Summary
The mechanisms that maintain human T cell memory during normal and perturbed homeostasis are not fully understood. The repeated induction of profound lymphocytopenia in patients undergoing multiple cycles of cytotoxic chemotherapy infrequently results in severe infections with viruses controlled by memory T cells, suggesting that some memory T cells survive chemotherapy and restore immunity. Here we identify a distinct subpopulation of memory CD8+ T cells with the ability to rapidly efflux and survive exposure to chemotherapy drugs in vitro and in vivo. T cells with high efflux capacity share expression of molecules with hematopoietic stem cells, are quiescent in non-lymphocytopenic individuals, and are induced to proliferate in patients rendered lymphocytopenic after chemotherapy. Effluxing T cells differentiate into non-effluxing subsets in response to antigen stimulation and inflammatory signals, thereby contributing to repopulation of memory cells after chemotherapy.
Introduction
A hallmark of adaptive immunity to pathogens is the establishment of long-lived memory T cells that are able to rapidly respond to re-infection and control reactivation of persistent pathogens. After clearance of primary viral infection in mice, CD8+ memory T cells remain for the life of the animal (Murali-Krishna et al., 1998). In humans, memory T cells elicited in response to smallpox vaccination persist for 75 years in the absence of re-exposure to the virus (Hammarlund et al., 2003). The durability of T cell memory under normal homeostasis is due in part to slow cell division mediated by cytokines such as IL-15 (Judge et al., 2002; Zhang et al., 1998), but the mechanisms by which T cell memory is maintained when homeostasis is perturbed by toxic environmental or iatrogenic insults that cause lymphocytopenia, have not been extensively studied.
CD8+ memory T cells are required to control reactivations of cytomegalovirus (CMV) and Epstein Barr virus (EBV) (Smets et al., 2002; Walter et al., 1995). Patients with acute myeloid leukemia (AML) receive repeated cycles of chemotherapy that induce severe but transient lymphocytopenia, yet rarely develop clinical infection with CMV or EBV either during the lymphocyte nadir or after recovery of lymphocyte numbers (Sung et al., 2009). The absence of infection in AML patients undergoing chemotherapy suggests that sufficient virus-specific memory T cells survive chemotherapy and reconstitute functional, long-lived immunity thereafter.
Two broad subsets of memory T cells, termed central memory (TCM) and effector memory (TEM), have been identified that differ in phenotype and function (Sallusto et al., 1999). In humans, these subsets have considerable heterogeneity, which could potentially include subpopulations that serve a distinct role in reconstituting memory T cells after chemotherapy, analogous to the reconstitution of hematopoiesis by hematopoietic stem cells (HSC). The mechanisms by which HSC are resistant to chemotherapy are related both to cell quiescence and the over-expression of ATP-binding cassette (ABC)-superfamily multidrug efflux proteins that protect cells from toxic xenobiotics and endogenous metabolites (Chaudhary and Roninson, 1991; Gottesman et al., 2002; Mizutani et al., 2008). We used ABCB1-mediated efflux of the fluorescent marker rhodamine-123 (Rh123) to determine whether CD8+ memory T cells might employ a similar mechanism. We identified a quiescent subpopulation of polyclonal memory CD8+ T cells in both TCM and TEM fractions that have high multidrug cotransporter activity and a distinct phenotype. The effluxing CD8+ T cells contain virus-specific cells, are induced to proliferate during lymphocytopenia, and can self-renew and differentiate into the more prevalent non-effluxing memory subsets. Thus, distinct CD8+ memory T cells employ conserved resistance mechanisms utilized by stem cells of diverse origin, and exhibit a stem cell like capacity for self-renewal and differentiation.
Results
CD8+ virus-specific T lymphocytes persist after cytotoxic chemotherapy
Patients with AML treated with chemotherapy that includes ABCB1 substrates such as daunorubicin and idarubicin, develop profound transient bone marrow hypoplasia and peripheral lymphocyte depletion (Figure 1a) (Berman et al., 1991). These patients rarely succumb to infection from acute or persistent viruses suggesting that some CD8+ memory T cells resist chemotherapy and replenish the memory T cell pool during lymphocyte recovery. We examined whether CD8+ T cells specific for CMV, EBV, and influenza were present in blood obtained from adults after recovery from chemotherapy that included an ABCB1 substrate drug, and induced a lymphocyte nadir to less than 100 cells/μl. CD8+ T cells specific for CMV, EBV or influenza virus were detected at a frequency of 12.5 +/− 3.9% (mean +/− standard error) after brief in vitro culture with autologous monocyte-derived dendritic cells (MoDC) pulsed with a pool of CMV-, EBV- and influenza-derived peptides (Figure 1b,c). What is uncertain is whether the survival of memory cells after chemotherapy is simply stochastic, or if a defined subset of memory T cells might preferentially resist chemotherapy.
Figure 1. CD8+ virus-specific memory T cells persist through profound chemotherapy-induced lymphocytopenia.
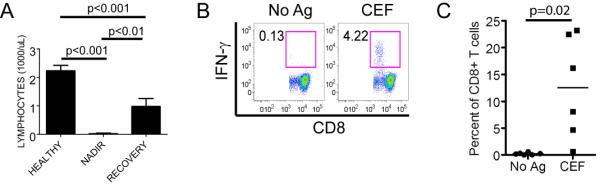
(A) Absolute blood lymphocyte counts (mean +/− SE) of healthy donors (n=11), and AML patients at the lymphocyte nadir (n=10) and after recovery (n=9, 1 died) from anthracycline containing induction chemotherapy.
(B) Cytokine flow cytometry (CFC) detects IFN-γ+ CD8+ virus-specific T cells in PBMC obtained after completion of induction and consolidation chemotherapy for AML. PBMC were stimulated with a pool of antigenic peptides from CMV, EBV and influenza viruses (CEF), and cultured with IL-2, IL-7 and IL-15 for 8 days. The percentage of CD8+ T cells that secrete IFN-γ in response to peptide restimulation (CEF) or without restimulation (No Ag) on day 8 is indicated. Data is shown for a representative patient sample obtained 2.5 months after chemotherapy.
(C) CFC assay for IFN-γ+ CD8+ T cells specific for CMV, EBV, and influenza in blood obtained from 6 AML patients after lymphocyte recovery from chemotherapy. The assay was performed on PBMC obtained 1.5–3 months after completing chemotherapy as described in (B). Each point represents data from an individual patient and the mean is represented by the bar.
Subsets of TCM and TEM CD8+ TcRαβ+ cells have high multidrug efflux capacity mediated by ABCB1
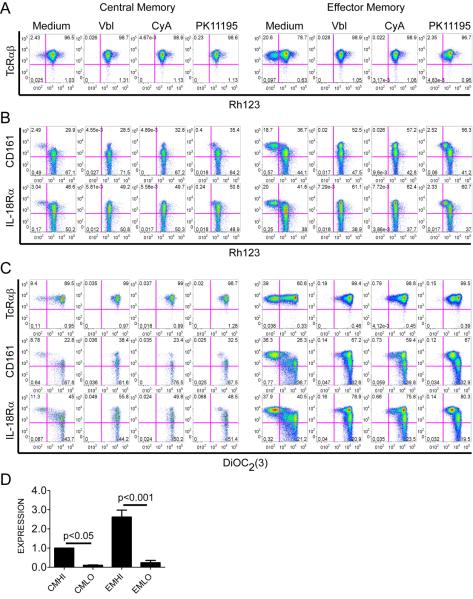
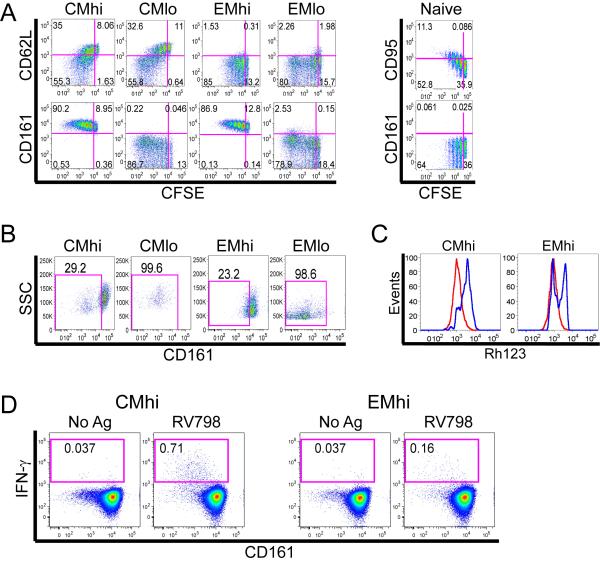
Overexpression of ABC-superfamily multidrug efflux proteins contributes to the chemotherapy resistance of HSC and malignant cells (Gottesman et al., 2002), and might explain the persistence of some CD8+ memory T cells after chemotherapy. We used multiparameter flow cytometry to measure the capacity of CD8+ naïve (TN), TCM and TEM cells from normal donors to efflux the fluorescent substrate Rh123, which is effluxed through the same ABC transporters that efflux drugs used to treat leukemia (Chaudhary and Roninson, 1991; Gottesman et al., 2002). We incorporated antibodies to CD4, TcRγδ, Vα24.1 and CD16 to exclude CD4+ T cells, γδ T cells, NKT cells and NK cells, allowing selective analysis of CD8+ TcRαβ+ T cells; and used expression of CD95 to distinguish memory cells from TN, and CD62L to divide memory cells into TCM and TEM subsets (Supplementary Figure 1). We identified a significant population of cells within each of the CD8+ TcRαβ+ TCM and TEM subsets that effluxed Rh123 at a rate far in excess of that observed for the majority of TCM and TEM (Figure 2a). The efflux of Rh123 was blocked by the addition of vinblastine, which acts as a competitive inhibitor of ABCB1 and ABCC1 transporters, and by cyclosporine A and PK11195, which inhibit ABCB1 (Figure 2a) (Schinkel and Jonker, 2003; Walter et al., 2004).
Figure 2. Subsets of TCM and TEM rapidly efflux Rh123 and DiOC2(3).
PBMC were loaded on ice with Rh123, cultured in medium alone (Medium) or with ABCB1 inhibitors as indicated, and labeled with mAbs. Gating is on TCM and TEM identified as CD62L+ and CD62L− events, respectively within the CD4−/CD16−/TcRγδ−/Vα24−/CD3+/CD8+/CD95+ population (Supplementary Figure 1). Data in (A-C) are representative of four separate experiments.
(A) A subset of TcRαβ+ TCM and TEM rapidly efflux Rh123 (left upper quadrant), and efflux is blocked by vinblastine (Vbl), cyclosporine A (CyA), and PK11195.
(B) Rapidly effluxing TCM and TEM express higher levels of CD161 and IL-18Rα than non-effluxing cells.
(C) TcRαβ+ CD161hi and IL-18Rαhi TCM and TEM efflux DiOC2(3), and efflux is blocked by Vbl, CyA or PK11195. PBMC were loaded on ice with DiOC2(3), cultured and labeled as in (A) and (B).
(D) Quantitative RT-PCR analysis of ABC transporter expression on sort-purified IL-18Rαhi CD8+ TCM or TEM. Expression of abcb1 is normalized to that of CMhi. Data represent the mean +/− SE of three separate experiments, each performed in triplicate.
It would be advantageous if surface markers could distinguish the subsets of TCM and TEM with high Rh123 efflux capacity (termed CMhi and EMhi) from slowly effluxing memory cells (termed CMlo and EMlo), and from TN cells. Thus, we analyzed the expression of cell surface molecules that were previously identified in microarray studies to be shared between memory T cells and HSC (Luckey et al., 2006); or to be differentially expressed between TN and memory cells (Haining et al., 2008; Holmes et al., 2005). We found that TCM and TEM that rapidly effluxed Rh123 could be distinguished from their non-effluxing counterparts by co-expression of high levels of IL-18Rα and CD161 (Figure 2b; Supplementary Figure 2a,b). CD117 (c-kit), which is expressed on HSC (Simmons et al., 1994), was also expressed on a significant fraction of CMhi and EMhi (Supplementary Figure 2c), however IL-18Rα or CD161 detected virtually all of the rapidly effluxing cells. A small subset of TN effluxed Rh123, but efflux was less rapid and complete than that observed for CMhi and EMhi, and the effluxing TN did not express high levels of IL-18Rα or CD161 (Supplementary Figure 3a,b).
The specificity of the efflux pathway utilized in CMhi and EMhi was interrogated further by measuring efflux of DiOC2(3), a substrate that has high specificity for ABCB1, minimal specificity for ABCG2, and no specificity for ABCC1 (Minderman et al., 1996). DiOC2(3) was rapidly effluxed by the subset of TCM and TEM that expressed high levels of IL-18Rα and CD161 suggesting efflux was mediated by ABCB1 (Figure 2c). Quantitative RT-PCR of ABC cotransporter expression in subsets of TCM and TEM separated based on expression of high levels of IL-18Rα confirmed high expression of abcb1 on the IL-18Rαhi subsets of TCM and TEM compared with IL-18Rαlo subsets (Figure 2d).
CMhi and EMhi are resistant to anthracycline cytotoxicity
The capacity of IL-18Rαhi CD161hi TCM and TEM to rapidly efflux Rh123 and DiOC2(3) suggested these cells may be preferentially protected from cytotoxic chemotherapy. We exposed PBMC to a fluorescent anthracycline (daunorubicin) and measured its efflux in CD8+ T cells over 30 minutes, using multiparameter flow cytometry to distinguish cells that expressed high levels of IL-18Rα and CD161. The IL-18Rαhi and CD161hi subsets of CD8+ TCM and TEM rapidly effluxed daunorubicin, and efflux was blocked by vinblastine, PK11195, and cyclosporine (Figure 3a, and data not shown).
Figure 3. CMhi and EMhi efflux daunorubicin and are protected from cytotoxicity during exposure to daunorubicin.
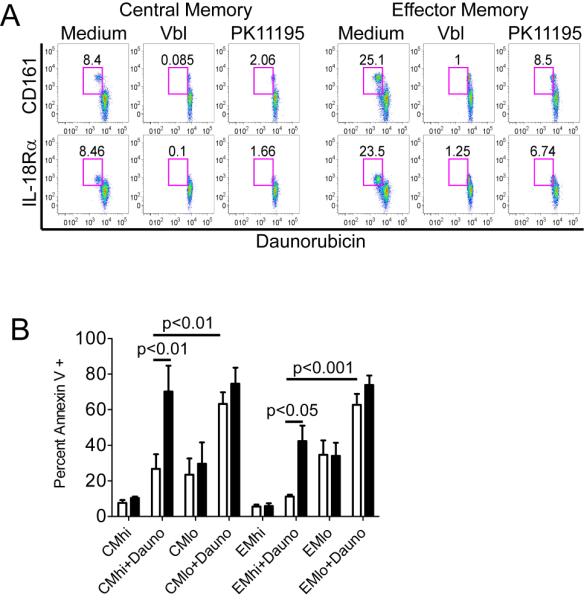
(A) CD161hi IL-18Rαhi TCM and TEM efflux daunorubicin. PBMC were loaded with daunorubicin, cultured in medium alone or with ABCB1 inhibitors (Vbl, PK11195), and surface labeled with mAbs. CD8+ TCM and TEM were identified as described in Figure 2 and the subset that effluxed daunorubicin is shown in the upper left gate in each panel. Data are representative of three separate experiments.
(B) Sort-purified IL-18Rαhi TCM and TEM are resistant to daunorubicin in vitro. IL-18Rαhi and IL-18Rαlo subsets of TCM and TEM were sort purified, cultured for 40 hours in the presence or absence of daunorubicin with (black bars) or without (white bars) PK11195 and then labeled with Annexin V. Data represent the mean +/− SE of four separate experiments.
We then sort-purified IL-18Rαhi and IL-18Rαlo CD8+ TCM and TEM without Rh123 loading to avoid competitive antagonism of efflux, cultured the sorted subsets for 40 hours in the presence or absence of daunorubicin alone or with PK11195, and evaluated apoptosis by Annexin V staining. IL-18Rαlo cells in both TCM and TEM subsets were highly susceptible to apoptosis when cultured in daunorubicin, and the addition of PK11195 only slightly increased apoptosis. By contrast, the IL-18Rαhi cells in both TCM and TEM subsets were remarkably resistant to daunorubicin-induced apoptosis, and resistance was abrogated by the addition of PK11195 or cyclosporine (Figure 3b, and data not shown).
CMhi and EMhi are distinct subsets of antigen-experienced memory T cells
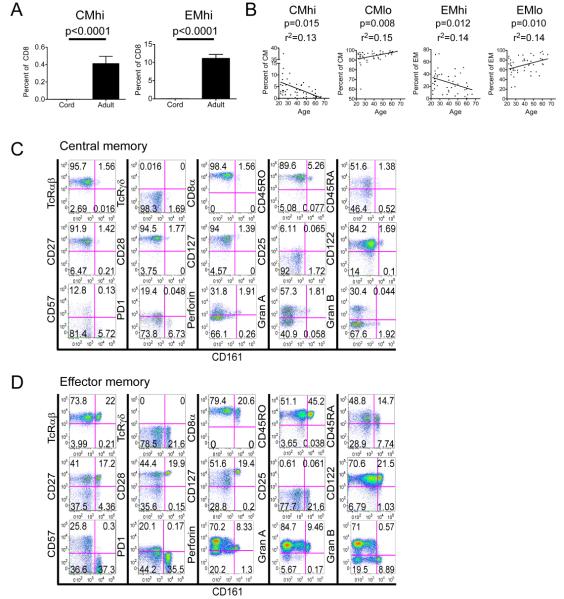
CD8+ CD161hi T cells with rapid Rh123 efflux capacity were either undetectable or exceedingly rare in cord blood, but were found in all 46 adult blood samples consistent with the emergence of these memory cells as a consequence of antigen exposure during the neonatal to adult transition (Figure 4a,b). The frequency of CD8+ CD161hi T cells as a component of total CD8+ T cells was low, particularly in the CD62L+ TCM subset and declined steadily with age (Figure 4b). CMhi and EMhi T cells could be distinguished from each other based on the expression of CD62L, but were otherwise similar in surface phenotype. CD161hi CD8+ CMhi and EMhi T cells were uniformly CD45RO+, expressed homogeneously high levels of CD127 and CD28, and were CD27+ and CD122+. These cells expressed low levels of CD45RA, perforin, and granzyme A, had lower levels of CD8α than their CD161lo counterparts, and were CD25−, CD57−, granzyme B− and PD-1− (Figure 4c-d).
Figure 4. CMhi and EMhi are absent from cord blood and exhibit a memory phenotype.
(A) Mononuclear cells from cord (n=6) and adult (n=46) blood were loaded with Rh123 and analyzed by flow cytometry as described in Figure 2. The mean +/− SE percentage of CMhi and EMhi in the total CD8+ T cell population is shown.
(B) The percentage of each subset in its parent population is shown according to the age of the donor. Linear regression is shown on each graph and r2 (derived from the Pearson correlation coefficient) and p values are as indicated.
(C-D) Phenotype of CD161hi TCM and TEM. PBMC from healthy adults were surface labeled with antibodies to CD4, CD16, TcRγδ, Vα24, CD3, CD8, CD95 and CD62L and CD8+ TCM and TEM identified as described in Figure 2. Expression of the indicated molecules on the CD161hi and CD161lo CD8+ TCM and TEM subsets is shown in (C) and (D) respectively. CMhi and EMhi are identified as CD161hi events, designated by the gate on the x-axis of each plot. Data are representative of four experiments.
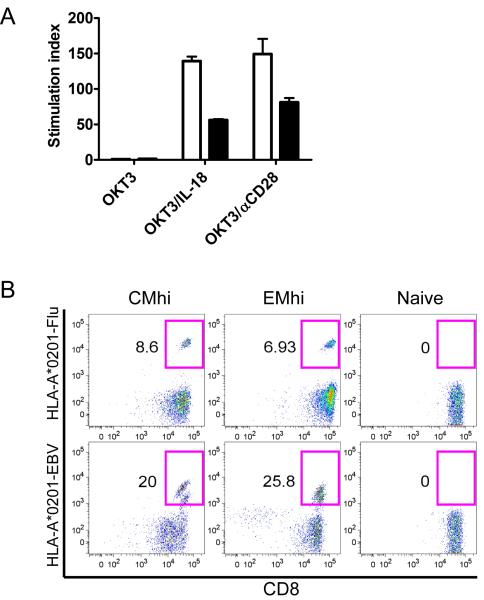
If CMhi and EMhi T cells contribute to recovery of antiviral immunity after chemotherapy, it should be possible to derive polyclonal virus-specific T cells from these subsets. A prior study suggested that CD161hi CD8+ T cells were less responsive to TcR ligation (Takahashi et al., 2006). Thus, we examined the requirements to induce proliferation of sort-purified CMhi and EMhi T cells and confirmed these cells proliferated poorly to anti-CD3 monoclonal antibody (mAb) alone, but proliferated well if IL-18 or anti-CD28 mAb were added to provide costimulation (Figure 5a). We then stimulated sort-purified CMhi, EMhi, and TN with autologous MoDC that expressed CD80/86 and were pulsed with peptides corresponding to epitopes of EBV or influenza, and elicited a population of tetramer positive T cells from each of the memory subsets in 3 of 3 donors (Figure 5b). Molecular spectratyping of expressed TcR Vβ genes was also performed to estimate the diversity of TcR gene usage by CD8+ TCM and TEM subsets sorted based on high and low CD161 expression and Rh123 efflux and by TN. CMhi and EMhi subsets had less diverse TcR Vβ usage than the TN subset, consistent with clonotype selection by prior antigen exposure. However, the TcR Vβ diversity of the CMhi and EMhi subsets was broad and similar to that observed in the CMlo and EMlo subsets (Supplementary Figure 4).
Figure 5. CMhi and EMhi proliferate to anti-CD3 mAb and costimulation and harbor virus-specific CTL.
(A) CMhi (white bars) and EMhi (black bars) were sort-purified and cultured for three days in wells with plate-bound anti-CD3 mAb with or without anti-CD28 mAb or IL-18, and tritiated thymidine incorporation measured for the last 18 hours. Data show the mean +/− SE of triplicate samples, and are representative of data from two donors.
(B) CMhi, EMhi and TN were sort-purified from HLA-A*0201+ donors, stimulated with autologous MoDC pulsed with epitopes from influenza (GILGFVFTL) or EBV (GLCTLVAML), and cultured for 8 days in the presence of IL-2, IL-7 and IL-15. Cells were stained with anti-CD8 and HLA-A*0201 tetramers folded with the influenza (left panel) or EBV (right panel) peptides. The percentage of tetramer+ events is shown. Data are representative of three (CMhi and EMhi) or two (TN) experiments.
CMhi and EMhi express high levels of bcl-2 and bcl-xL and have a low proliferative fraction
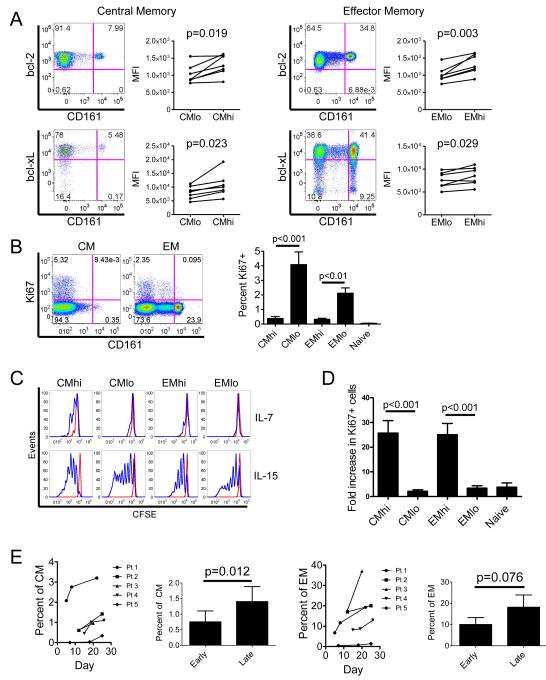
We noted that the fraction of IL-18Rαhi TCM and TEM cells that stained with Annexin V after culture in medium alone was significantly lower than for IL-18Rαlo cells (Figure 3b). This suggested that factors in addition to drug efflux might contribute to survival of IL-18Rαhi CD161hi T cells after exposure to daunorubicin. We compared the levels of bcl-2 and bcl-xL in effluxing TCM and TEM (identified by CD161hi expression) with their non-effluxing CD161lo counterparts and found that the CD161hi TCM and TEM constitutively expressed higher levels of bcl-2 and bcl-xL (Figure 6a). Cells that have a higher proliferative rate, which can be assessed by staining of intracellular Ki-67, are more sensitive to daunorubicin (Meyn et al., 1980; Scholzen and Gerdes, 2000). The proportion of CD161hi CD8+ TCM and TEM in blood that expressed Ki-67 was significantly lower than that of CD161lo CD8+ TCM and TEM (Figure 6b). Thus, CMhi and EMhi cells are endowed with several mechanisms including drug efflux, higher levels of anti-apoptotic molecules, and a lower fraction in cell cycle that enhance resistance to cytotoxic chemotherapy.
Figure 6. CMhi and EMhi are bcl-2hi, bcl-xLhi and quiescent in lymphoreplete individuals, but proliferate and are enriched under conditions of lymphocytopenia.
(A) CD161hi effluxing and CD161lo non-effluxing TCM and TEM were identified in PBMC as described in Figure 2, and stained for intracellular bcl-2 (upper panels) or bcl-xL (lower panels). Mean fluorescence intensity of bcl-2 and bcl-xL expression in effluxing and non-effluxing memory subsets from 7 healthy individuals are shown in the adjacent graphs.
(B) CD161hi and CD161lo TCM and TEM were identified in PBMC as described in Figure 2, and stained for intracellular Ki-67 expression. Representative plots of Ki-67 expression on CD161hi and CD161lo TCM and TEM are shown (left panel). The adjacent graph (right panel) shows the mean percentage +/− SE of cells that express Ki-67 in each subset in 8 healthy individuals.
(C) Subsets were isolated on the basis of CD161 expression and Rh123 efflux capacity, CFSE-loaded and cultured with IL-7 or IL-15 for 10 days before flow cytometry analysis. Histograms show viable events, gated by DAPI exclusion. Data are representative of experiments from 4 healthy individuals.
(D) Peripheral blood samples from AML patients during the lymphocyte nadir after completion of induction chemotherapy were surface labeled to identify memory T cell subsets and TN, followed by intracellular staining for Ki-67, as described in (B). The fold increase in the percentage of Ki-67+ events between healthy individuals (n=8) and AML patients after chemotherapy (n=10) for each subset is shown.
(E) The percentages of CMhi (left panels) and EMhi (right panels) in their respective parent populations are shown at variable times after commencement of anthracycline-containing induction chemotherapy for de novo AML. Data from 5 individuals are shown. Bar graphs show a comparison of the mean +/− SE percentages of CMhi (left) or EMhi (right) in their parent populations between the first sample drawn after completion of anthracycline infusion (Early) and the last sample drawn before day 28 (Late).
CMhi and EMhi proliferate in response to cytokines that maintain lymphocyte homeostasis
IL-7 and IL-15 promote the survival and intermittent proliferation of T cells under normal homeostasis, and drive their proliferation during lymphocytopenia to restore homeostasis (Schluns et al., 2000; Tan et al., 2002). To determine the responsiveness of CMhi and EMhi subsets to IL-7 and IL-15, CD161hi Rh123 effluxing and CD161lo non-effluxing TCM and TEM were sort-purified, labeled with CFSE, and cultured with IL-7 or IL-15 in the absence of TcR ligation. A greater proportion of CMhi underwent one or more divisions after culture in IL-7 compared to the CMlo, EMhi and EMlo subsets, although a significant fraction of cells in all subsets remained undivided (Figure 6c). Both effluxing and non-effluxing TCM and TEM proliferated vigorously in response to IL-15, demonstrating that CMhi and EMhi were responsive to homeostatic cytokines (Figure 6c).
CMhi and EMhi T cells are enriched and proliferate in patients undergoing chemotherapy for AML
Based on the in vitro studies, we reasoned that CMhi and EMhi T cells might preferentially survive cytotoxic chemotherapy in vivo, and proliferate during chemotherapy-induced lymphocytopenia when IL-7 and IL-15 are elevated (Gattinoni et al., 2005). We first confirmed that high expression of CD161 identified the subsets of TCM and TEM that rapidly effluxed Rh123 in chemotherapy-treated AML patients as in normal donors (data not shown). We then used CD161 to identify CMhi and EMhi in ten AML patients with lymphocytopenia after chemotherapy and compared the proportion of CD161hi and CD161lo T cells that expressed Ki-67 with that in healthy donors. We found a dramatic increase in the proportion of CD161hi TCM and TEM that expressed Ki-67 in lymphocytopenic patients (Figure 6d). CD161lo CD8+ memory T cells and CD161neg/int TN that survived chemotherapy were also recruited into the cell cycle, but to a lesser extent than the CD161hi subsets. The preferential survival of CMhi and EMhi and their recruitment into the cell cycle during lymphocytopenia resulted in an enrichment of CD161hi TCM and TEM as a component of the total TCM and TEM subsets in AML patients as lymphocyte numbers recovered from chemotherapy (Figure 6e). Thus, the phenotypically distinct subsets of CD8+ memory T cells with rapid efflux capacity survive cytotoxic chemotherapy in vivo, are induced to divide and are enriched during the lymphocyte nadir.
CD161hi effluxing T cells differentiate into CD161lo non-effluxing T cells
The accumulation of CD161hi T cells in the blood of AML patients after chemotherapy could result from the acquisition of a CD161hi phenotype by surviving CD161lo TCM, TEM or TN induced to proliferate during lymphocytopenia. Thus, we cultured sort-purified, CFSE-labeled CD161lo subsets in vitro with IL-7 and IL-15 in the absence of a TcR signal, and analyzed their phenotype during cell division. Infrequent cells converted to a CD161hi phenotype in CMlo (0.23 +/− 0.11%, mean +/− SE, n=7), EMlo (1.78 +/− 1.18%, n=7), and TN (0.33 +/− 0.17%, n=3) cultures, although a subset of TN acquired CD95 consistent with prior reports that TN may acquire memory markers in lymphocytopenia (Figure 7a). CMhi and CMlo exhibited reduced expression of CD62L, suggesting that CMhi and CMlo may convert to EMhi and EMlo respectively, in lymphocytopenia-induced proliferation (Figure 7a). Conversion from CD161hi effluxing to CD161lo non-effluxing subsets was not observed when proliferation was driven by cytokines alone.
Figure 7. CMhi and EMhi differentiate into CD161lo subsets after antigen stimulation.
(A) Sort-purified subsets were CFSE-loaded, cultured with IL-7 and IL-15 for 11 days and then analyzed for expression of CD161 and CD62L (CMhi, CMlo, EMhi and EMlo), or CD161 and CD95 (TN) by flow cytometry. Data are representative of experiments from 3 healthy individuals.
(B) Sort-purified CMhi, EMhi, CMlo and EMlo were cultured for 12 days with plate-bound anti-CD3 and anti-CD28 mAbs, and IL-7, and then examined for expression of CD161. Data are representative of experiments from 7 healthy individuals.
(C) Sort purified CMhi and EMhi were cultured with plate-bound anti-CD3 and anti-CD28 mAbs, and IL-7 for 12 days, then loaded and allowed to efflux Rh123 over 30 minutes and surface labeled with anti-CD161 mAb. Rh123 fluorescence was analyzed on CD161hi cells (red histogram) and CD161lo cells (blue histogram). Data are representative of experiments from 2 healthy individuals.
(D) Sort purified CMhi and EMhi were cultured for 11 days in IL-7 and IL-15, and then stimulated with RV798 CMV-infected autologous fibroblasts. After 9 days, the cultures of CMhi (left panels) and EMhi (right panels) were restimulated with autologous fibroblasts either infected with CMV (RV798) or mock infected (No Ag) for 5 hours and stained for intracellular IFN-γ and anti-CD161 mAb. Data are representative of experiments from three healthy individuals.
We reasoned that TcR and costimulatory signals might be required to differentiate CD161hi TCM and TEM to their CD161 counterparts and stimulated highly purified CMhi, EMhi, CMlo and EMlo from seven different donors with plate bound anti-CD3 and anti-CD28 mAbs in media with IL-7. After 11–14 days, 26.05 +/− 11.39% and 9.13 +/− 5.94% (mean +/− SE, n=7) of T cells in the cultures of CMhi and EMhi cells respectively, had acquired a CD161int/neg phenotype (Figure 7b). The proportion of cells that downregulated CD161 expression and the degree of downregulation was greater in cultures of CMhi than EMhi cells. However, the CD161lo T cells that emerged after TcR and costimulatory signaling from both subsets also lost the capacity to rapidly efflux Rh123 (Figure 7c).
We next investigated whether virus-specific T cells present in CMhi and EMhi subsets could differentiate to CD161lo non-effluxing cells in response to antigen stimulation. For these experiments, sort-purified CMhi and EMhi were first cultured for 11 days in IL-7 and IL-15 to mimic conditions during recovery from chemotherapy-induced lymphocytopenia and then stimulated with autologous fibroblasts infected with CMV. Following stimulation with virus-infected fibroblasts for eight days, virus-specific IFN-γ+ T cells were detected in CD161hi, CD161int and CD161neg subsets by cytokine flow cytometry (Figure 7d). Thus, CD161hi memory CD8+ T cells that preferentially survive chemotherapy retain the capacity to proliferate to homeostatic cytokines, respond to antigen, and differentiate into CD161lo subsets when provided with costimulatory and/or inflammatory signals from virus-infected antigen-presenting cells (APC). The data support a role for CD161hi CD8+ T cells in maintaining virus-specific memory after chemotherapy and repopulating the memory compartment after recovery from lymphocytopenia.
Discussion
Cytotoxic chemotherapy causes a profound reduction in neutrophils and platelets as a consequence of toxicity to committed hematopoietic progenitors, and restoration of hematopoiesis occurs because HSC are endowed with cell intrinsic mechanisms that enable their survival after drug exposure. T cell numbers are also transiently severely depleted after chemotherapy; and recovery, which occurs by proliferation of residual mature naïve and memory T cells, and by development of naïve T cells in the thymus, is often delayed in adults with age related involution of the thymus (Mackall et al., 1997).
Here we identify a novel compartment of human memory CD8+ T cells in both the CD62L+ TCM and CD62L− TEM subsets that shares resistance mechanisms with HSC, including the capacity to rapidly efflux ABCB1 substrate drugs, and to preferentially survive exposure to chemotherapy in vitro and in vivo. Memory T cells with high drug efflux capacity can be distinguished from their non-effluxing counterparts in normal individuals by the expression of higher levels of c-kit, IL-18Rα, CD161, bcl-2, CD28, CD127 and bcl-xL, and by a low proportion that are Ki-67+. Rapidly effluxing memory T cells are less frequent in peripheral blood than their non-effluxing counterparts, particularly the CMhi subset, which represents <0.05% of total PBMC in normal donors. CMhi and EMhi are quiescent when lymphocyte numbers are normal, but are induced to proliferate in patients rendered lymphocytopenic after chemotherapy, and can acquire a non-effluxing phenotype as a consequence of proliferation and stimulation with virus-infected APC.
The discovery of a subset of memory T cells with enhanced resistance to chemotherapy was facilitated by drug efflux assays that identify cells with high ABC transporter activity. Additional molecules such as c-kit, IL-18Rα and CD161 that are expressed on T cells with high efflux capacity were identified by analyzing surface expression of molecules previously shown by gene expression arrays to be shared by hematopoietic progenitors and memory T cells, and to be differentially expressed by TN and memory cells. The role of CD161 in T cells is controversial as CD161 ligation by the two known ligands, lectin-like transcript-1 (LLT1) or proliferation-induced lymphocyte-associated receptor (PILAR) can either inhibit or augment cytokine secretion and proliferation mediated by TcR signaling (Aldemir et al., 2005; Huarte et al., 2008; Rosen et al., 2005; Rosen et al., 2008). We found that effluxing CD8+ CD161+ T cells proliferated poorly to anti-CD3 mAb alone, and required costimulation through CD28 or IL-18Rα, demonstrating that these subsets of quiescent T cells may only respond to antigen under inflammatory conditions. In mice, IL-18Rα expression is upregulated on CD8+ T cells in the contraction phase, and incorporation of IL-18 into a tumor vaccine promoted long-lived memory T cells (Haring and Harty, 2009; Luo et al., 2005). However, in IL-18Rα−/− and IL-18−/− mice no defects in CD8+ T cell memory were apparent (Haring and Harty, 2009). Thus, additional studies will be required to determine whether IL-18 signaling serves a distinct function in human CD8 T cell memory formation, or is required for the selective derivation and/or maintenance of memory T cells with high drug efflux capacity.
Although differing in CD62L expression, CMhi and EMhi exhibit a phenotype typical of memory T cells and are CD127hi, CD28hi, CD27+, perforinlo, granzyme B−, CD25−, and PD-1−. This phenotype and the absence of Ki-67 expression in CMhi and EMhi suggests that T cells with high efflux capacity are not recently activated, and distinguishes these cells from anergic or exhausted CD8+ T cells, or those undergoing deletional tolerance described in murine models (Haining et al., 2008; Parish et al., 2009; Wherry et al., 2007). The TcR Vβ repertoire of CMhi and EMhi is similar in diversity to CMlo and EMlo subsets, and T cells specific for both persistent and cleared viruses were expanded from CMhi and EMhi subsets. The TcR diversity and decreased frequency of CMhi and EMhi with age is evidence that these subsets do not emerge as a consequence of age-associated clonal expansions (Messaoudi et al., 2006).
A very low fraction of CMhi and EMhi T cells are Ki-67+ in the blood of normal donors. In lymphocytopenic patients recovering from chemotherapy, IL-7 and IL-15 are elevated to promote recovery of lymphocyte numbers, and our studies show that CMhi and EMhi proliferate to IL-7 and IL-15 in vitro and during lymphocytopenia in vivo, supporting a contribution of these cells in reconstituting T cell memory after chemotherapy or other toxic agents that induce lymphocytopenia. Conversion of T cells from CD161lo memory and TN subsets to a CD161hi phenotype was not observed, suggesting that the accumulation of CD161hi cells after chemotherapy reflects their higher intrinsic resistance to cytotoxic agents and responsiveness to homeostatic cytokines. Proliferation of CMhi and EMhi induced in vitro by homeostatic cytokines alone did not result in loss of rapid drug efflux capacity or the CD161hi phenotype, but did result in a reduction of CD62L expression on CMhi cells, and their conversion to EMhi. However, stimulation of CMhi and EMhi with anti-CD3 and anti-CD28 or with virus-infected APC generated CD161int/neg cells that had both lost CD62L and the capacity to rapidly efflux Rh123. Cell transfer studies with marked T cells will be needed to definitively determine if differentiation of CMhi to EMhi, and from CD161hi to CD161lo subsets is unidirectional, and would be facilitated by the identification of similar subsets in animal models.
The mechanisms by which CD8+ T cell memory develops and is maintained for life, particularly in long-lived primate species remain elusive. Murine studies have shown that the initial division of naïve CD8+ T cells is asymmetric, and the daughter cell derived distal to the immune synapse is endowed with greater capacity to maintain memory than its proximally derived counterpart (Chang et al., 2007). Other murine studies have identified antigen-experienced putative ‘memory stem cells’ with the capacity for self-renewal and differentiation into TCM and TEM subsets (Zhang et al., 2005), or have derived candidate memory stem cells from naïve precursors in vitro through manipulation of Wnt signaling (Gattinoni et al., 2009). Our analysis focused on identifying human memory T cells formed in vivo that share properties with HSC and other stem cells. The CMhi cells identified based on drug efflux capacity exhibit a similar phenotype (CD45RAint/neg, CD62L+, CD127hi, CD122+, granzyme Blo and bcl-2hi) as the putative murine memory stem cell, and share expression of molecules with HSC including IL-18Rα and CD117 (c-kit). Like HSC, CMhi and EMhi survive cytotoxic chemotherapy and may play a critical role in protection of the host from viral infection during and after lymphocytopenia induced by chemotherapy or by exposure to environmental toxins that may have provided the evolutionary pressure to develop this property of T cell memory. The identification of this discrete functional and phenotypic subset of human CD8+ memory T cells provides insight into mechanisms that preserve immunity when homeostasis is severely perturbed, and tools to determine when these cells develop and how they contribute to maintenance of T cell memory under normal homeostasis. The results may have implications for vaccination and adoptive immunotherapy for infectious disease and cancer, where a goal is the induction of long-lived memory T cells.
Experimental procedures
Studies were performed in accordance with guidelines established by the Declaration of Helsinki and approval was obtained from the Institutional Review Board of the Fred Hutchinson Cancer Research Center.
Blood and tissue samples
All experiments using peripheral blood were performed using freshly drawn samples. Peripheral blood was obtained from healthy volunteer donors and from AML patients on up to three occasions during the lymphocyte nadir. Complete blood counts and white cell differential counts were performed on a Sysmex XE-2100. Cord blood was obtained with written informed maternal consent.
Antibodies and peptides
Monoclonal antibodies (mAbs) to the following molecules were obtained from BD Biosciences (San Diego, CA) and used in these experiments: CD3, CD8, CD4, CD16, TcRγδ, TcRαβ, CD45RO, CD45RA, CD27, CD28, CD117, CD127, CD161, bcl-2, perforin, granzyme A, granzyme B, Ki-67 and IFN-γ. Antibodies to IL-18Rα and CD95 were from eBioscience (San Diego, CA), V 24 from Coulter Immunotech (Fullerton, CA), bcl-xL from Genetex (Irvine, CA) and CD62L was from Biolegend (San Diego, CA). The following peptides were obtained from Genscript Corp. (Piscaway, NJ) at >80% purity by HPLC: NLVPMVATV from CMV pp65, GLCTLVAML from EBV BMLF-1, and GILGFVFTL from influenza M1. Pooled MHC class I-restricted peptides derived from CMV, EBV and influenza virus (CEF), and individual pools for each virus were obtained from Panatecs (Tubingen, Germany).
Multiparametric flow cytometry
Rh123, DiOC2(3) and daunorubicin efflux assays
Fresh, ficolled PBMC from healthy volunteer donors were loaded in efflux buffer containing RPMI 1640 (Gibco, Carlsbad, CA) and 1% bovine serum albumin (Sigma-Aldrich, St. Louis, MO) with either 10 μg/ml Rh123 or 30 ng/ml DiOC2(3) (Sigma) for 30 minutes on ice, or with 2.5 μM daunorubicin (Sigma) for 20 minutes at 37°C. They were then washed and cultured for 30 minutes at 37°C in the presence or absence of vinblastine, PK11195, or cyclosporine A (Sigma), as indicated, before surface labeling with appropriate antibodies. Samples for Rh123 or DiOC2(3) efflux assays were acquired on a FACS ARIA (BD Biosciences) equipped with 405 nm, 488 nm and 633 nm lasers. Rh123 and DiOC2(3) fluorescence were detected at 530/30 nm. Samples for daunorubicin efflux assay were acquired on a custom LSR-II (BD Biosciences) equipped with 405 nm, 488 nm, 532 nm and 633 nm lasers. Daunorubicin fluorescence was detected at 610/15 nm. Analysis was performed using FlowJo software (Treestar, Ashland, OR).
Surface and intracellular labeling
PBMC were surface labeled with antibodies for 20 minutes on ice. Samples for intracellular staining were additionally fixed in Cytofix/cytoperm before washing, permeabilization and antibody labeling in Perm/wash buffer (BD Biosciences). After washing, acquisition was performed on an LSR-II and analyzed with FlowJo software. Within the CD4−/CD16−/TcRγδ−/Vα24−/CD3+/CD8+/CD95+ T cell population, CMhi and EMhi subsets were identified as CD62L+/CD161hi or CD62L−/CD161hi events respectively, and CMlo and EMlo subsets were identified as CD62L+/CD161lo or CD62L−/CD161lo events respectively.
Isolation of effluxing and non-effluxing subsets
Effluxing and non-effluxing CD8+ T cell subsets were purified for functional and differentiation assays using magnetic bead separation and cell sorting to achieve >98% purity. CD8+ T cells were positively selected using CD8 Microbeads (Miltenyi Biotec), loaded with Rh123 and cultured for 30 min to allow Rh123 efflux, then labeled with fluorochrome-conjugated antibodies to CD4, CD16, TCRγδ, Vα24, CD8, CD95, CD62L and CD161. Labeled CD8+ T cells were sort-purified on a FACS ARIA into CMhi and EMhi subsets identified as CD62L+/Rh123lo/CD161hi and CD62L−/ Rh123lo/CD161hi respectively, in the CD4−/CD16−/TcRγδ−/Vα24−/CD8+/CD95+ population. CMlo and EMlo subsets were identified as CD62L+/Rh123hi/CD161int/neg and CD62L−/Rh123hi/CD161int/neg respectively, in the CD4−/CD16−/TcRγδ−/Vα24−/CD8+/CD95+ population. Naïve CD8+ T cells were identified as CD4−/CD16−/TcRγδ−/Vα24−/CD8+/CD95−/CD62L+/CD161int/neg.
Subsets for quantitative RT-PCR and the daunorubicin-induced apoptosis assay were isolated as follows. CD8+ T cells were negatively selected using the CD8+ T cell Isolation Kit II (Miltenyi Biotec). The CD8+ fraction was labeled with fluorochrome-labeled streptavidin (BD Biosciences) then antibodies to TcRγδ, CD95, CD62L and IL-18Rα. CMhi and EMhi were identified as streptavidin−/TcRγδ−/CD95+/IL-18Rαhi events, either positive or negative for CD62L, respectively. CMlo and EMlo were similarly identified as streptavidin−/TcRγδ−/CD95+/IL-18Rαlo events, either positive or negative for CD62L, respectively.
Quantitative RT-PCR
Quantitative RT-PCR for abcb1 expression in isolated subsets was performed using a duplex PCR with sequence-specific abcb1 and gapdh Taqman probes. Total RNA was extracted using the RNeasy kit (Qiagen, Valencia, CA), according to the manufacturer’s instructions, and cDNA generated using standard methods. The following primers and probes were used for abcb1 quantitative PCR, as previously described (Burger et al., 2003): ABCB1-forward, GGAAGCCAATGCCTATGACTTTA; ABCB1-reverse, GAACCACTGCTTCGCTTTCTG; ABCB1-probe, 6FAM-TGAAACTGCCTCATAAATTTGACACCCTGG-TAMRA. The Predeveloped Taqman Assay Reagent Human GAPDH (Applied Biosystems, Foster City, CA) served as an internal control. Reactions were run in triplicate on an ABI 7900-HT Real-time PCR System and analyzed using Sequence Detection Systems 2.2.2 software (Applied Biosystems). Relative gene expression is calculated as the ratio of abcb1:gapdh expression in each subset and normalized to that of CMhi.
Induction of daunorubicin-induced apoptosis
Effluxing and non-effluxing TCM and TEM subsets, isolated as above, were cultured for 40–44 hours in the presence or absence of 0.1 μM daunorubicin, with or without 50 μM PK11195. Cultures were harvested, washed twice with cold PBS and stained with Annexin V and DAPI before analysis.
In vitro culture of viral antigen-specific CD8+ T cells
Virus-specific T cell cultures were established from AML patients who were within 80 days of completion of induction and consolidation chemotherapy. PBMC were pulsed with pooled CEF, CMV, EBV or influenza peptides (Panatecs) at 1 μg/ml for 2 hours, washed and cultured with un-pulsed autologous PBMC. On day 8–10, the cultures were restimulated and analyzed by surface staining with anti-CD8 mAb followed by intracellular staining for IFN-γ.
Virus-specific CD8+ T cells were expanded from sort-purified CMhi and EMhi subsets from healthy HLA-A*0201+ donors by culture with autologous peptide-pulsed mature MoDC or RV798 CMV-infected fibroblasts (Manley et al., 2004). MoDC were generated as previously described (Thurner et al., 1999) and matured by culture with 2 ng/ml IL-1β, 1000 U/ml IL-6, 10 ng/ml TNFα (all from R and D Systems) and 1000 ng/ml PGE2 (MP Biomedicals, Solon, OH) for 48 hours, pulsed in RPMI 1640 for 2 hours with peptides (1 μg/ml), washed and irradiated (3500 cGy) before use. Autologous fibroblasts were cultured and infected for 48 hours with RV798 supernatant, as described (Manley et al., 2004), then irradiated (3500 cGy). CMhi, CMlo, EMhi and EMlo subsets were plated in 96 well plates with MoDC or fibroblasts at a T cell:APC ratio of 4:1. All cultures were supplemented with 10 U/ml IL-2 (Novartis, Basel, Switzerland), 1 ng/ml IL-7 and 1 ng/ml IL-15 (R and D Systems). Cytokine and half medium exchanges were performed on days 3–4 and 6–7. MoDC-stimulated cultures were labeled with DAPI (Sigma) for dead cell exclusion, and stained with CD8 antibody and appropriate tetramers (Beckman Coulter). Cultures stimulated with RV798-infected fibroblasts were surface labeled with anti-CD8 and anti-CD161 mAbs, then stained for intracellular IFN-γ expression.
Proliferation assays
CFSE-dilution assays were used to assess proliferation of isolated subsets stimulated with cytokines. Subsets were loaded with CFSE (Molecular Probes, Carlsbad, CA) and cultured for 10 days in cytokines, resuspended in DAPI, and analyzed by flow cytometry. Tritiated thymidine incorporation was used to assess proliferation in response to anti-CD3 stimulation. Subsets were cultured for 3 days with plate-bound anti-CD3 (1000 ng/ml) (OKT3, Ortho Biotech, Horsham, PA), with or without anti-CD28 (5 μg/ml) (FHCRC Shared Resources, Seattle, WA) mAbs or IL-18 (80 ng/ml) (MBL International, Woburn, MA), then pulsed overnight with 1 μCi tritiated thymidine before harvesting and scintillation counting (Perkin Elmer, Waltham, MA).
In vitro culture and differentiation
Sort-purified (>98% purity) CMhi, EMhi, CMlo and EMlo were CFSE-labeled and cultured for 11 days in the presence of 1 ng/ml IL-7 and 5 ng/ml IL-15, then washed and labeled with anti-CD161 and anti-CD62L mAbs before analysis. Alternatively, sort-purified subsets were cultured with plate-bound anti-CD3 and anti-CD28 mAbs, supplemented with 0.2 ng/ml IL-7. After 6 days, cells were transferred to new 96 well plates without anti-CD3 or anti-CD28 mAbs in medium supplemented with IL-2 50 U/ml. On day 11–14, cells were washed, labeled with anti-CD161 mAbs, resuspended in DAPI and analyzed. In some experiments, Rh123 efflux assay with surface staining for CD161 was performed, as described above.
Statistical methods
Statistical analysis was performed using Graphpad Prism 5 (Graphpad Software, La Jolla, CA). Data are shown as the mean +/− SE, unless otherwise indicated. One-way ANOVA with Bonferroni correction was used for comparison of three or more groups in a single condition. Two-tailed paired t-test was used for comparison between matched paired groups. Correlation was estimated by calculation of two-tailed Pearson coefficients and significance.
Supplementary Material
Acknowledgments
We acknowledge the assistance of Colette Chaney, Stephanie Crouch and Kelda Gardner in acquiring samples, and thank the patients and blood donors. We also acknowledge funding from the Thomsen Family, Komen for the Cure, the Avon Foundation, FHCRC BCRP, and NIH grants CA18029, CA114536, AI53193.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: None.
References
- Aldemir H, Prod’homme V, Dumaurier MJ, Retiere C, Poupon G, Cazareth J, Bihl F, Braud VM. Cutting edge: lectin-like transcript 1 is a ligand for the CD161 receptor. J Immunol. 2005;175:7791–7795. doi: 10.4049/jimmunol.175.12.7791. [DOI] [PubMed] [Google Scholar]
- Berman E, Heller G, Santorsa J, McKenzie S, Gee T, Kempin S, Gulati S, Andreeff M, Kolitz J, Gabrilove J. Results of a randomized trial comparing idarubicin and cytosine arabinoside with daunorubicin and cytosine arabinoside in adult patients with newly diagnosed acute myelogenous leukemia. Blood. 1991;77:1666–1674. [PubMed] [Google Scholar]
- Burger H, Foekens JA, Look MP, Meijer-van Gelder ME, Klijn JG, Wiemer EA, Stoter G, Nooter K. RNA expression of breast cancer resistance protein, lung resistance-related protein, multidrug resistance-associated proteins 1 and 2, and multidrug resistance gene 1 in breast cancer: correlation with chemotherapeutic response. Clin Cancer Res. 2003;9:827–836. [PubMed] [Google Scholar]
- Chang JT, Palanivel VR, Kinjyo I, Schambach F, Intlekofer AM, Banerjee A, Longworth SA, Vinup KE, Mrass P, Oliaro J, et al. Asymmetric T lymphocyte division in the initiation of adaptive immune responses. Science. 2007;315:1687–1691. doi: 10.1126/science.1139393. [DOI] [PubMed] [Google Scholar]
- Chaudhary PM, Roninson IB. Expression and activity of P-glycoprotein, a multidrug efflux pump, in human hematopoietic stem cells. Cell. 1991;66:85–94. doi: 10.1016/0092-8674(91)90141-k. [DOI] [PubMed] [Google Scholar]
- Gattinoni L, Finkelstein SE, Klebanoff CA, Antony PA, Palmer DC, Spiess PJ, Hwang LN, Yu Z, Wrzesinski C, Heimann DM, et al. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J Exp Med. 2005;202:907–912. doi: 10.1084/jem.20050732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattinoni L, Zhong XS, Palmer DC, Ji Y, Hinrichs CS, Yu Z, Wrzesinski C, Boni A, Cassard L, Garvin LM, et al. Wnt signaling arrests effector T cell differentiation and generates CD8(+) memory stem cells. Nat Med. 2009 doi: 10.1038/nm.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer. 2002;2:48–58. doi: 10.1038/nrc706. [DOI] [PubMed] [Google Scholar]
- Haining WN, Ebert BL, Subrmanian A, Wherry EJ, Eichbaum Q, Evans JW, Mak R, Rivoli S, Pretz J, Angelosanto J, et al. Identification of an evolutionarily conserved transcriptional signature of CD8 memory differentiation that is shared by T and B cells. J Immunol. 2008;181:1859–1868. doi: 10.4049/jimmunol.181.3.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammarlund E, Lewis MW, Hansen SG, Strelow LI, Nelson JA, Sexton GJ, Hanifin JM, Slifka MK. Duration of antiviral immunity after smallpox vaccination. Nat Med. 2003;9:1131–1137. doi: 10.1038/nm917. [DOI] [PubMed] [Google Scholar]
- Haring JS, Harty JT. Interleukin-18-related genes are induced during the contraction phase but do not play major roles in regulating the dynamics or function of the T-cell response to Listeria monocytogenes infection. Infect Immun. 2009;77:1894–1903. doi: 10.1128/IAI.01315-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes S, He M, Xu T, Lee PP. Memory T cells have gene expression patterns intermediate between and effector. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:5519–5523. doi: 10.1073/pnas.0501437102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huarte E, Cubillos-Ruiz JR, Nesbeth YC, Scarlett UK, Martinez DG, Engle XA, Rigby WF, Pioli PA, Guyre PM, Conejo-Garcia JR. PILAR is a novel modulator of human T-cell expansion. Blood. 2008;112:1259–1268. doi: 10.1182/blood-2007-12-130773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judge AD, Zhang X, Fujii H, Surh CD, Sprent J. Interleukin 15 Controls both Proliferation and Survival of a Subset of Memory-Phenotype CD8+ T Cells. J. Exp. Med. 2002;196:935–946. doi: 10.1084/jem.20020772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckey CJ, Bhattacharya D, Goldrath AW, Weissman IL, Benoist C, Mathis D. Memory T and memory B cells share a transcriptional program of self-renewal with long-term hematopoietic stem cells. Proc Natl Acad Sci U S A. 2006;103:3304–3309. doi: 10.1073/pnas.0511137103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, Zhou H, Mizutani M, Mizutani N, Liu C, Xiang R, Reisfeld RA. A DNA vaccine targeting Fos-related antigen 1 enhanced by IL-18 induces long-lived T-cell memory against tumor recurrence. Cancer Res. 2005;65:3419–3427. doi: 10.1158/0008-5472.CAN-04-3120. [DOI] [PubMed] [Google Scholar]
- Mackall CL, Fleisher TA, Brown MR, Andrich MP, Chen CC, Feuerstein IM, Magrath IT, Wexler LH, Dimitrov DS, Gress RE. Distinctions between CD8+ and CD4+ T-cell regenerative pathways result in prolonged T-cell subset imbalance after intensive chemotherapy. Blood. 1997;89:3700–3707. [PubMed] [Google Scholar]
- Manley TJ, Luy L, Jones T, Boeckh M, Mutimer H, Riddell SR. Immune evasion proteins of human cytomegalovirus do not prevent a diverse CD8+ cytotoxic T-cell response in natural infection. Blood. 2004;104:1075–1082. doi: 10.1182/blood-2003-06-1937. [DOI] [PubMed] [Google Scholar]
- Messaoudi I, Warner J, Nikolich-Zugich J. Age-related CD8+ T cell clonal expansions express elevated levels of CD122 and CD127 and display defects in perceiving homeostatic signals. J Immunol. 2006;177:2784–2792. doi: 10.4049/jimmunol.177.5.2784. [DOI] [PubMed] [Google Scholar]
- Meyn RE, Meistrich ML, White RA. Cycle-dependent anticancer drug cytotoxicity in mammalian cells synchronized by centrifugal elutriation. J Natl Cancer Inst. 1980;64:1215–1219. [PubMed] [Google Scholar]
- Minderman H, Vanhoefer U, Toth K, Yin MB, Minderman MD, Wrzosek C, Slovak ML, Rustum YM. DiOC2(3) is not a substrate for multidrug resistance protein (MRP)-mediated drug efflux. Cytometry. 1996;25:14–20. doi: 10.1002/(SICI)1097-0320(19960901)25:1<14::AID-CYTO2>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Mizutani T, Masuda M, Nakai E, Furumiya K, Togawa H, Nakamura Y, Kawai Y, Nakahira K, Shinkai S, Takahashi K. Genuine functions of P-glycoprotein (ABCB1) Curr Drug Metab. 2008;9:167–174. doi: 10.2174/138920008783571756. [DOI] [PubMed] [Google Scholar]
- Murali-Krishna K, Altman JD, Suresh M, Sourdive DJ, Zajac AJ, Miller JD, Slansky J, Ahmed R. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 1998;8:177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- Parish IA, Rao S, Smyth GK, Juelich T, Denyer GS, Davey GM, Strasser A, Heath WR. The molecular signature of CD8+ T cells undergoing deletional tolerance. Blood. 2009;113:4575–4585. doi: 10.1182/blood-2008-10-185223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen DB, Bettadapura J, Alsharifi M, Mathew PA, Warren HS, Lanier LL. Cutting edge: lectin-like transcript-1 is a ligand for the inhibitory human NKR-P1A receptor. J Immunol. 2005;175:7796–7799. doi: 10.4049/jimmunol.175.12.7796. [DOI] [PubMed] [Google Scholar]
- Rosen DB, Cao W, Avery DT, Tangye SG, Liu YJ, Houchins JP, Lanier LL. Functional consequences of interactions between human NKR-P1A and its ligand LLT1 expressed on activated dendritic cells and B cells. J Immunol. 2008;180:6508–6517. doi: 10.4049/jimmunol.180.10.6508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- Schinkel AH, Jonker JW. Mammalian drug efflux transporters of the ATP binding cassette (ABC) family: an overview. Adv Drug Deliv Rev. 2003;55:3–29. doi: 10.1016/s0169-409x(02)00169-2. [DOI] [PubMed] [Google Scholar]
- Schluns KS, Kieper WC, Jameson SC, Lefrancois L. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat Immunol. 2000;1:426–432. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol. 2000;182:311–322. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Simmons PJ, Aylett GW, Niutta S, To LB, Juttner CA, Ashman LK. c-kit is expressed by primitive human hematopoietic cells that give rise to colony-forming cells in stroma-dependent or cytokine-supplemented culture. Exp Hematol. 1994;22:157–165. [PubMed] [Google Scholar]
- Smets F, Latinne D, Bazin H, Reding R, Otte JB, Buts JP, Sokal EM. Ratio between Epstein-Barr viral load and anti-Epstein-Barr virus specific T-cell response as a predictive marker of posttransplant lymphoproliferative disease. Transplantation. 2002;73:1603–1610. doi: 10.1097/00007890-200205270-00014. [DOI] [PubMed] [Google Scholar]
- Sung L, Gamis A, Alonzo TA, Buxton A, Britton K, Deswarte-Wallace J, Woods WG. Infections and association with different intensity of chemotherapy in children with acute myeloid leukemia. Cancer. 2009;115:1100–1108. doi: 10.1002/cncr.24107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Dejbakhsh-Jones S, Strober S. Expression of CD161 (NKR-P1A) defines subsets of human CD4 and CD8 T cells with different functional activities. J Immunol. 2006;176:211–216. doi: 10.4049/jimmunol.176.1.211. [DOI] [PubMed] [Google Scholar]
- Tan JT, Ernst B, Kieper WC, LeRoy E, Sprent J, Surh CD. Interleukin (IL)-15 and IL-7 jointly regulate homeostatic proliferation of memory phenotype CD8+ cells but are not required for memory phenotype CD4+ cells. J Exp Med. 2002;195:1523–1532. doi: 10.1084/jem.20020066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurner B, Röder C, Dieckmann D, Heuer M, Kruse M, Glaser A, Keikavoussi P, Kämpgen E, Bender A, Schuler G. Generation of large numbers of fully mature and stable dendritic cells from leukapheresis products for clinical application. Journal of Immunological Methods. 1999;223:1–15. doi: 10.1016/s0022-1759(98)00208-7. [DOI] [PubMed] [Google Scholar]
- Walter EA, Greenberg PD, Gilbert MJ, Finch RJ, Watanabe KS, Thomas ED, Riddell SR. Reconstitution of cellular immunity against cytomegalovirus in recipients of allogeneic bone marrow by transfer of T-cell clones from the donor. N Engl J Med. 1995;333:1038–1044. doi: 10.1056/NEJM199510193331603. [DOI] [PubMed] [Google Scholar]
- Walter RB, Raden BW, Cronk MR, Bernstein ID, Appelbaum FR, Banker DE. The peripheral benzodiazepine receptor ligand PK11195 overcomes different resistance mechanisms to sensitize AML cells to gemtuzumab ozogamicin. Blood. 2004;103:4276–4284. doi: 10.1182/blood-2003-11-3825. [DOI] [PubMed] [Google Scholar]
- Wherry EJ, Ha SJ, Kaech SM, Haining WN, Sarkar S, Kalia V, Subramaniam S, Blattman JN, Barber DL, Ahmed R. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity. 2007;27:670–684. doi: 10.1016/j.immuni.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Zhang X, Sun S, Hwang I, Tough DF, Sprent J. Potent and Selective Stimulation of Memory-Phenotype CD8+ T Cells In Vivo by IL-15. Immunity. 1998;8:591–599. doi: 10.1016/s1074-7613(00)80564-6. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Joe G, Hexner E, Zhu J, Emerson SG. Host-reactive CD8+ memory stem cells in graft-versus-host disease. Nat Med. 2005;11:1299–1305. doi: 10.1038/nm1326. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.