Abstract
Objective
To examine the association between narrowly defined subsets of maternal ethnicity and birth outcomes.
Study Design
Analysis of 1995-2003 New York City birth certificates linked to hospital discharge data for 949,210 singleton births to examine the multivariable associations between maternal ethnicity and preterm birth, subsets of spontaneous and medically indicated preterm birth, term small for gestational age (SGA), and term birthweight.
Results
Compared to non-Hispanic whites, Puerto Ricans had an elevated odds ratio (OR 1.9, 95% CI 1.9-2.0) for delivering at 32-36 weeks (adjusted for nativity, maternal age, parity, education, tobacco use, pre-pregnancy weight, birth year). We found an excess of adverse outcomes among most Latino groups. Outcomes also varied within regions, with North African infants nearly 100g (adjusted) heavier than sub-Saharan Africans.
Conclusions
The considerable heterogeneity in risk of adverse perinatal outcomes is obscured in broad categorizations of maternal race/ethnicity, and may help to formulate etiologic hypotheses.
Keywords: Ethnic groups, Epidemiology, Outcomes, pregnancy
INTRODUCTION
Despite numerous social, behavioral, and biomedical interventions, the proportion of preterm births in the United States has been increasing.1 The rate of singleton preterm births rose 13 percent between 1990 and 2005.1 This increase is primarily due to births at 34-36 weeks. Preterm infants have higher rates of temperature instability, respiratory distress, apnea, hypoglycemia, seizures, jaundice, feeding difficulties, re-hospitalization, and death as compared to term infants.2 Preterm infants are also more likely to suffer from neurodevelopmental problems, such as cerebral palsy, mental retardation, and sensory impairments, with those born earliest at greatest risk.2 Preterm birth is a significant contributor to infant morbidity and mortality.3, 4
Within the United States, there is considerable variation in birth outcomes by maternal race. In 2005, 11.7% of births to non-Hispanic white women occurred at <37 completed weeks gestation compared to 18.4% of births to non-Hispanic black women.1 The pattern is similar for low birthweight, affecting 7.3% of births to non-Hispanic white women and 14.0% of births to non-Hispanic blacks.1 Birth outcomes vary by ethnicity as well. The Hispanic Paradox describes the favorable birth outcomes of Hispanic women relative to non-Hispanic white women despite the former's general socioeconomic disadvantage.5-12 This benefit, however, may not be universally experienced across Hispanic subgroups.13-15 Puerto Ricans, in particular, tend to have a higher risk of adverse birth outcomes as compared to other Hispanic groups.13, 14 Differences in birth outcomes among Asian subgroups have also been noted.16-20
Given the enormous challenge in improving birth outcomes, a better understanding of variation within a population may be informative. There is evidence that conventional categorizations of race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, Asian, Other) may obscure consequential differences in birth outcomes. Examining birth outcomes by detailed maternal ethnic ancestry may suggest etiologic hypotheses, highlight the implications of the changing demography of the United States as well as many other parts of the world, and help to better direct prenatal and postnatal care and resources. The large and ethnically diverse population of New York City makes it an ideal location for the study of ethnicity and adverse perinatal outcomes.
MATERIALS AND METHODS
Study population
Vital statistics birth data for 1995-2003 from the New York City Department of Health and Mental Hygiene were linked to the Statewide Planning and Research Cooperative System (SPARCS) hospital discharge record for delivery to examine the association between ethnicity and birth outcome in New York City. Of 1,133,020 vital records for singleton births, 1,067,356 (94.2%) were successfully linked to a hospital discharge record. Unmatched records resulted from missing personal data required for the matching algorithm. Further details on the matching methodology have been reported previously.21 We restricted our analyses to singleton births with plausible combinations of birthweight and gestational age.22
Measures
We studied four birth outcomes: preterm birth subdivided by gestational age, preterm birth subdivided by delivery indication, small for gestational age (SGA) among term births, and birthweight among term births. Delivery indication was determined from the hospital discharge record; data on the remaining outcomes and all maternal covariates were derived from the birth certificate file.
The clinical estimate of gestational age in completed weeks from the birth certificate was examined as a categorical variable (22-31, 32-34, 35-36, and 37-44 weeks). Small for gestational age (SGA) was defined for term births by identifying infants below the 10th percentile of weight for gestational age and gender.23 We examined birthweight (grams (g)) among term births as a continuous measure.
To identify differences in the underlying clinical presentations of preterm birth, we differentiated between medically indicated preterm births and spontaneous preterm births arising from either premature rupture of the membranes (PROM) or spontaneous preterm labor. Preterm birth subtypes were classified using International Classification of Diseases, Ninth Revision (ICD-9) hospital discharge diagnosis and procedure codes. Hospital discharge data are generally more accurate than birth certificate records for information on delivery indication.24 Women with artificial rupture of membranes, induction of labor by artificial rupture of membranes, or other surgical or medical induction of labor (ICD-9 codes 73.0, 73.01, 73.09, 73.1, 73.4) were categorized as medically indicated preterm births. From the remaining women, those with an ICD-9 code indicative of PROM (658.1x, 658.2x) were categorized as such. We then assigned pre-labor cesarean deliveries as medically indicated births by identifying deliveries by cesarean section (74.x), without codes indicating labor or spontaneous delivery (644.0x, 644.1x, 644.2x). The remaining preterm births were classified as spontaneous and grouped with preterm PROM.
The primary independent variable, maternal ethnic ancestry, was determined by selfreported ethnic ancestry from the birth certificate.21 Most women listed their ethnic ancestry as a country. These countries were then collapsed into geographic regions for analysis.25 The group Other Hispanics included Hispanic women from Spain or an unreported ancestral country, the majority of who are US-born. Ethnic groups with at least 500 preterm births were examined both individually and within their respective geographic region. This cutoff provided excellent power for examining a range of birth outcomes while maintaining a manageable number of ancestral countries. Additional maternal demographic characteristics were included as covariates. Maternal age and pre-pregnancy weight were included as continuous variables with squared and cubic transformations. Nativity (US or foreign born), parity (0, 1, 2, or ≥3 previous live births), education (≤8, 9-12, or ≥13 years), and tobacco use during pregnancy (smoker, non-smoker) were also included.
Statistical analysis
Analyses were restricted to singleton births with complete data on all measures and were performed using SAS Version 9.1 (SAS Institute, Cary, NC) and Stata Version 10 (Stata Corp, College Station, TX). Because of the large sample size, all covariates were retained in the model without formal testing for confounding,26 and all reported results are adjusted. There was minimal, and often no change between crude and adjusted effect estimates and there was no observed heterogeneity of effect by nativity. We used unconditional multiple logistic regression to estimate adjusted odds ratios (OR) and 95% confidence intervals (CI) for the relation between maternal ethnic ancestry and the dichotomous measure term SGA. For the multi-categorical outcomes (preterm birth and delivery indication), we used multinomial logistic regression to estimate the adjusted association between ethnic ancestry and outcome. Using linear regression, we estimated the adjusted difference in mean birthweight by maternal ethnic ancestry.
Institutional Review Board approval was granted from the Mount Sinai Program for the Protection of Human Subjects and the New York City Department of Health and Mental Hygiene Institutional Review Board.
RESULTS
Of the 949,210 singleton births included in this analysis, 70,997 (7.5%) occurred before 37 completed weeks gestation. Most preterm births (83.4%) occurred between 32-36 weeks. Preterm births resulting from PROM or spontaneous delivery (“spontaneous”) as opposed to medically indicated preterm births (“medical”) accounted for 78.0% of preterm births. The frequencies of spontaneous and medically indicated preterm births are within the range of what is commonly reported.27-29 The mean birthweight among term births was 3375 g (standard deviation (SD) 468) and 12.1% of term births were classified as SGA. The population of women giving birth was ethnically diverse, with non-Hispanic white women comprising only 28.8% of the total; the majority of women were born outside the US (51.2%).
As compared to term births among non-Hispanic white women, the effect of ethnicity on odds ratios for early preterm birth (22-31 weeks) was generally greater than for later preterm birth (32-36 weeks), adjusted for nativity, maternal age, parity, education, pre-pregnancy weight, and birth year (Table 1). For instance, not only were African Americans more likely to have a preterm birth as compared to non-Hispanic whites, but the odds ratio was greater for early preterm birth (OR 4.9, 95% CI 4.6, 5.3) than for late preterm birth (OR 2.1, 95% CI 2.0, 2.1). This pattern was also seen among non-Hispanic Caribbeans with the odds ratio for early preterm birth (OR 4.5, 95% CI 4.1, 4.9) more than twice that for late preterm birth (OR 2.1, 95% CI 2.0, 2.2) as compared to term births among non-Hispanic whites. Women from Mexico, Asia, and North Africa had similar effect estimates for both early and late preterm births. All Hispanic groups had an excess of preterm birth, although there was some regional variation. Among Hispanics, Puerto Ricans had the greatest odds ratios for both early (OR 3.2, 95% CI 3.0, 3.4) and late (OR 1.9, 95% CI 1.9, 2.0) preterm birth as compared to term births among non-Hispanic whites. The odds ratios for preterm birth among Chinese women were lower than for non-Hispanic white women, regardless of preterm birth severity. These low odds ratios for preterm birth among East Asians differed from other Asian subgroups.
TABLE 1.
Gestational Age by Maternal Ethnic Ancestry for Singleton Births, New York City, 1995 - 2003, n=949,210
| 22-31 weeks | 32-36 weeks | ||
|---|---|---|---|
| Maternal Ethnic Ancestrya | Total Births (N) | Adjustedb OR [95% CI] | Adjustedb OR [95% CI] |
| All women | 949,210 | ||
| Non-Hispanic White | 273,493 | 1.0 | 1.0 |
| African American | 147,449 | 4.9 [4.6, 5.3] | 2.1 [2.0, 2.1] |
| North Africac | 5,423 | 1.3 [0.9, 1.8] | 1.1 [1.0, 1.3] |
| Sub-Saharan Africad | 17,353 | 3.1 [2.7, 3.6] | 1.6 [1.5, 1.7] |
| Non-Hispanic Caribbean | 72,498 | 4.5 [4.1, 4.9] | 2.1 [2.0, 2.2] |
| Haiti | 15,540 | 4.2 [3.7, 4.8] | 2.0 [1.9, 2.1] |
| Jamaica | 25,946 | 4.3 [3.8, 4.8] | 1.9 [1.8, 2.0] |
| Trinidad and Tobago | 11,986 | 4.4 [3.8, 5.1] | 2.3 [2.1, 2.4] |
| Non-Hispanic Caribbean, Othere | 19,026 | 4.4 [3.9, 5.0] | 2.1 [2.0, 2.2] |
| Hispanic Caribbean | 179,407 | 2.9 [2.7, 3.1] | 1.8 [1.7, 1.8] |
| Dominican Republic | 82,837 | 2.5 [2.3, 2.8] | 1.6 [1.5, 1.6] |
| Puerto Rico | 93,957 | 3.2 [3.0, 3.4] | 1.9 [1.9, 2.0] |
| Cuba | 2,613 | 2.5 [1.8, 3.6] | 1.7 [1.4, 1.9] |
| Mexico | 44,300 | 1.8 [1.6, 2.1] | 1.5 [1.4, 1.6] |
| Central America | 22,960 | 2.8 [2.5, 3.2] | 1.6 [2.5, 1.7] |
| El Salvador | 6,086 | 2.5 [2.0, 3.2] | 1.5 [1.4, 1.7] |
| Honduras | 7,199 | 2.8 [2.3, 3.5] | 1.5 [1.4, 1.7] |
| Central America, Otherf | 9,675 | 2.8 [2.3, 3.4] | 1.7 [1.5, 1.8] |
| South America | 63,551 | 2.5 [2.3, 2.8] | 1.7 [1.6, 1.7] |
| Colombia | 10,840 | 1.5 [1.2, 1.9] | 1.2 [1.1, 1.3] |
| Ecuador | 21,475 | 1.7 [1.4, 2.0] | 1.4 [1.3, 1.5] |
| Guyana | 19,814 | 4.5 [3.9, 5.0] | 2.3 [2.1, 2.4] |
| South America, Otherg | 11,422 | 1.6 [1.3, 2.0] | 1.3 [1.2, 1.4] |
| Other Hispanich | 14,257 | 2.4 [2.0, 2.8] | 1.6 [1.5, 1.7] |
| East Asia | 56,382 | 0.9 [0.8, 1.0] | 0.9 [0.9, 1.0] |
| China | 42,058 | 0.9 [0.8, 1.1] | 0.9 [0.8, 0.9] |
| East Asia, Otheri | 14,324 | 0.8 [0.6, 1.0] | 1.0 [0.9, 1.1] |
| Southeast Asia & Pacific Islands | 11,956 | 1.7 [1.3, 2.0] | 1.6 [1.5, 1.8] |
| Philippines | 7,910 | 1.9 [1.5, 2.4] | 1.7 [1.6, 1.9] |
| Southeast Asia, Otherj | 4,046 | 1.1 [0.7, 1.6] | 1.3 [1.1, 1.5] |
| South Central Asia | 33,304 | 1.7 [1.5, 2.0] | 1.6 [1.5, 1.6] |
| Bangladesh | 7,627 | 1.9 [1.5, 2.5] | 1.6 [1.5, 1.8] |
| India | 13,641 | 1.7 [1.4, 2.1] | 1.6 [1.5, 1.7] |
| Pakistan | 7817 | 1.7 [1.3, 2.2] | 1.6 [1.4, 1.7] |
| South Central Asia, Otherk | 4,219 | 1.1 [0.7, 1.6] | 0.9 [0.7, 1.0] |
| Other | 6,492 | 3.4 [2.8, 4.1] | 1.8 [1.6, 1.9] |
Countries with at least 500 preterm births <37 weeks are examined individually
Adjusted for maternal age, nativity, parity, education, tobacco, pre-pregnancy weight, year (referent is ≥37 completed weeks gestation)
Algeria, Egypt, Libya, Morocco, Sudan, Tunisia
Angola, Benin, Botswana, Burkina Faso, Burundi, Cameroon, Cape Verde, Central African Republic, Chad, Comoro Islands, Congo, Equatorial Guinea, Eritrea, Ethiopia, Gabon, Gambia, Ghana, Guinea, Guinea-Bissau, Ivory Coast, Kenya, Lesotho, Liberia, Madagascar, Malawi, Mali, Mauritania, Mauritius, Mozambique, Namibia, Niger, Nigeria, Rwanda, Senegal, Seychelles, Sierra Leone, Somalia, South Africa, Swaziland, Tanzania, Togo, Uganda, Zaire, Zambia, Zimbabwe, 'Other African'
Aruba, Antigua and Barbuda, Bahamas, Barbados, Bermuda, Cayman Islands, Curacao, Dominica, Grenada, Guadalupe, Martinique, Montserrat, Nevis and St. Christopher, St. Kitts and Nevis, St. Lucia, St. Marartin, St. Martin, St. Vincent, Tortola, Turks and Cacaos, Virgin Islands, West Indies
Belize, Costa Rica, Guatemala, Nicaragua, Panama, 'Other Central America'
Argentina, Bolivia, Brazil, Chile, French Guiana, Paraguay, Peru, Suriname, Uruguay, Venezuela, 'Other South America'
Spain, Hispanic with no ancestral country reported
Hong Kong, Japan, Korea, Macao, Mongolia, Singapore, Taiwan, 'Other East Asian'
American Samoa, Brunei, Cambodia, Caroline Islands, Fiji, Guam, Indonesia, Kiribati, Laos, Malaysia, Mariana Islands, Marshall Islands, Micronesia, New Guinea, Papua New Guinea, Samoa, Vietnam, Solomon Islands, Tahiti, Thailand, Tonga, Truk Islands, 'Other Pacific Islands'
Afghanistan, Bhutan, Burma, East Indies, Iran, Kazakhstan, Kyrgyzstan, Nepal, Sri Lanka, Tajikistan, Turkmenistan, Uzbekistan
Among African Americans, the odds ratio for spontaneous preterm birth (OR 2.6, 95% CI 2.5, 2.7) were greater than the odds ratio for medically indicated preterm birth (OR 1.9, 95% CI 1.8, 2.0) as compared to non-Hispanic whites (data not shown). Except for women from Pakistan, the odds ratios for spontaneous preterm birth were always higher than for medically indicated preterm birth.
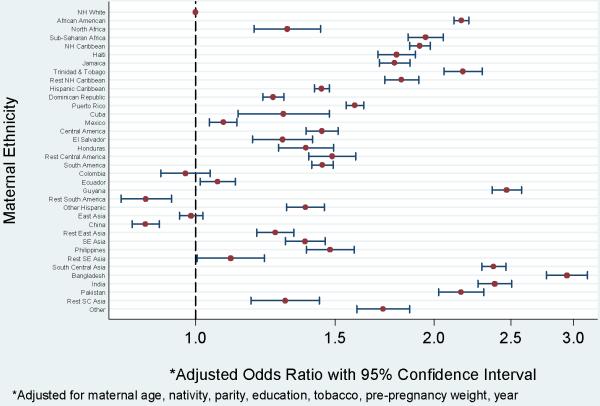
Women from Bangladesh had 2.9 (95% CI 2.8, 3.1) times higher odds of having a term SGA birth as compared to non-Hispanic whites, following adjustment for confounders (Fig 1). Women from Bangladesh, India, and Pakistan had odds ratios for SGA well above women from East or Southeast Asia. Guyanans (OR 2.5, 95% CI 2.4, 2.6), African Americans (OR 2.2, 95% CI 2.1, 2.2), and women from Trinidad and Tobago (OR 2.2, 95% CI 2.1, 2.3) also had elevated odds ratios for term SGA as compared to non-Hispanic whites. There was an excess of term SGA among most Hispanic subgroups as compared to non-Hispanic whites, although the excess was not as pronounced for preterm birth. Odds ratios for term SGA among women from Colombia were similar to those of non-Hispanic whites, while Hispanic Caribbeans and Central Americans had increased odds ratios (OR 1.4, 95% CI 1.4, 1.5).
Figure 1.
Term SGA by maternal ethnicity, New York City, 1995 - 2003. Odds ratios and 95% confidence intervals.
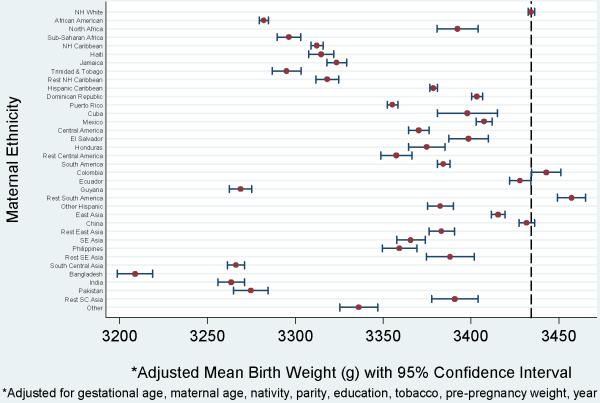
Mean birthweight at term exhibited patterns similar to those seen for term SGA (Fig 2). The adjusted mean birthweight for non-Hispanic whites was 3434 g. Only the group of Other South American women (23.0, 95% CI 14.6, 31.4) had adjusted mean birthweight greater than non-Hispanic whites. On average, Bangladeshi women delivered the lightest infants (-225.6 g, 95% CI -235.9, -215.3). There was a notable difference in birthweight between women from North (-42.0, 95% CI -53.9, -30.1) and sub-Saharan (-138.0, 95% CI -145.1, -130.9) Africa, each contrasted with non-Hispanic whites.
Figure 2.
Term birthweight by maternal ethnicity, New York City, 1995 - 2003. Odds ratios and 95% confidence intervals. The dashed vertical line at 3434.4 grams represents the adjusted mean birthweight for non-Hispanic whites.
COMMENT
There is pronounced ethnic heterogeneity in birth outcomes among women in New York City. African Americans and women from the non-Hispanic Caribbean and Guyana have less favorable birth outcomes compared to non-Hispanic white women, even after adjustment for nativity, maternal age, parity, education, tobacco use, pre-pregnancy weight, and birth year. In comparison to previous studies supporting the Hispanic Paradox, Hispanic women residing in New York City have birth outcomes that are comparable to non-Hispanic whites. Additionally, there are salient differences in birth outcomes within broad groupings of maternal ethnicity. For example, women from East Asia have lower odd ratios for delivering preterm whereas those from Southeast and South Central Asia have higher odds ratios for delivering preterm as compared to non-Hispanic whites.
Our findings reflect the continuing black-white disparity in birth outcomes in the United States.1, 3 The lack of confounding evidenced by the minimal change after adjustment highlights the need for continued exploration of the causes of these differences in adverse perinatal outcomes. In New York City, African Americans have nearly five times greater odds of having an early preterm birth and a four times greater odds of a spontaneous preterm birth as compared to non-Hispanic whites. For African Americans delivering at term, they have twice the odds of a SGA birth as compared to non-Hispanic whites. On average, term African American infants weigh 150g less (adjusted) than term non-Hispanic white infants. Women from the non-Hispanic Caribbean and Guyana experience birth outcomes most comparable to African Americans.
What is striking about these findings is the degree to which birth outcomes among ethnic groups socially recognized as black are similar. This is visually evident in Figure 1. For term births classified as SGA, African Americans and women from sub-Saharan Africa, the non-Hispanic Caribbean, Haiti, Trinidad and Tobago, Jamaica, and Guyana all have adjusted odds ratios grouped around 2. This finding is suggestive of the role racism may play in adverse perinatal outcomes.30-32 Racism is defined as prejudicial actions based on skin color or other physical characteristics.33 Racism can be individual, in which small numbers of people are affected, or institutional, when policies and traditions act against specific racial or ethnic groups.33 Broad based discrimination and disadvantage may result from institutional racism. The physical and psychological stresses of racism have been investigated to explain the increased risk of adverse perinatal outcomes among African American women in the United States.30, 32 In our study, these women with similarly elevated odds of SGA are from disparate regions with varying cultural traditions, diets, migration histories, and nativity. There is likely a range of socioeconomic positions represented by these women. Yet in New York City these groups have approximately the same increased odds of SGA as compared to non-Hispanic white women. Social recognition of “blackness” may have a meaningful effect on risk of adverse birth outcome, although with these data we are unable to directly examine the contribution of either racism or other unmeasured factors (e.g. diet, behavior, genes) that are correlated with geographic origin.34 This apparent clustering of risk seems similar among Hispanics, in that the degree of African admixture, and hence a recognizable “black” phenotype,35 appears to be associated with birth outcome. Among Caribbean populations with some African admixture, such as women from Puerto Rico, the Dominican Republic, and Cuba,36-38 adjusted odds ratios for term SGA cluster around 1.5. Odds ratios are lower for women from Mexico and South America where there is less African ancestry.39
Broad based discrimination and disadvantage - potentially experienced by any ethnic minority, not just those socially recognized as “black” - may also be a factor in the observed ethnic heterogeneity in birth outcomes. In addition to the step-wise clustering of the odds ratios for the groups noted above, there is an analogous situation for Asian subgroups. The odds of term SGA for women from South Central Asia are higher than for women from East Asia, as compared to non-Hispanic whites. New York City has recently experienced an influx of immigrants from South Asia, making South Asians one of the fastest growing ethnic groups in the city.40 The newness of the South Asian community, as compared to the well-established East Asian community, may mean that women from South Asia are subjected to more discrimination and suffer more disadvantages than their East Asian counterparts, irrespective of time since migration. While we cannot directly assess the effect of racism on birth outcome, our findings lend support to an association between them.
The Hispanic Paradox has been has been documented in the literature for over twenty years.5 Numerous studies report consistently favorable birth outcomes to Hispanic women despite their typical socioeconomic disadvantage as compared to non-Hispanic whites.5-12 The results of our study do not lend credence to this epidemiologic phenomenon. One major distinction of our study is the heterogeneity of the Hispanic population. Large studies of Hispanics in the US tend to focus on Mexicans in California or Cubans in Florida.7, 12, 41, 42 In New York City, while 34.2% of births were to women claiming Hispanic/Latino ancestry, only 13.7% of Hispanics were of Mexican origin. In fact, the most common maternal Hispanic heritage in New York City is either Puerto Rican (28.9%) or Dominican (25.5%). A study of 1996-97 New York City births also found less favorable birth outcomes among Puerto Ricans as compared to other Hispanic groups.13 As compared to California and Florida, the Hispanic population in New York City may differ with respect to selectivity and nativity. There may also be differences in the referent white population, which would impact our findings. The composition of a “Hispanic” ethnic group should be considered in future epidemiologic studies given the change in demographics over time and across geographic regions within the US.
Likewise, the standard categorization of race/ethnicity contains a single category for Asians and Pacific Islanders.43 This grouping combines people from distinct cultures, lifestyles, and migration histories as divergent as Pakistan and Japan. Consequently, we found distinct outcomes for Asian subgroups. Women from East Asia had birth outcomes as good as or better than non-Hispanic whites, whereas women from Southeast Asia/Pacific Islands and South Central Asia had less favorable outcomes than non-Hispanic whites. Similar findings have been reported previously.16-20 Even within these regional groupings, however, we still observed variation in risk, with women from Bangladesh having the poorest outcomes. The range of these outcomes is diluted in an overall “Asian” ethnic group. Cultural cohesiveness, timing of, and reasons for immigration may be key to understanding these differences.
The combination of birth certificates and hospital discharge records provides a rich resource for an analysis of this magnitude, although the data are understandably imperfect for defining the outcomes of interest. In general, however, vital statistics are widely used for monitoring trends in gestational age and birthweight. Restricting the analysis to births with plausible combinations of birthweight and gestational age likely alleviated much of the misclassification that may have resulted from incorrect estimates of gestational age. The lack of observed confounding by available covariates mitigates potential concerns that there is substantial residual confounding remaining after adjusting for maternal education, an imperfect marker of socioeconomic status. The large sample size, global representation of maternal ethnicity, and data from hospital discharge records that provided information on preterm subtypes are all notable strengths. Additionally, our approach to categorizing maternal ethnicity by reported ancestral country and geographic region25 is objective, albeit with some necessarily arbitrary decision rules. We objectively distinguished between East Asian and South Central Asian women, but then by geography, group women from Guyana with South Americans despite the fact that their risk profile and cultural experiences more closely matches those of women from the non-Hispanic Caribbean or African Americans. While we are unable to test hypotheses with these data, the large sample size and demographic diversity of the obstetrical population in New York City permitted exploration of birth outcomes by detailed maternal ethnicity not easily achieved elsewhere.
This study underscores the considerable risk heterogeneity in adverse perinatal outcomes by maternal race/ethnicity. Much of this heterogeneity is obscured by broad categorizations of race/ethnicity. Reported differences by country of origin may have direct implications for provision of community-level prenatal and postnatal health care for immigrant women in regions of ethnic concentration. Additionally, continued exploration of the causes of racial/ethnic disparity in adverse perinatal outcomes may help to formulate etiologic hypotheses that can support prevention efforts.
TABLE 2.
Delivery Indication by Maternal Ethnic Ancestry among Singleton Births, New York City, 1995 - 2003, n=949,210
| Delivery Indication | Preterm PROM or Spontaneous | Preterm Medically Indicated | ||
|---|---|---|---|---|
| Maternal Ethnic Ancestrya | N (percent) | Adjustedb Odds Ratio [95% CIc] | N (percent) | Adjustedb Odds Ratio [95% CIc] |
| Non-Hispanic White | 10,470 (3.8) | 1.0 | 3564 (1.3) | 1.0 |
| African American | 13,271 (9.0) | 2.6 [2.5, 2.7] | 3272 (2.2) | 1.9 [1.8, 2.0] |
| North Africad | 214 (3.9) | 1.2 [1.1, 1.4] | 59 (1.1) | 1.0 [0.7, 1.2] |
| Sub-Saharan Africae | 986 (5.7) | 1.8 [1.7, 1.9] | 306 (1.8) | 1.6 [1.4, 1.8] |
| Non-Hispanic Caribbean | 5,385 (7.4) | 2.5 [2.4, 2.6] | 1,609 (2.2) | 2.1 [1.9,2.2] |
| Haiti | 1133 (7.3) | 2.3 [2.2, 2.5] | 358 (2.3) | 2.0 [1.8, 2.3] |
| Jamaica | 1818 (7.0) | 2.3 [2.1, 2.4] | 549 (2.1) | 1.9 [1.7, 2.1] |
| Trinidad and Tobago | 962 (8.0) | 2.6 [2.4, 2.8] | 298 (2.5) | 2.2 [2.0, 2.5] |
| Non-Hispanic Caribbean, Otherf | 1472 (7.7) | 2.5 [2.4, 2.7] | 404 (2.1) | 1.9 [1.7, 2.1] |
| Hispanic Caribbean | 12,010 (6.7) | 2.0 [2.0, 2.1] | 3,217 (1.8) | 1.6 [1.5, 1.7] |
| Dominican Republic | 4636 (5.6) | 1.8 [1.7, 1.8] | 1240 (1.5) | 1.4 [1.3, 1.5] |
| Puerto Rico | 7197 (7.7) | 2.2 [2.1, 2.3] | 1934 (2.1) | 1.8 [1.7, 1.9] |
| Cuba | 177 (6.8) | 1.9 [1.6, 2.2] | 43 (1.7) | 1.3 [1.0, 1.8] |
| Mexico | 2311 (5.2) | 1.7 [1.6, 1.7] | 563 (1.3) | 1.2 [1.1, 1.3] |
| Central America | 1,298 (5.7) | 1.9 [1.7, 2.0] | 376 (1.6) | 1.5 [1.4, 1.7] |
| El Salvador | 320 (5.3) | 1.7 [1.5, 1.9] | 98 (1.6) | 1.5 [1.2, 1.8] |
| Honduras | 397 (5.5) | 1.7 [1.6, 1.9] | 116 (1.6) | 1.5 [1.2, 1.8] |
| Central America, Otherg | 581 (6.0) | 1.9 [1.7, 2.1] | 162 (1.7) | 1.5 [1.3, 1.8] |
| South America | 3,722 (5.9) | 1.9 [1.8, 1.9] | 1,012 (1.6) | 1.5 1.3, 1.6] |
| Colombia | 489 (4.5) | 1.3 [1.2, 1.5] | 120 (1.1) | 0.9 [0.8, 1.1] |
| Ecuador | 1070 (5.0) | 1.5 [1.4, 1.6] | 261 (1.2) | 1.1 [0.9, 1.2] |
| Guyana | 1622 (8.2) | 2.6 [2.5, 2.8] | 476 (2.4) | 2.2 [2.0, 2.5] |
| South America, Otherh | 541 (4.7) | 1.4 [1.3, 1.5] | 155 (1.4) | 1.1 [1.0, 1.3] |
| Other Hispanici | 872 (6.1) | 1.7 [1.6, 1.9] | 269 (1.9) | 1.6 [1.4, 1.8] |
| East Asia | 1,986 (3.5) | 1.0 [0.9, 1.1] | 527 (0.9) | 0.8 [0.7, 0.9] |
| China | 1437 (3.4) | 0.9 [0.9, 1.0] | 385 (0.9) | 0.8 [0.7, 0.8] |
| East Asia, Otherj | 549 (3.8) | 1.1 [1.0, 1.2] | 142 (1.0) | 0.8 [0.7, 1.0] |
| Southeast Asia & Pacific Islands | 708 (5.9) | 1.7 [1.6, 1.9] | 192 (1.6) | 1.4 [1.2, 1.6] |
| Philippines | 505 (6.4) | 1.8 [1.7, 2.0] | 143 (1.8) | 1.5 [1.2, 1.8] |
| Southeast Asia, Otherk | 203 (5.0) | 1.4 [1.2, 1.6] | 49 (1.2) | 1.0 [0.7, 1.3] |
| South Central Asia | 1,677 (5.0) | 1.6 [1.5, 1.7] | 500 (1.5) | 1.4 [1.3, 1.6] |
| Bangladesh | 419 (5.5) | 1.7 [1.6, 1.9] | 121 (1.6) | 1.5 [1.2, 1.8] |
| India | 740 (5.4) | 1.7 [1.6, 1.9] | 186 (1.4) | 1.3 [1.1,1.5] |
| Pakistan | 380 (4.9) | 1.5 [1.4, 1.7] | 157 (2.0) | 1.8 [1.6, 2.2] |
| South Central Asia, Otherl | 138 (3.3) | 1.0 [0.8, 1.1] | 36 (0.9) | 0.7 [0.5, 1.0] |
| Other | 450 (6.9) | 2.0 [1.8, 2.2] | 136(2.1) | 1.8 [1.5, 2.1] |
Countries with at least 500 preterm births <37 weeks are examined individually
Adjusted for nativity, maternal age, parity, education, tobacco, pre-pregnancy weight, and birth year (referent is ≥ 37 completed weeks gestation)
CI = confidence interval
Algeria, Egypt, Libya, Morocco, Sudan, Tunisia
Angola, Benin, Botswana, Burkina Faso, Burundi, Cameroon, Cape Verde, Central African Republic, Chad, Comoro Islands, Congo, Equatorial Guinea, Eritrea, Ethiopia, Gabon, Gambia, Ghana, Guinea, Guinea-Bissau, Ivory Coast, Kenya, Lesotho, Liberia, Madagascar, Malawi, Mali, Mauritania, Mauritius, Mozambique, Namibia, Niger, Nigeria, Rwanda, Senegal, Seychelles, Sierra Leone, Somalia, South Africa, Swaziland, Tanzania, Togo, Uganda, Zaire, Zambia, Zimbabwe, 'Other African'
Aruba, Antigua and Barbuda, Bahamas, Barbados, Bermuda, Cayman Islands, Curacao, Dominica, Grenada, Guadalupe, Martinique, Montserrat, Nevis and St. Christopher, St. Kitts and Nevis, St. Lucia, St. Marartin, St. Martin, St. Vincent, Tortola, Turks and Cacaos, Virgin Islands, West Indies
Belize, Costa Rica, Guatemala, Nicaragua, Panama, 'Other Central America'
Argentina, Bolivia, Brazil, Chile, French Guiana, Paraguay, Peru, Suriname, Uruguay, Venezuela, 'Other South America'
Spain, Hispanic with no ancestral country reported
Hong Kong, Japan, Korea, Macao, Mongolia, Singapore, Taiwan, 'Other East Asian'
American Samoa, Brunei, Cambodia, Caroline Islands, Fiji, Guam, Indonesia, Kiribati, Laos, Malaysia, Mariana Islands, Marshall Islands, Micronesia, New Guinea, Papua New Guinea, Samoa, Vietnam, Solomon Islands, Tahiti, Thailand, Tonga, Truk Islands, 'Other Pacific Islands'
Afghanistan, Bhutan, Burma, East Indies, Iran, Kazakhstan, Kyrgyzstan, Nepal, Sri Lanka, Tajikistan, Turkmenistan, Uzbekistan
Acknowledgements
This work was supported by the National Institute of Child Health and Human Development (R21-HD050739).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONDENSATION Racial/ethnic heterogeneity in perinatal outcomes is obscured by broad ethnic categorizations, and may directly affect provision of community-level prenatal and postnatal health care for immigrants.
Presentation information: Stein CR, Savitz DA, Janevic TM, Ananth CV, Kaufman JS, Herring AH, Engel SM. Ethnicity and birth outcome in New York City, 1995-2003. Poster presentation at the 21st Annual Meeting of the Society for Pediatric and Perinatal Epidemiologic Research, Chicago, Illinois, June 23-24, 2008.
REFERENCES
- 1.Artin JA, Hamilton BE, Sutton PD, et al. Births: final data for 2005. Natl Vital Stat Rep. 2007;56:1–103. [PubMed] [Google Scholar]
- 2.Saigal S, Doyle LW. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet. 2008;371:261–9. doi: 10.1016/S0140-6736(08)60136-1. [DOI] [PubMed] [Google Scholar]
- 3.Alexander GR, Wingate MS, Bader D, Kogan MD. The increasing racial disparity in infant mortality rates: composition and contributors to recent US trends. Am J Obstet Gynecol. 2008;198:51, e1–9. doi: 10.1016/j.ajog.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 4.Schempf AH, Branum AM, Lukacs SL, Schoendorf KC. The contribution of preterm birth to the Black-White infant mortality gap, 1990 and 2000. Am J Public Health. 2007;97:1255–60. doi: 10.2105/AJPH.2006.093708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Markides KS, Coreil J. The health of Hispanics in the southwestern United States: an epidemiologic paradox. Public Health Rep. 1986;101:253–65. [PMC free article] [PubMed] [Google Scholar]
- 6.Fuente-Safflick E, Lurie P. Low birth weight and Latino ethnicity. Examining the epidemiologic paradox. Arch Pediatr Adolesc Med. 1997;151:665–74. doi: 10.1001/archpedi.1997.02170440027005. [DOI] [PubMed] [Google Scholar]
- 7.Hessol NA, Fuentes-Afflick E. The perinatal advantage of Mexican-origin Latina women. Ann Epidemiol. 2000;10:516–23. doi: 10.1016/s1047-2797(00)00073-9. [DOI] [PubMed] [Google Scholar]
- 8.Buekens P, Notzon F, Kotelchuck M, Wilcox A. Why do Mexican Americans give birth to few low-birth-weight infants? Am J Epidemiol. 2000;152:347–51. doi: 10.1093/aje/152.4.347. [DOI] [PubMed] [Google Scholar]
- 9.Chung JH, Boscardin WJ, Garite TJ, Lagrew DC, Porto M. Ethnic differences in birth weight by gestational age: at least a partial explanation for the Hispanic epidemiologic paradox? Am J Obstet Gynecol. 2003;189:1058–62. doi: 10.1067/s0002-9378(03)00848-2. [DOI] [PubMed] [Google Scholar]
- 10.Mcglade MS, Saha S, Dahlstrom ME. The Latina paradox: an opportunity for restructuring prenatal care delivery. Am J Public Health. 2004;94:2062–5. doi: 10.2105/ajph.94.12.2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown HL, Chireau MV, Jallah Y, Howard D. The “Hispanic paradox”: an investigation of racial disparity in pregnancy outcomes at a tertiary care medical center. Am J Obstet Gynecol. 2007;197:197, e1–7. doi: 10.1016/j.ajog.2007.04.036. discussion 197 e7-9. [DOI] [PubMed] [Google Scholar]
- 12.Gonzalez-Quintero VH, Tolaymat L, Luke B, et al. Outcome of pregnancies among Hispanics: revisiting the epidemiologic paradox. J Reprod Med. 2006;51:10–4. [PubMed] [Google Scholar]
- 13.Rosenberg TJ, Raggio TP, Chiasson MA. A further examination of the “epidemiologic paradox”: birth outcomes among Latinas. J Natl Med Assoc. 2005;97:550–6. [PMC free article] [PubMed] [Google Scholar]
- 14.Mathews TJ, Macdorman MF. Infant mortality statistics from the 2005 period linked birth/infant death data set. Natl Vital Stat Rep. 2008;57:1–32. [PubMed] [Google Scholar]
- 15.Acevedo-Garcia D, Soobader MJ, Berkman LF. Low birthweight among US Hispanic/Latino subgroups: the effect of maternal foreign-born status and education. Soc Sci Med. 2007;65:2503–16. doi: 10.1016/j.socscimed.2007.06.033. [DOI] [PubMed] [Google Scholar]
- 16.Fuentes-Afflick E, Hessol NA. Impact of Asian ethnicity and national origin on infant birth weight. Am J Epidemiol. 1997;145:148–55. doi: 10.1093/oxfordjournals.aje.a009085. [DOI] [PubMed] [Google Scholar]
- 17.Wong LF, Caughey AB, Nakagawa S, Kaimal AJ, Tran SH, Cheng YW. Perinatal outcomes among different Asian-American subgroups. Am J Obstet Gynecol. 2008;199:382, e1–6. doi: 10.1016/j.ajog.2008.06.073. [DOI] [PubMed] [Google Scholar]
- 18.Baker LC, Afendulis CC, Chandra A, Mcconville S, Phibbs CS, Fuentes-Afflick E. Differences in neonatal mortality among whites and Asian American subgroups: evidence from California. Arch Pediatr Adolesc Med. 2007;161:69–76. doi: 10.1001/archpedi.161.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hayes DK, Lukacs SL, Schoendorf KC. Heterogeneity within Asian Subgroups: A Comparison of Birthweight Between Infants of US and Non-US Born Asian Indian and Chinese Mothers. Matern Child Health J. 2008;12:549–56. doi: 10.1007/s10995-007-0270-8. [DOI] [PubMed] [Google Scholar]
- 20.Qin C, Gould JB. The Asian birth outcome gap. Paediatr Perinat Epidemiol. 2006;20:279–89. doi: 10.1111/j.1365-3016.2006.00737.x. [DOI] [PubMed] [Google Scholar]
- 21.Savitz DA, Janevic T, Engel SM, Kaufman JS, Herring AH. Ethnicity and gestational diabetes in New York City, 1995 - 2003. British Journal of Obstetrics and Gyneacology. 2008;115:969–978. doi: 10.1111/j.1471-0528.2008.01763.x. [DOI] [PubMed] [Google Scholar]
- 22.Alexander GR, Kogan M, Bader D, Carlo W, Allen M, Mor J. US birth weight/gestational age-specific neonatal mortality: 1995-1997 rates for whites, hispanics, and blacks. Pediatrics. 2003;111:e61–6. doi: 10.1542/peds.111.1.e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oken E, Kleinman KP, Rich-Edwards J, Gillman MW. A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC Pediatr. 2003;3:6. doi: 10.1186/1471-2431-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lydon-Rochelle MT, Holt VL, Nelson JC, et al. Accuracy of reporting maternal in-hospital diagnoses and intrapartum procedures in Washington State linked birth records. Paediatr Perinat Epidemiol. 2005;19:460–71. doi: 10.1111/j.1365-3016.2005.00682.x. [DOI] [PubMed] [Google Scholar]
- 25.United Nations . Standard Country or Area Codes for Statistics Use. New York, NY: 1999. [Google Scholar]
- 26.Greenland S. Invited commentary: variable selection versus shrinkage in the control of multiple confounders. Am J Epidemiol. 2008;167:523–9. doi: 10.1093/aje/kwm355. discussion 530-1. [DOI] [PubMed] [Google Scholar]
- 27.Ananth CV, Vintzileos AM. Epidemiology of preterm birth and its clinical subtypes. J Matern Fetal Neonatal Med. 2006;19:773–82. doi: 10.1080/14767050600965882. [DOI] [PubMed] [Google Scholar]
- 28.Morken NH, Kallen K, Hagberg H, Jacobsson B. Preterm birth in Sweden 1973- 2001: rate, subgroups, and effect of changing patterns in multiple births, maternal age, and smoking. Acta Obstet Gynecol Scand. 2005;84:558–65. doi: 10.1111/j.0001-6349.2005.00765.x. [DOI] [PubMed] [Google Scholar]
- 29.Morken NH, Magnus P, Jacobsson B. Subgroups of preterm delivery in the Norwegian Mother and Child Cohort Study. Acta Obstet Gynecol Scand. 2008;87:1374–7. doi: 10.1080/00016340802491508. [DOI] [PubMed] [Google Scholar]
- 30.Collins JW, JR., David RJ, Handler A, Wall S, Andes S. Very low birthweight in African American infants: the role of maternal exposure to interpersonal racial discrimination. Am J Public Health. 2004;94:2132–8. doi: 10.2105/ajph.94.12.2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dominguez TP. Race, racism, and racial disparities in adverse birth outcomes. Clin Obstet Gynecol. 2008;51:360–70. doi: 10.1097/GRF.0b013e31816f28de. [DOI] [PubMed] [Google Scholar]
- 32.Rosenberg L, Palmer JR, Wise LA, Horton NJ, Corwin MJ. Perceptions of racial discrimination and the risk of preterm birth. Epidemiology. 2002;13:646–52. doi: 10.1097/00001648-200211000-00008. [DOI] [PubMed] [Google Scholar]
- 33.Bhopal R. Glossary of terms relating to ethnicity and race: for reflection and debate. J Epidemiol Community Health. 2004;58:441–5. doi: 10.1136/jech.2003.013466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anum EA, Springel EH, Shriver MD, Strauss JF., 3RD Genetic contributions to disparities in preterm birth. Pediatr Res. 2009;65:1–9. doi: 10.1203/PDR.0b013e31818912e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shriver MD, Parra EJ, Dios S, et al. Skin pigmentation, biogeographical ancestry and admixture mapping. Hum Genet. 2003;112:387–99. doi: 10.1007/s00439-002-0896-y. [DOI] [PubMed] [Google Scholar]
- 36.Bonilla C, Shriver MD, Parra EJ, Jones A, Fernandez JR. Ancestral proportions and their association with skin pigmentation and bone mineral density in Puerto Rican women from New York city. Hum Genet. 2004;115:57–68. doi: 10.1007/s00439-004-1125-7. [DOI] [PubMed] [Google Scholar]
- 37.Cintado A, Companioni O, Nazabal M, et al. Admixture estimates for the population of Havana City. Ann Hum Biol. 2009;36:350–60. doi: 10.1080/03014460902817984. [DOI] [PubMed] [Google Scholar]
- 38.Tajima A, Hamaguchi K, Terao H, et al. Genetic background of people in the Dominican Republic with or without obese type 2 diabetes revealed by mitochondrial DNA polymorphism. J Hum Genet. 2004;49:495–9. doi: 10.1007/s10038-004-0179-7. [DOI] [PubMed] [Google Scholar]
- 39.Mao X, Bigham AW, Mei R, et al. A genomewide admixture mapping panel for Hispanic/Latino populations. Am J Hum Genet. 2007;80:1171–8. doi: 10.1086/518564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.THE CITY OF NEW York . In: Immigrant New York in the new millenium briefing booklet. Department of City Planning, editor. New York: 2004. NYC DCP #04-09. [Google Scholar]
- 41.Guendelman S, Thornton D, Gould J, Hosang N. Mexican women in California: differentials in maternal morbidity between foreign and US-born populations. Paediatr Perinat Epidemiol. 2006;20:471–81. doi: 10.1111/j.1365-3016.2006.00751.x. [DOI] [PubMed] [Google Scholar]
- 42.Fuentes-Afflick E, Hessol NA, Perez-Stable EJ. Testing the epidemiologic paradox of low birth weight in Latinos. Arch Pediatr Adolesc Med. 1999;153:147–53. doi: 10.1001/archpedi.153.2.147. [DOI] [PubMed] [Google Scholar]
- 43.OFFICE OF MANAGEMENT AND Budget Revisions to the standards for the classification of federal data on race and ethnicity. Directive No. 15. 1997 [Google Scholar]