Introduction
In the present edition of Diabetologia, Nachnani and colleagues report that rats treated with the glucagon-like-peptide-1 (GLP-1) mimetic, exendin-4, for 75 days developed low-grade pancreatitis [1]. The obvious question is whether low-grade pancreatitis is also present in humans treated with synthetic exendin-4 (exenatide) or other GLP-1-based therapies? The paper by Nachnani et al. is a timely addition to an area of growing interest and controversy in which, at present, there are more questions than answers.
Pancreatitis induced by GLP-1 mimetic therapy: the case against
The US Food and Drug Administration (FDA) raised concerns over a possible association between treatment with exenatide and subsequent onset of acute pancreatitis in 2008 [2]. The manufacturer of exenatide, the Amylin Corporation, countered with two lines of argument [2].
First, they argued, there is no plausible biological mechanism by which the GLP-1 mimetic, exenatide, would induce pancreatitis. Given the paucity of experimental data available on GLP-1 actions on the exocrine pancreas at that time, the argument was a self-fulfilling prophecy. The paper by Nachnani and colleagues [1] undermines that argument.
Second, Amylin argued that since pancreatitis is more common in people with type 2 diabetes, the reported pancreatitis following therapy with exenatide was ‘guilt by association’ rather than a consequence of the therapy. This argument has been bolstered by a report sponsored by Lilly and the Amylin Corporation, which concludes that the incidence of pancreatitis in type 2 diabetes patients is comparable with the reported incidence of pancreatitis as an adverse event in patients treated with exenatide [3]. In an alternative approach, also sponsored by Amylin Pharmaceuticals, health insurance billing patterns in the USA revealed no association between billing for diabetes management with exenatide or with sitagliptin and subsequent billing for management of pancreatitis [4]. The second argument cannot be fully supported by the studies cited. First, adverse event reporting always greatly underestimates the frequency of events. Second, the insurance claims approach is indirect and provides no guarantee that patients who were prescribed and picked up a prescription for a given medication actually took that medication. The attrition rate for patients starting exenatide therapy is relatively high.
A study in mice might offer some reassurance that GLP-1-based therapy does not induce or exacerbate pancreatitis [5]. Koehler and colleagues treated C57Bl/6 J mice with exendin-4 (10 nmol/kg twice daily for 7 days) and then induced pancreatitis with caerulein. In another group of mice, the protocol was reversed, with caerulein preceding exendin-4 treatment at the same dose for up to 6 days. GLP-1 receptor activation increased exocrine pancreas mass and induced genes associated with cellular proliferation, but had no effect on chemically induced pancreatitis. In other studies in the same report, mRNA expression of a variety of genes was quantified in pancreas of mice on a high-fat diet followed by streptozotocin-induced diabetes treated with exenatide (3 nmol/kg) or sitagliptin (370 mg/kg/day). Transcription of genes considered important in the induction of acute pancreatitis was not increased by either of the GLP-1-based therapies. While the data of Koehler et al. are a welcome addition to the sparse information on potential actions of GLP-1 on the exocrine pancreas, the study was limited in three ways: (1) a relatively small number of animals was studied in each experiment (n ∼ 5); (2) GLP-1 mimetic therapy was of short duration; and (3) pancreatic histology was not reported.
Pancreatitis induced by GLP-1 mimetic therapy: the case for
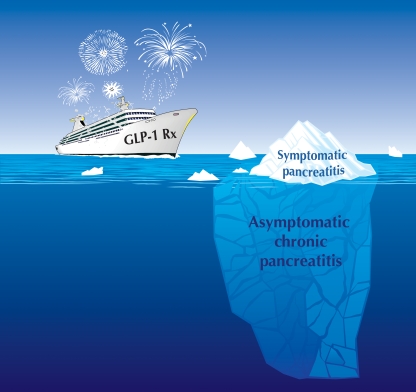
The FDA has now also announced that more than 80 cases of pancreatitis have been reported as adverse events in relation to treatment with sitagliptin and have introduced a label change to reflect this risk [6]. The number both of published reports of pancreatitis following treatment with exenatide and of cases reported to the FDA as adverse events is increasing [7–9]. This could be a consequence of increased awareness of the potential link. Prospective randomised trials are a more appropriate way to establish whether pancreatitis is indeed increased in connection with GLP-1-based therapy. In one such study, the incidence of pancreatitis was increased in patients with type 2 diabetes treated with the GLP-1 analogue liraglutide [10]. However, the incidence of symptomatic acute pancreatitis is relatively low in patients treated with exenatide or liraglutide [10]. Is this because the adverse effects (if any) of GLP-1 agonist therapy on the exocrine pancreas are a rare idiosyncratic event? Or, alternatively, is it because symptomatic pancreatitis in patients treated with GLP-1-based therapy is the visible tip of an iceberg, with varying degrees of more subtle asymptomatic changes in the exocrine pancreas being more commonly present (Fig. 1)?
Fig. 1.
The occasional acute episode of pancreatitis in some individuals treated with GLP-1-based therapy along with the suggestion from animal studies [1, 11] suggest that asymptomatic chronic pancreatitis may not be an uncommon consequence of GLP-1 based therapy. Rx, treatment
The report in the current issue of Diabetologia provides some insight in that regard [1]. Ten non-diabetic Sprague–Dawley male rats were treated from 8 weeks of age with daily injections of exendin-4 (10 μg/kg) for 75 days. The dose used exceeded that used in humans (10–20 μg per day). As expected, treated rats had lower circulating insulin levels with comparable glucose levels and decreased body weight, presumably due to less fat (leptin was lower). Evidence of chronic pancreatitis is presented histologically (loss of usual acinar structure, irregularly shaped and sized acinar cells, inflammation and fibrosis) and by increased circulating lipase (but not amylase) levels.
Given that numerous animal toxicology studies were presumably undertaken for exenatide to obtain approval from the FDA, why was pancreatitis not picked up in those studies? One possibility is that the low-grade pancreatitis detected and characterised in the present studies may have been insufficient to be noted in animal toxicology studies. The animals were apparently healthy despite the low-grade pancreatitis and macroscopic abnormalities of the pancreas may not have been visible at necropsy.
We also observed unexpected changes in the exocrine pancreas of 8-week-old Sprague–Dawley rats transgenic for human islet amyloid polypeptide (HIP rats) when on a high-fat diet and treated for 12 weeks with the dipeptidyl peptidase-4 inhibitor sitagliptin, 200 mg kg−1 day−1 [11]. One sitagliptin-treated rat had regional haemorrhagic pancreatitis. Others had acinar to ductal metaplasia; and all had increased ductal replication, both characteristics of chronic pancreatitis. As in the study presented in the current edition of Diabetologia [1], we did not observe behavioural changes suggestive of symptomatic pain in the treated rats. Food intake and growth were also normal. Again, it is unlikely that those changes would have been detected by routine toxicology screening, which does not include measures of cell turnover or screen for subtle changes such as ductal metaplasia.
These studies [1, 11] raise the possibility that GLP-1-based therapy by injection of GLP-1 agonist or use of a dipeptidyl peptidase-4 inhibitor to enhance endogenous GLP-1 levels could induce undetected low-grade asymptomatic chronic pancreatitis more commonly than the rare events of symptomatic pancreatitis. To further consider how GLP-1-based therapy might induce chronic pancreatitis, we first briefly consider the pathophysiology of chronic pancreatitis.
Pathophysiology of chronic pancreatitis?
Pancreas originates as ventral and dorsal buds of the primitive gut endoderm, which fuse to form the pancreas. Subsequently, through the actions of sequential programmes of development, definitive cell types arise. The pancreatic epithelium is marked by production of transcription factor pancreatic and duodenal homeobox 1 (PDX1; also referred to as insulin upstream factor 1 [IPF1]) and lineage tracing shows that all mature cell types in the pancreas originate from these PDX1-producing epithelial cells [12]. The branching tips of the growing pancreatic epithelium form domains of multipotent progenitor cells that give rise to differentiated cells. At mid-gestation, these branching tips undergo a developmental switch that converts them into exocrine cells. Endocrine cells emerge from duct-like structures that are centrally located within the trunk of the pancreatic epithelium and migrate to form islets. The regulation of cell proliferation of progenitor cells and differentiation of cell fates is coordinated by Notch homologue (NOTCH) signalling through activation of hairy and enhancer of split 1 [13]. The canonical wingless-type MMTV integration site family (WNT) pathway is essential for proliferation of pancreatic epithelium [14] and conditional disruption of pancreatic beta-catenin production, a downstream target of WNT signalling, resulted in pancreatic hypoplasia [15]. Gain of function experiments in pancreatic islets have shown that WNT signalling can promote islet proliferation [16]. Stabilisation of beta-catenin results in the formation of large pancreatic tumours [17].
The adult exocrine pancreas consists of pyramid-shaped acinar cells surrounding an acinus, into which the exocrine secretions are discharged. These secretions are then conveyed by small pancreatic ducts through the pancreatic ductal tree, eventually reaching the duodenum. Given the potent capacity for misdirected pancreatic enzymes to autodigest adjacent tissue, the integrity of the relationship between the acini and their attendant ducts is important [18].
Factors known to increase the risk of pancreatitis include excessive alcohol consumption, viral infection, obesity and diabetes [3, 19–21]. An important genetic component that enhances predisposition for the development of pancreatitis is emerging. Known predisposing genetic factors for pancreatitis can be broadly divided into those that enhance or prolong the activity of trypsin [22, 23], or those that increase the viscosity and impede the flow of ductal secretions [24]. Thus primacy to initiate pancreatitis can be attributed to either duct or acinar cells. If the microanatomy of the acinar–ductal relationship is disturbed, the basis for longer standing chronic pancreatitis is established.
In chronic pancreatitis increased ductal replication is a consistent feature [19–21]. It has been suggested that this represents attempted pancreas regeneration by recapitulating the original formation of pancreatic acini in relation to branching pancreatic ducts [25]. The concept that local damage and inflammation induces recruitment of somatic stem cells to permit repair was established in the transected flatworm [26] and is likely to be a general phenomenon. Increased ductal replication has also been proposed as contributing to chronic pancreatitis by helping distort and obstruct small pancreatic ducts [19–21].
In conclusion, pancreatitis may be initiated at the level of acinar cells or duct cells. Once the microanatomy of the acinar–ductal system has been disturbed, aberrant delivery of pancreatic enzymes leads to local inflammation and increased acinar cell death. The cytokines released from the latter are likely to be the signals that induce chronic increased ductal proliferation, which in turn leads to distorted ducts, obstructed flow and sustained chronic pancreatitis. How does the increased risk of chronic pancreatitis in patients with obesity and/or type 2 diabetes, and perhaps in those on GLP-1 therapy fit into this model?
Chronic pancreatitis in patients with diabetes and on GLP-1 therapy: ductal hyperplasia as a common precursor?
Pancreatic ductal replication is increased in humans with obesity and/or type 2 diabetes [27], providing a possible link between the increased risk of pancreatitis in individuals with obesity and/or type 2 diabetes.
The mechanisms that induce increased pancreatic ductal replication in individuals with obesity and/or type 2 diabetes are unknown. Excessive fat accumulation in pancreas could induce local inflammation [27]. Increased beta cell apoptosis in type 2 diabetes is also associated with inflammation [28] and may, through local cytokines, foster attempted islet regeneration via duct-related progenitors, comparable to the process proposed for acinar tissue in chronic pancreatitis. GLP-1 therapy has also been proposed to activate regenerative efforts in relation to pancreatic ducts, with increased duct cells positive for PDX-1 [29].
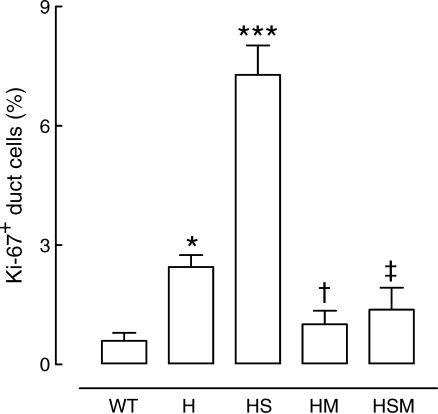
In common with humans with obesity and type 2 diabetes, pancreatic ductal replication was increased in the HIP rat model of type 2 diabetes (Fig. 2) [11]. Moreover, the increase in ductal replication in this model is amplified with sitagliptin therapy [11]. It is unknown if GLP-1 therapy also amplifies the increased ductal replication rates present in humans with obesity and type 2 diabetes. If so, this would be a potential mechanism to increase the risk of chronic pancreatitis by distortion of small pancreatic ducts.
Fig. 2.
Quantification of ductal cell replication in wild-type (WT) and HIP (H) rats, and in HIP rats treated with sitagliptin (HS), metformin (HM) or a combination of both (HSM) for 12 weeks. *p < 0.05 for H vs WT; ***p < 0.001 for HS vs all groups; †p < 0.05 for HM vs H and HS; ‡p < 0.001 for HSM vs HS. (Reprinted with permission of the American Diabetes Association, Inc., © 2009 from Matveyenko et al. [11])
GLP-1-based therapy may also induce pancreatitis by its known actions of altering enzyme secretion [30] and/or of promoting replication of acinar cells, thereby disturbing the acinar architecture. Interestingly, Nachnani and colleagues in the present edition of Diabetologia noted that acinar cells were abnormal in appearance and had a greater frequency of cell death [1]. In the shorter term studies of Koehler and colleagues, GLP-1 mimetic therapy led to an increase in pancreas size, implying increased cell formation [5].
Thus, while it remains unknown exactly how GLP-1 mimetic therapy might induce pancreatitis, plausible hypotheses as to how it might be mediated already exist and are worthy of further investigation.
Does low-grade pancreatitis matter?
There would indeed be grounds for concern if GLP-1 mimetic therapy were found to lead to chronic asymptomatic low-grade pancreatitis. Low-grade chronic pancreatitis is a well established risk factor for pancreatic adenocarcinoma [31, 32], a disease that is invariably diagnosed late with a 5 year life expectancy of less than 10%. Increased ductal replication, most frequently due to chronic pancreatitis, is considered to be the first stage in a progression of morphological stages that may lead to pancreatic adenocarcinoma [32]. The next stage is acinar to ductal metaplasia, observed in HIP rats treated for 12 weeks with sitagliptin [11]. With time, the increased ductal replication may lead to intraductal neoplasia, with progression thereafter to pancreatic cancer (the progression being classified by the advancing morphological stages known as pancreatic intra-epithelial neoplasias [PanIns]) [31].
Fortunately, the majority of individuals with chronic pancreatitis and increased ductal replication do not progress to pancreatic cancer. Both germ line and somatic mutations have been identified that predispose to pancreatic cancer [32]. However, these mutations do not seem to be required for the increased risk of pancreatic cancer seen in individuals with type 2 diabetes [33, 34]. The risk of chronic pancreatitis progressing to pancreatic adenocarcinoma increases with duration of pancreatitis. The cumulative risk for patients with hereditary pancreatitis of developing pancreatic cancer was reported as 10% at age 50 years, 19% at age 60 years and 54% at 75 years [35].
In conclusion, if GLP-1 mimetic therapy does induce low-grade pancreatitis superimposed on that present as a result of obesity and/or type 2 diabetes, then there would be grounds for concern that prolonged exposure to this class of drugs may lead to increased risk of pancreatic adenocarcinoma. While GLP-1-based drugs offer some advantages as therapies, no advantages could outweigh even a modestly increased risk of developing pancreatic adenocarcinoma. Moreover, metformin therapy decreases the risk of pancreatic cancer [36]. Indeed, several mechanisms have been proposed by which metformin may decrease the incidence of several cancers [37]. Intriguingly, metformin counters the actions by which sitagliptin amplifies pancreatic ductal replication in HIP rats (Fig. 2) [11]. On that basis, until more information is available on the effects of GLP-1-based therapy on the exocrine pancreas in humans, it would be prudent to use GLP-1 mimetic therapy only in addition to metformin.
Where to go from here?
The limitations of post-marketing adverse event reporting are well known. They have recently been highlighted by the debate on the risks of cancer in connection with use of insulin glargine (A21Gly,B31Arg,B32Arg human insulin) [34]. To make matters worse, the submerged part of the iceberg, asymptomatic pancreatitis (Fig. 1), is undetectable in clinical studies. One strategy is to wait until there is a clear sign of increased pancreatic adenocarcinoma in connection with GLP-1-based treatment, the usual approach of regulatory authorities.
Is that a wise strategy? How, for example, would we detect that signal with current post-marketing surveillance? Pancreatic cancer is already increased in type 2 diabetes and would predictably be underreported. Post-marketing databases maintained by pharmaceutical companies report rates of cancers far below those of the general population, so are of little value in tracking such an association. We agree with the recent proposal that new mechanisms are needed to track outcomes, expected and unexpected, in patients with diabetes on new and older therapies [34]. To avoid conflict, these data bases should be managed independently of the companies marketing the treatments.
Clearly we also need more information on the actions of GLP-1-based treatment on the exocrine pancreas and possibly on other cell types such as the C-cells of the thyroid gland, which also appeared to show increased tumour formation in animal testing of liraglutide [38]. The paper by Nachnani in the current edition of Diabetologia [1] is a welcome addition to these as yet limited data [31].
Acknowledgments
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Abbreviations
- GLP-1
Glucagon-like-peptide-1
- PDX1
Pancreatic and duodenal homeobox 1
- WNT
Wingless-type MMTV integration site family
References
- 1.Nachnani JS, Bulchandani DG, Nookala A, et al. Biochemical and histological effects of exendin-4 (exenatide) on the rat pancreas. Diabetologia. 2009 doi: 10.1007/s00125-009-1515-4. [DOI] [PubMed] [Google Scholar]
- 2.Cure P, Pileggi A, Alejandro R. Exenatide and rare adverse events. N Engl J Med. 2008;358:1969–1970. doi: 10.1056/NEJMc0707137. [DOI] [PubMed] [Google Scholar]
- 3.Noel RA, Braun DK, Patterson RE, Bloomgren GL. Increased risk of acute pancreatitis and biliary disease observed in patients with type 2 diabetes: a retrospective cohort study. Diabetes Care. 2009;32:834–838. doi: 10.2337/dc08-1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dore DD, Seeger JD, Arnold Chan K. Use of a claims-based active drug safety surveillance system to assess the risk of acute pancreatitis with exenatide or sitagliptin compared to metformin or glyburide. Curr Med Res Opin. 2009;25:1019–1027. doi: 10.1185/03007990902820519. [DOI] [PubMed] [Google Scholar]
- 5.Koehler JA, Baggio LL, Lamont BJ, Ali S, Drucker DJ. Glucagon-like peptide-1 receptor activation modulates pancreatitis-associated gene expression but does not modify the susceptibility to experimental pancreatitis in mice. Diabetes. 2009;58:2148–2161. doi: 10.2337/db09-0626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.US Food and Drug Administration, US Department of Health & Human Services (2009) Sitagliptin (marketed as Januvia and Janumet)—acute pancreatitis. Available from www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm183800.htm, accessed 12 October 2009
- 7.Denker PS, Dimarco PE. Exenatide (exendin-4)-induced pancreatitis: a case report. Diabetes Care. 2006;29:471. doi: 10.2337/diacare.29.02.06.dc05-2043. [DOI] [PubMed] [Google Scholar]
- 8.Tripathy NR, Basha S, Jain R, Shetty S, Ramachandran A. Exenatide and acute pancreatitis. J Assoc Physicians India. 2008;56:987–988. [PubMed] [Google Scholar]
- 9.Ayoub WA, Kumar AA, Naguib HS, Taylor HC (2009) Exenatide induced acute pancreatitis. Endocr Pract. doi:10.4158/EP09104.CRR [DOI] [PubMed]
- 10.Buse JB, Rosenstock J, Sesti G, et al. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6) Lancet. 2009;374:39–47. doi: 10.1016/S0140-6736(09)60659-0. [DOI] [PubMed] [Google Scholar]
- 11.Matveyenko AV, Dry S, Cox HI, et al. Beneficial endocrine but adverse exocrine effects of Sitagliptin in the HIP rat model of type 2 diabetes, interactions with metformin. Diabetes. 2009;58:1604–1615. doi: 10.2337/db09-0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herrera PL. Adult insulin- and glucagon-producing cells differentiate from two independent cell lineages. Development. 2000;127:2317–2322. doi: 10.1242/dev.127.11.2317. [DOI] [PubMed] [Google Scholar]
- 13.Apelqvist A, Li H, Sommer L, Beatus P, et al. Notch signalling controls pancreatic cell differentiation. Nature. 1999;400:877–881. doi: 10.1038/23716. [DOI] [PubMed] [Google Scholar]
- 14.Papadopoulou S, Edlund H. Attenuated Wnt signaling perturbs pancreatic growth but not pancreatic function. Diabetes. 2005;54:2844–2851. doi: 10.2337/diabetes.54.10.2844. [DOI] [PubMed] [Google Scholar]
- 15.Wells JM, Esni F, Boivin GP, et al. Wnt/beta-catenin signaling is required for development of the exocrine pancreas. BMC Dev Biol. 2007;7:4. doi: 10.1186/1471-213X-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rulifson IC, Karnik SK, Heiser PW, et al. Wnt signaling regulates pancreatic beta cell proliferation. Proc Natl Acad Sci U S A. 2007;104:6247–6252. doi: 10.1073/pnas.0701509104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heiser PW, Cano DA, Landsman L, et al. Stabilization of beta-catenin induces pancreas tumor formation. Gastroenterology. 2008;135:1288–1300. doi: 10.1053/j.gastro.2008.06.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gaisano HY, Gorelick FS. New insights into the mechanisms of pancreatitis. Gastroenterology. 2009;136:2040–2044. doi: 10.1053/j.gastro.2009.04.023. [DOI] [PubMed] [Google Scholar]
- 19.Bhanot UK, Moller P. Mechanisms of parenchymal injury and signaling pathways in ectatic ducts of chronic pancreatitis: implications for pancreatic carcinogenesis. Lab Invest. 2009;89:489–497. doi: 10.1038/labinvest.2009.19. [DOI] [PubMed] [Google Scholar]
- 20.Whitcomb DC. Mechanisms of disease: advances in understanding the mechanisms leading to chronic pancreatitis. Nat Clin Pract Gastroenterol Hepatol. 2004;1:46–52. doi: 10.1038/ncpgasthep0025. [DOI] [PubMed] [Google Scholar]
- 21.Stevens T, Conwell DL, Zuccaro G. Pathogenesis of chronic pancreatitis: an evidence-based review of past theories and recent developments. Am J Gastroenterol. 2004;99:2256–2270. doi: 10.1111/j.1572-0241.2004.40694.x. [DOI] [PubMed] [Google Scholar]
- 22.Whitcomb DC, Gorry MC, Preston RA, et al. Hereditary pancreatitis is caused by a mutation in the cationic trypsinogen gene. Nat Genet. 1996;14:141–145. doi: 10.1038/ng1096-141. [DOI] [PubMed] [Google Scholar]
- 23.Witt H, Luck W, Hennies HC, et al. Mutations in the gene encoding the serine protease inhibitor, Kazal type 1 are associated with chronic pancreatitis. Nat Genet. 2000;25:213–216. doi: 10.1038/76088. [DOI] [PubMed] [Google Scholar]
- 24.Sharer N, Schwarz M, Malone G, et al. Mutations of the cystic fibrosis gene in patients with chronic pancreatitis. N Engl J Med. 1998;339:645–652. doi: 10.1056/NEJM199809033391001. [DOI] [PubMed] [Google Scholar]
- 25.Taguchi M, Yamaguchi T, Otsuki M. Induction of PDX-1-positive cells in the main duct during regeneration after acute necrotizing pancreatitis in rats. J Pathol. 2002;197:638–646. doi: 10.1002/path.1134. [DOI] [PubMed] [Google Scholar]
- 26.Pellettieri J, Fitzgerald P, Watanabe S, Mancuso J, Green DR, Alvarado AS. Cell death and tissue remodeling in planarian regeneration. Dev Biol. 2009 doi: 10.1016/j.ydbio.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Butler AE, Galasso R, Matveyenko A, Rizza RA, Dry S, Butler PC. Pancreatic duct replication is increased with obesity and type 2 diabetes in humans. Diabetologia doi:10.1007/s00125-009-1556-8 [DOI] [PMC free article] [PubMed]
- 28.Ehses JA, Perren A, Eppler E, et al. Increased number of islet-associated macrophages in type 2 diabetes. Diabetes. 2007;56:2356–2370. doi: 10.2337/db06-1650. [DOI] [PubMed] [Google Scholar]
- 29.Stoffers DA, Kieffer TJ, Hussain MA, et al. Insulinotropic glucagon-like peptide 1 agonists stimulate expression of homeodomain protein IDX-1 and increase islet size in mouse pancreas. Diabetes. 2000;49:741–748. doi: 10.2337/diabetes.49.5.741. [DOI] [PubMed] [Google Scholar]
- 30.Wettergren A, Wojdemann M, Holst JJ. Glucagon-like peptide-1 inhibits gastropancreatic function by inhibiting central parasympathetic outflow. Am J Physiol. 1998;275:G984–G992. doi: 10.1152/ajpgi.1998.275.5.G984. [DOI] [PubMed] [Google Scholar]
- 31.Landi S. Genetic predisposition and environmental risk factors to pancreatic cancer: a review of the literature. Mutat Res. 2009;681:299–307. doi: 10.1016/j.mrrev.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 32.Jura N, Archer H, Bar-Sagi D. Chronic pancreatitis, pancreatic adenocarcinoma and the black box in-between. Cell Res. 2005;15:72–77. doi: 10.1038/sj.cr.7290269. [DOI] [PubMed] [Google Scholar]
- 33.Crous-Bou M, Porta M, Morales E, et al. Past medical conditions and K-ras mutations in pancreatic ductal adenocarcinoma: a hypothesis-generating study. Cancer Causes Control. 2009;20:591–599. doi: 10.1007/s10552-008-9267-x. [DOI] [PubMed] [Google Scholar]
- 34.Gale EA. Collateral damage: the conundrum of drug safety. Diabetologia. 2009;52:1975–1982. doi: 10.1007/s00125-009-1491-8. [DOI] [PubMed] [Google Scholar]
- 35.Rebours V, Boutron-Ruault MC, Schnee M, et al. The natural history of hereditary pancreatitis: a national series. Gut. 2009;58:97–103. doi: 10.1136/gut.2008.149179. [DOI] [PubMed] [Google Scholar]
- 36.Currie CJ, Poole CD, Gale EA. The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetologia. 2009;52:1766–1777. doi: 10.1007/s00125-009-1440-6. [DOI] [PubMed] [Google Scholar]
- 37.Yang YX. Do diabetes drugs modify the risk of pancreatic cancer? Gastroenterology. 2009;137:412–415. doi: 10.1053/j.gastro.2009.06.022. [DOI] [PubMed] [Google Scholar]
- 38.US Food and Drug Administration (2009) Liraglutide briefing materials. Available from www.fda.gov/ohrms/dockets/ac/09/briefing/2009-4422b2-01-FDA.pdf, accessed 12 October 2009