Abstract
Prenatal exposure to alcohol in humans can result in a wide range of deficits collectively referred to as Fetal Alcohol Spectrum Disorders. Of these deficits, cognitive impairments are among the most debilitating and long-lasting. Specifically, cognitive impairments in executive functioning suggest damage to the prefrontal cortex (PFC). Several external stimuli, such as morphine, chronic stress and maternal stress have been found to alter the dendritic structure of cells within the PFC. In this study, three groups of rat pups were used: intubated with alcohol (5.25g/kg/day; AE), sham intubated (SI), or suckle controls (SC) on PD4-9. On PD26-30 rats were anesthetized, perfused with saline and brains were processed for Golgi-Cox staining. Basilar dendrite complexity, spine density and spine phenotypes were evaluated for Layer II/III neurons in the medial PFC. Results indicate that AE rats have an altered basilar dendritic tree complexity due to a significant decrease in both length and number of intersections in proximity to the neuronal soma. Furthermore, spine density patterns of basilar dendrites remain unchanged while the density of mature versus immature spines significantly changes. These effects were not seen in the apical dendrites, indicating alcohol’s influence on different neuronal parts in a single cell. In addition, these results suggest that the innervations of the soma and basilar dendrites by thalamic projections may play a role. Thus, our data demonstrates that postnatal exposure to alcohol produces changes in the neuronal organization of rat adolescent PFC that may affect the performance on prefrontal-dependant behavioral tasks.
Keywords: pyramidal neurons, Golgi, prefrontal cortex, plasticity, fetal alcohol syndrome
INTRODUCTION
Prenatal alcohol exposure can result in Fetal Alcohol Spectrum Disorders (FASD), which have been diagnosed in nearly 10 out of 10,000 live births each year (Abel, 1998). Patients with FASD exhibit a wide range of effects including physical, cognitive, learning and behavioral disabilities all of which are prominent throughout a lifespan (Calhoun et al., 2006). Gross neuroanatomical effects are evidenced in a relationship between frontal brain size and maternal alcohol consumption as well gray matter asymmetry in the frontal lobe of patients with heavy prenatal alcohol exposure (Wass et al., 2001; Sowell et al., 2002; Rasmussen, 2005). Patients with FASD also show mental effects such as a lack of inhibition, logical reasoning and flexible thinking (Rasmussen, 2005; Connor 2000; Kuboshima-Amemori & Sawaguchi, 2007). Moreover, learning problems, involving working memory and behavioral flexibility, also are apparent (Streissguth et al., 1994; Connor et al, 2000; Rasmussen, 2005). Finally, patients with FASD exhibit behavioral disabilities that are common in patients suffering from frontal lobe damage (Connor et al., 2000) including, but not limited to, executive functioning deficits, such as planning, organized search, inhibition, working memory and flexible thinking (Rasmussen, 2005; Connor, 2000; Welsh & Pennington 1988), indicating the role of the prefrontal cortex in FASD behavioral disabilities.
Of particular interest is the medial PFC (mPFC), which is one of the last brain structures to develop in both humans and rats, yet its development occurs at different time points for each species. In humans, neurogenesis and migration of neocortical neurons occurs during the third trimester of fetal development, with the more superficial layers of the neocortex being formed last (Goldman-Rakic et al, 1983). Moreover, there is a rapid increase in the length of basilar dendrites of future Layer III and Layer V pyramidal neurons during the third trimester, while the number of basilar dendrites per pyramidal neuron appears to stabilize at the onset of the third trimester (Mrzljak et al., 1992). It is also during the third trimester when dendritic spines begin appearing on Layer III and V pyramidal neurons (Mrzljak et al., 1990). Other developmental processes, such as dendritic maturation and synaptogenesis, continue to occur postnatally, with the most rapid changes occurring in the first few years of life. (Goldman-Rakic et al., 1983; Anderson et al, 1995; Huttenlocher and Dabholkar, 1997). In rats, the medial prefrontal cortex (mPFC) consists of the anterior cingulate, prelimbic and infralimbic regions (Jerison, 1997; Uylings et al., 2003; Heidbreder & Groenewegen, 2003). Neurogenesis of the most superficial cortical cells occurs just prior to birth (Bayer and Altman, 2004) with some of the latest neurons still migrating to mature positions during the first few days of postnatal life (Van Eden et al., 1990). Although the process of entering the cortical plate starts just before birth (Kalsbeek et al., 1988), there is an increase in the number of dopaminergic fibers from the mediodorsal nucleus of the thalamus, the major afferent of the PFC, during the first postnatal week (Van Eden et al., 1986) suggesting that significant differentiation and maturation of prefrontal cortex occurs postnatally in the rat brain. These fibers reach and terminate in the developing Layer III and it is suggested that their early presence may play an important role in the laminar development of the prefrontal cortex (Van Eden et al., 1986). This is further supported by evidence of changes in the morphology of dopaminergic fibers beginning around postnatal day 4 (Kalsbeek et al., 1988). Thus, the rat model of third trimester alcohol exposure allows for a more direct examination of alcohol’s effects on the developing frontal cortex because many important stages of development occur after the animal is born.
In the developing cortex, pyramidal neurons are greatly affected by many external influences such as stress (Radley et al., 2006), environmental complexity (Kolb et al., 2003), amphetamines (Crombag et al., 2004) and alcohol exposure (Tu et al., 2008; Whitcher & Klintsova, 2008). Pyramidal neurons vary considerably in dendritic complexity (total length of dendrites, combined with the number of branches and bifurcations) between different layers and cortical regions, suggesting that different structural features may carry different functional loads (Spruston, 2008). In particular, Layer III pyramidal cells in the prefrontal cortex integrate and organize inputs from thalamocortical fibers, originating primarily in the mediodorsal (MD) nucleus (Van Eden, 1986). Furthermore, they influence the axonal competition of glutamatergic neurons originating in the MD nucleus (Wedzony et al, 2005). Thus, changes in dendritic complexity could affect the overall function of pyramidal neurons.
Along with dendritic complexity, dendritic spines in the prefrontal cortex are also greatly influenced by external factorssuch as stress (Radley et al., 2008), dopamine depletion (Wang & Deutch, 2008) and alcohol exposure (Whitcher & Klintsova, 2008). A postnatal-binge like alcohol exposure has been shown to affect spine density without affecting the dendritic morphology of apical dendrites in Layer III pyramidal cells, suggesting that the plasticity of spines on PFC neurons may be most vulnerable to alcohol exposure at this developmental stage (Whitcher & Klintsova, 2008). This harmful effect of alcohol exposure on the highly plastic pyramidal neurons in a still developing cortex is evident in both humans and in rats. In 1987, Ferrer and Galofré described a case study of a 4-month-old male, diagnosed with FAS, who died of pneumonia. His autopsy revealed that there was a decrease in spine density on the apical Layer V pyramidal neurons in his PFC as well as an increase in immature spines. Alcohol exposure in rat pups during postnatal days 2–6 has been shown to affect cortical areas, such as primary somatosensory and motor cortices (Granato et al., 2003). In particular, the postnatal exposure produced a decrease in dendritic length and a reduction in the number of end points in the basilar dendrites of Layers II/III pyramidal neurons (Granato et al., 2003). Thus, of these pyramidal neurons, it is their dendritic trees—in particular their dendritic complexity and their spines—that are the most affected by prenatal alcohol exposure.
The purpose of the current study was to extend the outcomes of our previous study on the persistency of the effects of neonatal exposure to alcohol on dendritic arborization, on spine density and on spine phenotypes in mPFC Layer II/III pyramidal neurons. Based on previous work in our lab, it was hypothesized that neonatal alcohol exposure would affect overall spine density as well as altering the density of mature and immature spines and reduce the complexity of basilar dendrites in Layer II/III pyramidal neurons.
MATERIALS AND METHODS
Subjects
All procedures were done in accordance with the University of Delaware Institutional Animal Care and Use Committee. Long Evans rat litters from timed pregnancies were obtained and litters were culled on postnatal day (PD) 3. Gestational age was used as a reference for the developmental timing of all treatments. Gestational Day (GD) 22 was considered to be the day of birth (PD 0) and thus, PD 4 would be GD 26.
One animal from the sham intubated (SI) group was removed from the data analysis. This was due to a lack of neurons that met criteria. Thus, the final number of animals per condition was suckle control (SC) = 5, SI = 4 and alcohol-exposed (AE) = 6.
Alcohol Exposure
At PD 4, litters were randomly assigned to one of three conditions: SC, SI or AE. SC animals were weighed daily but otherwise left undisturbed. The SC condition received no intubation treatment, as opposed to both the SI and the AE animals. Intragastric intubations occurred from PD4-9 for both the SI and AE groups. SI animals were intubated, on the same schedule as the AE animals, and the tube was removed after 10–15 seconds without the infusion of any solution. AE animals were intubated and given a daily dose of 5.25g/kg of alcohol, which was divided into two intubations each day, two hours apart. A third intubation of milk (no ethanol) was administered two hours after the second alcohol dose. Solely on PD 4, a fourth intubation of milk solution was given four hours after the second alcohol dose in order to compensate for reduced milk intake. All pups were weaned on PD 23 and subsequently housed in social conditions of 3–4 rats of the same sex per cage.
Blood Alcohol Concentrations
Blood samples were collected from a tail clip of each AE pup 90 minutes after the second alcohol intubation on PD 4 in order to determine the blood alcohol concentration (BAC). BACs were assayed from the plasma of each blood sample using an Analox GL5 Alcohol Analyzer (Analox Instruments, Boston, MA).
Tissue Preparation
On PD26-30, rats were deeply anesthetized and transcardially perfused with 0.9% saline. Brains were removed and transferred in Golgi-Cox solution in which they were left, in the dark, for three weeks. After which, they were transferred into 30% sucrose in saline. Two hundred micrometer coronal sections were cut through the entire extent of the PFC and collected in order on gelatinized slides. Slides were then processed as described previously (Gibb and Kolb, 1998; Whitcher & Klintsova, 2008).
Dendrite Analysis
Sholl Analysis
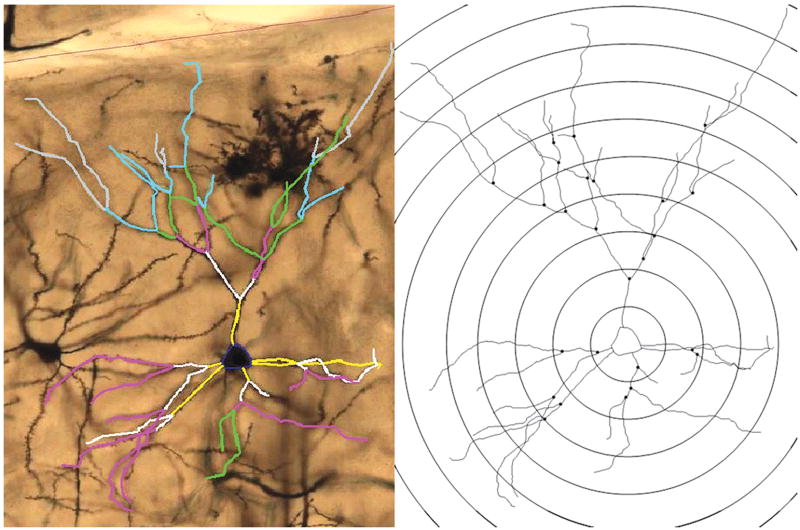
Tissue was coded to ensure that the experimenter remained blind to animal condition throughout the analysis procedure. Analysis of neurons occurred on the section closest to Bregma 3.7 as well as the next seven posterior sections in rostro-caudal direction, a total of eight sections per animal. Medial prefrontal cortex (mPFC) neurons were traced and measured using a computer-based neuron tracing system (NeuroLucida—Version 8.10.1; MBF, Bioscience, Williston, VT). For each section, the mPFC was identified by the experimenter at a low magnification (5× objective) and then outlined on the projected image. Next, the cell bodies of Layer II/III pyramidal neurons were marked and traced at a higher magnification (40× objective). Using Line Measure Tool in Neurolucida software the distance between the traced cell body and cortical surface was determined for every traced neuron (10×). Layer II/III pyramidal neurons were classified as such and included in the analysis if they were located within Zilles Cg1 or Cg3 region, demonstrated the characteristic pyramidal-shaped cell body and if they were located between 200–500 μm from the cortical surface. In addition, the following criteria had to be satisfied: the basilar tree’s branches were contained in the section being observed; branches could not be broken or obscured; the extent of the tree was evenly and fully impregnated, including dendritic spines. Basilar dendrites of each neuron were traced at high magnification (100× oil objective). While tracing the neuron, the software automatically assigned the branching order starting at the first bifurcation of the basilar dendrite. Next, in order to determine the dendritic complexity of the neurons, a Sholl analysis was performed (Figure 1) wherein the mean of all neurons in an individual animal was used to perform statistics. Dendritic complexity can be defined as the total length of dendrites, combined with the number of branches and bifurcations.
Figure 1.
Left: Magnified (20× objective) image and tracing of a Layer II/III pyramidal neuron in mPFC. The tracing demonstrates the designated ordered branches with yellow lines being first order branches, white lines being second order branches, pink lines being third order branches, green lines being fourth order branches, blue lines being fifth order branches and gray lines being sixth order branches. Right: Analysis of a stereotypical pyramidal neuron using the Sholl Analysis. Concentric spheres, which are centered in the neuron’s soma, expand outwards with increasing radii of 20μm. The Sholl Analysis counts the number of intersections with each sphere in order to determine dendritic complexity of an individual neuron.
Spine Density Analysis
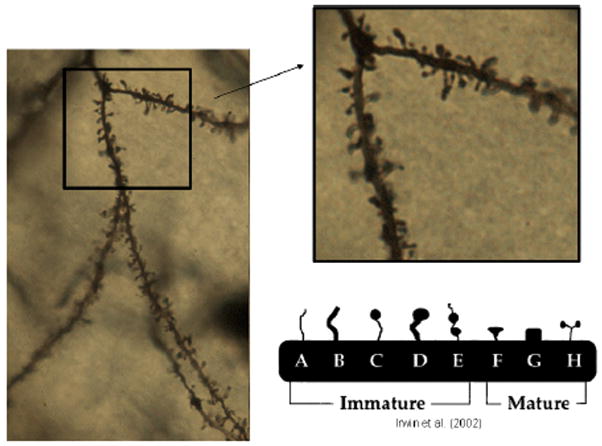
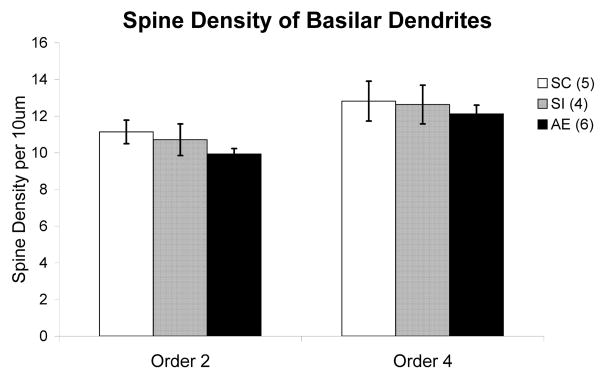
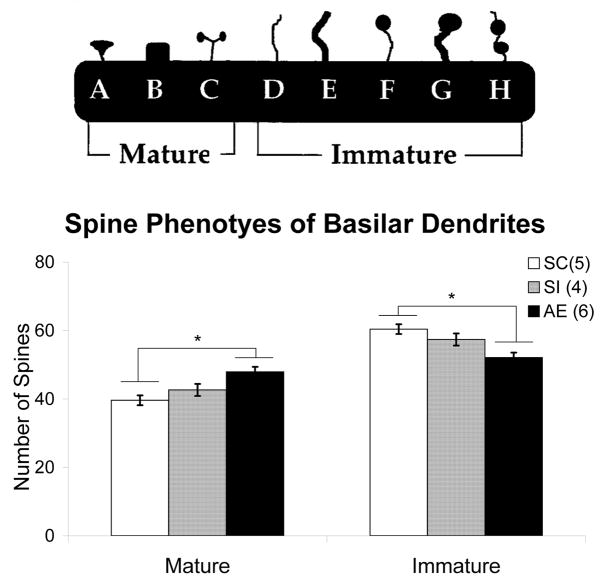
Spine density measurements consisted of marking all of the spines on two randomly selected Order 2 branches (due to their proximity to the soma) as well as two randomly selected Order 4 branches (due to their distal nature from the soma) for each cell.. Only branches over 20μm in length were included in the study. Spine density was calculated per 10μm of dendritic length. Finally, spine phenotyping was done on the first 7–13 spines of each order 2 and order 4 branches for a total of between one hundred to 110 phenotyped spines per animal. Spines were classified, based on the principles outlined in Irwin et al, 2002, as either immature or mature according to their shapes (Figure 2).The total density of mature versus immature spines was then calculated for each animal. In addition, at the site of each spine included in analysis, the dendritic width was measured to ensure that the thickness of dendritic branches did not differ between any of the three animal groups. No significant differences existed between groups in the width of dendrites used for spine phenotypes.
Figure 2.
Left: Magnified (40× objective) image of basilar branches from a Layer II/III pyramidal neuron in the mPFC. The boxed area is then magnified (right) to a 100× objective in order to determine the spine density of a given branch. Furthermore, the higher magnification allows for easier distinction of the different spines shapes (bottom right). Cartoon image is modified from Irwin et al. (2002).
Statistical Analysis
Repeated measures ANOVA was used to compare Sholl analyses between groups, looking in particular at ring × treatment. One-way ANOVA followed by Turkey post hoc test were used to evaluate the effect of postnatal condition on both the basilar dendrites’ spine density in mPFC and the percentage of mature or immature spine phenotypes. One-way ANOVA was also used to evaluate group differences for total dendritic length. The SPSS statistical package was used for all analyses. The level of significance was set at p < 0.05 for all tests.
RESULTS
Blood Alcohol Concentrations
Blood alcohol concentrations (BAC) were measured in samples obtained from each AE animal 1.5 hours after the second ethanol dose on PD4. The average BAC was 333.5 +/− 15.0 mg/dl.
Dendritic Complexity
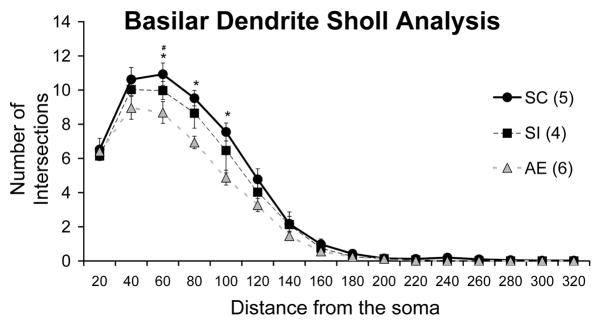
Repeated measures ANOVA was used to compare Sholl analyses between groups, in particular looking at ring (distance from soma) × treatment interaction. Results demonstrate a significant interaction between ring intersection and postnatal treatment (F(5.512, 30)=2.658, p=.036) Furthermore, T-tests for individual intersections found AE animals to have significantly less intersections than SC animals at 60μm (p <.05), at 80μm (p<.01) and at 100μm (p<.01). SI animals also have significantly less intersections than SC animals at 60μm (p<.05) (Figure 3).
Figure 3.
Number of dendritic tree intersections with Sholl radii in each animal group. Repeated Measure ANOVA demonstrated significant interaction between ring intersection and postnatal treatment (F(5.512, 30)=2.658, p=.036). Furthermore, T-tests for individual intersections found alcohol exposed (AE) animals to have significantly less intersections than suckle control (SC) animals at 60μm (p<.05), at 80μm (p<.01) and at 100μm (p<.01). Sham intubated (SI) animals also have significantly less intersections than SC animals at 60μm (p<.05). (AE – alcohol exposed; SI – sham intubated; SC – suckle control). Values indicate means +/− s.e.m.
Dendritic Length
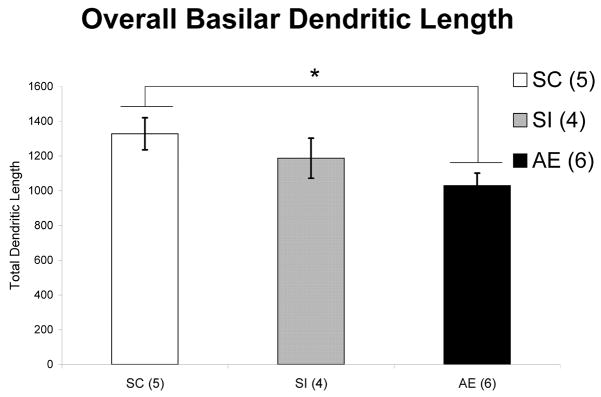
One-way ANOVA was used to analyze total length of all basilar dendrites in Layer II/III neurons. The total length of basilar dendrites in AE animals are significantly shorter than those of SC animals (F(2, 14) = 3.917, p = .049) (Figure 4).
Figure 4.
Total dendritic length per Layer II/III neuron in each animal group. Basilar dendrites of AE animals are significantly shorter than those of SC animals (F(2,14) = 3.917, p = .049). (AE – alcohol exposed; SI – sham intubated; SC – suckle control). Values indicate means + s.e.m.
Dendritic Spine Density
One way ANOVA followed by Turkey post hoc test revealed no significant difference in spine density across conditions in either Order 2 (F(2, 15) = 1.238, p = .322) or Order 4 (F(2,15) = .222, p = .804) branches (Figure 5).
Figure 5.
Spine density on order 2 and order 4 dendritic branches of Layer II/III pyramidal neurons in each animal group. No significant difference in spine density was found across conditions in either Order 2 or Order 4 branches. (AE – alcohol exposed; SI – sham intubated; SC – suckle control). Values indicate means + s.e.m.
Dendritic Spine Phenotypes
One-way ANOVA demonstrated significant differences in the spine phenotypes (Figure 6a) of both Order 2 (F (2, 14) = 8.175, p=.006) and Order 4 (F (2, 14) = 8.044, p=.006) dendritic spines. Turkey post hoc comparison revealed significant differences between the AE and SC animals in both the Order 2 (p=.005) and the Order 4 (p=.005) spines, wherein there was a significant increase in the density of mature spines compared to the percentage of immature spines (Figure 6b). Also, there was no difference between SC and SI in either Order 2 (p=.343) or Order 4 (p=.514).
Figure 6.
(a) Spine phenotype categorization. This scheme was used to categorize each spine (between 100–110 per animal) and assign it either mature or immature status. Cartoon modified from Irwin et al. (2002). (b) Density of mature vs. immature spine phenotypes in each animal group of Order 4 Branches. One-way ANOVA demonstrated significant differences in the spine phenotypes of both Order 2 (F (2, 14)= 8.175, p=.006) and Order 4 (F (2, 14) = 8.044, p=.006) dendrites. The graph represented is for Order 4 dendrites; only one graph is shown as Order 2 branches had almost identical results. Turkey post hoc comparison revealed significant differences between the alcohol exposed (AE) and suckle control (SC) animals in both the Order 2 (p<.01) and the Order 4 (p<.01) spines. Also, there was no difference between SC and sham intubated (SI) in either Order 2 (p=.343) or Order 4 (p=.514). Values indicate means + s.e.m.
DISCUSSION
The results of our study indicate that binge-like exposure to alcohol during a third-trimester equivalent has a long-lasting effect that impacts distinct dendritic parts of individual pyramidal cells in the mPFC differently, and that its effect is evident in adolescent rats. AE animals have significantly shorter dendrites as well as less dendritic branching and bifurcations in basilar dendrites of Layer II/III pyramidal neurons in the mPFC. Furthermore, the ratio of mature versus immature spines in AE animals is significantly higher compared with those of both the SC and SI animals. However, there is no significant evidence that the overall density of dendritic spines, in basilar branches of cortical neurons, is affected by postnatal alcohol exposure.
Few studies demonstrate decreased dendritic complexity resulting from developmental alcohol exposure in the mPFC. Granato and colleagues (Granato et al., 2003) administered ethanol through inhalation to PD2-PD6 neonatal rats, which resulted in a simplification of dendritic branching in mPFC layers II/III neurons, evidenced by a reduction in the number of dendritic end points and a significant decrease in the overall total length of basilar dendrites. Interestingly, an increased complexity in Layer V neurons was also seen in these animals, which could suggest a very specific time window in which ethanol exposure induces damage to specific neuronal regions.
Furthermore, previous work in our lab (Whitcher & Klintsova, 2008) explored the effects of third-trimester equivalent binge-like alcohol exposure on apical dendrites and found a significant decrease in spine density but no difference in dendritic complexity. Comparatively, the results of this study demonstrate that in the basilar dendrites of those same animals alcohol exposure reduces dendritic complexity as well changes the ratio of mature to immature spines without affecting spine density. These distinct effects of neonatal alcohol exposure on the apical versus basilar branches indicate a different outcome for connectivity or input on the two regions of the dendritic tree. Structural changes in mPFC neurons lead to changes in their function. These different structural effects also could support the possibility that the effects of developmental alcohol exposure on different neuronal layers in the cortex, and even unique neuronal parts of dendritic tree (i.e. apical versus basal), are secondary to the effects on afferent connections.
In the prefrontal cortex, apical dendrites of pyramidal neurons receive parallel input from other parts of the neocortex, whereas the basilar dendrites of Layer III neurons are primarily innervated by reciprocal connections from the mediodorsal and anterior nuclei in the thalamus (Heidbreder & Groenewegen, 2003; Rotaru et al., 2005; Wedzony et al., 2005). Reciprocal connections from the mediodorsal nucleus are directed to Layer III, whereas cortical projections to the mediodorsal nucleus originate from Layer VI (Heidbreder & Groenewegen, 2003). Inputs from the mediodorsal nucleus not only synapse directly onto the spines of pyramidal cells in the mPFC but they also have an indirect influence through the local cortical interneurons; in particular paravalbumin (PV) cells (Rotaru et al., 2005). These inhibitory cells synapse primarily onto the soma, proximal dendrites and axon initial segments of PFC cells. Still, not only do the majority of excitatory mediodorsal neuron axons innervate the basilar dendritic spines of Layer III PFC pyramidal cells (2005) but excitatory projections from the anterior thalamic nuclei also form synaptic contacts on the basilar dendrites of Layer III pyramidal cells (Shibata, 1993). A reduction in the dendritic complexity of proximal basilar dendrites in mPFC cells could result in a decreased influence of these projections, in particular through a lack of inhibitory signals from the PV cells. This would produce sensory information overload (Wedzony et al., 2005) resulting in only the direct excitatory signals being processed. An increase in excitatory connections is also found when deregulation of the thalamic filter occurs (Stahl, 2008). The thalamic filter serves to regulate the sensory input from afferent fibers traveling through the thalamus and then into the cortex. This allows information processing to occur in an orderly manner (Stahl, 2008). Disruption to this filter leads to a deregulation of information processing in the cortex. A marked reduction of thalamic afferents to the cortical layer III after postnatal alcohol exposure was demonstrated previously (Granato et al., 1995); suggesting modifications to the thalamic filter which result from postnatal alcohol exposure. The current study adds to the previous reports that suggest alterations to the thalamic filter may occur not only in thalamic afferents but also in the neurons to which they project.
Other factors have been shown to decrease dendritic complexity in the mPFC. For example, exposing a pregnant dam to stress significantly affects the development of Layer II/III pyramidal neurons in her offspring. In particular, there are stress-related changes in complexity, in which the number of dendritic intersections and total dendritic length are reduced (Murmu et al, 2006). Chronic stress has also been shown to produce a decrease in dendritic complexity, spine density and mature spines in the mPFC of rats (Radley et al., 2008). In particular, chronic restraint stress has been shown to decrease the length and branching of apical dendrites in the prefrontal cortex (Liston et al., 2006). Accordingly, animals with a decrease in mPFC dendritic material also showed a selective impairment in extradimensional attention shifting (mPFC dependent behavior), but not discrimination or reversal learning (mPFC-independent behavior) (2006). The mPFC is a target for glucocorticoids as evidenced by the growing literature that shows the damaging effects of stress and corticosteroid treatment on the structure of the mPFC (Cook & Wellman, 2003; Brown, Henning & Wellman, 2005; Cerqueira et al., 2007). Interestingly, the damaging effect of stress on the mPFC has been shown to be reversible in both humans (Liston et al., 2009) and rats (Radley et al., 2006). This growing body of literature indicates that the mPFC is a very plastic and thus very susceptible brain region which is markedly sensitive to the effects of teratogens and environmental factors.
The majority of work looking at prenatal alcohol exposure examines its teratogenic effects on the hippocampus. For example, providing pregnant dams with a liquid diet, in which 35% of their total daily calories are provided by the alcohol (35% EDC), produces abnormal branching of the mossy fibers in the ventral hippocampus of rat pups (West et al., 1981). Along with these morphological changes, hippocampal-dependent behavioral changes are also evident after prenatal alcohol exposure. In fact, prenatal alcohol exposure has been linked with impaired spatial response-dependent learning (for review, see Berman & Hannigan, 2000). In sum, this suggests that the loss of connections in the hippocampus could be playing a role in the behavioral deficit. This relationship between changes in hippocampal structure and hippocampal-dependent behavior as a result of prenatal alcohol exposure supports the hypothesis that mPFC structural alterations may lead to PFC-dependent behavioral disabilities in patients with FASD.
A novel finding in this study was that third-trimester equivalent binge-like alcohol exposure affected the density of mature and immature spines through a predominance of mature spines while not affecting total spine density. Moreover, it is interesting to note that we saw a decrease in dendritic complexity accompanying the increase in mature spines. Spines function as biomechanical (Nimchinsky et al., 2002; Ethell & Pasquale, 2005) and electrical dendritic compartments (Tsay & Yuste, 2004). Spine densities fluctuate across brain region, neuronal type, and location on an individual dendritic tree (Nimchinsky et al., 2002; Spruston, 2008). Spines vary considerably in shape and size and are highly plastic (Spruston, 2008). In fact, certain phenotypes, or shapes, have been associated with either immature or mature synapses (Zhang & Benson, 2000; Irwin et al., 2001; Portera-Cailiau et al., 2003; Ethell and Pasquale, 2005). Previous work in our lab found that in the mPFC, postnatal alcohol exposure produced a decrease in apical spine density without producing changes in spine phenotypes (Whitcher & Klintsova, 2008). This raises the question of why there is a significant increase in mature spine phenotype without any change to spine density in the basilar dendrites.
The majority of excitatory inputs on cortical pyramidal neurons are made on dendritic spines. The contribution of functionally immature spines has been shown to decrease significantly before adolescence begins. This decrease in immature spines is evident in adolescent monkeys, wherein there is also an increase in the AMPA/NMDA ratio (Gonzalez-Burgos et al., 2008). Mature spines have larger postsynaptic densities, containing more AMPA receptors (Harris et al., 1992); whereas, immature spines have smaller post synaptic densities, containing NMDA receptors but few AMPA receptors (Ganeshina et al., 2004). Ethanol acts by blocking NMDA receptors and activating GABAA receptors. Studies that explore the withdrawal effects from chronic alcohol exposure find hyperactivation of the NMDA receptors due to the lack of alcohol present (Hendricson et al., 2007). Other studies find that chronic alcohol exposure in vitro can result in regionally specific up-regulation of NR1/NR2A expression (Snell et al., 1996) while chronic alcohol exposure in vivo results in an increase in the number of binding sites for MK-801 ( Gulya et al., 1991). Therefore, the AMPA/NMDA ratio could be affected by ethanol through an increase in NMDA receptor presence or binding.
It is clear that neonatal alcohol exposure results in changes in the morphology of Layer II/III mPFC pyramidal cells, yet it remains to be shown, using a rat model, whether these changes are accompanied by the deficits in prefrontal cortex-dependent behavior. A human study, looking at the brains of alcoholics, shows significant decrease in basilar dendritic arborization of Layer III pyramidal neurons (Harper & Corbett, 1990). Moreover, studies using electrophysiology have shown that ethanol dose-dependently reduces spike activity in the PFC of an anesthetized rat (Tu et al., 2007), suggesting that ethanol-induced changes in the activity of deep-layer cortical neurons may underlie the behavior effects associated with alcohol intake.
Our results provide more evidence for a structural correlate of some of the behavioral deficits observed in FASD patients. FASD is associated with morphological changes in the PFC, including frontal brain size changes (Wass, Persutte & Hobbins, 2001) and frontal lobe gray matter asymmetry (Sowell et al., 2002). Decreased dendritic length and complexity in mPFC could add to the changes in the functioning of neurons in mPFC, which in turn would affect behavior. The lack of change in spine density on basilar dendrites accompanied by the significant decrease of spine density on apical dendrites in this animal model of FAS highlights how spines can be differentially affected by developmental alcohol exposure. It is not clear if these changes in dendritic and spine morphology result from the direct effect of alcohol on pyramidal neurons in the cortex or they are secondary to the alcohol-induced changes in the afferent connections. Future work with this animal model should attempt to further uncover changes in mPFC and its intrinsic and extrinsic connections, as well as identify potential ameliorative mechanisms.
Acknowledgments
This study was supported by the NIH AA009838.
References
- Abel EL. Fetal Alcohol Syndrome: The American paradox. Alcohol and Alcoholism. 1998;33(3):195–201. doi: 10.1093/oxfordjournals.alcalc.a008382. [DOI] [PubMed] [Google Scholar]
- Anderson SA, Classey JD, Condé F, Lund JS, Lewis DA. Synchronous development of pyramidal neuron dendritic spines and parvalbumin-immunoreactive chandelier neuron axon terminals in layer III of monkey prefrontal cortex. Neuroscience. 1995;67(1):7–22. doi: 10.1016/0306-4522(95)00051-j. [DOI] [PubMed] [Google Scholar]
- Bayer SA, Altman J. Development of the Telencephalon: Neural tem Cells, Neurogenesis, and Neuronal Migration. In: Paxinos G, editor. The Rat Nervous System. 3. Elsevier Academic Press; 2004. [Google Scholar]
- Berman RF, Hannigan JH. Effects of prenatal alcohol exposure on the hippocampus: spatial behavior, electrophysiology, and neuroanatomy. Hippocampus. 2000;10(1):94–110. doi: 10.1002/(SICI)1098-1063(2000)10:1<94::AID-HIPO11>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Brown SM, Henning S, Wellman CL. Mild, short-term stress alters dendritic morphology in rat medial prefrontal cortex. Cereb Cortex. 2005;15(11):1714–22. doi: 10.1093/cercor/bhi048. [DOI] [PubMed] [Google Scholar]
- Calhoun F, Attilia ML, Spagnolo PA, Rotondo C, Mancinelli R, Ceccanti M. National institute on alcohol abuse and alcoholism and the study of fetal alcohol spectrum disorders. The international consortium. Annual First Super Sanita. 2006;42(1):4–7. [PubMed] [Google Scholar]
- Cerqueira JJ, Taipa R, Uylings HB, Almeida OF, Sousa N. Specific configuration of dendritic degeneration in pyramidal neurons of the medial prefrontal cortex induced by differing corticosteroid regimens. Cereb Cortex. 2007;17(9):1998–2006. doi: 10.1093/cercor/bhl108. [DOI] [PubMed] [Google Scholar]
- Connor PD, Sampson PD, Bookstein FL, Barr HM, Streissguth AP. Direct and indirect effects of prenatal alcohol damage on executive function. Dev Neuropsychol. 2000;18(3):331–354. doi: 10.1207/S1532694204Connor. [DOI] [PubMed] [Google Scholar]
- Cook SC, Wellman CL. Chronic stress alters dendritic morphology in rat medial prefrontal cortex. Journal of Neurobiology. 2003;60(2):236–248. doi: 10.1002/neu.20025. [DOI] [PubMed] [Google Scholar]
- Crombag HS, Gorny G, Li Y, Kolb B, Robinson TE. Opposite Effects of Amphetamine Self-administration Experience on Dendritic Spines in the Medial and Orbital Prefrontal Cortex. Cereb Cortex. 2005;15(3):341–348. doi: 10.1093/cercor/bhh136. [DOI] [PubMed] [Google Scholar]
- Ethell IM, Pasquale EB. Molecular mechanisms of dendritic spine development and remodeling. Prog Neurobiology. 2005;75(3):161–205. doi: 10.1016/j.pneurobio.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Ferrer I, Galofré E. Dendritic spine anomalies in fetal alcohol syndrome. Neuropediatrics. 1987;18(3):161–3. doi: 10.1055/s-2008-1052472. [DOI] [PubMed] [Google Scholar]
- Ganeshina O, Berry RW, Petralia RS, Nicholson DA, Geinisman Y. Differences in the expression of AMPA and NMDA receptors between axospinous perforated and nonperforated synapses are related to the configuration and size of postsynaptic densities. J Comp Neurol. 2004;468:86–95. doi: 10.1002/cne.10950. [DOI] [PubMed] [Google Scholar]
- Gibb R, Kolb B. A method for vibratome sectioning of Golgi-Cox stained whole rat brain. Journal of Neuroscience Methods. 1998;79(1):1–4. doi: 10.1016/s0165-0270(97)00163-5. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Isseroff A, Schwartz ML, Bugbee NM. The Neurobiology of Cognitive Development. In: Mussen PM, editor. Handbook of Child Psychology. New York: John Wiley & Sons; 1983. [Google Scholar]
- Gonzalez-Burgos G, Kroener S, Zaitsev AV, Povysheva NV, Krimer LS, Barrionuevo G, Lewis DA. Functional maturation of excitatory synapses in layer 3 pyramidal neurons during postnatal development of the primate prefrontal cortex. Cereb Cortex. 2008;18(3):626–37. doi: 10.1093/cercor/bhm095. [DOI] [PubMed] [Google Scholar]
- Granato A, Santarelli M, Sbriccoli A, Minciacchi D. Multifaceted alterations of the thalamo-cortico-thalamic loop in adult rats prenatally exposed to ethanol. Anat Embryol (Berl) 1995;191(1):11–23. doi: 10.1007/BF00215293. [DOI] [PubMed] [Google Scholar]
- Granato A, Di Rocco F, Zumbo A, Toesca A, Giannetti S. Organization of cortico-cortical associative projections in rats exposed to ethanol during early postnatal life. Brain Research Bulletin. 2003;60(4):339–344. doi: 10.1016/s0361-9230(03)00052-2. [DOI] [PubMed] [Google Scholar]
- Gulya K, Grant KA, Valverius P, Hoffman PL, Tabakoff B. Brain regional specificity and time-course of changes in the NMDA receptor-ionophonre complex during ethanol withdrawal. Brain Research. 1991;547(1):130–134. [PubMed] [Google Scholar]
- Harper C, Corbett D. Changes in the basal dendrites of cortical pyramidal cells from alcoholic patients--a quantitative Golgi study. J Neurol Neurosurg Psychiatry. 1990;53(10):856–61. doi: 10.1136/jnnp.53.10.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KM, Jensen FE, Tsao B. Three-dimensional structure of dendritic spines and synapses in rat hippocampus (CA1) at postnatal day 15 and adult ages: implications for the maturation of synaptic physiology and long-term potentiation. J Neurosci. 1992;12:2685–2705. doi: 10.1523/JNEUROSCI.12-07-02685.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidbreder CA, Groenewegen HJ. The medial prefrontal cortex in the rat: Evidence for a dorso-ventral distinction based upon anatomical and functional characteristics. Neurosci Biobehav Rev. 2003;27:555–579. doi: 10.1016/j.neubiorev.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Hendricson AW, Maldve RE, Salinas AG, Theile JW, Zhang TA, Diaz LM, Morrisett RA. Aberrant Synaptic Activation of N-Methyl-D-aspartate Receptors Underlies Ethanol Withdrawal Hyperexcitability. The Journal of pharmacology and experimental therapeutics. 2007;321 (1):60–72. doi: 10.1124/jpet.106.111419. [DOI] [PubMed] [Google Scholar]
- Herman LE, Acosta MC, Chang PN. Gender and attention deficits in children diagnosed with a Fetal Alcohol Spectrum Disorder. Can J Clin Pharmacol. 2008;15(3):e411–9. [PubMed] [Google Scholar]
- Huttenlocher PR, Dabholkar AS. Developmental Anatomy of Prefrontal Cortex. In: Krasnegor NA, Lyon GR, Goldman-Rakic PS, editors. Development of the Prefrontal Cortex: Evolution, Neurobiology, and Behavior. 1. Baltimore, MD: Paul H. Brookes Publishing Company; 1997. [Google Scholar]
- Ikonomidou C, Bosch F, Miksa M, Bittigau P, Vockler J, Dikranian K, Tenkova TI, Stefovska V, Turski L, Olney JW. Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain. Science. 1999;283(5398):70–74. doi: 10.1126/science.283.5398.70. [DOI] [PubMed] [Google Scholar]
- Irwin SA, Idupulapati M, Gilbert ME, Harris JB, Chakravarti AB, Rogers EJ, Crisostomo RA, Larsen BP, Mehta A, Alcantara CJ, Patel B, Swain RA, Weiler IJ, Oostra BA, Greenough WT. Dendritic spine and dendritic field characteristics of layer V pyramidal neurons in the visual cortex of fragile-X knockout mice. American Journal of Medical Genetics. 2002;111(2):140–146. doi: 10.1002/ajmg.10500. [DOI] [PubMed] [Google Scholar]
- Jerison HJ. Evolution of prefrontal cortex. In: Krasnegor NA, Lyon R, Goldman-Rakic PS, editors. Development of the prefrontal cortex: Evolution, neurobiology, and behavior. Baltimore, Maryland: Paul H. Brookes Publishing Co; 1997. pp. 9–26. [Google Scholar]
- Kalsbeek A, Voorn P, Buijs R, Pool C, Uylings H. Development of the dopaminergic innervation in the prefrontal cortex of the rat. J Comp Neurol. 1988;269(1):58–72. doi: 10.1002/cne.902690105. [DOI] [PubMed] [Google Scholar]
- Kolb B, Gibb R, Robinson TE. Brain plasticity and behavior. Curr Dir Psychol Sci. 2003;12(1):1–5. [Google Scholar]
- Kuboshima-Amemori S, Sawaguchi T. Plasticity of the primate prefrontal cortex. Neuroscientist. 2007;13(3):229–40. doi: 10.1177/1073858406298554. [DOI] [PubMed] [Google Scholar]
- Liston C, McEwen BS, Casey BJ. Psychosocial stress reversibly disrupts prefrontal processing and attentional control. PNAS. 2009;109(3):912–917. doi: 10.1073/pnas.0807041106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston C, Miller MM, Goldwater DS, Radley JJ, Rocher AB, Hof PR, Morrison JH, McEwen BS. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. The Journal of Neuroscience. 2006;26(30):7870–7874. doi: 10.1523/JNEUROSCI.1184-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrzljak L, Uylings HBM, Vaneden CG, Judas M. Neuronal Development in Human Prefrontal Cortex in Prenatal and Postnatal Stages. Progress in Brain Research. 1990;85:185–222. doi: 10.1016/s0079-6123(08)62681-3. [DOI] [PubMed] [Google Scholar]
- Mrzljak L, Uylings HB, Kostovic I, van Eden CG. Prenatal development of neurons in the human prefrontal cortex. II. A quantitative Golgi study. J Comp Neurol. 1992;316(4):485–96. doi: 10.1002/cne.903160408. [DOI] [PubMed] [Google Scholar]
- Murmu MS, Salomon S, Biala Y, Weinstock M, Braun K, Bock J. Changes of spine density and dendritic complexity in the prefrontal cortex in offspring of mothers exposed to stress during pregnancy. Eur J Neuroscience. 2006;24(5):1477–87. doi: 10.1111/j.1460-9568.2006.05024.x. [DOI] [PubMed] [Google Scholar]
- Nimchinsky EA, Sabatini BL, Svoboda K. Structure and function of dendritic spines. Annu Rev Physiology. 2002;64:313–53. doi: 10.1146/annurev.physiol.64.081501.160008. [DOI] [PubMed] [Google Scholar]
- Portera-Cailliau C, Pan DT, Yuste R. Activity-regulated dynamic behavior of early dendritic protrusions: evidence for different types of dendritic filopodia. J Neuroscience. 2003;23(18):7129–42. doi: 10.1523/JNEUROSCI.23-18-07129.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley JJ, Rocher AB, Janssen WGM, Hof PR, McEwen BS, Morrison JH. Reversibility of apical dendritic retraction in the rat medial prefrontal cortex following repeated stress. Experimental Neurology. 2005;196:199–203. doi: 10.1016/j.expneurol.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Rocher AB, Miller M, Janssen WGM, Liston C, Hof PR, McEwen BS, Morrison JH. Repeated Stress Induces Dendritic Spine Loss in the Rat Medial Prefrontal Cortex. Cereb Cortex. 2006;16(3):313–320. doi: 10.1093/cercor/bhi104. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Rocher AB, Rodriguez A, Ehlenberger DB, Dammann M, McEwen BS, Morrison JH, Wearne SL, Hof PR. Repeated stress alters dendritic spine morphology in the rat medial prefrontal cortex. J Comp Neurol. 2008;507(1):1141–50. doi: 10.1002/cne.21588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen C. Executive functioning and working memory in fetal alcohol spectrum disorder. Alcoholism: Clinical and Experimental Research. 2005;29 (8):1359–1367. doi: 10.1097/01.alc.0000175040.91007.d0. [DOI] [PubMed] [Google Scholar]
- Rotaru DC, Barrionuevo G, Sesack SR. Mediodorsal thalamic afferents to layer III of the rat prefrontal cortex: synaptic relationships to subclasses of interneurons. The Journal of Comparative Neurology. 2005;490:220–238. doi: 10.1002/cne.20661. [DOI] [PubMed] [Google Scholar]
- Shibata H. Efferent projections from the anterior thalamic nuclei to the cingulate cortex in the rat. J Comp Neurology. 1993;330(4):533–42. doi: 10.1002/cne.903300409. [DOI] [PubMed] [Google Scholar]
- Snell LD, Nunley KR, Lickteig RL. Regional and subunit specific changes in NMDA receptor mRNA and immunoreactivity in mouse brain following chronic ethanol ingestion. Molecular Brain Research. 1996;40:71–78. doi: 10.1016/0169-328x(96)00038-1. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Mattson SN, Tessner KD, Jernigan TL, Riley EP, Toga AW. Regional Brain Shape Abnormalities Persist into Adolescence after Heavy Prenatal Alcohol Exposure. Cerebral Cortex. 2002;12(8):856–865. doi: 10.1093/cercor/12.8.856. [DOI] [PubMed] [Google Scholar]
- Spruston N. Pyramidal neurons: dendritic structure and synaptic integration. Nat Rev Neurosci. 2008;9(3):206–21. doi: 10.1038/nrn2286. [DOI] [PubMed] [Google Scholar]
- Stahl SM. Stahl’s Essential Psychopharmacology. 3. Cambridge University Press; 2008. [Google Scholar]
- Streissguth A, Sampson P, Carmichael Olson H, Bookstein F, Barr H, Scott M, Feldman J, Mirsky A. Maternal drinking during pregnancy: Attention and short-term memory in 14-year-old offspring - a longitudinal prospective study. Alcoholism: Clinical and Experimental Research. 1994;18:202–218. doi: 10.1111/j.1530-0277.1994.tb00904.x. [DOI] [PubMed] [Google Scholar]
- Tsay D, Yuste R. On the electrical function of dendritic spines. Trends Neuroscience. 2004;27(2):77–83. doi: 10.1016/j.tins.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Tu Y, Kroener S, Abernathy K, Lapish C, Seamans J, Chandler LJ, Woodward JJ. Ethanol Inhibits Persistent Activity in Prefrontal Cortical Neurons. J Neurosci. 2007;27(17):4765–4775. doi: 10.1523/JNEUROSCI.5378-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uylings H, Groenewegen H, Kolb B. Do rats have a prefrontal cortex? Behav Brain Res. 2003;146(1–2):3–17. doi: 10.1016/j.bbr.2003.09.028. [DOI] [PubMed] [Google Scholar]
- Van Eden C. Development of connections between the mediodorsal nucleus of the thalamus and the prefrontal cortex in the rat. J Comp Neurol. 1986;244(3):349–359. doi: 10.1002/cne.902440307. [DOI] [PubMed] [Google Scholar]
- Van Eden CG, Kros JM, Uylings HBM. The development of the rat prefrontal cortex. In: Uylings HBM, van Eden CG, De Bruin JCP, Corner MA, Feenstra MGP, editors. The Prefrontal Cortex: Its Structure, Function, and Pathology. New York: Elsevier; 1990. [Google Scholar]
- Wang H, Deutch A. Dopamine depletion of the prefrontal cortex induces dendritic spine loss: reversal by atypical antipsychotic drug treatment. Neuropsychopharmacology. 2008;33(6):1276–1286. doi: 10.1038/sj.npp.1301521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wass TS, Persutte WH, Hobbins JC. The impact of prenatal alcohol exposure on frontal cortex development in utero. American Journal of Obsteterics Gynecology. 2001;185(3):737–742. doi: 10.1067/mob.2001.117656. [DOI] [PubMed] [Google Scholar]
- Wedzony K, Fijał K, Chocyk A. Blockade of NMDA receptors in postnatal period decreased density of tyrosine hydroxylase immunoreactive axonal arbors in the medial prefrontal cortex of adult rats. J Physiol Pharmacol. 2005;56(2):205–221. [PubMed] [Google Scholar]
- Welsh MC, Pennington BF. Assessing frontal lobe functioniong in children: views from developmental psychology. Developmental neuropsychology. 1988;4(3):199–230. [Google Scholar]
- West JR, Hodges CA, Black AC., Jr Prenatal exposure to ethanol alters the organization of hippocampal mossy fibers in rats. Science. 1981;211(4485):957–9. doi: 10.1126/science.7466371. [DOI] [PubMed] [Google Scholar]
- Whitcher L, Klintsova A. Postnatal binge-like alcohol exposure reduces spine density without affecting dendritic morphology in rat mPFC. Synapse. 2008;62(8):566–573. doi: 10.1002/syn.20532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Benson DL. Development and molecular organization of dendritic spines and their synapses. Hippocampus. 2000;10(5):512–26. doi: 10.1002/1098-1063(2000)10:5<512::AID-HIPO2>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]