Abstract
BACKGROUND
Prenatal alcohol exposure has been consistently linked to neurocognitive deficits and structural brain abnormalities in affected individuals. Structural brain abnormalities observed in regions supporting spatial working memory (SWM) may contribute to observed deficits in visuospatial functioning in youth with fetal alcohol spectrum disorders (FASDs).
METHODS
We used functional neuroimaging (fMRI) to assess the blood oxygen level dependent (BOLD) response in alcohol-exposed individuals during a spatial working memory (SWM) task. Subjects were 22 youth (aged 10–18) with documented histories of heavy prenatal alcohol exposure (ALC, n=10), and age-, and sex-matched controls (CON, n=12). Subjects performed a SWM task during fMRI that alternated between 2-back location matching (SWM) and simple attention (vigilance) conditions.
RESULTS
Groups did not differ on task accuracy or reaction time to the SWM condition, although CON subjects had faster reaction times during the vigilance condition (617ms vs. 684ms, p=. 03). Both groups showed similar overall patterns of activation to the SWM condition in expected regions encompassing bilateral dorsolateral prefrontal lobes and parietal areas. However, ALC subjects showed greater BOLD response to the demands of the SWM relative to the vigilance condition in frontal, insular, superior and middle temporal, occipital, and subcortical regions. CON youth evidenced less increased brain activation to the SWM relative to the vigilance task in these areas (p<.05, clusters > 1,664 µl). These differences remained significant after including Full Scale IQ as a covariate. Similar qualitative results were obtained after subjects taking stimulant medication were excluded from the analysis.
CONCLUSIONS
In the context of equivalent performance to a SWM task, the current results suggest that widespread increases in BOLD response in youth with FASDs could either indicate decreased efficiency of relevant brain networks, or serve as a compensatory mechanism for deficiency at neural and/or cognitive levels. In context of existing fMRI evidence of heightened prefrontal activation in response to verbal working memory and inhibition demands, the present findings may indicate that frontal structures are taxed to a greater degree during cognitive demands in individuals with FASDs.
The negative effects of prenatal alcohol exposure on the developing embryo and fetus are well documented and include a wide range of physical anomalies and neurocognitive deficits. The diagnosis of fetal alcohol syndrome (FAS) requires a triad of characteristics: (1) pre- or post-natal growth deficiency, (2) cranio-facial abnormalities (e.g. indistinct philtrum, thin upper vermillion, small palpebral fissures) and (3) evidence of central nervous system (CNS) dysfunction (Jones and Smith, 1973; Jones et al., 1973). While FAS is considered to be at the most severe end of the outcome spectrum, it is recognized that prenatal alcohol exposure is associated with cognitive and behavioral deficits even in the absence of the facial features and growth deficiency required to make a diagnosis of FAS. Consequently, the National Task Force on Fetal Alcohol Syndrome and Fetal Alcohol Effects has adopted the non-diagnostic umbrella term Fetal Alcohol Spectrum Disorders (FASDs) to describe the range of effects resulting from gestational alcohol exposure (Bertrand et al., 2005). The incidence of FASDs has been estimated at 10 per 1,000 live births (May and Gossage, 2001) and impairments in general intelligence, attention, learning, memory, language, and several related domains have been demonstrated in such individuals (Mattson and Riley, 1998; Vaurio et al., 2008).
Neuropsychological studies have suggested that individuals with FASDs have visuospatial cognitive deficits (Coles et al., 1991; Hamilton et al., 2003; Kaemingk et al., 2002; Mattson and Riley, 1998; Mattson et al., 1996; Olson et al., 1998; Streissguth et al., 1989; Uecker and Nadel, 1996; Willford et al., 2004) and exhibit poor working memory abilities (Jacobson et al., 1998; Olson et al., 1998; Streissguth et al., 1990). These reports demonstrated impaired learning and recall for both simple and complex figures (Mattson and Roebuck, 2002), locations (Hamilton et al., 2003; Kaemingk et al., 2002; Uecker and Nadel, 1996) and digit span backwards (Jacobson et al., 1998; Olson et al., 1998; Streissguth et al., 1989). Evidence of poor visuospatial cognition and working memory suggests that spatial working memory (SWM), or the maintenance of the spatial location of a remembered visual stimulus (Constantinidis and Wang, 2004), may also be impaired in individuals with prenatal alcohol exposure.
Structural brain abnormalities observed in regions supporting SWM may contribute to observed deficits in visuospatial functioning in youth with FASDs. For example, alterations in the size and structure of frontal, parietal, and temporal cortices have been reported (Archibald et al., 2001; Sowell et al., 2001b; Sowell et al., 2002a; Sowell et al., 2002b). Disproportionate reduction in white matter relative to gray matter volumes in the parietal regions (Archibald et al., 2001), increased gray matter density in lateral temporal and inferior parietal lobes (Archibald et al., 2001; Sowell et al., 2001b; Sowell et al., 2002a), and abnormal glial cell metabolism (Fagerlund et al., 2006) suggest that white matter may be particularly vulnerable to alcohol-induced pathology. Observed increases in cortical thickness, that may reflect an abnormality in the process of white matter deposition or synaptic pruning (Sowell et al., 2008a), and reduced fiber integrity in the corpus callosum (Ma et al., 2005; Wozniak et al., 2006) suggest that microstructural abnormalities may contribute to poorer cognitive abilities in many alcohol-exposed individuals (Fryer et al., 2008; Sowell et al., 2008a). Thus, altered white matter integrity in regions important for SWM may contribute to the behavioral deficits observed in this population.
Patterns of brain response to cognitive demands in youth with FASDs may elucidate the neural mechanisms (perhaps stemming from structural abnormalities) of cognitive deficits. Functional neuroimaging (fMRI) studies have revealed altered patterns of BOLD response in alcohol-exposed individuals during cognitive task performance. In controlled comparisons, greater blood oxygen level dependent (BOLD) response during inhibition and verbal learning has been observed in prefrontal regions of youth with FASDs (Sowell et al., 2007a), while reduced response has been demonstrated in the right caudate during inhibition (Fryer et al., 2007) and in medial and posterior temporal cortices during verbal encoding and retrieval (Sowell et al., 2007). A previous fMRI study of BOLD response during a SWM task reported increased functional activity of inferior middle frontal brain regions in children and adults with FASDs, but relatively decreased brain response in superior frontal and superior parietal areas (Malisza et al., 2005). Due to the very small body of functional neuroimaging evidence in alcohol-exposed populations and limited overlap of the cognitive domains sampled, the neural mechanisms of cognitive deficits in this population remain speculative.
In light of alcohol-exposed individuals’ documented deficits in the component processes of SWM and evidence of structural and functional abnormalities in brain regions underlying SWM, we examined functional brain response to SWM demands in youth with FASDs. In the current study, age- and sex-matched youths performed a task that alternated between 2-back location matching (SWM) and baseline (vigilance) conditions during fMRI. Despite differences in task demands, greater prefrontal brain activation has been reported across the three previous fMRI studies of youth with FASDs (Fryer et al., 2007b; Malisza et al., 2005; Sowell et al., 2007). Therefore, we anticipated that alcohol-exposed individuals would have comparatively increased BOLD response in prefrontal brain regions during SWM relative to vigilance conditions. Any observed group differences in BOLD response during SWM might provide insight into the underpinnings of impaired cognitive function in youth with FASDs.
METHODS
Subjects
Subjects (Table 1) were 10 children and adolescents with heavy prenatal alcohol exposure (ALC) and 12 typically developing peers (CON); they were between 10- and 18-years-old, right-handed, and fluent in English. Subjects were excluded for history of head trauma, contraindication for MRI scanning, claustrophobia, serious medical conditions, or sensory problems that would interfere with the scanning procedure. Thirty-two percent of youth screened for the study were excluded, typically for MRI contraindications (e.g., orthodontic braces). On the day of the scan 7 of 10 ALC and 5 of 12 CON subjects were taking prescription medications. In the ALC group, 3 of 7 medicated subjects were taking CNS stimulants (i.e., Adderall, Concerta); other medications included Neurontin, Seroquel, Clonidine, Risperdal, Wellbutrin, Desipramine, Lithium, Abilify, Trazodone, Zoloft, Depakote, Cogentin, Strattera and Propanolol. One of the three unmedicated ALC subjects had discontinued use of Depakote ten days prior to the scan and the other two ALC subjects had no prescriptions. In the CON group, one subject was taking a stimulant (i.e., Ritalin), and the 4 other medicated youth were taking asthma and acne medications including Albuterol, Tetracycline, Doryx, and Accutane.
Table 1.
Demographics and intelligence scores for children with heavy prenatal alcohol exposure (ALC) and controls (CON).
| N(%) or mean ± SD | Control (n = 12) | ALC (n = 10) | Statistical test |
|---|---|---|---|
| Age at MRI scan | 13.6 ± 2.6 | 14.7 ± 1.9 | F=1.25, p = .28 |
| Male | 5 (42%) | 6 (60%) | χ2=. 73, p = .39 |
| Female | 7 (58%) | 4 (40%) | |
| Caucasian | 5 (42%) | 7 (70%) | χ2=1.76, p = 18 |
| Non-Caucasian | 7 (58%) | 3 (30%) | |
| Full Scale IQ (WISC-III) | 106 ± 15 | 87 ± 12 | F=11.11, p = .003 |
| Hollingshead SES scores | 52 ± 13 | 49 ± 5 | F=. 42, p = .52 |
Subjects were drawn from a larger multidisciplinary project assessing children and adolescents with histories of prenatal alcohol exposure at the Center for Behavioral Teratology (CBT), San Diego State University (SDSU). A detailed description of recruitment procedures can be found in earlier reports (Mattson et al., 2006). As part of this larger study, all subjects had recently undergone neuropsychological testing from which full-scale intelligence quotients (FSIQ) were collected using the Wechsler Intelligence Scale for Children – third edition (WISC-3) (Wechsler, 1991).
All individuals in the ALC group had documented histories of heavy prenatal alcohol exposure, and all but one of them were evaluated by Dr. Kenneth Lyon Jones, a dysmorphologist specializing in alcohol teratogenesis. The group included six individuals who met the full criteria for FAS, three individuals who did not meet full criteria for FAS but demonstrated some physical characteristics consistent with prenatal alcohol exposure, and one individual with a documented history of heavy prenatal alcohol exposure who had not yet had an evaluation with Dr. Jones. Subjects in both groups were matched for age, socioeconomic status, race, and sex. Estimates of FSIQ ranged from 61 to 128, and alcohol-exposed subjects were not excluded for psychiatric conditions or low IQ, as deficits in general intelligence (Aragon et al., 2008) and increased rates of psychopathology (Fryer et al., 2007a) are common in individuals with prenatal alcohol exposure.
Research protocols were approved by the Institutional Review Boards of SDSU and the University of California, San Diego (UCSD). Prior to participation, written informed parental consent and child assent were obtained. Subjects were compensated $50 for participation.
Procedures
Eligible subjects completed a 1-hour scan session during which structural and functional neuroimaging data were collected. Before the scan, directions were given in verbal and pictorial form, and pre-scan practice data was collected under administrator supervision. Images were acquired on a 3.0 Tesla General Electric Signa scanner at the UCSD Center for Functional Magnetic Resonance Imaging. A high-resolution structural image (TR = 8000 ms, TE = 3.1 ms, flip angle = 12 degrees, 256×192 matrix, 1-mm slice thickness, field of view = 24 cm, acquisition time = 7 minutes, 24 seconds) was collected in the sagittal plane for co-registration of functional data. Functional imaging was collected in the axial plane using echo planar imaging (TR = 3000 ms, TE = 32 ms, flip angle = 90 degrees, in-plane resolution = 3.43 mm × 3.43 mm, 4 mm slice thickness × 30 slices for whole brain coverage, 24 cm field of view, 156 repetitions, and acquisition time = 7 minutes, 48 seconds). The SWM task was administered last as part of the larger scan protocol. Subjects watched an animated movie during scans that did not require their response.
Subjects were placed comfortably in the scanner gurney with their heads positioned in an 8-channel head coil, stabilized with padding, and taped across the forehead to minimize motion. The task was administered from a laptop projected to a screen in the scan room near the foot of the scanner bed. Subjects viewed task stimuli through a mirror mounted on the head coil. The scan operator localized the head position, ensured the participant had full view of the display, provided short reminders of task instructions prior to their administration, and asked the participant to test the four-button fiber optic response box in their right hand.
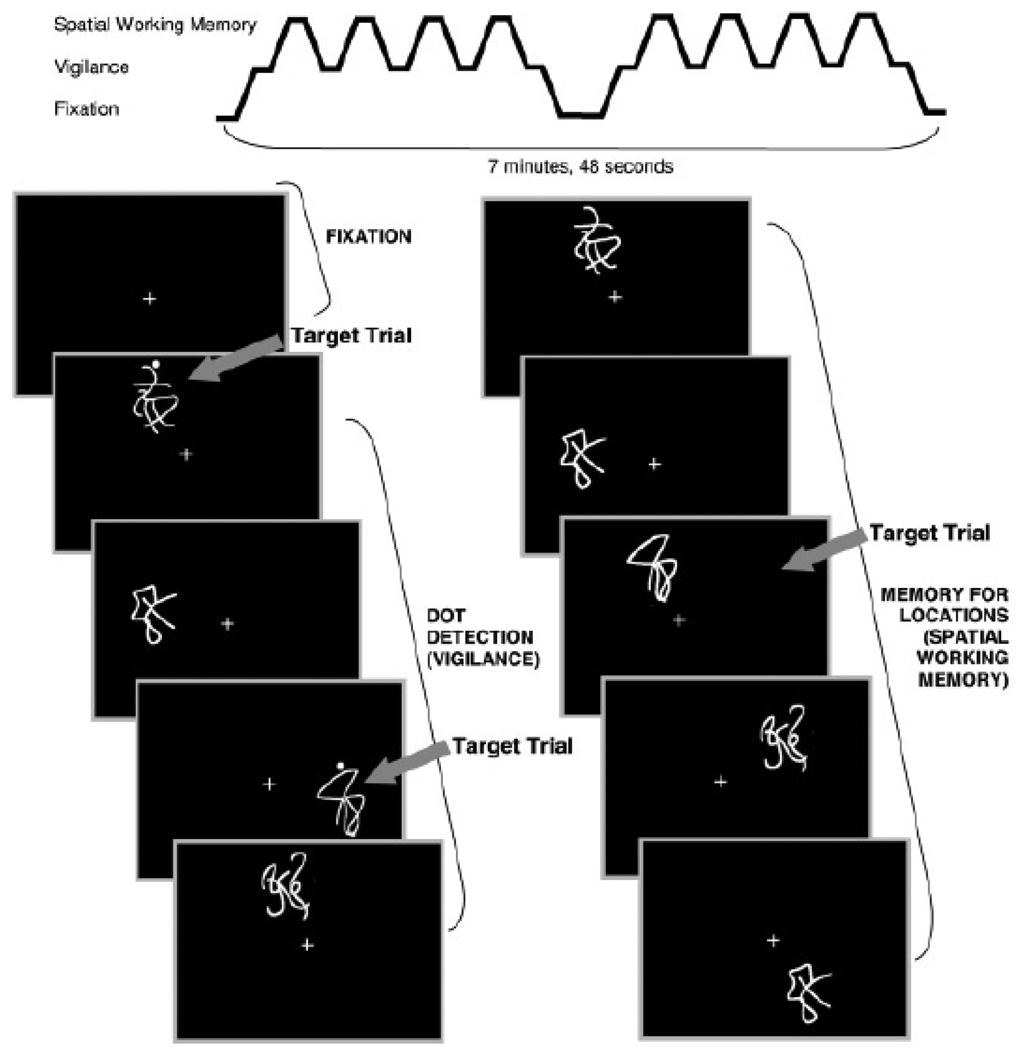
The SWM task (Kindermann et al., 2004; Tapert et al., 2001), adapted from McCarthy and colleagues (1994), was chosen to assess the integrity of brain regions subserving SWM. While we hoped to highlight relationships between brain activation and behavioral response, it was most important that the groups performed similarly on the task to facilitate BOLD comparison. Therefore, the restricted range of acceptable performances (69% to 100% total accuracy) somewhat limits the descriptive utility of behavioral performance. The task consisted of eighteen 20-sec blocks alternating between experimental (SWM) and baseline (vigilance) conditions (see Figure 1). Blocks of rest, during which a fixation cross appeared at the center of the screen, were interspersed at the beginning, middle, and end of the task. In the SWM condition, figures appeared serially in one of eight possible locations. Stimuli consisted of abstract line drawings at irregular angles from the center to minimize verbal memorization strategies. Subjects were asked to press a button with the index finger of their dominant hand when a design appeared in a location more than once during the block. Approximately 30% of the 10 total stimuli in each block were repeat locations of stimuli presented 2 prior (2-back). In the vigilance condition, the same stimuli were presented in the same manner as in the SWM condition (1 of 8 of the serial positions), but subjects were directed to press the button only when a figure with a dot appeared (30% of trials within that block). Stimuli were each presented for 1000 ms with 1000ms interstimulus intervals (20 sec/block, repetition time = 3000 ms, 156 repetitions). The vigilance condition was administered to control for simple motor and attention processes required by the experimental condition. Accuracy and response times from both conditions were recorded via a fiber-optic response system (LumiTouch response system; Vancouver, BC).
Figure 1.
Spatial working memory task stimuli
Data Analysis
Analysts blind to group classification processed and analyzed data using Analysis of Functional NeuroImages (AFNI; afni.nimh.nih.gov (Cox, 1996). Motion in the time series data was corrected by registering each acquisition to a representative repetition with an iterated least squares algorithm (Cox and Jesmanowicz, 1999) to estimate three rotational (roll, pitch, yaw) and three displacement (superior-to-inferior, left-to-right, posterior-to-anterior) parameters for each participant. Trained raters examined the time series data and removed repetitions that still contained excessive head motions. Subjects were excluded if > 20% of repetitions were removed (prenatal alcohol exposure (ALC=1), typically developing peers (CON=1) not described in this study). Of those subjects who met the criterion for inclusion, an average 6% of repetitions were removed due to movement. Time series data were deconvolved with a reference vector coding the alternating task conditions modeling typical delays in the hemodynamic response (Bandettini et al., 1993; Boynton et al., 1996) while covarying for linear trends and the degree of motion correction applied to control for spin history effects (Bandettini et al., 1993). This resulted in a fit coefficient for each voxel that represented BOLD response to SWM relative to the vigilance task condition. Functional data were transformed into standard space (Lancaster et al., 2000; Talairach and Tournoux, 1988), resampled into 4.0 mm3 voxels, and a spatial smoothing Gaussian filter (full-width half maximum = 5.0 mm) was applied. Only activations that consisted of at least 26 contiguous significantly (a=.05) activated voxels (1,664 µl) were interpreted to control for Type-1 error (Forman et al., 1995).
To determine if motion during the task differed between groups, each participant’s absolute mean for each of the six motion parameters (e.g., roll, pitch, and yaw rotations; superior, left, and posterior displacements) across the time series data were evaluated using non-parametric statistics. For estimating task-correlated motion, the six parameters were correlated with the task reference vector for each participant, and resulting values were compared between groups using non-parametric Mann-Whitney tests.
Mean reaction times and accuracy scores to SWM and vigilance conditions were measured. SWM accuracy reflects the percentage of correctly identified repeat locations. Accuracy in the vigilance condition was the total percentage of trials that were correctly identified as having a dot above the stimulus figure. Accuracy and reaction times were compared between groups using analysis of variance (ANOVA). To decrease the possibility that the results would be confounded by inattention or disengagement from the task, subjects were excluded from all subsequent analyses if they either scored below 69% on accuracy on SWM or vigilance tasks (total, first or second half means), or indicated during the post-scan questionnaire administration that he or she did not understand the purpose of the task (2 ALC). All subjects’ reaction times for each task (total, first and second half means) fell within 3SD of the mean, suggesting reliable responding. Subjects included in all further analyses were 10 children and adolescents with documented prenatal alcohol exposure (ALC) and 12 typically developing peers (CON) (Table 2). Of those included in the final sample, three subjects’ behavioral data was lost due to recording device failure (2 ALC, 1 CON). An additional two CON subjects’ accuracy scores were lost and replaced with practice data collected on the identical task during the training session just prior to the scan.
Table 2.
SWM Task Performance in children with heavy prenatal alcohol exposure (ALC) and controls. Data are presented as mean +/− standard deviation.
| Variable | Control (n = 12) | ALC (n = 10) | Statistical Test |
|---|---|---|---|
| Vigilance accuracy (%) | 93% ± 8 | 90% ± 11 | F=. 91; p=.35 |
| SWM accuracy (%) | 87% ± 10 | 84% ± 9 | F=. 41; p=.53 |
| Vigilance reaction time (ms)* | 617ms ± 47 | 684ms ± 73 | F=5.97; p=.03 |
| SWM reaction time (ms) | 569ms± 67 | 561ms± 125 | F=.03; p=.87 |
p ≡ .03
RESULTS
Behavioral Analyses
A one-way, between-subjects ANOVA found no significant differences due to group membership on mean task accuracy for the SWM or vigilance conditions; reaction times for the SWM condition were also statistically equal. Mean reaction time for the vigilance condition was slightly faster in CON subjects (Table 1). To increase the sensitivity of the behavioral analysis we also analyzed first and second half task performances. We hoped to identify individuals whose attention may have waned over one half of the task (e.g., fatigue) or subjects who perhaps improved with increasing numbers of trials. Therefore, we attempted to obtain information about cognitive style and to exclude subjects whose first or second half performances were deficient. First and second half of SWM and vigilance task accuracy and reaction times were statistically equal between groups. Average vigilance reaction times were slower for ALC individuals on both first and second halves of the task (p=.03).
For both groups, decreasing FSIQ was significantly inversely correlated to reaction times on the second half of the vigilance condition (r=−.54, p=.02) and directly correlated to accuracy on the first half of the vigilance condition (r=.49, p=.03). However, FSIQ was not significantly correlated to total mean reaction times or accuracy scores across subjects and did not mediate the effect of group on second half response times to the vigilance condition (ps>.05) (e.g., the relationship between first half accuracy scores on the vigilance condition and group reached near significance after controlling for FSIQ, p=.07).
Motion
To ensure that movement during the task did not affect results differently for each group, we performed Mann-Whitney tests comparing average movement in each direction (i.e., roll, pitch, yaw, superior, left, posterior) during the task and total number of repetitions removed between groups. The number of repetitions removed due to movement did not differ between groups (mean totals included were ALC=148.9±2.9, CON=144.4±2.7). Comparisons for bulk motion were also non-significant between groups (ps=0.3–0.7). Task-correlated motion values were also statistically comparable (ps≥.05). Motion corrections related to roll, pitch, yaw, superior-to-inferior, left-to-right, and posterior-to-anterior axis displacement for the CON group were −.06, −.16, .26, −.27, −.05, −.04, and for the ALC group were .07, .19, .20, .33, −.06, −.05. Between-group analyses on task-correlated motion artifact estimates were also non-significant (ps>.2), suggesting that movement artifact is not a significant concern for interpreting the data described in this study.
BOLD response
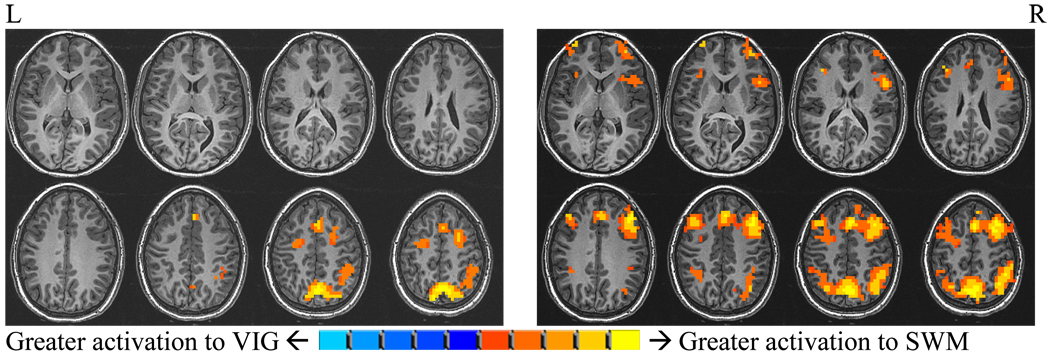
Single sample t-tests (1164µl, α=.05) examined SWM relative to vigilance response within ALC and CON groups and characterized each groups’ general pattern of activation (Figure 2). Each group demonstrated the expected pattern of dorsolateral fronto-parietal activation associated with the task in typically developing individuals (Nelson et al., 2000; Thomas et al., 1999). However, differences in the extent of activation were evident upon qualitative comparison, where ALC subjects had relatively greater activation within the expected regions, in addition to increased activation in less expected subcortical regions. Closer examination of these single-sample t-tests (i.e., unthresholded comparisons) indicated that group differences were primarily due to CON subjects’ relatively greater activation to the vigilance condition and ALC subjects’ greater response to the SWM task. Additional single-sample t-tests were conducted in ALC and CON groups for SWM and vigilance relative to fixation conditions (1164µl, α=.05). These comparisons confirmed greater BOLD activation in ALC subjects in response to both SWM and vigilance conditions across widespread brain regions.
Figure 2.
Within group t-tests (1,664µl, α=.05) demonstrate expected dorsolateral frontal parietal activation patterns to SWM relative to vigilance in both groups. ALC group (right) has relatively greater activation within the expected regions of activation as well as in subcortical regions than CON group (pictured left).
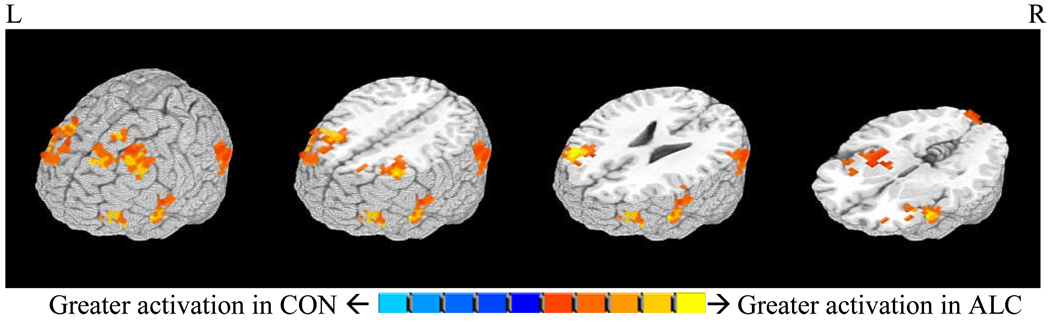
Independent samples t-tests were conducted to compare BOLD response contrast between groups on SWM relative to vigilance conditions to assess for statistically significant differences in BOLD response during the SWM condition. ALC individuals demonstrated significantly greater BOLD activation during SWM blocks relative to vigilance blocks compared to CON subjects across widespread cortical regions including bilateral middle, superior and inferior frontal gyri, left and right superior temporal gyri, left parahippocampal gyrus, right supramarginal, middle temporal, and precentral gyri, and left lingual gyrus and cuneus. Increased activation to the SWM relative to the vigilance condition was also observed in youth with FASDs in bilateral insulae, claustra, globus pallidum, and putamina (Table 3, Figure 3). We then assessed whether these differences in activation were secondary to the discrepancy across groups in FSIQ. While we found that increasing FSIQ was negatively related to each of the reported 6 clusters of brain activation (ps ≤ .10), when the clusters were regressed onto diagnosis, the addition of FSIQ to the model did not contribute a significant amount of variance to any relationship (partial regression coefficients ps>.10) (Baron and Kenny, 1986; Judd and Kenny, 1981). Therefore, FSIQ did not mediate differences in BOLD activation to SWM relative to vigilance conditions.
Table 3.
SWM relative to vigilance BOLD response. Cluster volumes (µL), locations (x, y, z), and effect sizes.
| µL | RL | AP | IS | R/L | Gyri and Associated Brodmann Areas | Cohen’s d |
|---|---|---|---|---|---|---|
| 19,968 | 33.4 | −22.7 | 20.6 | L | Middle frontal gyrus/BA 8, 10, superior frontal gyrus/BA 9, superior temporal gyrus/BA 13, parahippocampal gyrus, lentiform nucleus, insula, claustrum, inferior frontal gyrus |
2.67 |
| 11,840 | −27.2 | −20.6 | 45.2 | R | Middle frontal gyrus/ BA 8, 6, medial frontal and superior frontal gyri |
2.27 |
| 8,128 | −45 | −13.1 | 2.6 | R | Insula, lentiform nucleus, claustrum, inferior frontal gyrus/BA 13, 47, superior temporal gyrus and precentral gyrus/BA 44 |
2.13 |
| 3,904 | −52.8 | 55.4 | 18.1 | R | Superior temporal gyrus/BA 39, middle temporal gyrus, supramarginal gyrus/BA 40 |
1.67 |
| 3,264 | −36.9 | −45.9 | 8.6 | R | Middle frontal gyrus/BA 10 and inferior frontal gyrus | 1.82 |
| 1,920 | 12.9 | 93.9 | −7.5 | L | Cuneus, lingual gyrus/BA 18 | 1.88 |
Figure 3.
Regions of SWM BOLD response (i.e., bilateral inferior, middle, and superior frontal gyri, superior temporal and occipital gyri, lentiform nuclei, and insulae) that were significantly greater in the alcohol-exposed (ALC) group compared to control (CON) group in the SWM relative to vigilance comparison (1,664µl, α=.05).
Based on the evidence that central nervous system (CNS) stimulants (e.g., Ritalin) can positively skew BOLD activation (Bush et al., 2008; Shafritz et al., 2004), we conducted a separate comparison excluding the 2 ALC individuals and 1 CON participant who were taking stimulants. Although the total number of subjects for this new comparison was smaller (ALC=8, CON=11), we found strikingly similar results. Alcohol exposed subjects had qualitatively and statistically significantly greater BOLD activation in areas comparable to those demonstrated in the larger group comparison (1164µl, a=.05).
Behavioral Correlates of BOLD Response
Relationships between BOLD response and task performance can help to illustrate the behavioral relevance of observed BOLD response alterations in the ALC group. For instance, relationships between increased SWM accuracy and degree of BOLD response in particular brain regions might underscore areas supporting effective SWM, whereas associations between decreased SWM accuracy and degree of regional BOLD response might indicate less effective patterns of brain activation. Within each group, each participant’s average BOLD activation in the regions of significant difference was correlated with individual SWM and vigilance task accuracy and reaction times. For the CON group, increased BOLD response in the right middle frontal gyrus/ BA 8, 6, medial frontal and superior frontal gyri was related to decreased reaction times in the first half of the vigilance condition (r=− .64, p=.04). There were no significant correlations with performance in the ALC group.
Age, Behavioral Performance, and BOLD Response
As spatial working memory abilities have been demonstrated to improve over the course of development (Klingberg et al., 2002; Kwon et al., 2002; Schweinsburg et al., 2005; Smith et al., 1996; Thomas et al., 1999), we examined the relationship between age and task performance. With increasing age ALC subjects responded faster to the first half of the vigilance condition (r=−.72, p=.05), were more accurate on the second half of the same task (r=.74, p=.04), but less accurate on the second half of the SWM condition (2nd half range= 75 to 93%, r=−.74, p=.04). Age was not significantly correlated with any of the behavioral outcomes in the CON youth or to SWM relative to vigilance BOLD response in either group, although this was not surprising given the restricted range of performance (SWM accuracy SD=10; vigilance accuracy SD=8) and small sample size.
Evaluation of Total Brain Volume
Finally, structural abnormalities in alcohol-exposed individuals may complicate localization of BOLD response, therefore, total brain volume was considered as a potential factor accounting for between group BOLD response differences. In order to address this question, the high resolution anatomical image of each participant was processed with the FSL brain extraction and automated segmentation tools (FMRIB’s Software Library, http://www.frib.ox.ac.uk/fsl/index.html; (Smith, 2002; Zhuang et al., 2005) and whole-brain voxel counts were conducted using AFNI’s 3dROIstats function (Cox, 1996; Cox and Hyde, 1997). A post hoc between-group comparison of total brain volume indicated that brain size did not differ between alcohol-exposed and comparison subjects (F(1,21)=1.06, p = 0.32), suggesting that BOLD response patterns observed in this study are unlikely influenced by between-group differences in total brain volume.
DISCUSSION
This study employed fMRI methodology to compare BOLD response between youth with histories of prenatal alcohol exposure and non-exposed age, sex, and performance-matched controls in response to a SWM paradigm. Alcohol-exposed individuals had lower FSIQ scores (WISC-3), and these scores were related to response latency in the latter half and accuracy on the first portion of the baseline vigilance condition, although group differences were only significant for reaction time. These findings suggest that across the entire sample, individuals with higher FSIQ scores were better able to meet task demands, initially responding more accurately and with increased speed as the task progressed. Interestingly, this relationship with FSIQ was only noted in the control (vigilance) condition and there was no relationship between FSIQ and performance on the SWM condition, highlighting the importance of considering lower order task demands when assessing higher-order cognitive domains. In CON individuals, we also observed that increased BOLD response in the SWM relative to the vigilance condition in the right middle, medial, and superior frontal gyri predicted improved response times during the first half of the “dot-detection” or vigilance condition, suggesting that increased utilization of these regions supported faster response in the context of sustained attention. This relationship was not observed in ALC subjects, and is especially interesting considering prior evidence of hypoactivation of right prefrontal regions during motor-inhibition tasks in youth with ADHD (Rubia et al., 1998; Rubia et al., 2005; Smith et al., 2006). Finally, we did not observe improved SWM performance in relation to increasing age, although this was not surprising given our small sample size and restricted acceptable range of performance.
In terms of BOLD response to SWM, both groups showed similar overall patterns of activation to the SWM task condition in expected regions encompassing bilateral dorsolateral prefrontal lobes and parietal areas (Nelson et al., 2000; Thomas et al., 1999). However, ALC subjects activated more to the SWM relative to the vigilance condition in bilateral inferior, middle, and superior frontal gyri, superior temporal and occipital gyri, lentiform nuclei, and insulae. These results may suggest that increased brain response in prefrontal and subcortical areas was necessary to maintain adequate performance to the SWM condition in alcohol-exposed individuals. Indeed, previous studies of alcohol-exposed individuals have demonstrated differences compared to controls in the size and structure of frontal cortices (Archibald et al., 2001; Sowell et al., 2001b; Sowell et al., 2002a; Sowell et al., 2002b) and subcortical regions (Archibald et al., 2001; Mattson et al., 1994), suggesting that increased BOLD response may represent a compensatory mechanism for altered brain anatomy. However, our groups had no brain volume discrepancies. It also remains unclear as to whether changes in brain structure reliably predict BOLD response in any particular direction (Nagel et al., 2005). Alternatively, heightened activation may be required in the face of challenging SWM task demands. Previous studies suggest that regions of increased BOLD response might reflect greater recruitment of neural resources to compensate for poorer neurocognitive abilities. For example, in a study of typically developing adolescents, poorer neuropsychological performance on tests of working memory (e.g., WAIS-3 Digits backward and Arithmetic) positively predicted brain response to the SWM task used in the present study in many of the brain regions where greater activation was observed in alcohol-exposed subjects (i.e., bilateral inferior frontal gyri, insulae, claustra, superior frontal gyri, and right lingual gyrus (Nagel et al., 2005). While our intentionally easy task did not illustrate relationships between ALC group performance and BOLD response, there is evidence of poorer working memory and visuospatial abilities in youth with FASDs, and these deficits may be associated with increased BOLD activation during SWM.
Examination of neurofunctional development in typically developing youth suggests increased reliance on specialized networks in response to cognitive demands from childhood to adulthood (Casey et al., 2005). Interestingly, ALC subjects demonstrated greater BOLD response in regions outside recognized SWM regions (Ungerleider and Haxby, 1994) (i.e., insulae, temporal and occipital areas, putamina and globus pallidum), suggesting that youth with FASDs may have employed a “non-expert” network of activation in response to SWM. Also, whereas ALC subjects demonstrated greater activation in bilateral insulae, Scherf and colleagues reported decreasing reliance on the insula during a SWM task in adults versus adolescents and children (Scherf et al., 2006). These findings may indicate developmentally immature patterns of BOLD response in youth with FASDs, although future studies are needed to specifically test this hypothesis.
Previous fMRI studies in youth with FASDs also suggest alterations in functional response to cognitive demands. As with the current study, Malizsa and colleagues (2005) also observed greater brain response in ALC subjects relative to CON subjects during a SWM task in inferior frontal regions, although superior frontal and parietal regions demonstrated less response relative to a healthy comparison group in their study. Differences in methods may have contributed differential findings (for a commentary on this study see Bookheimer and Sowell, 2005). Furthermore, both studies detected group differences despite the lack of apparent structural anomalies in ALC subjects. Other fMRI studies examining verbal learning and response inhibition also reported increased BOLD activation in the prefrontal cortex compared to control groups despite comparable task performance (Fryer et al., 2007b; Sowell et al., 2007). Sowell and colleagues (2007a) suggested that alcohol-exposed youth may rely more extensively on prefrontal cortices to carry out the demands of verbal learning (i.e., encoding, retrieval) in the presence of compromised medial temporal regions, where relative decreases in BOLD activation and relative increases in gray matter have been observed. Our group also previously reported heightened BOLD response across frontal regions in conjunction with reduced striatal (caudate) response during a behavioral inhibition task (Fryer et al., 2007b) which could also reflect compensatory activation of frontal regions for deficient caudate function. While differences in task demands and methodologies constrain our ability to draw conclusions across studies, one might theorize that decreased activation in regions important for task demands (i.e., caudate during inhibition, medial temporal lobe during verbal learning and memory) necessitates hyperactivity in prefrontal regions. However, in the current study we did not observe any regions of decreased activation in the ALC group as compared to the CON youth. Alternatively, it may be the case that across verbal working memory, inhibition, and SWM tasks, frontal structures are taxed to a greater degree during cognitive demands (Sowell et al., 2007).
In the context of equivalent performance to a SWM task, the current results suggest that widespread increases in BOLD response in youth with FASDs could indicate either decreased efficiency of relevant brain networks, or compensation for deficiency at neural and/or cognitive levels. We observed greater brain activation in regions outside those “expert” in SWM processing (e.g., insula) in ALC youth, which could be viewed as evidence for less efficient coordination of neural resources (Casey et al., 2005). Also, previous studies have reported that decreased neuropsychological performance positively predicts brain activation to SWM in many of the regions in which we observed increased activation in our ALC group (Nagel et al., 2005), supporting the notion that heightened neural recruitment may be necessary to compensate for poorer neurocognitive abilities. Although our groups performed equally on SWM, the ALC group had lower FSIQ scores, and FSIQ moderated group differences in brain response. Therefore, compromised neuropsychological function may suggest a greater need for neural resources to adequately complete the task. Finally, although our groups were matched on total brain volume, we cannot rule out that microstructural abnormalities in alcohol-exposed individuals (Fryer et al., 2008) and their adverse impact on neurocognitive skills (Sowell et al., 2001a), (O'Hare et al., 2005; Sowell et al., 2008a; Sowell et al., 2008b) may have contributed to differential BOLD activation and neurofunctional changes.
Study Limitations
There are several limitations to the current study. Differences in BOLD activation in alcohol-exposed youth could be interpreted as a difference in either cognitive strategy or processing at the neuronal level (Price and Friston, 1999). Although we attempted to ascertain cognitive strategy using a post-scan questionnaire, the majority of subject responses were too vague to interpret any differences meaningfully. Therefore we cannot rule-out the possibility that the alcohol-exposed youth in our current sample employed different cognitive strategies from CON subjects, resulting in the observed differential BOLD activation.
In addition, small sample size (n=22) restricts the generalizability of these findings and maturational differences among the large age range sampled (10–18 years) may confound detection and interpretation of group differences. Also, we did not match subjects based on FSIQ scores, psychiatric diagnoses, or psychoactive mediations. However, despite between-group differences in intelligence, the BOLD response differences observed in this study were not mediated by FSIQ scores. In terms of medications, a separate comparison excluding subjects taking CNS stimulants confirmed that these results were not simply an artifact of stimulant prescriptions. However, subjects in the ALC group were also taking anti-depressants, anti-convulsants and mood stabilizers. Because of the high incidence of psychiatric comorbidities in individuals with prenatal alcohol exposure (Fryer et al., 2007a), excluding medicated subjects would limit the generalizability of these results. Although asking subjects to abstain from medication prior to scanning would have been an alternative option, we did not believe this was in the best interest of our subjects. Furthermore, we did not want to jeopardize subjects’ ability to tolerate the scanning procedure by removing them from their routine medication. If possible, future studies might consider excluding subjects who are prescribed stimulant medications that may affect baseline performances, and attempt to limit subjects prescribed other psychotropic medications whose effects on brain activation are less well characterized.
Conclusions
Differences in BOLD response to a SWM task, in which alcohol-exposed individuals performed as well as the comparison group, were significant above and beyond contributions of age, brain volume, FSIQ, and CNS stimulant medication use. In addition, activation in brain regions outside those typically thought to subserve SWM performance was observed in ALC youth, but not in CON youth. Current findings suggest that increased BOLD response in largely prefrontal and subcortical regions could mark decreased effectiveness of pertinent brain networks or compensation for deficiency at neural and/or cognitive levels. Future studies might employ parametric manipulation of task stimuli and utilize rapid event related designs to discriminate whether individuals with FASDs can compensate at more challenging levels of performance. Future studies might also ascertain whether heightened activation in task relevant areas or activation of less efficient brain networks, or both, result from paradigms with increasing task difficulty.
Acknowledgments
Research funded by NIAAA: R01 AA010417, T32 AA013525, R01 AA010820, U01 AA014834, R01 AA13419 and F31 F31 FAA016727A.
The authors thank Kristina E. Hubbard, Olivia Bjorkquist, and Andria Norman for assistance with subject recruitment and data management.
REFERENCES
- Aragon AS, Coriale G, Fiorentino D, Kalberg WO, Buckley D, Gossage JP, Ceccanti M, Mitchell ER, May PA. Neuropsychological characteristics of Italian children with fetal alcohol spectrum disorders. Alcohol Clin Exp Res. 2008;32(11):1909–1919. doi: 10.1111/j.1530-0277.2008.00775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archibald SL, Fennema-Notestine C, Gamst A, Riley EP, Mattson SN, Jernigan TL. Brain dysmorphology in individuals with severe prenatal alcohol exposure. Dev Med Child Neurol. 2001;43(3):148–154. [PubMed] [Google Scholar]
- Bandettini PA, Jesmanowicz A, Wong EC, Hyde JS. Processing strategies for time-course data sets in functional MRI of the human brain. Magnetic Resonance in Medicine. 1993;30:161–173. doi: 10.1002/mrm.1910300204. [DOI] [PubMed] [Google Scholar]
- Bertrand J, Floyd LL, Weber MK. Guidelines for identifying and referring persons with fetal alcohol syndrome. MMWR Recomm Rep. 2005;54(RR-11):1–14. [PubMed] [Google Scholar]
- Bookheimer SY, Sowell ER. Brain imaging in FAS: commentary on the article by Malisza et al. Pediatr Res. 2005;58(6):1148–1149. doi: 10.1203/01.pdr.0000188720.59781.b3. [DOI] [PubMed] [Google Scholar]
- Boynton GM, Engel SA, Glover GH, Heeger DJ. Linear systems analysis of functional magnetic resonance imaging in human V1. J Neurosci. 1996;16(13):4207–4221. doi: 10.1523/JNEUROSCI.16-13-04207.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Tottenham N, Liston C, Durston S. Imaging the developing brain: what have we learned about cognitive development? Trends Cogn Sci. 2005;9(3):104–110. doi: 10.1016/j.tics.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Coles CD, Brown RT, Smith IE, Platzman KA, Erickson S, Falek A. Effects of prenatal alcohol exposure at school age. I. Physical and cognitive development. Neurotoxicol Teratol. 1991;13(4):357–367. doi: 10.1016/0892-0362(91)90084-a. [DOI] [PubMed] [Google Scholar]
- Constantinidis C, Wang XJ. A neural circuit basis for spatial working memory. Neuroscientist. 2004;10(6):553–565. doi: 10.1177/1073858404268742. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Cox RW, Hyde JS. Software tools for analysis and visualization of fMRI data. NMR Biomed. 1997;10(4–5):171–178. doi: 10.1002/(sici)1099-1492(199706/08)10:4/5<171::aid-nbm453>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Cox RW, Jesmanowicz A. Real-time 3D image registration for functional MRI. Magnetic Resonance in Medicine. 1999;42(6):1014–1018. doi: 10.1002/(sici)1522-2594(199912)42:6<1014::aid-mrm4>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Fagerlund A, Heikkinen S, Autti-Ramo I, Korkman M, Timonen M, Kuusi T, Riley EP, Lundbom N. Brain metabolic alterations in adolescents and young adults with fetal alcohol spectrum disorders. Alcohol Clin Exp Res. 2006;30(12):2097–2104. doi: 10.1111/j.1530-0277.2006.00257.x. [DOI] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magnetic Resonance in Medicine. 1995;33(5):636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Fryer SL, McGee CL, Matt GE, Riley EP, Mattson SN. Evaluation of psychopathological conditions in children with heavy prenatal alcohol exposure. Pediatrics. 2007a;119(3):e733–e741. doi: 10.1542/peds.2006-1606. [DOI] [PubMed] [Google Scholar]
- Fryer SL, Schweinsburg BC, Bjorkquist OA, Frank LR, Mattson SN, Spadoni AD, Riley EP. Characterization of White Matter Microstructure in Fetal Alcohol Spectrum Disorders. Alcohol Clin Exp Res. 2008 doi: 10.1111/j.1530-0277.2008.00864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryer SL, Tapert SF, Mattson SN, Paulus MP, Spadoni AD, Riley EP. Prenatal alcohol exposure affects frontal-striatal BOLD response during inhibitory control. Alcohol Clin Exp Res. 2007b;31(8):1415–1424. doi: 10.1111/j.1530-0277.2007.00443.x. [DOI] [PubMed] [Google Scholar]
- Hamilton DA, Kodituwakku P, Sutherland RJ, Savage DD. Children with Fetal Alcohol Syndrome are impaired at place learning but not cued-navigation in a virtual Morris water task. Behav Brain Res. 2003;143(1):85–94. doi: 10.1016/s0166-4328(03)00028-7. [DOI] [PubMed] [Google Scholar]
- Jacobson JW, Jacobson JJ, Sokol LM, Chiodo RL, Berube S, Narang S. Preliminary evidence of working memory and attention deficits in 7-year-olds prenatally exposed to alcohol. Alcohol Clin Exp Res. 1998;22(61a) [Google Scholar]
- Jones KL, Smith DW. Recognition of the fetal alcohol syndrome in early infancy. Lancet. 1973;2(7836):999–1001. doi: 10.1016/s0140-6736(73)91092-1. [DOI] [PubMed] [Google Scholar]
- Jones KL, Smith DW, Ulleland CN, Streissguth P. Pattern of malformation in offspring of chronic alcoholic mothers. Lancet. 1973;1(7815):1267–1271. doi: 10.1016/s0140-6736(73)91291-9. [DOI] [PubMed] [Google Scholar]
- Kaemingk KL, Mulvaney S, Halverson PT. Learning following prenatal exposure: performance on verbal and visual multitrial tasks. Archives of Clinical Neuropsychology. 2002;18:33–47. [PubMed] [Google Scholar]
- Kindermann SS, Brown GG, Zorrilla LE, Olsen RK, Jeste DV. Spatial working memory among middle-aged and older patients with schizophrenia and volunteers using fMRI. Schizophrenia Research. 2004;68(2–3):203–216. doi: 10.1016/j.schres.2003.08.010. [DOI] [PubMed] [Google Scholar]
- Klingberg T, Forssberg H, Westerberg H. Increased brain activity in frontal and parietal cortex underlies the development of visuospatial working memory capacity during childhood. Journal of Cognitive Neuroscience. 2002;14(1):1–10. doi: 10.1162/089892902317205276. [DOI] [PubMed] [Google Scholar]
- Kwon H, Reiss AL, Menon V. Neural basis of protracted developmental changes in visuo-spatial working memory. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(20):13336–13341. doi: 10.1073/pnas.162486399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, Kochunov PV, Nickerson D, Mikiten SA, Fox PT. Automated Talairach atlas labels for functional brain mapping. Human Brain Mapping. 2000;10(3):120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Coles CD, Lynch ME, Laconte SM, Zurkiya O, Wang D, Hu X. Evaluation of corpus callosum anisotropy in young adults with fetal alcohol syndrome according to diffusion tensor imaging. Alcohol Clin Exp Res. 2005;29(7):1214–1222. doi: 10.1097/01.alc.0000171934.22755.6d. [DOI] [PubMed] [Google Scholar]
- Malisza KL, Allman AA, Shiloff D, Jakobson L, Longstaffe S, Chudley AE. Evaluation of spatial working memory function in children and adults with fetal alcohol spectrum disorders: a functional magnetic resonance imaging study. Pediatr Res. 2005;58(6):1150–1157. doi: 10.1203/01.pdr.0000185479.92484.a1. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Calarco KE, Lang AR. Focused and shifting attention in children with heavy prenatal alcohol exposure. Neuropsychology. 2006;20(3):361–369. doi: 10.1037/0894-4105.20.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson SN, Riley EP. A review of the neurobehavioral deficits in children with fetal alcohol syndrome or prenatal exposure to alcohol. Alcohol Clin Exp Res. 1998;22(2):279–294. doi: 10.1111/j.1530-0277.1998.tb03651.x. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Riley EP, Delis DC, Stern C, Jones KL. Verbal learning and memory in children with fetal alcohol syndrome. Alcohol Clin Exp Res. 1996;20(5):810–816. doi: 10.1111/j.1530-0277.1996.tb05256.x. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Riley EP, Jernigan TL, Garcia A, Kaneko WM, Ehlers CL, Jones KL. A decrease in the size of the basal ganglia following prenatal alcohol exposure: a preliminary report. Neurotoxicol Teratol. 1994;16(3):283–289. doi: 10.1016/0892-0362(94)90050-7. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Roebuck TM. Acquisition and retention of verbal and nonverbal information in children with heavy prenatal alcohol exposure. Alcohol Clin Exp Res. 2002;26(6):875–882. [PubMed] [Google Scholar]
- May PA, Gossage JP. Estimating the prevalence of fetal alcohol syndrome. A summary. Alcohol Res Health. 2001;25(3):159–167. [PMC free article] [PubMed] [Google Scholar]
- Nagel BJ, Barlett VC, Schweinsburg AD, Tapert SF. Neuropsychological predictors of BOLD response during a spatial working memory task in adolescents: what can performance tell us about fMRI response patterns? J Clin Exp Neuropsychol. 2005;27(7):823–839. doi: 10.1080/13803390490919038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson CA, Monk CS, Lin J, Carver LJ, Thomas KM, Truwit CL. Functional neuroanatomy of spatial working memory in children. Dev Psychol. 2000;36(1):109–116. doi: 10.1037//0012-1649.36.1.109. [DOI] [PubMed] [Google Scholar]
- O'Hare ED, Kan E, Yoshii J, Mattson SN, Riley EP, Thompson PM, Toga AW, Sowell ER. Mapping cerebellar vermal morphology and cognitive correlates in prenatal alcohol exposure. Neuroreport. 2005;16(12):1285–1290. doi: 10.1097/01.wnr.0000176515.11723.a2. [DOI] [PubMed] [Google Scholar]
- Olson HC, Feldman JJ, Streissguth AP, Sampson PD, Bookstein FL. Neuropsychological deficits in adolescents with fetal alcohol syndrome: clinical findings. Alcohol Clin Exp Res. 1998;22(9):1998–2012. [PubMed] [Google Scholar]
- Price CJ, Friston KJ. Scanning patients with tasks they can perform. Hum Brain Mapp. 1999;8(2–3):102–108. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<102::AID-HBM6>3.0.CO;2-J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K, Overmeyer S, Taylor E, Brammer M, Williams S, Simmons A, Andrew C, Bullmore E. Prefrontal involvement in "temporal bridging" and timing movement. Neuropsychologia. 1998;36(12):1283–1293. doi: 10.1016/s0028-3932(98)00038-4. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Brammer MJ, Toone B, Taylor E. Abnormal brain activation during inhibition and error detection in medication-naive adolescents with ADHD. Am J Psychiatry. 2005;162(6):1067–1075. doi: 10.1176/appi.ajp.162.6.1067. [DOI] [PubMed] [Google Scholar]
- Scherf KS, Sweeney JA, Luna B. Brain basis of developmental change in visuospatial working memory. J Cogn Neurosci. 2006;18(7):1045–1058. doi: 10.1162/jocn.2006.18.7.1045. [DOI] [PubMed] [Google Scholar]
- Schweinsburg AD, Nagel BJ, Tapert SF. fMRI reveals alteration of spatial working memory networks across adolescence. J Int Neuropsychol Soc. 2005;11(5):631–644. doi: 10.1017/S1355617705050757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AB, Taylor E, Brammer M, Toone B, Rubia K. Task-specific hypoactivation in prefrontal and temporoparietal brain regions during motor inhibition and task switching in medication-naive children and adolescents with attention deficit hyperactivity disorder. Am J Psychiatry. 2006;163(6):1044–1051. doi: 10.1176/ajp.2006.163.6.1044. [DOI] [PubMed] [Google Scholar]
- Smith EE, Jonides J, Koeppe RA. Dissociating verbal and spatial working memory using PET. Cereb Cortex. 1996;6(1):11–20. doi: 10.1093/cercor/6.1.11. [DOI] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17(3):143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Johnson A, Kan E, Lu LH, Van Horn JD, Toga AW, O'Connor MJ, Bookheimer SY. Mapping white matter integrity and neurobehavioral correlates in children with fetal alcohol spectrum disorders. J Neurosci. 2008a;28(6):1313–1319. doi: 10.1523/JNEUROSCI.5067-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Lu LH, O'Hare ED, McCourt ST, Mattson SN, O'Connor MJ, Bookheimer SY. Functional magnetic resonance imaging of verbal learning in children with heavy prenatal alcohol exposure. Neuroreport. 2007;18(7):635–639. doi: 10.1097/WNR.0b013e3280bad8dc. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Mattson SN, Kan E, Thompson PM, Riley EP, Toga AW. Abnormal cortical thickness and brain-behavior correlation patterns in individuals with heavy prenatal alcohol exposure. Cereb Cortex. 2008b;18(1):136–144. doi: 10.1093/cercor/bhm039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Mattson SN, Thompson PM, Jernigan TL, Riley EP, Toga AW. Mapping callosal morphology and cognitive correlates: effects of heavy prenatal alcohol exposure. Neurology. 2001a;57(2):235–244. doi: 10.1212/wnl.57.2.235. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Mattson SN, Tessner KD, Jernigan TL, Riley EP, Toga AW. Voxel-based morphometric analyses of the brain in children and adolescents prenatally exposed to alcohol. Neuroreport. 2001b;12(3):515–523. doi: 10.1097/00001756-200103050-00018. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Mattson SN, Tessner KD, Jernigan TL, Riley EP, Toga AW. Regional brain shape abnormalities persist into adolescence after heavy prenatal alcohol exposure. Cereb Cortex. 2002a;12(8):856–865. doi: 10.1093/cercor/12.8.856. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Peterson BS, Mattson SN, Welcome SE, Henkenius AL, Riley EP, Jernigan TL, Toga AW. Mapping cortical gray matter asymmetry patterns in adolescents with heavy prenatal alcohol exposure. Neuroimage. 2002b;17(4):1807–1819. doi: 10.1006/nimg.2002.1328. [DOI] [PubMed] [Google Scholar]
- Streissguth AP, Barr HM, Sampson PD. Moderate prenatal alcohol exposure: effects on child IQ and learning problems at age 7 1/2 years. Alcohol Clin Exp Res. 1990;14(5):662–669. doi: 10.1111/j.1530-0277.1990.tb01224.x. [DOI] [PubMed] [Google Scholar]
- Streissguth AP, Bookstein FL, Sampson PD, Barr HM. Neurobehavioral effects of prenatal alcohol: part III. PLS analyses of neuropsychologic tests. Neurotoxicology and Teratology. 1989;11:493–507. doi: 10.1016/0892-0362(89)90026-3. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Coplanar stereotaxic atlas of the human brain. Three-dimensional proportional system: An approach to cerebral imaging. New York: Thieme; 1988. [Google Scholar]
- Tapert SF, Brown GG, Kindermann SS, Cheung EH, Frank LR, Brown SA. fMRI measurement of brain dysfunction in alcohol-dependent young women. Alcohol Clin Exp Res. 2001;25(2):236–245. [PubMed] [Google Scholar]
- Thomas KM, King SW, Franzen PL, Welsh TF, Berkowitz AL, Noll DC, Birmaher V, Casey BJ. A developmental functional MRI study of spatial working memory. Neuroimage. 1999;10(3 Pt 1):327–338. doi: 10.1006/nimg.1999.0466. [DOI] [PubMed] [Google Scholar]
- Uecker A, Nadel L. Spatial locations gone awry: object and spatial memory deficits in children with fetal alcohol syndrome. Neuropsychologia. 1996;34(3):209–223. doi: 10.1016/0028-3932(95)00096-8. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG, Haxby JV. 'What' and 'where' in the human brain. Current Opinion in Neurobiology. 1994;4(2):157–165. doi: 10.1016/0959-4388(94)90066-3. [DOI] [PubMed] [Google Scholar]
- Vaurio L, Riley EP, Mattson SN. Differences in executive functioning in children with heavy prenatal alcohol exposure or attention-deficit/hyperactivity disorder. J Int Neuropsychol Soc. 2008;14(1):119–129. doi: 10.1017/S1355617708080144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willford JA, Richardson GA, Leech SL, Day NL. Verbal and visuospatial learning and memory function in children with moderate prenatal alcohol exposure. Alcohol Clin Exp Res. 2004;28(3):497–507. doi: 10.1097/01.alc.0000117868.97486.2d. [DOI] [PubMed] [Google Scholar]
- Wozniak JR, Mueller BA, Chang PN, Muetzel RL, Caros L, Lim KO. Diffusion tensor imaging in children with fetal alcohol spectrum disorders. Alcohol Clin Exp Res. 2006;30(10):1799–1806. doi: 10.1111/j.1530-0277.2006.00213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang J, LaConte S, Peltier S, Zhang K, Hu X. Connectivity exploration with structural equation modeling: an fMRI study of bimanual motor coordination. Neuroimage. 2005;25(2):462–470. doi: 10.1016/j.neuroimage.2004.11.007. [DOI] [PubMed] [Google Scholar]