Abstract
Background:
Thromboembolic events (TEs) are frequent after mechanical mitral valve replacement (MVR), but their association to anticoagulation quality is unclear and has never been studied in a population-based setting with patients who have a complete anticoagulation record.
Methods:
We compiled a complete record of all residents of Olmsted County, MN, who underwent mechanical MVR between 1981 and 2004, for all TE, bleeding episodes, and international normalized ratios (INRs) measured from prosthesis implantation.
Results:
In the 112 residents (mean [± SD] age, 57 ± 16 years; 60% female residents) who underwent mechanical MVR, 19,647 INR samples were obtained. While INR averaged 3.02 ± 0.57, almost 40% of INRs were < 2 or > 4.5. Thirty-four TEs and 28 bleeding episodes occurred during a mean duration of 8.2 ± 6.1 years of follow-up. There was no trend of association of INR (average, SD, growth variance rate, or intensity-specific incidence of events) with TE. Previous cardiac surgery (p = 0.014) and ball prosthesis (hazard ratio [HR], 2.92; 95% CI, 1.43 to 5.94; p = 0.003) independently determined TE. With MVR using a ball prosthesis, despite higher anticoagulation intensity (p = 0.002), the 8-year rate of freedom from TE was considerably lower (50 ± 9% vs 81 ± 5%, respectively; p < 0.0001). Compared with expected stroke rates in the population, stroke risk was elevated with non-ball prosthesis MVR (HR 2.6; 95% CI, 1.3 to 5.2; p = 0.007) but was considerable with ball prosthesis MVR (HR 11.7; 95% CI, 7.5 to 18.4; p < 0.0001). INR variability (SD) was higher with a higher mean INR value (p < 0.0001). INR variability (HR 2.485; 95% CI, 1.11 to 5.55; p = 0.027) and cancer history (p < 0.0001) independently determined bleeding rates.
Conclusion:
This population-based comprehensive study of anticoagulation and TE post-MVR shows that, in these closely anticoagulated patients, anticoagulation intensity was highly variable and not associated with TE incidence post-MVR. Higher anticoagulation intensity is linked to higher variability and, thus, to bleeding. The MVR-ball prosthesis design is associated with higher TE rates notwithstanding higher anticoagulation intensity, and its use should be retired worldwide.
Mitral valve diseases are frequent,1 and, despite repair attempts, mitral valve replacement (MVR) is required in numerous patients.2 Thromboembolic events (TEs) are frequent after mechanical MVR,3–6 particularly ischemic stroke,6,7 which results in poor quality of life and excess mortality.7 The ascertainment of TEs in tertiary care after MVR was often retrospective and of uncertain quality.8 Conversely, randomized trials4,9–12 enroll highly selected, often low-risk patients.10 Thus, the TE rates reported may not reflect those incurred in the community, and both those rates and the link to TE anticoagulation quality have never been analyzed in a population-based study.
The quality of anticoagulation is generally considered important in TE prevention after mechanical valve replacement.13 While high-visibility studies14 suggested that TEs were exceptional with optimal anticoagulation, TEs remain a major problem after mechanical MVR,6 and their link to anticoagulation quality remains controversial.7,10,14–19 Many studies3,7,9,10,18,20,21 have analyzed convenience samples of mechanical MVR, but incomplete or sparse anticoagulation data have been usual. Furthermore, anticoagulation regimens were assessed by the intention to treat and not by actual achieved results.22 Hence, clinical guidelines2,13,22–24 acknowledge scant evidence supporting anticoagulation recommendations and are remarkably discrepant (European vs American guidelines) regarding the thrombogenicity of mechanical prostheses and the tailoring of the goals of anticoagulation to patient and prosthesis.2,13,22,23
Therefore, we sought to evaluate the factors associated with TEs in patients who have undergone mechanical MVR in the population (not referral practice) with a complete record of all TEs and of the entire anticoagulation profile. We aimed at analyzing the link between TEs and the anticoagulation achieved and the prosthesis type.
Materials and Methods
Subjects
This was a population-based study identifying retrospectively all residents (≥ 10 years of age) in Olmsted County, MN, who underwent mechanical MVR from 1980 through 2004. Patients who underwent MVR elsewhere, four infants with MVR, and those patients denying research authorization were excluded. The study was approved by the Institutional Review Boards of the Mayo Clinic and Olmsted Medical Group. Linkage between county health-care providers25 allowed the detection of all events with inpatient and outpatient records and the collection of all anticoagulation data. Clinical data from 2006 to 2008 noted before and after surgery by the physicians of these patients were abstracted without alteration. During the study period, easy access has consistently been available for anticoagulation assessment throughout the county akin to that in anticoagulation clinics.
Follow-up and Events
Deaths were noted overall and were classified according to current guidelines.26 The main end point was TEs, including cerebral and peripheral embolic events. A secondary end point was bleeding complications. TEs and bleeding were defined in accordance with previous reports6,27,28 and current guidelines,26 along with a neurologic consultation to assess strokes. Bleeding within 48 h of cardiopulmonary bypass was not taken into account. Endocarditis, prosthesis dysfunction, and mitral valve reoperation were also assessed.26
Anticoagulation Assessment
Complete data sets of coagulation tests from surgery to the last follow-up were electronically downloaded or abstracted from all providers. The achieved anticoagulation was expressed as an international normalized ratio (INR). Prothrombin times were converted to INR values using the appropriate international sensitivity index. The main anticoagulation measures analyzed were the INR mean value (INR-average), which was reflective of anticoagulation intensity, the INR SD, which measured variability, and the mean interval between consecutive INR tests (INR-interval), which measured the frequency of testing. We also assessed the variability of anticoagulation using the variance growth rate methods described by Fihn and colleagues,29,30 the time spent inside and outside the expanded therapeutic range of 2.0 to 4.5, and the INR-specific incidence of events.14,31 These variables were analyzed for the entire follow-up period in all patients, up to the time of the event for those who had experienced events, or to the last follow-up for those who had not experienced events.
Statistical Analysis
Group characteristics are summarized as the mean ± SD or percentage, and were compared using the Student t test, Mann-Whitney U test, or χ2 test. Logistic regression assessed early postoperative (ie, within 30 days after surgery) events. Freedom from TEs, bleeding, or valve-related complications were estimated using the Kaplan-Meier method and were compared using the log-rank test. Univariate and multivariate associations of patient characteristics with end points used the Cox proportional hazards models. Because of sample size, we restricted explanatory variables to the following: patient characteristics (atrial fibrillation, atherosclerosis, history of cancer for bleeding events, left ventricle [LV] ejection fraction [EF], and preoperative embolism); surgical characteristics (previous cardiac surgery, MVR ball prosthesis or non-ball prosthesis MVR, and aortic valve replacement); and anticoagulation characteristics. The stroke rates observed were compared with county-expected rates (matching for age, sex, and rhythm).32 Candidate predictors with p < 0.20 were entered into multivariate models adjusted for age and sex. A p value < 0.05 was considered statistically significant.
Results
Population Characteristics
Baseline characteristics of 112 residents of Olmsted County who underwent mechanical MVR during the study period are shown Table 1. Etiology was rheumatic in 69 patients (62%), prolapse in 15 patients, endocarditis in 8 patients, functional regurgitation in 10 patients, and prosthesis dysfunction in 10 patients.
Table 1.
Characteristics of the Entire Patient Population (n = 112) and After Stratification According to the Occurrence of a TE During Follow-up in the 109 Patients Who Survived Surgery
| Variables | All Patients(n = 112) | Patients Without TE(n = 75) | Patients With TE(n = 34) | p Value |
|---|---|---|---|---|
| Clinical characteristics | ||||
| Age, yr | 57 ± 16 | 57 ± 17 | 57 ± 15 | 0.99 |
| Female gender | 67 (60) | 48 (62) | 19 (60) | 0.58 |
| Atrial fibrillation | 45 (40) | 32 (41) | 13 (38) | 0.78 |
| NYHA class 3–4 | 78 (70) | 53 (68) | 25 (74) | 0.56 |
| BMI, kg/m2 | 27 ± 6 | 27.1 ± 6.4 | 26.5 ± 4.7 | 0.62 |
| Hypertension | 66 (59) | 45 (58) | 21 (62) | 0.69 |
| Diabetes mellitus | 26 (23) | 18 (23) | 8 (24) | 0.99 |
| Coronary artery disease | 57 (51) | 39 (50) | 18 (53) | 0.78 |
| Atherosclerosis lesions | 69 (62) | 46 (59) | 23 (68) | 0.41 |
| Preoperative echocardiography | ||||
| LV end-diastolic diameter, mm | 52 ± 9 | 52 ± 9 | 53 ± 9 | 0.80 |
| LV EF, % | 58 ± 12 | 59 ± 12 | 56 ± 11 | 0.28 |
| LV EF ≤ 50% | 28 (25) | 16 (21) | 12 (35) | 0.10 |
| Left atrial diameter, mm | 54 ± 10 | 54 ± 10 | 54 ± 10 | 0.72 |
| Surgery | ||||
| Previous cardiac surgery | 41 (37) | 23 (30) | 18 (53) | 0.021 |
| Mitral ball prosthesis | 37 (33) | 16 (21) | 21 (62) | < 0.0001 |
| Aortic valve replacement | 41 (37) | 29 (37) | 12 (35) | 0.99 |
| Coronary artery bypass grafting | 23 (21) | 17 (22) | 6 (18) | 0.62 |
Values are given as the mean ± SD or No. (%), unless otherwise indicated. NYHA = New York Heart Association.
MVR used a ball prosthesis (Starr-Edwards) in 37 patients and a non-ball prosthesis in 75 patients (single-disk in 6 patients; bileaflet in 69 patients). The mean MVR prosthesis size was 29.6 ± 2.6 mm (range, 23 to 34 mm). Associated mechanical aortic valve replacement was performed in 41 patients (37%), and coronary bypass was performed in 23 patients (21%). The mean prosthetic gradient was 4.8 ± 1.9 mm Hg.
Mortality
Six patients died during the first 30 days after surgery (5.4%). The duration of follow-up averaged 8.2 ± 6.1 years (up to 26 years) for 109 patients surviving the operative day. Overall, 61 deaths occurred during follow-up, with a mean 8-year survival rate of 60 ± 5%. Death was cardiac related in 38 patients (62%) and MVR related in 19 patients (TEs, 5 patients; bleeding, 6 patients; and miscellaneous, 8 patients). The mean 8-year rate for freedom form MVR-related death was 85.3 ± 3.6%. The linearized rate of MVR-related death was 2.1% (range, 1.3% to 3.3%) per 100 patient-years overall, and 1.5% (range, 0.8% to 2.6%) per 100 patient-years after 30 days.
Anticoagulation Quality
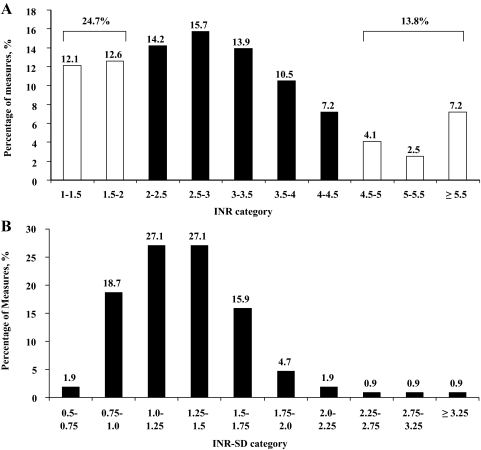
After surgery, 19,647 INRs were measured in 109 patients (180 ± 129 measurements per patient; range, 6 to 534 measurements). Analyzing all INRs in categories demonstrated that only 61.5% of measurements were inside an extended therapeutic range of 2.0 to 4.5 (based on the interpretation of Cannegieter et al14), and variability (INR SD) was considerable (Fig 1). Anticoagulation is summarized Table 2. Patients with higher INR-average values displayed higher variability (INR SD, 1.18 ± 0.27 vs 1.65 ± 0.41, respectively [for INR-average < 3.5 and ≥ 3.5, respectively]; p < 0.0001). INR-average was similar with and without postoperative atrial fibrillation (p = 0.39), LV EF ≤ 50% (p = 0.84), or associated aortic valve replacement (p = 0.33). Twenty-six patients received aspirin (with anticoagulation) with INRs identical to those not receiving aspirin (p = 0.77). Patients with MVR with a ball prosthesis had a higher mean INR-average than those with MVR with a non-ball prosthesis (3.22 ± 0.66 vs 2.85 ± 0.53, respectively; p = 0.002) and a higher mean INR SD (1.42 ± 0.48 vs 1.21 ± 0.33, respectively; p = 0.01), but the mean INR-interval was similar (21.3 ± 20.4 days vs 17.5 ± 17.9 days, respectively; p = 0.34).
Figure 1.
Anticoagulation variability in the entire patient population. A: distribution of all of INRs during long-term follow-up after mechanical MVR surgery. Note that almost 40% of INRs were outside of the extended therapeutic range. B: distribution of INR SD showing that most patients displayed a high variability of INR, reflecting a high variability of anticoagulation intensity.
Table 2.
Anticoagulation in the Entire Population and After Stratification According to Occurrence of TEs
| Variables | All Patients | Patients Without TE | Patients With TE | p Value |
|---|---|---|---|---|
| Entire follow-up | ||||
| INR-average | 3.02 ± 0.57 | 2.96 ± 0.61 | 3.16 ± 0.57 | 0.11 |
| INR SD | 1.32 ± 0.41 | 1.25 ± 0.36 | 1.46 ± 0.48 | 0.016 |
| INR-interval, d | 19.3 ± 17.6 | 18.7 ± 17.4 | 20.6 ± 18.4 | 0.62 |
| First 30 d | ||||
| INR-average | 2.57 ± 0.82 | 2.50 ± 0.87 | 2.71 ± 0.57 | 0.24 |
| INR SD | 1.23 ± 0.56 | 1.17 ± 0.49 | 1.36 ± 0.67 | 0.12 |
| INR-interval, d | 2.11 ± 0.83 | 2.08 ± 0.87 | 2.19 ± 0.76 | 0.49 |
| Up to TE or last follow-up* | ||||
| INR-average | 2.98 ± 0.59 | 2.96 ± 0.61 | 3.06 ± 0.69 | 0.36 |
| INR SD | 1.28 ± 0.39 | 1.25 ± 0.36 | 1.36 ± 0.53 | 0.16 |
| INR-interval, d | 18.7 ± 18.7 | 18.7 ± 17.4 | 18.7 ± 21.3 | 0.99 |
| Variance-growth rate 129 | 0.21 ± 0.11 | 0.20 ± 0.08 | 0.22 ± 0.16 | 0.39 |
| Variance-growth rate 230 | 0.69 ± 0.72 | 0.65 ± 0.36 | 0.78 ± 1.13 | 0.37 |
| Percent INR within 2–4.5 | 61 ± 14 | 62 ± 11 | 58 ± 20 | 0.18 |
| Before TE† | ||||
| Last INR before TE | 3.16 ± 1.18 | 2.96 ± 0.61 | 3.13 ± 1.51 | 0.86 |
| INR-average within 12 mo before TE‡ | 3.09 ± 0.63 | 3.02 ± 0.55 | 3.29 ± 0.79 | 0.074 |
| INR-SD within 12 mo before TE‡ | 1.19 ± 0.39 | 1.17 ± 0.35 | 1.29 ± 0.52 | 0.19 |
| INR-interval within 12 mo before TE, d‡ | 25.0 ± 27.2 | 24.1 ± 24.3 | 27.8 ± 34.8 | 0.56 |
Values are given as the mean ± SD, unless otherwise indicated.
*Calculation is up to the TE event (patients with TE) or for the entire follow-up (patients without TE).
†Averages of all INR for patients without TE.
‡Applies to patients with at least 12 mo of follow-up.
TEs After Mechanical MVR
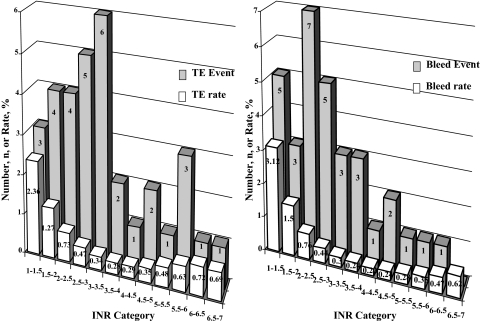
Thirty-four patients (31%) had experienced TEs, and the first event was ischemic stroke in 27 patients, coronary embolism in 4 patients, MVR thrombosis in 2 patients, and mesenteric embolism in 1 patient. TE number and INR-specific incidence rates are depicted in Figure 2 (left) according to each INR category. Linearized TE rates are stratified by postoperative period (Table 3). Compared with the rates of stroke expected in the general county population, patients with mechanical MVR displayed excess risk with an observed ischemic stroke/expected ischemic stroke hazard ratio (HR) of 5.7 (range, 3.9 to 8.4); p < 0.0001).
Figure 2.
The distribution of events (TE, left; bleeding, right) and of rates of events calculated as the ratio of events to time spent within 0.5 INR increments according to the Rosendaal method.31 Note that for an INR > 4.5, TE rates tend to be higher than for INRs within the 2 to 4.5 range; however, there are no significant differences among all ranges (p > 0.18). Note also that for an INR < 2, bleeding rates tend to be higher than INRs within the range of 2 to 4.5; however, there are no significant differences among all ranges (p > 0.48).
Table 3.
Mitral Valve-Related Complications Stratified by Postoperative Period (≤ 30 Days or > 30 Days)
| Postoperative Period |
|||
|---|---|---|---|
| Variables | Overall | ≤ 30 d | > 30 d |
| Thromboembolism | |||
| Events, No. | 34 | 6 | 28 |
| Linearized rate, %* | 4.9 (3.4–6.8) | 68.6 (25.2–149.3) | 4.0 (2.7–5.8) |
| Freedom from event, % | 8 yr: 69.8 ± 5.0 | 30 d: 94.3 ± 2.3 | 8 yr: 73.9 ± 5.0 |
| Ischemic stroke | |||
| Events, No. | 27 | 6 | 21 |
| Linearized rate, %* | 3.7 (2.4–5.4) | 68.6 (25.2–149.3) | 2.9 (1.8–4.4) |
| Freedom from event, % | 8 yr: 75.8 ± 4.6 | 30 d: 94.3 ± 2.3 | 8 yr: 80.4 ± 4.5 |
| Bleeding | |||
| Events, No. | 28 | 1 | 27 |
| Linearized rate, %* | 3.7 (2.4–5.3) | 11.2 (0.3–62.6) | 3.6 (2.3–5.7) |
| Freedom from event, % | 8 yr: 76 ± 5 | 30 d: 99 ± 1 | 8 yr: 77 ± 5 |
Values are given as the rate (95% CI) or mean ± SD.
*Expressed per 100 patient-years.
Determinants of TE After MVR
Table 1 shows baseline characteristics between patients with and without TEs, and most were identical. Among the variables selected as candidate predictors, few patient characteristics showed trends toward univariate Cox-proportional hazard association to TE (LV EF ≤ 50%, p = 0.029; atherosclerosis lesion, p = 0.11). Among operative characteristics, previous cardiac surgery (p = 0.003) and ball-valve prosthesis (p = 0.002) were associated with TEs. Atrial fibrillation (p > 0.5) and aortic valve replacement were not associated with TEs (p = 0.26).
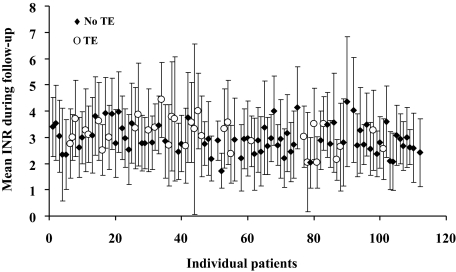
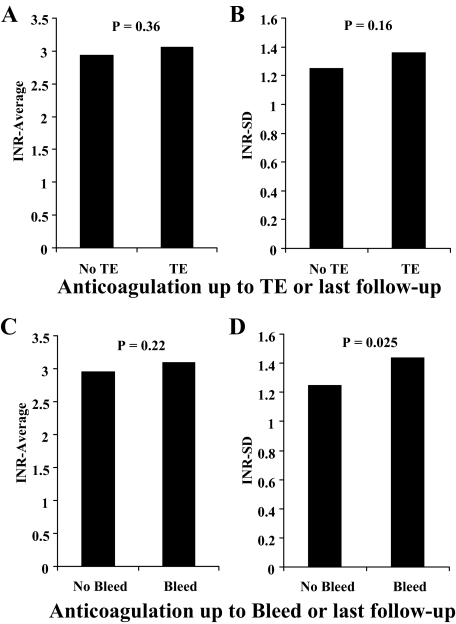
Individual values of INR-average and INR SD (Fig 3) show considerable overlap between those patients with and without TEs. A comparison of INR characteristics up to the time of the TEs or to the last follow-up (Fig 4) shows no difference between those patients with and without TEs. Aspirin therapy was not different between those with and without TEs (p = 0.42). Anticoagulation characteristics stratified by TE occurrence are summarized in Table 2. There was also no difference in any anticoagulation characteristics up to the time of an event, particularly the INR variance-growth rates and the percentage of time spent within the 2 to 4.5 range between patients with and without TEs. There was also no difference between patients with and without TEs, even when the last year or the last INR before TEs were considered. The mean INR on the day of TEs was 3.23 ± 1.90, which was not different from the remainder of INRs (p = 0.83).
Figure 3.
The distribution of INRs during the entire follow- up period in individual patients in the series. The solid symbols represent patients without TEs (no TE), and the open symbols show the patients with TEs. The INR SD is represented by the bar with each symbol. Note the considerable overlap between patients with and without TEs in terms of average INR and of SD.
Figure 4.
The plot of INR-average (A and C) and INR SD (B and D) calculated up to the time of the event for TE (A and B) and for bleeding (C and D). For TE, there was no difference in INR-average or INR SD between those patients who had and had not experienced events. For bleeding, there was no difference in INR-average, but there was a higher INR SD in those patients who had experienced events. In patients who had not experienced events, the INR was calculated over the entire follow-up period.
Independent predictors of TEs (Table 4) were atherosclerosis (p = 0.055), previous cardiac surgery (p = 0.014), and MVR ball prosthesis (HR, 2.92; 95% CI, 1.43 to 5.94; p = 0.003). Adjusting for age, sex, previous cardiac surgery (HR, 2.49; 95% CI, 1.22 to 5.08; p = 0.012), and MVR ball prosthesis (HR, 2.96; 95% CI, 1.45 to 6.06; p = 0.003) independently predicted TEs. TE rates stratified by MVR with ball prosthesis and non-ball prosthesis are shown in Table 5. MVR ball prosthesis was also independently predictive of late (ie, > 30-day) TEs or ischemic stroke (Table 4). A comparison of observed ischemic stroke rates vs expected ischemic stroke rates in the Olmsted County population showed moderate excess risk for MVR without ball prosthesis (HR, 2.6; 95% CI, 1.3 to 5.2; p = 0.007) but considerable excess risk for MVR with ball prosthesis (HR, 11.7; 95% CI, 7.5 to 18.4; p < 0.0001).
Table 4.
Predictors of Overall or Late TE, Ischemic Stroke, and Bleeding Event Using a Stepwise Multivariate Cox Model
| Conditions | HR | 95% CI | p Value |
|---|---|---|---|
| TE (n = 34) | |||
| Atherosclerosis | 2.11 | 0.98–4.53 | 0.055 |
| Previous cardiac surgery | 2.40 | 1.19–4.84 | 0.014 |
| Ball valve | 2.92 | 1.43–5.94 | 0.003 |
| Late TE > 30 d (n = 28) | |||
| LV EF ≤ 50% | 2.48 | 1.13–5.44 | 0.024 |
| Previous cardiac surgery | 3.30 | 1.51–7.20 | 0.003 |
| Ball valve | 3.03 | 1.38–6.67 | 0.006 |
| Ischemic stroke (n = 27) | |||
| LV EF ≤ 50% | 2.66 | 1.21–5.86 | 0.015 |
| Ball valve | 4.53 | 1.96–10.48 | < 0.0001 |
| Bleeding (n = 28) | |||
| History of cancer | 4.01 | 1.89–8.52 | < 0.0001 |
| INR-SD | 2.48 | 1.11–5.55 | 0.027 |
HR = hazard ratio.
Table 5.
Mitral Mechanical Valve-Related Complications According to the Type of Prosthesis
| Ball Valve (n = 37) |
Disk or Bileaflet Valve (n = 75) |
||||||
|---|---|---|---|---|---|---|---|
| Conditions | No. | Linearized Rate (95% CI) | 8-Yr Freedom, %* | No. | Linearized Rate (95% CI) | 8-Yr Freedom, %* | p Value |
| TE | 21 | 8.5 (5.3–13.0) | 50 ± 9 | 13 | 3.1 (1.7–5.3) | 81 ± 5 | < 0.0001 |
| Bleeding | 10 | 3.1 (1.4–5.3) | 80 ± 7.5 | 18 | 6.3 (4.1–9.2) | 75 ± 6 | 0.35 |
| Endocarditis | 1 | 0.3 (0.0–1.7) | 97 ± 3 | 3 | 0.6 (0.1–1.8) | 95 ± 4 | 0.49 |
| Periprosthetic regurgitation | 4 | 1.2 (0.3–3.1) | 90 ± 6 | 4 | 0.8 (0.2–2.0) | 94 ± 4 | 0.67 |
| Mitral valve reoperation | 3 | 0.8 (0.2–2.3) | 94 ± 4 | 4 | 0.8 (0.2–2.0) | 90 ± 5 | 0.99 |
| Any mitral valve complication | 27 | 12.3 (8.1–17.9) | 33 ± 8 | 29 | 8.8 (5.9–12.6) | 50 ± 8 | 0.03 |
Values are given as the mean ± SD.
No anticoagulation measure, including the variance- growth rate,29,30 independently predicted TEs, TEs at > 30 days, or ischemic stroke even after stratification by whether a ball prosthesis and non-ball prosthesis was used in MVR (all p > 0.30). Furthermore, there was no statistical interaction MVR type and anticoagulation characteristics for TE rates (all p > 0.29) TE INR-specific incidence rates (ratio of events to time spent within 0.5 INR increments) showed no significant differences in TEs according to anticoagulation intensity (p > 0.18) [Fig 2, left]. TE rates according to INR SD, INR-average, and patient-prosthetic characteristics (previous cardiac surgery and MVR ball prosthesis) are shown in Figure 5.
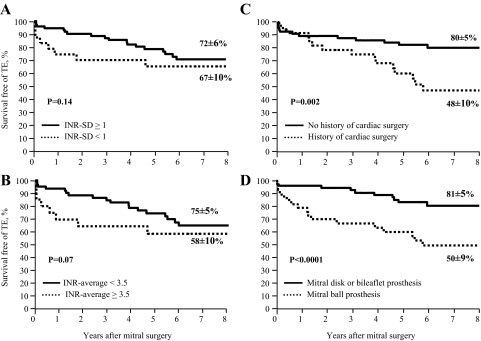
Figure 5.
Freedom from TE according to stratification by anticoagulation quality up to TEs (A and B) and by patients and prosthesis-related factors (C and D). Stratification was performed according to an INR SD ≥ 1 or < 1 (A), to INR-average ≥ 3.5 or < 3.5 (B), to a history of cardiac surgery (C), and to implantation of a ball prosthesis (D). Note the absence of a significant difference in TE rates between the strata defined by anticoagulation variability or intensity, whereas the large difference in TE rates is observed according to the implantation of a ball and non-ball mitral valve prosthesis.
Bleeding and Other Events
Bleeding occurred in 28 patients (26%) [Table 3] during follow-up, only 1 bleeding event (0.9%) occurring before the 30-day follow-up. Bleeding number and rates are depicted in Figure 2 according to each INR category. Bleeding rates overall and stratified by postoperative period are shown Table 3. Up to the time of the event or at the last follow-up, there was no difference between patients with and without bleeding regarding mean INR-average (3.16 ± 0.69 vs 2.95 ± 0.62, respectively; p = 0.16), but there was increased variability (INR SD) [1.49 ± 0.41 vs 1.26 ± 0.42, respectively; p = 0.025]. In multivariate analysis (Table 4), INR SD (p = 0.027) and a history of cancer (p < 0.0001) independently predicted bleeding, even after adjustment for age and sex. In Figure 4B and D, the INR-average and INR SD values are shown according to the occurrence of bleeding. Bleeding INR-specific incidence rates (ie, the ratio of events to time spent within the 0.5 INR increments) showed no significant differences according to anticoagulation intensity (p > 0.48) [Fig 2, right].
Other MVR complications included eight patients with periprosthetic leaks, four patients with endocarditis, and seven reoperations. Overall, 56 patients experienced at least one MVR-related complication, with a mean 8-year freedom from MVR-related complication of 44 ± 6%. When these events were stratified according to the type of valve (Table 5), patients who had undergone MVR with a ball prosthesis showed mostly a higher risk of TEs (p < 0.0001), resulting in a significantly higher MVR-related complication rate.
Discussion
The present study is, to our knowledge, the first to report TE risk after MVR in a population-based setting with a complete anticoagulation record. Our results emphasize a notable TE frequency after mechanical MVR, particularly strokes, despite an INR-average conforming to US guidelines.2 This on-target INR-average should not conceal the high variability of anticoagulation, often out of therapeutic range, despite frequent monitoring and easy access to INR assessment facilities. While no single anticoagulation characteristic determines TE risk, the variability (not intensity) of anticoagulation is strongly linked to bleeding events. The major implication is that large variations in anticoagulation should be carefully avoided. While previous cardiac surgery or low LV EF are TE risk factors, the concept of higher anticoagulation intensity compensating for higher risk is not supported by our data. Finally, while any mechanical prosthesis is associated with excess TE risk, MVR with a ball prosthesis is an important TE risk factor. This prosthetic design, which is a source of excess morbidity despite intense anticoagulation, should, in our opinion, be retired worldwide. For guidelines, our confirmation of part of the European guidelines that MVR mechanical prostheses vary in thrombogenicity is balanced by support of the American guidelines in aiming at relatively low-intensity, low-variability anticoagulation, as high-intensity anticoagulation is not associated with fewer TEs after mechanical MVR.
Thromboembolic Risk
Mechanical MVR causes TEs,2,22,23 with a lifelong excess stroke risk compared with the general population, while risk is only transient for the use of bioprostheses and valve repair.6 However, without a complete anticoagulation record, it was not possible to ascertain a relationship to anticoagulation or to mechanical MVR. Our complete population-based data, including data on anticoagulation and TEs (which can be underestimated in observational series8), indicate a high TE risk after mechanical MVR despite average on-target anticoagulation.15 While TE rates differ notably between series,3,10,21 comprehensive metaanalyses concur with our estimate.5 However, the indispensable anticoagulation has inherent substantial bleeding risk.6,22 High TE and bleeding risks suggest that mechanical MVR is the least desirable mitral procedure and should be selected in young patients only when mitral valve repair is not feasible.
Anticoagulation Effect
Our population-based study allowed complete collection of INRs with higher documentation than most attentive observational studies14,33 and comparable to prospective clinical trials.3,10,21 Moreover, anticoagulation is analyzed not as intention to treat but as achieved INRs. The influence of anticoagulation on TE risk has been debated. Although intermittent or inadequate anticoagulation leads to a high TE risk,7,15,16,21 randomized studies3,10,21 have not observed lower TE rates with higher INR goals. However, clinical trials often enroll low-risk patients, which is not reflective of community practice, emphasizing the importance of our population-based study. While anticoagulation is indispensable with mechanical MVR, the lack of association between TE rates and achieved anticoagulation reemphasizes the fact that aiming for higher INR goals to better prevent TEs is not appropriate.16
Our results extend observations about aortic replacement,10 that low-intensity anticoagulation is as effective as high-intensity anticoagulation in preventing TEs and should lead to recommending active but low-intensity anticoagulation after MVR.2,13,22,23 Lower INRs may decrease bleeding,10,16,17,19,34 while unstable anticoagulation predicts valve-related events.33,35,36 In the present study, INR variability (ie, INR SD) tended to univariately predict TE risk and independently predict bleeding.35,36 Thus, reducing INR variability is a stronger objective than INR level after MVR. Because high-intensity anticoagulation is associated with high variability, lower INR goals (possibly 2 to 3) would reduce variability. Also, higher testing frequency improves the length of time spent in the target range33,37–39 and is an incentive for anticoagulation clinics or INR self-management21,27,38 as tools to improve anticoagulation effectiveness.
Effect of Prosthesis Type
LV EF13,18,40,41 and MVR3,20,34 are recognized risk factors for TE after valve replacement that were also observed in our study. Atrial fibrillation precipitates TEs13,18,41 with tissue MVR but not mechanical MVR,6 which is similar to the results of the present study. Prosthesis type as a TE predictor is controversial.4,7,12,34,42,43 These uncertainties stem mostly from differences in TE definition between centers,5 and uniform TE definition may be an advantage of single-center studies. Recent prostheses are considered less thrombogenic than first-generation prostheses.2,13,23 However, proof of definite differences in TE rates between various types of prostheses is scant34 and disputable.5 The concept of a higher thrombogenicity for ball valves13,34 has led to the European guidelines23 recommendation of higher INRs as a presumed compensation for higher thrombogenicity. This recommendation should be reconsidered, as more intense anticoagulation in patients receiving MVR with ball prostheses did not compensate for higher thrombogenicity. Furthermore, higher intensity anticoagulation never reduced TEs, complicating the use of mechanical prostheses.10,16,17 The occurrence of excess TEs, particularly stroke, with use of a ball valve in MVR concurs with the results of small single-center studies12,44 and is quite considerable (8.5 vs 3.1 per 100 patient-year, respectively; p < 0.0001), leading to excess MVR-related events. An MVR ball prosthesis, the first successful prosthetic valve,45 was a life saver in its time and has economic advantages for developing countries,46 but it carries with it an unacceptable TE rate and should be retired from use worldwide. Safer valve substitutes should be available with similar cost-effectiveness. The management of the MVR ball valve in place is more conjectural. Whether target anticoagulation intensity should be an INR of 2.5 to 3.5,2,13,22–24 or whether an INR of 2 to 3 is also acceptable cannot be ascertained from our data. The absence of a link to the INR-average TE rate in our study may be an incentive for recommending an INR of 2 to 3. A lower goal would reduce variability and minimize bleeding. Finally, the recommendation of using low-dose aspirin in combination with anticoagulation is not affected by our study,2,23 and there is no rationale to alter this guideline.
Limitations
In analyzing TE rates after cardiac surgery, one has to balance sample size with data completeness. Our series is small, and careful analysis selected a limited number of a priori variables potentially linked to TEs to limit the possibility of statistical bias. Fortunately, our entire experience post- mitral surgery has already provided critical points in term of the superiority of tissue substitutes (repair or bioprostheses) and the lack of association of atrial fibrillation to TE post-mechanical MVR.6 Thus, the main issue to resolve focuses on anticoagulation quality, for which our series provides unique information. Furthermore, in comparing valve substitutes multicenter studies with their extreme variability in TE rate estimates5 are seriously limited, and our findings of excess TE rates in patients who have undergone MVR with ball prostheses concur with those of more uniform, small, single-center studies.12,44 Thus, despite its relatively small size, our population-based series with complete data contains sound statistical conclusions and novel information.
Conclusions
This first population-based study of TE post-MVR with a complete embolism and anticoagulation record shows that anticoagulation intensity post-MVR, although on target as an average, is highly variable and fails to be associated with TE prevention. Higher anticoagulation intensity is linked to higher variability and, thus, to bleeding. Despite higher INRs, the ball valve design for MVR is associated with higher TE rates, and the design should be retired worldwide.
Acknowledgments
Author contributions: Drs. Le Tourneau, Lim, and Inamo contributed to data collection. Drs. Let Tourneau, Lim, Inamo, Miller, Schaff, and Enriquez-Sarano, and Mr. Mahoney contributed to the drafting and review of the manuscript.
Financial/nonfinancial disclosures: Dr. Enriquez-Sarano is a consultant and receives research funding from Edwards LLC. The authors have reported to the ACCP that no significant conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Abbreviations:
- EF
ejection fraction
- HR
hazard ratio
- INR
international normalized ratio
- INR-average
international normalized ratio mean value
- INR-interval
mean interval between consecutive international normalized ratio tests
- LV
left ventricle
- MVR
mitral valve replacement
- TE
thromboembolic event
Footnotes
For editorial comment see page 1451
Funding/Support: This work was supported by the National Institutes of Health, Rochester Epidemiology Project Grant AR30582, Dr. Walter A. Rocca, Principal Investigator. This work was also supported by a grant from the French Foundation of Cardiology (Dr. Le Tourneau).
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (www.chestjournal.org/site/misc/reprints.xhtml).
References
- 1.Nkomo VT, Gardin JM, Skelton TN, et al. Burden of valvular heart diseases: a population-based study. Lancet. 2006;368:1005–1011. doi: 10.1016/S0140-6736(06)69208-8. [DOI] [PubMed] [Google Scholar]
- 2.Bonow RO, Carabello BA, Chatterjee K, et al. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing Committee to Revise the 1998 guidelines for the management of patients with valvular heart disease) developed in collaboration with the Society of Cardiovascular Anesthesiologists endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. J Am Coll Cardiol. 2006;48:e1–e148. doi: 10.1016/j.jacc.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 3.Hering D, Piper C, Bergemann R, et al. Thromboembolic and bleeding complications following St. Jude Medical valve replacement: results of the German experience with low-intensity anticoagulation study. Chest. 2005;127:53–59. doi: 10.1378/chest.127.1.53. [DOI] [PubMed] [Google Scholar]
- 4.Kuntze CE, Blackstone EH, Ebels T. Thromboembolism and mechanical heart valves: a randomized study revisited. Ann Thorac Surg. 1998;66:101–107. doi: 10.1016/s0003-4975(98)00313-0. [DOI] [PubMed] [Google Scholar]
- 5.Grunkemeier GL, Wu Y. “Our complication rates are lower than theirs”: statistical critique of heart valve comparisons. J Thorac Cardiovasc Surg. 2003;125:290–300. doi: 10.1067/mtc.2003.53. [DOI] [PubMed] [Google Scholar]
- 6.Russo A, Grigioni F, Avierinos JF, et al. Thrombo-embolic complications after surgical correction of mitral regurgitation: incidence, predictors and clinical implications. J Am Coll Cardiol. 2008;51:1203–1211. doi: 10.1016/j.jacc.2007.10.058. [DOI] [PubMed] [Google Scholar]
- 7.Fuster V, Pumphrey CW, McGoon MD, et al. Systemic thromboembolism in mitral and aortic Starr-Edwards prostheses: a 10–19 year follow-up. Circulation. 1982;66:I157–I161. [PubMed] [Google Scholar]
- 8.Bodnar E, Horstkotte D. Potential flaws in the assessment of minor cerebrovascular events after heart valve replacement. J Heart Valve Dis. 1993;2:287–290. [PubMed] [Google Scholar]
- 9.Murday AJ, Hochstitzky A, Mansfield J, et al. A prospective controlled trial of St. Jude versus Starr Edwards aortic and mitral valve prostheses. Ann Thorac Surg. 2003;76:66–74. doi: 10.1016/s0003-4975(03)00118-8. [DOI] [PubMed] [Google Scholar]
- 10.Acar J, Iung B, Boissel JP, et al. AREVA: Multicenter randomized comparison of low-dose versus standard-dose anticoagulation in patients with mechanical prosthetic heart valves. Circulation. 1996;94:2107–2112. doi: 10.1161/01.cir.94.9.2107. [DOI] [PubMed] [Google Scholar]
- 11.Kuntze CE, Ebels T, Eijgelaar A, et al. Rates of thromboembolism with three different mechanical heart valve prostheses: randomised study. Lancet. 1989;1:514–517. doi: 10.1016/s0140-6736(89)90065-2. [DOI] [PubMed] [Google Scholar]
- 12.Horstkotte D, Haerten K, Herzer JA, et al. Five-year results after randomized mitral valve replacement with Bjork-Shiley, Lillehei-Kaster, and Starr-Edwards prostheses. Thorac Cardiovasc Surg. 1983;31:206–214. doi: 10.1055/s-2007-1021981. [DOI] [PubMed] [Google Scholar]
- 13.Butchart EG, Gohlke-Barwolf C, Antunes MJ, et al. Recommendations for the management of patients after heart valve surgery. Eur Heart J. 2005;26:2463–2471. doi: 10.1093/eurheartj/ehi426. [DOI] [PubMed] [Google Scholar]
- 14.Cannegieter SC, Rosendaal FR, Wintzen AR, et al. Optimal oral anticoagulant therapy in patients with mechanical heart valves. N Engl J Med. 1995;333:11–17. doi: 10.1056/NEJM199507063330103. [DOI] [PubMed] [Google Scholar]
- 15.McGoon MD, Fuster V, McGoon DC, et al. Aortic and mitral valve incompetence: long-term follow-up (10 to 19 years) of patients treated with the Starr-Edwards prosthesis. J Am Coll Cardiol. 1984;3:930–938. doi: 10.1016/s0735-1097(84)80351-4. [DOI] [PubMed] [Google Scholar]
- 16.Saour JN, Sieck JO, Mamo LA, et al. Trial of different intensities of anticoagulation in patients with prosthetic heart valves. N Engl J Med. 1990;322:428–432. doi: 10.1056/NEJM199002153220703. [DOI] [PubMed] [Google Scholar]
- 17.Altman R, Rouvier J, Gurfinkel E, et al. Comparison of two levels of anticoagulant therapy in patients with substitute heart valves. J Thorac Cardiovasc Surg. 1991;101:427–431. [PubMed] [Google Scholar]
- 18.Horstkotte D, Schulte H, Bircks W, et al. Unexpected findings concerning thromboembolic complications and anticoagulation after complete 10 year follow-up of patients with St. Jude Medical prostheses. J Heart Valve Dis. 1993;2:291–301. [PubMed] [Google Scholar]
- 19.Wilson DB, Dunn MI, Hassanein K. Low-intensity anticoagulation in mechanical cardiac prosthetic valves. Chest. 1991;100:1553–1557. doi: 10.1378/chest.100.6.1553. [DOI] [PubMed] [Google Scholar]
- 20.Bryan AJ, Rogers CA, Bayliss K, et al. Prospective randomized comparison of CarboMedics and St. Jude Medical bileaflet mechanical heart valve prostheses: ten-year follow-up. J Thorac Cardiovasc Surg. 2007;133:614–622. doi: 10.1016/j.jtcvs.2006.08.075. [DOI] [PubMed] [Google Scholar]
- 21.Koertke H, Zittermann A, Tenderich G, et al. Low-dose oral anticoagulation in patients with mechanical heart valve prostheses: final report from the early self-management anticoagulation trial II. Eur Heart J. 2007;28:2479–2484. doi: 10.1093/eurheartj/ehm391. [DOI] [PubMed] [Google Scholar]
- 22.Salem DN, Stein PD, Al-Ahmad A, et al. Antithrombotic therapy in valvular heart disease–native and prosthetic: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126(suppl):457S–482S. doi: 10.1378/chest.126.3_suppl.457S. [DOI] [PubMed] [Google Scholar]
- 23.Vahanian A, Baumgartner H, Bax J, et al. Guidelines on the management of valvular heart disease: the Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology. Eur Heart J. 2007;28:230–268. doi: 10.1093/eurheartj/ehl428. [DOI] [PubMed] [Google Scholar]
- 24.Jamieson WR, Cartier PC, Allard M, et al. Surgical management of valvular heart disease 2004. Can J Cardiol. 2004;20(suppl):7E–120E. [PubMed] [Google Scholar]
- 25.Melton LJ., III History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71:266–274. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 26.Edmunds LH, Jr, Clark RE, Cohn LH, et al. Guidelines for reporting morbidity and mortality after cardiac valvular operations: Ad Hoc Liaison Committee for Standardizing Definitions of Prosthetic Heart Valve Morbidity of The American Association for Thoracic Surgery and The Society of Thoracic Surgeons. J Thorac Cardiovasc Surg. 1996;112:708–711. doi: 10.1016/s0022-5223(96)70055-7. [DOI] [PubMed] [Google Scholar]
- 27.Koertke H, Minami K, Boethig D, et al. INR self-management permits lower anticoagulation levels after mechanical heart valve replacement. Circulation. 2003;108(suppl):II75–II78. doi: 10.1161/01.cir.0000089185.80318.3f. [DOI] [PubMed] [Google Scholar]
- 28.Koertke H, Zittermann A, Minami K, et al. Low-dose INR self-management: a promising tool to achieve low complication rates after mechanical heart valve replacement. Ann Thorac Surg. 2005;79:1909–1914. doi: 10.1016/j.athoracsur.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 29.Fihn SD, McDonell M, Martin D, et al. Risk factors for complications of chronic anticoagulation: a multicenter study; warfarin optimized outpatient follow-up study group. Ann Intern Med. 1993;118:511–520. doi: 10.7326/0003-4819-118-7-199304010-00005. [DOI] [PubMed] [Google Scholar]
- 30.Fihn SD, Callahan CM, Martin DC, et al. The risk for and severity of bleeding complications in elderly patients treated with warfarin: the National Consortium of Anticoagulation Clinics. Ann Intern Med. 1996;124:970–979. doi: 10.7326/0003-4819-124-11-199606010-00004. [DOI] [PubMed] [Google Scholar]
- 31.Rosendaal FR, Cannegieter SC, van der Meer FJ, et al. A method to determine the optimal intensity of oral anticoagulant therapy. Thromb Haemost. 1993;69:236–239. [PubMed] [Google Scholar]
- 32.Brown RD, Whisnant JP, Sicks JD, et al. Stroke incidence, prevalence, and survival: secular trends in Rochester, Minnesota, through 1989. Stroke. 1996;27:373–380. [PubMed] [Google Scholar]
- 33.Butchart EG, Payne N, Li HH, et al. Better anticoagulation control improves survival after valve replacement. J Thorac Cardiovasc Surg. 2002;123:715–723. doi: 10.1067/mtc.2002.121162. [DOI] [PubMed] [Google Scholar]
- 34.Cannegieter SC, Rosendaal FR, Briet E. Thromboembolic and bleeding complications in patients with mechanical heart valve prostheses. Circulation. 1994;89:635–641. doi: 10.1161/01.cir.89.2.635. [DOI] [PubMed] [Google Scholar]
- 35.Bayliss A, Faber P, Dunning J, et al. What is the optimal level of anticoagulation in adult patients receiving warfarin following implantation of a mechanical prosthetic mitral valve? Interact Cardiovasc Thorac Surg. 2007;6:390–396. doi: 10.1510/icvts.2007.152819. [DOI] [PubMed] [Google Scholar]
- 36.van Leeuwen Y, Rosendaal FR, Cannegieter SC. Prediction of haemorrhagic and thrombotic events in patients with mechanical heart valve prostheses treated with oral anticoagulants. J Thromb Haemost. 2008;6:451–456. doi: 10.1111/j.1538-7836.2007.02874.x. [DOI] [PubMed] [Google Scholar]
- 37.Samsa GP, Matchar DB. Relationship between test frequency and outcomes of anticoagulation: a literature review and commentary with implications for the design of randomized trials of patient self-management. J Thromb Thrombolysis. 2000;9:283–292. doi: 10.1023/a:1018778914477. [DOI] [PubMed] [Google Scholar]
- 38.Chiquette E, Amato MG, Bussey HI. Comparison of an anticoagulation clinic with usual medical care: anticoagulation control, patient outcomes, and health care costs. Arch Intern Med. 1998;158:1641–1647. doi: 10.1001/archinte.158.15.1641. [DOI] [PubMed] [Google Scholar]
- 39.Hasenkam JM, Kimose HH, Knudsen L, et al. Self management of oral anticoagulant therapy after heart valve replacement. Eur J Cardiothorac Surg. 1997;11:935–942. doi: 10.1016/s1010-7940(97)01204-9. [DOI] [PubMed] [Google Scholar]
- 40.Ruel M, Masters RG, Rubens FD, et al. Late incidence and determinants of stroke after aortic and mitral valve replacement. Ann Thorac Surg. 2004;78:77–84. doi: 10.1016/j.athoracsur.2003.12.058. [DOI] [PubMed] [Google Scholar]
- 41.Acar J, Enriquez-Sarano M, Farah E, et al. Recurrent systemic embolic events with valve prosthesis. Eur Heart J. 1984;5(suppl):33–38. doi: 10.1093/eurheartj/5.suppl_d.33. [DOI] [PubMed] [Google Scholar]
- 42.Stein PD, Alpert JS, Copeland J, et al. Antithrombotic therapy in patients with mechanical and biological prosthetic heart valves. Chest. 1995;108(suppl):371S–379S. doi: 10.1378/chest.108.4_supplement.371s. [DOI] [PubMed] [Google Scholar]
- 43.Godje OL, Fischlein T, Adelhard K, et al. Thirty-year results of Starr-Edwards prostheses in the aortic and mitral position. Ann Thorac Surg. 1997;63:613–619. doi: 10.1016/s0003-4975(96)00945-9. [DOI] [PubMed] [Google Scholar]
- 44.Kafsi N, Trabelsi S, Acar P, et al. Comparison of long-term results of the Starr and Saint-Jude mitral valve prostheses. Arch Mal Coeur Vaiss. 1991;84:1523–1527. [PubMed] [Google Scholar]
- 45.Macmanus Q, Grunkemeier GL, Lambert LE, et al. Non-cloth-covered caged-ball prostheses: the second decade. J Thorac Cardiovasc Surg. 1978;76:788–794. [PubMed] [Google Scholar]
- 46.John S, Ravikumar E, John CN, et al. 25-year experience with 456 combined mitral and aortic valve replacement for rheumatic heart disease. Ann Thorac Surg. 2000;69:1167–1172. doi: 10.1016/s0003-4975(99)01559-3. [DOI] [PubMed] [Google Scholar]