Abstract
Background
Allopregnanolone (ALLO) is a progesterone derivative that rapidly potentiates γ-aminobutyric acidA (GABAA) receptor mediated inhibition and modulates symptoms of ethanol withdrawal. Since clinical and preclinical data indicate that ALLO levels are inversely related to symptoms of withdrawal, the present studies determined whether ethanol dependence and withdrawal differentially altered plasma and cortical ALLO levels in mice selectively bred for differences in ethanol withdrawal severity and determined whether the alterations in ALLO levels corresponded to a concomitant change in activity and expression of the biosynthetic enzyme 5α-reductase.
Methods
Male Withdrawal Seizure—Prone (WSP) and —Resistant (WSR) mice were exposed to 72 hr ethanol vapor or air and euthanized at select times following removal from the inhalation chambers. Blood was collected for analysis of ALLO and corticosterone levels by radioimmunoassay. Dissected amygdala, hippocampus, midbrain and cortex as well as adrenals were examined for 5α-reductase enzyme activity and expression levels.
Results
Plasma ALLO was decreased significantly only in WSP mice, and this corresponded to a decrease in adrenal 5α-reductase expression. Cortical ALLO was decreased up to 54% in WSP mice and up to 46% in WSR mice, with a similar decrease in cortical 5α-reductase activity during withdrawal in the lines. While cortical gene expression was significantly decreased during withdrawal in WSP mice, there was a 4-fold increase in expression in the WSR line during withdrawal. Hippocampal 5α-reductase activity and gene expression was decreased only in dependent WSP mice.
Conclusions
These results suggest that there are line and brain regional differences in the regulation of the neurosteroid biosynthetic enzyme 5α-reductase during ethanol dependence and withdrawal. In conjunction with the finding that WSP mice exhibit reduced sensitivity to ALLO during withdrawal, the present results are consistent with the hypothesis that genetic differences in ethanol withdrawal severity are due, in part, to modulatory effects of GABAergic neurosteroids such as ALLO.
Keywords: neurosteroid, GABAA receptors, radioimmunoassay, alcohol, mouse
Introduction
Allopregnanolone (ALLO; 3α-hydroxy-5α-pregnan-20-one) is a derivative of progesterone that is the most potent positive endogenous modulator of γ-aminobutyric acidA (GABAA) receptor mediated inhibition identified (Belelli et al., 1990; Purdy et al., 1990b; Rupprecht and Holsboer, 1999; Veleiro and Burton, 2009). Endogenous levels of ALLO are detectable in the brain independent of peripheral sources and they fluctuate in vivo within a range of concentrations that potentiate GABAergic inhibition in vitro (Barbaccia et al., 2001; Belelli and Lambert, 2005; Finn et al., 2004; Paul and Purdy, 1992). These findings suggest that endogenous ALLO levels may be important in retaining normal GABAergic brain function, and that alterations in endogenous levels could modify the functioning of central GABAA receptors in vivo. Support for this idea is provided by earlier findings that increased endogenous ALLO levels in the dentate gyrus in vitro revealed an endogenous neurosteroid tone that was sufficient to modulate GABAA receptors (Belelli and Herd, 2003) and that manipulation of hippocampal ALLO levels in vivo produced bi-directional effects on seizure susceptibility (Gililland-Kaufman et al., 2008).
It is well documented that ALLO shares a similar pharmacological profile to ethanol, with both exhibiting anxiolytic, anticonvulsant, locomotor stimulant and sedative/hypnotic properties (Gasior et al, 1999; Hirani et al., 2002, 2005; Van Doren et al., 2000 — also see reviews by Criswell and Breese, 2005; Finn et al., 2004; Grobin et al., 1998; Morrow et al., 1999, 2001). Additionally, ethanol and ALLO similarly influence hippocampal function (Murayama et al., 2006; Sanna et al., 2004; Silvers et al., 2003; Tokunaga et al., 2003), with evidence suggesting that an ethanol-induced steroidogenic effect to increase ALLO levels following acute administration contributes to ethanol’s effects on hippocampal neural activity (Sanna et al., 2004; Tokunaga et al., 2003).
Chronic ethanol withdrawal is known to alter ALLO concentrations in rodents and humans (Cagetti et al., 2004; Hill et al., 2005; Janis et al., 1998; Romeo et al., 1996). Additionally, an inverse relationship between plasma ALLO and tetrahydrodeoxycorticosterone (THDOC, 3α,21-dihydroxy-5α-pregnan-20-one; another GABAergic neurosteroid) levels and symptoms of alcohol withdrawal was demonstrated in small cohorts of alcoholic patients (Hill et al., 2005; Romeo et al., 1996, 2000). Specifically, a decrease in GABAergic neurosteroid levels corresponded to an increase in subjective ratings of anxiety and depression during the early withdrawal phase (i.e., days 4-5), when compared with control subjects. In mice selectively bred for differences in chronic ethanol withdrawal severity [i.e., Withdrawal Seizure-Prone (WSP) versus Withdrawal-Seizure Resistant (WSR)], preliminary data indicated that plasma ALLO levels were decreased during ethanol withdrawal only in WSP mice without producing a concomitant change in progesterone levels (Finn et al., 2004). Thus, the results in WSP mice and in humans are suggestive of a relationship between endogenous GABAergic neurosteroid levels and behavioral changes in excitability during ethanol withdrawal.
The WSP and WSR lines were selectively bred to have severe (WSP) or mild (WSR) handling-induced convulsions (HICs) after withdrawal from chronic ethanol exposure. This bi-directional selection was conducted in duplicate, resulting in two genetically independent lines of WSP (WSP-1, WSP-2) and WSR (WSR-1, WSR-2) mice (Crabbe et al., 1985). Withdrawal from exposure to 72 hr of ethanol vapor, measured by the increase in HICs following removal from the inhalation chambers was more than 10-fold greater in both WSP versus WSR replicate lines (Crabbe et al., 1985), even though both lines received equivalent ethanol exposure (see Finn and Crabbe, 1999). One advantage to testing selected lines is that they can be used to determine whether there is a genetic correlation between the selection phenotype and other traits of interest (discussed in Crabbe et al., 1990). When a pair of selected lines is found to differ significantly on a trait other than the one for which they were selected, this significant genetic correlation between two traits implies the action of a common set of genes on the two phenotypes. The strongest evidence for genetic codetermination of ethanol withdrawal severity and another trait is when both pairs of reciprocally selected lines differ in the correlated response (e.g., WSP-1 > WSR-1 and WSP-2 > WSR-2). In other words, if both replicate WSP lines differ from both replicate WSR lines on another trait, it is much more likely that there is a true genetic correlation between the two responses (i.e., withdrawal severity and the second trait). Given that selective breeding tends to fix alleles of trait-relevant genes in a homozygous manner without altering the frequencies of alleles at non-relevant genes, the use of replicate selected lines would avoid the potential interpretational confound of having the same trait-irrelevant alleles being fixed by chance in both replicate lines of WSP and WSR mice (discussed in Crabbe et al., 1990).
Chronic ethanol administration leads to the development of tolerance, or reduced sensitivity, to most of ethanol’s pharmacological effects (Kalant et al., 1971). The efficacy of GABA, benzodiazepines, and barbiturates also is reduced in animals following exposure to chronic ethanol (Buck and Harris, 1990; Morrow, 1995). However, the specific genes responsible for these adaptive changes have yet to be elucidated. Initial gene mapping studies found a significant genetic correlation between ethanol withdrawal severity and a region of chromosome 13 in which the murine gene for the enzyme 5α-reductase-1 is located (Srd5a1, Jenkins et al., 1991; Crabbe, 1998). A subsequent mapping study revealed an epistatic interaction between the chromosome 13 region and a region on chromosome 11 that contained several genes encoding proteins for GABAA receptor subunits α1, α6, and γ2, suggesting that an interaction between Srd5a1 and GABAA receptor subunit genes may occur (Bergeson et al., 2003). This epistatic interaction may be highly relevant to GABAergic neurosteroid synthesis, as the major route of progesterone metabolism in the rodent brain is via 5α-reduction, which is an irreversible reaction in mammalian cells. Consistent with the idea that ethanol withdrawal altered ALLO biosynthesis at a point downstream from progesterone, withdrawal from chronic intermittent ethanol exposure produced a significant decrease in hippocampal ALLO levels in genetically heterogeneous rats that was accompanied by a significant decrease in the expression of hippocampal 5α-reductase-1 (Cagetti et al., 2004).
Based on the above, the purpose of the present studies was to examine the genetic relationship between effects of chronic ethanol exposure and withdrawal on brain ALLO levels and 5α-reductase-1 activity and expression in WSP and WSR mice. Specific goals were to determine whether changes in ALLO levels corresponded to a concomitant alteration in 5α-reductase-1 enzyme activity and gene expression, and to determine whether there were line differences in the relationship between ALLO levels and 5α-reductase activity and expression during withdrawal. We hypothesized that brain ALLO levels and 5α-reductase-1 would be differentially altered in WSP and WSR mice during ethanol withdrawal, and that these line differences would represent correlated responses to selection. Additionally, the examination of enzyme activity and expression would allow us to draw conclusions about the regulation of 5α-reductase-1 in ethanol naïve, ethanol dependent, and ethanol-withdrawing WSP and WSR mice.
Methods
Animals
Two independent replicate lines of drug-naive male WSP (i.e.WSP-1 and-2) and WSR (i.e.WSR-1and -2) mice were used in all experiments. These lines were bred from a genetically heterogeneous stock of known composition (i.e., HS/Ibg) and proceeded by within-family, bi-directional selection with replicate and control lines. The genetic selection pressure used in developing these lines has been described (Crabbe et al., 1985). The animals were bred in the Veterinary Medical Unit at the Veterans Affairs Medical Center (Portland, Oregon) and were housed four per cage with ad libitum access to food and water. Subjects were acclimated to a 12:12 h light and dark cycle (lights on at 6:00 am). At the time of testing, the mice were from selected generation 26 [filial generations 79-81] and were 60-98 days old, consistent with other studies conducted with these mouse lines. Age ranges did not differ in the WSP and WSR mice. All procedures complied with the United States Public Health Service Institutes of Health guidelines in accordance with the Guide for the Care and Use of Laboratory Animals and were approved by the local Institutional Animal Care and Use Committee.
Chronic Ethanol Administration
Separate groups of animals were exposed to ethanol vapor or air for 72 hr using a standardized method for inducing ethanol dependence (Finn and Crabbe, 1999; Terdal and Crabbe, 1994). The objective was to expose WSP and WSR mice to an ethanol vapor concentration that would produce an equivalent chronic blood ethanol concentration (BEC) of 1.0 – 1.5 mg/ml in the lines. During the experiment, the animals were housed in a stainless steel ¼ inch hardware cloth cage inside a large Plexiglas chamber, with food and water freely available. Chamber temperature ranged from 28 – 30°C and airflow rate within the large Plexiglas chamber was 55 liter/min. The volume of each of the three large Plexiglas chamber was 538 liters; therefore, air or ethanol vapor was replaced every 10 min. Three separate chambers were available, which allowed for simultaneous treatment of the ethanol- and air-exposed mice.
The present studies used the alcohol dehydrogenate inhibitor pyrazole hydrochloride (pyrazole; Sigma Chemical Co., St. Louis, MO) to stabilize BEC. On day one, mice in the ethanol groups were weighed and injected intraperitoneally (i.p.) with a priming dose of ethanol (20% v/v, 1.5 g/kg for WSR-1, WSR-2 and WSP-1; and 1.75 g/kg for WSP-2) and pyrazole (68.1 mg/kg for WSR-1, WSR-2 and WSP-1 and 77.5 mg/kg for WSP-2) prior to placement into the vapor chambers. Vapor concentrations inside the inhalation chambers were 7.0 mg ethanol/liter air for WSPs and 9.0 mg ethanol/liter air for WSRs. Different vapor concentrations were used to achieve equivalent BECs for the two genotypes so that any genetic differences could not be ascribed to differences in ethanol pharmacokinetics. At 24 and 48 hr, the animals were briefly removed from the chamber, weighed, injected i.p. with pyrazole, and placed back into the inhalation chamber. Tail blood samples were taken from a subset of the ethanol-exposed animals each day to monitor BEC. Air-exposed animals also received daily pyrazole injections, but were injected with saline, instead of ethanol on day one, and were exposed to air inside the inhalation chamber.
Upon removal from the inhalation chamber at 72 hr, a tail blood sample was taken from all the ethanol-exposed mice to assess BEC. Tails were nicked in the air-exposed mice, but no blood samples were taken. Depending on the study, separate groups of air- and ethanol-exposed mice were euthanized at selected time points (i.e., t = 0, 4, and 8 hr post removal from the inhalation chamber). Trunk blood and dissected brain samples (details below) and adrenals were collected on ice. Due to limitations in sensitivity of the ALLO assay, only cortical ALLO levels were determined. Activity of the 5α-reductase-1 enzyme was examined in amygdala, hippocampus, midbrain, and cortex, based on the following pattern of 5α-reductase activity reported in mouse brain: midbrain tegmentum > hypothalamus > hippocampus > cerebral cortex (Roselli and Snipes, 1984) and was measured by the conversion of progesterone to 5α-dihydroprogesterone (5α-DHP; 5α-pregnane-3,20-dione). Expression of the 5α-reductase-1 transcript was measured by Real-time quantitative reverse transcriptase polymerase chain reaction (qRT-PCR) in the same tissues as for enzyme activity. We chose to examine the 5α-reductase type-1 isoform of the enzyme based on the data presented in the Introduction and because it is constitutively expressed in the rodent central nervous system and highly expressed in brain (Melcangi et al., 1998).
BEC Determination
Blood samples were analyzed for ethanol content by gas chromatography, utilizing a modification of the method described by Roach and Creaven (1968). Briefly, a 20 μl sample of blood was taken from the tip of the tail, added to 50 μl of chilled 5% ZnSO4, and stored on ice. Next, 50 μl of 0.3N Ba(OH)2 and 300 μl of distilled water were added to each sample. The samples were vortexed for 5 sec and centrifuged for 5 min at 12,000 rpm. The supernatant was transferred to a crimp-top glass vial and analyzed for ethanol concentration using an Agilent 6890N gas chromatograph with an Agilent 7683 autosampler and injector, injecting a 1 μl sample onto a capillary column (DB-ALC2, J & W Scientific, Folsom, CA). Sample peak area was interpolated from a standard curve that was derived from seven pairs of external standards of known concentration (0.5-4.0 mg/ml). A standard curve was defined as usable when the correlation between ethanol concentration and peak area was r2 = 0.9995 or greater.
Brain Dissections
Brain dissections were performed as described previously (Roselli and Snipes, 1984; Resko et al., 1986). Briefly, the whole brain was removed and placed ventral-side up on a cold platform. Sagittal cuts were made just rostral to the optic chiasm and on the rostral edge of the mamillary bodies. The amygdala was obtained from this section of tissue by removing a bilateral triangle of tissue just lateral to the hypothalamic sulci, which contained all the major amygdaloid nuclei as well as the overlying entorhineal cortex. The cortex was removed from the dorsolateral borders of the tissue section. Removing the cortex exposed the hippocampus, which was peeled away from the top of the thalamus and midbrain. The dissected tissues were frozen on dry ice (RIA and progesterone 5α-reductase assays) or in liquid nitrogen (qRT-PCR).
RIA
For plasma assays, trunk bloods were collected into heparinized tubes (BD Vacutainer, Franklin Lakes, NJ) and centrifuged at 2000 rpm for 20 minutes. Plasma was separated and stored at −12°C until the assays to measure ALLO and corticosterone (CORT) were performed. For brain assays, dissected cortical tissues were collected and stored in microcentrifuge tubes at −12°C until assays were conducted.
Brain Extraction of ALLO
ALLO was extracted from the cortical brain samples according to the method of Janis et al. (1998). Briefly, samples were digested with 385 μl of 0.3N NaOH, followed by the addition of 100 μl [3H] ALLO (2000 counts per min (cpm)/100 μl in ethanol) to monitor extraction efficiency (65 Ci/mmol; New England Nuclear, Boston, MA). Samples were extracted three times with 10% (v/v) ethyl acetate in heptane, sonicated for approximately 1 min, and centrifuged at 1,000 × g for 2 min. The supernatants from the three extractions were combined and then applied to solid phase silica columns (Honeywell, Burdick and Jackson, Muskegon, MI). The columns were then washed with heptane, and elution of ALLO occurred with 25% (v/v) acetone in pentane under gravity. The eluate was dried under nitrogen. To conduct the RIA, the steroid was reconstituted using 30 μl isopropanol + 170 μl RIA buffer (sodium phosphate/ bovine serum albumin (BSA) assay buffer). Extraction efficiency was determined using 50 μl of re-dissolved extract by liquid scintillation spectroscopy, while 100 μl was used in the RIA.
Plasma Extraction of ALLO
ALLO was extracted from plasma according to an adaptation of the method of Purdy et al. (1990a) and is described in detail elsewhere (Finn and Gee, 1994). The plasma sample (200 μl) was digested with 50 μl of 0.3N NaOH, and 100 μl [3H] ALLO (1500 cpm/100 μl in ethanol) was added to determine extraction efficiency. The steroid was extracted three times using anhydrous ethyl ether, and the combined supernatants from the three extractions were dried under nitrogen. To measure ALLO levels, the samples were re-dissolved in 2 μl of dimethyl sulfoxide and 198 μl of RIA buffer. Extraction efficiency was determined using 50 μl of re-dissolved extract by liquid scintillation spectroscopy, while 100 μl was used in the RIA.
ALLO RIA
The RIA was conducted according to the methods described in detail by Finn and Gee (1994). It utilized [3H]ALLO (10,000 cpm in 100 μl in RIA buffer) and a polyclonal antiserum that was a generous gift from Dr. Kelvin Gee (University of California, Irvine, CA) and that had minimal cross-reactivity (Finn and Gee, 1994). Results (cpm) were normalized and fit to a least-square regression equation produced by log-logit transformation of the standards (0.0195-20 ng). Mass of the samples was calculated by interpolation of the standards and correction for extraction efficiency. The minimum detectable limit in the present assay was 25 pg. The intra-assay coefficient of variation averaged 14%, and the inter-assay coefficient of variation in 7 assays averaged 15%.
CORT RIA
Plasma in 5 μl quantities was diluted with 100 μl of sterile water and stored at 4°C until assayed. Samples were immersed in boiling water for 5 min to denature CORT-binding globulin. The RIA was adapted from a previously reported procedure (Keith et al., 1978) and used [125I]CORT from ICN Pharmaceuticals (Cost Mesa, CA) and antiserum from Ventrex (Portland, MA). Results (cpm) were normalized and fit to a least-square regression equation produced by log-logit transformation of the standards. Mass of the samples was calculated by interpolation of the standards. The detectable range of the assay was from 0.1 to 400 μg CORT per 100 ml plasma. Intra- and inter-assay coefficients of variation were less than 10%. The specificity of the assay is very high, with only 4% cross-reactivity to deoxycorticosterone, 1% cross-reactivity to 5β-pregnanedione, and less than 0.6% cross-reactivity to other endogenous steroids.
Progesterone 5α-reductase Enzyme Activity
Progesterone 5α-reductase-1 enzyme activity was determined by measuring the amount of [3H]5α-DHP produced after incubating microsomal preparations of tissue samples with a saturating concentration (i.e., 33μM) of [3H]progesterone, supplemented with an NADPH-generating system (details below), according to previously published methods (Resko et al., 1988; Resko and Roselli, 1992; Roselli and Snipes, 1984; Roselli et al., 1987). Progesterone 5α-reductase activity is expressed as fmol 5α-DHP produced/mg protein/hr. Since this assay can be conducted on as little as 20 mg of tissue, dissected brain regions were not pooled.
Tissue Incubation and Extraction
Dissected brain samples were diluted 1:10 (w/v) in TEMGD buffer (10 mM Tris, 1.5 mM EDTA, 25 mM molybdate, 10% (v/v) glycerol, 1 mM DDT; pH = 7.4) and homogenized in ice-cold Dounce tissue grinders. The homogenates were centrifuged at 1000 × g for 10 min in a Beckman J-6B centrifuge. A microsomal fraction was prepared from the resultant supernatant by re-centrifugation at 106,800 × g for 10 min in a Beckman T100 ultracentrifuge. This fraction was used because progesterone 5α-reductase was shown to be concentrated in microsomes, whereas the NADPH-linked 3α-hydroxysteroid oxidoreductases (dehydrogenases) are found in the soluble fraction (Krause and Karavolas, 1980; Krieger et al., 1983).
Microsomal pellets were suspended in 10 volumes of phosphate buffer (100 mM KCl, 1 mM EDTA, and 0.01 M KH2PO4) using a tissue sonicator. Incubation tubes containing a saturating concentration of [3H]progesterone (i.e., 33 μM; New England Nuclear, specific activity adjusted to 534 Ci/mol) were dissolved in a phosphate buffer supplemented with an NADPH-generating system (1 mM NADP, 5 mM glucose-6-phosphate, and 2 units glucose-6-phosphate dehydrogenase Type XV). After a 30 min pre-incubation period, 25 μl of the suspended microsomal pellet was added to the tubes and shaken gently at 37°C for 1 hr in a Dubnoff incubator. A separate aliquot of microsomes was reserved for protein determination. Appropriate buffer and tissue blanks were run in each assay to correct for non-specific conversion. An additional set of tubes containing [3H]5αDHP (approximately 10,000 cpm) were carried through the procedure to monitor losses due to extraction and chromatography. The reactions were stopped by the addition of 500 μl ice-cold distilled water and extracted with 7 ml diethyl ether. The ether extracts were dried under nitrogen and concentrated in ethanol.
Chromatography
The ether extracts were fractionated on Eastman Kodak thin layer sheets (no. 13181; 100 μm silica gel with fluorescent indicator) in a 6:4 (v/v) benzene-ethyl acetate solvent system (Roselli et al., 1987). Authentic reference steroids were run in parallel lanes. The area with the mobility of 5αDHP was eluted with 5 ml of ethanol. The eluates were dried under a stream of air, dissolved in 50 μl of hexane:benzene:methanol (85:15:5, by vol), and applied in this solvent system to a column containing 2.6 g of Sephadex LH-20 (Resko and Roselli, 1992). In this chromatography system, 5αDHP eluates in the 10-17 ml fraction of solvent run through the system. Fractions with the mobility of authentic 5αDHP were then collected into scintillation vials, dried, and dissolved in 10 ml of scintillation fluid. The samples were counted by liquid scintillation spectrometry for a time sufficient to give < 2% counting error. The counts in the buffer blanks were subtracted from sample counts and then divided by the percent recovery to correct for procedural losses. To assure the identity of the radioactivity measured as 5αDHP, pools from several samples (5,000-10,000 dpm 3H) migrating with the chromatographic mobility of authentic 5αDHP were acetylated and re-crystallized to a constant specific activity after the addition of 20 mg radioinert 5αDHP-acetate.
Progesterone 5α-reductase Gene Expression
Total RNA was isolated from individual dissected brain samples, and analysis of 5α-reductase-1 transcript expression was performed using qRT-PCR with the iCycler IQ Real Time PCR detection system (Bio-Rad Laboratories, Inc.), as previously described (Hashimoto et al., 2004; Hashimoto and Wiren, 2008). PCR primers were designed based on the mRNA sequence by using OLIGO Software (v6.67; Oligo, Cascade, CO) and were synthesized by Fisher Scientific (Pittsburg, PA). Primers were chosen to generate an amplicon between 100 and 300 bp and to minimize dimerization. Normalization of real-time RT-PCR data was conducted for each transcript using the RNA-specific fluorescent dye RiboGreen® (Hashimoto et al., 2004).
RNA was isolated using the RNA Stat-60 kit (Tel-Test, Inc., Friendswood, TX). Contaminating DNA was removed by Zymo-spin column purification following manufacturer’s recommendations (Zymo Research, Orange, CA). RNA was quantified by spectrophotometric methods; lack of degradation was confirmed by agarose gel electrophoresis followed by staining with SYBR Gold. Twenty ng RNA was reverse transcribed and amplified in a 25 μl reaction mix using a one-step QuantiTect SYBR Green RT-PCR Master Mix (Qiagen, Valencia, CA) and 0.5 μM of primer. Relative expression of the RT-PCR product was determined using the comparative ΔΔCt method (Winer et al., 1999), after normalizing expression with fluorescence to RiboGreen®. Fold regulation then was determined by normalizing all values to the mean of the relative expression for the control group.
Real-time qRT-PCR efficiency was determined for each primer set using a five-fold dilution series of total RNA. Individual reaction kinetics also were analyzed to ensure that each real-time RT-PCR did not differ significantly from 100%. Following PCR, specificity of the PCR reaction was confirmed with melt curve analysis to ensure that only the expected PCR product was amplified per reaction. Reaction products were melted over the temperature range of 55°C to 95°C in 0.5°C increments, 10 seconds per increment. The optical data were collected over the duration of the temperature melt and evaluated with the negative first derivative of the change in fluorescence over time to ensure that only the expected PCR product was amplified in the reaction. Additionally, amplicons were sequenced for confirmation.
Statistical Analysis
Analysis of variance (ANOVA) was used to assess line (i.e., WSP vs. WSR), replicate (i.e., WSP-1 and WSR-1 vs. WSP-2 and WSR-2) and treatment/time (Air, ethanol 0 hr, ethanol 4 hr, ethanol 8 hr) effects on the dependent measures BEC, ALLO levels, CORT levels, 5α-reductase activity and 5α-reductase expression. For all experiments, there were no meaningful interactions involving genetic replicate; hence, data from the two replicates of each selected line were combined. When interactions were obtained, the data for each selected line were analyzed separately. Based on our a priori hypothesis that ALLO, CORT, and 5α-reductase activity and expression would be differentially altered in the WSP and WSR mice following chronic ethanol exposure and withdrawal, Simple Main Effects analysis were conducted to examine significant treatment/time effects within each selected line. Significance was set at P ≤ 0.05.
Results
Male WSP and WSR mice from both genetic replicates were exposed to 72 hr ethanol vapor or air, and separate groups of mice were euthanized at 0, 4, or 8 hrs following removal from the inhalation chambers. Chronic ethanol vapor exposure produced equivalent BEC upon removal from the inhalation chamber. Mean ± SEM BEC was 1.10 ± 0.05 mg/ml for WSP and 1.14 ± 0.02 mg/ml for WSR mice. While there was no significant line or replicate difference in BEC, the interaction was significant [F(1,104) = 11.68, P = 0.001]. This was due to the fact that BECs were slightly higher in WSP-1 vs. WSP-2 mice and slightly lower in WSR-1 vs. WSR-2 mice (not shown).
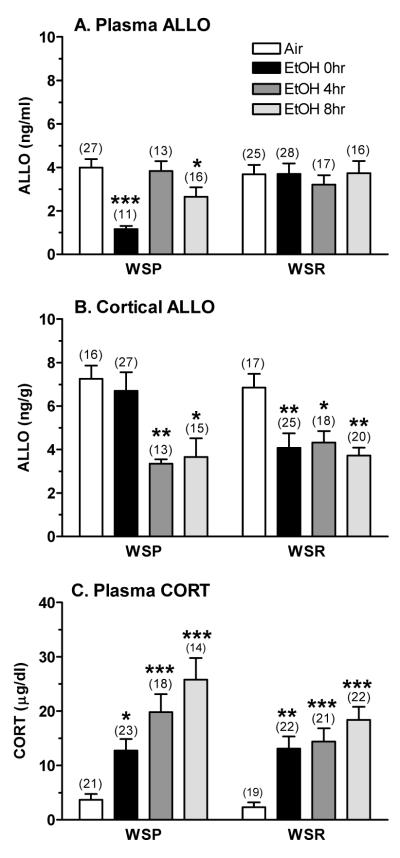
The initial analysis of plasma ALLO levels revealed that there were no main effects of genetic replicate, nor were there interactions with genetic replicate. When the analysis was conducted, collapsed across genetic replicate, plasma ALLO levels were lower in WSP vs. WSR mice and were significantly influenced by treatment/time [F(3,145) = 3.24, P < 0.05]. The significant interaction between line and treatment/time [F(3,145) = 4.21, P < 0.01] was due to the chronic ethanol-induced decrease in plasma ALLO levels that occurred only in WSP mice (Figure 1A). Post-hoc tests confirmed this conclusion. Plasma ALLO levels in WSP mice were decreased by 71% in dependent animals and by 34% during peak withdrawal, whereas values were unchanged in WSR mice.
Figure 1. The effect of exposure to 72 hr ethanol vapor and withdrawal on (A) plasma ALLO, (B) cortical ALLO, and (C) plasma CORT levels in WSP and WSR male mice.
Mice were exposed to ethanol vapor or air for 72 hrs and then euthanized at 0, 4, or 8 hrs following removal from the inhalation chambers. Steroid levels were measured by RIA. Values represent the mean ± SEM for the numbers of animals in parentheses, collapsed across genetic replicate. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001 vs. respective air control, Tukey’s post-hoc test
In contrast, cortical ALLO levels (Figure 1B) were decreased significantly in both WSP (by 50-54%) and WSR (by 37-46%) mice during ethanol withdrawal. As with plasma ALLO levels, there was no significant main effect of, or interaction with, genetic replicate. Subsequent analyses indicated that cortical ALLO levels were significantly altered by treatment/time [F(3,143) = 9.15, P < 0.001], with a significant interaction between line and treatment/time [F(3,143) = 2.78, P < 0.05]. The significant interaction appeared to be due to the fact that cortical ALLO levels were decreased significantly (by 40%) only in dependent WSR mice (i.e., 0 hr), whereas values were unchanged in WSP mice.
Plasma CORT levels (Figure 1C) were significantly increased in dependent mice and during withdrawal, with the WSP mice exhibiting a slightly higher elevation during withdrawal when compared to the WSR mice. ANOVA confirmed that plasma CORT levels were significantly higher in WSP vs. WSR mice [F(1,152) = 4.19, P < 0.05] and were significantly increased by chronic ethanol exposure and withdrawal [F(3,152) = 22.23, P < 0.001]. Although the interaction between line and treatment/time was not significant, post-hoc tests were conducted based on our a priori hypothesis that plasma CORT levels would be increased to a greater extent in the WSP vs. WSR mice. Although absolute plasma CORT levels were higher during peak withdrawal in the WSP vs. WSR mice, the fold increase was similar in the lines (~ 6-fold), due to the slightly lower basal CORT levels in the air-exposed WSR mice.
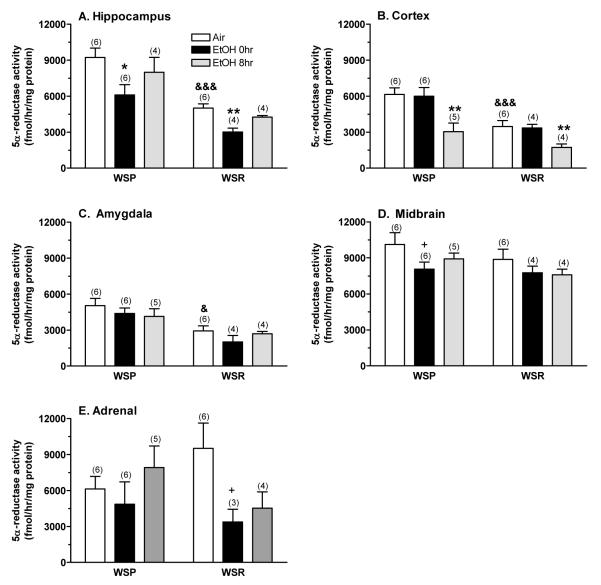
The effect of chronic ethanol exposure and withdrawal on 5α-reductase-1 activity is depicted in Figure 2. In general, there were some line differences in basal 5α-reductase activity, but the overall effects of treatment and time appeared to be similar in the lines. Hippocampal 5α-reductase activity (Figure 2A) was higher in WSP vs. WSR mice [F(1,24) = 36.53, P < 0.001] and was decreased significantly by chronic ethanol exposure [F(2,24) = 6.58, P < 0.01]. The lack of significant interaction between line and treatment/time was due to the fact that hippocampal enzyme activity was decreased similarly in dependent WSP (↓ 34%) and WSR (↓ 40%) mice, and only slightly decreased in both lines during ethanol withdrawal (↓ 13% in WSP, ↓ 15% in WSR). Cortical 5α-reductase activity (Figure 2B) also was higher in WSP vs. WSR mice [F(1,25) = 23.61, P < 0.001] and was decreased significantly during chronic ethanol withdrawal [F(2,25) = 11.30, P < 0.001]. The decrease in cortical enzyme activity during ethanol withdrawal was similar (↓ 50%) in both lines. Amygdala 5α-reductase activity (Figure 2C) only was significantly influenced by line [F(1,25) = 21.22, P < 0.001] (WSP > WSR), whereas midbrain 5α-reductase activity (Figure 2D) was slightly decreased by chronic ethanol exposure (↓ 20% in dependent WSP, ↓ 13% in dependent WSR), and adrenal 5α-reductase activity (Figure 2E) was a bit more variable.
Figure 2. The effect of chronic ethanol exposure and withdrawal on 5α-reductase-1 activity in (A) hippocampus, (B) cortex, (C) amygdala, (D) midbrain, and (E) adrenals from WSP and WSR mice.
Microsomal fractions from dissected tissues were incubated with a saturating concentration of [3H]progesterone. The amount of [3H]DHP formed was the index of 5α-reductase activity. Values represent the mean ± SEM for the number of animals in parentheses. Data were combined for the two replicates per line at each time point. +P ≤ 0.10, *P ≤ 0.05, **P ≤ 0.01 vs. respective air control, Tukey’s post-hoc test &P < 0.05, &&&P = 0.001 vs. respective WSP, t-test
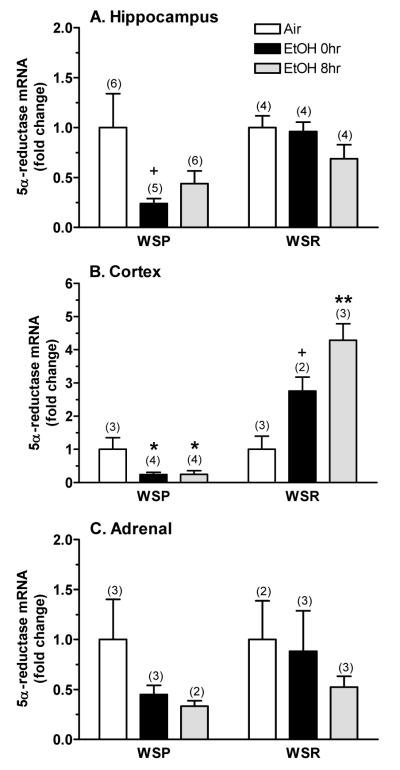
The effect of chronic ethanol exposure and withdrawal on expression of the 5α-reductase-1 transcript is depicted in Figure 3. Since transcript expression was normalized to the respective air control values, we only were able to analyze for treatment/time effects and for interaction with selected line. The overall pattern of the results suggests that there were some line differences in the effects of ethanol exposure and withdrawal on 5α-reductase expression. Hippocampal 5α-reductase mRNA levels (Figure 3A) appeared to be lower in WSP than in WSR mice and to be decreased by ethanol exposure and withdrawal. . In dependent mice, 5α-reductase expression was reduced only in WSP mice (↓76%). During withdrawal, mRNA levels remained decreased in WSP mice (↓55%) versus the change in WSR mice (↓31%). However, there was a much more profound line difference in the regulation of the cortical 5α-reductase transcript by chronic ethanol exposure and withdrawal (Figure 3B). Cortical mRNA expression was significantly lower in WSP versus WSR mice [F(1,13) = 75.12, P < 0.001] and was significantly altered by treatment/time [F(2,13) = 9.18, P < 0.01]. The significant interaction between line and treatment/time [F(2,13) = 23.03, P < 0.001] confirmed the differential regulation of the 5α-reductase transcript in WSP and WSR mice. In WSP mice, cortical expression was significantly decreased (by 75%) in dependent animals and during ethanol withdrawal. In contrast, 5α-reductase mRNA levels were increased 2.76-fold in dependent WSR mice, with a further significant increase (↑4.3-fold) during ethanol withdrawal. Treatment did not significantly alter mRNA levels in the amygdala (not shown), midbrain (not shown), or adrenal (Figure 3C). Planned comparisons of mRNA levels in adrenal tissue indicated that 5α-reductase expression was decreased to a greater extent in the WSP line (↓55% in dependent mice, ↓67% during withdrawal), when compared to the WSR line (↓47% during withdrawal).
Figure 3. Chronic ethanol exposure and withdrawal differentially altered expression of the 5α-reductase-1 transcript in (A) hippocampus, (B) cortex, and (C) adrenals from WSP and WSR mice.
Real-time qRT-PCR was conducted in dissected tissues. The fold-change in expression vs. respective air control mice is depicted. The y-axis for panel B differs from that shown in the other 2 panels. Values represent the mean ± SEM for the number of animals in parentheses. Assays were conducted in duplicate with similar values to those in the figure. +P ≤ 0.10, *P < 0.05, **P < 0.01 vs. respective air control, Tukey’s post-hoc test
Discussion
The purpose of the present studies was to examine the time course for the differential change in brain and plasma ALLO and plasma CORT levels following exposure to chronic ethanol as well as to determine whether a chronic ethanol-induced decrease in endogenous ALLO levels was due to a concomitant decrease in activity or expression of the rate-limiting neurosteroid biosynthetic enzyme 5α-reductase in WSP and WSR mice. We chose to examine regulation of the 5α-reductase-1 enzyme in both brain and peripheral tissues, due to the fact that the enzymes identified in classic steroidogenic tissues have been found in the nervous system, and it has been suggested that brain neurosteroid levels reflect a combination of neuroactive compounds produced de novo as well as peripherally derived steroids that are metabolized to neuroactive compounds in the brain (Mellon, 1994). In general, the results provide strong evidence that the 5α-reductase-1 enzyme was differentially regulated during chronic ethanol exposure and withdrawal in both peripheral tissues and select brain regions, and that the lines differed in these effects.
Basal levels of ALLO in the plasma and cortex did not differ between the lines, despite line differences in basal 5α-reductase activity in the cortex, hippocampus and amygdala. These brain regional differences in 5α-reductase-1 activity are intriguing and would predict line differences in local ALLO concentrations. Unfortunately, our RIA was sensitive enough to measure only cortical ALLO levels. The fact that cortical ALLO levels were slightly higher than those measured in plasma might suggest a minor contribution of brain 5α-reductase activity to brain ALLO concentrations. However, there were no line differences in enzyme activity in the adrenal, suggesting that peripheral steroidogenic tissues provide the major contribution to basal neurosteroid levels in the brain as well as in the plasma.
Chronic ethanol exposure and withdrawal produced a selective and persistent decrease in plasma ALLO levels only in WSP mice, which corresponded to a persistent decrease in adrenal 5α-reductase expression in this selected line. The fact that the change in plasma ALLO levels was consistent with the down regulation of the adrenal 5α-reductase transcript in dependent and withdrawing WSP mice provides support for the idea that the adrenal is a major source of plasma ALLO levels.
Cortical ALLO levels were significantly decreased during ethanol withdrawal in both WSP and WSR mice, and this reduction corresponded to a significant decrease in cortical 5α-reductase activity during withdrawal in both lines. Notably, the suppression of enzyme activity during ethanol withdrawal was consistent with the down regulation of the enzyme during withdrawal in WSP mice, but was completely opposite to the up regulation of the enzyme during withdrawal in WSR mice. These results imply an important discordance between mRNA and protein expression during ethanol withdrawal in the cortex, consistent with post-translational or allosteric regulation of 5α-reductase-1, in addition to transcriptional regulation. Future studies will examine more closely the brain regional differences in the effect of ethanol withdrawal on 5α-reductase-1 mRNA (in situ) and protein (immunohistochemistry) levels in order to better understand the regulation of this neurosteroid biosynthetic enzyme by chronic ethanol exposure and withdrawal in WSP and WSR mice.
Although we were unable to measure hippocampal ALLO levels, previous work has documented that withdrawal from chronic intermittent alcohol exposure significantly decreased hippocampal ALLO levels in rats (Cagetti et al., 2004). In this study by Cagetti et al. (2004), the decrease in hippocampal ALLO levels corresponded to a significant reduction in the expression of 5α-reductase in the hippocampus during withdrawal. Our results indicate that 5α-reductase activity and expression was decreased in dependent WSP mice, but that only enzyme activity was decreased in dependent WSR mice. Taken in conjunction with the down regulation of adrenal 5α-reductase mRNA following chronic ethanol exposure and withdrawal only in WSP mice, it is likely that hippocampal ALLO levels would be decreased during withdrawal in the WSP line.
A surprising finding was that the pattern of changes in cortical ALLO and plasma CORT levels during ethanol withdrawal was similar in WSP and WSR mice, with the proviso that the changes were slightly greater in the WSP line. However, with regard to the potential physiological relevance of these changes in steroid concentrations, it is important to consider that line differences in steroid sensitivity could impact the behavioral outcome of a change in steroid levels during ethanol withdrawal. For example, WSP mice are sensitive to the proconvulsant effect of CORT during acute ethanol withdrawal (Roberts et al., 1994) and exhibit tolerance to the anticonvulsant effect of ALLO or to the ability of ALLO to potentiate GABA-stimulated chloride flux during chronic ethanol withdrawal (Finn et al., 2006b). The DBA/2J inbred strain, which exhibits severe withdrawal comparable to the WSP line, also is sensitive to the proconvulsant effect of CORT (Roberts et al., 1992) and exhibits tolerance to the anticonvulsant effect of ALLO during chronic ethanol withdrawal (Finn et al., 2000). In animals that exhibit mild withdrawal, sensitivity to the anticonvulsant effect of alphaxalone, pregnanolone or ALLO was enhanced in rats (Alele and Devaud, 2007; Cagetti et al., 2004; Devaud et al., 1996) or C57BL/6J mice (Finn et al., 2000). Either enhancement or no change in sensitivity to the anticonvulsant effect of ALLO was observed in female and male WSR mice, respectively (Beckley et al., 2008; Finn et al., 2006b). Thus, it is likely that in seizure-prone versus -resistant genotypes, there is a balance between the alterations of concentrations of ALLO at GABAA receptors and the concomitant change in GABAA receptor sensitivity to ALLO during the ethanol withdrawal phase (as discussed in Finn et al., 2004), with a similar relationship for CORT at glucocorticoid and/or mineralocorticoid receptors.
Consistent with the notion regarding the physiological relevance of changes in endogenous steroid levels, evidence suggests that fluctuations in endogenous ALLO levels could modify the functioning of central GABAA receptors in vivo. The initial support for this idea was provided by the report that increasing endogenous ALLO levels in the dentate gyrus revealed a physiologically relevant neurosteroid tone that was sufficient to modulate GABAA receptors (Belelli and Herd, 2003). An additional consideration is that acute administration of either ethanol or ALLO exert similar effects on hippocampal function (Murayama et al., 2006; Sanna et al., 2004; Silvers et al., 2003; Tokunaga et al., 2003). For instance, acute ethanol administration induces a steroidogenic effect that includes increased ALLO levels. It is believed that ALLO contributes to ethanol’s effects on hippocampal neural activity (Sanna et al., 2004; Tokunaga et al., 2003) because the influence of ethanol was blocked by pretreatment with finasteride, a 5α-reductase inhibitor that reduces endogenous ALLO levels (discussed in Finn et al., 2006a). Thus, data to date suggest that acute and chronic ethanol administration can have marked effects on hippocampal neural function by altering endogenous neurosteroid tone.
With regard to WSP mice, we found that intra-hippocampal ALLO exhibited a potent anticonvulsant effect in ethanol naive WSP mice, but that mice were tolerant to the anticonvulsant effect of intra-hippocampal ALLO during ethanol withdrawal (Gililland-Kaufman et al., 2008), consistent with results seen following systemic administration (Finn et al., 2006b). Additionally, intra-hippocampal infusions of finasteride were proconvulsant in ethanol naive WSP mice, and significantly increased the magnitude and duration of ethanol withdrawal (Gililland-Kaufman et al., 2008). Our recent preliminary findings indicate that intra-hippocampal finasteride did not significantly increase ethanol withdrawal severity in WSR mice (Tanchuck and Finn, unpublished). Thus, manipulation of endogenous ALLO levels produces bidirectional changes in seizure susceptibility in ethanol naive WSP mice (↑ ALLO is anticonvulsant; ↓ ALLO with finasteride is proconvulsant), with a shift in sensitivity toward enhanced proconvulsant effects during ethanol withdrawal (↓ anticonvulsant effect of ALLO; ↑ proconvulsant effect of finasteride).
In conclusion, the present findings indicate that the correspondence between the ethanol-induced changes in ALLO levels and 5α-reductase enzyme activity and expression in dependent animals and during ethanol withdrawal is complex. Importantly, the results suggest that there are line differences in the regulation of the 5α-reductase enzyme by chronic ethanol exposure and withdrawal. Overall, WSP mice were more responsive to the chronic ethanol withdrawal-induced decreases in endogenous ALLO levels, and in 5α-reductase-1 enzyme activity and expression. Taken in conjunction with the reduced sensitivity to ALLO’s anticonvulsant effect, the reduced ability of ALLO to potentiate GABA-stimulated chloride uptake, as well as the enhanced proconvulsant effect of finasteride during ethanol withdrawal (Finn et al., 2006b; Gililland-Kaufman et al., 2008), the altered sensitivity of GABAA receptors to ALLO in the WSP line may render them more sensitive to fluctuations in endogenous ALLO levels during ethanol withdrawal. These results are consistent with the hypothesis that genetic differences in chronic ethanol withdrawal severity are due, in part, to modulatory effects of GABAergic steroids such as ALLO in the WSP and WSR selected lines. It may be that the genotypic differences in the modulatory effects of ALLO on ethanol withdrawal severity reflect a balance between the changes in local concentration of ALLO at GABAA receptors and the change in sensitivity of the GABAA receptors to ALLO that are occurring concomitantly during ethanol withdrawal. Increasing our understanding of this relationship may aid in the identification of new neurosteroid analogs or in targeting aspects of neurosteroid biosynthesis for the treatment of ethanol dependence and withdrawal.
Acknowledgements
We thank the Portland Alcohol Dependence Core, particularly Dr. Pamela Metten, Janet Dorow, and Jason Sibert, for assistance with the induction of physical dependence and the measurement of BECs. We also thank Stacy Matthews and Henry Stadelman for their expert technical assistance on other technical aspects of these studies.
This research was supported by USPHS grants AA12439 and AA10760 from the National Institute on Alcohol Abuse and Alcoholism and by grants from the Department of Veterans Affairs.
References
- Alele PE, Devaud LL. Sex differences in steroid modulation of ethanol withdrawal in male and female rats. J Pharmacol Exp Ther. 2007;320:427–436. doi: 10.1124/jpet.106.107896. [DOI] [PubMed] [Google Scholar]
- Barbaccia ML, Serra M, Purdy RH, Biggio G. Stress and neuroactive steroids. Int Rev Neurobiol. 2001;46:243–272. doi: 10.1016/s0074-7742(01)46065-x. [DOI] [PubMed] [Google Scholar]
- Beckley EH, Fretwell AM, Tanchuck MA, Gililland KR, Crabbe JC, Finn DA. Decreased anticonvulsant efficacy of allopregnanolone during ethanol withdrawal in female Withdrawal Seizure-Prone vs. Withdrawal Seizure-Resistant mice. Neuropharmacology. 2008;54:365–374. doi: 10.1016/j.neuropharm.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belelli D, Herd MB. The contraceptive agent Provera enhances GABAA receptor-mediated inhibitory neurotransmission in the rat hippocampus: Evidence for endogenous neurosteroids? J Neurosci. 2003;23:10013–10020. doi: 10.1523/JNEUROSCI.23-31-10013.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belelli D, Lambert JJ. Neurosteroids: Endogenous regulators of the GABAA receptor. Nature Rev Neurosci. 2005;6:565–575. doi: 10.1038/nrn1703. [DOI] [PubMed] [Google Scholar]
- Belelli D, Lan NC, Gee KW. Anticonvulsant steroids and the GABA/benzodiazepine receptor-chloride ionophore complex. Neurosci Biobehav Rev. 1990;14:315–322. doi: 10.1016/s0149-7634(05)80041-7. [DOI] [PubMed] [Google Scholar]
- Bergeson SE, Warren RK, Crabbe JC, Metten P, Erwin VG, Belknap JK. Chromosomal loci influencing chronic alcohol withdrawal severity. Mamm Genome. 2003;14:454–463. doi: 10.1007/s00335-002-2254-4. [DOI] [PubMed] [Google Scholar]
- Buck KJ, Harris RA. Benzodiazepine agonist and inverse agonist actions on GABAA receptor-operated chloride channels. II. Chronic effects of ethanol. J Pharmacol Exp Ther. 1990;253:713–719. [PubMed] [Google Scholar]
- Cagetti E, Pinna G, Guidotti A, Baicy K, Olsen RW. Chronic intermittent ethanol (CIE) administration in rats decreases levels of neurosteroids in hippocampus, accompanied by altered behavioral responses to neurosteroids and memory function. Neuropharmacology. 2004;46:570–579. doi: 10.1016/j.neuropharm.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Crabbe JC. Provisional mapping of quantitative trait loci for chronic ethanol withdrawal severity in BXD Recombinant Inbred mice. J Pharmacol Exp Ther. 1998;286:263–271. [PubMed] [Google Scholar]
- Crabbe JC, Kosobud A, Young ER, Tam BR, McSwigan JD. Bidirectional selection for susceptibility to ethanol withdrawal seizures in Mus musculus. Behav Genet. 1985;15:521–536. doi: 10.1007/BF01065448. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Phillips TJ, Kosobud A, Belknap JK. Estimation of genetic correlation: Interpretation of experiments using selectively bred and inbred animals. Alcohol Clin Exp Res. 1990;14:141–151. doi: 10.1111/j.1530-0277.1990.tb00461.x. [DOI] [PubMed] [Google Scholar]
- Criswell HE, Breese GR. A conceptualization of integrated actions of ethanol contributing to its GABAmimetic profile: A commentary. Neuropsychopharmacology. 2005;30:1407–1425. doi: 10.1038/sj.npp.1300750. [DOI] [PubMed] [Google Scholar]
- Devaud LL, Purdy RH, Finn DA, Morrow AL. Sensitization of γ-aminobutyric acidA receptors to neuroactive steroids in rats during ethanol withdrawal. J Pharmacol Exp Ther. 1996;278:510–517. [PubMed] [Google Scholar]
- Finn DA, Crabbe JC. Chronic ethanol differentially alters susceptibility to chemically-induced convulsions in Withdrawal Seizure-Prone and -Resistant mice. J Pharmacol Exp Ther. 1999;288:782–790. [PubMed] [Google Scholar]
- Finn DA, Gee KW. The estrus cycle, sensitivity to convulsants and the anticonvulsant effect of a neuroactive steroid. J Pharmacol Exp Ther. 1994;271:164–170. [PubMed] [Google Scholar]
- Finn DA, Beadles-Bohling AS, Beckley EH, Ford MM, Gililland KR, Gorin-Meyer RE, Wiren KM. A new look at the 5α-reductase inhibitor finasteride. CNS Drug Rev. 2006a;12:53–76. doi: 10.1111/j.1527-3458.2006.00053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn DA, Douglass AD, Tanchuck MA, Long SL, Crabbe JC. Selected line difference in sensitivity to a GABAergic neurosteroid during ethanol withdrawal. Genes Brain Behav. 2006b;5:53–63. doi: 10.1111/j.1601-183X.2005.00137.x. [DOI] [PubMed] [Google Scholar]
- Finn DA, Ford MM, Wiren KW, Roselli CE, Crabbe JC. The role of pregnane neurosteroids in ethanol withdrawal: Behavioral genetic approaches. Pharmacol Ther. 2004;101:91–112. doi: 10.1016/j.pharmthera.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Finn DA, Gallaher EJ, Crabbe JC. Differential change in neuroactive steroid sensitivity during ethanol withdrawal. J Pharmacol Exp Ther. 2000;292:394–405. [PubMed] [Google Scholar]
- Gasior M, Carter RB, Witkin JM. Neuroactive steroids: potential therapeutic use in neurological and psychiatric disorders. Trends Pharmacol Sci. 1999;20:107–112. doi: 10.1016/s0165-6147(99)01318-8. [DOI] [PubMed] [Google Scholar]
- Gililland-Kaufman KR, Tanchuck MA, Ford MM, Crabbe JC, Beadles-Bohling AS, Snelling C, Mark GP, Finn DA. The neurosteroid environment in the hippocampus exerts bidirectional effects on seizure susceptibility in mice. Brain Res. 2008;1243:113–123. doi: 10.1016/j.brainres.2008.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grobin AC, Matthew DB, Devaud LL, Morrow AL. The role of GABAA receptors in the acute and chronic effects of ethanol. Psychopharmacology. 1998;139:2–19. doi: 10.1007/s002130050685. [DOI] [PubMed] [Google Scholar]
- Hashimoto JG, Beadles-Bohling AS, Wiren KM. Comparison of RiboGreen® and 18S tRNA quantitation for normalizing real-time RT-PCR expression analysis. Biotechniques. 2004;36:54–60. doi: 10.2144/04361BM06. [DOI] [PubMed] [Google Scholar]
- Hashimoto JG, Wiren KM. Neurotoxic consequences of chronic alcohol withdrawal: expression profiling reveals importance of gender over withdrawal severity. Neuropsychopharmacology. 2008;33:1084–1096. doi: 10.1038/sj.npp.1301494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill M, Popov P, Havlíková H, Lyudmila K, Vrbíková J, Kancheva R, Pouzar V, Černý I, Stárka L. Altered profiles of serum neuroactive steroids in premenopausal women treated for alcohol addiction. Steroids. 2005;70:515–524. doi: 10.1016/j.steroids.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Hirani K, Khisti RT, Chopde CT. Behavioral action of ethanol in Porsolt’s forced swim test: modulation by 3alpha-hydroxy-5alpha-pregnan-20-one. Neuropharmacology. 2002;43:1339–1350. doi: 10.1016/s0028-3908(02)00330-1. [DOI] [PubMed] [Google Scholar]
- Hirani K, Sharma AN, Jain NS, Ugale RR, Chopde CT. Evaluation of GABAergic neuroactive steroid 3α-hydroxy-5α-pregnane-20-one as a neurobiological substrate for the anti-anxiety effect of ethanol in rats. Psychopharmacology. 2005;180:267–278. doi: 10.1007/s00213-005-2169-7. [DOI] [PubMed] [Google Scholar]
- Janis GC, Devaud LL, Mitsuyama H, Morrow AL. Effects of chronic ethanol consumption and withdrawal on the neuroactive steroid 3α-hydroxy-5α-pregnan-20-one in male and female rats. Alcohol Clin Exp Res. 1998;22:2055–2061. [PubMed] [Google Scholar]
- Jenkins EP, Hseih CL, Milantovich A, Normington K, Berman DM, Francke U, Russell DW. Characterization and chromosomal mapping of a human steroid 5α-reductase gene and pseudogene and mapping of the mouse homologue. Genomics. 1991;11:1102–1112. doi: 10.1016/0888-7543(91)90038-g. [DOI] [PubMed] [Google Scholar]
- Kalant H, LeBlanc AE, Gibbins RJ. Tolerance to, and dependence on, some non-opiate psychotropic drugs. Pharmacol Rev. 1971;23:135–191. [PubMed] [Google Scholar]
- Keith LD, Winslow JR, Reynolds RW. A general procedure for estimation of corticosteroid response in individual rats. Steroids. 1978;31:523–531. doi: 10.1016/0039-128x(78)90034-x. [DOI] [PubMed] [Google Scholar]
- Krause JE, Karavolas HJ. Subcellular location of hypothalamic progesterone metabolizing enzymes and evidence for distinct NADH- and NADPH-linked 3α-hydroxysteroid oxidoreductase activities. J Steroid Biochem. 1980;13:271–280. doi: 10.1016/0022-4731(80)90005-9. [DOI] [PubMed] [Google Scholar]
- Krieger NR, Scott RG, Jurman ME. Testosterone 5α-reductase in rat brain. J Neurochem. 1983;40:1460–1464. doi: 10.1111/j.1471-4159.1983.tb13591.x. [DOI] [PubMed] [Google Scholar]
- Murayama K, Zorumski CR, Izumi Y. Effects of neurosteroid 3α-hydroxy-5α-pregnan-20-one on ethanol-mediated paired-pulse depression of population spikes in the CA1 region of rat hippocampal slices. Neurosci Lett. 2006;394:28–32. doi: 10.1016/j.neulet.2005.09.062. [DOI] [PubMed] [Google Scholar]
- Melcangi RC, Poletti A, Cavarretta I, Celotti F, Colciago A, Magnaghi V, Motta M, Negri-Cesi P, Martini L. The 5α-reductase in the central nervous system: expression and modes of control. J Steroid Biochem Molec Biol. 1998;65:295–299. doi: 10.1016/s0960-0760(98)00030-2. [DOI] [PubMed] [Google Scholar]
- Mellon S. Neurosteroids: Biochemistry, modes of action, and clinical relevance. J Clin Endo Metab. 1994;78:1003–1008. doi: 10.1210/jcem.78.5.8175951. [DOI] [PubMed] [Google Scholar]
- Morrow AL. Regulation of GABAA receptor function and gene expression in the central nervous system. Int Rev Neurobiol. 1995;38:1–41. doi: 10.1016/s0074-7742(08)60523-1. [DOI] [PubMed] [Google Scholar]
- Morrow AL, Janis GC, VanDoren MJ, Matthews DB, Samson HH, Janak PH, Grant KA. Neurosteroids mediate pharmacological effects of ethanol: a new mechanism of ethanol action? Alcohol Clin Exp Res. 1999;23:1933–1940. doi: 10.1111/j.1530-0277.1999.tb04094.x. [DOI] [PubMed] [Google Scholar]
- Morrow AL, VanDoren MJ, Penland SN, Matthews DB. The role of GABAergic neuroactive steroids in ethanol action, tolerance and dependence. Brain Res Rev. 2001;37:98–109. doi: 10.1016/s0165-0173(01)00127-8. [DOI] [PubMed] [Google Scholar]
- Paul SM, Purdy RH. Neuroactive steroids. FASEB J. 1992;6:2311–2322. [PubMed] [Google Scholar]
- Purdy RH, Moore PH, Jr, Rao N, Hagino N, Yamaguchi T, Schmidt P, Rubinow DR, Morrow AL, Paul SM. Radioimmunoassay of 3α-hydroxy-5α-pregnan-20-one in rat and human plasma. Steroids. 1990a;55:290–296. doi: 10.1016/0039-128x(90)90031-6. [DOI] [PubMed] [Google Scholar]
- Purdy RH, Morrow AL, Blinn JR, Paul SM. Synthesis, metabolism, and pharmacological activity of 3α-hydroxy steroids which potentiate GABA-receptor-mediated chloride ion uptake in rat cerebral cortical synaptoneurosomes. J Med Chem. 1990b;33:1572–1581. doi: 10.1021/jm00168a008. [DOI] [PubMed] [Google Scholar]
- Resko JA, Connolly PB, Roselli CE. Testosterone 5α-reductase activity in neural tissue of fetal rhesus macaques. J Steroid Biochem. 1988;29:429–434. doi: 10.1016/0022-4731(88)90253-1. [DOI] [PubMed] [Google Scholar]
- Resko JA, Roselli CE. Brain steroid synthesis and metabolism. Neuroprotocols. 1992;1:27–34. [Google Scholar]
- Resko JA, Stadelman H, Handa RJ. Control of 5α-reduction of testosterone in neuroendocrine tissues of female rats. Biol Reprod. 1986;34:870–877. doi: 10.1095/biolreprod34.5.870. [DOI] [PubMed] [Google Scholar]
- Roach M, Creaven P. A micro-method for the determination of acetaldehyde and ethanol in blood. Clin Chim Acta. 1968;21:275–278. doi: 10.1016/0009-8981(68)90138-1. [DOI] [PubMed] [Google Scholar]
- Roberts AJ, Crabbe JC, Keith LD. Genetic differences in hypothalamic-pituitary-adrenal axis responsiveness to acute ethanol and acute ethanol withdrawal. Brain Res. 1992;579:296–302. doi: 10.1016/0006-8993(92)90064-g. [DOI] [PubMed] [Google Scholar]
- Roberts AJ, Crabbe JC, Keith LD. Corticosterone increases severity of acute withdrawal from ethanol, pentobarbital, and diazepam in mice. Psychopharmacology. 1994;115:278–284. doi: 10.1007/BF02244784. [DOI] [PubMed] [Google Scholar]
- Romeo E, Brancati A, De Lorenzo A, Fucci P, Furnari C, Pompili E, Sasso GF, Spalletta G, Troisi A, Pasini A. Marked decrease of plasma neuroactive steroids during alcohol withdrawal. Clin Neuropharmacology. 1996;19:366–369. doi: 10.1097/00002826-199619040-00011. [DOI] [PubMed] [Google Scholar]
- Romeo E, Pompili E, di Michele F, Pace M, Rupprecht R, Bernardi G, Pasinib A. Effects of fluoxetine, indomethacine and placebo on 3alpha, 5alpha tetrahydroprogesterone (THP) plasma levels in uncomplicated alcohol withdrawal. World J Biol Psychiatry. 2000;1:101–104. doi: 10.3109/15622970009150572. [DOI] [PubMed] [Google Scholar]
- Roselli CE, Snipes CA. Progesterone 5α-reductase in mouse brain. Brain Res. 1984;305:197–202. doi: 10.1016/0006-8993(84)90425-6. [DOI] [PubMed] [Google Scholar]
- Roselli CE, Stadelman H, Horton LE, Resko JA. Regulation of androgen metabolism and luteinizing hormone-releasing hormone content in discrete hypothalamic and limbic areas of male rhesus macaques. Endocrinology. 1987;120:97–106. doi: 10.1210/endo-120-1-97. [DOI] [PubMed] [Google Scholar]
- Rupprecht R, Holsboer F. Neuroactive steroids: Mechanisms of action and neuropsychopharmacological perspectives. Trends Neurosci. 1999;22:410–416. doi: 10.1016/s0166-2236(99)01399-5. [DOI] [PubMed] [Google Scholar]
- Sanna E, Talani G, Busonero F, Pisu MG, Purdy RH, Serra M, Biggio G. Brain steroidogenesis mediates ethanol modulation of GABAA receptor activity in rat hippocampus. J Neurosci. 2004;24:6521–6530. doi: 10.1523/JNEUROSCI.0075-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvers JM, Tokunaga S, Berry RB, White AM, Matthews DB. Impairments in spatial learning and memory: ethanol, allopregnanolone, and the hippocampus. Brain Res Rev. 2003;43:275–284. doi: 10.1016/j.brainresrev.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Terdal ES, Crabbe JC. Indexing withdrawal in mice: matching genotypes for exposure in studies using ethanol vapor inhalation. Alcohol Clin Exp Res. 1994;18:542–547. doi: 10.1111/j.1530-0277.1994.tb00907.x. [DOI] [PubMed] [Google Scholar]
- Tokunaga S, McDaniel JR, Morrow AL, Matthew DB. Effect of acute ethanol administration and acute allopregnanolone administration on spontaneous hippocampal pyramidal cell neural activity. Brain Res. 2003;967:273–280. doi: 10.1016/s0006-8993(02)04266-x. [DOI] [PubMed] [Google Scholar]
- VanDoren MJ, Matthews DB, Janis GC, Grobin AC, Devaud LL, Morrow AL. Neuroactive steroid 3α-hydroxy-5α-pregnan-20-one modulates electrophysiological and behavioral actions of ethanol. J Neurosci. 2000;20:1982–1989. doi: 10.1523/JNEUROSCI.20-05-01982.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veleiro AS, Burton G. Structure-activity relationships of neuroactive steroids acting on the GABAA receptor. Curr Med Chem. 2009;16:455–472. doi: 10.2174/092986709787315522. [DOI] [PubMed] [Google Scholar]
- Winer J, Jung C, Shackel I, Williams P. Development and validation of real-time quantitative reverse transcriptase-polymerase chain reaction for monitoring gene expression in cardiac myocytes in vitro. Anal Biochem. 1999;270:41–49. doi: 10.1006/abio.1999.4085. [DOI] [PubMed] [Google Scholar]