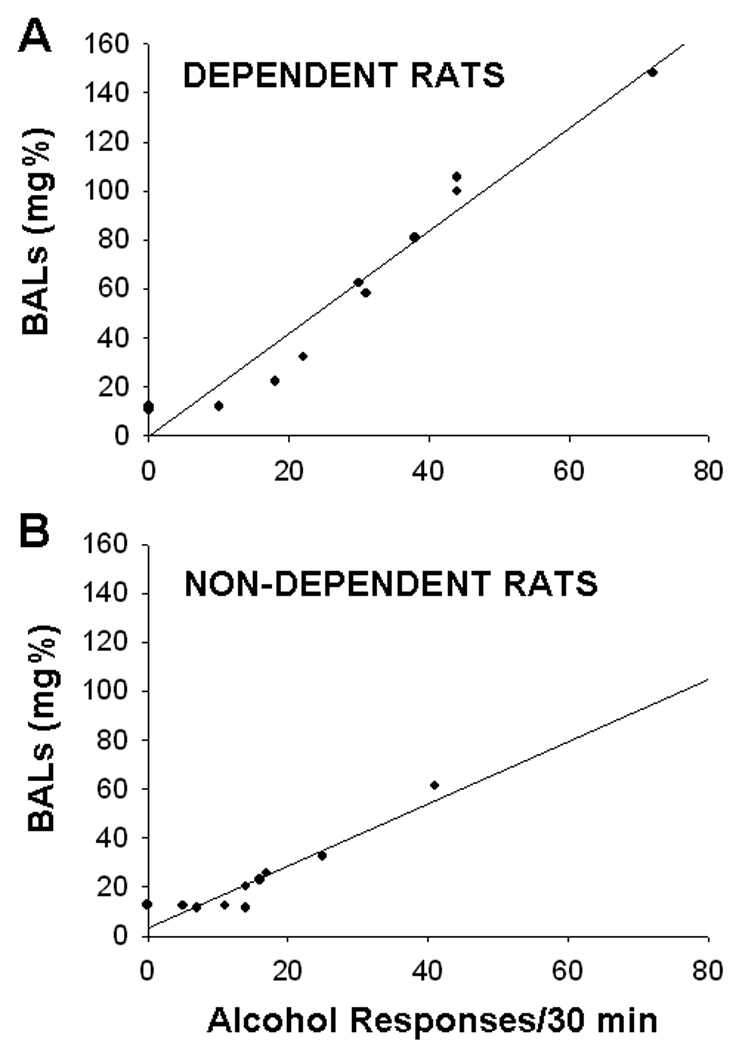
Scatter plot of operant responses for 10% w/v alcohol and resultant BALs for (A) dependent and (B) non-dependent rats during the 30-min test session that occurred 4 hrs into withdrawal from alcohol-liquid diet. The scatter plot for the 4-hr time point is representative of scatter plots at the 2-hr and 6-hr time points. Operant alcohol responses were significantly correlated with BALs at all three time points (see text)
DISCUSSION
The present results show that blood-alcohol levels are representative of brain-alcohol levels during alcohol vapor exposure and subsequent withdrawal; also, lower brain-alcohol levels in alcohol-experienced rats relative to alcohol-naïve rats indicate the development of metabolic tolerance to alcohol vapor (Experiment 1). Vapor exposure produces an upward shift in operant alcohol responding and resultant blood-alcohol levels of dependent rats relative to non-dependent controls (Experiment 2). Finally, alcohol-liquid diet consumption throughout the circadian cycle produces higher operant ethanol responding and resultant BALs at multiple withdrawal time points (Experiment 3).
For many years, our lab has used alcohol vapor inhalation to produce symptoms of alcohol dependence in rats (Rogers et al., 1979). The present study illustrates comparable time courses of blood- and brain-alcohol levels during and following alcohol vapor exposure. Also, animals that have previously been exposed to alcohol vapor for 14 days exhibit a downward shift in brain-alcohol levels relative to alcohol-naïve rats. Collectively, these results confirm that blood-alcohol levels in vapor-exposed animals are representative of brain-alcohol levels in those animals, and that rats develop metabolic tolerance to alcohol vapor with repeated exposure. These results are in agreement with previous findings that blood- and brain-alcohol levels are similar following alcohol vapor exposure (Griffin et al., 2009) and acute alcohol injection (Smolen & Smolen, 1989) in mice. The brain metabolic tolerance observed in Experiment 1 reflects previously reported development of blood metabolic tolerance and functional tolerance following chronic alcohol vapor exposure (Rimondini et al., 2008; Rogers et al., 1979). However, this metabolic tolerance is only partial in that vapor inhalation continues to produce significant blood-alcohol levels in vapor-experienced rats, and vapor-exposed animals self-administer alcohol to produce high BALs (Experiment 2).
Recently, the alcohol vapor procedure has been refined to model the motivational (O’Dell et al., 2004; Roberts et al., 1996, 2000; Walker & Koob, 2007) and affective (Zhao et al., 2007) aspects of alcohol dependence in the absence of severe physical withdrawal signs. At the same time, alcohol-liquid diet procedures have been refined to model the affective aspects of alcohol dependence (Overstreet et al., 2002). Here, we employed operant procedures to test the motivational aspects of alcohol dependence using these two models. Alcohol vapor inhalation and alcohol-liquid diet produce similar increases in operant alcohol responding that are highly correlated with post-operant BALs. Using vapor inhalation and liquid diet procedures, Experiments 2 and 3 illustrate representative operant alcohol responding during withdrawal, the high BALs produced by withdrawal-induced elevations in operant alcohol consumption, and the strong correlation between responding and BALs (at multiple time points during the operant session in Experiment 2).
Rats in Experiments 2 and 3 were tested for alcohol responding in 30-min operant sessions during acute withdrawal. Rats in Experiment 2 were withdrawn from alcohol vapor, whereas rats in Experiment 3 were withdrawn from alcohol-liquid diet. In drawing comparisons between the 2 experiments, it should be reiterated here that low responders were removed from Experiment 2 prior to the start of vapor exposure (as is customary in our lab), so the relevant comparison group in Experiment 3 is the “high responder” group. Alcohol-dependent rats in Experiments 2 and 3 exhibited similarly elevated alcohol response rates relative to respective non-dependent controls (Figure 3). Furthermore, alcohol-dependent rats in the two experiments exhibited similarly elevated post-operant BALs relative to respective non-dependent controls (Figure 4). These data underscore the similar effects of chronic alcohol vapor inhalation and liquid diet on the subsequent motivation of rats to consume alcohol. More importantly, these results emphasize that the key element driving withdrawal-induced alcohol drinking is the range of blood-alcohol levels maintained during chronic alcohol exposure, and not the method of BAL induction.
Because repeated withdrawal periods accelerate and intensify the development of dependence-related symptomatology (Becker & Hale, 1993; Breese et al., 2005; Clemmesen and Hemmingsen, 1984), it is important to note differences in the patterns of vapor and liquid-diet alcohol exposure. Chronic intermittent alcohol vapor exposure (Experiment 2), by definition, incorporates experimenter-imposed periods of alcohol abstinence, but BALs gradually rise and vapor settings are uniform throughout the exposure period. In contrast, alcohol-liquid diet access is usually continuous (although this procedure may incorporate entire days off between multi-day blocks of diet access; Overstreet et al., 2002), and BALs are likely variable during the exposure period. Data from Experiment 3 illustrate the drastically different BALs produced by liquid diet consumption across the circadian cycle, however, those data also show that rats consume enough alcohol, even during the “inactive” phase, to avoid complete elimination of alcohol from blood and presumably the manifestation of a withdrawal syndrome (Figure 5). Therefore, alcohol-liquid diet produces oscillating BALs that are never interrupted by periods when BALs return to zero, whereas vapor procedures expose animals to steady and constant amounts of alcohol with precise and scheduled periods of abstinence during which BALs return to zero. Different from both of these procedures, oral intubation and intragastric infusion techniques have been used largely to model neural changes associated with alcohol dependence (Cagetti et al., 2004; Crews et al., 2000; Kokka et al., 1993). In those procedures, rats are administered often sedative doses of ethanol once or more daily and BALs are marked by sudden upward spikes followed by a long and gradual return to zero. Chronic injection procedures also produce somatic (i.e. decreased seizure threshold) and affective (i.e. increased anxiety-like behavior in elevated plus-maze) signs of alcohol dependence (Cagetti et al., 2004).
While acute withdrawal is often conceptually tied with physical disturbances, the vapor exposure dosing and pattern employed here are moderate enough to avoid a severe physical withdrawal syndrome in rats (Richardson et al., 2008b). Physical withdrawal symptoms are variable with alcohol-liquid diet because individual animals control the dose and pattern of alcohol exposure. Conversely, the motivational symptoms of withdrawal are traditionally linked with both acute and protracted abstinence because they persist at protracted time points long after physical symptoms have subsided (Gilpin et al., 2008a; Roberts et al., 2000; Zhao et al., 2007). Indeed, in the chronic intermittent alcohol vapor model, motivational symptoms of dependence are reliably present in rats at acute withdrawal time points as evidenced by increased anxiety-like behavior, increased alcohol drinking, and increased willingness to work for alcohol early during acute withdrawal, even when animals still have alcohol in blood from vapor exposure (Funk et al., 2007; O’Dell et al., 2004; Roberts et al., 1996; Valdez et al., 2002; Walker et al., 2007; Zhao et al., 2007). Rats also exhibit increased operant alcohol responding early during acute withdrawal from alcohol-liquid diet (Experiment 3). Therefore, there is a weak temporal correlation between physical withdrawal signs and elevations in alcohol consumption by dependent animals following termination of chronic alcohol exposure.
All animal models of alcohol dependence are, in actuality, models of components of alcohol dependence. In this context, face validity (how much the animal model “looks like” the human condition) is far less important than construct validity, and more specifically, predictive validity (how well the animal model predicts mechanisms of and treatments for the human condition). Despite shortcomings, both procedures utilize forced alcohol administration to produce chronic BALs that are representative of the BALs maintained by human alcoholics and in this sense, both models contain a certain degree of face validity. The more crucial factor in these animal models of alcohol dependence is their predictive validity. Acamprosate, a drug that blocks relapse drinking in human alcoholics via suppression of craving, effectively suppress alcohol drinking by rats made dependent on alcohol via vapor inhalation, but not in non-dependent controls (Le Magnen et al., 1987; Morse & Koob, 2002; Rimondini et al., 2002). More recently, it was shown that an indirect GABA agonist, gabapentin suppresses alcohol drinking in rats made dependent on alcohol via vapor inhalation and liquid diet (Roberto et al., 2008), and a subsequent human laboratory study confirmed that gabapentin reduces alcohol craving in dependent individuals (Mason et al., 2009). Other labs have developed elegant models for dependence induction via alcohol liquid-diet, and use this model for high-throughput screening of compounds that target the high anxiety state produced by chronic alcohol exposure and repeated withdrawals (Breese et al., 2004, 2005; Overstreet et al. 2002, 2003, 2004). For example, the GABAB agonist baclofen, an effective anxiolytic in PTSD and alcoholic humans (Addolorato et al., 2002; Drake et al., 2003), blocks sensitization of anxiety-like behavior in rats consuming intermittent alcohol liquid-diet (Knapp et al., 2007), and also selectively suppresses alcohol drinking in rats made dependent on alcohol via vapor inhalation (Walker & Koob, 2007).
The present series of experiments relates commonly measured blood-alcohol levels to less frequently measured brain-alcohol levels and highlights several factors that determine alcohol self-administration in rats. The single most important factor in withdrawal-induced alcohol drinking is the blood-alcohol level range maintained during chronic alcohol exposure, regardless of route of administration or method of induction. Another important factor is the pattern of alcohol exposure and BALs; intermittent alcohol exposure patterns, whether experimenter-imposed (vapor) or self-imposed by rats (liquid diet), produce BALs that oscillate between high and low values, and accelerate the development of excessive alcohol drinking associated with alcohol dependence (O’Dell et al., 2004). Prior training in operant self-administration procedures is imperative for rats to experience changes in the reinforcement value of alcohol during dependence induction. These factors should all be considered when designing experiments aimed at testing the effects of chronic alcohol exposure on subsequent alcohol self-administration behavior.
Acknowledgements
The authors thank Yanabel Grant for excellent technical assistance and Mike Arends for his skilled assistance in preparing this manuscript. This is manuscript number 19712 from The Scripps Research Institute. Supported by the Pearson Center for Alcoholism and Addiction Research and NIAAA grants AA06420, AA08459, and AA12018.
REFERENCES
- Addolorato G, Caputo F, Capristo E, Domenicali M, Bernardi M, Janiri L, et al. Baclofen efficacy in reducing alcohol craving and intake: a preliminary double-blind randomized controlled study. Alcohol Alcohol. 2002;37:504–508. doi: 10.1093/alcalc/37.5.504. [DOI] [PubMed] [Google Scholar]
- Becker HC, Hale RL. Repeated episodes of ethanol withdrawal potentiate the severity of subsequent withdrawal seizures: an animal model of alcohol withdrawal “kindling”. Alcohol Clin Exp Res. 1993;17:94–98. doi: 10.1111/j.1530-0277.1993.tb00731.x. [DOI] [PubMed] [Google Scholar]
- Breese GR, Knapp DJ, Overstreet DH. Stress sensitization of ethanol withdrawal-induced reduction in social interaction: inhibition by CRF-1 and benzodiazepine receptor antagonists and a 5-HT1A-receptor agonist. Neuropsychopharmacol. 2004;29:470–482. doi: 10.1038/sj.npp.1300282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese GR, Overstreet DH, Knapp DJ, Navarro M. Prior multiple ethanol withdrawals enhance stress-induced anxiety-like behavior: inhibition by CRF1- and benzodiazepine-receptor antagonists and a 5-HT1a-receptor agonist. Neuropsychopharmacol. 2005;30:1662–1669. doi: 10.1038/sj.npp.1300706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagetti E, Baicy KJ, Olsen RW. Topiramate attenuates withdrawal signs after chronic intermittent ethanol in rats. Neuroreport. 2004;15:207–210. doi: 10.1097/00001756-200401190-00040. [DOI] [PubMed] [Google Scholar]
- Clemmesen L, Hemmingsen R. Physical dependence on ethanol during multiple intoxication and withdrawal episodes in the rat: evidence of a potentiation. Acta Pharmacol Toxicol. 1984;55:345–350. doi: 10.1111/j.1600-0773.1984.tb01993.x. [DOI] [PubMed] [Google Scholar]
- Crews FT, Braun CJ, Hoplight B, Switzer RC, 3rd, Knapp DJ. Binge ethanol consumption causes differential brain damage in young adolescent rats compared with adult rats. Alcohol Clin Exp Res. 2000;24:1712–1723. [PubMed] [Google Scholar]
- Drake RG, Davis LL, Cates ME, Jewell ME, Ambrose SM, Lowe JS. Baclofen treatment for chronic posttraumatic stress disorder. Ann Pharmacother. 2003;37:1177–1181. doi: 10.1345/aph.1C465. [DOI] [PubMed] [Google Scholar]
- Frye GD, Chapin RE, Vogel RA, Mailman RB, Kilts CD, Mueller RA, Breese GR. Effects of acute and chronic 1,3-butanediol treatment on central nervous system function: a comparison with ethanol. J Pharmacol Exp Therapeutics. 1981;216:306–314. [PubMed] [Google Scholar]
- Funk CK, Zorrilla EP, Lee M-J, Rice KC, Koob GF. Corticotropin-releasing factor 1 antagonists selectively reduce ethanol self-administration in ethanol-dependent rats. Biol Psychiatry. 2007;61:78–86. doi: 10.1016/j.biopsych.2006.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehlert DR, Cippitelli A, Thorsell A, Lê AD, Hipskind PA, Hamdouchi C, Lu J, Hembre EJ, Cramer J, Song M, McKinzie D, Morin M, Ciccocioppo R, Heilig M. 3-(4-Chloro-2-morpholin-4-yl-thiazol-5-yl)-8-(1-ethylpropyl)-2,6-dimethyl-imidazo[1,2-b] pyridazine: a novel brain-penetrant, orally available corticotropin-releasing factor receptor 1 antagonist with efficacy in animal models of alcoholism. J Neurosci. 2007;27:2718–2726. doi: 10.1523/JNEUROSCI.4985-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Richardson HN, Lumeng L, Koob GF. Dependence-induced alcohol drinking by alcohol-preferring (P) rats and outbred Wistar rats. Alcohol Clin Exp Res. 2008a;32:1688–1696. doi: 10.1111/j.1530-0277.2008.00678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Richardson HN, Cole M, Koob GF. Vapor inhalation of alcohol in rats. Curr Protocol Neurosci. 2008b;44:9.29.1–9.29.19. doi: 10.1002/0471142301.ns0929s44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Stewart RB, Badia-Elder NE. Neuropeptide Y suppresses ethanol drinking in ethanol-abstinent, but not non-ethanol-abstinent. Wistar rats. Alcohol. 2008c;42:541–551. doi: 10.1016/j.alcohol.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin WC, III, Lopez MF, Yanke AB, Middaugh LD, Becker HC. Repeated cycles of chronic intermittent ethanol exposure in mice increases voluntary ethanol drinking and ethanol concentrations in the nucleus accumbens. Psychopharmacol. 2009;201:569–580. doi: 10.1007/s00213-008-1324-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isbell H, Fraser H, Wikler A, Belleville R, Eisenman A. An experimental study of the etiology of rum fits and delirium tremens. Quarterly J Stud Alcohol. 1955;16:1–33. [PubMed] [Google Scholar]
- Knapp DJ, Overstreet DH, Breese GR. Baclofen blocks expression and sensitization of anxiety-like behavior in an animal model of repeated stress and ethanol withdrawal. Alcohol Clin Exp Res. 2007;31:582–595. doi: 10.1111/j.1530-0277.2007.00342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokka N, Sapp DW, Taylor AM, Olsen RW. The kindling model of alcohol dependence: similar persistent reduction in seizure threshold to pentylenetetrazol in animals receiving chronic ethanol or chronic pentylenetetrazol. Alcohol Clin Exp Res. 1993;17:525–531. doi: 10.1111/j.1530-0277.1993.tb00793.x. [DOI] [PubMed] [Google Scholar]
- Le Magnen J, Tran G, Durlach J, Martin C. Dose-dependent suppression of the high alcohol intake of chronically intoxicated rats by Ca-acetyl homotaurinate. Alcohol. 1987;4:97–102. doi: 10.1016/0741-8329(87)90005-x. [DOI] [PubMed] [Google Scholar]
- Lieber CS, DeCarli LM. Ethanol dependence and tolerance: A nutritionally controlled experimental model in the rat. Res Comm Chem Pathol Pharmacol. 1973;6:983–991. [PubMed] [Google Scholar]
- Majchrowicz E. Induction of physical dependence upon ethanol and the associated behavioral changes in rats. Psychopharmacol. 1975;43:245–254. doi: 10.1007/BF00429258. [DOI] [PubMed] [Google Scholar]
- Mason BJ, Light JM, Williams LD, Drobes DJ. Proof-of-concept human laboratory study for protracted abstinence in alcohol dependence: effects of gabapentin. Addiction Biol. 2009;14:73–83. doi: 10.1111/j.1369-1600.2008.00133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse AC, Koob GF. Intra-BNST acamprosate attenuates withdrawal-induced increases in ethanol consumption in dependent rats. Washington, DC: Abstract Society for Neuroscience; 2002. [Google Scholar]
- O’Dell LE, Roberts AJ, Smith RT, Koob GF. Enhanced alcohol self-administration after intermittent versus continuous alcohol vapor exposure. Alcohol Clin Exp Res. 2004;28:1676–1682. doi: 10.1097/01.alc.0000145781.11923.4e. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Breese GR. Accentuated decrease in social interaction in rats subjected to repeated ethanol withdrawals. Alcohol Clin Exp Res. 2002;26:1259–1268. doi: 10.1097/01.ALC.0000023983.10615.D7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Breese GR. Similar anxiety-like responses in male and female rats exposed to repeated withdrawals from ethanol. Pharmacol Biochem Behav. 2004;78:459–463. doi: 10.1016/j.pbb.2004.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Moy SS, Breese GR. A 5-HT1A agonist and a 5-HT2c antagonist reduce social interaction deficit induced by multiple ethanol withdrawals in rats. Psychopharmacol. 2003;167:344–352. doi: 10.1007/s00213-003-1425-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4th ed. San Diego, CA: Academic Press; 1998. [Google Scholar]
- Richardson HN, Fekete EM, Zhao Y, Funk CK, Zorrilla EP, Koob GF. A novel small molecule antagonist of the corticotropin-releasing factor type 1 receptor (CRF1) is a potent anxiolytic and reduces excessive alcohol intake in dependent male rats. Pharmacol Biochem Behav. 2008a;88:497–510. doi: 10.1016/j.pbb.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson HN, Lee SY, O’Dell LE, Koob GF, Rivier CL. Alcohol selfadministration acutely stimulates the hypothalamic-pituitary-adrenal (HPA) axis, but alcohol dependence leads to a dampened neuroendocrine state. Eur J Neuro. 2008b;28:1641–1653. doi: 10.1111/j.1460-9568.2008.06455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimondini R, Arlinde C, Sommer W, Heilig M. Long-lasting increase in voluntary ethanol consumption and transcriptional regulation in the rat brain after intermittent exposure to alcohol. FASEB J. 2002;16:27–35. doi: 10.1096/fj.01-0593com. [DOI] [PubMed] [Google Scholar]
- Rimondini R, Sommer WH, Dall'Olio R, Heilig M. Long-lasting tolerance to alcohol following a history of dependence. Addict Biol. 2008;13:26–30. doi: 10.1111/j.1369-1600.2007.00079.x. [DOI] [PubMed] [Google Scholar]
- Roberto M, Gilpin NW, O’Dell LE, Cruz MT, Morse AC, Siggins GR, Koob GF. Cellular and behavioral interactions of gabapentin with alcohol dependence. J Neurosci. 2008;28:5762–5771. doi: 10.1523/JNEUROSCI.0575-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AJ, Cole M, Koob GF. Intra-amygdala muscimol decreases operant ethanol self-administration in dependent rats. Alcohol Clin Exp Res. 1996;20:1289–1298. doi: 10.1111/j.1530-0277.1996.tb01125.x. [DOI] [PubMed] [Google Scholar]
- Roberts AJ, Heyser CJ, Cole M, Griffin P, Koob GF. Excessive ethanol drinking following a history of dependence: animal model of allostasis. Neuropsychopharmacol. 2000;22:581–594. doi: 10.1016/S0893-133X(99)00167-0. [DOI] [PubMed] [Google Scholar]
- Rogers J, Wiener SG, Bloom FE. Long-term ethanol administration methods for rats: advantages of inhalation over intubation or liquid diets. Behav Neural Biol. 1979;27:466–486. doi: 10.1016/s0163-1047(79)92061-2. [DOI] [PubMed] [Google Scholar]
- Smith AD, Justice JB. The effect of inhibition of synthesis, release, metabolism and uptake on the in vivo recovery of dopamine using quantitative microdialysis. J Neurosci Methods. 1993;54:75–82. doi: 10.1016/0165-0270(94)90161-9. [DOI] [PubMed] [Google Scholar]
- Smolen TN, Smolen A. Blood and brain ethanol concentrations during absorption and distribution in long-sleep and short-sleep mice. Alcohol. 1989;6:33–38. doi: 10.1016/0741-8329(89)90070-0. [DOI] [PubMed] [Google Scholar]
- Valdez GR, Roberts AJ, Chan K, Davis H, Brennan M, Zorrilla EP, Koob GF. Increased ethanol self-administration and anxiety-like behavior during acute withdrawal and protracted abstinence: regulation by corticotropin-releasing factor. Alcohol Clin Exp Res. 2002;26:1494–1501. doi: 10.1097/01.ALC.0000033120.51856.F0. [DOI] [PubMed] [Google Scholar]
- Victor M, Adams RD. The effect of alcohol on the nervous system. Research Publications of the Association for Research in Nervous & Mental Disease. 1953;32:526–573. [PubMed] [Google Scholar]
- Walker BM, Koob GF. The γ-aminobutyric acid-B receptor Agonist baclofen attenuates responding for ethanol in ethanol-dependent rats. Alcohol Clin Exp Res. 2007;31:11–18. doi: 10.1111/j.1530-0277.2006.00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Weiss F, Zorrilla EP. Remission and resurgence of anxiety-like behavior across protracted withdrawal stages in ethanol-dependent rats. Alcohol Clin Exp Res. 2007;31:1505–1515. doi: 10.1111/j.1530-0277.2007.00456.x. [DOI] [PubMed] [Google Scholar]


