Abstract
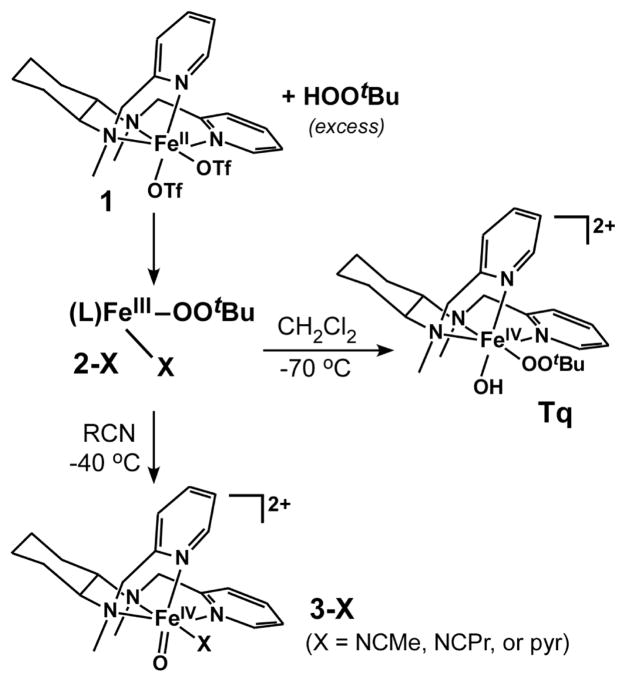
In a previous paper (Jensen et al., J. Am. Chem. Soc. 2005, 127, 10512), we reported the synthesis of the turquoise-colored intermediate [FeIV(β-BPMCN)(OOtBu)(OH)]2+ (Tq; BPMCN = N,N′-bis(2-pyridylmethyl)-N,N′-dimethyl-trans-1,2-diaminocyclohexane). The structure of Tq is unprecedented, as it represents the only synthetic example to date of a non-heme FeIV complex with both alkylperoxo and hydroxide ligands. Given the significance of similar high-valent Fe intermediates in the mechanisms of oxygenase enzymes, we have explored the reactivity of Tq at −70 °C, a temperature at which it is stable, and found that it is capable of activating weak X-H bonds (X = C, O) with bond dissociation energies ≤~80 kcal/mol. The FeIV-OH unit of Tq, and not the alkylperoxo moiety, performs the initial H-atom abstraction. However at −45 °C, Tq decays at a rate that is independent of substrate identity and concentration, forming a species capable of oxidizing substrates with stronger C-H bonds. Parallel reactivity studies were also conducted with the related oxoiron(IV) complexes [FeIV(β-BPMCN)(O)(X)]2+ (3-X; X = pyridine or nitrile), thereby permitting a direct comparison of the reactivity of FeIV centers with oxo and hydroxide ligands. We found that the H-atom abstracting ability of the FeIV=O species greatly exceeds that of the FeIV-OH species, generally by greater than 100-fold. Examination of the electronic structures of Tq and 3-X with density functional theory (DFT) provides a rationale for their differing reactivities.
Introduction
Nature employs a number of heme and nonheme Fe-dependent enzymes to carry out the selective oxidation of H-X bonds (X = C, N, or O). Such enzymes typically require dioxygen to generate a reactive Fe-containing intermediate, which then abstracts a hydrogen-atom from substrate.1,2 The active intermediates are often mononuclear FeIV species with terminal oxo ligands, such as compound I of cytochrome P4503 and intermediate J of the α-ketoacid-dependent enzyme TauD.4 Alternatively, the oxidant can be a high-valent diiron species with bridging oxo ligands, such as the putative FeIV 2(μ-O)2 core of intermediate Q in methane monooxygenase (MMO) or the mixed-valent FeIII(μ-O)FeIV intermediate X of ribonucleotide reductase (RNR).5 Finally, it has been proposed that FeIII-OH and FeIII-OOH species serve as the active oxidants in lipoxygenase6 and bleomycin,7 respectively, indicating that ferric intermediates are also viable oxidants.
Each of the Fe intermediates described above contains an Fe/O unit that directly participates in the H-atom abstraction, as shown in the general equations 1–4:
| (1) |
| (2) |
| (3) |
| (4) |
Given that such a wide variety of Fe intermediates are involved in H-X bond activation, it is crucial to identify the structural and electronic features that control their reactivity. A particularly important issue concerns the relative H-atom abstracting abilities of FeIV=O, FeIV-OH, and FeIV-O- M species (M = metal ion), as all three types of structures have been invoked in biological and synthetic systems. Of course, direct comparisons of such reactivities are greatly facilitated by the use of similar supporting ligand environments and reaction conditions, which is easiest to achieve with synthetic complexes. To this end, Busch and coworkers recently employed a series of MnIV(OH)x(O)y complexes that are supported by the same tetradentate macrocyclic ligand to compare the H-atom abstracting abilities of MnIV-OH and MnIV=O units.8,9 However, no study to date has explored the comparative reactivity of FeIV =O and FeIV–OH complexes, despite the fact that such species are relevant to a number of biological systems.
In 2005, we reported that treatment of [FeII(β-BPMCN)(OTf)2] (1, BPMCN = N,N′-bis(2- pyridylmethyl)-N,N′-dimethyl-trans-1,2-diaminocyclohexane) with tert-butyl hydroperoxide (TBHP) yields two distinct iron(IV) complexes, depending upon solvent.10 In both MeCN and CH2Cl2 solutions, the initial reaction results in formation of an FeIII-OOtBu species (2; Scheme 1). In MeCN at −40 °C, 2-MeCN slowly converts via O-O bond homolysis to the oxoiron(IV) complex [FeIV(β-BPMCN)(O)(NCMe)]2+ (3-MeCN), which exhibits a pale green color. By contrast, in CH2Cl2 at −70 °C, the reaction gives rise to a complex with a deep turquoise color; detailed spectroscopic studies identified this species as [FeIV(β-BPMCN)(OOtBu)(OH)]2+ (Tq; Scheme 1).10 The structure of Tq is remarkable; it represents the only example to date of a well-characterized alkylperoxoiron(IV) complex, and one of only two isolable nonheme FeIV-OH compounds (the other example is [FeIV(O)(Me4-cyclam)(OH)]2+).11 Furthermore, the chemistry shown in Scheme 1 is unique in that, with the same supporting ligand, we can generate metastable FeIV complexes with oxo, hydroxo, and alkylperoxo ligands.
Scheme 1.
Clearly, the Fe(β-BPMCN) framework provides an unprecedented opportunity to explore the role of ligand identity in modulating the oxidizing power of high-valent Fe centers. We have therefore sought to measure and compare the reactivities of complexes Tq and 3 towards various organic substrates. While the H-atom abstracting ability of FeIV=O complexes has been studied extensively,12–15 there are no extant reports on the chemistry of nonheme FeIV-OH complexes. Tq is also interesting due to the fact that it possesses two reactive sites – the hydroxo and alkylperoxo ligands. Indeed, we will demonstrate below that both functionalities are capable of participating in the oxidation of substrates, depending upon reaction conditions. Our results also indicate that the H-atom abstracting ability of the FeIV=O species (3) greatly exceeds that Tq. Examination of the electronic structures of these two FeIV complexes with computational methods provides a rationale for their differing reactivities.
Experimental Section
Materials and Synthesis
All reagents were purchased from commercial sources and used as received, unless noted otherwise. Butyronitrile (PrCN) was purified by heating over Na2CO3 and KMnO4 for several hours, followed by distillation and drying over molecular sieves. MeCN and CH2Cl2 were purified by distillation from CaH2. The β-BPMCN ligand and complex 1 were synthesized following literature procedures.16 Prior to their use in reactivity studies, cyclohexene, styrene, 1,4-cyclohexadiene, and stilbene were purified by passage through basic alumina. 9,10-dihydroanthracene (DHA) and 2,4,6-tri-tert-butylphenol (TTBP) were both recrystallized from EtOH. The deuterated analogues of DHA and TTBP were prepared following literature procedures.17
Physical Methods
Electronic absorption spectra were measured using a Hewlett-Packard 8453 diode array spectrophotometer equipped with liquid nitrogen cryostat (Unisoku) for temperature control. Resonance Raman spectra were recorded on an Acton AM-506 spectrophotometer, using a Kaiser Optical holographic supernotch filter with a Princeton Instruments LN/CCD-1100-PB/UVAR detector cooled with liquid nitrogen. Laser excitation was provided by a Spectra Physics 2060 Kr+ laser. The spectra were obtained at 77 K and the Raman frequencies were referenced to indene. Electron paramagnetic resonance spectra were recorded on a Bruker EPP 300 spectrometer equipped with a liquid He cryostat.
Reactivity Studies
Tq samples for reactivity studies were prepared by first adding ~10 equiv of TBHP to 7 mM solutions of 1 in CH2Cl2 at −78 °C. The resulting solutions were placed overnight in a −80 °C freezer and then layer with cold pentane, resulting in precipitation of Tq after several days. After removal of solvent at −78 °C, the Tq solid was washed with pentane and then redissolved in cold PrCN. Small amounts (~0.1–0.2 mL) of this concentrated Tq solution were then directly transferred to UV-vis cuvettes (1 cm path length) cooled to −78 °C containing 2 mL of degassed PrCN, resulting in Tq concentrations of 0.3–0.7 mM. The resulting solutions were then subjected to several vacuum/backfill cycles with Ar to remove dissolved dioxygen. The cuvette was transferred to the low-temperature cryostat (set at either −70 or −45 °C) and substrate was added via syringe. Reaction progress was monitored with the UV-vis spectrometer described above.
The corresponding FeIV=O complexes (3-X) were generated in quartz cuvettes via two different procedures. The first involved addition of 4 equiv of TBHP to 1 in PrCN at −70 °C to give 2-PrCN, followed by addition of excess pyridine to yield 3-pyr. The second method involved addition of 4 equiv of TBHP to 1 in MeCN at −40 °C to give 2-MeCN, followed by three-fold dilution with cold PrCN to yield 3-NCR. After lowering the temperature to −70 °C, substrate was added via syringe and the reaction progress was monitored with UV-vis absorption spectroscopy.
Determination of reaction products and yields was carried out using gas chromatography, after first filtering solutions through silica gel to remove Fe salts. Naphthalene was employed as the internal standard.
Density Functional Theory Calculations
Geometry optimizations were performed with the Amsterdam Density Functional (ADF) 2006.01 software package18 using ADF basis set IV (triple-ζ with single polarization), an integration constant of 4.0, and the Vosko-Wilk-Nusair local density approximation19 with the nonlocal gradient corrections of Becke20 and Perdew.21 Cartesian coordinates for the DFT geometry-optimized models of Tq and 3-NCMe are provided in Table S1(a–d).
Single-point DFT calculations (spin-unrestricted) were performed using the ORCA 2.4 software package developed by Dr. F. Neese.22 The computations utilized Ahlrichs’ valence triple-ζ basis set with one set of polarization functions (TZV/P) on all atoms, in conjunction with the corresponding auxiliary basis set (VTZ/C).23 Extra polarization functions were included on the Fe and first-sphere ligand atoms. The calculations employed Becke’s three-parameter hybrid functional for exchange24,25 along with the Lee-Yang-Parr correlation functional26 (B3LYP) with an integration grid of 4.0. The gOpenMol program developed by Laaksonen27 was used to generate isosurface plots of molecular orbitals.
Results and Discussion
1. General Properties of Tq
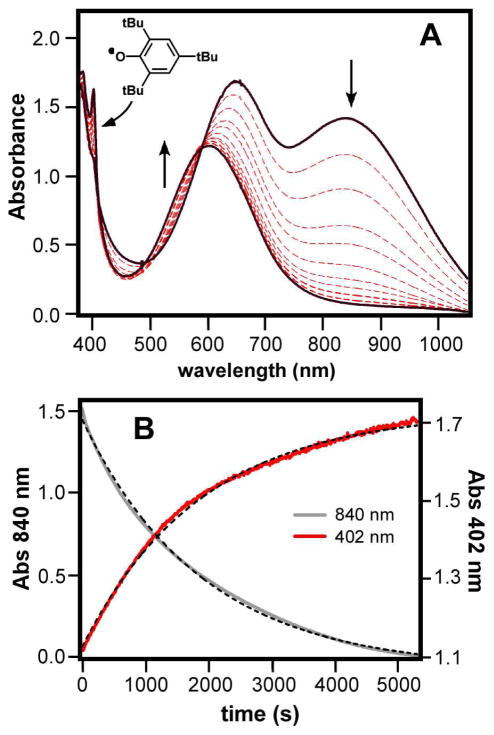
As shown in Figure 1A, Tq exhibits two intense absorption features in the visible region at 645 and 840 nm (ε = 4.0 and 3.6 mM−1cm−1, respectively), which give the complex its bright turquoise color. Both bands likely arise from alkylperoxo→FeIV charge transfer transitions, as resonance Raman (rR) peaks corresponding to the ν(Fe-O) and ν(O-O) alkylperoxo modes are enhanced by excitation into these features.10 Mössbauer studies of Tq confirmed the +4 oxidation state of the Fe center (δ = 0.10 mm/s; ΔEQ = 1.76 mm/s) and revealed that the complex is low-spin (S = 1). For comparison, the corresponding FeIV=O complex (3-MeCN) is also low-spin with Mössbauer parameters δ = 0.07 mm/s and ΔEQ = 1.02 mm/s.10
Figure 1.
(A) UV-vis absorption spectra of the reaction of Tq with TTBP at −70 °C in PrCN ([TTBP] = 150 mM, [Tq]0 = 0.43 mM). (B) Plot of absorption intensities at 840 nm (solid black) and 402 nm (solid red) as a function of time for the reaction shown above. The dashed lines represent first-order kinetic fits (k1 = 5.3 × 10−4 s−1 for both traces).
The redox potential of Tq was estimated with reductants of known oxidation potential. Tq is rapidly reduced by 1,1′-diacetylferrocene (490 mV vs. Fc/Fc+) but fails to react with higher-potential reductants like tris(4-bromophenyl)amine (670 mV). Interestingly, 3-pyr exhibits the same reactivity, suggesting that the redox potentials of both FeIV complexes lie between 490 and 670 mV.
2. Reaction of Tq with Phenols
The reactivity of Tq was first explored using the substrate 2,4,6-tri-tert-butylphenol (TTBP), which has proven a useful substrate for probing the H-atom abstracting ability of transition-metal complexes (the O-H bond dissociation energy, BDE, of TTBP is 81 kcal/mol28). While formation of Tq requires CH2Cl2 as solvent and a large excess of TBHP (Scheme 1), it is possible to isolate the complex by precipitation with pentane at −80 °C. The resulting solid can then be redissolved (without decomposition) into various other solvents, thereby yielding solutions of Tq with no excess oxidant. All samples employed in our reactivity studies were prepared in this manner, and the reactions were conducted under an inert atmosphere in butyronitrile (PrCN) at −70 °C – the highest temperature at which Tq is stable.
Figure 1A exhibits the changes observed in the UV-visible spectra upon addition of excess TTBP to Tq. The characteristic Tq absorption features at 840 and 645 nm decrease in intensity with concomitant formation of the phenoxyl radical peak at 402 nm. Based on the intensity of this feature (ε = 1.25 mM−1cm−1), the yield of the TTBP radical was determined to be 1.0 ± 0.1 equivalent, indicating that Tq functions as a one-electron oxidant. As evident in Figure 1B, both the decay of Tq and the formation of the phenoxyl radical follow pseudo first-order kinetics; variation of [TTBP] yielded a second-order rate constant of 3(1) × 10−3 M−1s−1 (Table 1; Figure S1A). Using d1-TTBP as the substrate, a kinetic isotope effect (KIE) of ~2 was measured (Figure S1B), indicating that H-O bond cleavage is involved in the rate-limiting step. Tq reacts in a similar fashion with other substituted phenols, such as 2,6-di-tert-butylphenol (BDE = 83 kcal/mol) and 2,6-di-tert-butyl-4-methoxyphenol (BDE = 78 kcal/mol),28 with reaction rates increasing with decreasing O-H BDE (Figure S2).29
Table 1.
Kinetic Parameters for the Reaction of H-atom Donors with Tq and 3, and Comparison to Previously-Reported Results for FeIII and MnIV Complexes.
| substrate | oxidant | T (°C) | K2 (M−1s−1) | KIE a | K2(O)/K2(OH) | Reference |
|---|---|---|---|---|---|---|
| TTBP | Tq | −70 | (3 ± 1) × 10−3 | 2 | > 1000 | d |
| 3-RCN | −70 | 25 ± 5 | 4 | d | ||
| 3-pyr | −70 | 4 ± 1 | d | |||
| [(PY5)FeIII(OMe)]2+, b | 25 | 0.60 | 2.0 | 17 | ||
| DHA | Tq | −70 | (1.5 ± 0.5) × 10−3 | 3 | 100 | d |
| 3-pyr | −70 | 0.14 ± 0.01 | 200 | d | ||
| [(EBC)MnIV(OH)2] 2+, c | 15 | 3.52 × 10−4 | 3.3 | 43 | 9 | |
| [(EBC)MnIV (O)2] c | 15 | 1.50 × 10−2 | 3.8 | 9 | ||
| 1,4-CHD | Tq | −70 | (4 ± 1) × 10−4 | 200 | d | |
| 3-pyr | −70 | (8 ± 2) × 10−2 | d | |||
| [(EBC)MnIV(OH)2] 2+, c | 15 | 3.64 × 10−4 | 44 | 9 | ||
| [(EBC)MnIV (O)2] c | 15 | 1.59 × 10−2 | 9 | |||
KIE = k2,H/k2,D
PY5 = 2,6-bis(bis(2-pyridyl)methoxymethane)pyridine.
EBC = 4,11-dimethyl-1,4,8,11-tetraazabicyclo[6.6.2]hexadecane.
This work.
While a concerted H-atom transfer mechanism is supported by the strong dependence of rates on H-O BDE and the observed KIE for TTBP oxidation, two alternatives were also considered. The first involves fast electron transfer followed by rate-limiting proton transfer; however, this possibility seems unlikely given that Tq fails to oxidize tris(4-bromophenyl)amine, which has a much lower potential (0.67 V) than the substrates (1.0 V for TTBP,30 ~1.4 V for DHA31). The second alternative mechanism involves rate-limiting proton transfer followed by fast electron-transfer. Yet this mechanism is disfavored by the low basicity of Tq, as evidenced by the fact the addition of excess HBF4 at −70 °C results in no spectral changes.
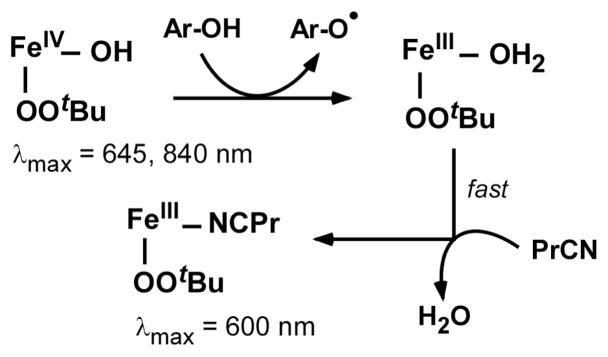
The isosbestic point at 590 nm (Figure 1A) indicates that TTBP cleanly reduces Tq to the product complex, which exhibits a single intense visible band at 600 nm (ε = 2.5 mM−1cm−1). Significantly, the absorption spectrum of the product is identical to the one reported, in our previous study, for [FeIII(β-BPMCN)(OOtBu)(PrCN)]2+ (2-PrCN).10 EPR and rR spectra obtained with the product solutions (Figures S3 and S4, respectively) provide further evidence that 2-PrCN is the sole Fe-containing product of the reaction of Tq with phenols. Thus, the alkylperoxo ligand remains intact and coordinated to the Fe center during the course of the reaction. We therefore conclude that abstraction of the phenolic H is carried out by the FeIV-OH unit of Tq to yield the complex [(L)FeIII(OOtBu)(OH2)]2+, which in PrCN undergoes rapid ligand substitution to give 2-PrCN (Scheme 2).
Scheme 2.
3. Reaction of Tq Intermediate with Hydrocarbons
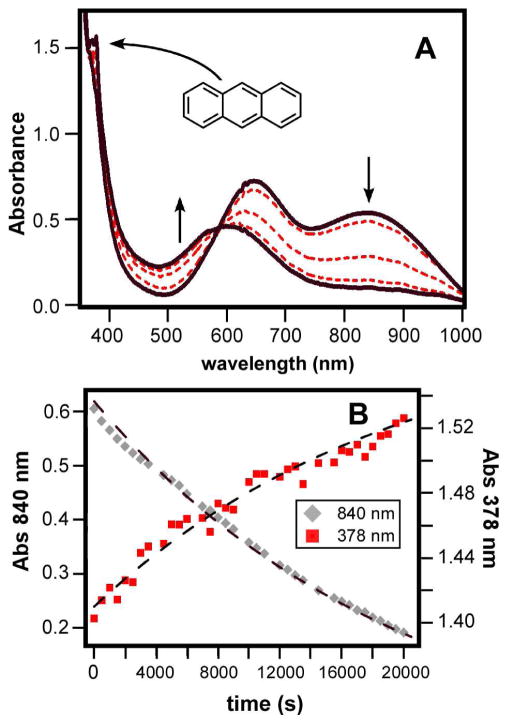
At −70 °C, Tq exhibits only sluggish reactivity towards hydrocarbons with weak C-H bonds, such as 9,10-dihydroanthracene (DHA; BDE = 78 kcal/mol) and 1,4-cyclohexadiene (1,4-CHD; BDE = 77 kcal/mol).32 Both substrates reduce Tq to the corresponding alkylperoxoiron(III) complex 2-PrCN, as was found to be the case with phenols (vide supra). In the case of DHA, the reaction yields 0.53 ± 0.08 equivalents of anthracene per Tq as determined by the intensity of the absorption feature at 378 nm (Figure 2), providing further evidence that Tq behaves as a one-electron oxidant. When the reaction with DHA is performed in CH2Cl2, a non-coordinating solvent, the absorption feature of the product complex is blue-shifted to 530 nm (Figure S5). This chromophore corresponds to the high-spin complex 2-OH2,10 thus proving that H-atom transfer initially generates an FeIII-OH2 complex (Scheme 2).
Figure 2.
(A) UV-vis absorption spectra of the reaction of Tq with DHA at −70 °C in PrCN ([DHA] = 24 mM, [Tq]0 = 0.15 mM). (B) Plot of absorption intensities at 840 nm (solid black) and 378 nm (solid red) as a function of time for the reaction shown above; both traces were adjusted to remove changes in baseline intensity. The dashed lines represent first-order kinetic fits (k1 = 5 × 10−5 s−1 for both traces).
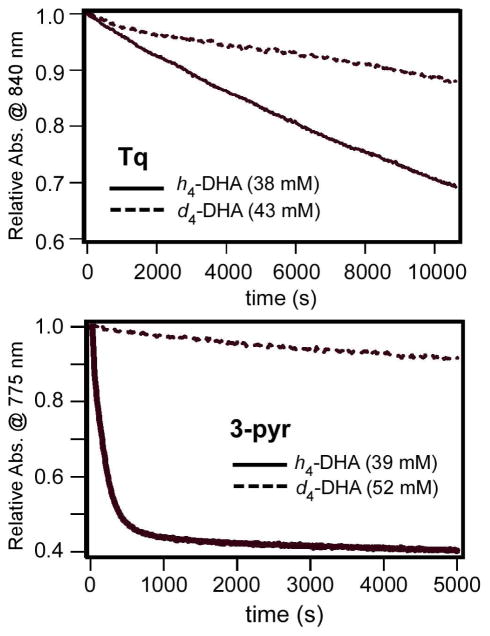
A detailed kinetic analysis of the Tq + DHA reaction was hindered by the exceptionally slow nature of the reaction rate, combined with the low solubility of DHA in PrCN at −70 °C. Nevertheless, using the method of initial rates, it was possible to estimate a rate constant of k2 = (1.5 ± 0.5) × 10−3 M−1s−1 (Table 1). With d4-DHA as substrate, a KIE value of approximately 3 was measured (Figure 3), indicating that C-H bond activation is the rate-limiting step. For both DHA and 1,4-CHD, the reaction with Tq is first-order with respect to substrate concentration. Interestingly, Tq is more reactive towards DHA than 1,4-CHD, despite the fact that the two substrates possess nearly identical C-H BDE’s.
Figure 3.
Kinetic isotope effects for the reaction of Tq (top) and 3-pyr (bottom) with h4-DHA (solid lines) and d4-DHA (dashed line) at similar concentrations. The reactions were performed in PrCN at −70 °C. Kinetic traces were measured at 840 nm for Tq and 775 nm for 3-pyr, which reflect the concentration of the Fe oxidants. The traces were normalized and adjusted for minor baseline shifts. Rate constants for Tq: k1 = 4.4 × 10−5 s−1 (h4-DHA) and k1 = 1.1 × 10−5 s−1 (d4- DHA). For 3-pyr: k1 = 5.7 × 10−3 s−1 (h4-DHA) and k1 = 3 × 10−5 s−1 (d4-DHA)
At −70 °C, Tq is unreactive towards hydrocarbons with C-H BDE’s greater than 80 kcal/mol, such as cyclohexene and toluene. In contrast, it has been shown that these substrates are readily oxidized by various FeIV=O complexes, albeit at higher temperatures.12 Thus, the FeIV-OH moiety of Tq appears to exhibit diminished reactivity relative to FeIV=O units – a result that is confirmed in the following section.
4. Comparison with [FeIV(β-BPMCN)(O)]2+ (3) Reactivity
As shown in Scheme 1, treatment of FeII(β-BPMCN)(OTf)2 (1) with TBHP at −40 °C in MeCN generates the corresponding alkylperoxoiron(III) complex, which slowly converts to the oxoiron(IV) complex 3-MeCN via O-O bond homolysis. To directly compare its reactivity with Tq at −70 °C, though, it was necessary to prepare 3 in PrCN. This was accomplished with two different methods. The first involved direct addition of TBHP to solutions of 1 in PrCN. However, it was found that smooth conversion of 2-NCPr to 3 required addition of pyridine, presumably to yield 3-pyr. This “push effect” of Lewis bases was observed previously in the homolysis of FeIII-OOR intermediates generated with TPA – another tetradentate supporting ligand that permits cis-coordination of exogenous ligands.33 The second method involved first generating 3 in MeCN and then diluting the solution with excess PrCN to give 3-NCR.
Both oxoiron(IV) complexes (3-pyr and 3-NCR) react rapidly with TTBP even at −70 °C, as determined by decay of the NIR feature at 750 nm and concomitant formation of the TTBP radical signal (Figure S6). The reaction is first-order with respect to 3 and TTBP, although accurate assessment of rates was complicated by the rapid nature of the reaction, which may introduce errors due to mixing times. Despite this, an overall second-order rate constant of ~15 M−1s−1 was determined, indicating that the FeIV=O unit of 3 is more than 103 times more reactive towards TTBP than the FeIV-OH unit of Tq (Table 1). Slightly higher rates were measured for 3- NCR than 3-pyr, indicating that the reaction does not operate through a base-assisted pathway (3-pyr is prepared in the presence of a large excess of pyridine). Further support for a direct H-atom transfer mechanism comes from the KIE value of 4 measured for 3-NCR with TTBP (Table 1; Figure S7).
The much greater H-atom abstracting ability of 3 relative to Tq was also observed for hydrocarbon substrates. The second-order rate constant for the reaction of 3-pyr with DHA was found to be 0.14(1) M−1s−1 – a nearly 100-fold increase over the Tq value (Table 1). This reaction also exhibits a large nonclassical KIE of 200 (Figure 3), which is a common feature of FeIV=O reactivity towards C-H bonds.12,13,15 For 1,4-CHD, the disparity in oxo-versus-hydroxo rates is even greater than for DHA, with a k(3)/k(Tq) value of 200 (Table 1). Moreover, unlike Tq, 3-pyr is capable of oxidizing substrates with C-H bonds > 80 kcal/mol. For instance, 3-pyr reacts with cyclohexene (BDE = 87 kcal/mol) at −70 °C (k2 = 2 × 10−4 M−1s−1) to yield the allylic oxidation products 2-cyclohexen-1-ol and 2-cyclohexen-1-one.
Our results are generally consistent with kinetic data published by Busch and coworkers for the series of MnIV complexes [(EBC)MnIV(OH)2]2+, [(EBC)MnIV(O)(OH)]+, and (EBC)MnIV(O)2 (where EBC is the cross-bridged cyclam ligand 4,11-dimethyl-1,4,8,11-tetraazabicyclo- [6.6.2]hexadecane). Using various substrates, it was found that the mono- and dioxo MnIV complexes perform H-atom abstractions approximately 15 and 40 times faster, respectively, than the corresponding dihydroxo complex (Table 1).8,9 Interestingly, our findings indicate that the disparity in oxo/hydroxo rates is even larger in FeIV systems. Furthermore, the reactivity of Tq towards hydrocarbons at −70 °C is roughly the same as the reactivity of [MnIV(OH)2]2+ at room temperature, pointing to greater intrinsic oxidizing power of FeIV relative to MnIV.
5. Reactivity of Tq at Elevated Temperatures
As noted above, Tq exhibits no reactivity at −70 °C towards hydrocarbons with C-H BDE’s > 80 kcal/mol. To determine whether this lack of reactivity was merely due to the low temperatures employed, experiments were carried out at −45 °C in PrCN under an Ar atmosphere. At this higher temperature, Tq self-decays at a rate of 1.3(2) × 10−3 s−1. However, the reaction does not yield the alkylperoxoiron(III) complex 2-PrCN; instead, the absorption spectrum of the final solution is nearly featureless (Figure S8), indicating loss of the alkylperoxo-to-iron charge transfer transitions.
At −45 °C Tq decay in the presence of hydrocarbons with BDE’s > 80 kcal/mol results in their oxidation (Table 2). For example, for cyclohexene (BDE = 87 kcal/mol), the reaction carried out under Ar yielded 1.0(2) equiv 2-cyclohexen-1-ol and 0.4(1) equiv 2-cyclohexen-1- one per Tq (and no epoxide). The same reaction performed in air afforded ketone as the dominant product and nearly 5 equiv cyclohexene oxidized per Tq (Table 2). The dramatic increase in the ketone yield in air and the corresponding change in the alcohol/ketone ratio indicate that long-lived cyclohexenyl radicals are formed initially. More importantly, it is clear that more than 1 equiv cyclohexenyl radical is produced under Ar, suggesting that Tq decomposition creates more than one equivalent of oxidant capable of H-atom abstraction from cyclohexene. Tq decomposition also generates species capable of oxo transfer to styrene and cis-stilbene yielding the corresponding epoxides (Table 2). The epoxide yields were lower than the allylic oxidation yields for cyclohexene, and some loss of stereochemistry was observed in the case of cis-stilbene. The fact that cyclohexene oxide is not formed at all in the course of cyclohexene oxidation suggests that the oxidants formed during Tq decay appear to favor H-atom abstraction over oxo transfer.
Table 2.
Product Yields for the Reaction of Tq Solutions with Hydrocarbons at −45°C in PrCN.
| substrate | atmosphere | product | Yield (equiv./Tq) | A/K ratio |
|---|---|---|---|---|
| cyclohexene | Ar | 2-cyclohexen-1-ol | 1.0 ± 0.2 | 2.5 ± 0.7 |
| 2-cyclohexen-1-one | 0.4 ± 0.1 | |||
| cyclohexene oxide | trace | |||
| cyclohexene | Air | 2-cyclohexen-1-ol | 1.3 ± 0.3 | 0.4 ± 0.1 |
| 2-cyclohexen-1-one | 3.6 ± 0.1 | |||
| cyclohexene oxide | 0.0 | |||
| adamantanea | Ar | 1-adamantanol | 0.56 | 2.8 |
| 2-adamantanone | 0.20 | |||
| styrene | Ar | styrene oxide | 0.33 ± 0.04 | N/A |
| benzaldehyde | 0.2 ± 0.1 | |||
| cis-stilbene | Ar | cis-stilbene oxide | 0.09 ± 0.02 | N/A |
| trans-stilbene oxide | 0.05 ± 0.01 |
Adamantane results were originally reported in reference 16.
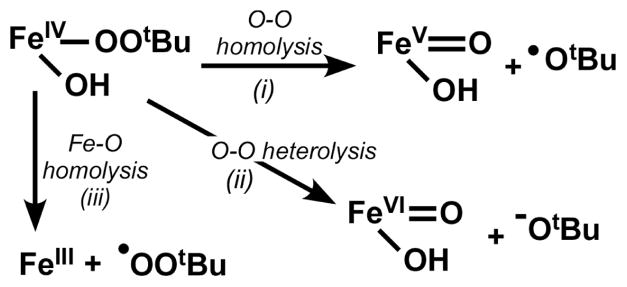
Kinetic studies in the presence of substrates show that Tq decay is independent of both substrate identity and concentration. Thus, Tq itself is not the active species in hydrocarbon oxidation. Instead, Tq must first convert – in the rate-limiting step – to more reactive species that can carry out H-atom abstraction and olefin epoxidation. The nature of the active oxidant(s) is currently unknown. We demonstrated above that at −70 °C the Fe-OOtBu unit is stable and unreactive. However, this unit decomposes at − 45 °C with loss of the alkylperoxo-to-iron charge transfer band, so it is likely that the FeIV-OOR unit fragments upon warming. Fragmentation of the alkylperoxoiron(IV) unit can proceed along three possible pathways, as shown in Scheme 3: (i) homolytic O-O cleavage to yield tert-butoxyl radical and an FeV(O)(OH) species, (ii) heterolytic O-O cleavage to give an FeVI(O)(OH) species, or (iii) Fe-OOR cleavage to provide an alkylperoxyl radical. Previous studies of transition-metal/TBHP systems have demonstrated that alkoxyl and alkylperoxyl radicals are often the active species in hydrocarbon oxidation and responsible for the generation of long-lived alkyl radicals.34–36 Such chemistry is consistent with that observed for cyclohexene oxidation by the Tq system at −45 °C, yet the formation of cis-stilbene oxide from cis-stilbene (Table 2) requires formation of an Fe=O species capable of direct O-atom transfer to substrate with retention of configuration. We thus favor option (i) above as the dominant decomposition mechanism for Tq at −45 °C that accounts for all the experimental observations. This mechanism would produce two oxidants, both of which can act as H-atom abstraction agents, and thus rationalize the formation of more than one equivalent of oxidized product from cyclohexene.
Scheme 3.
6. Electronic Structure Analysis
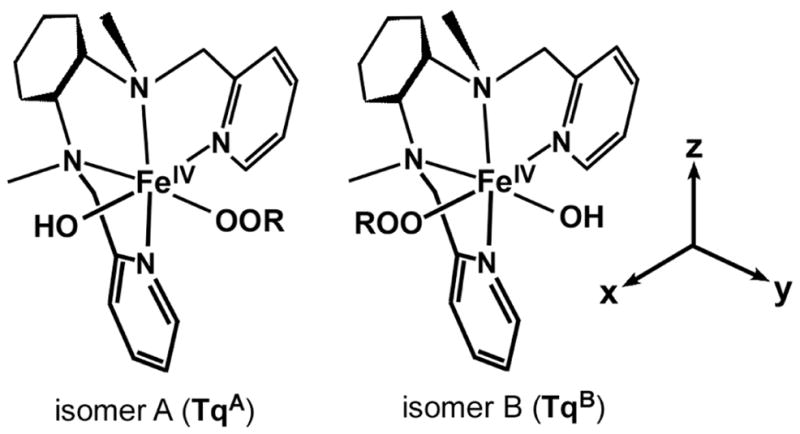
The electronic structures the (S = 1) FeIV complexes Tq and 3 were analyzed with density functional theory (DFT) with the aim of gaining insight into their contrasting reactivities. As the BPMCN ligand is the cis-β conformation, the two labile coordination sites are distinct and two positional isomers exist for each complex. For Tq (and 3), the isomers have been labeled A and B depending on whether the hydroxo (or oxo) ligand is trans to the pyridyl (A) or the amino (B) moiety (see Scheme 4). Full geometry optimizations were performed for both Tq isomers, although the cyclohexane ring was replaced with a simple –CH2CH2– bridge. The resulting Fe-ligand bond distances, as well as the orientations of the tBuOO group, are nearly identical for both isomers. While isomer B is slightly more stable (0.7 kcal/mol), the difference is well within the error of DFT, further supporting the earlier conclusion (derived from spectroscopic data) that Tq solutions contain roughly equal amounts of both isomers.10 In the optimized structures (TqA and TqB), the two Fe-O bond distances are approximately 1.8 Å and the four Fe-N bond distances were found to be 2.05 ± 0.07 Å, in excellent agreement with the EXAFS data (2 N/O scatterers at 1.82 Å, 3 N/O scatterers at 2.01 Å). The short FeIV-Op bond distance of ~1.82 Å (Op = proximal O of alkylperoxo ligand) and O-O distance of ~1.42 Å are similar to values calculated for low-spin FeIII-alkylperoxo complexes.37 The calculations also suggest that the hydroxo ligand is capable of forming a weak hydrogen-bond with the distal oxygen (Od) of the alkylperoxo ligand, which may control the relative orientation of the OtBu group.
Scheme 4.
Geometry optimizations were also carried out for the FeIV=O complex 3-NCMe. As with Tq, the resulting structural parameters and molecular energies were virtually identical for the two isomers (3A-NCMe and 3B-NCMe). The computed Fe=O and Fe-N bond distances of 1.65 and ~2.06 Å, respectively, are consistent with the 3-NCMe EXAFS data as well as previously reported experimental and computational structures of FeIV=O complexes.11,38,39
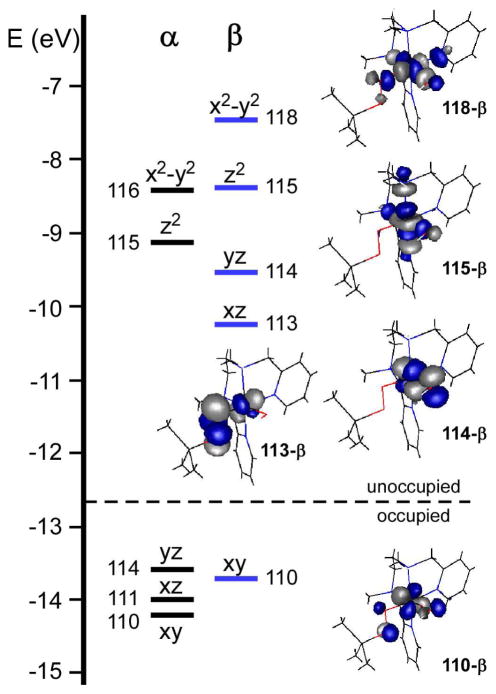
Figure 4 depicts the relevant portion of molecular orbital (MO) energy level diagram for model TqB. The most appropriate molecular orientation places the z-axis along the Npyr-Fe- Namine bonds, while the hydroxo and alkylperoxo ligands lie in the xy-plane (Scheme 4). As Tq is a low-spin (S = 1) complex, the Fe(xy)-based MO is doubly-occupied, while the high-energy Fe(x2−y2)- and Fe(z2)-based MOs are unoccupied. The two unpaired electrons lie in orbitals with Fe(xz/yz) character that participate in π-interactions with either the alkylperoxo (113-β) or hydroxo (114-β) ligands (Figure 4). The π-interaction between the Fe(xz) and peroxo(πv *) orbitals is particularly strong and covalent, with MO 113-β containing 39% Fe and 53% OOtBu character (a similarly covalent Fe/O π-interaction is characteristic of low-spin ferric-alkylperoxo systems37). By comparison, the Fe-OH bond in Tq is more ionic, with MO 114-β containing 73% Fe and only 20% OH character.
Figure 4.
Molecular orbital energy-level diagram obtained from DFT calculations on the TqB model. MOs are labeled according to their principal Fe d-orbital contributor. DFT-generated isosurface plots of the spin-down (β) Fe d-based orbitals are also shown.
H-atom abstraction reactions proceed via donation of electron density from the substrate H-X bond into a low-lying unoccupied orbital on the oxidant. For this reason, it is possible to rationalize the H-atom abstracting ability of transition-metal complexes by analysis of their frontier molecular orbitals (FMOs), particularly those lowest-energy unoccupied MOs (LUMOs) that can serve as acceptor orbitals. Indeed, a recent study of transition-metal oxo species by Michel et al. demonstrated that the activation barrier for H-atom abstraction is directly controlled by the energy and shape of the acceptor orbital(s).40 More specifically, Solomon and coworkers have shown that the reactivity of low-spin FeIV=O complexes towards C-H bonds is proportional to the amount of oxo character in the spin-down (β) Fe/O(π*) orbitals, which are the LUMOs.41,42 The oxo character is important because FMO theory requires the acceptor orbital to have significant electron density on the reactive atom in order to maximize overlap between the donor and acceptor orbitals.
For the Tq models, the LUMO is the π* orbital (113-β) localized on the Fe-O-O unit. Thus, it might be expected that Tq would react with H-X bonds to yield the FeIII complex, HOOtBu, and the substrate radical. However, it appears that 113-β is not a viable acceptor orbital for steric reasons. Access to the proximal O-atom, which contains the largest lobe of electron density (35% of MO β-113), is blocked by both the tert-butyl group and substituents on the BPMCN ligand (a pyridyl ring for TqA and a methyl group for TqB). And while the FeIII-OOH intermediate of activated bleomycin has been postulated to directly abstract a H-atom from DNA7,42 the acceptor orbital in that system is the O-O σ* orbital, not the π* orbital. For Tq, the O-O σ* orbital is high in energy (4.7 eV above the LUMO) and its access to substrate is also blocked by the tBu group.
The second lowest unoccupied MO for Tq is the π* orbital of the Fe-OH bond (114- β), which lies 0.7 eV higher in energy than 113-β. The hydroxide ligand is well-exposed to solvent, but this accessibility is partially offset by the small amount of OH character in the MO (20%). Thus, the elevated energy of the acceptor MO and the low MO coefficients on the hydroxide ligand combine to limit the oxidizing power of the FeIV-OH unit.
Like all low-spin FeIV=O complexes studied to date, 3B-NCMe possesses a (xy)2(xz)1(yz)1 configuration in which Fe(xz/yz) orbitals participate in highly covalent π-interactions with the O(px/py) orbitals (Figure 5). Indeed, our calculations indicate that the two spin-down Fe/O(π*) orbitals (99-β and 100-β), which are nearly isoenergetic, contain approximately 50% Fe and 40% O character. These orbitals are the LUMOs and thus serve as the acceptor orbitals in H-atom abstraction reactions. As noted above, the large degree of oxo character in these orbitals facilitates the H-atom abstraction and accounts for the remarkable oxidizing ability of 3B-NCMe. The contrast between the electronic structures of TqB and 3B-NCMe is especially evident in Figure 6, which displays contour plots of the total unpaired spin density for both complexes (note that since the putative acceptor orbitals are the singly-occupied MOs in both cases, plots of spin density correspond to the spatial distribution of the FMOs). 3B-NCMe has a large lobe of unpaired spin density (43% of the total) that is localized on the oxo ligand and easily accessible to substrates – the ideal scenario for facile H-atom abstraction. By contrast, the unpaired spin density in Tq is much more diffuse, with no ligand atom carrying more than 18% of the total; indeed, only 10% is localized on the reactive hydroxo ligand. Collectively, these results indicate that protonation of the ferryl unit substantially decreases the covalency of the Fe/O bond, thereby diminishing the amount of oxygen character in the LUMOs. As these orbitals serve as the acceptor orbitals for H-atom abstraction, this dramatic change in Fe-O bonding explains the massive disparities in reactivity reported above.
Figure 5.
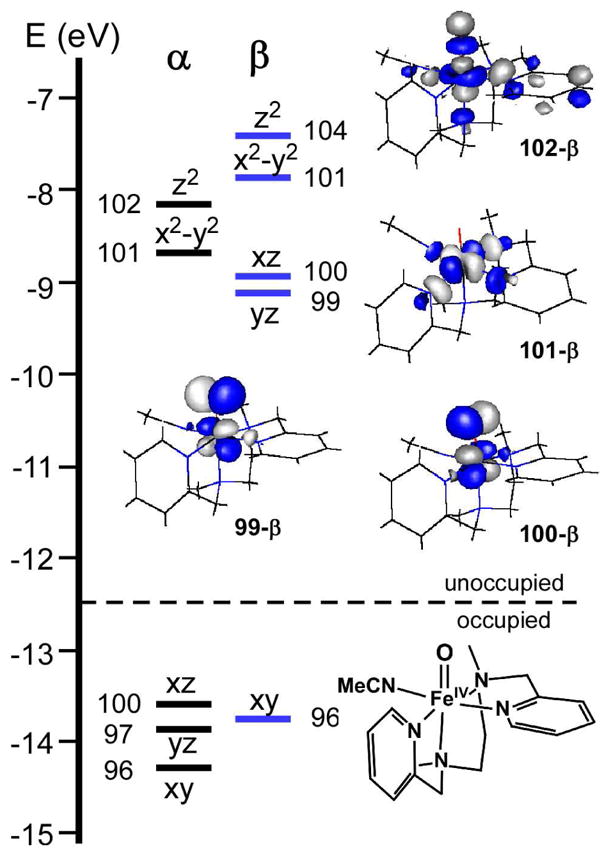
Molecular orbital energy-level diagram obtained from DFT calculations on the 3BNCMe model. MOs are labeled according to their principal Fe d-orbital contributor. DFTgenerated isosurface plots of the unoccupied spin-down (β) Fe d-based orbitals are also shown.
Figure 6.
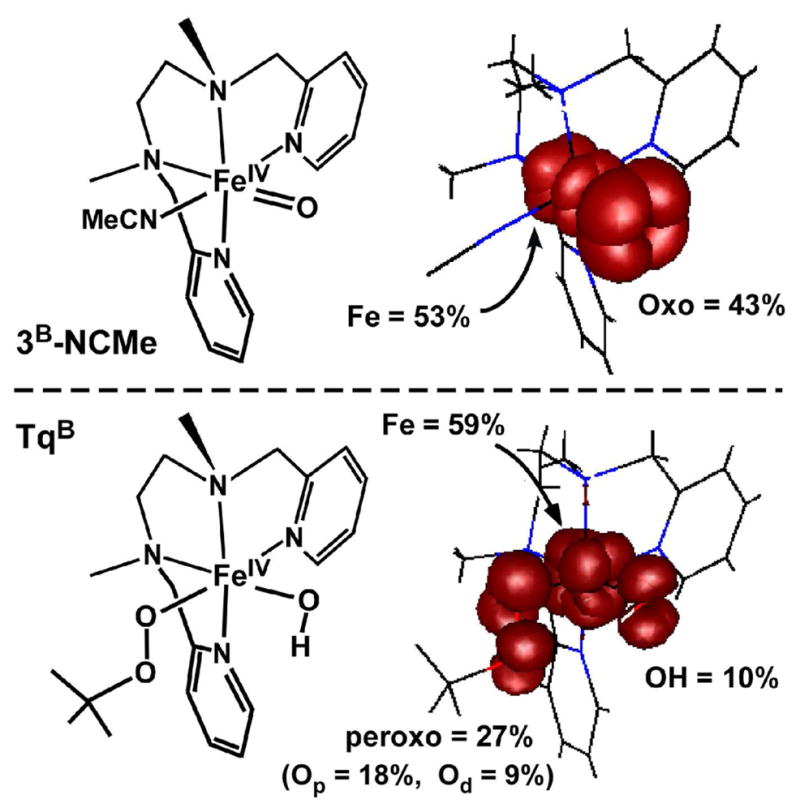
DFT-generated isosurface plots representing the unpaired electronic spin-density of models 3B-NCMe (top) and TqB (bottom). Percentage of the total spin-density localized on specific atoms/ligands is also provided.
While FMO theory accounts for the increased kinetic reactivity of 3 relative to Tq, it does not address the related thermodynamic issue; i.e., the fact that Tq cannot oxidize C-H bonds stronger than ~80 kcal/mol whereas 3-pyr oxidizes the stronger C-H bonds of cyclohexene at −70 °C. This matter is determined by the dissociation energy of the H-O bonds of the resulting FeIII species. For the complexes discussed here, the strength of the H-O bond formed during H-atom abstraction is proportional to (i) the one-electron redox potential of the FeIV oxidant, and (ii) the pKa of the FeIII-OH(1 or 2) product complex. The exact formula is provided below:43–45
| (5) |
While precise E0 values for Tq and 3 are not known, our results indicate that both potentials fall within the range of 490–670 mV, suggesting that the observed differences in H-atom abstracting power are not determined by redox potential. Instead, the second term in eq. 5 likely plays the decisive role, as the [FeIII-OH2] unit formed by Tq + H• is undoubtedly much more acidic than the [FeIII-OH] unit formed by 3 + H•. Thus, the basicity of the one-electron reduced FeIII-O− complex provides much of the driving force for the remarkable H-atom abstracting ability of 3. The importance of oxo ligand basicity in H-atom abstraction reactions has been highlighted by others in recent studies of Mn-oxo reactivity.9,46,47
Summary
Tq is a novel FeIV complex with both alkylperoxo and hydroxo ligands. Here, we showed that Tq reacts at −70 °C with substrates with weak H-X bonds (like TTBP and DHA) to yield the corresponding alkylperoxoiron(III) complex 2-PrCN, indicating that an H-atom from substrate is transferred to the FeIV-OH unit (Scheme 2). We then compared the reactivity of Tq with that of complex 3, which features an FeIV=O moiety supported by the same ligand. Even with a limited number of substrates, our kinetic studies clearly demonstrate that 3 is far more reactive than Tq towards H-atom donors, by as much as a factor of 103 (Table 1). The differing H-atom abstracting abilities of 3 and Tq are related to their underlying electronic structures, as elucidated with DFT methods. Due to the highly covalent nature of the FeIV=O π-bonds, 3 features two low-lying acceptor MOs with substantial oxo-character, thereby facilitating the electrophilic attack of H-X bonds. However, protonation of the ferryl to give an FeIV-OH unit disrupts this felicitous situation in two respects: (i) the hydroxo ligand can have only one π-interaction with the Fe center (versus two for the oxo ligand), and (ii) the proton lowers the energy of the remaining O(p) orbital, resulting in diminished Fe-O covalency and less OH character in the acceptor MO. Hence, we have the (counterintuitive) result that protonation of the ferryl unit makes it less electrophilic towards H-atom donors. This trend is also observed by Busch and co-workers in their study of MnIV(EBC) complexes.9
In contrast to the remarkable oxidizing power of 3 and other FeIV=O complexes, Tq is only capable of oxidizing hydrocarbons with weak C-H bonds (< 80 kcal/mol). However, at elevated temperatures, Tq decays to form one or more reactive species that oxidize more demanding substrates (Table 2). Based on spectroscopic and reactivity data, we conclude that the active species is generated by fragmentation of the alkylperoxoiron(IV) unit (Scheme 3). Indeed, this scenario is consistent with previous studies where FeIII- and CuII-alkylperoxo complexes were found to be inert but gave rise to very reactive intermediates via O-O bond homolysis.48–50
The results presented here have several implications for metalloenzyme systems involved in H-atom abstraction. First, and most important, is the conclusion that FeIV-OH intermediates are significantly less reactive than FeIV=O intermediates. Thus, it is not surprising that only one class of enzymes – the lipoxygenases – is known to employ an Fe-OH species as the reactive intermediate for H-atom abstraction. And, in this case, the target C-H bond of the fatty acid substrate is quite weak (estimated BDE ~76 kcal/mol) due to its bis-allylic position.6 Interestingly, despite its higher oxidation state, the reactivity of Tq is comparable to that of the lipoxygenase model complex [(PY5)FeIII-OH] (PY5 = 2,6-bis(bis(2-pyridyl)methoxy-methane) pyridine), which is also limited to oxidizing weak C-H bonds.51 While the oxidation potential of Tq is at least 0.25 V more positive than the value measured for [(PY5)FeIII-OH], this factor is likely offset by the increased basicity of the hydroxo ligand in the more reduced complex. Thus, it appears that Fe-OH species exhibit sluggish H-atom abstraction reactivity regardless of oxidation state.
Secondly, our study of the FeIV-OH unit of Tq has implications for diiron enzymes, such as MMO and RNR, in which the putative reactive oxo ligand is located in a bridging position between the two Fe centers. This comparison is valid because the second Fe center can be thought of as a weakly-bound proton (i.e., FeIV –O–H ≈ FeIV –O–Fe). Inspection of DFT results published by Solomon and coworkers for the hypothetical complex [FeIIIFeIV(μ-O)(μ-OAc)2(L)2]3+ (L = 9-crown-3-ether), suggests that FeIII centers perturb the FeIV-oxo unit in a manner similar to that described here for protons.52 For instance, relative to the mononuclear FeIV=O system, coordination of the second Fe center lengthens the FeIV-O bond and diminishes the amount of O(p) character in the LUMOs. However, it is also clear that metal binding does not disrupt the oxoiron(IV) unit to the same extent as proton binding.53 Whereas the additional Fe center merely perturbs the π-interactions of the oxoiron(IV) unit, the O→OH conversion fundamentally destroys the covalent nature of the FeIV=O bond. Thus, based on our analysis, we predict that bridging oxo ligands are generally more reactive than terminal hydroxo ligands but less reactive than terminal oxo ligands. Support for this conclusion comes from our recent study of a synthetic model that, like intermediate Q, possesses a [FeIV 2(μ-O)2] diamond core. This complex was found to be 80-fold less reactive toward DHA than the corresponding mononuclear FeIV=O complex.54 This finding, combined with the results presented here regarding an FeIV-OH species, suggests the following order of reactivity for FeIV intermediates:
We therefore conclude that the coordination of Lewis acids, whether protons or metals ions, to oxoiron(IV) units dramatically reduces their oxidizing power.
Supplementary Material
Acknowledgments
This research was supported by the National Institutes of Health (grant GM-33162 to L.Q. and postdoctoral fellowship GM-079839 to A.T.F.). We thank Professor Thomas Brunold of the University of Wisconsin-Madison for graciously providing access to his computer cluster.
Footnotes
Supporting Information Available: Detailed kinetic data for the reaction of Tq with TTBP (Figure S1) and various phenols (Figure S2). EPR and Resonance Raman spectra of product solutions generated by reaction of Tq with phenols (Figures S3 and S4, respectively). Absorption spectra of the reaction of Tq with DHA in CH2Cl2 (Figure S5) and the reaction of 3- NCR with TTBP in PrCN (Figure S6). Detailed kinetic data for the reaction of 3-NCR with TTBP (Figure S7). Absorption spectra for the reaction of Tq at −45°C with cyclohexene (Figure S8). Cartesian coordinates for the DFT geometry-optimized models TqA, TqB, 3A-NCMe, and 3B-NCMe (Table S1(a–d)). This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Costas M, Mehn MP, Jensen MP, Que L., Jr Chem Rev. 2004;104:939–986. doi: 10.1021/cr020628n. [DOI] [PubMed] [Google Scholar]
- 2.Groves JT. J Inorg Biochem. 2006;100:434–447. doi: 10.1016/j.jinorgbio.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 3.Sono M, Roach MP, Coulter ED, Dawson JH. Chem Rev. 1996;96:2841–2887. doi: 10.1021/cr9500500. [DOI] [PubMed] [Google Scholar]
- 4.Krebs C, Galonić Fujimori D, Walsh CT, Bollinger JM., Jr Acc Chem Res. 2007;40:484–492. doi: 10.1021/ar700066p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tshuva EY, Lippard SJ. Chem Rev. 2004;104:987–1012. doi: 10.1021/cr020622y. [DOI] [PubMed] [Google Scholar]
- 6.Nelson MJ, Seitz SP. In: Active Oxygen in Biochemistry. Valentine JS, Foote CS, Greenberg A, Liebman JF, editors. Chapman & Hall; London: 1995. pp. 276–312. [Google Scholar]
- 7.Decker A, Chow MS, Kemsley JN, Lehnert N, Solomon EI. J Am Chem Soc. 2006;128:4719–4733. doi: 10.1021/ja057378n. [DOI] [PubMed] [Google Scholar]
- 8.Yin G, Danby AM, Kitko D, Carter JD, Scheper WM, Busch DH. J Am Chem Soc. 2007;129:1512–1513. doi: 10.1021/ja0673229. [DOI] [PubMed] [Google Scholar]
- 9.Yin GC, Danby AM, Kitko D, Carter JD, Scheper WM, Busch DH. J Am Chem Soc. 2008;130:16245–16253. doi: 10.1021/ja804305x. [DOI] [PubMed] [Google Scholar]
- 10.Jensen MP, Costas M, Ho RYN, Kaizer J, Mairata i Payeras A, Münck E, Que L, Jr, Rohde J-U, Stubna A. J Am Chem Soc. 2005;127:10512–10525. doi: 10.1021/ja0438765. [DOI] [PubMed] [Google Scholar]
- 11.Jackson TA, Rohde JU, Seo MS, Sastri CV, DeHont R, Stubna A, Ohta T, Kitagawa T, Munck E, Nam W, Que L. J Am Chem Soc. 2008;130:12394–12407. doi: 10.1021/ja8022576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaizer J, Klinker EJ, Oh NY, Rohde JU, Song WJ, Stubna A, Kim J, Münck E, Nam W, Que L., Jr J Am Chem Soc. 2004;126:472–473. doi: 10.1021/ja037288n. [DOI] [PubMed] [Google Scholar]
- 13.Oh NY, Suh Y, Park MJ, Seo MS, Kim J, Nam W. Angew Chem Int Ed. 2005;44:4235–4239. doi: 10.1002/anie.200500623. [DOI] [PubMed] [Google Scholar]
- 14.Sastri CV, Lee J, Oh K, Lee YJ, Lee J, Jackson TA, Ray K, Hirao H, Shin W, Halfen JA, Kim J, Que L, Jr, Shaik S, Nam W. Proc Natl Acad Sci USA. 2007;104:19181–19186. doi: 10.1073/pnas.0709471104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klinker EJ, Shaik S, Hirao H, Que L. Angew Chem Int Ed. 2009;48:1291–1295. doi: 10.1002/anie.200804029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Costas M, Rohde JU, Stubna A, Ho RYN, Quaroni L, Münck E, Que L., Jr J Am Chem Soc. 2001;123:12931–12932. doi: 10.1021/ja017204f. [DOI] [PubMed] [Google Scholar]
- 17.Goldsmith CR, Jonas RT, Stack TDP. J Am Chem Soc. 2002;124:83–96. doi: 10.1021/ja016451g. [DOI] [PubMed] [Google Scholar]
- 18.ADF2006.01; SCM, Theoretical Chemistry. Vrije Universiteit; Amsterdam, The Netherlands: http://www.scm.com. [Google Scholar]
- 19.Vosko SH, Wilk L, Nusair M. Can J Phys. 1980;58:1200. [Google Scholar]
- 20.Becke AD. J Chem Phys. 1986;84:4524–4529. [Google Scholar]
- 21.Perdew JP. Phys Rev B. 1986;33:8822–8824. doi: 10.1103/physrevb.33.8822. [DOI] [PubMed] [Google Scholar]
- 22.Neese F. version 2.5 ed. University of Bonn; Bonn, Germany: 2006. [Google Scholar]
- 23.Schafer A, Horn H, Ahlrichs R. J Chem Phys. 1992;97:2571–2577. [Google Scholar]
- 24.Becke AD. J Chem Phys. 1993;98:5648–5652. [Google Scholar]
- 25.Becke AD. J Chem Phys. 1993;98:1372–1377. [Google Scholar]
- 26.Lee CT, Yang WT, Parr RG. Phys Rev B. 1988;37:785–789. doi: 10.1103/physrevb.37.785. [DOI] [PubMed] [Google Scholar]
- 27.Laaksonen L. J Mol Graph. 1992;10:33. doi: 10.1016/0263-7855(92)80007-z. [DOI] [PubMed] [Google Scholar]
- 28.Lucarini M, Pedrielli P, Pedulli GF, Cabiddu S, Fattuoni C. J Org Chem. 1996;61:9259–9263. [Google Scholar]
- 29.Pseudo first-order rate constants for these 2,6-di-tert-butyl-4-X-phenols were measured at similar substrate concentrations. For X = H, k1 = 0.99 × 10−4 s−1 at 50 mM; for X = tBu, k1 = 2.7 × 10−4 s−1 at 59 mM; for X = OMe, k1 = 27 × 10−4 s−1 at 39 mM.
- 30.Bordwell FG, Cheng JP. J Am Chem Soc. 1991;113:1736–1743. [Google Scholar]
- 31.Schlesener CJ, Amatore C, Kochi JK. J Am Chem Soc. 1984;106:3567–3577. [Google Scholar]
- 32.Laarhoven LJJ, Mulder P, Wayner DDM. Acc Chem Res. 1999;32:342–349. [Google Scholar]
- 33.Kaizer J, Costas M, Que L., Jr Angew Chem Int Ed. 2003;42:3671–3673. doi: 10.1002/anie.200351694. [DOI] [PubMed] [Google Scholar]
- 34.MacFaul PA, Arends IWCE, Ingold KI, Wayner DDM. J Chem Soc, Perkin Trans. 1997;2:135–145. [Google Scholar]
- 35.MacFaul PA, Ingold KU, Wayner DDM, Que L., Jr J Am Chem Soc. 1997;119:10594–10598. [Google Scholar]
- 36.Caudle MT, Riggs-Gelasco P, Gelasco AK, Penner-Hahn JE, Pecoraro VL. Inorg Chem. 1996;35:3577–3584. [Google Scholar]
- 37.Lehnert N, Ho RYN, Que L, Jr, Solomon EI. J Am Chem Soc. 2001;123:8271–8290. doi: 10.1021/ja010165n. [DOI] [PubMed] [Google Scholar]
- 38.Rohde JU, In JH, Lim MH, Brennessel WW, Bukowski MR, Stubna A, Münck E, Nam W, Que L., Jr Science. 2003;299:1037–1039. doi: 10.1126/science.299.5609.1037. [DOI] [PubMed] [Google Scholar]
- 39.Klinker EJ, Kaizer J, Brennessel WW, Woodrum NL, Cramer CJ, Que L., Jr Angew Chem Int Ed. 2005;44:3690–3694. doi: 10.1002/anie.200500485. [DOI] [PubMed] [Google Scholar]
- 40.Michel C, Baerends EJ. Inorg Chem. 2009;48:3628–3638. doi: 10.1021/ic802095m. [DOI] [PubMed] [Google Scholar]
- 41.Decker A, Rohde J-U, Klinker EJ, Wong SD, Que L, Jr, Solomon EI. J Am Chem Soc. 2007;129:15983–15996. doi: 10.1021/ja074900s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Solomon EI, Wong SD, Liu LV, Decker A, Chow MS. Curr Opin Chem Biol. 2009;13:99–113. doi: 10.1016/j.cbpa.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bordwell FG, Cheng JP, Ji GZ, Satish AV, Zhang X. J Am Chem Soc. 1991;113:9790–9795. [Google Scholar]
- 44.Parker VD, Jr, Handoo KL, Roness F, Tilset M. J Am Chem Soc. 1991;113:7493–7498. [Google Scholar]
- 45.Gardner KA, Mayer JM. Science. 1995;269:1849–1851. doi: 10.1126/science.7569922. [DOI] [PubMed] [Google Scholar]
- 46.Parsell TH, Yang MY, Borovik AS. J Am Chem Soc. 2009;131:2762–2763. doi: 10.1021/ja8100825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lansky DE, Goldberg DP. Inorg Chem. 2006;45:5119–5125. doi: 10.1021/ic060491+. [DOI] [PubMed] [Google Scholar]
- 48.Seo MS, Kamachi T, Kouno T, Murata K, JPM, Yoshizawa K, Nam W. Angew Chem Int Ed. 2007;46:2291–2294. doi: 10.1002/anie.200604219. [DOI] [PubMed] [Google Scholar]
- 49.Kunishita A, Ishimaru H, Nakashima S, Ogura T, Itoh S. J Am Chem Soc. 2008;130:4244–4245. doi: 10.1021/ja800443s. [DOI] [PubMed] [Google Scholar]
- 50.Kitajima N, Katayama T, Fujisawa K, Iwata Y, Morooka Y. J Am Chem Soc. 1993;115:7872–7873. [Google Scholar]
- 51.Goldsmith CR, Stack TDP. Inorg Chem. 2006;45:6048–6055. doi: 10.1021/ic060621e. [DOI] [PubMed] [Google Scholar]
- 52.Decker A, Clay MD, Solomon EI. J Inorg Biochem. 2006;100:697–706. doi: 10.1016/j.jinorgbio.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 53.In the [FeIII-O-FeIV] complex, the O(p) orbital directed towards the second Fe center still participates in π-bonding with the FeIV center, whereas the corresponding orbital in the FeIV-OH unit interacts too strongly with the H to allow for significant bonding to the Fe center.
- 54.Xue G, Wang D, De Hont R, Fiedler AT, Shan X, Münck E, Que L., Jr Proc Natl Acad Sci USA. 2007;104:20713–20718. doi: 10.1073/pnas.0708516105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.