Abstract
The ADAMTS (a disintegrin and metalloproteinase domain with thrombospondin motifs) family includes 19 secreted proteinases in man. ADAMTS16 is a recently cloned gene expressed at high levels in fetal lung and kidney and adult brain and ovary. The ADAMTS-16 protein currently has no known function. ADAMTS16 is also expressed in human cartilage and synovium where its expression is increased in tissues from osteoarthritis patients compared to normal tissues. In this study, we ascertained that the full length ADAMTS16 mRNA was expressed in chondrocytes and cloned the appropriate cDNA. Stable over-expression of ADAMTS16 in chondrosarcoma cells led to a decrease in cell proliferation and migration, though not adhesion, as well as a decrease in the expression of matrix metalloproteinase-13 (MMP13). The transcription start point of the human ADAMTS16 gene was experimentally identified as 138 bp upstream of the translation start ATG and the basal promoter was mapped out to − 1802 bp. Overexpression of Egr1 induced ADAMTS16 promoter constructs of − 157/+138 or longer whilst Sp1 induced all ADAMTS16 promoter constructs. Transforming growth factor beta (TGFβ) stimulated expression of endogenous ADAMTS16 gene expression in chondrocyte cell lines.
Abbreviations: ADAMTS, a disintegrin and metalloproteinase domain with thrombospondin motif; MMP, matrix metalloproteinase; RACE, rapid amplification of cDNA ends; TGFβ, transforming growth factor beta; TIMP, tissue inhibitor of metalloproteinases
Keywords: ADAMTS, Metalloproteinase, Chondrocyte, Cartilage, Promoter, Transcription
1. Introduction
The ADAMTS (a disintegrin and metalloproteinase domain with thrombospondin motifs) family includes 19 secreted proteinases in man. These enzymes have a complex domain structure consisting of at least a signal peptide, pro domain, metalloproteinase domain, disintegrin domain, thrombospondin type I motif and cysteine rich domain (Porter et al., 2005). Phylogenetically, the enzyme family separates into eight clades which to some extent correlate with function where assigned (Huxley-Jones et al., 2005). ADAMTS-1, -4, -5, -8, -9 and -15 are all capable of degrading aggrecan at a specific set of loci with ADAMTS-5 being the major aggrecanase in cartilage destruction, at least in mice (Clark and Parker, 2003; Collins-Racie et al., 2004; Glasson et al., 2005; Stanton et al., 2005). At least three ADAMTS enzymes (ADAMTS-4, -7 and -12) can degrade cartilage oligomeric matrix protein, another component of cartilage ECM (Dickinson et al., 2003; Liu et al., 2006a,b). Three ADAMTS enzymes, ADAMTS-2, -3 and -14, are procollagen N-propeptidases (PCNPs), which have roles in collagen biosynthesis. ADAMTS-2 acts preferentially on type I collagen and ADAMTS-3 on type II collagen, whereas the preference of ADAMTS-14 is currently unknown (Colige et al., 2002). Mutation of the ADAMTS2 gene causes Ehlers–Danlos syndrome Type VIIC in man (Colige et al., 1999). ADAMTS-13 cleaves von Willebrand factor with mutations in this enzyme leading to an inherited thrombotic thrombocytopenia (Tsai, 2007). A form of Weill–Marchesani syndrome is caused by mutation in the ADAMTS10 gene (Dagoneau et al., 2004).
ADAMTS-16 is a recently described member of the ADAMTS gene family (Porter et al., 2005). The cDNA was cloned using a combination of bioinformatics and degenerate RT-PCR, which also identified ADAMTS-13, -14, -15, -17, -18 and -19 (Cal et al., 2002). Amino acid sequence alignment showed a significant percentage of identity between ADAMTS-16 and ADAMTS-18 (overall identity, 57%), particularly in the catalytic domain where identity reaches 85% (Porter et al., 2005). Indeed, the zinc-binding motif is identical between these two proteinases (HESGHNFGMIHD) (Somerville et al., 2003a). ADAMTS-16 and -18 form a phylogenetic clade, with the nearest evolutionary neighbours being ADAMTS-6, -7, -10 and -12.
Whilst the substrates for ADAMTS-16 are currently unknown, a recombinant truncated form of ADAMTS-16 shows weak aggrecanase activity (Zeng et al., 2006) and full length recombinant ADAMTS-16 is capable of cleaving the proteinase inhibitor α2-macroglobulin (Gao et al., 2007).
An initial expression analysis in a selection of human tissues shows high expression of ADAMTS16 mRNA in fetal lung and kidney, and adult brain and ovary (Cal et al., 2002). In this latter tissue, ADAMTS16 is expressed predominantly in the parietal granulosa cells of pre-ovulatory follicles. Expression of the gene can be induced by follicle-stimulating hormone and forskolin in granulosa cells, suggesting that the cAMP pathway may be involved in its regulation in this system (Gao et al., 2007). ADAMTS16 has also recently been genetically linked to inherited hypertension (Joe et al., 2009).
We undertook the expression profiling of all ADAMTS genes in cartilage and synovium. We compared expression in patients undergoing hip replacement for osteoarthritis (OA) to phenotypically normal tissues from patients undergoing hip replacement following fracture to the neck of femur. In cartilage, the expression of ADAMTS16 increased in the OA samples with a significance of p < 0.001, comparable to the increase in expression of MMP13, a collagenase whose activity is pathognomic with cartilage destruction in OA. This was backed up with a preliminary study in the knee using cartilage from OA patients compared to normal cartilage from post-mortem, where a similar increase in expression of these two genes was measured (Kevorkian et al., 2004). Similarly, in synovium, the expression of ADAMTS16 was significantly increased in OA with the absolute level of ADAMTS16 mRNA approximately 10-fold higher in synovium than in cartilage (Davidson et al., 2006).
In order to provide some insight into the regulation and function of ADAMTS-16 in the joint, the current study examines the expression of ADAMTS-16 by chondrocytes, the regulation of the gene and the consequence of stable over-expression of the gene in chondrosarcoma and immortalized chondrocyte cell lines.
2. Results
2.1. Characterisation of ADAMTS16 and creation of stably expressing cell lines
Using PCR primer pairs to amplify overlapping sections of the ADAMTS16 gene, the entire transcript was amplified from cDNA reverse transcribed from mRNA purified from an immortalized human chondrocyte cell line, C28/I2 (Loeser et al., 2000) (data not shown). No evidence for expression of splice variants was found (though it is possible that some truncated forms of the transcript might not have been detected if they were not amplified at all by selected primers). Based on these data, a full length cDNA was cloned and the final cDNA verified by sequencing in both directions. It should be noted that, despite using high fidelity polymerases and low numbers of amplifications cycles, frequent mutations were introduced by the PCR. The correct version of ADAMTS16 could only be created by amplifying sections of the gene (up to approximately 1000 bp), verifying sequence, then assembling via restriction digestion and ligation into an error-free cDNA.
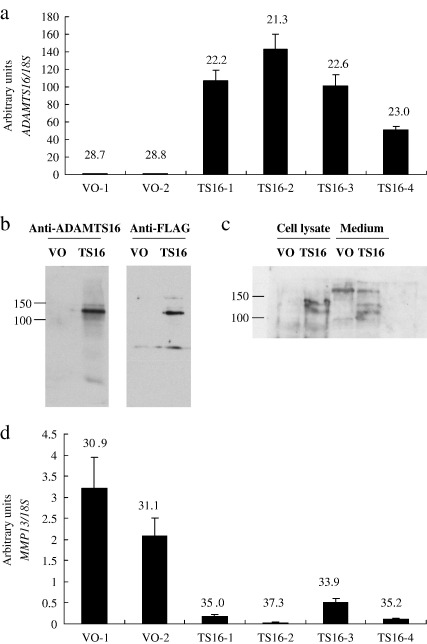
Initially, both immortalized chondrocytes (C28/I2) and chondrosarcoma (SW1353) cells were transfected with the full length ADAMTS16 construct (with a C-terminal FLAG tag). However, the C28/I2 transfectants did not grow robustly after selection and experiments were pursued in the SW1353 line alone. Following cloning by limiting dilution, four different clonal populations expressing ADAMTS16 (TS16) were compared to two clonal populations transfected with vector only control constructs (VO). Expression of ADAMTS16 at the mRNA level was confirmed using qRT-PCR (Fig. 1a). The ADAMTS16 expressing clones had an average of approximately 95-fold higher expression than the vector only controls. Western blot using both an anti-ADAMTS-16 antibody and an anti-FLAG antibody showed expression of a high molecular weight (~ 130 kDa) protein predominantly in the extracellular matrix fraction of the ADAMTS16 transfectants (Fig. 1b). Two bands of similar size were also detected in the conditioned medium and cell lysate fractions (Fig. 1c).
Fig. 1.
Stable over-expression of ADAMTS16 in SW1353 cells. SW1353 cells were stably transfected with either vector only (VO) or ADAMTS16 (TS16) expression constructs. Cells were harvested and total RNA isolated and subjected to qRT-PCR for expression of (a) ADAMTS16 or (d) MMP13. Data are normalized to 18 S and expressed as mean ± s.e.m. Mean threshold cycle (Ct) is given above each bar. Extracellular matrix (b) or cell lysate and conditioned medium (c) were harvested (VO vs. TS16) and subjected to western blot analysis using anti-ADAMTS-16 and/or anti-FLAG primary antibodies.
2.2. Influence of ADAMTS-16 on expression levels of other metzincin genes
Expression levels of all 19 members of the ADAMTS family, all four members of the TIMP gene family and MMP2, MMP9, MMP13 and MMP28 were determined in cells stably over-expressing ADAMTS16 compared to vector only controls using qRT-PCR. The representatives of the MMP family were chosen because of their expression pattern in tissues of the OA joint (Davidson et al., 2006; Kevorkian et al., 2004). MMP13 expression levels were significantly reduced in ADAMTS16-expressing clones compared to vector only controls (p < 0.005; Fig. 1d) with a mean of approximately 9-fold lower expression. Interestingly, the level of MMP13 expression was inversely correlated with that of ADAMTS16 (compare Fig. 1a and d). Expression levels of the other genes analysed did not change significantly between over-expressing cells and vector only controls (data not shown).
2.3. Effects of ADAMTS16 expression on cell phenotype
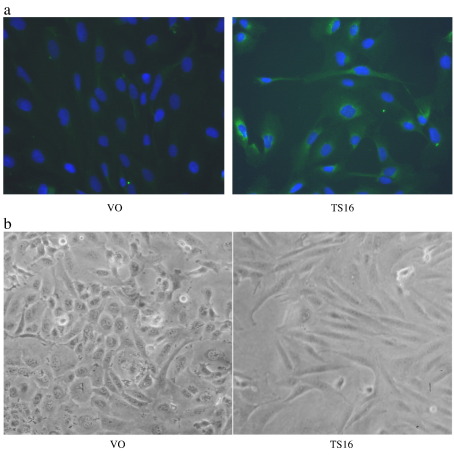
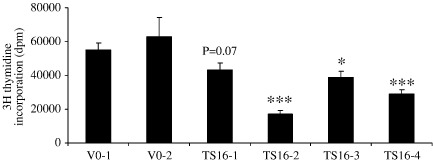
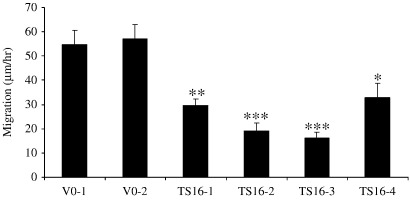
Expression of ADAMTS-16 by stably expressing clones was confirmed by immunocytochemistry using anti-FLAG antibodies (Fig. 2a). Protein was detected only in permeabilized cells, with cytoplasmic and some perinuclear staining. No staining was observed in vector only controls or non-immune IgG controls. Cells stably over-expressing ADAMTS16 were larger and more elongated than vector only controls (Fig. 2b) which were not significantly different from non-transfected SW1353 cells. These differences were observed at all stages of confluence. The ability of cells to adhere to extracellular matrix proteins (collagen I, collagen II, fibronectin and vitronectin) was measured and no significant difference was observed between ADAMTS16 over-expressing cells and vector only controls (data not shown). Cell proliferation was measured by uptake of tritiated thymidine, a direct measure of DNA replication. This showed that proliferation of over-expressing cells was significantly reduced in all four clones in medium containing 0.5% FCS compared to vector only controls (p < 0.001 comparing ADAMTS16-expressing cells vs. vector only; Fig. 3). Proliferation was similarly reduced in cells growing in medium containing 10% FCS though with lower significance (p = 0.01). Migration of cells measured using time-lapse microscopy also showed that ADAMTS16 over-expressing cells had significantly reduced migration on plastic compared to vector only controls (p < 0.005, comparing mean of ADAMTS16-expressing cells vs. vector only; Fig. 4).
Fig. 2.
Effect of ADAMTS-16 on cell phenotype. SW1353 cells were stably transfected with either vector only (VO) or ADAMTS16 (TS16) expression constructs. (a) Cells were fixed in 4% paraformaldehyde and stained with a 1:5000 dilution of mouse anti-FLAG antibody followed by FITC-linked second antibody; nuclei were counterstained with DAPI. (b) Cells were visualised under phase contrast 72 h after plating on plastic (×10 objective).
Fig. 3.
Proliferation of SW1353 cells stably expressing ADAMTS16. SW1353 cells were stably transfected with either vector only (VO) or ADAMTS16 (TS16) expression constructs. Cells were allowed to adhere overnight in DMEM/10% FCS then treated with 3H-thymidine in DMEM/0.5% FCS for 6 h followed by an overnight cold chase. Cell lysates were harvested and counted for tritium. Data are expressed as mean ± s.e.m., ⁎p < 0.05; ⁎⁎p < 0.01, ⁎⁎⁎p<0.001 comparing TS16 with either VO-1 or VO-2.
Fig. 4.
Cell migration of SW1353 cells stably expressing ADAMTS16. SW1353 cells were stably transfected with either vector only (VO) or ADAMTS16 (TS16) expression constructs. Eight hours after plating in DMEM/10% FCS, cells were subjected to time-lapse microscopy at 15 min intervals for 13 h. Distance moved per cell was averaged for 10 cells. Data are expressed as mean ± s.e.m., ⁎p < 0.05; ⁎⁎p < 0.01, ⁎⁎⁎p<0.001 comparing TS16 with VO-1 or VO-2.
2.4. Regulation of ADAMTS16 by growth factors and cytokines
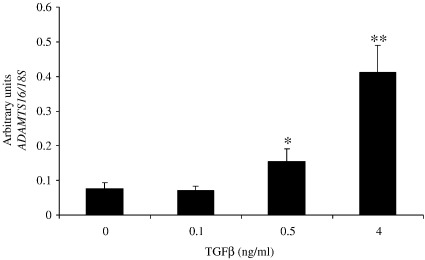
Of a number of growth factors and cytokines tested (IL-1, TNF-α, TGF-β1, IGF-1, IFN-γ, IL-6, IL-6, IL-4, IL-10, IL-11, IL-17, OSM, EGF, BMP-2, BMP-7), only TGFβ was found to regulate levels of endogenous ADAMTS16 expression in chondrocyte cell lines. TGFβ induced mRNA expression at the steady state mRNA level in both SW1353 and C28/I2 cells in a dose-dependent manner (Fig. 5).
Fig. 5.
Induction of ADAMTS16 expression by TGFβ. Confluent C28/I2 cells were serum starved for 24 h prior to addition of TGFβ for 6 h followed by harvest of RNA and qRT-PCR for ADAMTS16. Data are expressed as mean ± s.e.m., ⁎p < 0.05; ⁎⁎p < 0.01.
2.5. Identification of the transcription start site and minimal promoter
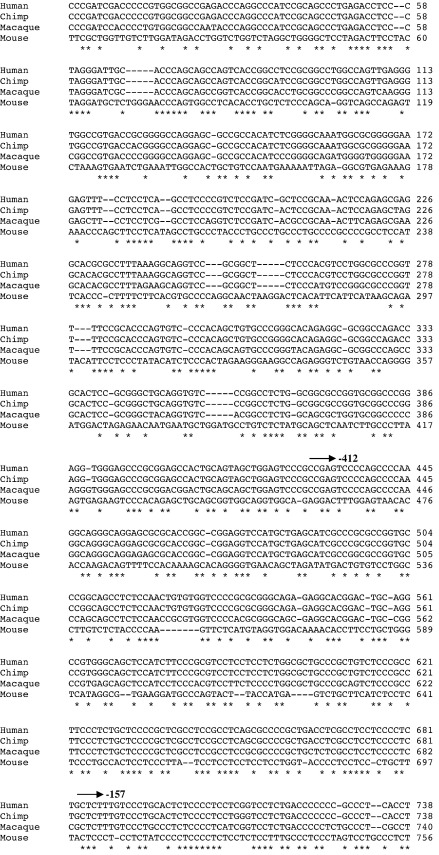
Fig. 6 shows the alignment of 1000 base pairs (bp) of sequence upstream of the translation start site of ADAMTS16 for human, chimp, macaque and mouse. This alignment revealed a number of conserved regions (indicated ‘⁎’ in Fig. 6) that potentially indicate regulatory sequences, with no strong TATA sequence. The predicted translation start site (ATG) is conserved amongst the primates, but not in mouse which appears to use a downstream ATG. This latter sequence is conserved in primates, but not in frame with the upstream primate ATG.
Fig. 6.
Sequence alignment for ADAMTS16 putative promoter region. Sequence alignment of 1000 bp upstream of ADAMTS16 ATG from human, macaque, chimp and mouse (www.ensembl.org). ⁎Indicates conserved regions. Translation start codon is shaded in grey with transcription start point (TSP) also marked. The 5′ positions of the deletion set of promoter–reporter constructs are also shown (arrows).
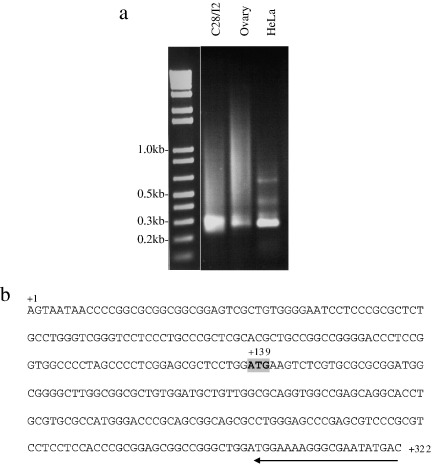
The longest PCR products obtained using 5′ RLM-RACE indicate that the transcription start site lies 138 bp upstream of the ATG in the human gene. Fig. 7a shows the PCR products obtained by performing 5′ RLM-RACE on RNA from three different sources (C28/I2 cells stimulated with TGFβ, ovary and HeLa cells). Fig. 7b shows the position of the primer SP2 used for RLM-RACE and the predicted transcription start point. This site is in agreement with an mRNA transcript of ADAMTS16 deposited in Genbank (NM_139056). Primer extension using primer SP2 also detected a band of approximately the same size as the RACE products (data not shown).
Fig. 7.
Identification of transcription start point in ADAMTS16 gene. 5′ RNA ligase-mediated (RLM) RACE was undertaken using C28/I2, human ovary or HeLa cell cDNA templates. (a) An agarose gel of the PCR products is shown; (b) following sequencing of RACE products, the transcription start point is marked as +1, translation start codon is shaded grey and position of RACE primer is shown (arrow).
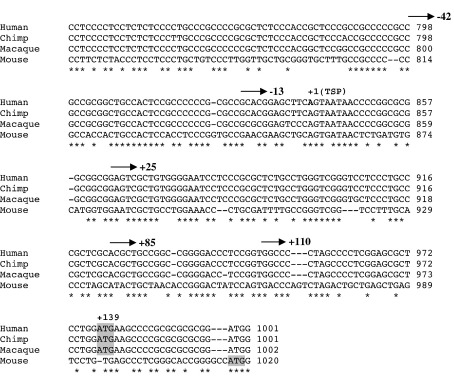
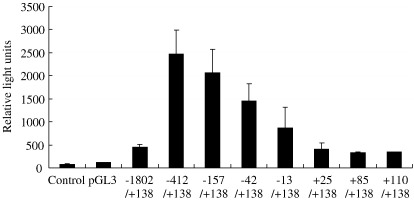
Eight fragments of the 5′ region of ADAMTS16 ranging from +110/+138 to − 1802/+138 (marked on Fig. 6) were cloned into a luciferase reporter vector. Analysis of reporter activity (Fig. 8) indicated that the minimal promoter lies between − 13 and + 25, encompassing the transcription start point identified above. Activity increased greatly in deletions from − 1802 to − 412 indicating the presence of a repressive element within this region. Successive deletions between − 412 and − 13 resulted in incremental decreases in activity, suggesting the presence of a number of transcription factor binding sites in these regions.
Fig. 8.
Promoter activity in the ADAMTS16 gene. SW1353 cells were transiently transfected with promoter–reporter fragments in pGL3-basic as shown. Luciferase activity (measured as relative light units) of untransfected cells (control) and transient transfections of empty vector (pGL3) and eight deletion constructs ranging from − 1802/+138 to +110/+138 are plotted as mean ± s.e.m.
2.6. Identification of transcription factors activating the ADAMTS16 promoter
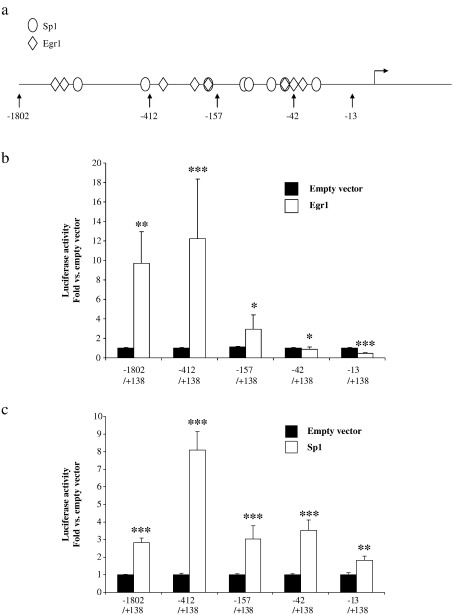
The proximal promoter is GC rich and a search for transcription factor consensus sequences (MatInspector, Genomatix) revealed a number of both Sp1 and Egr1 binding sites (Fig. 9a). These factors have also been implicated in TGFβ signalling to extracellular matrix genes (Chen et al., 2006; Zhang et al., 2000). Co-transfection of the ADAMTS16 promoter constructs with expression vectors for each of these factors showed responsiveness to both transcription factors (Fig. 9b and c). Induction of the promoter by Egr1 is reduced by deletion from − 412 to − 157 and lost by further deletion to − 42 (with Egr1 repressing smaller constructs). All promoter constructs were induced by Sp1 and indeed there are further Sp1 sites downstream of the transcription start point.
Fig. 9.
Activation of the ADAMTS16 promoter by Egr1 and Sp1. SW1353 cells were transiently transfected with promoter–reporter fragments in pGL3-basic as shown and co-transfected with expression constructs for Egr1, Sp1 or empty vector. Luciferase activity is plotted as fold compared to empty vector, mean ± s.e.m.; ⁎p < 0.05; ⁎⁎p < 0.01; ⁎⁎⁎p < 0.001. (a) schematic representation of Egr1 and Sp1 consensus sequences in the ADAMTS16 promoter; (b) induction of promoter by Egr1; (c) induction of promoter by Sp1.
3. Discussion
ADAMTS-16 is a multi-domain protein encoded by 23 exons. Initially in this study, we verified that the full length transcript was expressed in chondrocyte cell lines and then cloned this cDNA into an expression construct with a C-terminal FLAG tag. Chondrosarcoma cells stably over-expressing ADAMTS-16 were selected and cloned. Over-expression of ADAMTS16 was verified at the mRNA level by qRT-PCR and at the protein level by immunocytochemistry and western blot using the anti-FLAG antibody and/or an anti-ADAMTS-16 antibody (raised against a C-terminal peptide). Western blot showed an approximately 130 kDa band predominantly associated with the extracellular matrix fraction, with an additional higher molecular weight band faintly detected. The full length ADAMTS-16 polypeptide has a predicted molecular weight of 136 kDa, in good agreement with the experimental finding. A doublet around this molecular weight was also weakly detected in the cell lysate and conditioned medium of the ADAMTS16 transfects. The lower molecular weight band corresponds to the major band in the ECM, suggesting that this is a processed form. Two ‘in-house’ anti-peptide antibodies raised against sequences in the propeptide and catalytic domain respectively recognise a band of approximately 65 kDa in conditioned medium (data not shown), though the specificity of these antibodies remains to be determined.
Whilst stably transfected SW1353 cells could be maintained and expanded, this wasn't the case for the C28/I2 transformed chondrocytes. In the SW1353 cells, over-expression of ADAMTS-16 lead to a reduction in cell proliferation and this change in phenotype may, in part, explain why the C28/I2 cells were not successfully expanded. SW1353 cells over-expressing ADAMTS-16 also showed a change in cell shape and a decrease in cell migration on plastic, though no difference in cell adhesion to a number of extracellular matrix proteins. This does not appear to be the consequence solely of stable transfection since similar cell lines over-expressing MMP-28 display a different phenotype (Rodgers et al., 2009). Interestingly, ADAMTS-1 modifies both cell proliferation and migration dependent on cell type, domain content and concentration of the enzyme used (Krampert et al., 2005; Liu et al., 2006c).
Members of the metzincin family are known to process a number of growth factors, cytokines and signalling molecules in addition to matrix substrates (Somerville et al., 2003b). Since the over-expression of ADAMTS-16 has no effect on expression levels of most of the ADAMTS, TIMP and MMP genes tested, it is unlikely that ADAMTS-16 processes factors that regulate these genes. However, our initial interests in ADAMTS-16 centred around the fact that in end stage osteoarthritis, the expression of the ADAMTS16 gene is increased in cartilage and synovium in a way similar to that of MMP-13, a collagenolytic enzyme known to be involved in the destruction of tissues in the joint [17, 18]. Interestingly, the SW1353 cells over-expressing ADAMTS-16 display reduced expression of MMP13 mRNA, though the mechanism or functional significance of this observation is unknown. There is no clear link in the literature between decreased MMP13 expression and decreased proliferation or migration (as observed for ADAMTS16 over-expressing cells). Indeed one agent, pleiotrophin, has recently been reported to induce proliferation and migration of chondrocytes whilst repressing MMP13 expression (Pufe et al., 2007). In OA cartilage, other factors must override the repression of MMP13 expression by increased ADAMTS-16 since both enzymes show increased expression compared to that in normal cartilage. Given the importance of MMP-13 as a collagenase in OA, the mechanistic link between ADAMTS-16 and MMP13 expression is worthy of further investigation.
Since ADAMTS16 expression was increased in OA tissues, we tested a number of growth factors and cytokines implicated in this disease for their ability to regulate expression of the ADAMTS16 gene in vitro. In both C28/I2 and SW1353 cells, TGFβ induced expression of ADAMTS16 in a dose-dependent fashion. TGFβ has been reported to induce MMP13 expression in chondrocytes (Moldovan et al., 1997) and there is evidence to demonstrate a role for this growth factor in the development or progression of osteoarthritis (Blaney Davidson et al., 2007).
Sequence alignment shows a high level of conservation between primates and mouse in the 5′ upstream region of the ADAMTS16 gene. 5′ RACE gives a transcription start point 138 bp upstream of the translation start point and this is in agreement with both primer extension data and the end of exon 1 given in Ensembl (www.ensembl.org). Interestingly, the sequence across the transcription start point (− 2/+5, 5′TCAGTAA3′) is a putative AP-1 binding site. This is contrary to the computer prediction of the transcription start point at 560 bp upstream of the translation start point (− 422 from the transcription start point) and the associated promoter reported in Gao et al. (Gao et al., 2007), however it is possible that the gene has greater than one transcription start point.
A deletion analysis from − 1802 (relative to the transcription start point) showed that constructs expressed promoter activity only when they encompassed the transcription start point. Promoter activity increased with the increasing length to − 412, a region which is GC rich and contains many putative binding sites for transcription factors such as Sp1 and Egr1. Interestingly, these factors have previously been implicated in TGFβ signalling, with both factors involved in the induction of type I collagen by TGFβ (Chen et al., 2006; Zhang et al., 2000). Forced over-expression of these factors demonstrates that both factors are capable of inducing the ADAMTS16 promoter. Egr1 induction appears to be mediated mainly by the − 412/− 157 region, with Sp1 induction also mediated through further downstream sequences.
In summary, over-expression of ADAMTS-16 leads to decreased cell proliferation and migration either directly, or indirectly via changes in gene expression e.g. MMP13. Expression of ADAMTS16 is induced by TGFβ and we have experimentally identified the transcription start point of the gene, its promoter, and two relevant activating transcription factors. Overall, these data provide new information on the function and regulation of ADAMTS-16 in chondrocyte cell lines.
4. Experimental procedures
4.1. PCR and cloning of full length transcript
RNA was extracted from C28/I2 immortalised human chondrocyte cells (Loeser et al., 2000) using TRIzol® reagent (Invitrogen) and adding 0.5× volume of chloroform. The aqueous phase was recovered after centrifugation at 12,000 rpm for 15 min at 4 °C and added to an equal volume of isopropanol. RNA was pelleted by centrifugation at 12,000 rpm for 30 min at 4 °C and washed with 70% ethanol. RNA was eluted into distilled water and quantified using a NanoDrop® spectrophotometer (Nanodrop Technologies). cDNA was synthesized from 1 µg of RNA using SuperscriptTM II reverse transcriptase (Invitrogen) and random hexamers, according to the manufacturer's recommendations. PCRs were performed using 50 ng of cDNA template, 0.5 µM each primer, 200 µM dNTPs and 0.5 U of AccuTaqTM LA polymerase (Sigma-Aldrich) in the manufacturer's buffer, in a PTC-100 thermal cycler (MJ Research). PCR products were visualised on 1% agarose gels stained with ethidium bromide. For cloning, PCR primers at each end of the gene were tagged with four random nucleotides (italics) and an EcoRI (5′) and NotI (3′) restriction enzyme recognition site (underlined). The 5′ forward primer also contained a consensus Kozak sequence (bold; (Kozak, 1987)). Primer sequences were as follows: 5′-ACGTGAATTCGCCGCCACCATGAAGCCCCGCGCG-3′ and 5′-ACGTGCGGCCGCCCAAGTTGGACTTAGAGCAAG-3′ for forward and reverse primers respectively. PCR products were digested with EcoRI and NotI and ligated into a modified version of the pcDNA4TM expression vector (Invitrogen) expressing an in frame C-terminal FLAG-tag. Sequencing of the cloned gene was performed in both directions using BigDyeTM v.3.1 terminator sequencing chemistry (Applied Biosytems) on an ABI Prism® 3730 capillary sequencer (Applied Biosystems).
4.2. Creation of stably expressing cell lines
SW1353 human chondrosarcoma cells (ATCC) or immortalized C28/I2 chondrocytes were cultured in DMEM plus GlutaMAXTM (Invitrogen) with 10% fetal calf serum (FCS; Invitrogen). 100,000 cells per well were plated into 6-well plates and incubated overnight. The ADAMTS16/pcDNA4-FLAG construct was linearised by digesting with BglII and 1 µg was transfected into cells using FuGENE®6 transfection reagent (Roche) according to the manufacturer's recommendations. After 48 h, cells were switched to culture medium containing 200 µg/ml zeocin (Melford Laboratories). After 7 days, surviving cells were plated into 96-well plates containing three, one and 0.3 cells/well. Wells containing cells were expanded and plated again into 96-well plates containing three, one and 0.3 cells/well to select for clonal colonies originating from single cells. Cells were maintained in zeocin-containing media.
4.3. Quantitative real-time PCR
Cells were plated into 6-well plates containing 200,000 cells/well in triplicate. After 24 h, cells were changed to serum-free media and incubated for a further 24 h. RNA extraction and cDNA synthesis were performed as before. Primers and fluorescent probes for ADAMTS16 and all of the ADAMTSs, MMPs and TIMPs were designed using Primer Express 1.0 software (Applied Biosystems) and are described elsewhere (Nuttall et al., 2003; Porter et al., 2004). The 18S rRNA gene was used as an endogenous control to normalise for differences in the amount of RNA between samples. Primers and probe for 18S were obtained from Applied Biosystems. Quantitative PCRs were performed and analysed as described previously (Davidson et al., 2006).
4.4. Immunocytochemistry
Stably transfected SW1353 cells were plated into chamber slides at a density of 10,000 cells/well. After incubating for 24 h, cells were switched to serum-free media for a further 24 h. Cells were fixed using 4% paraformaldehyde, blocked with 3% BSA and incubated with a 1:5000 dilution of mouse ANTI-FLAG® M2 IgG monoclonal antibody (Sigma) and a 1:1000 dilution of FITC-labelled rabbit anti-mouse IgG secondary antibody (Dako). Nuclei were stained with 2.5 µg/ml DAPI. Cells were also incubated with mouse IgG and secondary antibody as before as a control. Cells were visualised using an AxioPlan 2ie confocal microscope with Axiovision software (Carl Zeiss).
4.5. Western blot analysis
Stably transfected SW1353 cells were plated at 100,000/well of a 6-well plate. After 24 h cells were switched to serum-free medium for a further 48 h. The conditioned media was collected and proteins precipitated with 0.5 vol. of 10% (v/v) trichloroacetic acid (TCA) for 1 h on ice. Samples were centrifuged for 15 min at 13,000 rpm, and pellets washed with cold acetone and pelleted for a further 15 min at 13,000 rpm. Protein pellets were air-dried and resuspended in 1 × SDS final sample buffer (0.058 M Tris-HCl, pH 6.8; 5% v/v glycerol; 1.7% w/v SDS and 0.002% w/v bromophenol blue) containing 50 mM DTT and 6 M urea (FSB/DTT/urea). Cells were detached with 0.5 mM EDTA in PBS. Cell pellets were collected by centrifugation at full speed for 5 min, resuspended in FSB/DTT/urea and sonicated prior to analysis. ECM proteins were harvested by scraping each well with FSB/DTT/urea for approximately 30 s/well. All samples were boiled for 5 min and subjected to western blotting. Proteins were separated by SDS-PAGE, followed by transfer to PVDF membrane. ADAMTS-16 was probed using a 1:5000 dilution of mouse ANTI-FLAG® M2 IgG monoclonal antibody (Sigma-Aldrich) followed by a 1:1000 dilution of HRP-labelled rabbit anti-mouse IgG secondary antibody (Dako, Denmark). An antibody raised against a 75 amino acid peptide from the C-terminal thrombospondin repeat domain of human ADAMTS-16 (Santa Cruz SC-50490) were used to verify expression. LumiGLO reagent and peroxide (Cell Signalling Technology, Beverley, MA) were used for detection on Kodak Biomax MS film (Sigma-Aldrich) by chemiluminescence.
4.6. Adhesion, proliferation and migration assays
96-well culture plates were coated with 50 µg/ml of vitronectin or fibronectin or 100 µg/ml of collagens I or II overnight at 4 °C. Plates were blocked with 1% BSA for 1 h at 37 °C before adding 30,000 cells/well. After 15 min, plates were washed six times with PBS. Cell adhesion was measured using a Cell Titer 96® AQueous One Solution Cell Proliferation Assay kit (Promega), according to the manufacturer's recommendations. For proliferation assays, cells were plated in medium containing 10% FCS at 25,000 cells/well in 24 well plates in replicates of six and allowed to adhere overnight. Each well was labelled with 0.5 µCi per well [6-3H]-thymidine for 6 h in either medium containing 10% or 0.5% FCS, washed twice with HBSS and fresh medium added containing 3 µM cold thymidine. After overnight incubation, medium was aspirated and 300 µl/well 0.25 M ammonia added. Plates were rocked for 2 h at room temperature and lysate from each well transferred into scintillant for counting tritium. For migration assays, cells were plated at 10,000 cells/well in 24-well plates. After 8 h cells were subjected to time-lapse microscopy on an AxioVert 200 M microscope that was enclosed in an Incubator XL (Carl Zeiss) for temperature and CO2 control. Cells were photographed at 15 min intervals for 13 h. Total distance moved (in µm) for 10 cells/well was measured using Axiovision Software (Carl Zeiss).
4.7. Induction with cytokines and growth factors
SW1353 cells were plated into six-well plates at 200,000 cells per well. After 24 h cells were transferred into serum-free media. Cells were incubated with each factor in triplicate for 6 h for Taqman analysis. RNA extraction, cDNA synthesis and TaqMan® real-time PCR were performed as described above for analysis of ADAMTS16.
4.8. Promoter alignment
The 5′ upstream region of human ADAMTS16 was obtained and aligned with chimp, macaque and mouse using the Ensembl database www.ensembl.org.
4.9. 5′ RACE
5′ RLM-RACE was performed using a GeneRacerTM kit (Invitrogen) according to the manufacturer's recommendations. The primer 5′TGGAAAAGGGCGAATATGAC3′ (SP2) was used to generate amplified products which were cloned using a TOPO-TA cloning kit (Invitrogen) according to the manufacturer's recommendations. DNA sequencing was performed using the M13 universal primers supplied with the kit.
4.10. Cloning of a deletion set and luciferase reporter assays
Genomic DNA was extracted from cultured SW1353 cells using a QIAamp DNA Mini Kit (Qiagen) according to the manufacturer's recommendations. PCR products were obtained using 50 ng of genomic DNA template, 0.5 µM of each primer, (synthesised with a 5′ MluI recognition site) 200 µM dNTPs and 0.5 U of AccuTaqTM LA polymerase (Sigma-Aldrich) in the manufacturer's buffer, in a PTC-100 thermal cycler (MJ Research). For fragments of 29 bp and 54 bp, synthetic oligonucleotides were manufactured. Fragments were run on 1% agarose gels stained with ethidium bromide, digested with MluI and cloned into the MluI site of the pGL3-basic luciferase reporter vector (Promega). Insert orientation was verified by sequencing using pGL3 sequencing primers. SW1353 cells were plated into 24-well plates at 20,000 cells/well. Each pGL3 construct was transfected in triplicate using 200 ng DNA and FuGENE®6 transfection reagent (Roche), according to the manufacturer's recommendations. After 24 h cells were lysed and assayed using a Luciferase Reporter Gene Assay kit (Roche) following the manufacturer's recommendations. Transfections were performed with at least two different isolates of each plasmid. The Sp1 cDNA was a kind gift from Professor G. Suske (Marburg, Germany) and was subcloned into the pcDNA4 vector (above). The Egr1 expression construct was as previously described (Tan et al., 2003).
Acknowledgement
AKS was funded by a grant from the Arthritis Research Campaign UK.
References
- Blaney Davidson E.N., van der Kraan P.M., van den Berg W.B. TGF-beta and osteoarthritis. Osteoarthr. Cartil. 2007;15:597–604. doi: 10.1016/j.joca.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Cal S., Obaya A.J., Llamazares M., Garabaya C., Quesada V., Lopez-Otin C. Cloning, expression analysis, and structural characterization of seven novel human ADAMTSs, a family of metalloproteinases with disintegrin and thrombospondin-1 domains. Gene. 2002;283:49–62. doi: 10.1016/s0378-1119(01)00861-7. [DOI] [PubMed] [Google Scholar]
- Chen S.J., Ning H., Ishida W., Sodin-Semrl S., Takagawa S., Mori Y., Varga J. The early-immediate gene EGR-1 is induced by transforming growth factor-beta and mediates stimulation of collagen gene expression. J. Biol. Chem. 2006;281:21183–21197. doi: 10.1074/jbc.M603270200. [DOI] [PubMed] [Google Scholar]
- Clark I.M., Parker A.E. Metalloproteinases: their role in arthritis and potential as therapeutic targets. Expert Opin. Ther. Targets. 2003;7:19–34. doi: 10.1517/14728222.7.1.19. [DOI] [PubMed] [Google Scholar]
- Colige A., Sieron A.L., Li S.W., Schwarze U., Petty E., Wertelecki W., Wilcox W., Krakow D., Cohn D.H., Reardon W., Byers P.H., Lapiere C.M., Prockop D.J., Nusgens B.V. Human Ehlers–Danlos syndrome type VII C and bovine dermatosparaxis are caused by mutations in the procollagen I N-proteinase gene. Am. J. Hum. Genet. 1999;65:308–317. doi: 10.1086/302504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colige A., Vandenberghe I., Thiry M., Lambert C.A., Van Beeumen J., Li S.W., Prockop D.J., Lapiere C.M., Nusgens B.V. Cloning and characterization of ADAMTS-14, a novel ADAMTS displaying high homology with ADAMTS-2 and ADAMTS-3. J. Biol. Chem. 2002;277:5756–5766. doi: 10.1074/jbc.M105601200. [DOI] [PubMed] [Google Scholar]
- Collins-Racie L.A., Flannery C.R., Zeng W., Corcoran C., Annis-Freeman B., Agostino M.J., Arai M., DiBlasio-Smith E., Dorner A.J., Georgiadis K.E., Jin M., Tan X.Y., Morris E.A., LaVallie E.R. ADAMTS-8 exhibits aggrecanase activity and is expressed in human articular cartilage. Matrix Biol. 2004;23:219–230. doi: 10.1016/j.matbio.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Dagoneau N., Benoist-Lasselin C., Huber C., Faivre L., Megarbane A., Alswaid A., Dollfus H., Alembik Y., Munnich A., Legeai-Mallet L., Cormier-Daire V. ADAMTS10 mutations in autosomal recessive Weill–Marchesani syndrome. Am. J. Hum. Genet. 2004;75:801–806. doi: 10.1086/425231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson R.K., Waters J.G., Kevorkian L., Darrah C., Cooper A., Donell S.T., Clark I.M. Expression profiling of metalloproteinases and their inhibitors in synovium and cartilage. Arthritis Res. Ther. 2006;8:R124. doi: 10.1186/ar2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson S.C., Vankemmelbeke M.N., Buttle D.J., Rosenberg K., Heinegard D., Hollander A.P. Cleavage of cartilage oligomeric matrix protein (thrombospondin-5) by matrix metalloproteinases and a disintegrin and metalloproteinase with thrombospondin motifs. Matrix Biol. 2003;22:267–278. doi: 10.1016/s0945-053x(03)00034-9. [DOI] [PubMed] [Google Scholar]
- Gao S., De Geyter C., Kossowska K., Zhang H. FSH stimulates the expression of the ADAMTS-16 protease in mature human ovarian follicles. Mol. Hum. Reprod. 2007;13:465–471. doi: 10.1093/molehr/gam037. [DOI] [PubMed] [Google Scholar]
- Glasson S.S., Askew R., Sheppard B., Carito B., Blanchet T., Ma H.L., Flannery C.R., Peluso D., Kanki K., Yang Z., Majumdar M.K., Morris E.A. Deletion of active ADAMTS5 prevents cartilage degradation in a murine model of osteoarthritis. Nature. 2005;434:644–648. doi: 10.1038/nature03369. [DOI] [PubMed] [Google Scholar]
- Huxley-Jones J., Apte S.S., Robertson D.L., Boot-Handford R.P. The characterisation of six ADAMTS proteases in the basal chordate Ciona intestinalis provides new insights into the vertebrate ADAMTS family. Int. J. Biochem. Cell Biol. 2005;37:1838–1845. doi: 10.1016/j.biocel.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Joe B., Saad Y., Lee N., Frank B., Achinike O., Luu T., Gopalakrishnan K., Toland E., Farms P., Yerga-Woolwine S., Manickavasagam E., Rapp J., Garrett M., Coe D., Apte S., Rankinen T., Perusse L., Ehret G., Ganesh S., Cooper R., Connor A., Rice T., Weder A., Chakravarti A., Rao D., Bouchard C. Positional identification of variants of ADAMTS16 linked to inherited hypertension. Hum. Mol. Genet. 2009;18:2825–2838. doi: 10.1093/hmg/ddp218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kevorkian L., Young D.A., Darrah C., Donell S.T., Shepstone L., Porter S., Brockbank S.M., Edwards D.R., Parker A.E., Clark I.M. Expression profiling of metalloproteinases and their inhibitors in cartilage. Arthritis Rheum. 2004;50:131–141. doi: 10.1002/art.11433. [DOI] [PubMed] [Google Scholar]
- Kozak M. An analysis of 5′-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 1987;15:8125–8148. doi: 10.1093/nar/15.20.8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krampert M., Kuenzle S., Thai S.N., Lee N., Iruela-Arispe M.L., Werner S. ADAMTS1 proteinase is up-regulated in wounded skin and regulates migration of fibroblasts and endothelial cells. J. Biol. Chem. 2005;280:23844–23852. doi: 10.1074/jbc.M412212200. [DOI] [PubMed] [Google Scholar]
- Liu C.J., Kong W., Ilalov K., Yu S., Xu K., Prazak L., Fajardo M., Sehgal B., Di Cesare P.E. ADAMTS-7: a metalloproteinase that directly binds to and degrades cartilage oligomeric matrix protein. FASEB J. 2006;20:988–990. doi: 10.1096/fj.05-3877fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C.J., Kong W., Xu K., Luan Y., Ilalov K., Sehgal B., Yu S., Howell R.D., Di Cesare P.E. ADAMTS-12 associates with and degrades cartilage oligomeric matrix protein. J. Biol. Chem. 2006;281:15800–15808. doi: 10.1074/jbc.M513433200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.J., Xu Y., Yu Q. Full-length ADAMTS-1 and the ADAMTS-1 fragments display pro- and antimetastatic activity, respectively. Oncogene. 2006;25:2452–2467. doi: 10.1038/sj.onc.1209287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeser R.F., Sadiev S., Tan L., Goldring M.B. Integrin expression by primary and immortalized human chondrocytes: evidence of a differential role for alpha1beta1 and alpha2beta1 integrins in mediating chondrocyte adhesion to types II and VI collagen. Osteoarthr. Cartil. 2000;8:96–105. doi: 10.1053/joca.1999.0277. [DOI] [PubMed] [Google Scholar]
- Moldovan F., Pelletier J.P., Hambor J., Cloutier J.M., Martel-Pelletier J. Collagenase-3 (matrix metalloprotease 13) is preferentially localized in the deep layer of human arthritic cartilage in situ: in vitro mimicking effect by transforming growth factor beta. Arthritis Rheum. 1997;40:1653–1661. doi: 10.1002/art.1780400915. [DOI] [PubMed] [Google Scholar]
- Nuttall R.K., Pennington C.J., Taplin J., Wheal A., Yong V.W., Forsyth P.A., Edwards D.R. Elevated membrane-type matrix metalloproteinases in gliomas revealed by profiling proteases and inhibitors in human cancer cells. Mol. Cancer Res. 2003;1:333–345. [PubMed] [Google Scholar]
- Porter S., Scott S.D., Sassoon E.M., Williams M.R., Jones J.L., Girling A.C., Ball R.Y., Edwards D.R. Dysregulated expression of adamalysin-thrombospondin genes in human breast carcinoma. Clin. Cancer Res. 2004;10:2429–2440. doi: 10.1158/1078-0432.ccr-0398-3. [DOI] [PubMed] [Google Scholar]
- Porter S., Clark I.M., Kevorkian L., Edwards D.R. The ADAMTS metalloproteinases. Biochem. J. 2005;386:15–27. doi: 10.1042/BJ20040424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pufe T., Groth G., Goldring M.B., Tillmann B., Mentlein R. Effects of pleiotrophin, a heparin-binding growth factor, on human primary and immortalized chondrocytes. Osteoarthr. Cartil. 2007;15:155–162. doi: 10.1016/j.joca.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Rodgers U.R., Kevorkian L., Surridge A.K., Waters J.G., Swingler T.E., Culley K., Illman S., Lohi J., Parker A.E., Clark I.M. Expression and function of matrix metalloproteinase (MMP)-28. Matrix Biol. 2009;28:263–272. doi: 10.1016/j.matbio.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville R.P., Longpre J.M., Jungers K.A., Engle J.M., Ross M., Evanko S., Wight T.N., Leduc R., Apte S.S. Characterization of ADAMTS-9 and ADAMTS-20 as a distinct ADAMTS subfamily related to Caenorhabditis elegans GON-1. J. Biol. Chem. 2003;278:9503–9513. doi: 10.1074/jbc.M211009200. [DOI] [PubMed] [Google Scholar]
- Somerville R.P., Oblander S.A., Apte S.S. Matrix metalloproteinases: old dogs with new tricks. GenomeBiology. 2003;4:216. doi: 10.1186/gb-2003-4-6-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton H., Rogerson F.M., East C.J., Golub S.B., Lawlor K.E., Meeker C.T., Little C.B., Last K., Farmer P.J., Campbell I.K., Fourie A.M., Fosang A.J. ADAMTS5 is the major aggrecanase in mouse cartilage in vivo and in vitro. Nature. 2005;434:648–652. doi: 10.1038/nature03417. [DOI] [PubMed] [Google Scholar]
- Tan L., Peng H., Osaki M., Choy B.K., Auron P.E., Sandell L.J., Goldring M.B. Egr-1 mediates transcriptional repression of COL2A1 promoter activity by interleukin-1beta. J. Biol. Chem. 2003;278:17688–17700. doi: 10.1074/jbc.M301676200. [DOI] [PubMed] [Google Scholar]
- Tsai H.M. Thrombotic thrombocytopenic purpura: a thrombotic disorder caused by ADAMTS13 deficiency. Hematol. Oncol. Clin. North Am. 2007;21:609–632. doi: 10.1016/j.hoc.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng W., Corcoran C., Collins-Racie L.A., Lavallie E.R., Morris E.A., Flannery C.R. Glycosaminoglycan-binding properties and aggrecanase activities of truncated ADAMTSs: comparative analyses with ADAMTS-5, -9, -16 and -18. Biochim. Biophys. Acta. 2006;1760:517–524. doi: 10.1016/j.bbagen.2006.01.013. [DOI] [PubMed] [Google Scholar]
- Zhang W., Ou J., Inagaki Y., Greenwel P., Ramirez F. Synergistic cooperation between Sp1 and Smad3/Smad4 mediates transforming growth factor beta1 stimulation of alpha 2(I)-collagen (COL1A2) transcription. J. Biol. Chem. 2000;275:39237–39245. doi: 10.1074/jbc.M003339200. [DOI] [PubMed] [Google Scholar]