Abstract
Background& Aims
A history of early adverse life events (EAL) is associated with a poorer outcome and higher levels of distress in adult patients with functional gastrointestinal disorders. EAL is thought to predispose individuals to develop a range of chronic illnesses by inducing persistent changes in the central stress response systems, including the hypothalamic-pituitary-adrenal (HPA) axis. We sought to determine if EAL affects the HPA axis response to a visceral stressor in IBS patients and healthy controls, and to determine if this is affected by sex or related to symptoms or quality of life (QOL).
Methods
44 IBS patients (25 women, 19 men) and 39 healthy controls (21 women, 18 men) were assessed for gastrointestinal and psychological symptoms and EAL by validated questionnaires and interview. All subjects underwent a visceral stressor (sigmoidoscopy). Salivary cortisol was collected at baseline and serially for 1 hour post-stressor.
Results
21 IBS patients and 18 controls had EAL. In subjects with and without IBS, EAL was associated with higher mean (±SD) cortisol levels (0.32±0.2 vs. 0.20±0.1 μg/dl; p =0.003) and higher area under the curve (28.1±17 vs. 18.6±13 (μg·min/dL; p=0.005) following the stressor compared to subjects without EAL. In IBS, a faster resolution of cortisol to basal values corresponded to lower symptom-severity (r=−0.36, p<0.05) and better disease-specific QOL (r=0.33, p<0.05).
Conclusions
HPA axis hyperresponsiveness to a visceral stressor is more related to a history of EAL than to the presence of IBS. However, HPA axis reactivity has a moderating effect on IBS symptoms.
Keywords: irritable bowel syndrome, early adverse life events, abuse, trauma, cortisol, epigenetics
Introduction
Extensive preclinical and clinical evidence supports the concept that irritable bowel syndrome (IBS) is a stress-related disorder. For example, stressful life events are associated with first symptom onset and symptom exacerbation in a majority of patients1 and predict development of post-infectious IBS.2, 3 In addition, experimental stress increases visceral sensitivity4 and alters gut physiology.5 However, evidence from IBS patients remains inconclusive regarding alterations in the peripheral output from the two arms of the central stress response system—the hypothalamic-pituitary-adrenal (HPA) axis and the sympathetic nervous system (SNS). While there is more agreement about an increase in SNS tone and/or responsiveness,6, 7 data about the HPA axis are less consistent. Different laboratories have reported increased basal cortisol levels, 8–10 enhanced responses to physiologic11 or psychological4 stressors, blunted HPA axis responses,12, 13 or no difference between IBS and control groups.14, 15 These differences may be due in part to differences in study methodology (e.g., basal vs. stimulated cortisol levels, free vs. bound cortisol, time of day samples are collected) or in the patient populations studied (including presence or absence of psychiatric comorbidity, gender, history of stressful life events).
Interpretation of these findings is complicated by the presence of other modifiers of the HPA axis response in the IBS population. Epidemiological studies have found an increased prevalence of early adverse life events (EAL) in patients with IBS when compared to healthy controls and individuals with organic gastrointestinal disease.16–20 EAL refers to traumatic experience or significant perturbations in the quality of the family environment or relationship of the primary caregiver with the child before the age of 14,21 and includes but is not limited to abuse and loss or severe illness of a parent.17, 22 When defined in this way, EAL are surprisingly common. In a retrospective study of over 17,000 adults in a U.S. health maintenance organization, over 60% reported at least one EAL before the age of 18.23
A growing body of evidence from both animal and human studies supports the hypothesis that EAL represents an important epigenetic factor, which can alter glucocorticoid receptor (GR) expression, thereby affecting the responsiveness of the HPA axis, and resulting in life-long changes that predispose individuals with EAL to develop stress-related disorders later in life.17, 21, 24–26 Specifically, EAL has been associated with exaggerated HPA axis responses to corticotropin releasing factor stimulation in women27 and psychological stress in adolescents. 28 However, in HPA axis studies conducted in IBS patients, EAL have generally not been evaluated. In the one exception,8 the prevalence of EAL was too low in both the IBS and control groups to detect an impact. We therefore sought to test the general hypothesis that stress-induced changes in salivary cortisol responses are associated with the presence of EAL in IBS patients and controls. We measured the salivary (free) cortisol response to a visceral stressor (flexible sigmoidoscopy) to test the following hypotheses: 1) IBS patients have a higher or more prolonged cortisol response to the visceral stressor compared to controls; and 2) The response would be highest in IBS patients with EAL. We also sought to determine whether this response was related to sex, symptom ratings or disease-specific quality of life (QOL).
Methods
Study Subjects and Recruitment
IBS patients 18–55 years of age, who were recruited from specialty clinics and community advertisements, fulfilled ROME II29 diagnostic criteria and diagnosis was confirmed by a gastroenterologist with expertise in IBS (LC). Healthy controls were recruited by newspaper or internet advertisement from the community, did not have a history of IBS, other chronic pain conditions, or psychiatric illness, and were not taking centrally acting drugs (anxiolytics, narcotics, antidepressants). None of the subjects had taken corticosteroids or other medications that could affect neuroendocrine function in the past two months or had a current history of tobacco or alcohol abuse. For premenopausal women not taking oral contraceptive agents or provera, menstrual cycle phase was determined by the count forward/backward method (menses: first three days of menses; follicular: days 4–14; luteal: day 14 - onset of menses). Serum progesterone was collected to help confirm cycle phase. Subjects were compensated $25 for an initial screening visit and $125 for completing the flexible sigmoidoscopy.
Symptom Measures
Validated questionnaires were used for IBS symptoms (UCLA Bowel Symptom Questionnaire),30 depression and anxiety (Hospital Anxiety and Depression [HAD] scale), 31 and disease-specific QOL assessment.32 History of trauma or abuse was assessed by the Trauma History Questionnaire33 which inventoried the presence and age(s) of occurrence of traumatic events including crime, general trauma and disaster, and physical and sexual abuse. “Early life” was defined as under the age of 14. Subjects were screened by interview for abuse history and psychiatric disorders using the structured clinical interview for the DSM-IV (SCID) by a licensed therapist (MM).34
Protocol
Subjects first completed a screening visit. On a separate day, sigmoidoscopy to at least 40 cm from the anal verge was performed in the Medical Procedures Unit between 12:00pm–2:00pm. Subjects were instructed to use two tap-water enemas as the bowel preparation. Saliva samples were collected at 7 time-points: before sigmoidoscopy (baseline), immediately following sigmoidoscopy, and 10, 20, 30, 45, and 60 minutes after sigmoidoscopy.
Salivary cortisol measurements
Salivary cortisol levels were measured using a high sensitivity enzyme immunoassay system (Salimatrics; State College, PA).35, 36 Intra-assay and inter-assay variation is less than 5% and 7%, respectively.
Statistical analysis
Missing values
For all analyses excluding mixed models, missing values were replaced with the mean value for the subgroup based on disease (IBS, control) and sex (female, male), as long as there was data for the cortisol level at 10 minutes post-stressor, which was the peak cortisol for most subjects.
Baseline characteristics
Groups were compared by chi-square tests for categorical variables, and t-tests for continuous variables.
Cortisol data
All statistical tests requiring normally distributed data were performed on square-root transformations of the data. Data presented in tables and figures is raw un-transformed data. For an analysis of cortisol change relative to baseline, cortisol values were divided by the baseline cortisol for that subject.
Group differences
Cortisol response was evaluated by repeated measures mixed-effects analyses using the ProcMixed procedure of Statistical Analysis Software (SAS). Analyses were performed with the 7 cortisol samples as the within-subjects factor and disease, EAL or sex as the between-subjects (group) factor. The model yielding the best fit among the covariance structures was determined by the lowest Akaike’s Information Criteria.
Cortisol response summary measures
In order to summarize the response in one variable, area under the curve (AUC) was calculated.37 Several formulas for calculation of AUC have been described. 38 AUC was calculated with respect to the x-axis (ground) in order to represent the overall magnitude of response (AUCg) and with respect to the minimum value for each subject (AUCm). Another summary measure, rate of change, is usually calculated as the difference between the initial value and the maximum value divided by the elapsed time.37 Because the initial cortisol was sampled prior to sigmoidoscopy, the final cortisol values may represent a more physiological baseline, so the rate of decline was determined by dividing the difference between the maximum cortisol occurring up to 30 minutes post-sigmoidoscopy and the minimum after this time point by the time elapsed between those samples.
Correlation with symptoms
The relationship between AUC and symptoms or QOL in IBS patients was evaluated by bivariate correlation analysis.
Results
Subject characteristics
The characteristics of the subjects are summarized in Table 1. The IBS patients included 26 women and 19 men, and the control group included 22 healthy women and 19 healthy men. The IBS patients had higher HAD anxiety and depression scores than controls. There were 3 subjects without data on EAL, and there were 7 additional subjects who were eliminated from AUC analysis due to incomplete cortisol data (Women: 1 control without EAL, 2 IBS [1 without EAL, 1 with sexual abuse]; Men: 2 controls without EAL, 2 IBS [1 without EAL, 1 with loss]). Three subjects missing all cortisol data (Women: 1 control; Men: 1 control, 1 IBS; all without EAL) were eliminated from mixed-effects analysis. Eight IBS patients were taking psychotropic medications. Three of the 4 patients on tricyclic antidepressants were on doses (<50 mg/day) generally considered too low to significantly affect mood. Menstrual cycle phases were similar for pre-menopausal IBS and control women as well as for EAL and no EAL groups. Details of the EAL are listed in Table 2. The types of EAL did not differ significantly by diagnosis or sex.
Table 1.
Subject characteristics
| IBS (n=44) | Controls (n=39) | EAL (n=39) | No EAL (n=44) | ||
|---|---|---|---|---|---|
| Presence of IBS or EAL | 21 EAL | 18 EAL | 21 IBS | 23 IBS | |
| Men/Women | 19/25 | 18/21 | 19/20 | 18/26 | |
| Mean age (SD) | 40.4 (10.6) | 37.3 (10.9) | 38.5 (10.1) | 39.3 (11.5) | |
| HAD depression, 0–20 scale (SD) | 5.0 (4.4)** | 1.4 (2.0) | 3.7 (4.5) | 2.9 (3.2) | |
| HAD anxiety, 0–20 scale (SD) | 7.7 (5.1)** | 3.9 (3.0) | 6.5 (4.7) | 5.3 (4.5) | |
| Current Axis I disorder | MDD (M/F) | 4 (3/1) | 0 | 4 | 0 |
| DD (M/F) | 4 (4/0) | 0 | 2 | 2 | |
| Bowel Habit Subtype* | IBS C (M/F) | 15 (0/15) | 9 (0/9) | 6 (0/6) | |
| IBS D (M/F) | 15 (10/5) | 5 (5/0) | 10 (5/5) | ||
| IBS A/M (M/F) | 13 (8/5) | 6 (5/1) | 7 (3/4) | ||
| Baseline cortisol (μg/dl, SD) | 0.23 (0.16) | 0.20 (0.13) | 0.21 (0.1) | 0.22 (0.2) | |
| Gynecologic history | |||||
| Premenopausal phase | Follicular | 5 | 6 | 5 | 6 |
| Luteal | 8 | 8 | 8 | 8 | |
| Menses | 3 | 2 | 1 | 4 | |
| Perimenopausal | 1 | 1 | 1 | 1 | |
| Post-menopausal | 4 | 1 | 1 | 4 | |
| OCP | 5 | 3 | 4 | 4 | |
| Provera | 1 | 0 | 0 | 1 | |
| HRT | 3 | 0 | 0 | 3 | |
| Hysterectomy | 5 | 0 | 2 | 3 | |
| Medications | |||||
| TCA | 4(9.1) | 0 | 1 (2.6) | 3 (6.8) | |
| SSRI | 3 (6.8) | 0 | 2 (5.1) | 1 (2.3) | |
| Benzodiazepine | 4 (9.1) | 0 | 0 | 4 (9.1) | |
| TCA, SSRI and/or benzodiazepine | 8 (18.2) | 0 | 2 (5.1) | 6 (13.6) | |
p < 0.001 (IBS vs Control only)
(by Rome III classification)63
Abbreviations: EAL, early adverse life events; IBS, irritable bowel syndrome; MDD, major depressive disorder; DD, dysthymic disorder; OCP, oral contraceptive pills; HRT, hormone replacement therapy; TCA, tricyclic antidepressant; SSRI, selective serotonin reuptake inhibitor
Table 2.
Early adverse life events
| IBS | Control | Total | |||||
|---|---|---|---|---|---|---|---|
| Total (44) | M (19) | W (25) | Total (39) | M (18) | W (21) | 83 | |
| Any EAL | 21 | 11 | 10 | 18 | 8 | 10 | 39 |
| Physical Abuse | 5 | 2 | 3 | 7 | 3 | 4 | 12 |
| Sexual Abuse | 7 | 3 | 4 | 6 | 0 | 6 | 13 |
| Verbal Abuse | 3 | 2 | 1 | 4 | 0 | 4 | 7 |
| Trauma* | 12 | 9 | 3 | 11 | 4 | 7 | 23 |
Trauma included (N): victim of crime (8), accident or disaster (11), injury or fear of (4), witness of death or injury (7), illness (1), loss (6), assault (6). M=men, W=women
IBS vs. Controls
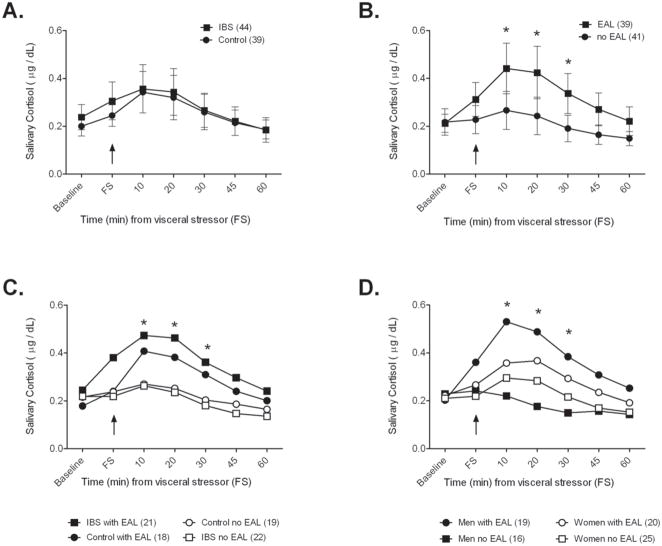
The salivary cortisol response to flexible sigmoidoscopy with respect to the presence of IBS, EAL and sex is shown in Figures 1A–D. Salivary cortisol response was similar between IBS patients and controls (Figure 1A). The effect of diagnosis on mean cortisol as analyzed by repeated mixed-effects analyses was not significant (p = 0.89). There was also no significant effect of diagnosis on cortisol response after normalizing for baseline levels (p = 0.59). Additionally, IBS and controls had similar cortisol response as measured by AUC (Table 3). The addition of sex and age to the model did not affect the results or have independent effects on cortisol response, and there was no interaction effect of disease and sex.
Figure 1.
Salivary cortisol response to a visceral stressor (flexible sigmoidoscopy [FS]) for IBS and controls (A), subjects with and without EAL (B), subjects broken intro groups by diagnosis and presence of EAL (C), and by sex and EAL (D). Presence of EAL (B) had a significant effect on the cortisol response (p < 0.05). * indicates that there is a significant difference between the groups at that time point in post-hoc analyses. In C, the EAL groups were significantly greater than IBS and controls without EAL, and in D, the men with EAL were significantly greater than men without EAL. Where shown, error bars represent the 95% confidence interval around the mean.
Table 3.
Summary measures of cortisol response
| IBS (36) | Control (40) | EAL (37) | No EAL (39) | |
|---|---|---|---|---|
| AUCg (μg·min/dL, SD) | 24.4(18) | 22.1(13) | 28.1(17)* | 18.7(13) |
| AUCm (μg· min/dL, SD) | 12.2(12) | 11.2(11) | 14.2(12)* | 9.3(11) |
| Rate of decline (ng/dL·min, SD) | 5.6(6) | 5.2(5) | 6.7(6)* | 4.1(5) |
p < 0.05 compared to No EAL..
Presence vs. absence of EAL
Subjects with EAL had significantly higher cortisol levels following flexible sigmoidoscopy than those without EAL (Figure 1B). There was an overall group effect of EAL on cortisol (p = 0.005), and post-hoc comparisons yielded significant differences at 10, 20 and 30 minutes following flexible sigmoidoscopy. This effect was also present when the values were normalized by baseline (p = 0.0009). There was no significant interaction effect for EAL and disease, EAL and sex or a three-way interaction.
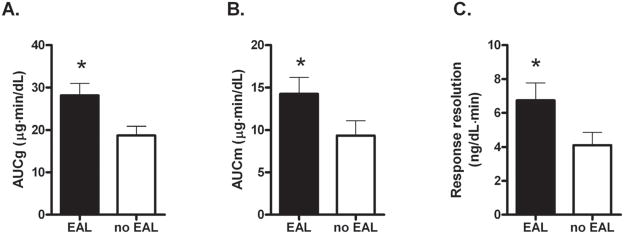
The AUC measures were significantly greater for subjects with EAL than those without EAL (AUCg: p = 0.005, AUCm: p = 0.024, rate of decline: p = 0.014) (Table 3, Figure 2). There were too few individuals that met criteria for psychiatric disorders to analyze as a separate group, but HAD anxiety and depression scores were included in the model as covariates and the effect of EAL on these three summary measures remained significant (p = 0.007, 0.027, 0.012, respectively).
Figure 2.
Summary measures of cortisol response. The AUCg (A) is the area under the curve to the x-axis, and the AUCm (B) is the area under the curve to a horizontal line at the minimum value for that subject. The response resolution rate (C) is the decrease in cortisol from peak to baseline after the stressor divided by the time elapsed between the two points. All measures are greater in individuals with EAL (p < 0.05).
The 8 IBS patients who were taking a psychotropic agent were compared to the rest of the IBS group using Mann-Whitney U tests, and were found to have similar baseline cortisol levels (p = 0.69), AUCg (p = 0.97), and AUCm (p = 0.55). Results were similar when compared to the entire study sample. When the main analyses of the study (mixed-models, AUC comparisons) were repeated without these subjects, the results were unchanged.
Although there was no overall interaction effect of EAL and IBS, there was a trend for IBS patients with EAL to have higher cortisol immediately following the stressor when compared to controls with EAL (p = 0.056) (Figure 1C).
Sex
There was no effect of sex or its interaction with disease on the cortisol response. However, sex differences in stress-induced HPA axis hormones have been reported39 and therefore, we analyzed cortisol levels separately by sex. There was a significant group effect of EAL on cortisol among men (p = 0.019), but not among women (p = 0.12). Effect of IBS or interaction effect of IBS and EAL were non-significant in men and women (Figure 1D).
Summary measures of cortisol response and relation to IBS symptoms and QOL
Univariate ANOVA with diagnosis, sex and presence of EAL as independent variables showed that subjects with EAL had greater AUCg (with respect to x-axis), AUCm (with respect to minimum cortisol) and rate of decline than those without EAL (Table 3, Figure 2). There was not a significant effect of diagnosis or sex on any of the summary measures and there were no two or three-way interaction effects. Within the IBS group, the resolution of cortisol response as measured by the rate of decline from peak cortisol negatively correlated with recent (24 hours) and longer-term (3 months) symptom intensity (r = −0.36, p = 0.03 for both) and positively correlated with IBS-related QOL (r = 0.33, p = 0.045). In other words, the slower the cortisol level returned to baseline, the lower the IBS-related QOL. The magnitude of cortisol response as measured by AUCg and AUCm did not significantly correlate with IBS symptoms or QOLIBS. There was not a significant effect of bowel habit on cortisol response resolution or magnitude.
Discussion
In the present study, we have demonstrated that in response to a visceral stressor: 1) IBS patients and controls with EAL have a greater cortisol response compared to individuals without EAL; 2) This difference was more pronounced among men than women; 3) IBS patients and controls have similar salivary cortisol responses; 4) Among IBS patients with EAL, there was a trend to have higher cortisol levels immediately following the stressor; and 5) In IBS, a slower return of stimulated cortisol to basal levels was associated with increased IBS symptom intensity and lower QOL.
The impact of EAL on cortisol response
The effect of EAL on cortisol response in the current study supports a role for EAL in the development and life-long functioning of the HPA axis. This may implicate EAL as a vulnerability factor for the development of stress-related disorders; however, the presence of increased cortisol response in healthy controls with EAL suggests that there are other mechanisms, including genetic factors, which play a role in the development of these disorders. Our findings of an exaggerated HPA axis response to a visceral stressor are in agreement with those reported in other populations to hormone challenge or mental stress, including adults without depression with history of childhood sexual abuse,27 adults with parental loss during childhood,40 and adolescents with EAL and high levels of chronic stress.28 In addition, in preclinical studies, early life stress in the form of intermittent maternal separation predisposes adult rats to greater and more prolonged plasma corticosterone levels.21, 41, 42
However, the findings in the current study are not in agreement with several other studies. Both Elzinga et al43 and Carpenter et al44 report a blunted HPA axis response to a mental stressor. Although Heim et al.27 found increased HPA axis response in non-depressed women with a history of childhood abuse, depressed women with abuse demonstrated a blunted response. In our patient population, only 4 of 37 patients with EAL met diagnostic criteria for depression, and none of those without EAL, and therefore we were not able to assess the interaction of EAL and depression on cortisol response. However, studies have supported that an exaggerated HPA axis response in subjects with childhood abuse is independent of the presence of any psychiatric disorder including borderline personality disorder, major depression and post-traumatic stress disorder (PTSD).28, 45 In Rao et al.’s study examining the cortisol response to a mental stressor in depressed adolescents, the best predictor of an elevated response was a history of EAL combined with current stress. 28
Review of the genetic and epigenetic factors at play in the development of the stress response lends insight into the phenotypic variability that is seen. At the most basic level, genetics play a role in predisposing individuals to develop illness. For example, specific alleles of the GR gene have been associated with an increased risk for depression.24 Epigenetics refers to modification in gene expression resulting in a change in phenotype without change to the genetic sequence (genotype). The most well-studied forms of this modification are DNA methylation and histone modification. These changes can be passed down meiotically and can permanently alter the expression of genes in somatic cells, an effect referred to as epigenetic programming.46 In animal studies, fetal exposure to cortisol results in reduced expression of GR in the hippocampus, which decreases negative feedback and results in a greater and more prolonged HPA axis response compared to that in animals not exposed to cortisol.47 Meaney and colleagues were the first to demonstrate methylation of the GR promoter in rodents associated with perinatal stress, a phenomenon that they subsequently linked to a prolonged HPA response in the adult animals.48
A recent study published by Meaney’s group provides strong evidence to support a similar mechanism in humans by demonstrating decreased hippocampal GR expression and increased methylation of the neuron-specific GR gene promotor NR3C1 in suicide victims with a history of childhood abuse when compared to suicide victims without abuse and to controls without abuse who died of unrelated causes.49
There was a high prevalence of EAL (46%) among our healthy controls. This prevalence is similar to that in a large HMO,50 but may not be representative of the general population. A measure of somatization, which has been associated with EAL,51 would have helped to clarify whether the increased cortisol response in the controls with EAL was associated with presence of non-gastrointestinal symptoms. However, the controls with EAL did not have elevated HAD scores. Data from studies described above28, 45, 52 also suggests that an alteration in HPA response or GR receptor expression may be a vulnerability factor but is not predictive of the presence of symptoms or psychiatric disorder.
The role of EAL and cortisol response in IBS
Evidence that perturbations during the pre- and perinatal period might contribute to the development of IBS in adults is summarized in a recent systematic review.17 Examples include a twin study, in which IBS was more prevalent and occurred at an earlier age in the twin with the lower birth weight,53 and a study associating gastric suction at birth with a diagnosis of a functional intestinal disorder in adulthood.54 A number of studies have examined the association of traumatic events during childhood including, but not limited to, abuse and loss of a parent, with the development of IBS.17 Furthermore, an animal model of IBS is based on the occurrence of early life stress (i.e., maternal separation), which predisposes adult rats to develop stress-induced visceral hypersensitivity, enhanced defecation, intestinal mucosal dysfunction, enhanced HPA axis responses and anxiety-like behavior.41, 55
While there are conflicting reports in the literature regarding alterations in HPA axis responsiveness in IBS,8 the role of EAL in the development of IBS has never been systematically studied in relation to HPA axis response. In the current study, there was no significant difference in stress-induced salivary cortisol levels or in the time course of the response between IBS patients and healthy controls. There was a trend for IBS patients with EAL to have higher cortisol levels immediately following the visceral stressor. Walter et al56 saw a similar effect when they found elevated salivary cortisol levels before and after rectal distention in IBS patients compared to controls. This could represent disease-related anticipatory anxiety to the procedure leading to an HPA axis response that is greater in magnitude and perhaps occurs sooner in IBS patients.
While HPA axis response to a visceral stressor does not appear to predict the presence of IBS in adults, we found an association between cortisol response and IBS symptoms. A faster resolution of cortisol to basal levels was associated with less symptom intensity and increased IBS-related QOL, suggesting that HPA axis reactivity has a moderating effect on IBS symptoms. One may speculate that faster resolution reflects a more adaptive phenotype in which GR expression, and therefore negative feedback of the HPA axis, is adequate and a stress response can be quickly attenuated. In contrast, a reduced ability to maintain homeostasis may render IBS patients more vulnerable to severe symptoms and poorer overall well-being. This is supported by a study in rats, in which repeated maternal separation in the neonatal period was associated with reduced glucocorticoid negative feedback as an adult.57 Further studies are warranted to examine this further.
In the present study, the effect of EAL on cortisol response was most pronounced in men. This is in line with consistent reports in the literature of increased HPA axis responsiveness in men compared to women in the healthy population.39 However, there is little data on sex differences in HPA axis response in individuals with EAL. The more robust response in men may help explain the decreased incidence of IBS and other pain disorders in men as a reactive HPA axis seems to correspond to a healthier, more adaptive phenotype in disease.
Based on preclinical data, the effect of EAL is not limited to the HPA axis, but affects a range of neurobiological systems, including those involved in antinociception, autonomic responsiveness and behavior.58 In view of previous reports on an association of EAL with a variety of persistent pain syndromes,59 including fibromyalgia,60 chronic pelvic pain,61 and associated psychiatric conditions, including anxiety, somatization and depression, it is likely that the primary effect that mediates the influence of EAL on morbidity involves other brain systems, including cortical systems underlying cognition (attention, coping) and emotional regulation17, 62 and central autonomic circuits.
Limitations of the current study include that SNS responsiveness was not measured and therefore it is not known if EAL is also associated with dysregulation of this arm the central stress response. Trait anxiety and somatization were also not assessed. Creed et al.51 have shown that the level of somatization in IBS patients is associated with symptom severity, QOL and history of sexual abuse. A measure of somatization would have determined whether the effect of EAL on cortisol response is independent of or mediated by somatization. Future studies should also evaluate additional EAL beyond trauma and abuse (e.g., neglect, difficult peer interactions, etc.), and severity of trauma or degree to which the subject perceived events as traumatic.
In summary, we have found that HPA axis response to a visceral stressor is more related to the presence of EAL rather than the presence of IBS. Yet, the presence of IBS has a slight enhancing effect on the immediate stress-induced cortisol response to a visceral stressor. Interestingly in the IBS patients, the faster cortisol returned to basal levels, the lower the IBS symptom ratings and the higher the disease-specific QOL. These results suggest that, analogous to preclinical findings, the early environment can modify stress responsiveness (as indexed by HPA axis responsiveness), possibly via epigenetic programming.
Acknowledgments
Grant support: Supported by NIH grants P50 DK64539 (EAM, LC), R01 AR46122 (LC), R24 AT002681 (EAM), and GCRC M01-RR00865.
Abbreviations
- HPA
hypothalamic-pituitary-adrenal
- IBS
irritable bowel syndrome
- EAL
early adverse life events
- QOL
quality of life
- HAD
hospital anxiety and depression scale
- SCID
structured clinical interview for the diagnostic and statistical manual of psychiatric disorders
- AUC
area under the curve
- PTSD
post traumatic stress disorder
- GR
glucocorticoid receptor
Footnotes
Author roles: EV: Data analysis & interpretation, drafting of manuscript, revision of manuscript
MA: Data analysis & interpretation, mixed models statistical analysis, assistance with manuscript preparation and revision
AL: Study Coordinator, data acquisition
MH: Data acquisition, technical support
GO: Salivary cortisol measurements
MM: Psychological screening for abuse history, SCID
EA: Funding, concept and design, critical review
LC: Principal Investigator, funding, concept and design, performed medical history and physical examinations, performed sigmoidoscopy with biopsies, analysis and interpretation of data, manuscript preparation
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mayer EA, Naliboff BD, Chang L, Coutinho SVV. Stress and irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2001;280:G519–24. doi: 10.1152/ajpgi.2001.280.4.G519. [DOI] [PubMed] [Google Scholar]
- 2.Spiller RC. Postinfectious irritable bowel syndrome. Gastroenterology. 2003;124:1662–1671. doi: 10.1016/s0016-5085(03)00324-x. [DOI] [PubMed] [Google Scholar]
- 3.Gwee KA, Leong YL, Graham C, McKendrick MW, Collins SM, Walters SJ, Underwood JE, Read NW. The role of psychological and biological factors in postinfective gut dysfunction. Gut. 1999;44:400–6. doi: 10.1136/gut.44.3.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Posserud I, Agerforz P, Ekman R, Bjornsson ES, Abrahamsson H, Simren M. Altered visceral perceptual and neuroendocrine response in patients with irritable bowel syndrome during mental stress. Gut. 2004;53:1102–8. doi: 10.1136/gut.2003.017962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alonso C, Guilarte M, Vicario M, Ramos L, Ramadan Z, Antolin M, Martinez C, Rezzi S, Saperas E, Kochhar S, Santos J, Malagelada JR. Maladaptive intestinal epithelial responses to life stress may predispose healthy women to gut mucosal inflammation. Gastroenterology. 2008;135:163–172. e1. doi: 10.1053/j.gastro.2008.03.036. [DOI] [PubMed] [Google Scholar]
- 6.Tillisch K, Mayer EA, Labus JS, Stains J, Chang L, Naliboff BD. Sex specific alterations in autonomic function among patients with irritable bowel syndrome. Gut. 2005;54:1396–401. doi: 10.1136/gut.2004.058685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cain KC, Jarrett ME, Burr RL, Hertig VL, Heitkemper MM. Heart rate variability is related to pain severity and predominant bowel pattern in women with irritable bowel syndrome. Neurogastroenterology and Motility. 2007;19:110–8. doi: 10.1111/j.1365-2982.2006.00877.x. [DOI] [PubMed] [Google Scholar]
- 8.Chang L, Sundaresh S, Elliott J, Anton PA, Baldi P, Licudine A, Mayer M, Vuong T, Hirano M, Naliboff BD, Ameen VZ, Mayer EA. Dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis in irritable bowel syndrome. Neurogastroenterol Motil. 2009;21:149–59. doi: 10.1111/j.1365-2982.2008.01171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patacchioli FR, Angelucci L, Dellerba G, Monnazzi P, Leri O. Actual stress, psychopathology and salivary cortisol levels in the irritable bowel syndrome (IBS) J Endocrinol Invest. 2001;24:173–7. doi: 10.1007/BF03343838. [DOI] [PubMed] [Google Scholar]
- 10.Heitkemper M, Jarrett M, Cain K, Shaver J, Bond E, Woods NF, Walker E. Increased urine catecholamines and cortisol in women with irritable bowel syndrome. Am J Gastroenterol. 1996;91:906–13. [PubMed] [Google Scholar]
- 11.Dinan TG, Quigley EM, Ahmed SM, Scully P, O’Brien S, O’Mahony L, O’Mahony S, Shanahan F, Keeling PW. Hypothalamic-pituitary-gut axis dysregulation in irritable bowel syndrome: plasma cytokines as a potential biomarker? Gastroenterology. 2006;130:304–11. doi: 10.1053/j.gastro.2005.11.033. [DOI] [PubMed] [Google Scholar]
- 12.Bohmelt AH, Nater UM, Franke S, Hellhammer DH, Ehlert U. Basal and stimulated hypothalamic-pituitary-adrenal axis activity in patients with functional gastrointestinal disorders and healthy controls. Psychosom Med. 2005;67:288–94. doi: 10.1097/01.psy.0000157064.72831.ba. [DOI] [PubMed] [Google Scholar]
- 13.Ehlert U, Nater UM, Bohmelt A. High and low unstimulated salivary cortisol levels correspond to different symptoms of functional gastrointestinal disorders. J Psychosom Res. 2005;59:7–10. doi: 10.1016/j.jpsychores.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 14.Elsenbruch S, Lucas A, Holtmann G, Haag S, Gerken G, Riemenschneider N, Langhorst J, Kavelaars A, Heijnen CJ, Schedlowski M. Public speaking stress-induced neuroendocrine responses and circulating immune cell redistribution in irritable bowel syndrome. Am J Gastroenterol. 2006;101:2300–7. doi: 10.1111/j.1572-0241.2006.00837.x. [DOI] [PubMed] [Google Scholar]
- 15.Tak LM, Bakker SJ, Rosmalen JG. Dysfunction of the hypothalamic-pituitary-adrenal axis and functional somatic symptoms: A longitudinal cohort study in the general population. Psychoneuroendocrinology. 2009 doi: 10.1016/j.psyneuen.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 16.Drossman DA, Leserman J, Nachman G, Li ZM, Gluck H, Toomey TC, Mitchell CM. Sexual and physical abuse in women with functional or organic gastrointestinal disorders. Ann Intern Med. 1990;113:828–33. doi: 10.7326/0003-4819-113-11-828. [DOI] [PubMed] [Google Scholar]
- 17.Chitkara DK, van Tilburg MA, Blois-Martin N, Whitehead WE. Early life risk factors that contribute to irritable bowel syndrome in adults: a systematic review. Am J Gastroenterol. 2008;103:765–74. doi: 10.1111/j.1572-0241.2007.01722.x. quiz 775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Talley NJ, Fett SL, Zinsmeister AR, Melton LJ., 3rd Gastrointestinal tract symptoms and self-reported abuse: a population-based study. Gastroenterology. 1994;107:1040–9. doi: 10.1016/0016-5085(94)90228-3. [DOI] [PubMed] [Google Scholar]
- 19.Salmon P, Skaife K, Rhodes J. Abuse, dissociation, and somatization in irritable bowel syndrome: towards an explanatory model. J Behav Med. 2003;26:1–18. doi: 10.1023/a:1021718304633. [DOI] [PubMed] [Google Scholar]
- 20.Ross CA. Childhood sexual abuse and psychosomatic symptoms in irritable bowel syndrome. J Child Sex Abus. 2005;14:27–38. doi: 10.1300/J070v14n01_02. [DOI] [PubMed] [Google Scholar]
- 21.Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol Psychiatry. 2001;49:1023–39. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- 22.Bremner JD, Bolus R, Mayer EA. Psychometric properties of the Early Trauma Inventory-Self Report. The Journal of nervous and mental disease. 2007;195:211–8. doi: 10.1097/01.nmd.0000243824.84651.6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dube SR, Felitti VJ, Dong M, Giles WH, Anda RF. The impact of adverse childhood experiences on health problems: evidence from four birth cohorts dating back to 1900. Prev Med. 2003;37:268–77. doi: 10.1016/s0091-7435(03)00123-3. [DOI] [PubMed] [Google Scholar]
- 24.McEwen BS. Understanding the potency of stressful early life experiences on brain and body function. Metabolism. 2008;57 (Suppl 2):S11–5. doi: 10.1016/j.metabol.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sanchez MM. The impact of early adverse care on HPA axis development: nonhuman primate models. Horm Behav. 2006;50:623–31. doi: 10.1016/j.yhbeh.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 26.Turner-Cobb JM. Psychological and stress hormone correlates in early life: A key to HPA-axis dysregulation and normalisation. Stress. 2005;8:47 – 57. doi: 10.1080/10253890500095200. [DOI] [PubMed] [Google Scholar]
- 27.Heim C, Newport DJ, Bonsall R, Miller AH, Nemeroff CB. Altered pituitary-adrenal axis responses to provocative challenge tests in adult survivors of childhood abuse. Am J Psychiatry. 2001;158:575–81. doi: 10.1176/appi.ajp.158.4.575. [DOI] [PubMed] [Google Scholar]
- 28.Rao U, Hammen C, Ortiz LR, Chen L-A, Poland RE. Effects of Early and Recent Adverse Experiences on Adrenal Response to Psychosocial Stress in Depressed Adolescents. Biological Psychiatry. 2008;64:521–526. doi: 10.1016/j.biopsych.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thompson WG, Longstreth GF, Drossman DA, Heaton KW, Irvine EJ, Muller-Lissner SA. Functional bowel disorders and functional abdominal pain. Gut. 1999;45(Suppl 2):II43–7. doi: 10.1136/gut.45.2008.ii43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Munakata J, Naliboff B, Harraf F, Kodner A, Lembo T, Chang L, Silverman DH, Mayer EA. Repetitive sigmoid stimulation induces rectal hyperalgesia in patients with irritable bowel syndrome. Gastroenterology. 1997;112:55–63. doi: 10.1016/s0016-5085(97)70219-1. [DOI] [PubMed] [Google Scholar]
- 31.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 32.Longstreth GF, Bolus R, Naliboff B, Chang L, Kulich KR, Carlsson J, Mayer EA, Naesdal J, Wiklund IK. Impact of irritable bowel syndrome on patients’ lives: development and psychometric documentation of a disease-specific measure for use in clinical trials. Eur J Gastroenterol Hepatol. 2005;17:411–20. doi: 10.1097/00042737-200504000-00004. [DOI] [PubMed] [Google Scholar]
- 33.Green BL. Trauma History Questionnaire. In: Stamm B, Varra E, editors. Measurement of stress, trauma and adaptation. Lutherville, MD: Sidran; 1996. pp. 366–368. [Google Scholar]
- 34.Spitzer R, Williams J, Gibbon M, First M. Structured Clinical Interview for DSM-III-R. American Psychiatric Press; 1990. [Google Scholar]
- 35.McCracken JT, Poland RE. Saliva and serum cortisol dynamics following intravenous dexamethasone in normal volunteers. Life Sci. 1989;45:1781–5. doi: 10.1016/0024-3205(89)90517-1. [DOI] [PubMed] [Google Scholar]
- 36.Poland RE, Rubin RT. Saliva cortisol levels following dexamethasone administration in endogenously depressed patients. Life Sci. 1982;30:177–81. doi: 10.1016/0024-3205(82)90650-6. [DOI] [PubMed] [Google Scholar]
- 37.Matthews JN, Altman DG, Campbell MJ, Royston P. Analysis of serial measurements in medical research. BMJ. 1990;300:230–5. doi: 10.1136/bmj.300.6719.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28:916–31. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- 39.Kudielka BM, Hellhammer DH, Wst S. Why do we respond so differently? Reviewing determinants of human salivary cortisol responses to challenge. Psychoneuroendocrinology. 2009;34:2–18. doi: 10.1016/j.psyneuen.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 40.Tyrka AR, Wier L, Price LH, Ross N, Anderson GM, Wilkinson CW, Carpenter LL. Childhood parental loss and adult hypothalamic-pituitary-adrenal function. Biol Psychiatry. 2008;63:1147–54. doi: 10.1016/j.biopsych.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gareau MG, Jury J, Yang PC, MacQueen G, Perdue MH. Neonatal maternal separation causes colonic dysfunction in rat pups including impaired host resistance. Pediatr Res. 2006;59:83–8. doi: 10.1203/01.pdr.0000190577.62426.45. [DOI] [PubMed] [Google Scholar]
- 42.O’Mahony SM, Marchesi JR, Scully P, Codling C, Ceolho AM, Quigley EM, Cryan JF, Dinan TG. Early life stress alters behavior, immunity, and microbiota in rats: implications for irritable bowel syndrome and psychiatric illnesses. Biol Psychiatry. 2009;65:263–7. doi: 10.1016/j.biopsych.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 43.Elzinga BM, Roelofs K, Tollenaar MS, Bakvis P, van Pelt J, Spinhoven P. Diminished cortisol responses to psychosocial stress associated with lifetime adverse events a study among healthy young subjects. Psychoneuroendocrinology. 2008;33:227–37. doi: 10.1016/j.psyneuen.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 44.Carpenter LL, Carvalho JP, Tyrka AR, Wier LM, Mello AF, Mello MF, Anderson GM, Wilkinson CW, Price LH. Decreased adrenocorticotropic hormone and cortisol responses to stress in healthy adults reporting significant childhood maltreatment. Biol Psychiatry. 2007;62:1080–7. doi: 10.1016/j.biopsych.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rinne T, de Kloet ER, Wouters L, Goekoop JG, DeRijk RH, van den Brink W. Hyperresponsiveness of hypothalamic-pituitary-adrenal axis to combined dexamethasone/corticotropin-releasing hormone challenge in female borderline personality disorder subjects with a history of sustained childhood abuse. Biol Psychiatry. 2002;52:1102–12. doi: 10.1016/s0006-3223(02)01395-1. [DOI] [PubMed] [Google Scholar]
- 46.Wolffe AP, Matzke MA. Epigenetics: regulation through repression. Science. 1999;286:481–6. doi: 10.1126/science.286.5439.481. [DOI] [PubMed] [Google Scholar]
- 47.Meaney MJ, Szyf M, Seckl JR. Epigenetic mechanisms of perinatal programming of hypothalamic-pituitary-adrenal function and health. Trends Mol Med. 2007;13:269–77. doi: 10.1016/j.molmed.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 48.Fish E. Epigenetic Programming of Stress Responses through Variations in Maternal Care. Annals of the New York Academy of Sciences. 2004;1036:167–180. doi: 10.1196/annals.1330.011. [DOI] [PubMed] [Google Scholar]
- 49.McGowan PO, Sasaki A, D’Alessio AC, Dymov S, Labonte B, Szyf M, Turecki G, Meaney MJ. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat Neurosci. 2009;12:342–8. doi: 10.1038/nn.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Anda RF, Felitti VJ, Bremner JD, Walker JD, Whitfield C, Perry BD, Dube SR, Giles WH. The enduring effects of abuse and related adverse experiences in childhood. A convergence of evidence from neurobiology and epidemiology. Eur Arch Psychiatry Clin Neurosci. 2006;256:174–86. doi: 10.1007/s00406-005-0624-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Creed F, Tomenson B, Guthrie E, Ratcliffe J, Fernandes L, Read N, Palmer S, Thompson DG. The relationship between somatisation and outcome in patients with severe irritable bowel syndrome. J Psychosom Res. 2008;64:613–20. doi: 10.1016/j.jpsychores.2008.02.016. [DOI] [PubMed] [Google Scholar]
- 52.Bremner JD, Vythilingam M, Anderson G, Vermetten E, McGlashan T, Heninger G, Rasmusson A, Southwick SM, Charney DS. Assessment of the hypothalamic-pituitary-adrenal axis over a 24-hour diurnal period and in response to neuroendocrine challenges in women with and without childhood sexual abuse and posttraumatic stress disorder. Biol Psychiatry. 2003;54:710–8. doi: 10.1016/s0006-3223(02)01912-1. [DOI] [PubMed] [Google Scholar]
- 53.Bengtson MB, Ronning T, Vatn MH, Harris JR. Irritable bowel syndrome in twins: genes and environment. Gut. 2006;55:1754–9. doi: 10.1136/gut.2006.097287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Anand KJ, Runeson B, Jacobson B. Gastric suction at birth associated with long-term risk for functional intestinal disorders in later life. J Pediatr. 2004;144:449–54. doi: 10.1016/j.jpeds.2003.12.035. [DOI] [PubMed] [Google Scholar]
- 55.Coutinho SV, Plotsky PM, Sablad M, Miller JC, Zhou H, Bayati AI, McRoberts JA, Mayer EA. Neonatal maternal separation alters stress-induced responses to viscerosomatic nociceptive stimuli in rat. Am J Physiol Gastrointest Liver Physiol. 2002;282:G307–16. doi: 10.1152/ajpgi.00240.2001. [DOI] [PubMed] [Google Scholar]
- 56.Walter SA, Aardal-Eriksson E, Thorell LH, Bodemar G, Hallbook O. Pre-experimental stress in patients with irritable bowel syndrome: high cortisol values already before symptom provocation with rectal distensions. Neurogastroenterol Motil. 2006;18:1069–77. doi: 10.1111/j.1365-2982.2006.00833.x. [DOI] [PubMed] [Google Scholar]
- 57.Meaney MJ, Diorio J, Francis D, Widdowson J, LaPlante P, Caldji C, Sharma S, Seckl JR, Plotsky PM. Early environmental regulation of forebrain glucocorticoid receptor gene expression: implications for adrenocortical responses to stress. Dev Neurosci. 1996;18:49–72. doi: 10.1159/000111395. [DOI] [PubMed] [Google Scholar]
- 58.Mayer EA, Collins SM. Evolving pathophysiologic models of functional gastrointestinal disorders. Gastroenterology. 2002;122:2032–48. doi: 10.1053/gast.2002.33584. [DOI] [PubMed] [Google Scholar]
- 59.Mayer EA, Bushnell MC. Functional Pain Syndromes: Presentation and Pathophysiology. Intl Assoc for the Study of Pain. 2009 [Google Scholar]
- 60.Weissbecker I, Floyd A, Dedert E, Salmon P, Sephton S. Childhood trauma and diurnal cortisol disruption in fibromyalgia syndrome. Psychoneuroendocrinology. 2006;31:312–24. doi: 10.1016/j.psyneuen.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 61.Heim C, Ehlert U, Hanker JP, Hellhammer DH. Abuse-related posttraumatic stress disorder and alterations of the hypothalamic-pituitary-adrenal axis in women with chronic pelvic pain. Psychosom Med. 1998;60:309–18. doi: 10.1097/00006842-199805000-00017. [DOI] [PubMed] [Google Scholar]
- 62.Whitehead WE, Crowell MD, Robinson JC, Heller BR, Schuster MM. Effects of stressful life events on bowel symptoms: subjects with irritable bowel syndrome compared with subjects without bowel dysfunction. Gut. 1992;33:825–30. doi: 10.1136/gut.33.6.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130:1480–91. doi: 10.1053/j.gastro.2005.11.061. [DOI] [PubMed] [Google Scholar]