Abstract
Genome integrity appears to be a fundamental issue in both cancer and stem cell biology. A series of recent studies reveal that a tumor suppressor p53, which is required for genome integrity, is also critical for stem cell pluripotency and reprogramming, thus further strengthening the fundamental link between cancer and pluripotency. p53 and other tumor suppressors might be roadblocks of reprogramming somatic cells for the generation of induced pluripotent stem cells (iPSCs), and simultaneously and systematically destroying these roadblocks would improve reprogramming efficiency. However, it is also crucial to determine the tumorigenecity of the cells derived from iPSCs for any future therapeutic use.
The tumor suppressor p53 as a barrier to induced pluripotency
The generation of induced pluripotent stem cells (iPSCs) by reprogramming somatic cells with defined factors has raised the hope that iPSCs, which are similar to embryonic stem cells (ESCs) in the context of self-renewal and pluripotency, could become a renewable source of autologous cells for transplantation into patients [1]. These disease-specific iPSCs could provide a unique opportunity to develop new much needed disease models to address an unmet challenge in drug discovery. Although the ‘triggers’ of cellular reprogramming, including a panel of transcription factors such as Oct4, Sox2, Klf4 and c-Myc – the four most commonly used ‘reprogramming factors’ – have been identified, the rate at which differentiated cells can be successfully reprogrammed into the pluoripotent state is very low, suggesting the existence of barriers limiting reprogramming efficiency. Several chemical compounds have been identified to improve reprogramming efficiency by modulating the global epigenetics of the genome [2,3]. Last year, a link between p53 and transforming the efficiency of human iPSC generation was discovered, when silencing the expression of p53 in somatic cells was found to increase the reprogramming frequency [4]. Considering the critical role of p53 in tumor suppression, this finding suggests a functional link between tumor suppression and reprogramming.
Now a series of papers [5–9] extend the previous observation [4], and demonstrate that the ARF–p53 pathways are a barrier to induced pluripotency, and that inactivating these pathways enables an approximately 100-fold increase in cell reprogramming efficiency. The expression of the four reprogramming factors triggers senescence by up-regulating p53, p16(INK4a), and p21(CIP1) as a result of the induction of DNA-damage response and chromatin remodeling of the INK4a/ARF locus, identifying senescence as a barrier to induced pluripotency [10]. Consistent with this idea, the efficiency of iPSC generation is decreased with the increased passages of somatic cells and the onset of senescence [6]. In addition, the INK4/ARF locus is upregulated with age, and reprogramming is less efficient in older cells [8]. The barriers that keep the genome intact and prevent ocongenesis in older age might be the same barriers preventing reprogramming to pluripotency.
Possible mechanisms underlying ARF-p53 impediment of cellular reprogramming
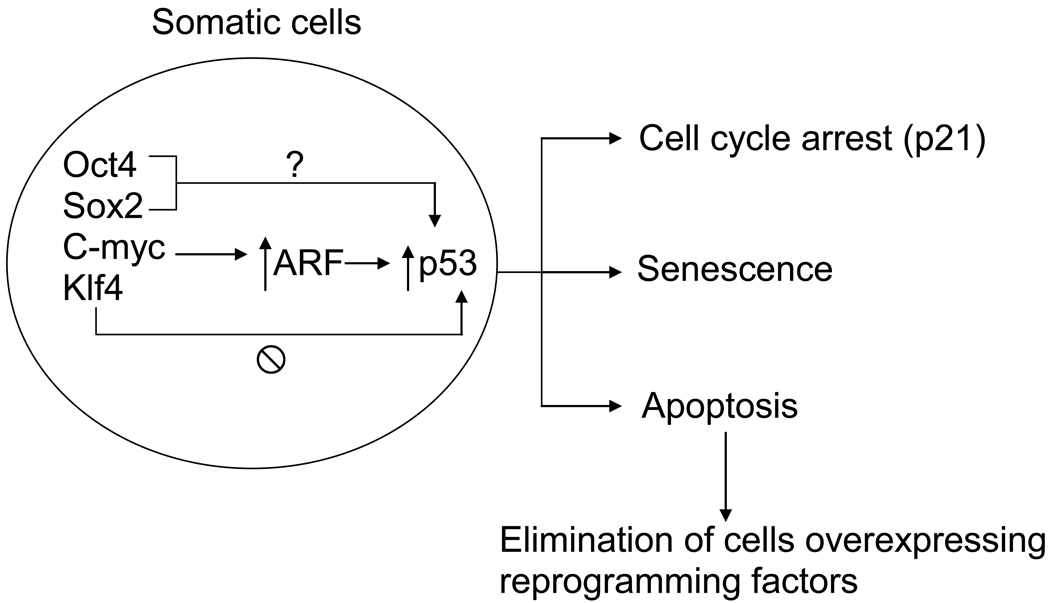
How does p53 repress the reprogramming? p53 – the “guardian of the genome” – plays several roles in protecting the mammalian genome from genetic mutations. As a transcription factor, its activation in somatic cells by genotoxic or oncogenic stresses induces the expression of hundreds of genes, leading to cell cycle arrest, apoptosis and senescence [11]. After oncogenic stresses, ARF plays a critical role in activating p53 [11]. Since all reprogramming factors discovered so far have oncogenic potential, and c-Myc and Klf4 are well-established oncogenes, the ectopic expression of the reprogramming factors in somatic cells activates ARF-p53 pathways, leading to the cell cycle arrest, apoptosis and senescence of these cells, all of which could inhibit successful reprogramming (Figure 1). In support of this notion, three of the five Nature papers demonstrate that depletion of p21, the transcriptional target of p53 required for cell cycle arrest, improves the reprogramming efficiency [5,7,9]. In addition, the other two reports indicate that oncogene-induced senescence suppresses reprogramming [8,10]. It remains, however, unclear whether p53-dependent apoptosis is involved in mediating the suppression of reprogramming.
Figure 1. Possible mechanisms for impediment of cellular reprogramming by ARF-p53.
The overexpression of reprogramming factors induces ARF–p53 pathways in somatic cells. Overexpression of c-Myc is known to activate ARF–p53 in human somatic cells. Although Oct4 and Sox2 have oncogenic potential, it remains unclear whether these two reprogramming factors can activate p53. Klf4 is an oncoprotein that inactivates p53. Once activated, p53 activates the transcription of hundreds of target genes, leading to cell cycle arrest, senescence or apoptosis, depending on the levels of genotoxic and oncogenic strtreess, all of which might hinder cellular reprogramming to pluripotency.
p53 has an important role in maintaining the genetic stability of ESCs, by coordinating their DNA damage response and self-renewal [12]. In this context, activated p53 directly suppresses the expression of Nanog, which is required for the self-renewal of ESCs, leading to the elimination of DNA damaged ESCs from the self-renewing pool. Nanog is likely to be an important factor for the self-renewal of iPSCs, the lack of p53 during reprogramming could promote the establishment and self-renewal of the newly reprogrammed iPSCs.
In the absence of p53, would the reprogrammed cells actually be useful? Reprogramming methods coupled with the inactivation or deletion of p53 enables damaged cells to be turned into iPSCs [5,7]. Although the methods might not seem desirable for therapeutic use of iPSCs, they could help to establish useful cellular models for a variety of diseases in which somatic cells other than fibroblasts might need to be reprogrammed but are more difficult to reprogram. Because the persistent inactivation of p53 during reprogramming leads to apparent genomic instability and tumorigenesis of iPSCs [5,7], transient p53 inactivation by small molecule inhibitors or siRNAs might be useful to reduce the trading of the cancer risk for reprogramming efficiency.
To attempt to understand the logic of genomic reprogramming, it is useful to conceptualize each cell as having reached a distinct molecular steady state through as-yet-incompletely understood genome dynamics involving gene regulation, epigenetic modifications and molecular cell physiology. Considering the critical role of p53 in suppressing reprogramming, the extremely low efficiency of reprogramming could be due to the possibility that only the cells with spontaneously mutated or epigenetically silenced ARF–p53 can be successfully reprogrammed. It would be interesting to determine whether genes involved in ARF–p53 pathways are preferentially mutated in iPSCs. Alternatively, it is possible that the action of p53 is required to prevent the generation of iPSCs containing DNA damage or DNA repair deficiency [7]. Cells closer to pluripotency might have a lower tolerance for DNA damage, and the removal of p53 might allow these abnormalities to be passed on to reprogrammed cells. Thus, p53 could be an important regulator preventing the generation of iPSCs from damaged sources.
Concluding remarks
These new studies support an idea that molecules critically involved in genome stability function as not only guardians of the genome, but also barriers to pluripotency. In addition to p53 and ARF, it would be interesting to determine whether other guardians of the genome, such as retinoblastoma protein (RB) and PTEN, are also involved in suppressing reprogramming. Genome integrity might indeed be a fundamental issue in stem cell pluripotency and reprogramming. Although some iPSCs have normal karyotypes, the levels of genomic mutations in iPSCs remain to be examined by more detailed genomic analysis such as the whole genome sequencing. If genetic mutations that disrupt the ARF-p53 or other tumor suppressor pathways are accumulated in iPSCs, this would suggest that the loss of these tumor suppression pathways is a prerequisite for successful reprogramming, and iPSCs would be intrinsically genetic unstable. All this further underscores the importance to determine the tumorigenecity of the cells derived from iPSCs for any therapeutic use.
It would appear as if p53 and ARF are roadblocks for iPSC generation, and simultaneously and systematically destroying these roadblocks would truly improve reprogramming efficiency. Altogether, these new insights into key players involved in genome integrity and pluripotency starts to link cancer with stem cell biology. The reprogramming factors are overexpressed in certain human cancers and have the potential to reprogram human cancer cells into stem/precursor stages that are difficult to treat. Together with the findings that p53 is functionally or genetically inactivated in most human cancers, the findings that p53 suppresses reprogramming suggest an important role of p53 in preventing the dedifferentiation of human cancer cells that could give rise to cancer stem cells.
Acknowledgements
W.D. is supported by grants from National Institutes of Health (RO1 NS059043 and RO1 ES015988), National Multiple Sclerosis Society, Feldstein Medical Foundation, and Shriners Hospitals for Children. Y.X. is supported by a grant (RC1-00148) from California Institute of Regenerative Medicine.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest that relate to this article.
References
- 1.Lewitzky M, Yamanaka S. Reprogramming somatic cells towards pluripotency by defined factors. Current Opinion in Biotechnology. 2007;18:467–473. doi: 10.1016/j.copbio.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 2.Huangfu D, et al. Induction of pluripotent stem cells from primary human fibroblasts with only Oct4 and Sox2. Nat Biotech. 2008;26:1269–1275. doi: 10.1038/nbt.1502. [DOI] [PubMed] [Google Scholar]
- 3.Shi Y, et al. A combined chemical and genetic approach for the generation of induced pluripotent stem cells. Cell Stem Cell. 2008;2:525–528. doi: 10.1016/j.stem.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 4.Zhao Y, et al. Two supporting factors greatly improve the efficiency of human iPSC generation. Cell Stem Cell. 2008;3:475–479. doi: 10.1016/j.stem.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 5.Hong H, et al. Suppression of induced pluripotent stem cell generation by the p53-p21 pathway. Nature. 2009;460(7259):1132–1135. doi: 10.1038/nature08235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Utikal J, et al. Immortalization eliminates a roadblock during cellular reprogramming into iPS cells. Nature. 2009;460(7259):1145–1148. doi: 10.1038/nature08285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marión RM, et al. A p53-mediated DNA damage response limits reprogramming to ensure iPS cell genomic integrity. Nature. 2009;460(7259):1149–1153. doi: 10.1038/nature08287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li H, et al. The Ink4/ARF locus is a barrier for iPS cell reprogramming. Nature. 2009;460(7259):1136–1139. doi: 10.1038/nature08290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kawamura T, et al. Linking the p53 tumour suppressor pathway to somatic cell reprogramming. Nature. 2009;460(7259):1140–1144. doi: 10.1038/nature08311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Banito A, et al. Senescence impairs successful reprogramming to pluripotent stem cells. Genes Dev. 2009 doi: 10.1101/gad.1811609. Published in Advance August 20, 2009, doi: 10.1101/gad.1811609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prives C, Hall PA. The p53 pathway. J. Pathol. 1999;187:112–126. doi: 10.1002/(SICI)1096-9896(199901)187:1<112::AID-PATH250>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 12.Lin T, et al. p53 induces differentiation of mouse embryonic stem cells by suppressing Nanog expression. Nat. Cell Biol. 2005;7:165–171. doi: 10.1038/ncb1211. [DOI] [PubMed] [Google Scholar]