Abstract
Recent studies have demonstrated that neuropilin 1 (NP-1) is involved in HTLV-1 entry; however, the role NP-1 plays in this process is not understood. We demonstrated that ectopic expression of human NP-1 but not NP-2 cDNA increased susceptibility to HTLV-1. SiRNA-mediated inhibition of NP-1 expression correlated with significant reduction of HTLV-1 Env-mediated fusion. The vascular endothelial growth factor (VEGF165) caused downmodulation of surface NP-1 and inhibited HTLV-1 infection of U87 cells. In contrast, VEGF165 partially inhibited infection of primary astrocytes and had no significant effect on infection of HeLa cells. VEGF165 and antibodies to the glucose transporter protein 1 (anti-GLUT-1) were both needed to block infection of primary astrocytes, however, only anti-GLUT-1 antibodies were sufficient to block infection of HeLa cells. HTLV-1 Env forms complexes with both NP-1 and GLUT-1 in primary human astrocytes. The alternate usage of these two cellular receptors may have important implications regarding HTLV-1 neuro-tropism.
Keywords: Virus entry, HTLV-1, neurotropism, Neuropilin 1
INTRODUCTION
Human T-cell leukemia virus type 1(HTLV-1) is the etiologic agent of adult T-cell leukemia/lymphoma (ATLL) and a progressive demyelinating disease known as tropical spastic paraparesis/HTLV-1 associated myelopathy (TSP/HAM) (reviewed in (Feuer and Green, 2005; Gallo, 2005; Yoshida, 2005)). HTLV-1 was the first human retrovirus to be isolated and characterized (Gallo, 2005). Retroviral infection is associated with a number of pathologic abnormalities, including a variety of cancers, immunologic diseases, and neurological disorders. ATLL is characterized by T cell oncogenesis, whereas HAM/TSP involves cellular destruction and inflammation of the upper motor neurons. ATL and HAM are the most devastating diseases associated with HTLV-1 infection. Vaccines or cures to these diseases are not available. While clinical symptoms of ATL and HAM are well defined, the molecular determinants involved in this differential disease pathogenesis are unknown. This highlights the importance of developing new strategies aimed at preventing HTLV-1 infection.
Manel et al have identified the glucose transporter type 1(GLUT-1) as a receptor for HTLV-1 and HTLV-2 (Manel et al., 2003). The results by Manel et al have suggested that, in addition to GLUT-1, other cofactors/coreceptors might be involved in HTLV-1 infection (reviewed in (Manel et al., 2005)). However, the lack of a cell system that does not express GLUT-1 has made it difficult to provide experimental evidence for the existence of alternative receptors or coreceptors. The literature reported a number of observations that favor the alternate receptor usage by HTLV-1, however, evidence for the utilization of these receptors in primary cells is lacking. Previous studies demonstrated that DC-SIGN facilitates fusion of dendritic cells with HTLV-1-infected cells (Ceccaldi et al., 2006). Other studies claimed that heparan sulfate proteoglycans (HSPGs) might play a role in HTLV-1 entry (Jones et al., 2006; Jones et al., 2005; Pinon et al., 2003; Takenouchi et al., 2007). Recent work by Lambert et al demonstrated an important role for NP-1 and HSPG in HTLV-1 infection of dendritic cells (Lambert et al., 2009). Jones et al demonstrated that blocking interactions with either NP-1 or HSPGs decreased the infection of CD4+ T cells (Jones et al., 2008). It is possible that HSPGs are utilized by HTLV-1 in certain cell culture conditions and may have a role in viral attachment, however, the exact contribution of HSPGs and their relevance for the observed in vivo tropism of HTLV-1 to CD4+ T lymphocytes remain controversial. We have previously provided evidence for the utilization of GLUT-1 for HTLV-1 infection of CD4+ T lymphocytes, the targets for HTLV-1 in vivo (Jin et al., 2006a). We have also described a 5 bp deletion in the GLUT-1 gene of the astrocytoma/astroglioma U87 cell line that caused these cells to express very low levels of GLUT-1 (Jin et al., 2006a). We have proposed a GLUT-1-independent pathway for the observed efficient HTLV-1 infection of U87 cells (Jin et al., 2006a).
Recent studies reported neuropilin 1 (NP-1), a receptor for Semaphorin 3A(Sema3A) and vascular endothelial growth factor, as a co-factor for HTLV-1 (Ghez et al., 2006). NP-1 is a transmembrane protein initially identified as an epitope recognized by a monoclonal antibody (A5) that labels specific subsets of axons in the developing Xenopus nervous system (Takagi et al., 1991). NP-1 is a cell surface receptor that has been implicated to function in the development of both the cardiovascular and nervous systems. NP-1 is a receptor for the axonal chemo repellent semaphorin III (Sema III) (He and Tessier-Lavigne, 1997; Kolodkin et al., 1997). Sema III is a secreted protein that in vitro causes neuronal growth and in vivo is required for correct sensory afferent innervations and other aspects of development. NP-1 can also bind with high affinity to select isoforms of vascular endothelial growth factor (VEGF) including VEGF165 (Soker et al., 1998). VEGF is a highly secreted polypeptide growth factor with 5 alternatively spliced isoforms of which VEGF165 and VEGF121 are the most abundant (Neufeld et al., 1999).
In the present study, we examined the role of human NP-1 in HTLV-1 Env-mediated fusion and HTLV-1 infection. We demonstrate that HTLV-1 utilizes NP-1 and GLUT-1 as receptors to infect the astroglioma/astroblastoma U87 cells and human primary astrocytes. The results may provide important insight into HTLV-1 neuro-tropism and pathogenesis.
MATERIALS AND METHODS
Cells and viruses
HeLa cells, 293 cells, and CHO cells were obtained from the American Type Culture Collection. U87 cells were obtained from NIH AIDS Research & Reference Reagent Program (lot # 4 021998). The U87 cells were subjected to multiple rounds of cell cloning in the presence of 10% calf serum supplemented with glial cell medium with G-5 supplement (Invitrogen Corporation, Grand Island, NY). Cells were grown in DMEM containing 10% cosmic calf serum (U87) or 10% fetal bovine serum (293, HeLa and CHO), penicillin G (100 units/ml) and streptomycin sulfate (0.1 mg/ml) in a humidified incubator held at 5% CO2. All cell culture media were purchased from GIBCO. Normal Human Astrocytes (NHA) and their basal medium (with 10% FBS) were purchased from AllCells (AllCells, LLC., Emeryville, CA, Cat#NHA-001F).
The recombinant vaccinia viruses used in the Env-mediated fusion assay have been previously described (Agrawal et al., 2004a; Agrawal, Vanhorn-Ali, and Alkhatib, 2002; Agrawal et al., 2004b). The Western Reserve (WR) refers to the vaccinia virus empty vector used to generate all our recombinant viruses. We The recombinant vaccinia virus encoding the HTLV-1 cleavage mutant (Unc63) has been used as a negative control in the Env-mediated fusion assays (Jin et al., 2006b). The cleavage site of the HTLV-1 Env at residues 309–312 (RSRR) was changed to GLGL in Unc63. The Unc63 was used as an envelope control to measure the background fusion since it can bind target cells but does not engage in cell fusion activity.
Human and rat neuropilin 1 cDNA clones
We utilized poly A+ RNA isolated from U87 cells as a template in an RT-PCR reaction to clone the human NP-1 from U87 cells. We obtained the rat NP-1 from the laboratory of Dr. David Ginty, Department of Neuroscience, The Johns Hopkins University School of Medicine, Baltimore, MD. Human NP-2 cDNA was obtained by RT-PCR cloning from U373 cells using NP-2 specific primers and mRNA isolated from U373 cells. The 3kb PCR product was cloned into pCDNA3.1 downstream of T7 and CMV promoters. The cDNA sequencing confirmed that the cloned fragment encodes human NP-2.
RT-PCR analysis of RNA expression
RT-PCR was performed using freshly isolated total RNA (RNeasy mini kit, Qiagen) from U87, U373, HeLa, and 293 cells. The cDNA synthesis was accomplished by using the superscript III first-strand synthesis system that is part of the RT-PCR protocol (Invitrogen). Approximately, 5µg RNA was used to synthesize first-strand cDNA in a volume of 20µl. A 2 µl aliquote of RT product was used as template in a 25-µl PCR reaction. The PCR conditions were as follows: 35 cycles, 1 min/94°C, 1 min/annealing temperature, 1–3 min/72°C. Primer sequences and annealing temperatures were as follows: NP-1cDNA forward primer: 5`-atggagagggggctgccgc-3` and reverse primer: 5`-ctcgagttcatctctgtctgccttca-3`, annealing at 60°C. NP-2 cDNA forward primer: 5’-atggatatgtttcctctcacctgg-3’ and reverse primer: 5’-tcatgcctcggagcagcacttttg-3’, annealing at 65°C.
For RT-PCR analysis the primer sequences are the following: the β-actin forward primer: 5`-gagcacagagcctcgcctttgc-3` and reverse primer: 5`- tcttctcgcggttggccttggg-3’, annealing at 55°C. NP-1 forward primer: 5’-atggagagggggctgccg-3’ and reverse primer: 5’-caacatcagggaatccatccc-3’ annealing at 57°C. Glut-1 forward primer: 5’-atggagcccagcagcaagaa-3’ and reverse primer: 5’-agggaccagagcgtggtgag-3’ annealing at 57°C. Glut-3 forward primer: 5’-atggggacacagaaggtcac-3’ and reverse primer: 5’-acagacaaggaccagagaga-3’ annealing at 55°C.
HTLV-1 Env-mediated fusion and VEGF165 blocking experiments
Env-mediated cell fusion was measured by a vaccinia-based reporter gene assay (Jin et al., 2006b). The assay analyzes fusion between two distinct cell populations, one that expresses the potential receptor along with the lacZ gene linked to the T7 promoter and another that expresses HTLV-1 Env and T7 RNA polymerase encoded by a recombinant vaccinia virus. All cell populations were incubated overnight at 31°C to allow expression of the vaccinia-encoded proteins. Duplicate samples containing 105 target cells and the same number of effector cells were mixed in 96 well micro titer plates and incubated at 37°C for 2.5 hours. The extent of Env-mediated fusion was assayed by colorimetric measurement of β-galactosidase activity in detergent-treated cell lysates (Nussbaum, Broder, and Berger, 1994). For blocking experiments, recombinant human VEGF165 (PeproTech, Inc, Rocky Hill, NJ. Cat#100-20) at increasing concentrations (0,10,100,500 ng/ml) was added to the target cells (105 HeLa or U87 cells). The cells and VEGF165 mixture was incubated for 30 minutes at 37°C. The cell mixture was then mixed with the corresponding Env-expressing cell population and incubated at 37°C for 2.5 hours. The blocking effect was evaluated by a colorimetric measurement of β-glycosidase produced as a result of cell fusion. Control wells incubated with irrelevant bacterial proteins were included.
SiRNA experiments
We purchased the human neuropilin siRNA molecules from Santa Cruz Biotechnolgy, Inc, CA (Cat# sc-36038). The Glut-1 siRNA was purchased from Integrated DNA Technologies (Coralville, IA) and used as previously described (Jin et al., 2006a; Jin et al., 2006b). Briefly, U87 cells were plated at 1.5×105 cells/well in a 6-well plate using serum-free medium without antibiotics. The siRNA (10 nM) was transfected by oligofectamine reagent (Invitrogen, Grand Island, NY, Cat#12252-011). After a 4 hr incubation period at 37°C, the cells were washed and changed with fresh media containing 10% FBS. Following incubation for 72 hr in a 37°C incubator the transfected cells were infected with pT7-lacZ reporter. Separate populations of 293 cells were infected with vaccinia viruses encoding T7 RNA polymerase and either the wild type HTLV-1 envelope glycoprotein (Env63) or the uncleaved HTLV-1 Env (Unc63). At 16 hr post-infection the Env-expressing cells were mixed (1:1) with the siRNA-transfected cells and incubated for 2.5 hr to allow cell fusion. The effect of siRNA on Env-mediated fusion was assayed by measuring β-galactosidase produced. The negative and positive control siRNA were also purchased from Integrated DNA Technologies.
Pseudotyped virus infection
We adapted the improved pseudotyping assay developed by Sutton and Littman (Sutton and Littman, 1996) to prepare pseudotyped virus particles that carry the HTLV-1 Env protein. 293T cells were cotransfected with 10 µg of PcREV and 10 µg of the envelope-defective luciferase transducing HIV proviral clone pNL4-3. Luc+env- (Catalog # 3418, AIDS Reagent Program) in the presence or absence of 10 µg of pHTLV-env-rre using standard calcium phosphate. Fifteen hours following transfection, 20 mM sodium butyrate and fresh media were added. The supernatants were harvested after 48–72 hours by low-speed centrifugation and filtrated through a 0.22um filter. Pseudotyped vesicular stomatitis virus G glycoprotein (VSV-G) was used as a positive control to verify the competence of all cell types in cell fusion.
HTLV-1 infection assays were performed by incubating 3×105 cells/ml containing 5 µg/ml polybrene mixed with 1 ml of pseudotyped HTLV-1 virus stock and plated into the wells of a six well plate. The mixture was incubated for 4–5 hours at 37°C in a 5% CO2 incubator. Following incubation the cells were washed with 10% FBS, fresh medium was added and incubation continued for another 48 hours. The cells were harvested and luciferase assay was performed using the luciferase assay system as recommended by the manufacturer (Promega, Madison, WI).
Cell surface expression of receptors and protein analysis
To detect GLUT-1 at the cell surface, we used previously described chicken polyclonal antibodies generated against a synthetic peptide corresponding to the large extracellular domain of GLUT-1 (ECL1) (Agrawal et al., 2006). We will refer to these antibodies as GLUT-IgY to indicate the source of these antibodies. Other antibodies used for cell surface staining of GLUT-1 were mouse monoclonal anti-human Glut-1, Glut-3 (Alpha Diagnostic International, Inc, San Antonio, TX, Cat#GT15-M and Cat#GT-34-M). For VEGF165-induced downmodulation we used the mouse anti-human BDCA-4 (neuropilin-1) monoclonal antibody (Miltenyi Biotec, Cat#130-090-533), and monoclonal anti-rat neuropilin-1 antibody (R & D System, Cat#AF566). The U87 cells were incubated with the VEGF165, washed with cold PBS containing 0.1% PBS/BSA, pelleted by centrifugation for 5 min at 500xg at 4°C. After fixing with 4% formaldehyde, the cells, either permeabilized (0.05% Saponin for 15 minutes on ice) or non-permeabilized, were resuspended in 100 µl of 1% PBS/BSA containing a 1:1000 diluted chicken anti-human GLUT-IgY antibody or a mouse monoclonal antibody at a concentration of 10 µg/ml. The mixtures were then incubated on ice for 30 min. The cells were then washed with 1% PBS/BSA, pelleted, and resuspended in 100 µl of 1% PBS/BSA containing 2 µg of FITC-conjugated rabbit anti-chicken IgY (Biomeda, Foster City, CA) or FITC-conjugated rat anti-mouse IgG1 (A85-1, BD Biosciences Pharmingen). The cells were then incubated on ice for 30 min, washed three times with 1% PBS/BSA solution, and resuspended in 1 ml of PBS and analyzed in a FACS Vantage flow cytometer (Becton- Dickinson, San Jose, CA).
Immunoblot analysis was performed using rabbit antibodies raised against the C-terminal domain of GLUT-1 (Dakocytomation, Carpinteria, CA). Rabbit polyclonal anti-human neuropilin antibodies (H-286) were purchased from Santa Cruz Biotechnology, Inc, CA (Cat#sc-5541). Dr. Steven Foung provided the human monoclonal antibody to HTLV-1. The mouse monoclonal antibody to Glyceraldehyde-3-PDH (GAPDH) was purchased from Biodesign International, Portland, ME (Cat#H86504M). Cells were rinsed with PBS, scraped on ice in PBS and centrifuged at 500xg. The cell pellets were resuspended in protein lysis buffer (300mM NaCL, 50mM Tris.HCL pH 7.6, 0.1% SDS, Aprotonin 10ug/ml, PMSF 1mM) and incubated for 30 min on ice then sonicated for 1 min to shear the genomic DNA. After centrifugation, the supernatants were mixed with same volume of protein loading buffer and fractionated in a 12% SDS-PAGE (0.1×106 cells/lane) and transferred into PVDF membrane blots. Blots were incubated with rabbit antibodies directed against C-terminal domain (1:2000) and left rocking overnight at 4°C. The blots were developed by BM Chemiluminescence using peroxidase-conjugated antibody against mouse/rabbit IgG (Roche, Indianapolis, IN).
For cell membrane preparations, cells were homogenized in 10 mM Tris, 1 mM EDTA, 1mM phenlmethylsulphonyl fluoride (PMSF) and 250 mM sucrose, pH 7.4, in Potter-Elvehjem glass-Teflon type homogenizer at 4°C. The homogenates were centrifuged at 900xg for 10 minutes to sediment the fraction containing nuclei and the supernatants were centrifuged at 17,000xg for 75 minutes at 4°C. The pellets were subjected to 12% SDS-PAGE. An equivalent of 0.1×106 cells were loaded per lane.
Co-immunoprecipitation
Human Astrocytes were infected with either an empty WR vaccinia vector or a recombinant vaccinia virus expressing the HTLV-1 envelope glycoprotein (gp63) at 10pfu/cell for 1 hour then incubated overnight at 31°C. After washing 3 times with PBS, the cells were pelleted by centrifuging and suspended in lysis buffer [300mM NaCL, 50mM Tris-HCL pH7.6, 0.1% SDS, Aprotonin 10ug/ml, PMSF 1mM, and protease inhibitors] at 4°C for 40 min with gentle mixing. The protease inhibitors were purchased from Roche, Indianapolis, IN (Complete Mini, Cat#11 836 153 001). The nuclei were pelleted by centrifugation at 17,000 g for 25 min in a refrigerated Eppendorf centrifuge. Immobilized Protein A beads (Pierce, Rockford, IL), pre-washed with PBS, were added to the immunoprecipitating antibody (neuropilin A-12, 2ug/ml) and incubated at 4°C for 2 hours. The Protein A beads and antibody mixture was then added to the cell lysates and incubated at 4°C for 16 hours. The beads were then washed 4 times with 1 ml of ice-cold lysis buffer and eluted by adding 2x laemmli buffer (Sigma, St. Louis, MO). They were run on 12%SDS-PAGE without boiling for Glut-1 detection and were electrophoretically transferred to PVDF membranes. The membranes were blocked with 20mM Tris-HCL (pH7.6) buffer containing 140mM NaCl, 0.1% Tween-20, and 5% nonfat powered milk and incubated with their respective antibodies. The membranes were washed and reacted with horseradish peroxidase-conjugated secondary antibodies and developed by using the supersignal chemiluminescent substrate (Pierce, Rockford, IL).
RESULTS
Human neuropilin 1 (NP-1) is abundantly expressed in U87 cells and primary human astrocytes
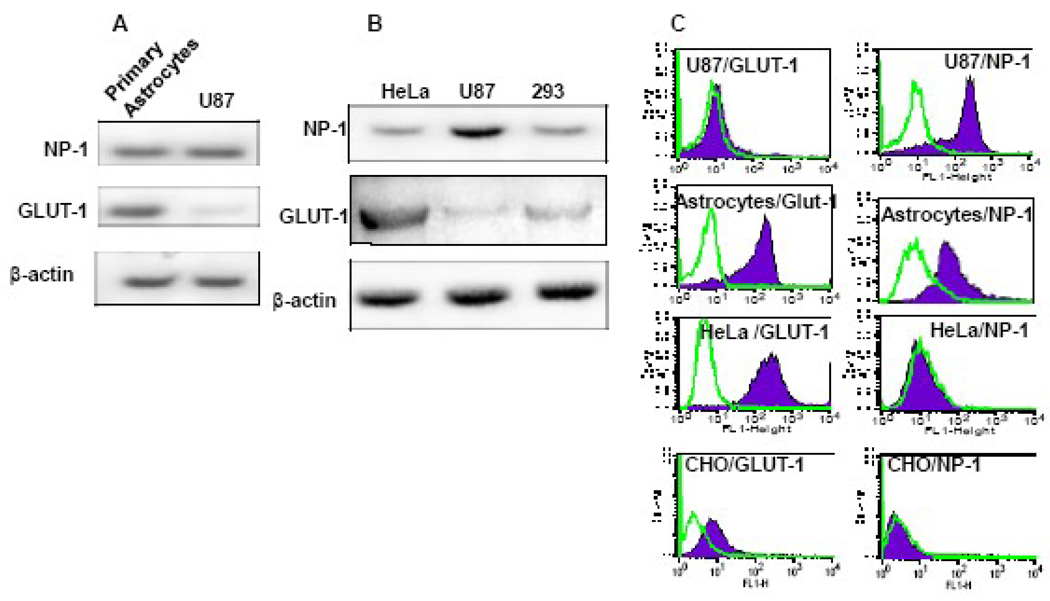
We have previously demonstrated that HTLV-1 Env-mediated fusion and infection of U87 cells is not sensitive to GLUT-IgY antibodies suggesting a GLUT-1-independent pathway of viral entry (Jin et al., 2006a). To examine GLUT-1 and NP-1 RNA expression in U87 cells, we performed RT-PCR using freshly isolated total RNA from U87, HeLa cells, 293 cells, or normal human astrocytes (NHA). The results show that NHA expressed both GLUT-1 and NP-1 RNA (Figure 1A). The expression pattern of GLUT-1 and NP-1 was different in the cell lines. The U87 cell line expressed relatively high levels of NP-1 and very low levels of GLUT-1 (Figure 1B). The relative expression levels were compared to the endogenous expression of β-actin. In contrast, Hela cells expressed the highest levels of GLUT-1 (Figure 1B). The 293 cells expressed both NP-1 and GLUT-1 proteins at lower levels compared to the NP-1 in U87 cells and GLUT-1 in HeLa cells (Figure 1B). We have previously demonstrated that most of HTLV-1 Env-mediated fusion with HeLa cells can be inhibited using GLUT-IgY (Jin et al., 2006a).
Figure 1. Expression of NP-1 and GLUT-1 in primary human astrocytes and cell lines.
A&B) RT-PCR analysis of NP-1 and GLUT-1 mRNA expression was performed as described in text. Amplification of β-actin was used as a control for gel loading. The data represent scans of agarose gels stained with ethidium bromide. C) FACS analysis of surface NP-1 and GLUT-1 in cell lines and primary human astrocytes. Polyclonal antibodies were used to analyze NP-1 expression while expression of GLUT-1 was analyzed using GLUT-IgY antibodies. Staining of CHO was performed to provide evidence for the low expression levels of GLUT-1 and NP-1 proteins.
FACS analysis was performed to analyze NP-1 and GLUT-1 surface expression. We used mouse monoclonal anti-human GLUT-1 to stain GLUT-1 and anti-NP-1 monoclonal antibodies (Miltenyi Biotec and R & D System) to stain cell surface NP-1. High GLUT-1 staining was always detected on NHA and HeLa cells but only background levels detected on U87 cells (Figure 1C). We consistently observed abundant expression of human NP-1 in both U87 cells and NHA but almost background levels detected on HeLa cells (Figure 1C). Since we used CHO cells for ectopic expression of NP-1 and GLUT-1 the CHO cells were stained to verify the low endogenous levels of surface GLUT-1 and NP-1 proteins (Figure 1C). These results show differential expression of NP-1 and GLUT-1 in the four cell populations and confirm our previous observations of low levels of GLUT-1 protein expression in U87 cells (Jin et al., 2006a).
Human NP-1 acts as a fusion receptor for HTLV-1 in transfected CHO cells
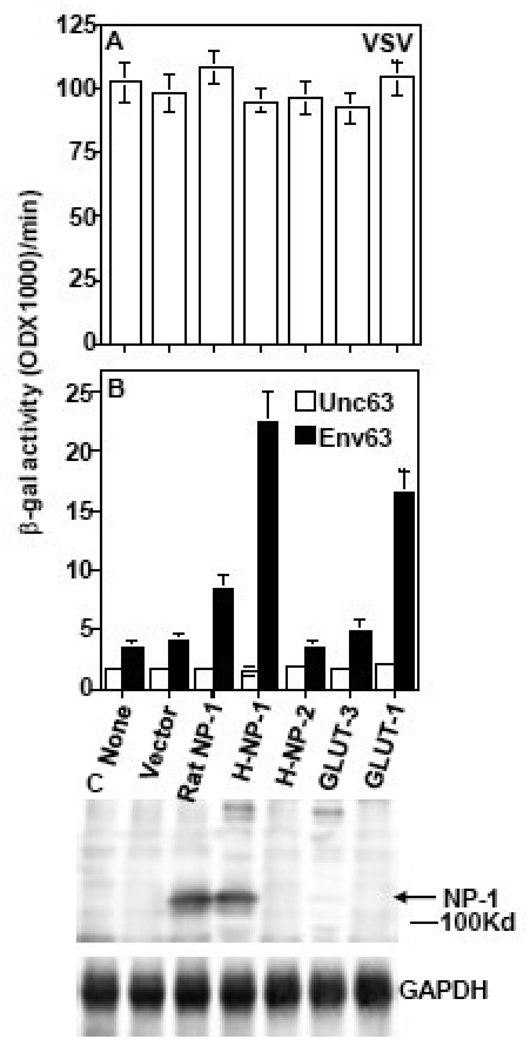
To determine whether neuropilins could serve as HTLV-1 receptors we used CHO cells for ectopic expression of human NP-1 or NP-2, rat NP-1, and GLUT-1 or GLUT-3 as cellular targets in the Env-mediated fusion assay. The rationale for using CHO cells is they express low levels of GLUT-1 and trace levels of NP-1 (Figure 1C). The competence of the transfected targets in Env-mediated fusion was verified by VSV fusions (Figure 2A). The CHO cells expressing recombinant NP-1 showed higher fusion signal than those expressing recombinant GLUT-1 (Figure 2B). Furthermore, the fusion signal was significantly higher with cells expressing recombinant NP-1 compared to cells expressing recombinant NP-2 (Figure 2B). The rat NP-1 showed some activity in this assay but was significantly lower than the human NP-1. Low signals above the background were observed with GLUT-3 or empty vector transfected CHO cells (Figure 2B). Expression of the human and rat NP-1 proteins was verified by western blotting using commercially available rabbit polyclonal antibodies against human NP-1 which cross- react with the rat NP-1 but not with the human NP-2 (Figure 2C). The results demonstrated that human NP-1 acts as a fusion receptor for HTLV-1.
Figure 2. Human Neuropilin 1 (NP-1) acts as a fusion receptor for HTLV-1.
CHO cells were transfected with pCNDA3 plasmids encoding either vector, rat NP-1, human NP-1, human NP-2, GLUT-3 or GLUT1 under T7 promoter. Expression of the proteins was accomplished by infection with vaccinia virus encoding T7 RNA polymerase. The CHO cells expressing the different proteins were mixed with HTLV-1 Env-expressing 293 cells that co-express T7-lacZ reporter. Following incubation the extent of Env-mediated cell fusion was quantified by measuring β-galactosidase production. The competence of the transfected cells to undergo cell fusion was verified by VSV fusion (A). The ability of the transfected cells to fuse with the HTLV-1 Env63 is shown in panel B. Expression of rat and human NP-1 was verified by western blotting using a polyclonal antibody against the human NP-1 that cross-reacts with rat NP-1 but not with human NP-2 (C). The results were reproduced at least four times. The control envelope Unc63 is a cleavage mutant of HTLV-1 Env63 that does not promote cell fusion.
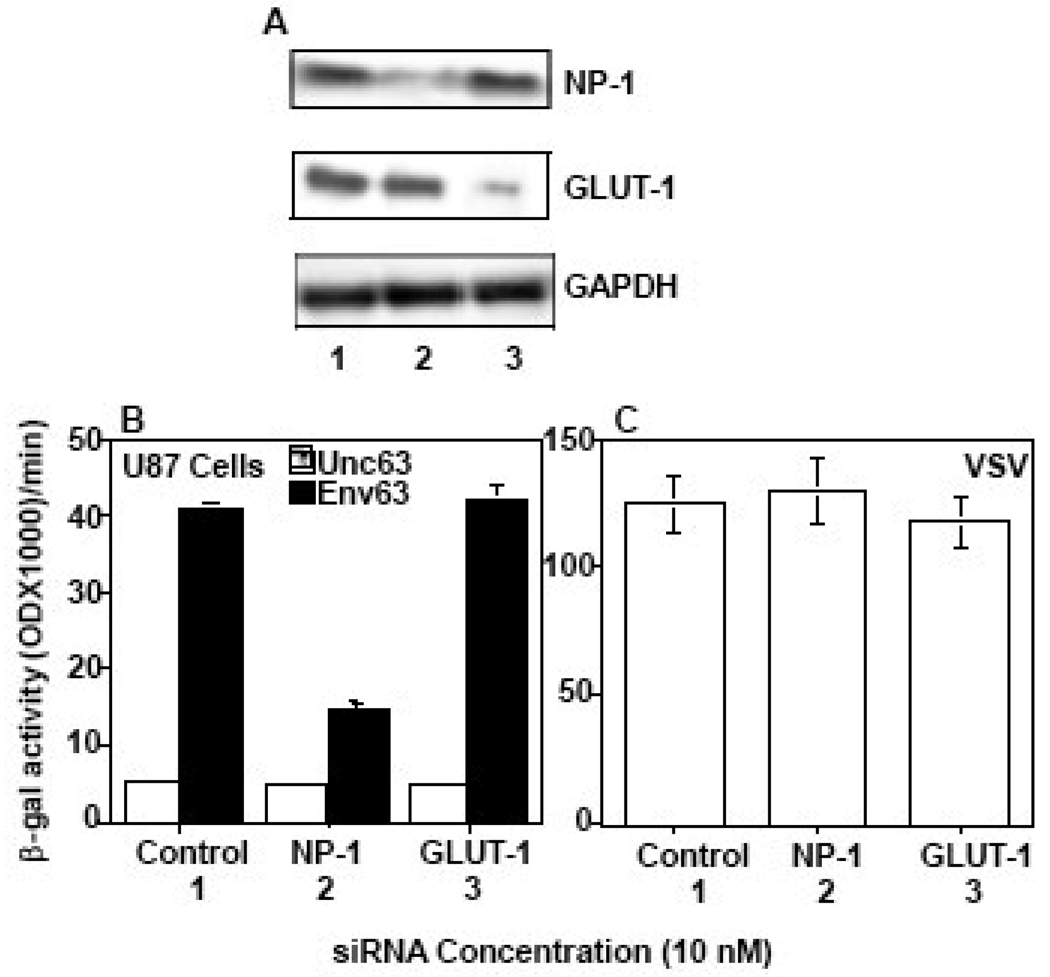
SiRNA-mediated reduction of NP-1 protein expression blocks HTLV-1 Env-mediated fusion with U87 cells
To examine the role of NP-1 in HTLV-1 Env-mediated fusion with U87 cells we compared the effect of reducing the expression levels of GLUT-1 versus NP-1 by using siRNA specific to each gene. Significant reduction of NP-1 and GLUT-1 protein expression in siRNA-transfected cells was confirmed by western blotting (Figure 3A). The GLUT-1 gel image was obtained by over-exposure of the gel containing the RT-PCR reaction since U87 express relatively low levels of GLUT-1 (Figure 3A). The effect of siRNA on Env-mediated fusion was assayed by measuring β-galactosidase produced as a result of mixing siRNA-transfected target cells with HTLV-1 Env-expressing 293 cells. The results demonstrate that NP-1 siRNA-transfected U87 cells had a significant reduction in their ability to fuse with HTLV-1 Env (Figure 3B). In contrast, GLUT-1 siRNA-transfected U87 cells (corresponding to lanes 3) were indistinguishable from control vector-transfected cells in terms of their Env-mediated fusion signal (Figure 3B, compare graphs and lanes 1 & 3). The competence of the transfected cell populations to undergo cell fusion was confirmed with VSV Env-expressing cells (Figure 3C).
Figure 3. SiRNA-mediated reduction of NP-1 expression correlates with significant blocking of HTLV-1 Env-mediated fusion.
The siRNA molecules were transfected into U87 cells and the reduction of gene expression was verified by western blot analysis (A). The GLUT-1 gel was exposed 5 times the exposure time of the NP-1 gel (A). The transfected cells were infected with vaccinia recombinant encoding T7 RNA polymerase and mixed with Env-expressing 293 cells expressing T7-lacZ reporter. The potency of the transfected cells to promote HTLV-1 Env-mediated fusion was examined by measuring the amount of β-galactosidase produced (B). VSV Env-mediated fusion was used as a positive control to verify the competence of the transfected cells to undergo cell fusion (C). The numbers 1, 2 and 3 below the lanes correspond to the transfected control, NP-1 or GLUT-1 siRNA respectively. The GLUT-1 gel images shown in panel A was over-exposed to visualize the siRNA effect in U87 cells that express low levels of GLUT-1.
VEGF165 downmodulates NP-1 expression
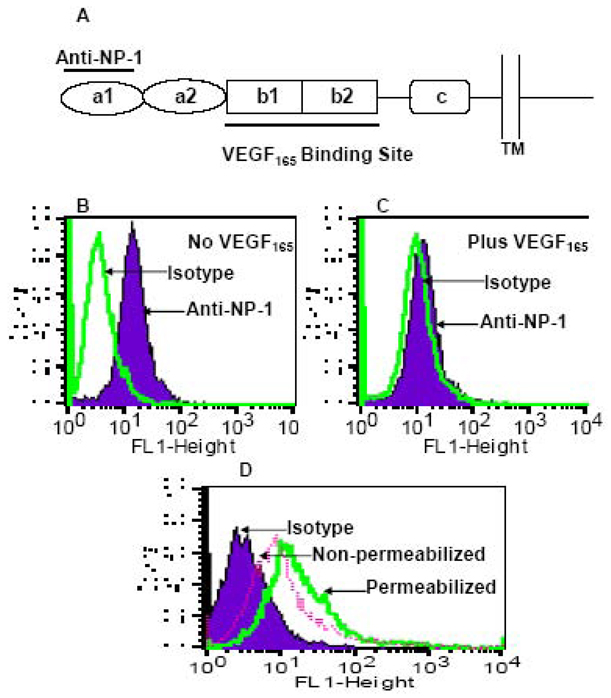
NP-1 was identified as a functional receptor for VEGF165 (Soker et al., 1998). Since VEGF165 binds with high affinity to NP-1 we examined the effect of incubating VEGF165 with U87 cells expressing endogenous NP-1. We utilized a similar strategy to that previously reported by Narazaki and Tosato who were the first to provide experimental evidence for VEGF165-induced internalization of NP-1 in endothelial cells (Narazaki, Segarra, and Tosato, 2008; Narazaki and Tosato, 2006). The U87 cells were pre-incubated with or without 500ng/ml VEGF165 and then stained with a monoclonal antibody specific to human NP-1. This is a commercially available antibody generated against the a1 domain of NP-1 that is distant from the well-known VEGF165 binding site (Figure 4A). Cell surface expression of NP-1 on U87 cells was consistently obtained using this antibody (Figure 4B). Significant reduction in cell surface expression of NP-1 with U87 cells pre-incubated with VEGF165 (Figure 4C). This suggested that NP-1 surface expression was down-modulated by its specific ligand VEGF165. Down-modulation of NP-1 was only observed when antibodies distant from the VEGF165 binding site were used. To confirm these results we repeated the experiment and divided the VEGF165-treated U87 cells into two equal portions, one was directly incubated with the anti-NP-1 antibody (Non-permeabilized); the other was treated with Triton X-100 (permeabilized) before antibody treatment. The results demonstrated an increase in NP-1 staining in permeabilized cells that could account for the internalized NP-1 (Figure 4D). This suggests receptor downmodulation as a potential mechanism for the observed antiviral activity of VEGF165.
Figure 4. Down-modulation of human NP-1 by VEGF165.
A) Schematic diagram illustrating the different domains of the human NP-1, the binding site of VEGF165, and the anti-NP-1 antibodies used in this analysis. The mouse anti-human BDCA-4 (Neuropilin-1) monoclonal antibody recognizes the a1 domain of the human NP-1. B&C) Flow cytometry analysis of surface expression of the human NP-1 on U87 cells, pre-incubated at 37°C for 30 min in the absence of VEGF165 (B) or in the presence of VEGF165 (C). Panel D shows another experiment where surface expression of the human NP-1 was analyzed in the presence of VEGF165. Following treatment with VEGF165 the U87 cells were divided into two aliquots, one was directly stained with anti-NP-1 (Non-permeabilized); the other was treated with 0.3% Triton-X100 for 20 min at 4°C then stained with anti-NP-1 antibodies (Permeabilized).
HTLV-1 infection of primary astrocytes and U87 cells is sensitive to VEGF165
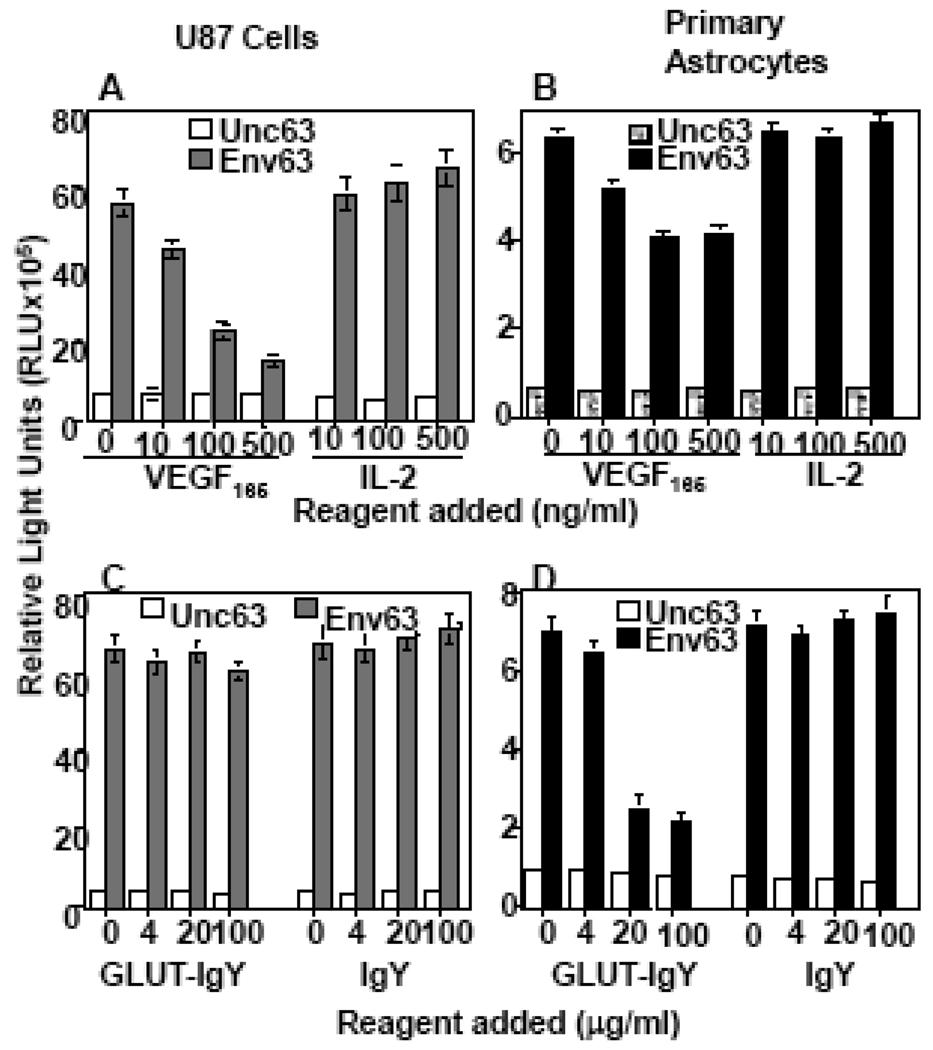
VEGF165-induced specific downmodulation of NP-1 suggested that VEGF165 may compete with HTLV-1 Env for binding to NP-1. To confirm this we added recombinant VEGF165 at increasing concentrations to U87 cells or NHA then infected them with HTLV-1 pseudotyped virus. We found that VEGF165 specifically inhibited HTLV-1 infection of both U87 cells (Figure 5A) and NHA primary astrocytes (Figure 5B). We consistently observed more potent VEGF165 inhibitory effect in U87 cells (Figure 5A) than in NHA primary astrocytes (Figure 5B). Since the VEGF165 source was bacterial, we used similar concentrations of an affinity-purified CC chemokine MIP-1β purified from bacteria as a negative control. This control protein had no significant effect on HTLV-1 infection of either U87 cells (Figure 5A) or NHA primary astrocytes (Figure 5B). GLUT-IgY or the control IgY antibodies also had no significant effect on HTLV-1 infection of U87 cells (Figure 5C). In contrast, GLUT-IgY but not the control IgY antibodies caused significant inhibition of infection of primary astrocytes (Figure 5D). These results demonstrate that HTLV-1 utilizes NP-1 as a major receptor for entry into U87 cells but uses both GLUT-1 and NP-1 for entry into NHA primary astrocytes.
Figure 5. VEGF165 blocks HTLV-1 infection of U87 cells and primary human astrocytes.
To examine NP-1-mediated infection the commercially available VEGF165 or IL-2 were added to the indicated cell populations at escalating concentrations and incubated for 1 hr at 37°C before infection with the pseudotyped HTLV-1 virus (A&B). To examine the effect of GLUT-1-mediated infection other portions of the same cell populations were incubated with escalating doses of either GLUT-IgY or control IgY antibodies before HTLV-1 infection (C&D). Seventy-two hours post-infection, the cells were lysed and infectivity measured by the amount of luciferase produced (RLU). Unc63 is a control with a mutated envelope protein that is defective in membrane fusion. IL-2 was used as a negative control for the VEGF165 activity. The control for GLUT-IgY activity was the normal chicken IgY. This experiment was reproduced at least three times.
Alternate receptor usage of NP-1 and GLUT-1
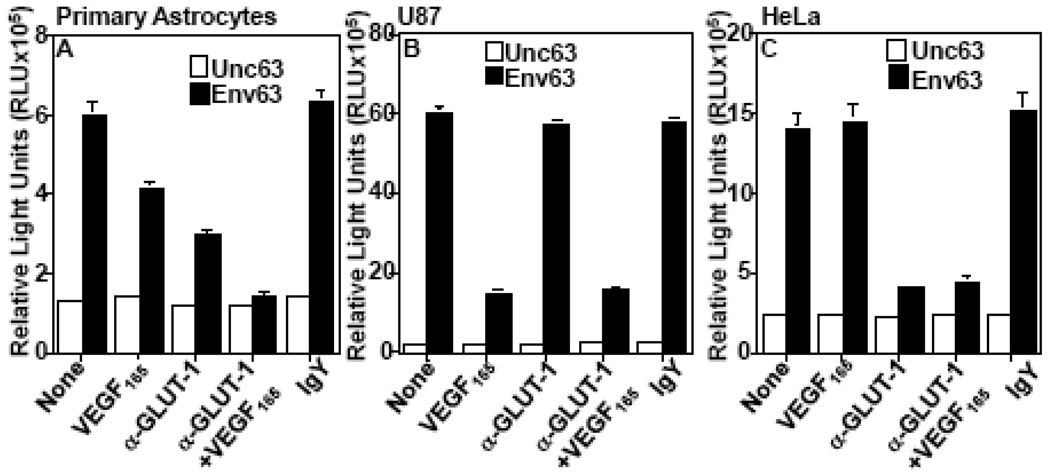
The observed potent VEGF165 inhibition of HTLV-1 Env fusion with U87 cells prompted us to analyze whether NP-1 and GLUT-1 are both utilized for HTLV-1 entry in NHA. We reasoned that if GLUT-1 were utilized as a major receptor then the VEGF165 would have little or no effect on HTLV-1 entry. We used GLUT-IgY to block GLUT-1-mediated infection (Jin et al., 2006a; Jin et al., 2006b) and VEGF165 to block NP-1-mediated infection. The HTLV-1 pseudotyped virus infection of NHA was sensitive to both GLUT-IgY and VEGF165 (Figure 6A). The blocking activity was consistently higher with GLUT-IgY than VEGF165 (Figure 6A). However, when used together the blocking effect was enhanced (Figure 6A). The VEGF165 alone had efficient blocking effect on infection of U87 cells while GLUT-IgY had little effect (Figure 6B). In contrast, infection of HeLa cells was efficiently blocked by GLUT-IgY but was insensitive to VEGF165 (Figure 6C). The results suggested that GLUT-1 and NP-1 are both utilized as cellular receptors for HTLV-1 infection of primary astrocytes but only one receptor is utilized in HeLa or U87 cell lines.
Figure 6. HTLV-1 Infection of primary human astrocytes is sensitive to both VEGF165 and anti-GLUT-1 antibodies.
GLUT-IgY antibodies, VEGF165, or both were pre-incubated with primary astrocytes (A), U87 cells (B), or HeLa cells (C) before infection with pseudotyped HTLV-1. The effect on HTLV-1 infection was examined 48 hr post-infection by measuring luciferase production (RLU). Normal chicken IgY was used as a negative control. The results are from one experiment performed in duplicates and are representative of at least three different experiments.
Physical interaction of HTLV-1 Env with human NP-1 and GLUT-1 proteins in normal human astrocytes
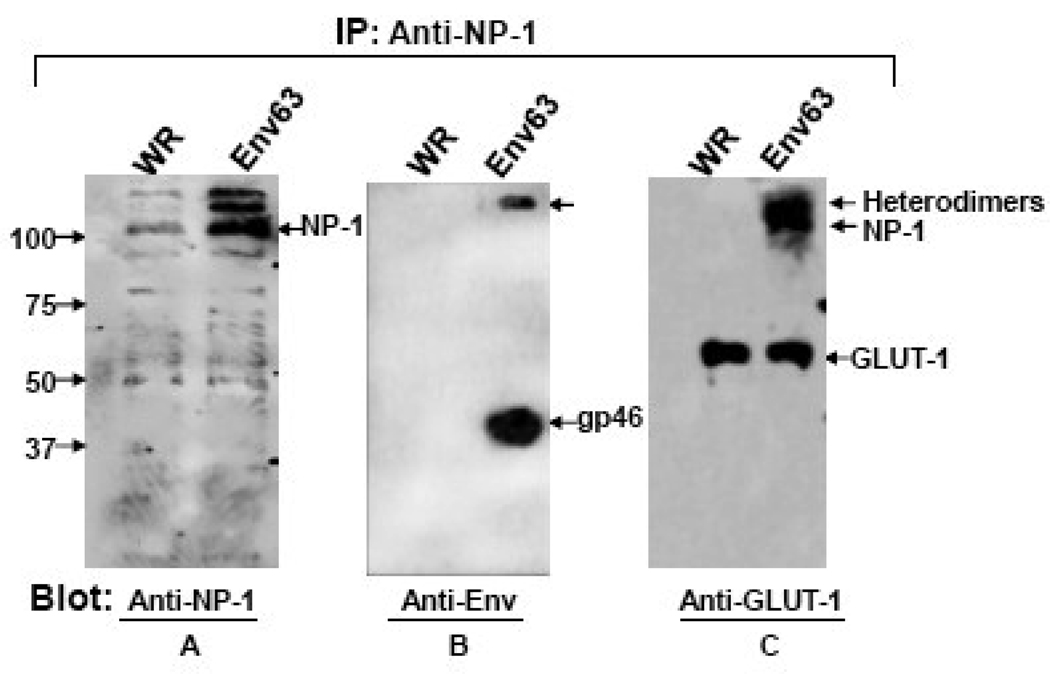
Previous studies have demonstrated physical interaction of HTLV-1 Env, NP-1 and GLUT-1 in 293 cells (Ghez et al., 2006). To demonstrate this interaction in primary astrocytes we infected NHA (endogenous NP-1 and GLUT-1) with either an empty vaccinia virus vector (WR) or a recombinant vaccinia virus encoding the Env63. Total cell lysates were used for immunoprecipitation with anti-NP-1 antibodies. The immunoprecipitated proteins were analyzed in immunoblots that have been reacted with antibodies to either GLUT-1 or HTLV-1 Env. As expected, probing the blot with anti-NP-1 antibodies detected the NP-1 protein in both the control and Env63-expressing NHA with more NP-1 protein detected in the presence of Env63 (Figure 7A). The higher molecular weight (MW) protein band seen above the NP-1 band may represent protein oligomers. Immunoblotting with anti-Env monoclonal antibodies detected the soluble subunit gp46 protein band with no bands detected in the control WR lane (Figure 7B). Immunoblotting with anti-GLUT-1 antibody revealed the GLUT-1 protein bands in both NHA cell samples expressing either control WR vector or Env63 (Figure 7C). This suggested the physical association of NP-1 and GLUT-1 proteins without the presence of HTLV-1 Env. Higher molecular weight bands were detected in the Env-63-expressing cells but not in the control (Fig. 7C). The high MW protein bands detected in all three panels might represent incompletely denatured protein complexes between Env, NP-1 and GLUT-1. The results demonstrate physical interaction between HTLV-1 Env, NP-1, and GLUT-1 in primary human astrocytes.
Figure 7. Co-immunoprecipitation of HTLV-1 Env by antibodies to human NP-1.
Primary human astrocytes infected with either the WR vaccinia control or with a recombinant vaccinia virus encoding the HTLV-1 Env63 were used to prepare cellular lysates for immunoprecipitation with Anti-NP-1 antibodies. The precipitated proteins were fractionated in 10% SDS-PAGE and blotted. The blot was first probed with anti-NP-1, stripped, reprobed with anti-gp46 (anti-Env), stripped, and then re-probed with anti-GLUT-1 antibodies. Arrows at the right side mark the positions of the different proteins. The positions of the molecular weight markers are indicated at the left side.
DISSCUSION
This study utilized three cellular models to show that GLUT-1 and NP-1 can serve as cellular receptors for HTLV-1. HTLV-1 exclusively utilizes human NP-1 as a cellular receptor to infect the astroglioma/astroblastoma U87 cell line that expresses low levels of cell surface GLUT-1 (Jin et al., 2006a). In contrast, HTLV-1 strictly utilizes GLUT-1 on HeLa cells as a cellular receptor despite the observed low expression levels of NP-1. Interestingly, HTLV-1 utilizes both GLUT-1 and NP-1 to infect human primary astrocytes. This conclusion is based on the observed additive blocking effect by GLUT-IgY and VEGF165, two reagents that bind to GLUT-1 and NP-1 respectively. The optimum number of receptor molecules required for HTLV-1 entry is currently unknown. We hypothesize that the relative expression levels of GLUT-1 and NP-1 proteins might determine the choice of their utilization by HTLV-1.
Several lines of experimental evidence were presented to show that NP-1 is utilized as a cellular receptor by HTLV-1. First, recombinant expression of NP-1 but not NP-2 significantly increased the susceptibility of CHO cells to Env-mediated fusion and HTLV-1 infection. Second, siRNA-mediated reduction of NP-1 but not GLUT-1 in U87 cells was associated with a significant reduction in HTLV-1 Env-mediated fusion. Third, HTLV-1 infection of U87 cells was efficiently blocked by VEGF165, a high affinity ligand specific for NP-1. Finally, FACS analysis demonstrated a high-level expression of cell surface NP-1 and trace amounts of GLUT-1 on U87 cells. Our results are consistent with previous data in the literature showing high levels of NP-1 expression in U87 cells (Rieger, Wick, and Weller, 2003).
Previous studies reported that HSPG is the receptor that allows HTLV-1 to infect CD4+ T lymphocytes (Jones et al., 2005). It is well known that HSPG enhances attachment by almost all viruses (reviewed in (Manel et al., 2005)). The HSPG involvement in this seems very similar to the role of HSPG in HIV-1 infection; that is, promoting attachment without being involved in the membrane fusion events. Previous studies have shown that HSPG has promoted attachment of HIV-1 on macrophages that express low levels of CD4 (Saphire et al., 2001). The current model of HIV-1 entry argues against the involvement of HSPG in the membrane fusion events that result in the internalization of HIV-1 virions into the host cell. Nonetheless, the possible involvement of HSPG can be considered in a broader context since the internalization of enveloped viruses from many families can be strongly influenced by HSPG (Liu and Thorp, 2002; Shukla and Spear, 2001).
The recent report that DC-SIGN facilitates HTLV-1 entry into myeloid dendritic cells (Ceccaldi et al., 2006) is also noteworthy in view of emerging evidence implicating C-type lectins in interactions of many viruses with the cell surface of target cells (Altmeyer, 2004). These surface entities play important roles in facilitating the entry of many viruses, in some cases by serving as attachment factors to enhance virus adsorption to the cell. In addition, they may participate in signaling pathways that influence infectivity in ways that do not directly involve the specific entry mechanism. These considerations highlight the difficulties in defining the molecular elements that comprise the essential entry machinery and in distinguishing them from factors that play important accessory roles. The involvement of HSPGs, GLUT-1, and NP-1 proteins in HTLV-1 entry suggested a complex method of viral entry. The sequence of the molecular events involved in HTLV-1 entry is unknown.
NP-1 is a cell surface receptor that has been implicated to function in the development of both the cardiovascular and nervous systems. Additionally, NP-1 is involved in regulation of cell migration, morphogenesis, and membrane protrusions (recently reviewed in (Mendes-da-Cruz et al., 2009). Previous studies demonstrated that HTLV-1 Env-mediated fusion is enhanced by increased cell adhesion (Daenke, McCracken, and Booth, 1999; Hildreth, Subramanium, and Hampton, 1997). At the present time, it is not clear whether increased Env-mediated fusion is merely due to increased ruffling of the plasma membrane or increased cellular adhesion in NP-1-expressing cells. It is possible that high levels of NP-1 expression could compensate for the low levels of GLUT-1 expression by increasing the adhesive properties of U87 cells.
The neuropilin genes, NP-1 and NP-2, share similar protein structure but differ in their expression patterns, regulation, and ligand-binding specificities. Human NP-1 and NP-2 show 43% amino acid homology while human and rat NP-1 show 87% homology (reviewed in (Guttmann-Raviv et al., 2006)). The high degree of amino acid homology between the human and rat NP-1 may explain our observed positive fusion signal with the rat NP-1. Early studies have demonstrated that titers of HTLV-1 Env pseudotyped viruses were nearly always higher on human cells than on rat or murine cell lines (Sutton and Littman, 1996; Trejo and Ratner, 2000). Additionally, the observed low levels of GLUT-1 and NP-1 in CHO cells might explain previous observations that the levels of HSPGs on CHO cells correlate with the titer of HTLV-1 pseudotyped viruses and other Env-mediated interactions on those cells (Pinon et al JV 77:9922).
We demonstrated physical interaction between NP-1, GLUT-1, and HTLV-1 Env in primary human astrocytes. Our results confirm previous studies showing that GLUT-1 and NP-1 form intracellular complexes in 293 cells that are increased in the presence of HTLV-1 Env (Ghez et al., 2006). Ghez et al have demonstrated that NP-1 and GLUT-1 colocalize in membrane junctions formed between infected and uninfected T cells. Our study demonstrated for the first time the physical association of endogenous NP-1 and GLUT-1 in primary astrocytes without the presence of HTLV-1 Env (Figure 7, Panel C). These results are different from those reported earlier showing that the co-immunoprecipitation of NP-1 and GLUT-1 was dependent on the presence of Env in transfected 293 or HeLa cells (Ghez et al., 2006; Lambert et al., 2009). The different cell type utilized in the different studies might explain this discrepancy. Our results were obtained by using primary astrocytes where the levels of NP-1 and GLUT-1 are different from those in human cell lines. The usage of both NP-1 and GLUT-1 in primary astrocytes suggests the possibility of a receptor/coreceptor system utilized by HTLV-1 for specific entry into the brain. This possibility needs further investigation.
Ligand-induced down modulation of cell surface receptors represents an obvious mechanism by which VEGF165 inhibits HTLV-1 Env-mediated fusion and virus infection. In support of this conclusion, Narazaki and Tosato have provided strong experimental evidence for VEGF165-induced internalization of NP-1 in endothelial cells (Narazaki, Segarra, and Tosato, 2008; Narazaki and Tosato, 2006). Additionally, Lamber et al have recently demonstrated that the VEGF165-homolgous region on HTLV-1 Env directly binds NP-1 and is essential for internalization and infection of CD4+ T cells and dendritic cells (Lambert et al., 2009). An alternative mechanism would be direct blocking of the Env interaction site(s). In view of our results it seems that the first mechanism may be in effect although we cannot disregard the mechanism of steric blockade. Recent studies proposed that VEGF165 is a selective competitor of HTLV-1 entry and that the b domain of NP-1 is required for HTLV-1 binding (Lambert et al., 2009). This is in agreement with our finding that VEGF165 blocked HTLV-1 envelope-mediated entry by a receptor downmodulation mechanism. Binding of VEGF165 to HSPGs (Krilleke et al., 2007) has previously been demonstrated, therefore, it cannot be ruled out that at least part of the observed inhibition by VEGF165 might be due to blocking interaction with HSPGs. However, as discussed earlier it is hard to designate a specific role for HSPGs in the membrane fusion events or in receptor downmodulation. These results have implications for designing novel VEGF165 NP-1-targeted therapies for HTLV-1 since they suggest that an agent capable of both down modulating NP-1and physically blocking its interaction with Env may be most efficacious.
Analysis of the mechanism of HTLV-1 infection of primary astrocytes is critical for the fundamental understanding of virus-host interaction and will provide important insight into the mechanism of HTLV-1 neuro-tropism. The ability to explore the biochemical basis of Env/GLUT-1/NP-1 interactions in direct binding assays and the availability of antibodies to study the GLUT-1 and NP-1 proteins at the cellular level will provide important new information on the role of these molecules in viral infection and their contribution to tropism and cytopathic effects of retroviral infections.
ACKNOWLEDGEMENTS
We thank Jon Marsh and the Indiana University Vector Production Facility for assistance in constructing the Unc63 mutant and Zainab VanHorn-Ali for excellent technical assistance. We also thank Dr. Steven Foung for providing the human monoclonal antibody to HTLV-1. This work was supported by a grant from the National Cancer Institute 1R21CA98095-01 awarded to GA.
Abbreviations
- Env
envelope glycoprotein
- Env63
wild type HTLV-1 envelope glycoprotein
- Unc63
a cleavage mutant of HTLV-1 Env63 defective in syncytium formation
- wt
wild type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Agrawal L, Lu X, Jin Q, Alkhatib G. Anti-HIV Therapy: Current and Future Directions. Curr Pharm Des. 2006;12(16):2031–2055. doi: 10.2174/138161206777442100. [DOI] [PubMed] [Google Scholar]
- Agrawal L, Lu X, Qingwen J, VanHorn-Ali Z, Nicolescue V, McDermott D, Murphy PM, Alkhatib G. Role for CCR5Δ32 protein in resistance to R5, R5X4, and X4 human immunodeficiency virus type 1 in primary CD4+ cells. Journal of Virology. 2004a;78(5):2277–2287. doi: 10.1128/JVI.78.5.2277-2287.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal L, Vanhorn-Ali Z, Alkhatib G. Multiple determinants are involved in HIV coreceptor use as demonstrated by CCR4/CCL22 interaction in peripheral blood mononuclear cells (PBMCs) J Leukoc Biol. 2002;72(5):1063–1074. [PubMed] [Google Scholar]
- Agrawal L, VanHorn-Ali Z, Berger EA, Alkhatib G. Specific inhibition of HIV-1 coreceptor activity by synthetic peptides corresponding to the predicted extracellular loops of CCR5. Blood. 2004b;103(4):1211–1217. doi: 10.1182/blood-2003-08-2669. [DOI] [PubMed] [Google Scholar]
- Altmeyer R. Virus attachment and entry offer numerous targets for antiviral therapy. Curr Pharm Des. 2004;10(30):3701–3712. doi: 10.2174/1381612043382729. [DOI] [PubMed] [Google Scholar]
- Ceccaldi PE, Delebecque F, Prevost MC, Moris A, Abastado JP, Gessain A, Schwartz O, Ozden S. DC-SIGN facilitates fusion of dendritic cells with human T-cell leukemia virus type 1-infected cells. J Virol. 2006;80(10):4771–4780. doi: 10.1128/JVI.80.10.4771-4780.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daenke S, McCracken SA, Booth S. Human T-cell leukaemia/lymphoma virus type 1 syncytium formation is regulated in a cell-specific manner by ICAM-1, ICAM-3 and VCAM-1 and can be inhibited by antibodies to integrin beta2 or beta7. J Gen Virol. 1999;80(Pt 6):1429–1436. doi: 10.1099/0022-1317-80-6-1429. [DOI] [PubMed] [Google Scholar]
- Feuer G, Green PL. Comparative biology of human T-cell lymphotropic virus type 1 (HTLV-1) and HTLV-2. Oncogene. 2005;24(39):5996–6004. doi: 10.1038/sj.onc.1208971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo RC. History of the discoveries of the first human retroviruses: HTLV-1 and HTLV-2. Oncogene. 2005;24(39):5926–5930. doi: 10.1038/sj.onc.1208980. [DOI] [PubMed] [Google Scholar]
- Ghez D, Lepelletier Y, Lambert S, Fourneau J-M, Blot V, Janvier S, Arnulf B, Endert PM, Heveker N, Pique C, Hermine O. Neuropilin-1 Is Involved in Human T-Cell Lymphotropic Virus Type 1 Entry. Journal of Virology. 2006;80(14):6844–6854. doi: 10.1128/JVI.02719-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttmann-Raviv N, Kessler O, Shraga-Heled N, Lange T, Herzog Y, Neufeld G. The neuropilins and their role in tumorigenesis and tumor progression. Cancer Lett. 2006;231(1):1–11. doi: 10.1016/j.canlet.2004.12.047. [DOI] [PubMed] [Google Scholar]
- He Z, Tessier-Lavigne M. Neuropilin is a receptor for the axonal chemorepellent Semaphorin III. Cell. 1997;90(4):739–751. doi: 10.1016/s0092-8674(00)80534-6. [DOI] [PubMed] [Google Scholar]
- Hildreth JE, Subramanium A, Hampton RA. Human T-cell lymphotropic virus type 1 (HTLV-1)-induced syncytium formation mediated by vascular cell adhesion molecule-1: evidence for involvement of cell adhesion molecules in HTLV-1 biology. Journal of Virology. 1997;71(2):1173–1180. doi: 10.1128/jvi.71.2.1173-1180.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Q, Agrawal L, Vanhorn-Ali Z, Alkhatib G. GLUT-1-independent infection of the glioblastoma/astroglioma U87 cells by the human T cell leukemia virus type 1. Virology. 2006a doi: 10.1016/j.virol.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Jin Q, Agrawal L, Vanhorn-Ali Z, Alkhatib G. Infection of CD4(+) T lymphocytes by the human T cell leukemia virus type 1 is mediated by the glucose transporter GLUT-1: Evidence using antibodies specific to the receptor's large extracellular domain. Virology. 2006b;349(1):184–196. doi: 10.1016/j.virol.2006.01.045. [DOI] [PubMed] [Google Scholar]
- Jones KS, Fugo K, Petrow-Sadowski C, Huang Y, Bertolette DC, Lisinski I, Cushman SW, Jacobson S, Ruscetti FW. Human T-cell leukemia virus type 1 (HTLV-1) and HTLV-2 use different receptor complexes to enter T cells. J Virol. 2006;80(17):8291–8302. doi: 10.1128/JVI.00389-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KS, Petrow-Sadowski C, Bertolette DC, Huang Y, Ruscetti FW. Heparan sulfate proteoglycans mediate attachment and entry of human T-cell leukemia virus type 1 virions into CD4+ T cells. J Virol. 2005;79(20):12692–12702. doi: 10.1128/JVI.79.20.12692-12702.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KS, Petrow-Sadowski C, Huang YK, Bertolette DC, Ruscetti FW. Cell-free HTLV-1 infects dendritic cells leading to transmission and transformation of CD4(+) T cells. Nat Med. 2008;14(4):429–436. doi: 10.1038/nm1745. [DOI] [PubMed] [Google Scholar]
- Kolodkin AL, Levengood DV, Rowe EG, Tai YT, Giger RJ, Ginty DD. Neuropilin is a semaphorin III receptor. Cell. 1997;90(4):753–762. doi: 10.1016/s0092-8674(00)80535-8. [DOI] [PubMed] [Google Scholar]
- Krilleke D, DeErkenez A, Schubert W, Giri I, Robinson GS, Ng YS, Shima DT. Molecular mapping and functional characterization of the VEGF164 heparin-binding domain. J Biol Chem. 2007;282(38):28045–28056. doi: 10.1074/jbc.M700319200. [DOI] [PubMed] [Google Scholar]
- Lambert S, Bouttier M, Vassy R, Seigneuret M, Petrow-Sadowski C, Janvier S, Heveker N, Ruscetti FW, Perret G, Jones KS, Pique C. HTLV-1 uses HSPG and neuropilin-1 for entry by molecular mimicry of VEGF165. Blood. 2009;113(21):5176–5185. doi: 10.1182/blood-2008-04-150342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Thorp SC. Cell surface heparan sulfate and its roles in assisting viral infections. Med Res Rev. 2002;22(1):1–25. doi: 10.1002/med.1026. [DOI] [PubMed] [Google Scholar]
- Manel N, Battini JL, Taylor N, Sitbon M. HTLV-1 tropism and envelope receptor. Oncogene. 2005;24(39):6016–6025. doi: 10.1038/sj.onc.1208972. [DOI] [PubMed] [Google Scholar]
- Manel N, Kim FJ, Kinet S, Taylor N, Sitbon M, Battini JL. The ubiquitous glucose transporter GLUT-1 is a receptor for HTLV. Cell. 2003;115(4):449–459. doi: 10.1016/s0092-8674(03)00881-x. [DOI] [PubMed] [Google Scholar]
- Mendes-da-Cruz DA, Lepelletier Y, Brignier AC, Smaniotto S, Renand A, Milpied P, Dardenne M, Hermine O, Savino W. Neuropilins, semaphorins, and their role in thymocyte development. Ann N Y Acad Sci. 2009;1153:20–28. doi: 10.1111/j.1749-6632.2008.03980.x. [DOI] [PubMed] [Google Scholar]
- Narazaki M, Segarra M, Tosato G. Sulfated polysaccharides identified as inducers of neuropilin-1 internalization and functional inhibition of VEGF165 and semaphorin3A. Blood. 2008;111(8):4126–4136. doi: 10.1182/blood-2007-09-112474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narazaki M, Tosato G. Ligand-induced internalization selects use of common receptor neuropilin-1 by VEGF165 and semaphorin3A. Blood. 2006;107(10):3892–3901. doi: 10.1182/blood-2005-10-4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z. Vascular endothelial growth factor (VEGF) and its receptors. Faseb J. 1999;13(1):9–22. [PubMed] [Google Scholar]
- Nussbaum O, Broder CC, Berger EA. Fusogenic mechanisms of enveloped-virus glycoproteins analyzed by a novel recombinant vaccinia virus-based assay quantitating cell fusion-dependent reporter gene activation. J.Virol. 1994;68:5411–5422. doi: 10.1128/jvi.68.9.5411-5422.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinon JD, Klasse PJ, Jassal SR, Welson S, Weber J, Brighty DW, Sattentau QJ. Human T-Cell Leukemia Virus Type 1 Envelope Glycoprotein gp46 Interacts with Cell Surface Heparan Sulfate Proteoglycans. J. Virol. 2003;77(18):9922–9930. doi: 10.1128/JVI.77.18.9922-9930.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieger J, Wick W, Weller M. Human malignant glioma cells express semaphorins and their receptors, neuropilins and plexins. Glia. 2003;42(4):379–389. doi: 10.1002/glia.10210. [DOI] [PubMed] [Google Scholar]
- Saphire AC, Bobardt MD, Zhang Z, David G, Gallay PA. Syndecans serve as attachment receptors for human immunodeficiency virus type 1 on macrophages. J Virol. 2001;75(19):9187–9200. doi: 10.1128/JVI.75.19.9187-9200.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla D, Spear PG. Herpesviruses and heparan sulfate: an intimate relationship in aid of viral entry. J Clin Invest. 2001;108(4):503–510. doi: 10.1172/JCI13799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soker S, Takashima S, Miao HQ, Neufeld G, Klagsbrun M. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell. 1998;92(6):735–745. doi: 10.1016/s0092-8674(00)81402-6. [DOI] [PubMed] [Google Scholar]
- Sutton RE, Littman DR. Broad host range of human T-cell leukemia virus type 1 demonstrated with an improved pseudotyping system. Journal of Virology. 1996;70(10):7322–7326. doi: 10.1128/jvi.70.10.7322-7326.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi S, Hirata T, Agata K, Mochii M, Eguchi G, Fujisawa H. The A5 antigen, a candidate for the neuronal recognition molecule, has homologies to complement components and coagulation factors. Neuron. 1991;7(2):295–307. doi: 10.1016/0896-6273(91)90268-5. [DOI] [PubMed] [Google Scholar]
- Takenouchi N, Jones KS, Lisinski I, Fugo K, Yao K, Cushman SW, Ruscetti FW, Jacobson S. GLUT1 is not the primary binding receptor but is associated with cell-to-cell transmission of human T-cell leukemia virus type 1. J Virol. 2007;81(3):1506–1510. doi: 10.1128/JVI.01522-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trejo SR, Ratner L. The HTLV receptor is a widely expressed protein. Virology. 2000;268(1):41–48. doi: 10.1006/viro.2000.0143. [DOI] [PubMed] [Google Scholar]
- Yoshida M. Discovery of HTLV-1, the first human retrovirus, its unique regulatory mechanisms, and insights into pathogenesis. Oncogene. 2005;24(39):5931–5937. doi: 10.1038/sj.onc.1208981. [DOI] [PubMed] [Google Scholar]