Abstract
Objectives
The main goal was to develop a reproducible method for estimating the diffusion of water in human fetal lung tissue using diffusion weighted imaging. A secondary objective was to determine the relationship of the apparent diffusion coefficients (ADC) in the fetal lung to menstrual age and total lung volume.
Methods
Normal pregnant volunteers were scanned on a 1.5 Tesla MRI system. The MR system was equipped with 40 mT/m gradients (slew rate 200 T/m/s, rise time 0.2 msecs A 6-channel body array coil was used for signal reception. Single shot DWI utilized TE/TR = 125/3400 ms, slice thickness = 4 mm, field of view 280 mm × 280 mm, inter-slice gap 0.8 mm and a matrix of 128 × 128. The voxel size was 2.5 mm × 2.5 mm × 4.0 mm. Two b-values (0 and 1000) were chosen along three orthogonal directions. Apparent diffusion coefficient (ADC) maps were created using assigned b-values. Simple linear regression was performed with Pearson correlation coefficient. Inter- and intra- examiner bias and 95% limits of agreement (LOA) were determined using Bland-Altman plots.
Results
Forty-seven scans were performed at a mean of at 29.2 ± 4.5 weeks’ (1 SD). The median coefficient of variation for ADC was 5.6% [IQR 4.0 – 8.1%]. No differences in ADC values were found between right and left lungs. ADC values of the right lung were not significantly correlated with either total lung volume (r2 = 0.0001, p = 0.94, n.s) or menstrual age (r2 = 0.003, p = 0.70, n.s). The mean ADC value was 1.75 [95% CI, 1.63 – 1.86]. Intra-examiner bias was −0.15 ± 2.3 [95% limits of agreement, −4.7 to +4.4]. Inter-examiner bias was 2.2 ± 3.5 [95% limit of agreement, −4.7 to +9.1].
Conclusion
Our findings suggest that ADC measurements of the fetal lung are reproducible between blinded examiners and are independent of menstrual age, as well as lung volume.
Keywords: diffusion weighted imaging, magnetic resonance imaging, fetus, lung
Introduction
Fetal magnetic resonance imaging (MR) is useful for the prenatal detection of selected congenital anomalies because it provides images with superior tissue contrast resolution (1–3). The MR technology offers potential advantages over obstetrical ultrasonography because a wider field of view is obtained without the usual acoustic shadowing that is often encountered during pregnancy. Diffusion-weighted MRI (DWI) provides another type of image contrast that is based on the molecular motion of water - a process that can be altered by the presence of disease (4,5). The apparent diffusion coefficient (ADC) is based on these principles and reflects water movement within the tissue environment. In this context, ADC values have been well described for non-obstetrical applications that include the evaluation of newborns (6,7), adult brain ischemia (8) and tumors (9).
Only three other published studies have investigated the use of DWI to characterize water diffusion in human fetal lung tissue. In 2001, Moore and colleagues (10) first introduced this concept with a very low static magnetic field strength of only 0.5 Tesla to demonstrate that the ADC increased with gestational age. Two other studies, however, subsequently reported conflicting results regarding the relationship between ADC and gestational age under a higher magnetic field strength of 1.5 Tesla (11,12). The current investigation describes our experience with DWI during the second and third trimester of pregnancy. We document the range of ADC signal intensities for the fetal lung, reproducibility of ADC measurements, and the relationship of this parameter to menstrual age.
Methods
Research subjects were invited to participate under informed consent that was approved by the Human Investigation Committee at William Beaumont Hospital and the Institutional Review Board at the Eunice Shriver Kennedy National Institutes of Child Health and Human Development. All subjects had a previously normal prenatal ultrasound screening examination upon entry into this protocol. Pregnant volunteers were primarily scanned from the supine position on a 1.5 Tesla MRI system (Sonata, Siemens Medical Solutions). No maternal or fetal sedation was used. The MR system was equipped with 40 mT/m gradients with a rise time of 0.2 msecs (slew rate 200 T/m/s). A 6-channel body array coil was positioned near the fetus for the optimal signal reception.
For conventional imaging, non breath-hold HASTE and TRUE-FISP sequences provided proper localization and selection of a plane before implementing a rapid echo-planar sequence with DWI. Each DWI sequence took approximately 2 minutes. Single shot DWI utilized TE/TR = 125/3400 ms, slice thickness = 4 mm, field of view 280 mm × 280 mm, inter-slice gap 0.8 mm and a matrix of 128 × 128. The voxel size was 2.5 mm × 2.5 mm × 4.0 mm. Two b-values (0 and 1000) were chosen along three orthogonal directions. Fat suppression was used with a pixel bandwidth of 1500 Hz. With a parallel imaging factor (GRAPPA) of 2, the scan time with three averages was reduced to 63 seconds. Apparent diffusion coefficient (ADC) maps were created using assigned b-values.
The highest quality parallel slice acquisition series was chosen that utilized ADC maps of the fetal lung for each subject. Offline MR software was used to analyze signal intensities from traced regions of interest (ROI) (OsiriX DICOM software, WACOM Intuos graphics tablet). Total lung volume was calculated by manually tracing the best defined T2-weighted images for a given MR acquisition sequence (Figure 1). Next, the traced diffusion maps were used to estimate the coefficient of variation in the ADC measurement between slices for each subject. This information was used to decide whether or not it was really necessary to average the ADC values from all parallel image slices or to more simply use the largest image slice as a representative sample of the lung ADC value. If only the largest image slice was necessary, then the mean ADC value from this representative slice would be used to compare the right versus left lung as well as the relationship between ADC and menstrual age or total lung volume.
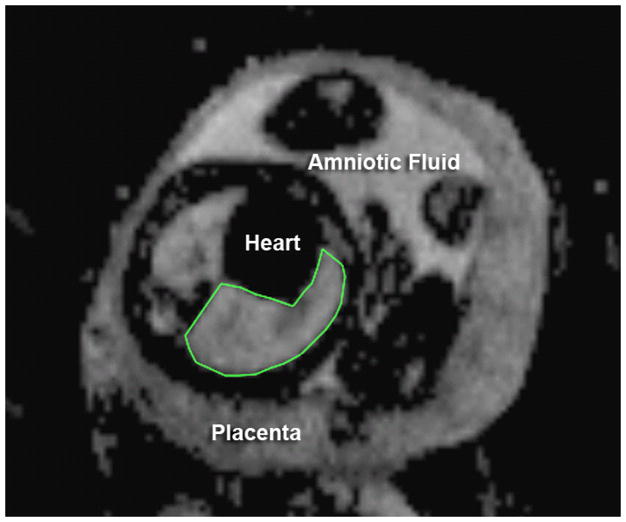
Figure 1.
Apparent diffusion coefficient (ADC) map of the axial fetal chest at 35 weeks, menstrual age. The right lung has been manually traced to estimate the mean signal intensity.
The Shapiro-Wilk normality test was used to evaluate the distribution of measurements. Simple linear regression was performed with Pearson correlation coefficient. Measurement bias and agreement were examined with their 95% limits of agreement using Bland-Altman plots (13). A paired t-test was used to evaluate possible differences in ADC measurements between left and right lungs. Statistical significance was taken at p < 0.05 level.
Results
Forty-seven MRI examinations were performed from January, 2006 to September, 2008 at a mean menstrual age of 29.2 ± 4.5 weeks (range 20.9 to 37.1 weeks). Newborn infants were delivered at 39.2 ± 1.5 weeks with a mean birth weight of 3,277 ± 455 grams. All infants had a normal newborn physical examination.
Each subject was examined for consistency of ADC measurements across fetal lung image slices. A median number of 5 slices (IQR 4 – 7] were acquired for each subject and the median coefficient of variation for ADC measurements among slices was 5.6% ([IQR 4.0 – 8.1]. Since the coefficient of variation was small, subsequent analysis of ADC values was based on the single largest lung slice from each fetus that was scanned using DWI. The mean ADC value was 1.75 [95% CI, 1.63 – 1.86].
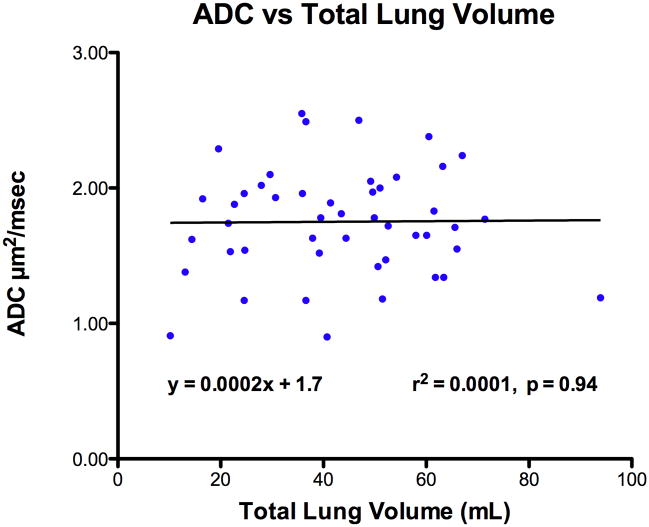
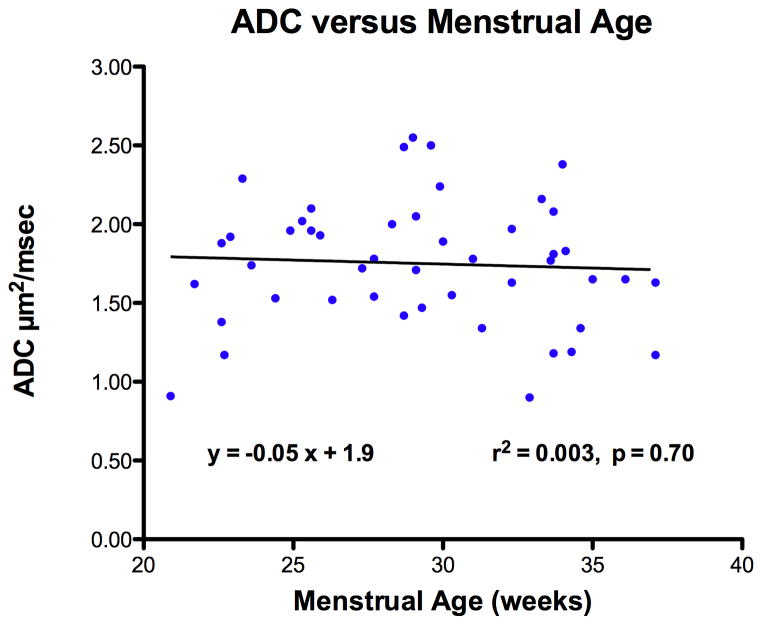
Total lung volume significantly increased with menstrual age (r2 = 0.57, p < 0.001). However, normally distributed ADC measurements were not significantly correlated with either total lung volume (r2 = 0.0001, p = 0.94, n.s) or menstrual age (r2 = 0.003, p = 0.70, n.s) (Figures 2 and 3). The mean right lung ADC value (1.75 ± 0.40) was not statistically different from the mean left lung measurements (1.71 ± 0.32) (p = 0.10, n.s.). Hence, subsequent analysis was based on ADC values from the right lung since it was easier to perform tracings without considering the fetal heart on the left side.
Figure 2.
Relationship of apparent diffusion coefficient (ADC) in fetal lung tissue to total tung volume.
Figure 3.
Relationship of apparent diffusion coefficient (ADC) in fetal lung tissue to menstrual age.
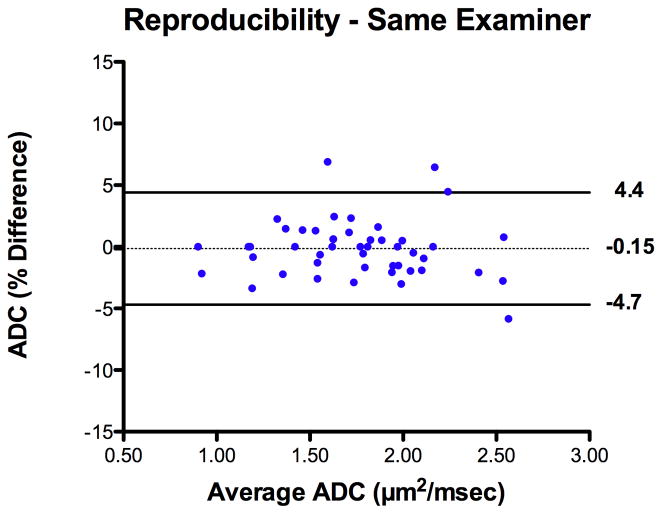
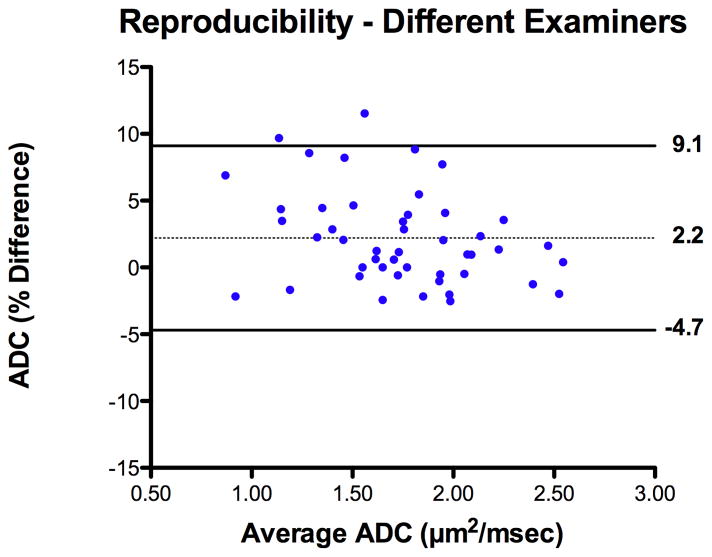
Reproducibility studies were performed for ADC measurements on the right lung. The intra-examiner bias and agreement was −0.1 ± 2.3% [95% limits of agreement −4.7 to 4.4%] (Figure 4). The inter-examiner bias and agreement was 2.2 ± 3.5% [95% limits of agreement −4.7 to 9.1%] (Figure 5).
Figure 4.
Bland Altman plot of intra-examiner measurement bias with 95% limits of agreement for fetal lung ADC measurements.
Figure 5.
Bland Altman plot of inter-examiner measurement bias with 95% limits of agreement for fetal lung ADC measurements.
Discussion
Simple diffusion can be described as the random movement of molecules from areas of high to low concentration within a medium. The process also causes signal loss during conventional T1 and T2 weighted MR sequences, although such effects are insignificant unless the relative signal change is amplified (14). This is achieved by applying a strong magnetic field gradient since the movement of water molecules attenuates the observed signal within a magnetic field gradient. The DWI methodology applies a T2-weighted sequence with two extra gradient pulses with equal amplitude and width. They are placed on either side of a 180-degree radiofrequency pulse. Static proton spins are de-phased by the first pulse and then completely re-phased by a second pulse. If the net protons have been moved by diffusion along that axis during the time interval between pulses, they will not experience the same amount of phase shift during the presence of a magnetic field gradient. As a result, the returning signal will be attenuated. The degree of signal attenuation is proportional to the strength of the diffusion weighting which is denoted by a “b-value”, a factor that is dependent on gradient length, amplitude and the spacing between them.
The diffusion of protons in free water is actually greater than what occurs in biological tissue. Such a process is influenced by the presence of natural barriers to translational motion, such as cell membranes and myelin fibers (i.e. anisotropy). The ADC calculation in biological tissue accounts for quantitative diffusion that varies from the orientation of restricting barriers in relation to the diffusion-sensitive-gradient direction. At least two b-values are required to estimate the water ADC value in a specific tissue of interest. If the b-value is too low, the diffusion induced signal attenuation will be comparable to variance of the DW data and this result will underestimate the ADC value. When an excessively large b value is chosen, the high signal attenuation and signal intensity may drop below the system noise level. The b value is commonly set to 1,000 s/mm2 in clinical practice and allows for sensitivity for changes in the diffusion coefficient while retaining an adequate signal to noise ratio (15). As a final technical consideration, changes in lung perfusion can theoretically affect the ADC value and mask the effects of water diffusion. However, the echo-planar nature of our rapid measurement sequence (spin echo with echo-planar read-out) provides temporal resolution that is sufficient enough to minimize any effects from flow, perfusion and gross body movement.
Relatively few studies have examined the application of DWI for ADC measurements in fetal lung. Moore and colleagues (10) were the first to use a low field strength MR system (0.5 T) for establishing a normal range of fetal ADC values in 26 subjects. Their results suggested a trend for ADC to increase with gestational age at a rate of 0.07 × 10−9 m2/sec per week. A second study by Balassy and co-workers (11) found small regional ADC differences within the right fetal lung that ranged from 1.91 to 2.13 μm2/ms when using a stronger 1.5 T MR system. Similar to the present study, ADC values did not significantly correlate with either menstrual age or lung volume. They proposed that the “relatively stable relation of the future air-space volume and the interstitium” was the reason why ADC values did not correlate with menstrual age. A third investigation by Manganaro et al. (12) also used a 1.5 T MR system on 50 subjects between 18–36 weeks gestation. Thirty-five of their fetuses had non-pulmonary anomalies. Their ADC values ranged from 1.2 μm2/ms at 18 weeks gestation to 3.9 μm2/ms at 36 weeks gestation with a significant correlation between ADC values and age (r = 0.82, p < 0.001).
Some of these discrepancies may be related to methodological differences in the manner by which ADC measurements were obtained and compared to gestational age. For example, Moore et al. (10) used a very low magnetic field strength gradient and globally averaged all ADC measurements from both lungs as they appeared on various images. Manganaro and colleagues (12) averaged ADC values from “all the lung parenchyma in all axial sections”. Our results were most consistent with the findings of Balassy and coworkers (11) who used the “best motion-artifact-free coronal slice that displayed the large surface of the lungs” to estimate ADC of the right fetal lung. Our study analyzed the largest image slice, regardless of the scanning plane, that provided good quality data that could be used as a representative sample of fetal lung tissue.
The reproducibility of these measurements has not been extensively examined for fetal lung tissue. Moore and colleagues did not specifically address this issue using a lower static magnetic field strength. Manganaro and co-workers (12) also did not examine the reproducibility of DWI measurements. Balassy et al. (11) reported that only 2 of the 30 average ADC measurements from 3 different lung regions exceeded the 95 percent limits of agreement although these measurements were obtained from only 10 subjects and gestational ages were not provided. Similar to their results, we used the largest lung image slice for the ADC calculation. Our technique for averaging ADC values from a single image slice of the right lung simplified the alternative requirement for multiple manual tracings and was associated with acceptable bias and agreement between examiners.
Validation of a reproducible ADC measurement technique for the fetal lung may provide a potential non-invasive biomarker of fetal lung maturity that is independent of both menstrual age and lung volume. Moore and colleagues (10) investigated the in-vitro effects of varying surfactant concentrations on T1 and T2 weighted imaging relaxation times as well as ADC values. None of these parameters, however, were significantly correlated with surfactant solution concentrations. They also examined a three-compartment in-vitro model that included intra-lung amniotic fluid, intra-tissue water, and vascular blood. The introduction of a vascular component to the lung model was associated with a dramatic increase in ADC. The model predicted the in-vivo data reasonably well and they proposed that fetal lung diffusion measurements could represent a novel marker for development of capillaries surrounding the terminal lung tubules. They also proposed that ADC measurements could be used to study fetal lung maturation. Although Balassy and co-workers (11) concluded that ADC was not a good indicator of lung maturity, their study design did not specifically examine outcome variables that could be used to directly test this hypothesis. Manganaro et al. (12) also hypothesized that lung ADC measurements could be used to study fetal pulmonary maturity because of the correlation that was found between ADC and menstrual age.
Diffusion weighted imaging can be used to characterize movement of water molecules through fetal tissue. Other investigators have described the use of this technology to evaluate the fetal brain (16–19), kidneys (20, 21), and twin-twin transfusion (22). Our findings indicate that ADC measurements of the fetal lung are reproducible between blinded examiners and are independent of menstrual age. A simplified approach for tracing a well-defined section of right lung appears to satisfactorily provide a representative sample of ADC values in the normal fetus. Future studies will need to correlate ADC measurements with amniotic fluid pulmonary surfactant and neonatal outcome to further examine the potential use of this non-invasive parameter for predicting the risk of respiratory distress syndrome.
Acknowledgments
The Authors wish to acknowledge the technical assistance of Melissa Powell, RDMS and Beverley McNie, BS, CCRP. This research was supported (in part) by the Perinatology Research Branch, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, DHHS.
References
- 1.Pugash D, Brugger PC, Bettelheim D, Prayer D. Prenatal ultrasound and fetal MRI: the comparative value of each modality in prenatal diagnosis. Eur J Radiol. 2008;68:214–26. doi: 10.1016/j.ejrad.2008.06.031. [DOI] [PubMed] [Google Scholar]
- 2.Benacerraf BR, Shipp TD, Bromley B, Levine D. What does magnetic resonance imaging add to the prenatal sonographic diagnosis of ventriculomegaly? J Ultrasound Med. 2007;26:1513–22. doi: 10.7863/jum.2007.26.11.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levine D. Obstetric MRI. J Magn Reson Imaging. 2006;24:1–15. doi: 10.1002/jmri.20608. [DOI] [PubMed] [Google Scholar]
- 4.Roberts TPL, Schwartz ES. Principles and implementation of diffusion-weighted and diffusion tensor imaging. Pediatr Radiol. 2007;37:739–48. doi: 10.1007/s00247-007-0516-z. [DOI] [PubMed] [Google Scholar]
- 5.Hagmann P, Jonasson L, Maeder P, Thiran JP, Wedeen VJ, Meuli R. Understanding diffusion MR imaging techniques: from scalar diffusion-weighted imaging to diffusion tensor imaging and beyond. Radiographics. 2006;26(Suppl 1):S205–23. doi: 10.1148/rg.26si065510. [DOI] [PubMed] [Google Scholar]
- 6.Beaulieu C, D’Arceuil H, Hedehus M, de Crespigny A, Kastrup A, Moseley ME. Diffusion-weighted magnetic resonance imaging: theory and potential applications to child neurology. Semin Pediatr Neurol. 1999;6:87–100. doi: 10.1016/s1071-9091(99)80035-7. [DOI] [PubMed] [Google Scholar]
- 7.Sagar P, Grant PE. Diffusion-weighted MR imaging: pediatric clinical applications. Neuroimaging Clin N Am. 2006;16:45–74. doi: 10.1016/j.nic.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 8.Schaefer PW, Copen WA, Lev MH, Gonzalez RG. Diffusion weighted imaging in acute stroke. Neuroimaging Clin N Am. 2005;15:503–530. doi: 10.1016/j.nic.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 9.Lim HK, Kim JK, Kim KA, Cho KS. Prostate cancer: apparent diffusion coefficient map with T2-weighted images for detection--a multireader study. Radiology. 2009;250:145–51. doi: 10.1148/radiol.2501080207. [DOI] [PubMed] [Google Scholar]
- 10.Moore RJ, Strachan B, Tyler DJ, Baker PN, Gowland PA. In vivo diffusion measurements as an indication of fetal lung maturation using echo planar imaging at 0.5 T. Magn Reson Med. 2001;45:247–53. doi: 10.1002/1522-2594(200102)45:2<247::aid-mrm1033>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 11.Balassy C, Kasprian G, Brugger PC, Csapo B, Weber M, Hormann M, Bankier A, Bammer R, Herold CJ, Prayer D. Diffusion-weighted MR imaging of the normal fetal lung. Eur J Radiol. 2006;57:261–70. doi: 10.1007/s00330-007-0784-x. [DOI] [PubMed] [Google Scholar]
- 12.Manganaro L, Perrone A, Sassi S, Fierro F, Savelli S, Di Maurizio M, Tomei A, Francioso A, La Barbera L, Giancotti A, Ballesio L. Diffusion-weighted MR imaging and apparent diffusion coefficient of the normal fetal lung: preliminary experience. Prenat Diagn. 2008;28:745–8. doi: 10.1002/pd.2041. [DOI] [PubMed] [Google Scholar]
- 13.Bland JM, Altman DG. Applying the right statistics: analyses of measurement studies. Ultrasound Obstet Gynecol. 2003;22:85–93. doi: 10.1002/uog.122. [DOI] [PubMed] [Google Scholar]
- 14.Prayer D, Prayer L. Diffusion-weighted magnetic resonance imaging of cerebral white matter development. Eur J Radiol. 2003;45:235–43. doi: 10.1016/s0720-048x(02)00312-1. [DOI] [PubMed] [Google Scholar]
- 15.Roberts TPL, Schwartz ES. Principles and implementation of diffusion-weighted and diffusion tensor imaging. Pediatr Radiol. 2007;37:739–748. doi: 10.1007/s00247-007-0516-z. [DOI] [PubMed] [Google Scholar]
- 16.Righini A, Bianchini E, Parazzini C, Gementi P, Ramenghi L, Baldoli C, Nicolini U, Mosca F, Triulzi F. Apparent diffusion coefficient determination in normal fetal brain: a prenatal MR imaging study. AJNR Am J Neuroradiol. 2003;24:799–804. [PMC free article] [PubMed] [Google Scholar]
- 17.Garel C. New advances in fetal MR neuroimaging. Pediatr Radiol. 2006;36:621–25. doi: 10.1007/s00247-006-0200-8. [DOI] [PubMed] [Google Scholar]
- 18.Cannie M, De Keyzer F, Meersschaert J, Jani J, Lewi L, Deprest J, Dymarkowski S, Demaerel P. A diffusion-weighted template for gestational age-related apparent diffusion coefficient values in the developing fetal brain. Ultrasound Obstet Gynecol. 2007;30:318–24. doi: 10.1002/uog.4078. [DOI] [PubMed] [Google Scholar]
- 19.Kim DH, Chung S, Vigneron DB, Barkovich AJ, Glenn OA. Diffusion-weighted imaging of the fetal brain in vivo. Magn Reson Med. 2008;59:216–20. doi: 10.1002/mrm.21459. [DOI] [PubMed] [Google Scholar]
- 20.Savelli S, Di Maurizio M, Perrone A, Tesei J, Francioso A, Angeletti M, La Barbera L, Ballesio L, de Felice C, Porfiri LM, Manganaro L. MRI with diffusion-weighted imaging (DWI) and apparent diffusion coefficient (ADC) assessment in the evaluation of normal and abnormal fetal kidneys: preliminary experience. Prenat Diagn. 2007;27:1104–11. doi: 10.1002/pd.1839. [DOI] [PubMed] [Google Scholar]
- 21.Chaumoitre K, Colavolpe N, Shojai R, Sarran A, D’ Ercole C, Panuel M. Diffusion-weighted magnetic resonance imaging with apparent diffusion coefficient (ADC) determination in normal and pathological fetal kidneys. Ultrasound Obstet Gynecol. 2007;29:22–31. doi: 10.1002/uog.3892. [DOI] [PubMed] [Google Scholar]
- 22.Righini A, Kustermann A, Parazzini C, Fogliani R, Ceriani F, Triulzi F. Diffusion-weighted magnetic resonance imaging of acute hypoxic-ischemic cerebral lesions in the survivor of a monochorionic twin pregnancy: case report. Ultrasound Obstet Gynecol. 2007;29:453–6. doi: 10.1002/uog.3967. [DOI] [PubMed] [Google Scholar]