Abstract
Objective
To study the effect of anterior cruciate ligament (ACL) injury on lubricin concentration in synovial fluid (SF) and its correlation with time post-injury, inflammatory cytokines, lubricin degrading enzymes, and SF proteoglycan content.
Methods
SF samples were obtained from both knees of 30 patients with a unilateral ACL insufficiency 32–364 days post-injury. Lubricin, inflammatory cytokines [interleukin-1 beta (IL-1β), tumor necrosis factor alpha (TNF-α) and interleukin-6 (IL-6)] and catabolic enzymes [procathepsin-B and neutrophil elastase (NE)] were quantified in the SF of injured and contralateral (uninjured) joints using ELISAs. Sulfated glycosaminoglycans (sGAG) levels in SF were measured by Alcian blue binding assay.
Results
SF lubricin concentrations were significantly (p<0.001) reduced following ACL injury when compared to the contralateral joint. Within 12-months, the lubricin concentration of the injured knee (slope=0..006, SE=0.00010, p<0.001) approached that of the contralateral knee, which did not change with time (slope=−0.0002, SE=0.00050, p=0.71). TNF-α levels showed a significant negative relationship with log2 lubricin levels. IL-1β, TNF-α, IL-6, procathepsin-B and NE concentrations in injured SF were greater in samples whose injuries were recent compared to those that were chronic. There were no detectable cytokines or enzymes in SF of contralateral joints. sGAG concentrations were significantly (p<0.01) higher in injured SF compared to contralateral joints.
Conclusions
The decrease in SF lubricin concentrations following ACL injury may place the joint at an increased risk of wear-induced damage, as a consequence of lack of boundary lubrication, potentially leading to secondary osteoarthritis. The decrease in SF lubricin was associated with elevation of inflammatory cytokines.
Keywords: ACL, Injury, Lubricin, Inflammation, Osteoarthritis
INTRODUCTION
Lubricin, also known as superficial zone protein (SZP) or proteoglycan 4 (Prg4), is a mucinous glycoprotein secreted from synovial fibroblasts and the superficial zone articular chondrocytes (1-3). Lubricin is a factor responsible, in part, for the lubrication of apposed and pressurized cartilage surfaces (4). Lubricin provides essential chondroprotective properties to articular cartilage as evidenced by lack of cartilage surface integrity and surface disruption in Prg4 knockout mice (5). Previously, we have demonstrated that decreased synovial fluid (SF) lubricating properties, indicative of decreased SF lubricin concentrations, in patients diagnosed with traumatic synovitis (6). Altered joint mechanics leads to decreased lubricin expression in the superficial layer and decreased lubricin coating of the surface of articular cartilage, as shown in a sheep meniscectomy model (7). Lubricin expression is down regulated by pro-inflammatory cytokines e.g. interleukin 1 beta (IL-1β), tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6) (8-10). Moreover, lubricin was shown to be proteolytically susceptible to the effects of cysteine proteases e.g. cathepsin B and serine proteases e.g. neutrophil elastase (NE) (11, 12).
Anterior cruciate ligament (ACL) injury is a significant risk factor for the development of secondary osteoarthritis (OA), however, the mechanism by which it develops is still under investigation (13, 14). We postulate that following a traumatic knee injury, changes in joint lubricating mechanisms, mediated by lubricin catabolism and down regulation due to the elevated inflammatory cytokines levels, predispose articular cartilage surfaces to wear-induced damage. The objective of this investigation is to study the effect of anterior cruciate ligament (ACL) injury on lubricin concentrations in SF and its correlation with time from injury, inflammatory cytokines, lubricin degrading enzymes, and a marker of cartilage turnover (sGAG).
MATERIALS & METHODS
Patients
The SF samples were obtained from both knees of 30 patients (11 female/19 males) who suffered a unilateral ACL injury from an ongoing clinical trial of ACL reconstruction surgery. On average, the aspirations were performed 103 days (range: 32–364 days) after injury. The mean age of the patients was 24 years (range: 15–47 years). Patients were included in the study if they sustained an isolated ACL injury, had no history of a previous injury (either knee), meniscal damage involving less than 1/3 of a meniscus, and no arthroscopic signs of chondral lesions. In our ongoing prospective studies of ACL reconstruction, 40% of the patients seeking treatment for an ACL injury meet these inclusion criteria. The study was approved by the Institutional Review Board and all subjects granted their informed consent prior to participating.
Synovial Fluid Samples
The SF samples were obtained without lavage just prior to reconstruction surgery. The samples were centrifuged at 2500rpms for 10min, and the aliquot was removed and stored at −80°C until assayed.
SF lubricin (sandwich ELISA)
A sandwich ELISA using peanut agglutinin (PNA) and anti-lubricin Mab S6.89 (15) was designed and validated. High binding 96 well plates were coated overnight with PNA in 50mM sodium bicarbonate buffer, pH 9.5 at a final concentration of 10μg/ml. The following day, serial dilutions of purified human lubricin and aspirated SF were incubated on the PNA-coated plates for 60min at room temperature. The plate was subsequently washed with phosphate buffer saline (PBS)+0.1% Tween 20. S6.89 was subsequently added at a 1:10,000 dilution and incubated for 60 min at room temperature, followed by washing with PBS-0.1% Tween 20. Goat anti-mouse IgG-alkaline phosphatase was added at 1:1,000 dilution to the plate and incubated for 60min at room temperature and followed by washing with PBS-0.1% Tween 20 and PBS. Finally, 4-methylumbelliferyl phosphate (4-MUP) (Sigma-Aldrich) was added and the fluorescence was measured using 465nm and 550nm as emission and excitation wavelengths, respectively.
Inflammatory cytokines, procathepsin B and NE
Quantification of inflammatory cytokines e.g. IL-1β, TNF-α and IL-6 in SF from ACL injured and contralateral joints was performed using commercially available ELISAs (R&D systems, Minneapolis, MN). The reported minimum detection limits of the IL-1β, TNF-α and IL-6 assays were 3, 0.1 and 0.04pg/ml, respectively.
Procathepsin B was measured by a commercially available ELISA (R&D systems). The assay is a sandwich ELISA and the reported mean minimum detectable procathepsin B was reported to be 16pg/ml. NE was measured by a commercially available ELISA (Hbt Technologies, Netherlands). The assay is a sandwich ELISA and the detection range of the assay is reported to be between 0.4 and 25ng/ml.
Sulfated glycosaminoglycan (sGAG)
Quantification of sGAG levels in SF in both knees was performed using Alcian Blue dye binding assay (Alpco Diagnostics, Windham, NH). The assay is based on the formation of an insoluble blue colored complex between sGAG and Alcian blue that was quantified at a 590nm absorbance wavelength.
Data Analysis
Detectable concentrations of lubricin and sGAG concentrations were collected from both the injured and contralateral limb. These were analyzed using mixed linear models for repeated measures with an unstructured variance-covariance structure and fit by residual estimation of maximum likelihood. Limb was a within-subject’s effect, and time from injury was a continuous between-subjects effect. Independent linear functions were fit to log2 lubricin concentrations in injured and contralateral limbs as a function of days after injury and their parameters tested against both zero and between limbs using orthogonal contrasts. In this way, the difference between the slope parameters represented the change in lubricin concentrations over time since injury in the injured limb, adjusting for any systemic effects that may be present in the contralateral limb. The difference between the intercepts of the linear functions were used as an estimate for initial difference between limbs at the time of the injury or soon thereafter. The same data were used to calculate a contralateral-normalized injured limb lubricin concentration (injured concentration divided by contralateral concentration). This was also fit via a mixed linear model to days since injury (between-subjects effect only, variance component structure).
Catabolic factors (TNF-α, IL-1β, IL-6, NE, and procathepsin B) had detectable concentrations in the injured limbs only, restricting analysis to their log2 concentrations used as predictors of log2 lubricin concentrations in the injured limb. These were carried out using mixed linear models between-subjects effect only, variance components structure). The sample sizes in these regressions varied substantially owing to frequent values lying below the detectable range of the assays (n=18 for TNF-α, n=15 for IL-1β, n=29 for IL-6, n=19 for NE, and n=30 for procathepsin B).
Detectable concentrations of sGAG were obtained from both limbs and analyzed using analogous mixed linear models to that used to asses changes in log2 lubricin concentration over time. Further, an additional model used sGAG as a within-limb predictor of log2 lubricin concentrations based on both injured and contralateral limbs, and checking for their interaction.
The distribution of each variable was checked for the assumptions of normality and homogeneity of regression using visualization techniques. Previous experience with several of these variables concurs with the observation by Bland and Altman (1996) regarding the common occurrence of biological data which is better represented on a log scale owing to complex or multiplicative mechanism which become additive with symmetrical variance when transformed with logarithms (16). Lubricin and sGAG concentrations from injured and contralateral limbs were examined separately.
RESULTS
SF lubricin (sandwich ELISA)
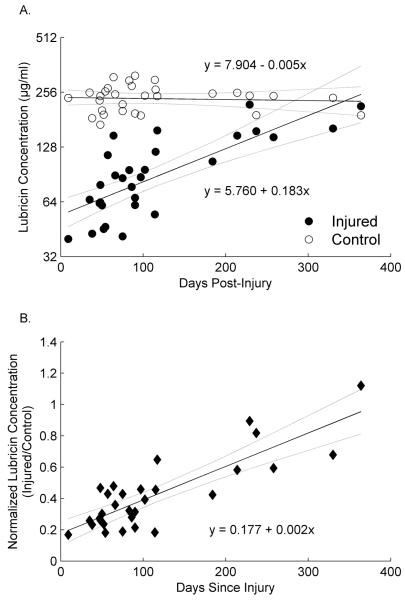
SF lubricin levels were significantly lower in the injured joints compared to the contralateral joints at the early stage following injury, as evidenced by a significant difference between intercepts for the linear functions describing log2 lubricin as a function of time post-injury in injured and contralateral limbs (diff=2.14, SE=0.14, T(28)=15.33, p<0.001). These functions intersected between 11 and 12 months following injury (Fig. 1a) suggesting that normal lubricin levels were approach within 12 months. When the lubricin levels were normalized to the contralateral knee, there was a significant increase in the lubricin concentration with time post-injury (Fig. 1B). The contralateral-normalized injured limb concentration was low shortly after injury but increased as a function of days since injury (Fig. 1B. slope=0.002, SE=0.0003, F(1,28)=65.89, p<.0001).
Fig. 1.
(A) The log2 SF lubricin concentrations following ACL injury in the injured and contralateral joints expressed as a function of time, and (B) the SF lubricin concentrations of the injured knee normalized to that of the contralateral joint also expressed as a function of time. The solid line represents the regression equations for each knee and the dashed lines represent the associated 95% confidence intervals.
Inflammatory cytokines, procathepsin B and NE levels
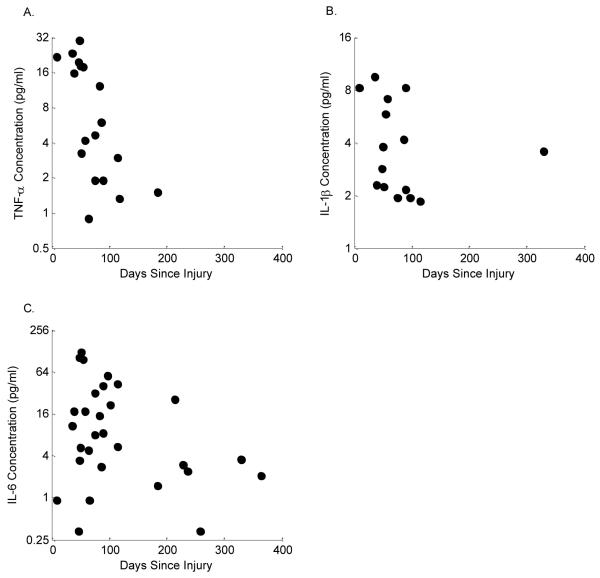
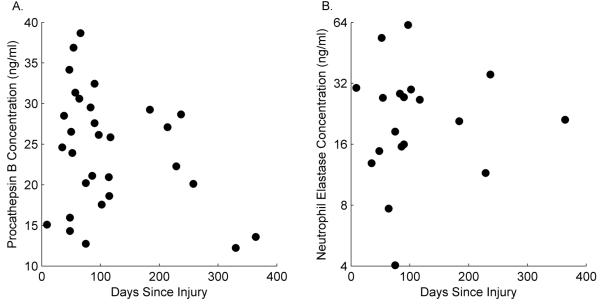
The SF IL-1β, TNF-α, IL-6, procathepsin B and NE in injured joints and their relationships to time post-injury are depicted in Figures 2 and 3. The likelihood of attaining detectable levels of TNF-α, IL-1β, IL-6, procathepsin B, and NE was significantly higher in the injured knee than in the contralateral knee (p<0.0001 for each). In fact, there were no detectable levels of any of these cytokines or enzymes found in contralateral knees. Log2 TNF-α levels showed a statistically significant negative relationship with log2 lubricin concentrations where levels were detectable (slope=−0.23, SE=0.08, T(16)=−3.00, p=0.0085)(Fig. 4A). There was no statistically significant relationship between log2 IL-1β, log2 IL6, procathepsin B, or NE and log2 lubricin (slope=−0.12, SE=0.19, T(13)=−0.62, p=0.5474; slope=−0.08, SE=0.06, T(27)=−1.52, p=0.1402; slope=−0.09, SE=0.29, T(28)=−0.30, p=0.7629; and slope=−0.1717, SE=0.1931, T(17)=−0.89, p=0.3865, respectively).
Fig. 2.
Cytokine concentrations in synovial fluid: (A) TNF-α (slope=−0.23, SE=0.08, p=0.0085), (B) IL-1β (slope=−0.12, SE=0.19, p=0.5474), and (C) IL-6 (slope=−0.08, SE=0.06, p=0.1402) in injured joints by ELISA. From the injured joints, 50% of the samples had detectable IL-1β levels, 60% had detectable TNF-α levels, and 95% had detectable IL-6 levels. 100% of the samples from the contralateral joints had no detectable cytokine levels.
Fig. 3.
Catabolic enzyme concentrations in synovial fluid: (A) Procathepsin B (slope=−0.1717, SE=0.1931, T(17)=−0.89, p=0.3865), and (B) NE (slope=−0.09, SE=0.29, T(28)=−0.30, p=0.7629) in injured joints by ELISA. From the injured joints, 100% of the samples had detectable procathepsin B levels, and 80% had detectable NE levels. 100% of the samples from the contralateral joints had no detectable NE and procathepsin B levels.
Fig. 4.
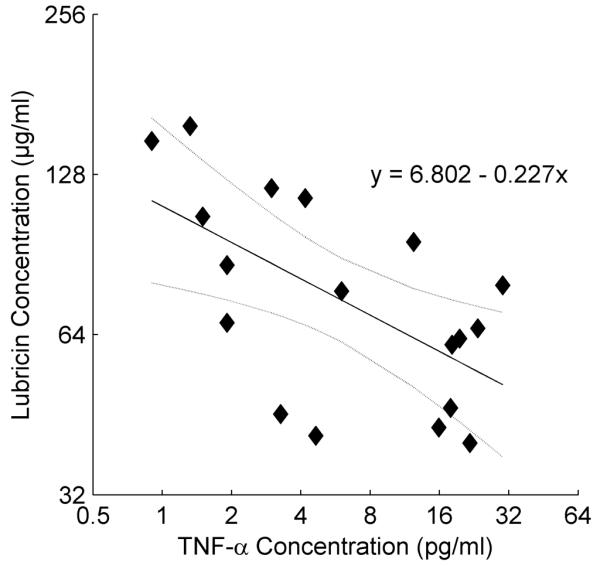
Regression analysis of log2 lubricin vs log2 TNF-α concentration in SF from patients diagnosed with ACL injury. There was no detectable TNF-a in the SF from the contralateral knee. No other significant regressions were found with the other cytokines, NE, and procathepsin B.
Sulfated glycosaminoglycan (sGAG) levels
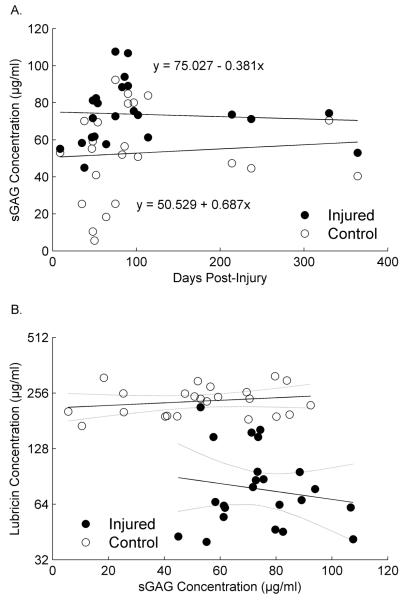
There was no statistically significant trend in SF sGAG concentrations in either the injured or contralateral limb with time post-injury, nor any difference between their trends (Fig. 5A). However, the injured limbs had significantly higher concentrations than contralateral limbs (lsmean=73.7, SE=3.5 and lsmean=52.9, SE=5.2, respectively, diff=20.82, SE=4.70, T(21)=4.43, p=0.0002). There was also no statistically significant relationship between sGAG and lubricin (Main effect F(1,22)=0.25, p=0.6195), and no evidence of a statistically significant difference between limbs in the relationship between sGAG and lubricin (Interaction F(1,22)=1.02, p=0.3245). It is interesting to note that sGAG concentrations were highly variable in the contralateral knee when the lubricin levels were high, where as the sGAG concentrations were always high in the injured knee when the lubricin concentrations were low (Fig. 5B).
Fig. 5.
(A) Mean SF sGAG concentrations in injured and contralateral joints of patients following an isolated ACL injury. Error bars represent 1 standard deviation. *Indicates that SF sGAG concentrations were significantly (p=0.003) higher in injured joints compared to contralateral joints. (B) SF sGAG concentrations expressed as a function of time following ACL injury in both joints. The sGAG concentration did not change as a function of time in either knee.
DISCUSSION
Lubricin is a surface-active mucin glycoprotein which physiosorbs to surfaces providing chondroprotective and anti-adhesive properties. Lubricin protects the cartilage surface, inhibits synoviocyte overgrowth (17) and prevents wear of articular cartilage. Camptodactyly-arthropathy-coxa-vara pericarditis (CACP) syndrome is an autosomal recessive disorder characterized by non-inflammatory arthropathy and synovial hyperplasia attributable to absent lubricin expression (18). SF boundary lubrication provided by lubricin studied in vitro, was decreased in a patient population with traumatic joint synovitis and RA (6). In contrast, SF from patients with OA appears to provide near normal lubricating ability in vitro (6). The observation of compromised joint lubrication also extends to patients with an acute ACL injury.
The significant decrease in SF lubricin levels of the ACL deficient joint was marked within the early days post-injury. As inflammatory cytokines diminished, SF lubricin levels in injured joints approached that of the contralateral control over the course of a year (Fig. 1B), though we can not predict how the lubricin concentrations would continue to change after 12 months. The decrease in SF lubricin following ACL injury has been corroborated in animal models. In the guinea pig, transection of the ACL significantly reduced SF lubricin levels 9-months after surgery, and this corresponded to an increase in the coefficient of friction of the articulating surfaces (19). Following rabbit ACL and PCL transection, SF lubricin concentrations in lavage progressively decreased, lacked full-length lubricin on Western blot and appeared associated with increased cartilage damage (20)
As expected, there were higher SF concentrations of IL-1β, TNF-α and IL-6 at an early stage following the injury. Other investigators have reported that the levels of these cytokines were highest within 24 hours after injury and that they decreased there after (21). In our study, IL-6 and TNF-α remained detectable for almost 6 months following injury, while IL-1β SF levels quickly declined at 3 months following the injury (Fig. 2). The injured SF contained higher levels of IL-6 and TNF-α compared to IL-1β when the injury was 60 days old or less. Our findings also demonstrate that these cytokines were undetected in the contralateral joint. Similar findings were reported in a study of the natural history of ACL-deficient knees at different stages of injury acuity (22).
There were many cytokine assays for which concentration estimates either fell below the manufacturer’s recommended minimum detectable limit, or the individual assay’s estimate fell below that of the zero concentration control estimate. Both these scenarios are likely generated by “truly” low or zero concentrations. This is supported by the finding that the probability of a detectable cytokine concentration was significantly higher for injured than contralateral knees for all three cytokines (60% vs. zero for TNF-a, 50% vs. zero for IL1-B, and 97% vs. zero for IL-6; Fisher’s exact p<.0001 for each). When conservatively treated as missing data, this non-random effect may bias results, particularly in terms of our primary hypothesis; cytokines catabolize lubricin, and therefore their concentrations are inversely related to lubricin concentrations. To evaluate the impact, three approaches were performed when handling “truly low” values when analyzing cytokines relative to lubricin concentrations: 1) considered them missing, 2) imputed as 3, .01 or 0.04 pg/ml (the minimum detection limit for IL-1β, TNF-α, and IL-6, respectively), and 3) imputed as the minimum value detected. Neither, the first approach nor the latter two suggested contradictory results but rather reaffirmed the positive findings or tempered the negative findings (i.e. lower p-values, marginally significant for IL-1β using minimum detectable, but not minimum observed), so the results of the first method were reported here.
The cytokines IL-1β, TNF-α and IL-6 upregulate proteolytic enzymes, that may degrade lubricin and lead to the loss of SF chondroprotection. IL-1β and TNF-α can stimulate the secretion of latent cysteine proteases e.g. cathepsin B from cultured synovial fibroblasts from patients with OA and RA (23). In an antigen-induced arthritis model, a decrease in synovial lubricin expression was found to be associated with an increase expression of cathepsin B and IL-1β (24). Inhibition of cathepsin B activity in pooled SF from patients with RA inhibited the proteolytic degradation of re-introduced human lubricin in these fluids (25). In the present study, procathepsin B levels were elevated in SF from injured joints while remaining undetectable in the contralateral joint SF. Procathepsin B is the precursor to cathepsin B. SF cathepsin B activity was not measured in this study, but it seems plausible to assume that an increase in SF procathepsin B levels would be associated with increased SF cathepsin B activity (26). Similarly, NE was detected in SF from the injured joints and not the contralateral joints. NE is a serine protease that was shown to completely degrade lubricin (4, 12, 20) and increase cartilage friction, and hence wear, in vivo (5).
Changes in SF sGAG levels were evident at an early stage following ACL injury. The significant increase in SF sGAG levels following the injury maybe indicative of articular cartilage damage. Within 3 months of the injury, there was overlap of SF sGAG levels between the injured and contralateral joints, indicating that proteoglycan catabolism may be contained. This could be consistent with a superficial injury to cartilage characterized by larger differences in SF lubricin, than SF GAG, between injured and contralateral joints. Following ACL and PCL transection in the rabbit model, low lubricin levels and the appearance of collagen type II peptides appeared to precede the appearance of sGAG in the SF (20). This highlights an important difference between lubricin and sGAG metabolism following injury that might connect an ACL injury to a nidus leading to secondary OA.
In the present study we used sGAG as a biomarker for proteoglycan turnover, and we found that the sGAG concentrations were elevated in the injured knee when compared to the contralateral knee. Various markers of cartilage matrix turnover have been previously evaluated following ACL injury (27-31). Lohmander et al reported a marked increase in SF proteoglycan fragments within 3–4 weeks of ACL injury that remained high in many patients out to 4 years after injury (29). Likewise, the proteoglycan turnover epitope 846 has also been shown to be elevated following ACL injury, and this was correlated with collagen turnover markers including cartilage oligomeric matrix protein (COMP) and C-propeptide of type II collagen (CPII) (30). In addition, procollagen type II C-propeptide (PIICP), a marker of collagen type II synthesis, and the release of cross-linked collagen type II into SF have been shown to increase following ACL injury (28, 31). These studies support the finding that cartilage metabolism is altered following injury and may be indicators for OA in this patient population. In the current study, the pattern of lubricin SF changes following ACL injury shows an early decrease in SF lubricin levels, which appear to recover within 1 year after injury, however the metabolic changes appear to persist. It is interesting to note that the SF sGAG concentrations were variable when the lubricin concentrations were high in the contralateral knee (Fig 5B). However, in the injured knee, low lubricin levels were consistently associated with high sGAG concentrations (Fig. 5B). The implications of this remain to be proven but suggest that proteoglycan turnover is increased in the presence of low lubricin values. Thus, it is our hypothesis that the pro-inflammatory cytokines initiate a cascade of events that lead to a decrease in joint lubrication and an increase in cartilage wear. Although the lubricin levels eventually return to normal, ongoing damage may have been initiated.
In this study, the patient population was relatively young with no history of degenerative joint disease. Each patient served as his own control by aspirating undiluted SF from the contralateral joint allowing for meaningful comparison between the injured and the contralateral joint. We attempted to limit confounding factors by the study inclusion criteria. Nonetheless, other factors such as bone bruising, minor meniscal damage, and unseen trauma could also influence the results. Other limitations of this study include the cross-sectional nature of the study, and the possible dilution of lubricin and other markers in the injured joint due to transudate. To prevent the dilution effect, lubricin levels were also compared after normalizing them to SF total protein levels without changing the results. It is also unknown what happens to the lubricin concentrations after the 12 month time period. The true concentration must eventually asymptote at a biologically plausible value. Unfortunately we do not have lubricin concentration data from patients more than one year from injury since the focus of the study was on the acute injury state. Future long-term longitudinal studies will be directed at measuring the temporal changes in lubricin levels following ACL injury and its treatment.
In addition, alterations in joint kinematics in the contralateral joint may occur, and it is not completely known how it may affect lubricin metabolism. It was assumed that the contralateral knee was not affected, an assumption supported by the finding that the lubricin concentration did not significantly change with time (Fig. 1B). The dynamic shear of cartilage appears important in expression of lubricin by superficial zone chondrocytes (32, 33). Relieving load could lead to lower expression of articular lubricin by cartilage. This could mean that normal lubricin levels would have been higher than we observed in the contralateral joints. Finally, the relationship between lubricin in SF and lubricin bound to the articular cartilage is not completely known. However, there is evidence that equilibrium may exist between SF and surface-associated lubricin (34).
In this study, we have examined the effects of an ACL injury on SF lubricin and its relationship to markers of inflammation and cartilage damage. SF lubricin level was significantly decreased following the injury and subsequently increased as the inflammation subsided. The decrease in SF lubricin in the early stages places the joint at elevated risk of wear-induced damage. Blocking the effects of TNF-α, IL-1β, and IL-6 after injury may prove valuable in preventing the decrease in SF lubricin, which in turn could possibly preserve chondroprotection. The results of this study also suggest that lubricin levels can be used as a marker of cartilage health, especially in the early stages of an acute disease process.
ACKNOWLEDGEMENTS
The authors would like to thank Dr. Tom Schmid of Rush Medical School for providing anti-lubricin monoclonal antibody S6.89. This research was funded by NIH RO1-AR051080, RO1-AR047910, RO1-AR049199, P20-RR024484.
REFERENCES
- 1.Jay GD, Britt DE, Cha CJ. Lubricin is a product of megakaryocyte stimulating factor gene expression by human synovial fibroblasts. J Rheumatol. 2000;27:594–600. [PubMed] [Google Scholar]
- 2.Jay GD, Tantravahi U, Britt D, Barrach HJ, Cha CJ. Homology of lubricin and superficial zone protein (SZP): Products of megakaryocyte stimulating factor (MSF) gene expression by human synovial fibroblasts and articular chondrocytes localized to chromosome 1q25. J Orthop Res. 2001;19:9–19. doi: 10.1016/S0736-0266(00)00040-1. [DOI] [PubMed] [Google Scholar]
- 3.Flannery CR, Hughes CE, Schumacher BL, Tudor D, Aydelotte MB, Kuettner KE, et al. Articular cartilage superficial zone protein (SZP) is homologous to megakaryocyte stimulating factor precursor and Is a multifunctional proteoglycan with potential growth-promoting, cytoprotective, and lubricating properties in cartilage metabolism. Biochem Biophys Res Comm. 1999;254:535–541. doi: 10.1006/bbrc.1998.0104. [DOI] [PubMed] [Google Scholar]
- 4.Jay GD. Characterization of a bovine synovial fluid lubricating factor. I. Chemical, surface activity and lubricating properties. Connect Tissue Res. 1992;28:71–88. doi: 10.3109/03008209209014228. [DOI] [PubMed] [Google Scholar]
- 5.Jay GD, Torres JR, Rhee DK, Helminen HJ, Hytinnen MM, Cha CJ, et al. Association between friction and wear in diarthrodial joints lacking lubricin. Arthritis Rheum. 2007;56:3662–3669. doi: 10.1002/art.22974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jay GD, Elsaid KA, Zack J, Robinson K, Trespalacios F, Cha CJ, et al. Lubricating ability of aspirated synovial fluid from emergency department patients with knee joint synovitis. J Rheumatol. 2004;31:557–64. [PubMed] [Google Scholar]
- 7.Young AA, McLennan S, Smith MM, Smith SM, Cake MA, Read RA, et al. Proteoglycan 4 downregulation in a sheep meniscectomy model of early osteoarthritis. Arthritis Res Ther. 2006;8:R41. doi: 10.1186/ar1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmidt TA, Gastelum NS, Han EH, Nugent-Derfus GE, Schumacher BL, Sah RL. Differential regulation of proteoglycan 4 metabolism in cartilage by IL-1alpha, IGF-I, and TGF-beta1. Osteoarthritis Cartilage. 2007 doi: 10.1016/j.joca.2007.05.009. Epub. [DOI] [PubMed] [Google Scholar]
- 9.Jones ARC, Flannery CR. Differential regulation of lubricin biosynthesis by chondrocytes and synoviocytes. Trans Orthop Res Soc. 2007;32:547. [Google Scholar]
- 10.Jones ARC, Sheldon R, Keohan C, Majumader M, Morris E, Flannery CR. Cytokine regulated metabolism of lubricin in situ in cartilage explant culture. Trans Orthop Res Soc. 2006;31:143. [Google Scholar]
- 11.Elsaid KA, Chichester CO, Jay GD. Cathepsin B and neurtophil elastase degrade lubricin and increase friction in excised murine joints. Trans Orthop Res Soc. 2005;30:0924. [Google Scholar]
- 12.Jones ARC, Hughes CE, Wainwright SD, Flannery CR, Little CB, Caterson B. Degradation of PRG4/SZP by matrix proteases. Trans Orthop Res Soc. 2003;28:133. [Google Scholar]
- 13.Gelber AC, Hochberg MC, Mead LA, Wang NY, Wigley FM, Klag MJ. Joint injury in young adults and risk for subsequent knee and hip osteoarthritis. Ann Intern Med. 2000;133:321–328. doi: 10.7326/0003-4819-133-5-200009050-00007. [DOI] [PubMed] [Google Scholar]
- 14.Fleming BC, Hulstyn MJ, Oksendahl HL, Fadale PD. Ligament injury, reconstruction, and osteoarthritis. Curr Opin Orthop. 2005;16:354–362. doi: 10.1097/01.bco.0000176423.07865.d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Su JL, Schumacher BL, Lindley KM, Soloveychik V, Burkhart W, Triantafillou JA, et al. Detection of superficial zone protein in human and animal body fluids by cross-species monoclonal antibodies specific to superficial zone protein. Hybridoma. 2001;20:149–157. doi: 10.1089/027245701750293475. [DOI] [PubMed] [Google Scholar]
- 16.Bland JM, Altman JM. Statistical Notes: Transforming Data. British Medical Journal. 1996;312:770. doi: 10.1136/bmj.312.7033.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rhee DK, Marcelino J, Baker M, Gong Y, Smits P, Lefebvre V, et al. The secreted glycoprotein lubricin protects cartilage surfaces and inhibits synovial cell overgrowth. J Clin Invest. 2005;115:622–631. doi: 10.1172/JCI200522263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marcelino J, Carpten JD, Suwairi WM, Gutierrez OM, Schwartz S, Robbins C, et al. CACP, encoding a secreted proteoglycan, is mutated in camptodactyly-arthropathy-coxa vara-pericarditis syndrome. Nat Genet. 1999;23:319–322. doi: 10.1038/15496. [DOI] [PubMed] [Google Scholar]
- 19.Teeple E, Elsaid KA, Fleming BC, Jay GD, Aslani K, Crisco JJ, et al. Coefficients of friction and cartilage damage in the guinea pig knee. J Orthop Res. 2007 doi: 10.1002/jor.20492. EPub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elsaid KA, Jay GD, Warman ML, Rhee DK, Chichester CO. Association of articular cartilage degradation and loss of boundary-lubricating ability of synovial fluid following injury and inflammatory arthritis. Arthritis Rheum. 2005;52:1632–1633. doi: 10.1002/art.21038. [DOI] [PubMed] [Google Scholar]
- 21.Irie K, Uchiyama E, Iwaso H. Intraarticular inflammatory cytokines in acute anterior cruciate ligament injured knee. Knee. 2003;10:93–96. doi: 10.1016/s0968-0160(02)00083-2. [DOI] [PubMed] [Google Scholar]
- 22.Cameron M, Buchgraber A, Passler H, Vogt M, Thonar E, Fu FH, et al. The natural history of the anterior cruciate ligament-deficient knee - Changes in synovial fluid cytokine and keratan sulfate concentrations. Am J Sports Med. 1997;25:751–754. doi: 10.1177/036354659702500605. [DOI] [PubMed] [Google Scholar]
- 23.Huet G, Flipo RM, Colin C, Janin A, Hemon B, Collyn-d’Hooghe M, et al. Stimulation of the secretion of latent cysteine proteinase activity by tumor necrosis factor alpha and interleukin-1. Arthritis Rheum. 1993;36:772–80. doi: 10.1002/art.1780360606. [DOI] [PubMed] [Google Scholar]
- 24.Elsaid KA, Jay GD, Chichester CO. Reduced expression and proteolytic susceptibility of lubricin/superficial zone protein may explain early elevation in the coefficient of friction in the joints of rats with antigen-induced arthritis. Arthritis Rheum. 2007;56:108–116. doi: 10.1002/art.22321. [DOI] [PubMed] [Google Scholar]
- 25.Elsaid KA, Jay GD, Chichester CO. Inhibition of cathepsin B and L retards the loss of boundary lubrication of rheumatoid arthritis synovial fluid aspirates in vitro. Trans Orthop Res Soc. 2005;30:340. [Google Scholar]
- 26.Mach L, Schwihla H, Stuwe K, Rowan AD, Mort JS, Glossl J. Activation of procathepsin B in human hepatoma cells: the conversion into the mature enzyme relies on the action of cathepsin B itself. Biochem J. 1993;293:437–42. doi: 10.1042/bj2930437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fang C, Johnson D, Leslie MP, Carlson CS, Robbins M, Di Cesare PE. Tissue distribution and measurement of cartilage oligomeric matrix protein in patients with magnetic resonance imaging-detected bone bruises after acute anterior cruciate ligament tears. J Orthop Res. 2001;19:634–641. doi: 10.1016/S0736-0266(00)00039-5. [DOI] [PubMed] [Google Scholar]
- 28.Lohmander LS, Atley L, Pietka TA, Eyre DR. The release of cross linked proteins from type II collagen in human synovial fluid is increased soon after injury. Arthritis Rheum. 2003;48:3130–3139. doi: 10.1002/art.11326. [DOI] [PubMed] [Google Scholar]
- 29.Lohmander LS, Dahlberg L, Ryd L, Heinegard D. Increased levels of proteoglycan fragments in knee joint fluid after injury. Arthritis Rheum. 1989;32:1434–1442. doi: 10.1002/anr.1780321113. [DOI] [PubMed] [Google Scholar]
- 30.Lohmander LS, Ionescu M, Jugessur H, Poole AR. Changes in joint cartilage aggrecan after knee injury and in osteoarthritis. Arthritis Rheum. 1999;42:534–544. doi: 10.1002/1529-0131(199904)42:3<534::AID-ANR19>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 31.Lohmander LS, Yoshihara Y, Roos H, Kobayashi T, Yamada H, Shinmei M. Procollagen II C-propeptide in joint fluid: changes in concentration with age, time after knee injury, and osteoarthritis. J Rheumatol. 1996;23(10):765–769. [PubMed] [Google Scholar]
- 32.Nugent GE, Schmidt TA, Schumacher BL, Voegtline MS, Bae WC, Jadin KD, et al. Static and dynamic compression regulate cartilage metabolism of ProteoGlycan 4 (PRG4) Biorheology. 2006;43:191–200. [PubMed] [Google Scholar]
- 33.Grad S, Lee CR, Wimmer MA, Alini M. Chondrocyte gene expression under applied surface motion. Biorheology. 2006;43:259–69. [PubMed] [Google Scholar]
- 34.Nugent-Derfus GE, Chan AH, Schumacher BL, Sah RL. PRG4 exchange between the articular cartilage surface and synovial fluid. J Orthop Res. 2007;25:1269–76. doi: 10.1002/jor.20431. [DOI] [PubMed] [Google Scholar]