Abstract
Objectives
We hypothesized that maternal treatments with betamethasone acetate (Beta-Ac) induce fetal lung maturation comparably to the betamethasone phosphate (Beta-PO4) + Beta-Ac (Celestone®) used clinically.
Study Design
Ewes with singleton pregnancies were treated with single doses of 0.25 mg/kg or 0.5 mg/kg Beta-Ac, 4 doses of 0.25 mg/kg Beta-PO4, a single dose of 0.5 mg/kg Beta-Ac + 0.25 mg/kg Beta-PO4, 2 doses of 0.25 mg/kg Beta-Ac + 0.25 mg Beta-PO4 or vehicle beginning 48 h before preterm delivery. Fetal lung maturation was evaluated.
Results
All treatments induced lung maturation relative to vehicle controls. The relatively insoluble Beta-Ac resulted in low maternal blood Beta and no detectable fetal blood Beta in 2 of 3 fetuses, but induced fetal lung maturation comparable to the 2 dose Beta-Ac + Beta-PO4 or 4 doses of Beta-PO4.
Conclusions
A single maternal dose of Beta-Ac effectively induces fetal lung maturation in sheep with minimal fetal exposure.
Keywords: Corticosteroid, Fetal Therapy, Preterm, Respiratory Distress Syndrome, Sheep 7
INTRODUCTION
Single treatment courses with the fluorinated corticosteroids betamethasone (Beta) or dexamethasone (Dex) are standard of care for women at high risk of preterm delivery before 32–34 wks gestation 1. Despite 21 randomized trials for single course treatments, and more recently 6 trials of repeated courses of corticosteroids 2, 3, the type and dosing schedules for the antenatal treatments remain empiric and are not based on a sound pharmacologic understanding of how maternal corticosteroids induce fetal lung maturation 4, 5. The principal formulation of Beta used worldwide is a 1 to 1 mixture of two pro-drugs, the soluble Beta-phosphate, and the less soluble suspension of Beta-acetate formulated as Celestone® 1, 2. The Beta-PO4 is dephosphorylated and the Beta-Ac is deacylated to release the active Beta. We have reported a number of observations that suggest the relationships between types of corticosteroids, the formulation of the corticosteroids, and route of administration can result in quite different lung maturational responses in fetal sheep. Single fetal doses of cortisol, Beta-PO4 or Dex-PO4 or a single maternal dose of Beta-PO4 do not induce fetal lung maturation 4, 6, 7, while repeated doses of cortisol do 7, suggesting a prolonged exposure is required. One dose of Beta-PO4 + Beta-Ac causes lung maturation in fetal sheep, but a second dose given 24 h later, as is used clinically, increases the lung maturation response 4. However, fetal exposure to these corticosteroids does not explain all of the fetal effects. Fetal treatment with Beta-PO4 + Beta-Ac achieves high fetal plasma Beta levels and causes some lung maturation, but no growth restriction 8, 9. In contrast, maternal treatment with Beta-PO4 + Beta-Ac results in lower fetal plasma Beta levels, but more lung maturation and fetal growth restriction 8, 10. Low dose Dex-PO4 given to the ewe also causes fetal growth restriction 11. We initially thought that the Beta-Ac component of the mixture used clinically would not contribute to the clinical responses because of its insolubility 12. However, a single maternal dose of 0.25 mg Beta-Ac/kg maternal weight induced some maturation, while a single dose of 0.5 mg/kg Beta-PO4 had no maturational effect 4. Therefore, we hypothesized that Beta-Ac may be the more active agent in the Beta-PO4 + Beta-Ac mixture. We report measurements of maternal and fetal plasma Beta levels and fetal lung maturational responses to Beta-Ac and Beta-PO4 in different doses and combinations in sheep.
MATERIALS and METHODS
Plasma Levels of Betamethasone
The studies were approved by the animal ethics committees of Cincinnati Children's Hospital and The University of Western Australia. The initial study was a measurement of plasma Beta levels in the ewe and fetus. Ewes with singleton fetuses had maternal and fetal arterial and venous catheters placed by standard surgical techniques 13. Three ewes were treated with 0.25 mg/kg Beta-Ac and 2 ewes received 0.5 mg/kg of a 1:1 mixture of Beta-PO4 and Beta-Ac (see formulations of Beta below). Maternal and fetal blood samples were collected over 48 h into chilled tubes containing EDTA and sodium azide and plasma was recovered after centrifugation. Potassium fluoride was added and the plasma was frozen until analysis. The azide and fluoride were added to prevent the Beta-PO4 and Beta-Ac prodrugs from enzymatic cleavage to free Beta 14, 15. Analyses for Beta, Beta-PO4 and Beta-Ac were performed by mass spectroscopic techniques by Analpharm, Quebec, Canada. The detection limits were 1 ng/ml for Beta and Beta-Ac, and 5 ng/ml for Beta-PO4.
Betamethasone Treatments for Lung Maturation
Merino ewes with singleton fetuses were weighed and treated with Beta provided by Schering-Plough (Summit, NJ) as Beta-PO4, Beta-Ac milled to a mean particle size of 4 to 12 microns or as Beta-Ac + Beta-PO4, in a 1:1 mixture as Celestone Soluspan®. The Beta-Ac milled suspension was the same material used to formulate Celestone Soluspan. The characteristics of the suspension influences solubility characteristics. Ewes were weighed and randomized for IM injections with the vehicle or the Beta as follows: Vehicle 2 d and 1 d before delivery (control), 0.25 mg Beta-Ac/kg 2 d and vehicle 1 d before delivery (Beta-Ac 0.25), 0.50 mg Beta-Ac/kg 2 d and vehicle day 1 before delivery (Beta-Ac 0.50), four doses of 0.25 mg Beta-PO4/kg given every 12 h beginning 2 d before delivery (Beta-PO4 x 4), a mixture of 0.5 mg/kg Beta-Ac and 0.25 mg/kg Beta-PO4 given 2 d and vehicle 1 d before delivery (Beta- Ac + Beta- PO4), and 0.5 mg Celestone Soluspan/kg 2 d and 1 d before delivery (Celestone x 2) (Table 1). All lambs were delivered 48 h after the initial treatment at 123 or 124 d gestation.
TABLE 1.
Summary of Animals
| Group | Number | Birth Wt (kg) | Sex M/F | Cord pH | Cord Paco2 (mmHg) |
|---|---|---|---|---|---|
| Control | 11 | 2.7±0.1 | 7/4 | 7.20±0.03 | 72±4 |
| Beta-Ac 0.25 | 11 | 2.5±0.1 | 6/5 | 7.22±0.02 | 68±3 |
| Beta-Ac 0.50 | 11 | 2.5±0.1 | 5/6 | 7.26±0.03 | 64±3 |
| Beta-Phos × 4 | 11 | 2.5±0.1 | 8/3 | 7.28±0.02 | 62±3 |
| Beta-Ac + Phos | 12 | 2.5±0.1 | 8/3 | 7.31±0.01a | 60±2a |
| Celestone × 2 | 12 | 2.5±0.1 | 7/5 | 7.32±0.02a | 62±2 |
p<0.05 vs. control
Assessment of Lung Function
The ewes were heavily sedated with intravenous ketamine (12 mg/kg) and medetomidine (0.12 mg/kg) and given spinal anesthesia with lidocaine. The head of each fetus was delivered through abdominal and uterine incisions. The fetus received an intramuscular injection of 10 mg ketamine/kg estimated fetal weight, and the skin over the trachea was infused with lidocaine. A 4 mm endotracheal tube was placed through a tracheotomy and secured 4. The fetus was delivered, weighed, and placed on a radiant warmer (Cozy Cot™, Fisher & Paykel Healthcare, NZ) with a plastic cover (Neowrap™, Fisher & Paykel, NZ) to maintain temperature. Mechanical ventilation was initiated with 100% heated and humidified oxygen. The initial ventilator settings were a rate of 40 breaths/minute, a peak inspiratory pressure of 35 cmH2O, a positive end expiratory pressure of 5 cmH2O, and an inspiratory time of 0.7 seconds. An umbilical artery catheter was placed to permit blood sampling. An umbilical venous catheter was used for supplemental anesthesia with propofol (0.1 mg/kg/min) and remifentanyl (0.05 µg/kg/min) infusions. The lambs were ventilated for 30 minutes with blood gas measurements at 10, 20, and 30 minutes. Tidal volume (VT) was measured continuously with a Florian Infant Monitor (Acutronic Medical Systems, Hirzel, Switzerland). Only peak inspiratory pressure was changed to target Paco2 at 50 to 60 mm Hg, with a tidal volume of <10 ml/kg. Peak inspiratory pressures were limited to 40 cmH2O pressure to avoid pneumothorax. Compliance per kilogram body weight was calculated as: VT/ΔP/kg, where ΔP is peak minus end expiratory pressure. Ventilation efficiency index is an integrated measure of ventilation that is calculated as: Ventilation efficiency index = 3800/(ΔP.f.Paco2), where 3800 is a CO2 production constant, and f is ventilator rate for these lambs without spontaneous breathing 16. The investigators who delivered and ventilated the lambs and assessed the lungs were not aware of the treatment that the ewe had received.
Assessments of Lung
After the collection of a final arterial blood sample at 30 minutes of age, the lambs were anesthetized with pentobarbital, and the tracheal tube was clamped for 3 minutes to achieve atelectasis by oxygen absorption. The lamb received a lethal dose of pentobarbital, was weighed and exanguinated. The chest was opened, and the lungs were evaluated visually for lung injury. A deflation pressure-volume curve was measured after air inflation of the lungs to a pressure of 40 cmH2O 17.
Surfactant Protein mRNA
The messenger RNAs (mRNAs) for surfactant proteins A, B, and C were measured with S1 nuclease protection assays, as described previously 18.
Statistical Analysis
Data are presented as mean±SEM. Statistical comparisons were performed with Instat software (GraphPad Software Inc, San Diego, CA). Data were compared between controls and each of the 5 corticosteroid treatment groups with 1-way analysis of variance (ANOVA) with the Tukey post hoc test for multiple comparisons. Selected group comparisons with controls by 2-tailed t tests were also made. A difference between groups was considered significant at p<.05.
RESULTS
Betamethasone in Maternal and Fetal Blood
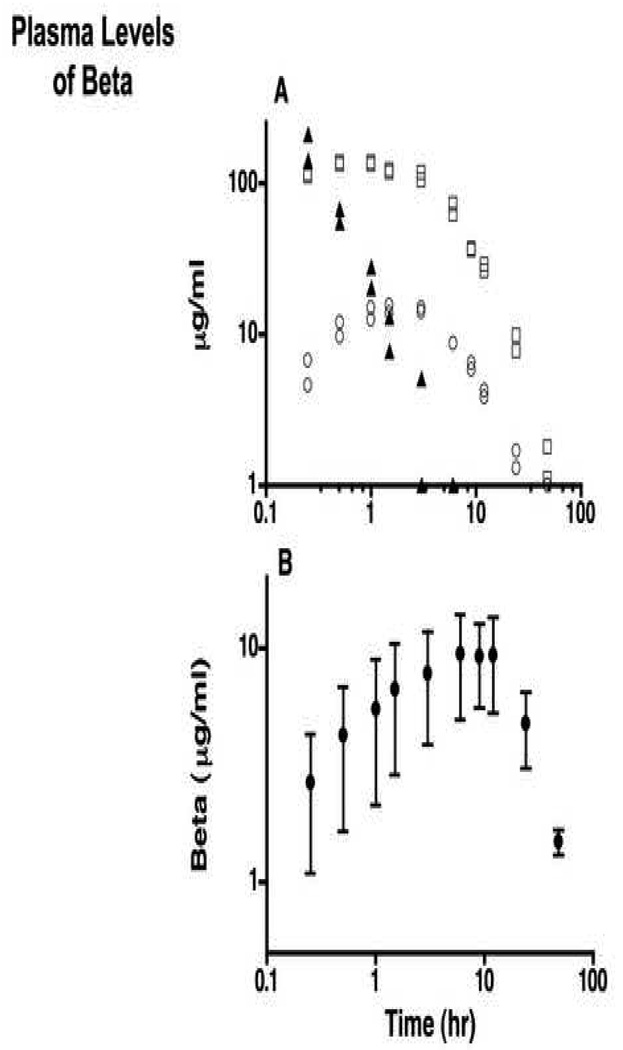
Two ewes received 0.5 mg/kg Beta-Ac + Beta - PO4 (Celestone Soluspan) (Fig. 1A). The Beta-PO4 prodrug was detectable in maternal blood for 3 h with rapid clearance half-life of <0.5 h. Beta peaked at about 130 ng/ml at about 0.5 to 1 h with an initial clearance with a half-life of about 5 h. Beta levels were just detectable in the plasma from the ewe at 48 h. No Beta-Ac was detectable in the maternal blood at any time. Fetal blood contained no Beta-PO4 or Beta-Ac. Beta was detected in fetal blood for 24 h with a peak of about 15 ng/ml at 1.5 h. In contrast in 3 animals given 0.25 mg/kg Beta-Ac, maternal Beta increased to a peak average value of about 9 ng/ml at 6–12 h. Beta was detected at low levels to 48 h (Fig. 1B). Beta was not detected in two fetuses and in the third Beta was detected at low levels between 3 and 24 h (maximum value 2.4 µg/ml). No Beta-Ac was detected in ewe or fetal plasma. The maternal treatments with Beta-Ac resulted in low maternal and fetal exposures to Beta.
Fig. 1.
Plasma levels of Beta and Beta-PO4. A. Two ewes received 0.25mg Beta-Ac/kg plus 0.25 mg Beta-PO4/kg (Celestone) by intramuscular injection. Plasma levels of Beta-PO4 in each ewe (▲), plasma levels of Beta in each ewe (□), and fetus (○) were measured to 48 h. The individual values are shown. B. Three ewes received 0.25 mg Beta-Ac/kg by intramuscular injection and plasma levels of Beta in the plasma of the ewe were measured (mean±SE).
Description of Preterm Lambs
The numbers, birth weights, sex distributions, and cord arterial pH and Paco2 values for the lambs assessed for lung maturation are given in Table 1. There were no differences in birth weights between groups. The differences in pH in cord blood reflect the Paco2 values resulting from the anesthesia and the position of the ewe for delivery.
Ventilation Outcomes
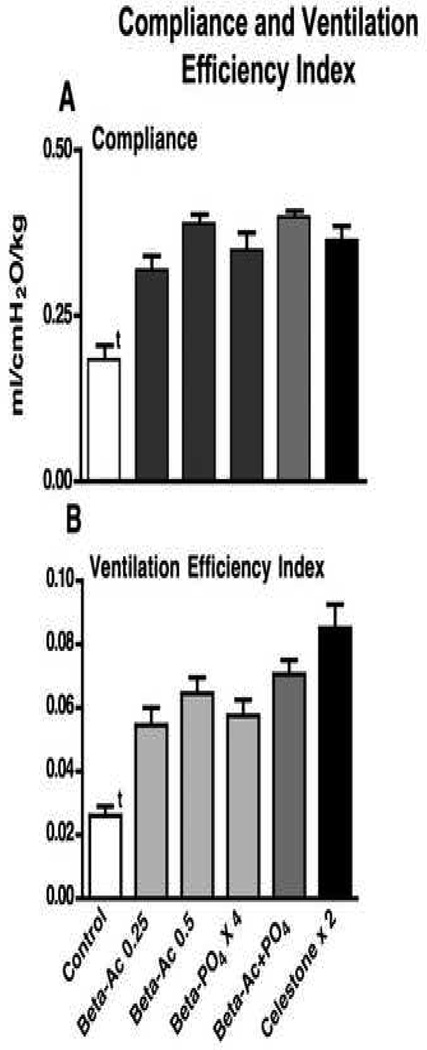
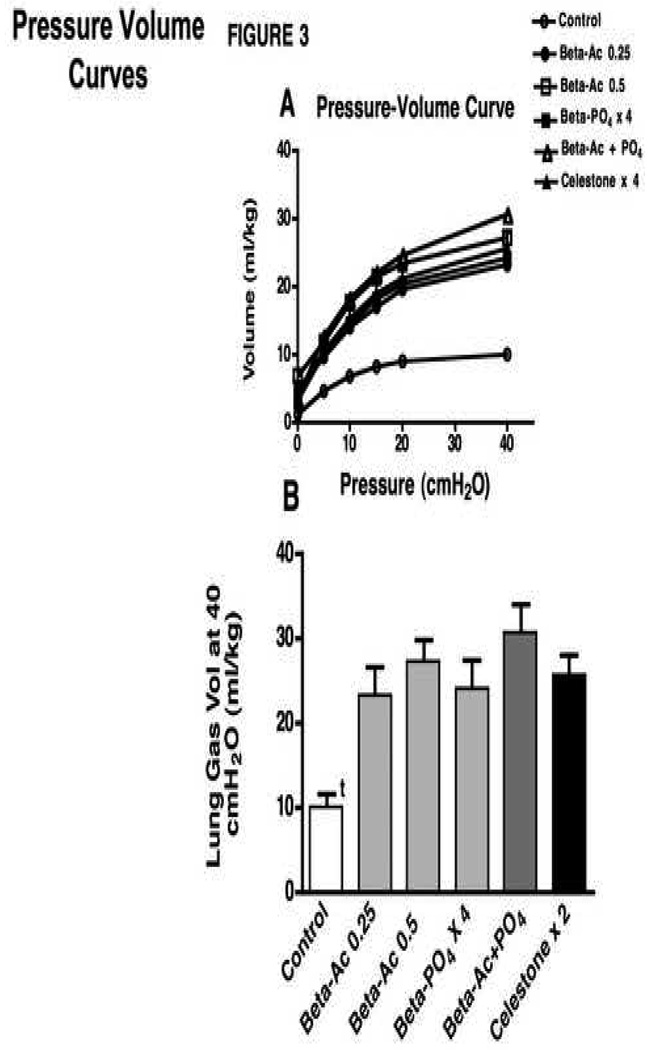
The newborn lambs were ventilated for 30 minutes in a standardized fashion to evaluate lung mechanics and gas exchange (Table 2). All Beta treatments resulted in better pH and Paco2 values than for controls. Oxygenation was significantly higher for the Beta-Ac groups and the 4 dose Beta-PO4 group relative to control by ANOVA. The Pao2 values were higher than control values for the Celestone and Beta-Ac plus Beta-PO4 groups by t-tests (p<0.05). Higher VT and better gas exchange were achieved despite lower ventilatory pressures. Dynamic compliance, the integrated assessment of lung mechanics, increased consistently for all treatment groups relative to controls with no differences between groups (Fig. 2A). Similarly, the Ventilation Efficiency Index, which combines the ventilatory pressure and rate with Paco2 to assess gas exchange, was improved for all animals treated with any of the Beta formulations relative to controls (Fig. 2B). The deflation limb of the static pressure volume curve quantifies the changes in fetal lung gas volume as a result of maternal Beta treatment. The curves from the Beta treated lambs overlap and were not different from each other, but were different from the control curve (Fig. 3A). The return of all curves to close to 0 ml volume at 0 cmH2O pressure indicated a minimal contribution of surfactant to these curves. The maximal volumes at 40 cmH2O pressure were higher for all treatment groups relative to controls (Fig. 3B). These increased volumes were large and similar for all treatment groups. The Beta treatments, and especially the two dose Celestone treatment, did tend to increase the amount of lung damage that was evident on examination of the lungs (Table 2).
TABLE 2.
Ventilatory Variables after 30 min Ventilation
| Group | Blood Gas-30 min | |||||
|---|---|---|---|---|---|---|
| pH | Paco2 (mmHg) | Pao2 (mmHg) | Vent.Pressure cmH2O | VT/kg (ml/kg) | Gross Lung Injury (PIE, Bleeding, Blebs) | |
| Vehicle Control | 6.96±0.04 | 117±9 | 94±20 | 34.3±0.4 | 6.2±0.7 | 3/11 |
| Beta-Ac 0.25 | 7.18±0.03a | 63±5a | 259±38a | 30.3±1.0 | 9.4±0.4a | 6/11 |
| Beta-Ac 0.50 | 7.21±0.03a | 62±3a | 269±48a | 25.9±1.2a | 9.9±0.2a | 6/11 |
| Beta-Phos x 4 | 7.22±0.03a | 62±5a | 330±28a | 28.1±1.6a | 9.4±0.5a | 4/11 |
| Beta-Ac + Phos | 7.25±0.03a | 56±2a | 214±36 | 24.8±1.2a | 9.7±0.2a | 4/12 |
| Celestone x 2 | 7.33±0.02a | 52±4a | 212±42 | 26.0±1.4a | 9.1±0.2a | 9/12 |
p<0.05 vs. control
Fig. 2.
Compliance and ventilation efficiency index after 30 min of ventilation. A Compliance values increased for all treatment groups relative to control and there were not differences between treatment groups. B Ventilation efficiency index increased for all treatment groups relative to control. The value for the Beta-Ac 0.25 group was less than for the Celestone × 2 group by t test (p<0.02). t p<0.01 for control less than for all treated groups.
Fig. 3.
Pressure volume curves after 30 min of ventilation. A Curves for the Beta treated groups overlap and are different from the control curve (p<0.01). B Lung gas volumes measured at 40 cmH2O for the Beta treated groups are different from the control value but are not different from each other. t p<0.01, control relative to other groups.
Surfactant Protein mRNA
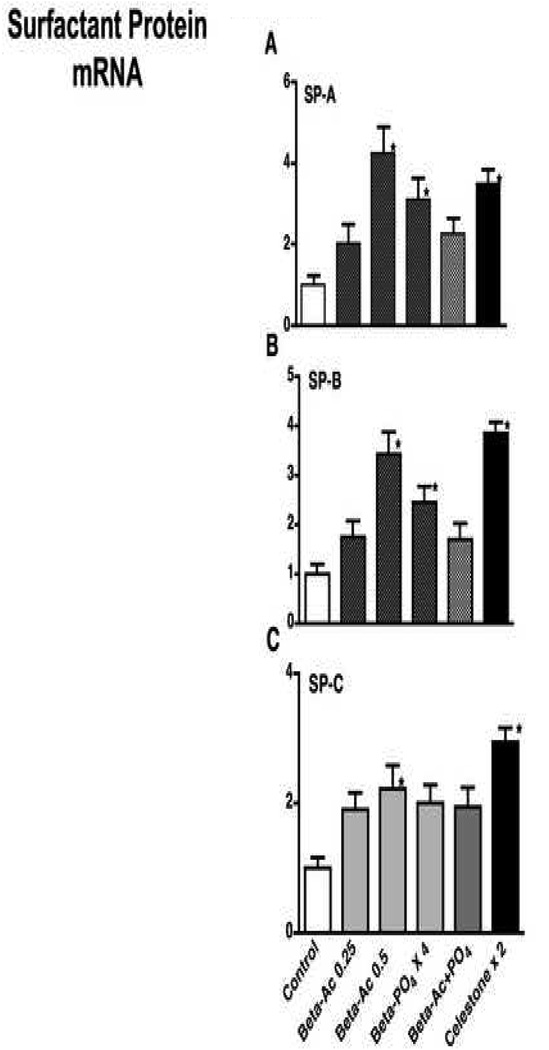
All Beta exposures qualitatively increased the mRNA levels for SP-A, SP-B, and SP-C relative to controls with many of the increases significant by ANOVA relative to controls (Fig. 4). There were no significant differences between the Beta treatment groups.
Fig. 4.
Surfactant protein (SP) mRNA in lung tissue. A Values for SP-A mRNA. B SP-B mRNA. C. SP-C mRNA. Bar graphs marked with an asterisk are different from controls by ANOVA with 5 RNA samples tested for each group. All other measurements are different from controls (p<0.05) by t-test except SP-A and SP-B for 0.25-Beta-Ac, and SP-B for Beta-Ac + PO4.
COMMENT
These are extraordinary results in terms of the amount and formulation of Beta-Ac that matured the fetal sheep lung within 48 h. A single dose of 0.25 mg/kg maternal weight resulted in very low maternal and fetal Beta levels. Yet the lung maturational effects for the one 0.25 mg Beta-Ac/kg dose were equivalent for most of the assessments and the 0.5 mg Beta-Ac/kg dose was as effective as 2 doses of Celestone (total Beta dose - 1 mg/kg). The use of Beta-Ac has two potential benefits - lower total maternal dose of Beta and much lower fetal exposures to Beta. We included the 4-dose Beta-PO4 group to test if we could induce lung maturation with Beta-PO4 alone. We reported previously that single doses of 0.25 mg/kg or 0.5 mg/kg Beta-PO4 given to the ewe or a single fetal dose of 0.5 mg/kg estimated fetal weight of Dex did not induce fetal lung maturation 4, 6, 7. The 4-dose Beta-PO4 exposure did induce equivalent lung maturation to the other treatments, but at a much higher Beta exposure for the fetus. There was no advantage of using the mixture of 0.25 mg Beta-PO4/kg and 0.5 mg Beta-Ac/kg. These results suggest that although the current dosing strategies of 2 doses of Celestone or 4 doses of Dex are effective, there may be dosing strategies using only Beta-Ac or other corticosteroid preparations that might be safer because of lower fetal exposures to the corticosteroids.
We reported previously that a maternal dose of 0.25 mg/kg Beta-Ac had only modest effects on lung maturation 4. That result probably differs from this result because we used an uncharacterized suspension of Beta-Ac precipitated from alcohol. The pharmacological preparation used in Celestone is milled to a uniform particle size of 4 to 12 microns and was the material used for these experiments. We have no other explanation for less fetal response to Beta-Ac in the previous report 4. We tested groups of animals with two different doses of Beta-Ac in this study and found consistent responses. These disparate results illustrate that investigators and pharmaceutical companies must work together to explore treatment strategies, as precise formulations may be as or more important than dose.
The discussion as to the best corticosteroid to use for maternal treatments remains unsettled. The observational and cohort studies comparing Beta with Dex suggest that Beta is associated with fewer postnatal deaths and less brain injury with perhaps better acute respiratory effects 12, 19, 20. In contrast, a recent randomized control trial comparing Dex-PO4 and Celestone suggests more brain injury and comparable other outcomes with the Beta 21, a conclusion that is supported by a meta-analysis that is dominated by this one trial 5. However, the recent trials of repeated courses of corticosteroids have used Beta 2. Several of those randomized-controlled trials of repeated courses of Beta were stopped early because of concerns about the adverse neurodevelopmental effects of repeated courses 22, 23, and a recent trial found repeated courses caused small, but significant effects on fetal growth 3. In contrast, a recent trial reported significant respiratory benefit from a single "rescue" treatment with Celestone, and without adverse effects 24. Kutzler, et al.11 reported that repeated courses of four 2 mg doses of Dex caused growth restriction in fetal sheep. We do not know if the Beta-Ac doses that we used caused growth restriction, because we assessed the animals just 2 d after the initial treatment. We also do not know if there would be differences in the magnitude or persistence of effects for treatment to delivery intervals longer than 48 h. The lung responses to the different doses were not uniform in that all Beta treatments caused similar improvements in compliance and the pressure-volume curves, but a single dose of 0.5 mg Beta-Ac and two doses of 0.5 mg/kg Celestone qualitatively caused the largest increase in SP-B mRNA. These responses probably result from the different time courses of responses after treatment. Longer treatment to delivery intervals will be needed to assess the final effects of the fetal responses to these corticosteroid treatments. An hypothesis is that the low fetal exposure to Beta following maternal Beta-Ac treatment might decrease risks of adverse neurodevelopmental and growth effects. That hypothesis needs to be tested because high fetal Beta levels do not cause growth restriction in sheep if the Beta is given to the fetus, while maternal Beta treatments cause fetal growth restriction 9. Our results demonstrate that very low maternal and fetal exposures to Beta are associated with fetal lung maturation, but it is unknown if these effects are a direct effect of the corticosteroid or secondary to other maternal/placental responses to the corticosteroid.
For historical reasons, the clinical trials of single course or repeated courses of corticosteroids continue to be conducted with the same Beta and Dex formulations and doses 1, 2. These trials done over a period of 38 years were difficult and expensive to do, and it no longer is possible to randomize patients to a no treatment/placebo group for the initial corticosteroid treatment. However, we suggest that the pharmacology of maternal-fetal responses to corticosteroids is poorly understood - particularly with respect to differing outcomes such as growth effects and lung maturation. Clinically relevant studies can only be performed in long gestation animals or humans. A safer dose and formulation of corticosteroid for maternal treatments should be evaluated.
ACKNOWLEDGEMENTS
None
Funding: This work was funded in part by grant HL-65397 and by a grant from Schering Plough, Summit, New Jersey. JJP is supported by a Sylvia and Charles Viertel Senior Medical Research Fellowship and GRP is supported by a NHFA and NHMRC Fellowship. Infrastructure support was provided by the Women and Infants’ Research Foundation, WA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Roberts D, Dalziel S. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2006;3:CD004454. doi: 10.1002/14651858.CD004454.pub2. [DOI] [PubMed] [Google Scholar]
- 2.Crowther CA, Harding JE. Repeat doses of prenatal corticosteroids for women at risk of preterm birth for preventing neonatal respiratory disease. Cochrane Database Syst Rev. 2007:CD003935. doi: 10.1002/14651858.CD003935.pub2. [DOI] [PubMed] [Google Scholar]
- 3.Murphy KE, Hannah ME, Willan AR, et al. Multiple courses of antenatal corticosteroids for preterm birth (MACS): a randomised controlled trial. Lancet. 2009;372:2143–2151. doi: 10.1016/S0140-6736(08)61929-7. [DOI] [PubMed] [Google Scholar]
- 4.Jobe A, Moss TJM, Nitsos I, Ikegami M, Kallapur SG, Newnham JP. Betamethasone for lung maturation: Testing dose and formulation in fetal sheep. American Journal of Obstetrics and Gynecology. 2007;97:523–526. doi: 10.1016/j.ajog.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brownfoot FC, Crowther CA, Middleton P. Different corticosteroids and regimens for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2008:CD006764. doi: 10.1002/14651858.CD006764.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jobe AH, Polk D, Ikegami M, et al. Lung responses to ultrasound-guided fetal treatments with corticosteroids in preterm lambs. J. Appl. Physiol. 1993;75:2099–2105. doi: 10.1152/jappl.1993.75.5.2099. [DOI] [PubMed] [Google Scholar]
- 7.Jobe AH, Newnham J, Moss TJ, Ikegami M. Differential effects of maternal betamethasone and cortisol on lung maturation and growth in fetal sheep. American Journal of Obstetrics and Gynecology. 2003;188:22–28. doi: 10.1067/mob.2003.61. [DOI] [PubMed] [Google Scholar]
- 8.Berry LM, Polk DH, Ikegami M, Jobe AH, Padbury JF, Ervin MG. Preterm newborn lamb renal and cardiovascular responses after fetal or maternal antenatal betamethasone. Am. J. Physiol. 1997;272:R1972–R1979. doi: 10.1152/ajpregu.1997.272.6.R1972. [DOI] [PubMed] [Google Scholar]
- 9.Jobe AH, Newnham J, WilletK, Sly P, Ikegami M. Fetal Versus Maternal and Gestational Age Effects of Repetitive Antenatal Glucocorticoids. Pediatrics. 1998;102:1116–1125. doi: 10.1542/peds.102.5.1116. [DOI] [PubMed] [Google Scholar]
- 10.Jobe AH, Wada N, Berry LM, Ikegami M, Ervin MG. Single and repetitive maternal glucocorticoid exposures reduce fetal growth in sheep. Am J Obstet Gynecol. 1998;178:880–885. doi: 10.1016/s0002-9378(98)70518-6. [DOI] [PubMed] [Google Scholar]
- 11.Kutzler MA, Ruane EK, Coksaygan T, Vincent SE, Nathanielsz PW. Effects of three courses of maternally administered dexamethasone at 0.7, 0.75, and 0.8 of gestation on prenatal and postnatal growth in sheep. Pediatrics. 2004;113:313–319. doi: 10.1542/peds.113.2.313. [DOI] [PubMed] [Google Scholar]
- 12.Jobe AH, Soll RF. Choice and dose of corticosteroid for antenatal treatments. American Journal of Obstetrics and Gynecology. 2004;190:878–881. doi: 10.1016/j.ajog.2004.01.044. [DOI] [PubMed] [Google Scholar]
- 13.Newnham JP, Polk DH, Kelly RW, et al. Catecholamine response to ultrasonographically guided percutaneous blood sampling in fetal sheep. Am. J. Obstet. Gynecol. 1994;171:460–465. doi: 10.1016/0002-9378(94)90283-6. [DOI] [PubMed] [Google Scholar]
- 14.Samtani MN, Lohle M, Grant A, Nathanielsz PW, Jusko WJ. Betamethasone pharmacokinetics after two prodrug formulations in sheep: implications for antenatal corticosteroid use. Drug Metab Dispos. 2005;33:1124–1130. doi: 10.1124/dmd.105.004309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smtani MN, Schwab M, Nathanielsz PW, Jusko WJ. Stabilization and HPLC analysis of betamethasone sodium phosphate in plasma. J Pharm Sci. 2004;93:726–732. doi: 10.1002/jps.10577. [DOI] [PubMed] [Google Scholar]
- 16.Notter RH, Egan EA, Kwong MS, Holm BA, Shapiro DL. Lung surfactant replacement in premature lambs with extracted lipids from bovine lung lavage: effects of dose, dispersion technique, and gestational age. Pediatr Res. 1985;19:569–577. doi: 10.1203/00006450-198506000-00014. [DOI] [PubMed] [Google Scholar]
- 17.Jobe AH, Newnham JP, Willet KE, et al. Endotoxin induced lung maturation in preterm lambs is not mediated by cortisol. Am. J. Respirt. Crit. Care Med. 2000;162:1656–1661. doi: 10.1164/ajrccm.162.5.2003044. [DOI] [PubMed] [Google Scholar]
- 18.Bachurski CJ, Ross GF, Ikegami M, Kramer BW, Jobe AH. Intra-amniotic endotoxin increases pulmonary surfactant components and induces SP-B processing in fetal sheep. Am J Physiol Lung Cell Mol Physiol. 2001;280:L279–L285. doi: 10.1152/ajplung.2001.280.2.L279. [DOI] [PubMed] [Google Scholar]
- 19.Lee BH, Stoll BJ, McDonald SA, Higgins RD. Adverse neonatal outcomes associated with antenatal dexamethasone versus antenatal betamethasone. Pediatrics. 2006;117:1503–1510. doi: 10.1542/peds.2005-1749. [DOI] [PubMed] [Google Scholar]
- 20.Feldman DM, Carbone J, Belden L, Borgida AF, Herson V. Betamethasone vs dexamethasone for the prevention of morbidity in very-low-birthweight neonates. Am J Obstet Gynecol. 2007;197:284. doi: 10.1016/j.ajog.2007.07.010. e1–4. [DOI] [PubMed] [Google Scholar]
- 21.Elimian A, Garry D, Figueroa R, Spitzer A, Wiencek V, Quirk JG. Antenatal betamethasone compared with dexamethasone (betacode trial): a randomized controlled trial. Obstet Gynecol. 2007;110:26–30. doi: 10.1097/01.AOG.0000268281.36788.81. [DOI] [PubMed] [Google Scholar]
- 22.Guinn DA, Atkinson MW, Sullivan L, et al. Single vs Weekly Courses of Antenatal Corticosteroids for Women at Risk of Preterm Delivery: A Randomized Controlled Trial. Jama. 2001;286:1581–1587. doi: 10.1001/jama.286.13.1581. [DOI] [PubMed] [Google Scholar]
- 23.Wapner RJ, Sorokin Y, Thom EA, et al. Single versus weekly courses of antenatal corticosteroids: evaluation of safety and efficacy. Am J Obstet Gynecol. 2006;195:633–642. doi: 10.1016/j.ajog.2006.03.087. [DOI] [PubMed] [Google Scholar]
- 24.Kurtzman J, Garite T, Clark R, Maurel K. Network TOCR. Impact of a "rescue" course of antenatal corticosteroids (ACS): A multi-center randomized placebo controlled trial. American Journal of Obstetrics and Gynecology. 2008 doi: 10.1016/j.ajog.2009.01.021. Abstract, SMFM:S2 - December Supplement. [DOI] [PubMed] [Google Scholar]