Abstract
CONDENSATION
Body mass index is not associated with latency or the occurrence of maternal infectious complications during conservative management of preterm premature rupture of fetal membranes.
OBJECTIVE
Obesity has been associated with chronic inflammation. We hypothesized that body mass index (BMI) may be inversely related to latency and directly related to infectious complications following preterm premature rupture of membranes (pPROM).
STUDY DESIGN
This secondary analysis of a randomized trial of antibiotics for pPROM had information available for 562 subjects. We analyzed the association between BMI and latency, the occurrence of chorioamnionitis, endometritis and maternal infectious morbidity, after controlling for gestational age (GA) at rupture and treatment group. Survival analysis, regression and test of proportions were used as appropriate.
RESULTS
When evaluated as a categorical or continuous variable, BMI did not reveal any significant associations. Latency to delivery was affected by GA at rupture of membrane and antibiotic therapy, but not by BMI group.
CONCLUSION
BMI was not associated with latency or the occurrence of maternal infectious complications during conservative management of PROM before 32 weeks gestation.
Keywords: BMI, Body mass index, Obesity, Premature rupture of membranes, PROM
Introduction
Obesity continues to increase in prevalence nationally approaching fifty percent in some ethnic groups 1. Furthermore, pre-pregnancy obesity is becoming a common occurrence in obstetric management as more than 60% of women of child bearing age are considered overweight or obese 2. Overweight (body mass index [BMI] = 25-29.9 kg/m2) and obese individuals (BMI > 30) have a 50-100% increased risk of death from all causes, especially cardiovascular causes, compared with normal-weight individuals 3. Furthermore, obesity and overweight have been identified as major modifiable risk factors for adverse pregnancy outcomes with strong associations to maternal, fetal and neonatal complications.
Preterm premature rupture of membranes (pPROM) complicates 3% of all pregnancies annually in the United States, affecting over 120,000 pregnancies each year. Preterm PROM is responsible for up to 33% of all preterm births 4, yet, there are limited data evaluating the relationship between obesity and pPROM. Specifically, whether or not the latency period after pPROM is affected by BMI has not previously been explored. In a recent study, the risk of neonatal death after pPROM was greater for overweight women than for women of normal weight. The authors of this study point out that the effect of infection on the fetus may be much more severe when the fetal membranes have ruptured, especially with long time spans between rupture of membranes and labor. 5 Given that several studies have shown that obesity represents a low-grade chronic inflammatory state 6;7, it is biologically plausible that obesity may shorten the latency period after pPROM.
Our primary objective was to explore the relationship between BMI and latency period following pPROM during conservative management of pPROM remote from term. Secondly, we wanted to determine if overweight or obesity in the setting of pPROM is associated with an increased risk of chorioamnionitis, postpartum endometritis or other infectious morbidity in the mother.
Materials and Methods
This is a secondary analysis from women with singleton gestations complicated by pPROM at 240 to 320 weeks. These participants, from 11 clinical centers, were recruited to participate in a randomized trial of antibiotic therapy to prolong pregnancy and reduce morbidity. Candidates with pPROM, no contraindication to pregnancy prolongation (non-reassuring fetal status, severe pre-eclampsia, etc.) and who had not received corticosteroids for fetal maturation or antibiotic treatment within 1 week of randomization were included in the trial. Detailed methodology for the trial is reported elsewhere 8. The protocol was approved by the Human Research committees of all participating institutions. A total of 614 gravidas with pPROM (585 singleton gestations) who were not in labor, and were considered to be candidates for conservative management, were randomized to either antibiotic therapy or a matching placebo regimen. The antibiotic regimen consisted of intravenous ampicillin (2g q6h) and erythromycin (250 mg q6h) for 48 hours, followed by oral amoxicillin (250 mg q8h) and erythromycin-base (333 mg q8h) for 5 days unless delivery occurred. Participating women were expectantly managed in hospital unless fluid leakage stopped and amniotic fluid volume returned to normal. Elective labor induction was prohibited before 34 weeks and discouraged subsequently.
Body mass index (BMI) was divided into five categories based on the accepted CDC definitions: underweight (BMI <18.5), normal weight (18.5 ≤ BMI ≤ 24.9), overweight (25 ≤ BMI ≤ 29.9), obese (30 ≤ BMI ≤ 39.9) and morbid obesity (BMI ≥ 40). Some comparisons looked only at obese (BMI ≥ 30) versus non-obese (18.5 ≤ BMI < 30). The impact of BMI on latency from randomization to delivery, clinically diagnosed chorioamnionitis, endometritis and maternal infectious morbidity were evaluated. The diagnosis of clinical chorioamnionitis required at least 2 of: antepartum temperature > 100.4 F, uterine tenderness, foul smelling vaginal discharge or amniotic fluid, maternal tachycardia (> 100 bpm), fetal tachycardia (> 160 bpm), or white blood cell count > 20,000. Endometritis was clinically diagnosed with postpartum temperature > 100.4 F and uterine tenderness with other signs and symptoms to support the diagnosis that included foul smelling lochia, chills, and lower abdominal pain. Maternal infectious morbidity included Cesarean delivery wound infection, urinary tract infection diagnosed post-partum, episiotomy wound infection, pyelonephritis, and first line antibiotics failure. The relationship between BMI categories and neonatal composite outcome (one or more of: fetal/neonatal death, neonatal sepsis within 72 hours of birth, grade 3-4 intraventricular hemorrhage, stage 2-3 necrotizing enterocolitis, or respiratory distress syndrome) by treatment groups was also evaluated.
Statistical analyses were performed using SAS statistical software (SAS Institute Inc, Cary, NC). To assess the relationship between obstetric outcomes and five categories of BMI, the Chi-square test or Fisher’s exact test was used for categorical variables and the Kruskal wallis test was used for continuous outcomes. Logistic regression analysis controlling for GBS status, antibiotic therapy, and gestational age at ROM was also performed to evaluate the independent effects of body mass index (BMI) on obstetric outcomes. Survival analysis, using the Kaplan-Meier method, and the Ccox proportional hazard model was performed to determine if body mass index was associated with shortened latency to delivery.
Results
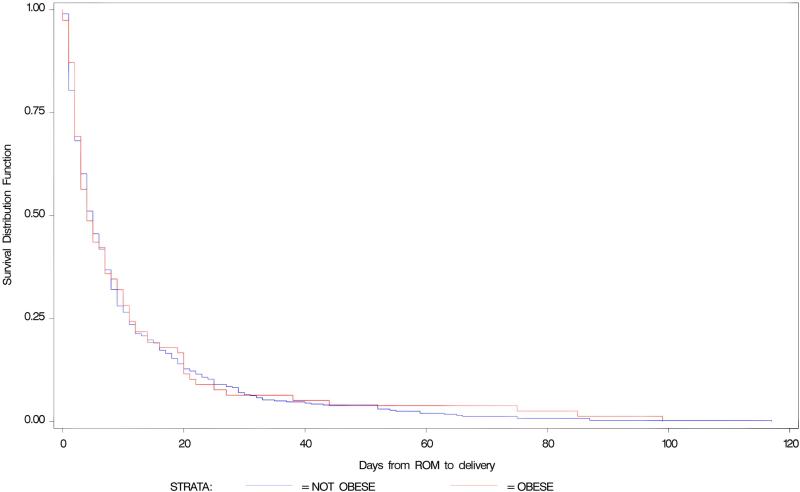
Between February 1992 and January 1995, a total of 562 of the enrolled 614 patients met eligibility requirements and were included in this analysis. Of the 52 patients excluded, 32 patients did not have a singleton gestation and the remaining 20 patients had incomplete data related to prepregnancy weight for BMI calculation. The demographic characteristics for these patients are listed in Table I. There were 85 underweight (BMI < 18.5 kg/m2), 287 normal weight (BMI = 18.5-24.9 kg/m2), 112 overweight (BMI = 25-29.9 kg/m2), 67 obese (BMI = 30-39.9 kg/m2) and 11 morbidly obese women (BMI ≥ 40 kg/m2) in the cohort. Table II reveals the cases of chorioamnionitis, endomyometritis, maternal infectious morbidity identified in the study and latency to delivery stratified by each BMI group. No significant differences were found among five BMI groups. When comparing obese (BMI ≥ 30) to non-obese (excluding the underweight patients), the median days from rupture to delivery in the obese group was 4 days with interquartile range 2-11 days, and was 5 days with interquartile range 2-11 days in the non-obese group. There was no significant difference between the latency to delivery (survival) curves of obese and non-obese group (p= 0.60) when controlling for GA at ROM, antibiotic treatment assignment and GBS (Figure I). However, increased latency was associated with decreased gestational age at randomization (p < 0.0001) and with antibiotic therapy (p = 0.002).
Table 1. Patient demographics.
| Underweight | Normal | Overweight | Obese | Morbid Obesity | P value | |
|---|---|---|---|---|---|---|
| Maternal Age | 23.7 (5.7) | 24.5 (6.1) | 25.6 (6.0) | 26.9 (5.7) | 28.5 (6.7) | 0.0009 |
|
GA at Randomization |
28.8 (2.3) | 28.7 (2.2) | 28.5 (2.3) | 27.8 (2.5) | 27.3 (2.2) | 0.0125 |
| Race (%) | ||||||
| Caucasian | 28 (32.9) | 86 (30.0) | 23 (20.5) | 24 (31.3) | 3 (27.2) | 0.1868 |
| Black | 52 (61.2) | 166 (57.8) | 69 (61.6) | 38 (56.7) | 7 (63.6) | |
| Hispanic | 3 (3.5) | 28 (9.8) | 19 (17.0) | 8 (11.9) | 1 (9.1) | |
| Others | 2 (2.4) | 7 (2.4) | 1 (0.9) | 0 | 0 | |
| Previous preterm births (#) | ||||||
| 1 | 54 (63.5) | 201 (70.0) | 68 (60.7) | 50 (74.6) | 8 (72.7) | 0.2402 |
| 2 or more | 31 (36.5) | 86 (30.0) | 44 (39.3) | 17 (25.4) | 3 (27.3) | |
| Diabetes (%) | ||||||
| Yes | 1 (1.2) | 5 (1.7) | 5 (5.4) | 8 (11.9) | 1 (4.8) | 0.0008 |
| Treatment Group (%) | ||||||
| Active | 41 (48.2) | 142 (49.5) | 60 (53.6) | 31 (46.3) | 6 (54.6) | 0.8837 |
Table II. Outcomes stratified by Body Mass Index category.
| Underweight (n = 85) |
Normal (n = 287) |
Overweight (n = 112) |
Obese (n = 67) |
Morbid Obesity (n = 11) |
P value* | |
|---|---|---|---|---|---|---|
| Chorioamnionitis | ||||||
| N (%) | 13 (15.3%) | 44 (15.3%) | 24 (21.4%) | 14 (20.9%) | 1 (9.09%) | 0.4793 |
| OR (95% CI) | 0.98 (0.51-1.95) | - | 1.51 (0.87-2.62) | 1.46 (0.75-2.85) | 0.55 (0.01-4.07)¶ | |
| Postpartum Endometritis | ||||||
| N (%) | 7 (8.3%) | 36 (12.5%) | 12 (10.7%) | 11 (16.7%) | 2 (18.18%) | 0.4794 |
| OR (95% CI) | 0.63 (0.27 - 1.48) | - | 0.83 (0.42 - 1.67) | 1.39 (0.67 -2.90) | 1.54 (0.16 - 7.88) ¶ | |
| Infectious Morbidity | ||||||
| N (%) | 12 (14.3%) | 41 (14.3%) | 16 (14.3%) | 16 (23.9%) | 2 (18.2%) | 0.3846 |
| OR (95% CI) | 1.00 (0.50 - 2.00) | - | 1.00 (0.53 - 1.86) | 1.87 (0.98 - 3.60) | 1.33 (0.13 - 6.74) ¶ | |
| Neonatal Composite Outcome | ||||||
| N (%) | 42 (49.4%) | 126 (44.1%) | 52 (46.4%) | 37 (55.2%) | 8 (72.7%) | 0.2092 |
| OR (95% CI) | 1.24 (0.76 - 2.01) | - | 1.10 (0.71- 1.71) | 1.57 (0.92 - 2.67) | 3.39 (0.79 - 20.14) ¶ | |
| Days from rupture to delivery | ||||||
| Median (interquartile range) | 5 (2-11) | 4 (2-11) | 6 (2-12) | 4 (2-12) | 7 (2-11) | 0.8869§ |
| Hazard Ratio (95% CL) | 1.04 (0.82 - 1.33) | - | 0.92 (0.74 - 1.14) | 0.97 (0.85 - 1.11) | 1.01 (0.82 - 1.23) |
P values were calculated from chi-square test across all BMI groups.
95% exact confidence intervals.
P value was calculated from Kruskal-Wallis non-parametric test across all BMI groups.
Fig. 1.
Survival Analysis on latency of obese and nonobese patients.
The Kruskal-Wallis test revealed no difference in the median latency stratified by treatment group. Logistic regression analysis controlling for GBS status, antibiotic therapy, and gestational age at ROM did not show that BMI evaluated as a continuous variable was significantly associated with chorioamnionitis (OR = 1.01, 95%CI 0.97-1.04, p = 0.73), endometritis (OR = 1.02, 95%CI 0.98-1.06, p = 0.38) or any maternal postpartum infectious morbidity (OR = 1.03, 95%CI 0.99-1.07, p = 0.10). Similarly, this logistic model did not reveal a significant relationship between the outcome and BMI (OR = 1.00, 95%CI 0.97-1.04, p = 0.84). When evaluating the relationship between BMI categories and neonatal composite outcome by treatment groups, neither was found significant (placebo p = 0.21, antibiotic group p = 0.39).
Comment
As obesity increases in prevalence nationally approaching fifty percent in some ethnic groups, it has become a public health concern of significant proportions. Prepregnancy obesity has been associated with a range of metabolic, inflammatory, and vascular abnormalities during pregnancy, even in pregnant women without clinical disease.9;10 Some authors have speculated that endothelial dysfunction and inflammatory up-regulation due to obesity accelerate the timing and severity of some pregnancy complications and may lead to the birth of more vulnerable infants among obese women. 5 Other studies have evaluated the role of obesity in the setting of preterm birth. These studies have revealed a risk reduction for spontaneous preterm birth in obese women, but a high percentage of the indicated preterm births, often in association with preeclampsia. 11-13
We did not demonstrate an association with BMI and infectious morbidities.A limitation of this study is the small sample size with only 14.4% and 1.9% of patients being obese and morbidly obese, respectively. Because we considered our analysis to be exploratory in nature, we did not conduct a power analysis in advance. While it is not useful to conduct an ad-hoc power analysis based on the observed results, the information from the analysis does allow us to estimate the prevailing rate of the outcomes in the normal weight group, and to evaluate the sample size needed to adequately evaluate a clinically meaningful difference from that baseline for future studies. For example, a sample size of approximately 400 patients would be needed to detect a 1/3 increase in composite neonatal outcome in obese women compared with normal weight women, with 80% power and type 1 error of 5% 2-sided.
Several studies have shown that obesity is associated with increased production of systemic proinflammatory cytokines,9 therefore it is biologically plausible that obesity may shorten the latency period after pPROM. Although this study controlled for maternal infectious morbidities, levels of cytokines were not available for evaluation. Other factors such as malnutrition, vitamin D deficiency, etc. were also not available for comparison.
This study suggests that BMI does not affect latency to delivery or the occurrence of maternal infectious complications during conservative management of PROM before 32 weeks gestation. Maternal obesity should not alter decision-making regarding conservative management of PROM remote from term.
Acknowledgments
This work was supported by grants HD21434, HD21410, HD27917, HD27915, HD27869, HD27905, HD27861, HD27860, HD27889, HD27883, HD21414 and HD36801 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, Maryland.
Appendix
In addition to the authors, participating members of the Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network were as follows
University of Alabama at Birmingham: Hauth JC, Goldenberg RL, Copper RL
University of Chicago: Moawad AH, Lindheimer M, Jones P, Brown M
The Ohio State University: Landon MB, Johnson F, Meadows S
University of Oklahoma: Thurnau GR, Carey JC, Meier A, Minton V
Medical University of South Carolina: Newman R, Collins B, Stramm S
University of Tennessee: Sibai BM, Manners L
Wake Forest University Health Sciences: Meis PJ, Mueller-Heubach E, Swain M, Phillips G
University of Cincinnati: Siddiqi TA, Elder N
University of Pittsburgh: Caritis SN, Harger JH, Cotroneo M
National Institute of Child Health and Human Development: McNellis D, Yaffe SJ, Catz C, Klebanoff M
University of Southern California: Kovacs C, McCart D
Medical College of Virginia: Dinsmoor M, McCoy S
Wayne State University: Dombrowski MR, Norman GS, Wilson-Lacey D
George Washington University Biostatistics Center: Thom E, Das A, Leuchtenburg L, Rowland B
Footnotes
Presented at the 28th Annual Meeting of the Society for Maternal-Fetal Medicine, Dallas, TX.
Reference List
- 1.ACOG - Obesity Obesity in pregnancy. Obstet Gynecol. 2005;106:671–75. doi: 10.1097/00006250-200509000-00054. ACOG Committee Opinion number 315, September 2005. [DOI] [PubMed] [Google Scholar]
- 2.Ogden CL, Carroll MD, McDowell MA, Flegal KM. Obesity among adults in the United States - no change since 2003-2004. 2007. NCHS data brief no 1.Ref Type: Journal (Full)
- 3.Reece EA. Perspectives on obesity, pregnancy and birth outcomes in the United States: the scope of the problem. Am J Obstet Gynecol. 2008;198:23–27. doi: 10.1016/j.ajog.2007.06.076. [DOI] [PubMed] [Google Scholar]
- 4.Meis PJ, Ernest JM, Moore ML. Causes of low birth weight births in public and private patients. Am J Obstet Gynecol. 1987;156:1165–68. doi: 10.1016/0002-9378(87)90133-5. [DOI] [PubMed] [Google Scholar]
- 5.Nohr EA, Vaeth M, Bech BH, Henricksen TB, Cnattingius S, Olsen J. Maternal Obesity and Neonatal Mortality According to Subtypes of Preterm Birth. Obstet Gynecol. 2007;110:1083–1090. doi: 10.1097/01.AOG.0000286760.46679.f8.Ref Type: Journal (Full)
- 6.Cottam DR, Mattar SG, Barinas-Mitchell E, Eid G, Kuller L, Kelley DE, Schauer PR. The chronic inflammatory hypothesis for the morbidity associated with morbid obesity: implications and effects of weight loss. Obes Surg. 2004;14:589–600. doi: 10.1381/096089204323093345.Ref Type: Journal (Full)
- 7.Lin Y, Lee H, Berg AH, Lisanti MP, Shapiro L, Scherer PE. The lipopolysaccharide-activated toll-like receptor (TLR)-4 induces synthesis of the closely related receptor TLR-2 in adipocytes. J Biol Chem. 2000;275:24255–24263. doi: 10.1074/jbc.M002137200.Ref Type: Journal (Full)
- 8.Mercer B, Miodovnik M, Thurnau G, Goldenberg R, Das A, Ramsey RD, et al. the NICHD-MFMU Network Antibiotic therapy for reduction of infant morbidity after preterm premature rupture of the membranes. JAMA. 1997;278:989–995.Ref Type: Journal (Full)
- 9.Ramsay JE, Ferrell WR, Crawford L, Wallace AM, Greer IA, Sattar N. Maternal obesity is associated with dysregulation of metabolic, vascular, and inflammatory pathways. J Clin.Endocrinol.Metab. 2002;87:4231–37. doi: 10.1210/jc.2002-020311. [DOI] [PubMed] [Google Scholar]
- 10.Retnakaran R, Hanley AJ, Raif N, Connelly PW, Sermer M, Zinman B. C-reactive protein and gestational diabetes: the central role of maternal obesity. J Clin.Endocrinol.Metab. 2003;88:3507–12. doi: 10.1210/jc.2003-030186. [DOI] [PubMed] [Google Scholar]
- 11.Smith GC, Shah I, Pell JP, Crossley JA, Dobbie R. Maternal obesity in early pregnancy and risk of spontaneous and elective preterm deliveries: a retrospective cohort study. Am J Public Health. 2007;97:157–62. doi: 10.2105/AJPH.2005.074294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hendler I, Schatz M, Momirova V, Wise R, Landon M, Mabie W, et al. Association of obesity with pulmonary and nonpulmonary complications of pregnancy in asthmatic women. Obstet Gynecol. 2006;108:77–82. doi: 10.1097/01.AOG.0000223180.53113.0f. [DOI] [PubMed] [Google Scholar]
- 13.Hendler I, Goldenberg RL, Mercer BM, Iams JD, Meis PJ, Moawad AH, et al. The Preterm Prediction Study: association between maternal body mass index and spontaneous and indicated preterm birth. Am J Obstet Gynecol. 2005;192:882–86. doi: 10.1016/j.ajog.2004.09.021. [DOI] [PubMed] [Google Scholar]