Abstract
Two sensorimotor tasks that share neither sensory nor motor modality can interfere with one another when they are performed simultaneously. A possible cause for this interference is the recruitment of common brain regions by these two tasks, thereby creating a bottleneck of information processing. This hypothesis predicts that such “bottleneck” regions would be activated by each task even when they are performed separately. To test this prediction, we sought to identify, with fMRI, brain regions commonly activated by sensorimotor tasks that share neither sensory input nor motor output. One group of subjects was scanned while they performed in separate runs an auditory‐vocal (AVo) task and a visuo‐manual (ViM) task, while a second group of subjects performed the reversed sensorimotor mapping tasks (AM and ViVo). The results revealed strong activation preferences in specific sensory and motor cortical areas for each sensory and motor modality. By contrast, the posterior portion of the lateral prefrontal cortex (pLPFC), anterior insula, and, less consistently, the anterior cingulate, presupplementary and supplementary motor areas, and subcortical areas were commonly activated across all four sensorimotor tasks. These results were observed in both blocked and event‐related fMRI designs, in both 3D‐group averaged and 2D‐individual subject analyses, and were replicated within individuals across scanning sessions. These findings not only suggest that these brain regions serve a common amodal function in sensorimotor tasks, they also point to these regions—particularly, the pLPFC and anterior insula—as candidate neural substrates underlying a central hub of information processing in the human brain. Hum Brain Mapp, 2009. © 2009 Wiley‐Liss, Inc.
Keywords: fMRI, bottleneck of information processing, response selection, dual‐tasking, psychological refractory period
INTRODUCTION
Effective interaction with one's environment requires the ability to select appropriate responses to particular stimulus situations. Although some stimulus‐response (S–R) ensembles are more compatible than others [e.g., Fitts and Seeger, 1953], humans are endowed with the remarkable ability of making arbitrary responses to stimuli purely based on a given set of rules and instructions. This ability to quickly and efficiently pair any motor response to any stimulus affords us with an immensely adaptive way to respond to environmental changes.
Although we can efficiently execute a proper response to any arbitrary stimuli, performance deteriorates greatly under conditions of multitasking. This is well illustrated by the psychological refractory period (PRP) paradigm, which requires making two distinct motor responses to two successively presented stimuli [Pashler, 1994a]. The response to the second stimulus is greatly slowed as the stimulus‐onset asynchrony (SOA) between the two stimuli decreases. This dual‐task slowing is thought to result from the inability to select two responses simultaneously. Specifically, the PRP effect has been attributed to the existence of a response selection bottleneck, in which only one response selection process can be executed at a time, leading to the serial postponement of response selection associated with the second task [see Pashler and Johnston (1998) for a review]. According to this central‐bottleneck account, information arising from distinct sensorimotor pathways ultimately converge onto a common structural hub, thereby creating a bottleneck of information processing [see Klingberg (1998) and Marois and Ivanoff (2005) for a discussion of alternative accounts of neural correlates of multitasking limitations]. Despite the fact that dual‐tasking has been the subject of numerous functional neuroimaging studies [e.g., Adcock et al., 2000; Bunge et al., 2000; Collette et al., 2005; D'Esposito et al., 1995; Dreher and Grafman, 2003; Erickson et al., 2005, 2007; Jaeggi et al., 2003; Just et al., 2001; Klingberg and Roland, 1997; Sigman and Dehaene, 2008; Stelzel et al., 2008], there is little consensus regarding the neural mechanism responsible for dual‐task limitations. This is the case even when only considering studies that have investigated one classic form of dual‐task interference, the PRP. Some PRP studies have compared dual‐task and single‐task conditions [Herath et al., 2001; Schubert and Szameitat, 2003; Stelzel et al., 2006; Szameitat et al., 2002]. These studies have identified middle frontal, inferior frontal, and intraparietal cortical regions as being involved in the coordination of dual‐tasking. However, this methodological approach to dual‐task interference may not necessarily yield activations specific to the executive control needed to co‐ordinate dual‐task performance, as they could also be related to executive processes (e.g. task‐switching) that are not directly responsible to the dual‐task slowing observed in the PRP. In addition, other studies have failed to show any evidence of dual‐task specific activations [Adcock et al., 2000; Erickson et al., 2005; Dux et al., 2006].
Another neuroimaging approach to dual‐task interference consists in taking the same experimental principle used to demonstrate the PRP effect in behavioral studies, which is to compare brain activity levels at short versus long SOAs. Such efforts have isolated the right inferior frontal gyrus (IFG) as a potential neural candidate of the PRP bottleneck [e.g., Herath et al., 2001; Jiang et al., 2003]. However, the strength of the activation within this region was negatively correlated with the magnitude of the PRP effect [Jiang et al., 2004, but see Herath et al. (2001), who observed a positive correlation], suggesting that this region was related to efforts to reduce the PRP effect. Furthermore, while some studies [Herath et al., 2001; Jiang, 2004; Jiang et al., 2004] have found the right IFG with a short SOA—long SOA subtraction, one study [Marois et al., 2006] observed no significant differences with this comparison. If the PRP is the result of a queuing of response selection for Task 2 as suggested by the central bottleneck model [Pashler, 1994a], then one would actually not expect to find any region whose magnitude of activation differs between short and long SOAs [Jiang et al., 2004; Marois and Ivanoff, 2005]. Rather, the SOA manipulation would be expected to affect the duration of neural activity in the brain regions subserving this central bottleneck, and recent time‐resolved fMRI studies of the PRP provide support for this hypothesis [Dux et al., 2006; Sigman and Dehaene, 2008].
Given that behavioral studies have pointed to the stage of response selection as the source of the bottleneck underlying the PRP [Pashler, 1994a], a fruitful approach to isolating that neural substrates of the central bottleneck is to identify brain regions associated with response selection. Using such approach, Jiang and Kanwisher [ 2003a] noted that a fronto‐parietal network [including the intraparietal sulcus (IPS), percutaneous, frontal eye fields (FEF), and lateral frontal areas in inferior and middle frontal gyrus (IFG and MFG, respectively)] was commonly activated in S–R compatibility tasks that did not share a common sensory or motor modality. In their task, spatial stimulus‐response mapping was natural (i.e., stimuli were spatially aligned in the same order as response output) or unnatural (i.e., stimuli and responses were not arranged similarly). Jiang and Kanwisher [ 2003a] concluded that response selection is implemented by a common neural network irrespective of task details. In a similar vein, but with different conclusions, Schumacher et al. [ 2003] used a parametric manipulation of response selection (by increasing the number of alternative responses and the spatial compatibility of the S–R pairing) to isolate response selection processes. Schumacher et al. [ 2003] observed a frontal‐parietal network (including dorsal premotor, middle frontal, and superior parietal areas) whose activation pattern depended upon the task (spatial compatibility vs. nonspatial numerosity) and therefore concluded that response selection is representation‐specific. The discrepancy between these studies may have been the result of different kinds of response selection operations involved in S–R compatibility and S–R numerosity [Schumacher and Jiang, 2003], suggesting that response selection is not a simple unitary process.
The conflicting results of the latter studies may be resolved by avoiding compatibility manipulations all together as a means of isolating brain regions involved in simple sensorimotor response selection tasks. To that effect, the goal of this study was to localize brain regions that are commonly activated when performing two tasks that share neither sensory nor motor modalities. Our goal is conceptually similar to that of Jiang and Kanwisher [ 2003a] in that we sought regions that are commonly activated irrespective of the particular sensory input or motor output. However, Jiang and Kanwisher [ 2003a] and Schumacher et al. [ 2003] relied on a particular behavioral manipulation (S–R compatibility or S–R numerosity [Hick, 1952; see also Woo and Lee, 2007], respectively) to uncover brain regions specifically involved in response selection. By contrast, we adopted a simpler and more inclusive approach to identifying brain regions commonly activated across four sets of sensorimotor mappings. Specifically, to isolate most (if not all) brain regions that may be activated across sensorimotor tasks, we compared activation of each sensorimotor task to a simple baseline condition and then searched for regions of overlapping activation between these tasks. According to the response selection bottleneck model [Pashler, 1994a], any brain regions taking part in the structural bottleneck revealed by the PRP should be activated even when each task is carried out in isolation. Thus, in this study, we scanned two groups of subjects who each performed two distinct sensorimotor tasks under single‐task situations: one group of participants performed an auditory‐manual (AM) task and a visual‐vocal (ViVo) task. The other group of participants performed the opposite pairing (AVo and ViM). Any brain regions supporting a central, capacity‐limited stage of information processing should be commonly activated by all four pairings. Although this approach does not allow us to unambiguously claim that the “convergence” areas are involved in response selection per se, it has the advantage of revealing brain areas that are commonly involved in the performance of simple sensorimotor tasks irrespective of the sensory or motor modalities. Given that response selection is not the only process that taps into this central bottleneck of information processing [e.g. Carrier and Pashler, 1995; Ruthruff et al., 1995; see Marois and Ivanoff (2005) for review], our approach therefore has the potential to reveal all the brain regions that support common, amodal stages of information processing.
Two recent studies have used a similar experimental approach to isolate brain regions commonly activated across sensorimotor tasks. For one of these studies, this approach was used to identify candidate central processing regions that could then be probed under other experimental manipulations [Dux et al., 2006]. However, that study only examined one sensorimotor pair of tasks (i.e., AM and ViVo). The other study included the reciprocal combination of sensorimotor pairs (i.e., AM‐ViVo and AVo‐ViM) but used those pairs to specifically isolate putative brain regions involved in dual‐task condition relative to single‐task conditions [Stelzel et al., 2006]. As mentioned earlier, a dual‐ versus single‐task comparison is not expected to specifically isolate brain regions that may underly a central bottleneck of information processing at the stage of response selection. Moreover, this study differs from these previous studies in several technical respects. First, we used both blocked and event‐related (ER) designs to isolate such brain regions to provide a replication of results with two experimentally distinct paradigms, thereby making it unlikely that the results are paradigm‐specific. In addition, we examined all brain regions that showed convergence of activation not only at the group‐average level, but also at the individual level. The latter approach allows one to assess whether commonly activated brain regions are simply an artifact of the lower resolution afforded by group averages. Finally, this single‐subject analysis included rescanning a subset of our participants to determine whether the task‐specific activation was replicable across fMRI sessions.
METHODS
Subjects
Twenty‐eight adult volunteers, aged 18–39 and recruited from the Vanderbilt community, participated in the study for financial compensation. All participants filled out informed consent forms. This study was approved by Vanderbilt's Internal Review Board.
General Design
All subjects performed two separate sensorimotor tasks under single‐task condition, with each of the two tasks consisting of a three alternative‐discrimination. Half of the subjects were randomly assigned to the “AVo/ViM” condition in which they were to make vocal utterances in response to auditory stimuli (auditory‐vocal; AVo) and manual key‐presses in response to visual stimuli (visual‐manual; ViM). The remaining 14 subjects were assigned to the “AM/ViVo” group: manual key‐press responses were made to auditory stimuli (AM) and vocal responses to the visual stimuli (ViVo). Different subjects were used for each pairings (AVo/ViM and AM/ViVo) to avoid prepotency interference (e.g. interference during ViVo task from previous association of a visual stimulus with a manual response in ViM task).
The scanning session included four blocked and four ER runs. The blocked and fast‐event runs were counterbalanced between subjects. In the blocked runs, each of the two tasks were presented in separate blocks of trials (see below), and task order was counterbalanced between runs, whereas, in the ER runs, each of the two tasks were presented in separate runs.
Behavioral paradigm
Task design
The three visual stimuli were colored checkered shapes (circle, square, and triangle, all 3.7° wide) presented on a light gray background. To improve visual cortex activity, the visual shapes were filled with red and green alternating (10 Hz) checkers (∼0.37° per checker). The auditory stimuli were complex frequency‐modulated sounds created by resampling segments of bird songs such that they were unrecognizable as bird sounds. Stimuli (visual and auditory) were presented for 200 ms for the first eight participants, and for 500 ms for the last 20 participants (to further amplify activity in sensory cortex). This stimulus duration change was offset by changing the intertrial interval (ITI), so that the length of a trial remained constant (see below). The ITI was the same for both auditory and visual stimuli (see below). Manual key‐press responses were recorded with the index, middle, and ring finger of the right hand. Vocal responses were monosyllabic utterances “ba,” “bo,” and “be” recorded with an MRI compatible microphone (Resonance Technology, Northridge, CA). To minimize motion and vocal artifacts within the scanner, participants were practiced making the vocal responses with minimal jaw movement. The pairings between specific sensory and motor responses were counterbalanced between subjects. Participants were instructed to respond as soon as they felt confident of their responses (i.e., both accuracy and speed was emphasized).
Trial design
Block runs. These runs included distinct blocks of trials for each of the two sensorimotor tasks. Each 24.75‐s block contained the presentation of a cue followed by 10 trials of a given task. Each trial started with the presentation of the stimulus (auditory or visual) and followed by a white fixation cross (0.5°). The trial onset asynchrony (TOA) was 2.25 s for both stimuli durations (200 and 500 ms). At the beginning of each block, subjects were cued to the modality of the task by means of a visual cue (i.e., the fixation cross turning green) or an auditory cue (i.e., a pure tone of 880 Hz) for 1000 ms for the visual and auditory tasks, respectively. The cues were followed by a 1.25‐s fixation period before onset of the first trial. These two block types alternated trice in each fMRI run and were separated from each other by 24.75‐s long periods of fixation, during which subjects were only required to fixate the cross. Thus, there were six fixation, three visual, and three auditory blocks per 4.5‐min‐long fMRI run, and four of such runs per fMRI session.
ER runs. Each 4.5‐min ER run included 42 trials of the same stimulus type (auditory or visual). The presentation of the stimulus (for 200 or 500 ms) preceded the presentation of a fixation cross. For both stimulus durations, the TOAs were 2.25, 4.5, 6.75, or 8.5 s. There were two runs of each of the two sensorimotor tasks, with the order of these runs alternating within subjects and counterbalanced between subjects. The task identity was introduced to the participant over the scanner intercom system by the experimenter prior to each fMRI run.
Practice. Participants were trained and practiced on the tasks within the scanner and prior to the fMRI runs. The practice block of trials was similar to the experimental blocks runs with the exception that the correct responses were presented visually (centrally, approximately within 1°) during the ITI. Subjects were instructed to use this information to learn the appropriate S–R mappings, and they made responses during the presentation of the visual presentation of the correct answer. For the vocal responses the letters “BO,” “BE,” or “BA”, and for the key‐press responses the number corresponding to the key (1, 2, or 3), were displayed at fixation. Subjects were instructed to use the answers to learn the appropriate responses to the stimuli. Responses were monitored online by the experimenter to assure subjects had made the appropriate response. Performance measures (RT, accuracy) were not saved for the practice runs.
fMRI parameters
Imaging data were acquired on a GE LX 3T (Madison, WI) scanner. The 2D anatomical T1‐weighted images were acquired using conventional parameters. The T2*‐weighted transverse echoplanar images (EPI) slices (64 × 64 matrix) were interleaved and acquired axially (aligned to the AC‐PC plane; 17 slices; 3.75 mm2 in‐plane; 6 mm thick, 0 mm gap) for the first four (2 AVo‐ViM, 2 AM‐ViVo) participants and sagittally (22 slices; 3.75 mm2 in‐plane; 7 mm thick; 0 mm gap) for the remaining 24 participants (12 AVo‐ViM and 12 AM‐ViVo). After the first four participants were scanned, we suspected that medial frontal regions may play a key role in response selection [e.g., Dux et al., 2006; Marois et al., 2006]. Thus, sagittal sectioning was adopted, because it afforded the optimal orientation for high‐resolution 2D analysis of these areas. Adding the participants with axial slice acquisition did not change the pattern of results, thus they were included in the analysis. The T2* parameters for the axial prescription were the following: TR = 1.75 s, TE = 25 ms, and FA = 70°. For the sagittal prescription, it was the following: TR = 2.25 s; TE = 25 ms; and FA = 70. Finally, 124 high‐resolution 3D‐SPGR images were taken in the axial plane (along the AC–PC plane) at the end of each scanning session.
Data Analysis
Behavioral data
Because of technical problems, incomplete vocal and/or manual reaction times (RTs) were acquired from seven participants. The group behavioral data was therefore calculated from 21 participants. Responses faster than 200 ms (anticipations) and slower than 1500 ms were excluded from the behavioral analyses. Performances in the sensory tasks were analyzed by combining the auditory tasks (AVo and AM) and contrasting them with the visual tasks (ViVo and ViM). Likewise, performances in the motor tasks were analyzed by combining the manual tasks (AM and ViM) and contrasting them with the vocal tasks (AVo and ViVo). Last, performances in the four sensorimotor tasks were compared to each other with four unpaired t‐tests (AVo vs. AM, AVo vs. ViVo, ViM vs. ViVo, and ViM vs. AM), and two‐paired t‐tests (AVo vs. ViM and ViVo vs. AM) owing to the nonorthogonality of the contrasts and therefore preventing the usual 2 × 2 ANOVA.
fMRI data
Brainvoyager software (versions 2000 and QX, BV Innovation, Maastricht, The Netherlands) was used for preprocessing and data analysis. All images were transformed into interpolated 1 mm3 Talairach space [Talairach and Tournoux, 1988] for each participant. Preprocessing included motion correction, slice scan time correction (relative to the first volume of the first run), and linear trend removal for both the ER and block runs. Participants were explicitly instructed to restrict their movement while being scanned, and the degree of movement was acceptable (i.e., < 5 mm translation [x,y,z] or rotation [pitch, yaw, and roll]). Temporal smoothing (1.75‐s FWHM for the axial data and 2.25‐s FWHM for the sagittal data) was used for the block runs only. ER analyses were corrected for serial correlations. Within each run, the first seven volumes were discarded for the MR signal to reach its steady state. To combine data for timecourse analyses from both the axial and sagittal group of subjects, the axial data sets were down‐sampled using MATLAB's (The Mathworks, Natick, MA) signal processing toolbox to the sampling rate (TR = 2.25 s) acquired with the sagittal images. All subsequent analyses were performed on the resampled data for these participants.
Three main analyses were performed on the fMRI data set. The first analysis served to identify brain regions associated with each sensorimotor task from sensation to response. The second analysis aimed at distinguishing the sensory and motor neural components of each sensorimotor task. The third analysis isolated regions that were commonly activated across all sensorimotor tasks.
Activated Regions for Each Sensorimotor Task
The first analysis aimed at isolating, for each sensorimotor task, the brain regions associated with the entire flow of information processing from sensation to motor response. Only the block runs were used for this analysis, because only the block runs had appropriate baseline contrast (i.e. the fixation blocks). Voxels whose activity correlated with each task were isolated using a voxel‐based multiple regression analysis by convolving a canonical hemodynamic response [Boynton et al., 1996]. Activity in each sensorimotor task (AM, AVo, ViVo, and ViM) was contrasted with activity during the fixation blocks. The resulting maps were overlaid to create composite activation maps. The overall model fit was assessed with a t‐statistic using a random effects model, and the thresholded P‐values were set using the false discovery rate method [Genovese et al., 2002]. Unless otherwise indicated, all SPMs were thresholded with a minimal value of q(FDR) = 0.05, c(V) = 1. The activation maps were projected onto an inflated brain of a representative subject's anatomy (see Fig. 1).
Figure 1.
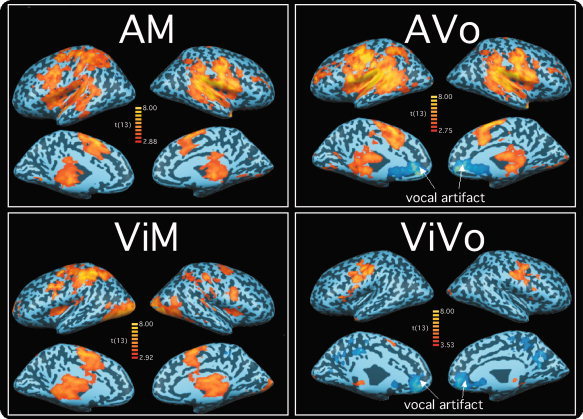
Brain regions activated for each sensorimotor task in the blocked design fMRI runs (AVo, auditory‐vocal; AM, auditory‐manual; ViVo, visual‐vocal; ViM, visuo‐manual). The group‐averaged activation maps are projected onto an inflated brain of a representative subject. All maps thresholded at q(FDR) < 0.05.
Sensory and Motor Regions
The second analysis aimed at highlighting brain regions specifically or preferentially activated to each sensory and motor modality. Sensory areas were identified using the following contrast:
The areas associated with auditory processing are isolated as “positive” activations and those with the visual processing are “negative.” Likewise, the motor areas were identified with the following contrast:
Here, brain regions preferentially associated with vocal responses are identified with “positive” activations, whereas the “negative” activity reflects brain regions preferentially responsive to the manual response.
Separate SPMs for the block and ER runs were computed using the standard random effects GLM. The SPMs for the block runs were computed as described in (1) but using the contrasts mentioned earlier. The SPMs for the ER runs were constructed as in the block runs with the exception that only the peak volume (i.e. fourth volume) was used for the sensory‐motor contrasts. This contrast analysis offered results that were very similar to the result from an analysis that used the entire timecourse. Only voxels that were significantly [q(FDR) = 0.05] activated in both maps were considered truly activated and are illustrated in Figure 3. This “method” ensured that isolated voxels are most likely those that show reliable differences in activation between the two sensory modalities and between the two motor modalities.
Figure 3.
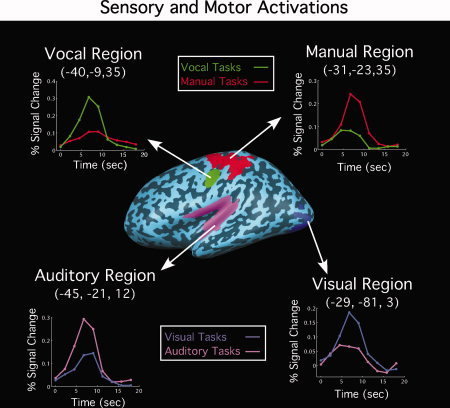
Brain regions, in the left hemisphere, exhibiting sensory (auditory or visual) or motor (vocal or manual) activation preferences in group‐averaged SPMs. These regions were activated in both the block and fast‐event runs [q(FDR) < 0.05]. The time courses from the event‐related runs contrasting sensory or motor modalities are also illustrated. The activation foci and time courses in the right hemisphere were similar to those in the left.
Timecourse estimates for each sensory and motor conditions in the isolated ROIs were then obtained with deconvolution analysis of the ER data. Thus, for each sensory (or motor) areas, the estimated timecourses for the visual and auditory conditions (or manual and vocal) were plotted by averaging the response to tasks that share the same modality (e.g., the timecourse of visual stimulation in visual cortex represent the average of the timecourses of the ViM and ViVo conditions in that ROI). Finally, two t‐tests were carried out on the timecourses at peak amplitude (i.e., the fourth volume). The first t‐test determined whether the hemodynamic responses in the opposite‐modality trials (e.g., ViVo/ViM trial in auditory cortex) were smaller than those in the proper‐modality trial (e.g., AM/AVo trial in auditory cortex), to confirm the SPM analysis regarding modality preference. The second t‐test assessed whether the peak responses in the opposite‐modality trial were greater than baseline, thereby establishing whether the ROI specifically responds to one modality and not the other.
Identification of Commonly Activated Regions Across Sensorimotor Tasks
To identify regions commonly activated by all sensorimotor tasks, we adopted several, multistep criteria. A flow chart of our analysis approach is illustrated in Figure 2. An initial conjunction analysis isolated potential convergence brain regions at the group‐averaged level. We ran the analysis on both blocked and ER data to ensure that the activation results were consistent across these two methodological approaches. Thus, only regions that were identified in both designs were considered for further analyses. It is possible, however, that some of the putative “conjunctive” regions demonstrate coactivation only because of the spatial blurring of activation foci during intersubject averaging. Because true conjunctive activity ought to be seen within the data of individual participants, the ROIs isolated in group‐averaged SPMs were also probed for overlap in activation across sensorimotor tasks in the unsmoothed, original 2D slices of individual participants. If a region passed these analysis steps, we further explored the reliability of activation in a second scanning session performed in a subset of participants.
Figure 2.
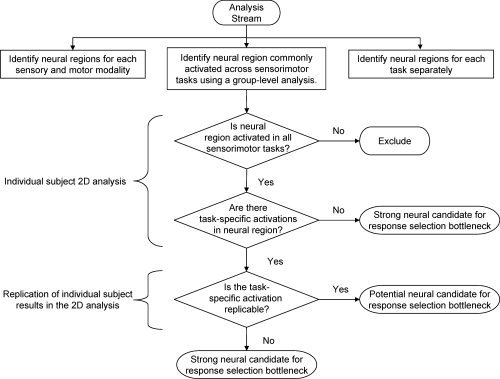
Flow diagram highlighting the analysis stream for identifying convergence regions. See main text for details.
Group‐level conjunction analysis
A region was identified as a convergence area if it was significantly activated in all the four sensorimotor tasks (i.e., using a logical AND; Nichols et al., 2005) in both the block and ER data sets. Thus, any particular voxel had to be significantly activated [q(FDR) < 0.05, random‐effects model] in all eight SPMs to be considered a candidate convergence area in group composites. Subsequent, confirmatory, paired sample t‐tests on peak activations from the ER timecourses assessed whether there were any sensory or motor preferences in the conjunction areas.
Individual subject 2D analysis
This analysis was carried out on the 2D slice data of each individual participant who underwent a sagittal scan (n = 24). This analysis was performed on unfiltered, nonsmoothed original 2D data to minimize blurring of activation across voxels.
For each ROI isolated in the group average, the corresponding area in each hemisphere was first isolated in individual subjects' 3D anatomical (i.e. SPGR) data. The corresponding area in the 2D slices of the same subject was then determined, using anatomical landmarks (gyri and sulci) to delineate the boundaries of the ROI. The large medial wall ROI isolated in the group analysis was broken down into supplementary motor area (SMA), pre‐SMA, and anterior cingulate cortex (ACC) sub‐ROIs for the left and right hemispheres for the 2D analysis. Similarly, the large cerebellar ROI was subdivided into left and right hemispheres and into the anterior lobule and superior–posterior lobule ROI. The 2D SPMs for each of the two sensorimotor tasks (because each subject performed only two of the four sensorimotor tasks) were then thresholded [q(FDR) < 0.05] using a fixed‐effect GLM applied to the ER data of each individual subject, and activation overlap between the two tasks in each of the ROI voxels was assessed for conjunction of activation. The timecourses from the voxels with activation overlap were then examined to verify that they exhibited gamma function—like hemodynamic responses to each of the two sensorimotor tasks. The number of such voxels was then recorded for every participant in each ROI.
To be considered a strong candidate for convergence of activations across all tasks, an ROI should (1) have a significant number of participants showing voxel‐based activation overlap and (2) a nonsignificant number of participants with task‐specific activation. Thus, the convergence ROIs were also probed for voxels exhibiting task‐specific activation (i.e., activated by one task, but not by the other). Any participant was considered to have conjunction activation and/or task‐specific activation if there was at least one voxel within the ROI exhibiting significant activation in both tasks (AVo and ViM or AM and ViVo) and/or only in one task, respectively. Although a one‐voxel criterion may appear liberal, it should be stressed that these voxels had to pass several statistical criteria (described above and below) to be considered significantly activated and that the number of voxels per ROI in individual subjects' Talairach space was small (range, 2–16 voxels).
We used the Clopper–Pearson's “exact” confidence intervals (CI) [Agresti, 2002; Clopper and Pearson, 1934] to determine whether the proportion of subjects demonstrating “activation” for a particular category (task‐specific or conjunction) was significantly above zero. Our confidence limits were Bonferroni corrected to account for multiple comparisons (i.e., nine ROIs and two hemispheres = 18 samples per group; 0.05 Bonferroni corrected CI: lower bound = 0.14%, upper‐bound = 99.86%). For each ROI and sensorimotor tasks, there are 12 participants. A brain region could be confidently (99.72% probability) claimed to contain at least one participant with either conjunction or task‐specific activation if the lower bound of the CI was greater than 1/12. To obtain a lower bound corresponding to a 0.14% Clopper‐Pearson CI greater than 1/12, at least 6 of 12 participants needed to show activated voxels.
Replication of individual subject results in the 2D analysis
In a final analysis, we sought to assess the reliability of the conjunction and task‐specific activity observed in the individual subject 2D analysis described earlier. Four of the participants demonstrating conjunction and task‐specific activity were rescanned in an ER experiment that was identical to the ER runs of the first scanning session. To match voxels across scans, care was taken with slice prescription of the second scan to ensure that it matched closely the prescription of the first scan. To determine whether voxels in the regions of interest showed either conjunction or task‐specific activation in both fMRI sessions, the FDR threshold, q, for each session was set to 0.22 as the combined FDR satisfies E(V 2/R 2) ≤ q 1 q 2 [Reiner et al., 2003], giving rise to an omnibus q level of 0.05. The timecourses in the surviving voxels were then examined for the presence of gamma function‐like hemodynamic response. Regions that survive this three‐stage analysis (group random effects analysis for both Block and ER data, individual 2D analysis, and the 2D replication analysis) were deemed to be strong candidates for neural areas exhibiting amodal response selection processing.
RESULTS
Behavioral Results
As seen from Table I, there were no differences in accuracy between all sensorimotor tasks. With RTs, there was no overall difference between manual (AMViM) and vocal (AVoViVo) responses, F(1,19) = 0.90. However, responses to auditory (AM and AVo; 849 ms) stimuli were overall slower than responses to visual (ViM and ViVo; 675 ms) stimuli, F(1,19) = 16.06, P < 0.001. AM responses were marginally slower than ViVo responses [t(11) = 2.19, P = 0.051], and AVo responses were slower than responses in the three other tasks (see Table I).
Table I.
Behavioral performance
| Vocal response | Manual response | ||
|---|---|---|---|
| Visual stimulus | 647 msec (96.3%) | 712 msec (98.2%) | ns (ns) |
| Auditory stimulus | 935 msec (97.9%) | 785 msec (96.0%) | * (ns) |
| * (ns) | ns (ns) |
The diagonal comparison, using paired t‐tests, between ViVo and AM revealed no significant differences in reaction time and accuracy measures. Although the accuracy difference between ViM and AVo was not significant, responses were significantly (P < 0.05) faster in the ViM condition than they were in the AVo condition. A * denotes a significant difference, using unpaired t‐tests (P < 0.05).
Reaction time (and percentage accuracy) for sensorimotor tasks.
The slower responses to auditory tasks might be due to the presence of background noise from the scanner and/or the discriminative difficulty of the auditory stimuli. Whatever the cause for the slower RTs in auditory tasks, it should have little bearing on isolating brain regions coactivated by all tasks.
We also considered whether overall performance differed between the AVo/ViM and AM/ViVo groups. The RT difference was significant [F(1,19) = 4.64, P < 0.05] owing to faster average responses for the AM/ViVo group (716 ms) than the AVo/ViM group (824 ms). However, this difference may have been the result of a speed‐accuracy tradeoff as the slower AVo/ViM group (98%) responded marginally [t(19) = 2.05, P = 0.055] more accurately than the ViVo/AM group (96%).
FMRI Results
Activated regions for each sensorimotor task
SPMs for the AVo‐fixation, AM‐fixation, ViVo‐fixation, and ViM‐fixation contrasts from the blocked fMRI runs are illustrated in Figure 1. Extensive activation foci were not only observed in primary sensory and motor cortices, but also in association areas of all cortical lobes. Although all but the ViVo tasks recruited large expanses of cortex, auditory cortex activation was more prominent in the auditory tasks, whereas visual cortex activation was more readily observed in the visual tasks. Similarly, manual responses recruited more dorsal and slightly more posterior regions of the frontal lobe than vocal responses. In addition, subcortical (thalamus and superior colliculus) activations (not shown) were also common across tasks.
Although this analysis reveals that each sensorimotor task recruits a relatively large extent of cortical and subcortical tissue, it does not identify brain regions that are either commonly activated across all four tasks or that are specific to each sensory and motor modalities. The following analyses serve these purposes.
Identification of sensory and motor areas
We sought to identify brain regions that were more activated for a particular sensory (e.g. auditory) or motor (e.g. manual) modality than for the other (i.e., visual and vocal, respectively). Regions that exhibited modality‐preferred activation in both blocked and ER runs are illustrated in Figure 3.
Not surprisingly, visual stimulation preferentially recruited Brodmann areas (BAs) 18 and 19 of the occipital lobe, whereas auditory stimuli preferentially recruited the superior temporal cortex and adjacent areas. In addition, the vocal responses preferentially recruited lateral regions of the precentral gyrus, whereas manual responses engaged more dorsal regions of the pre‐ and postcentral gyrus. Examination of the time courses from the ER runs in these areas confirms that they demonstrate preferential, but not specific responses to a given sensory or motor modality (see Fig. 3). That is to say, foci showed significant activation (all Ps < 0.01) for the nonpreferred modality (e.g., auditory activation in visual cortex or manual response activation in vocal ROI). However, these nonpreferred activations were significantly weaker than the preferred modality activations (Fig. 3 and Table II). Because these group‐defined ROIs were relatively large, it is not possible to ascertain whether the present results were obtained, because these sensory or motor ROIs do not exhibit strict modality specificity or because they are composed of a mixture of multimodal and modality‐specific areas.
Table II.
Average Talaraich coordinates and size of regions showing sensory‐ (auditory vs. visual) or motor‐ (manual vs. vocal) preferred activation
| Side | Brodmann areas | x, y, z | Voxels (mm3) | Sensory/motor preference t‐value | |
|---|---|---|---|---|---|
| Visual | Left | 18,19 | −29, −81, 3 | 1706 | 3.71** |
| (ViM‐AVo) − (AM ‐ViVo) | Right | 18,19 | 29, −82, 4 | 1472 | 3.32** |
| Auditory | Left | 13, 22, 40, 41, 42 | −45, −21, 12 | 11667 | 5.67** |
| (AVo‐ViM) − (ViVo‐AM) | Right | 13, 22, 40, 41, 42 | 52, −20, 10 | 17943 | 4.73** |
| Manual | Left | 2, 3, 4, 5, 6, 40 | −31, −23, 55 | 5531 | 6.31** |
| (ViM‐AVo) − (ViVo‐AM) | Right | 4 | 41, −25, 43 | 4 | 2.90* |
| Vocal | Left | 4, 6 | −40, −9, 35 | 2202 | 6.91** |
| (AVo‐ViM) − (AM‐ViVo) | Right | 4, 6 | 46, −9, 38 | 2607 | 5.63** |
| Right | 6, 43 | 57, −7, 13 | 89 | 3.62** |
The equations below the modality names describe the contrasts used to isolate the modality‐preferred activations. A * denotes a significant t‐value uncorrected (P < 0.05) and a ** denotes a significant t‐value using the Bonferroni adjustments for multiple comparisons (P < 0.006).
Identification of brain regions commonly activated across sensorimotor tasks
Group‐level conjunction analysis
We first sought to identify candidate convergence zones for all sensorimotor tasks in group‐averaged SPMs. To achieve this, we identified, for the block and fast‐ER runs, voxels that were conjointly activated by all four tasks (AVo, ViM, ViVo, and AM) in a random‐effects analysis. Thus, only regions that were significantly activated for all four tasks and in both designs (block and fast ER) were accepted. This analysis revealed the following regions as commonly activated across the four tasks (Fig. 4; Table III): a region of the medial wall comprising the bilateral SMA, pre‐SMA, and ACC; the right anterior insula; the posterior lateral frontal/prefrontal cortex (pLPFC) in BA 6 and 9 intersecting the inferior precentral sulcus and inferior frontal sulcus [Brass et al., 2005]; a more dorsal bilateral premotor (PM) cortex region in BA6; left thalamus; and a segment of the brainstem near the superior colliculus. All these brain regions showed significant peak volume activity relative to baseline in all four sensorimotor tasks (AVo, ViM, ViVo, and AM). Except for the left pLPFC, right PM, and brainstem, which responded more to the vocal than the manual tasks, these brain regions did not demonstrate sensory or motor preference (ANOVAs on the percent signal change at peak amplitude) (Table III). Thus, these brain regions are distinct from the sensory and motor regions in that they generally respond strongly and similarly to all sensory and motor modalities tested.
Figure 4.
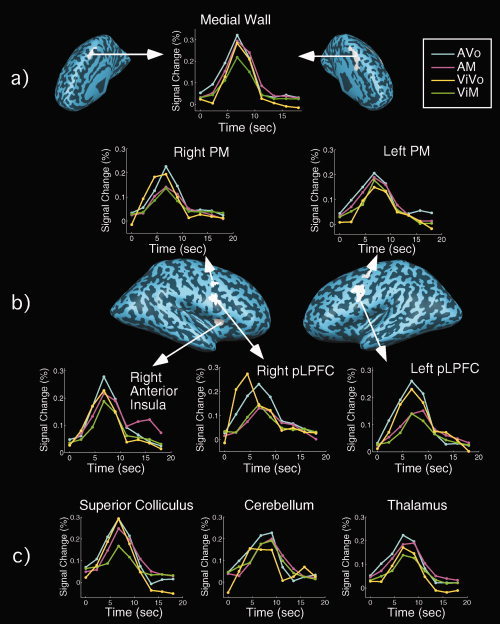
Brain regions exhibiting coactivation across all four sensorimotor tasks (AVo, AM, ViVo, and ViM) in both the block and fast‐event runs [q(FDR) < 0.05] in group‐averaged SPMs. The timecourses are from the fast‐event‐related runs. (a) Medial regions; (b) Lateral ROIs; (c) Time courses in subcortical structures (cerebellum, brainstem, and thalamus).
Table III.
Average Talaraich coordinates and size of brain regions showing conjunction of task activation
| x,y,z | Voxels (mm3) | Sensory preference t‐value | Motor preference t‐value | |
|---|---|---|---|---|
| Left pLPFC (BAs 6,9) | −40, 0, 36 | 824 | 1.64 | 4.54** |
| Right pLPFC (BAs 6,9) | 48,2,23 | 172 | 1.20 | 1.61 |
| Left PM (BA 6) | −31, −5, 48 | 45 | 1.46 | 0.24 |
| Right PM (BA 6) | 41, −3, 45 | 75 | 0.67 | 2.94* |
| Right anterior insula | 37,8,8 | 105 | 1.15 | 1.50 |
| Medial wall (BAs 6, 24, 32) | −2, 4, 51 | 1257 | 1.64 | 1.33 |
| Brainstem | −2, −29, 0 | 365 | 1.11 | 2.57* |
| Left thalamus | −14, −14, 10 | 830 | 1.88 | 1.33 |
Group‐defined ROIs with random effects analysis. A * denotes a significant t‐value (P < 0.05), and ** denotes a significant t‐value using a Bonferroni adjustment for 12 comparisons (P < 0.004). The medial wall ROI consists of pre‐SMA, SMA, and anterior cingulate. A right precentral gyrus ROI (BAs 3,4) was removed from further analysis due to its close proximity between auditory and vocal ROIs. pLPFC, posterior lateral prefrontal cortex; PM, premotor.
Although these results are consistent with the notion that these regions may constitute neural convergence zones for all sensorimotor tasks, they may also result from the blurring of adjacent foci displaying sensory‐ and/or motor‐specific activation in this standardized group‐averaged data. To address this issue, we carried out an analysis on individual subject's nonstandardized data.
Individual 2D analysis
Within each convergence ROI, using in‐plane 2D images, we assessed whether the majority of subjects exhibited at least one voxel of conjunction and/or task‐specific activity (see Methods section and Table IV). Given that this brain region is thought to be functionally heterogeneous (Picard and Strick, 2001), the frontal medial wall ROI was divided into a ventral ACC ROI and a dorsal pre‐SMA/SMA ROI. It was also divided according to the left and right hemisphere. Regions that demonstrated conjunction‐related activation for all conditions in most subjects consisted of the right anterior insula, bilateral pLPFC, bilateral pre‐SMA/SMA, bilateral ACC, and the left thalamus. The PM cortex demonstrated conjunction‐related activation in only the AVo/ViM tasks, whereas the brainstem did not exhibit robust conjunction activity. In addition, the pLPFC and the pre‐SMA/SMA showed task‐specific activation in at least one of the four sensorimotor tasks (AVo, ViM, AM, and ViVo).
Table IV.
Number of participants demonstrating conjunction and/or task‐specific activation
| Side | ROI | AM/ViVo | AVo/ViM | ||||
|---|---|---|---|---|---|---|---|
| Conjunction | AM | ViVo | Conjunction | AVo | ViM | ||
| Left | Thalamus | 6* | 4 | 2 | 10* | 1 | 1 |
| Brainstem | 2 | 0 | 1 | 5 | 1 | 0 | |
| pLPFC | 7* | 4 | 3 | 8* | 6* | 3 | |
| PM | 5 | 3 | 5 | 7* | 3 | 2 | |
| Pre‐SMA/SMA | 9* | 4 | 7* | 9* | 7* | 3 | |
| Anterior Cingulate | 8* | 5 | 3 | 11* | 3 | 5 | |
| Right | Brainstem | 3 | 0 | 1 | 3 | 2 | 0 |
| Anterior Insula | 6* | 4 | 3 | 7* | 2 | 2 | |
| pLPFC | 6* | 2 | 4 | 7* | 7* | 3 | |
| PM | 5 | 3 | 4 | 8* | 5 | 5 | |
| Pre‐SMA/SMA | 10* | 6* | 5 | 10* | 3 | 3 | |
| Anterior Cingulate | 7* | 5 | 5 | 9* | 0 | 3 | |
The total number of participants per cell is 12 (i.e., only subjects with sagittal prescriptions were analyzed in 2D). Cells with asterisks (*) have six or more participants, indicating that there is 99.72% confidence, using the Bonferroni corrected Clopper–Pearson test (see Methods section), that there is at least one participant with conjunction or task‐specific activation. SP, superior–posterior; pLPFC, posterior lateral prefrontal cortex; PM, premotor; SMA, supplementary motor area.
Thus, several of the conjunction ROIs defined at the group level also demonstrated robust conjunction activity in 2D analysis of single subjects. However, some of these ROIs, namely the pLPFC and pre‐SMA/SMA, also showed task‐specific activation. The latter finding can be interpreted in at least two ways. First, the conjunction activity observed both at the group and individual levels could be an artifact of the hemodynamic blurring of distinct single‐task activation foci. Alternatively, the conjunction ROIs may include distinct subregions demonstrating true task‐specific and conjunction‐related activity. Importantly, although the pLPFC showed both conjunction activity and task‐specific activity, the latter was limited to a vocal task (AVo; see Table IV). These vocal‐specific voxels were not only posterior to the conjunction voxels in the individual subject 2D analysis, they were also immediately anterior to the vocal motor ROI (cf. Figs. 3 and 4), suggesting that they belong to the vocal motor/premotor cortex. Thus, the posterior extent of the pLPFC ROI may have been tainted by vocal‐specific activity from the adjacent vocal motor cortex. This finding, together with the fact that no significant manual‐specific activation was observed in pLPFC, suggests that this brain region contains genuine conjunction‐related activity. By contrast, the pre‐SMA/SMA showed significant task‐specific activity in both vocal and manual tasks, casting doubt as to whether this brain region truly contains conjunction foci.
Replication of individual subject analysis
For three of the ROIs that exhibited conjunction activity at the group level, the individual‐level analysis revealed a more complex pattern of activation. Specifically, examination of individual subjects' activity profile in pLPFC and pre‐SMA/SMA showed both conjunction‐related and task‐specific activations, whereas the PM ROI showed conjunction‐related activity in the AVo/ViM, but not in the AM/ViVo, pairing. To determine whether the complex activation patterns in these brain regions are robust, we rescanned four of the participants who showed conjunction and task‐specific activations in these three ROIs in a fast ER experiment that was identical to the first scanning session. The conjunction and task‐specific voxels were separately identified in each session of each subject and assessed for overlap of activation across sessions using a conjunction analysis that required voxels to be significantly activated in each of the two sessions (see Methods section).
The analysis revealed that both conjunction and task‐specific activations replicated in three or all the subjects for the pre‐SMA/SMA and pLPFC, whereas the replication for task‐specific activation held for about half the subjects in PM cortex (Table V). Figure 5 illustrates the replication of activation across sessions for single‐task activity in the pre‐SMA/SMA and for conjunction‐related activity in the pLPFC. Thus, the replication analysis provides additional support for the presence of genuine conjunction‐related activity in pLPFC and of task‐specific activity in pre‐SMA/SMA. By contrast, the pattern of activation in PM proved to be less consistent.
Table V.
Number of participants exhibiting replicable conjunction and task‐specific activation
| Side | ROI | Conjunction replications | Single‐task replications |
|---|---|---|---|
| Left | pLPFC | 4/4 | 4/4 |
| Right | pLPFC | 4/4 | 3/4 |
| Left | PM | 3/3 | 1/3 |
| Right | PM | 3/4 | 2/4 |
| Left | Pre‐SMA/SMA | 4/4 | 3/4 |
| Right | Pre‐SMA/SMA | 4/4 | 3/3 |
Three participants performed the AVo/ViM tasks and the other participant performed the AM/ViVo tasks. The numerator indicates the number of subjects who demonstrated intersession replicability of activation (i.e., significant conjunction or task‐specific activation in Sessions 1 and 2), whereas the denominator indicates the number of subjects who showed conjunction or task‐specific activation in Session 1 (as activation in session 1 is a prerequisite for assessing reliability). Note that one participant did not demonstrate any activity in left PM in Session 1, and another did not demonstrate single‐task activity in right pre‐SMA/SMA in Session 1, so the denominator in those cases is three, rather than four. pLPFC, posterior lateral prefrontal cortex; PM, premotor; SMA, supplementary motor area.
Figure 5.
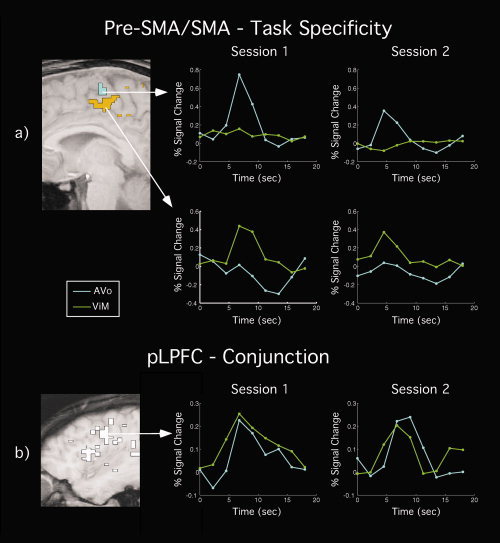
Replication of activation patterns across fMRI sessions in a representative subject. Left, 2D SPMs and right, time courses. (a) Replication of task‐specific (AVo/ViM) activation across sessions in pre‐SMA/SMA. (b) Replication of conjunction activation across fMRI sessions in pLPFC. White voxels were those that demonstrated replicable coactivation for the AVo/ViM tasks across sessions. Maps were thresholded at q(FDR) < 0.22 for an omnibus q(FDR) < 0.05 across sessions.
Summary
The results of the group‐average and individual‐level analyses specifically highlight the anterior insula, pLPFC, ACC, and thalamus as candidate neural substrates subserving response selection irrespective of the sensory and motor modalities. The pre‐SMA/SMA is another candidate brain region, but the presence of task‐related activity neighboring the conjunction activity does not allow us to rule out the possibility that the conjunction activation is a result of overlap of the hemodynamic spread of task‐specific activity. A similar issue limits the interpretability of the PM activation, especially considering that the conjunction activation for one set of tasks (i.e. AM/ViVo) failed to demonstrate a sufficient number of participants with conjunctive activation.
Comparison of Sensorimotor Pairings
In a final analysis, we considered whether the AVo/ViM and ViVo/AM pairings differentially affected brain activations. It has been argued that some sensorimotor mappings are more naturally compatible than others [Hazeltine and Ruthruff, 2006; McCleod and Posner, 1984; Stelzel et al., 2006; Wickens, 1984] and that this compatibility effect may differentially recruit the (left) prefrontal cortex [Stelzel et al., 2006]. Specifically, the AVo/ViM mapping may be less demanding on limited resources than the ViVo/AM pairing given that in the former pairing the input and outputs may be preferentially linked: we tend to respond verbally to auditory input and often make manual movements toward visual objects. However, our behavioral results are inconsistent with this hypothesis, as subjects were generally faster in the AM/ViVo pairing than in the AVo/ViM pairing [t(19) = 2.15, P < 0.05]. Furthermore, a between‐group comparison of these two sensorimotor mappings ([ViVo + AM] − [AVo + ViM]) revealed no activation differences at q(FDR) < 0.05 or even with an uncorrected threshold of P < 0.001. Thus, even if sensorimotor pairings differ in their compatibilities, it does not appear that these compatibility differences are expressed by differential activation of brain tissue. These results apply, at least, under conditions in which the sensorimotor tasks are performed separately. However, it is possible that different results could be obtained under dual‐task situations [Stelzel et al., 2006].
DISCUSSION
The goal of this study was to identify brain regions that are commonly activated across sensorimotor tasks that share neither sensory nor motor modalities. Our results highlight a number of frontal/prefrontal and subcortical areas that are coactivated across sensorimotor tasks and that are distinct from brain regions showing clear preferences for distinct sensory or motor modalities. These findings were replicated across experimental paradigms, fMRI sessions, and obtained under both group‐level and individual‐level analyses. In the following, we first discuss those regions that showed sensitivity to the sensory or motor modalities and then turn to the brain regions that demonstrated a multi‐modal pattern of activation.
Brain Regions Sensitive to Sensory or Motor Modalities
We identified sensory and motor areas preferentially associated with perceiving auditory or visual stimuli and executing vocal or manual responses. The areas activated by visual stimuli correspond primarily to extra‐striate cortex (BAs 18 and 19), whereas the broad area activated by auditory stimuli includes primary (BA 41) and secondary (BAs 22 and 42) auditory cortex [Binder et al., 1994; Galaburda and Sanides, 1980]. The motoric foci identified in the manual task consist of the pre‐ and postcentral gyrus and a small dorsal portion of the middle frontal gyrus (BAs 4 and 6). This activated region includes the primary motor cortex, but may also contain somatosensory cortex [cf. Indovina and Sanes, 2001]. The motor region activated by the vocal task corresponds to BAs 4p and 4a/6, areas that are strongly activated during speech production [Wilson et al., 2004] and may also include more inferior vocal region (BA 43) associated with tongue movements [Corfield et al., 1999]. The fact that these sensory and motoric activation foci match very well those previously described in the literature validates our methodological approach to isolating sensory and motor areas.
Although these sensory and motor ROIs showed strong modality preferences, in that they were significantly more activated by one modality than by another, none showed strict modality specificity. For instance, the visual ROIs responded slightly to auditory stimulus presentations. It is possible that the cross‐modal activation reflects genuine interactions between the different modalities and their neural substrates [Calvert et al., 1997; Falchier et al., 2002; Shimojo and Shams, 2001; Wallace et al., 2004]. However, such interactions are rather limited in the absence of sensory integration [Shimojo and Shams, 2001; Wallace et al., 2004], and sensory specificity has been observed in other neuroimaging studies under unimodal presentations [Johnson and Zatorre, 2005]. Alternatively, the cross‐modal activation could have resulted from the inclusion of both truly unimodal areas and higher‐level polymodal areas in each of the large sensory and motor ROIs. Indeed, when we restricted the spatial extent of these sensory or motor areas by defining them in individual subjects, greater evidence for modality specificity was evident (data not shown). It is important to emphasize, however, that the central aim of this study was not to isolate sensory‐ or motor‐specific areas, but to identify regions that demonstrated little or no sensitivity preferences in sensory or motor modality. The sensory and motor regions described here, by virtue of their strong modality preference, do not meet this criterion.
Brain Regions Commonly Activated Across Sensorimotor Mapping Tasks
Our methodology has isolated several frontal, prefrontal, and subcortical regions that were coactivated by all four tasks. This convergence of activations was found across experimental paradigms (blocked and ER designs), levels of analysis (3D group‐average and 2D individual‐level), and fMRI sessions (replication of activations across two scan sessions). As such, our study provides a highly rigorous test of the hypothesis that specific brain regions serve as neural nodes of information convergence across sensorimotor tasks. Nonetheless, two caveats are worth discussing here. First, although the ROIs we identified may be candidate regions for a central bottleneck of information processing, we cannot claim that these brain regions are specifically involved in the capacity‐limited stage of response selection. Coactivation may have resulted from any amodal process that is shared across tasks, such as posttrial performance evaluation, response readiness, or attentional processes, to name a few. These coactivations may also be limited to the type of sensorimotor mapping tasks (i.e. choice‐RT) we have used. Thus, additional research is necessary to help identify the precise roles that each region plays in dual‐task performance [e.g., Dux et al., 2006]. Nevertheless, the present results are highly helpful in narrowing down the number of brain areas that may act as structural bottlenecks of information processing. Second, before discussing the ROIs activated across all sensorimotor tasks, it should be kept in mind that while we observed conjunction of activations at the single‐voxel level in unsmoothened, unfiltered 2D data of individual subjects, these findings are ultimately limited by the spatial resolution of our voxel size. Thus, it remains possible that different neuronal ensembles within the same voxel encode distinct sensorimotor pairings. This caveat likely applies to brain regions (e.g. thalamus) that contain tightly compact and functionally distinct subnuclei. However, it may be less pertinent for such cortical regions as the lateral prefrontal cortex (pLPFC), especially considering that LPFC cells can flexibly encode task‐relevant information that is neither strictly sensory nor motor in a distributed manner across large extents of this brain region [Duncan, 2001; Duncan and Owen, 2001; Miller and Cohen, 2001; Passingham and Sakai, 2004].
Subcortical Regions
The thalamus demonstrated robust activity across all four tasks. Given that the thalamus is a relatively small brain structure that contains densely packed subnuclei involved in processing distinct types of sensory information, it is difficult to ascertain that the convergence of activation is not a result of the limited spatial resolution of fMRI. Furthermore, Jiang and Kanwisher [ 2003b] have specifically argued that this brain region is not the source of central processing limitations, because it was not commonly activated across a set of visual and auditory tasks, casting further doubt on its central involvement in response selection. However, other studies have observed thalamic activation in response selection [Schumacher et al., 2003] and dual‐tasking [e.g., Herath et al., 2001; Szameitat et al., 2002, 2006], and some thalamic nuclei, such as the pulvinar, are thought to subserve more general attention functions [cf. Michael and Buron, 2005] that may be called upon by all sensorimotor tasks. Clearly, further work is necessary to establish whether certain thalamic nuclei act as a central hub of sensorimotor information processing.
Medial Frontal Cortex
In the group analysis, a large medial frontal ROI demonstrated coactivation across all tasks. In subsequent analysis (i.e., 2D, individual subjects, and fixed‐effects analyses), we broke this large ROI down into the ACC and pre‐SMA/SMA complex. In the following sections, we discuss these two components of the medial wall.
pre‐SMA/SMA
The group‐level analysis identified the SMA/pre‐SMA as a potential region where sensorimotor information converges. These brain regions are considered higher motor areas involved in motor preparation and the selection of response sets [Rushworth et al., 2004]. Correspondingly, they are activated in advance of an overt motor response [Lee et al., 1999; van Eimeren et al., 2006] and before primary motor cortex (M1) activity [Cunnington et al., 2002]. The pre‐SMA and SMA could not be functionally distinguished in our study. However, despite their close proximity and frequent coactivations, the SMA and pre‐SMA have been proposed to exert different roles. Specifically, the pre‐SMA is thought to play a more general role in response preparation and/or selection [Lau et al., 2004; Rushworth et al., 2004; Sakai et al., 2000], whereas the SMA may be more involved in sequencing motor responses than in selecting responses per se [Deiber et al., 1999].
The observation that the SMA/pre‐SMA was activated across all sensorimotor tasks is consistent with this brain region playing an important role in response selection [Lau et al., 2004, 2006; Marois et al., 2006], dual‐task interference [Dux et al., 2006; Herath et al., 2001; Klingberg, 1998; Marois et al., 2006; Schubert and Szameitat, 2003; Szameitat et al., 2002], and task‐set implementation [Dosenbach et al. 2006]. However, there is neurophysiological and neuroimaging evidence that distinct regions of the SMA/pre‐SMA may control the movement of different motor effectors [Dum and Strick, 2002; Picard and Strick, 1996]. Moreover, we observed robust task selective activation in the SMA/pre‐SMA. These results cast doubts on the notions that the SMA/pre‐SMA serve a nonselective role in response selection and form a central bottleneck of information processing.
Anterior cingulate cortex
The anterior cingulate cortex (ACC) was reliably activated by all four sensorimotor tasks, suggesting that this brain region is not sensitive to the sensory or motor modality of a response selection task. Although this finding is consistent with an fMRI study failing to find any response specificity in the ACC [Barch et al., 2001], it does not provide compelling evidence against the view, based on neurophysiological [Picard and Strick, 1996], neuroimaging [Paus, 2001; Paus et al., 1998], and neuropsychological evidence [Turken and Swick, 1999] that different regions of the ACC control motoric behavior of different effectors. Thus, the evidence suggesting an amodal role for the ACC in simple sensorimotor tasks is, at present, conflicting. Resolution of this issue will have to await further studies that will specifically investigate the functional neuroanatomy of this brain region with novel techniques, such as multivariate pattern analysis [e.g., Kamitani and Tong, 2005] that can overcome the relatively poor spatial resolution of fMRI.
It should be added that not all studies of response selection have reported ACC activation [cf. Jiang and Kanwisher, 2003; Schumacher and D'Esposito, 2003]. By contrast, the ACC has been frequently observed in dual‐task studies [Herath et al., 2001; Klingberg, 1998; Schubert and Szameitat, 2003; Szmeitat, 2002; Marois et al., 2006]. Several lines of evidence suggest that this brain region may be involved in cognitive control [Posner et al., 1988; Weissman et al., 2005], error or conflict monitoring [Botvinick et al., 2004; Carter et al., 1998; Dreher and Grafman, 2003; see also Rushworth et al. (2007)] or, more broadly speaking, in relating actions to their outcome [Rushworth et al., 2004, 2007]. These functions may be particularly important for efficient dual‐tasking [Dreher and Grafman, 2003], as performing two tasks at once is likely to generate conflict. In the context of the present single sensorimotor mapping tasks, although demands for conflict resolution and cognitive control were not strong, the ACC activity may have reflected an evaluative process of the response choice or performance. If the function of the ACC is primarily related to postresponse processing, then it is unlikely to constitute the bottleneck of information processing revealed by the PRP. Clearly, elucidation of the role of the ACC in response selection and dual‐tasking would benefit from using a methodological approach with a higher temporal resolution to precisely pinpoint the temporal stage of its involvement under single‐ and dual‐task conditions.
Lateral Frontal and Prefrontal Cortex
Anterior insula
The anterior insula was not only activated across all sensorimotor tasks, it also showed little evidence of task‐specific activity. This is consistent with the proposed role of the insula in crossmodal processing [e.g., Bushara et al., 2003; Calvert, 2001] and general task‐set implementation [Dosenbach et al., 2007]. This brain region has also been implicated in decision‐making [Sanfrey et al., 2003] and response‐related processes, including response inhibition [Bunge et al., 2002a; Wager et al., 2005] and response switching [Paulus et al., 2005]. Other work has shown that anterior insula activity significantly correlates with response time performance [Binder et al., 2004; but see van Eimeren et al. (2006)], again supporting a response‐related function for this area. On the other hand, anterior insula activity has been inconsistently observed in response selection experiments [e.g. Jiang and Kanwisher 2003; Marois et al., 2006; Schumacher et al., 2003; van Eimeren et al., 2006].
Although some dual‐tasking studies do not report insular activation in their investigations of dual‐tasking [e.g., Bunge et al., 2000; Klingberg, 1998], a few others [Jiang, 2004; Schubert and Szameitat, 2003; Szameitat et al., 2003] have found modest evidence of the insula's role in dual‐tasking. That the anterior insula is involved in response inhibition and switching connects well with a purported role in dual‐tasking. Inhibiting a response to the second target while processing the first may be necessary to efficiently execute both responses independently. However, cross‐task inhibition does not easily account for the insula activation seen under our single‐task conditions [but see Burle et al. (2004) for potential evidence of inhibition in single tasks].
In summary, while the involvement of the anterior insula in response selection and dual‐tasking has been inconsistently observed in previous studies, the present results clearly point to this brain region as a potential neural node of information convergence across distinct sensorimotor tasks. What function(s) the anterior insula may ultimately fulfill in our tasks will, however, require further investigation.
Premotor cortex
The premotor cortex was another brain region that showed some evidence of activation across sensory and motor modalities in our response selection tasks. The premotor cortex, located within BA 6, is divided into two regions, dorsal PM and ventral PM, which are separated by the superior demarcation of the frontal eye fields (z = 51) [Rizzolatti et al., 2002]. According to this boundary, our PM activation falls within the dorsal part of PMv, just shy of the z = 51 boundary. In addition to this dorso‐ventral division, the PM cortex may be anatomically distinguished into a caudal and rostral section [Boussaoud, 2001; Picard and Stick, 2001; Schubotz and von Cramon, 2003]. This caudal/rostral division may relate to an intention/attention [Boussaoud, 2001], motor preparation/attention [Simon et al., 2001], or motor/cognitive [Matsumoto et al., 2003] functional dissociation. Our activation foci lie closer to the caudal region involved in motor‐ and intention‐related functions.
The premotor cortex has been linked to several response‐related processes. Specifically, it has been implicated in motor preparation and the sensory guidance of movement [Rizzolatti et al., 2002; Schubotz and von Crammon, 2003; Simon et al., 2002; Wise, 1985], in stimulus‐response compatibility [Dassonville et al., 2001; von Eimeren et al., 2005], temporal adjustments of responding [Sakai et al., 2000], and response selection per se [e.g. Jiang and Kanwisher, 2003; Marois et al., 2006]. Moreover, this brain region has been directly implicated in dual‐task interference [Marois et al., 2006; Stelzel et al., 2006; Szameitat et al., 2006].
Although there is substantial evidence that the premotor cortex is involved in response selection and dual‐tasking, it is much less evident that this involvement is amodal. Indeed, the premotor cortex demonstrates motor and effector specificity [Fink et al., 1997; Schubotz and von Crammon, 2001] and somatotopy in the observation of actions [Buccino et al., 2001]. Furthermore, our study garnered only equivocal evidence that the premotor cortex is commonly activated across all sensorimotor tasks. Specifically, the group‐defined ROI analysis revealed an activation preference for vocal tasks in the right PM cortex, and the 2D individual‐level analysis failed to reveal significant conjunction‐related activation in left and right PM for the AM and ViVo tasks. Taken together with studies demonstrating response specificity in premotor cortex, these findings do not support the view that this brain region represents a central, amodal bottleneck of information processing, although it certainly could represent a bottleneck at later, response‐related stages of information processing [Marois and Ivanoff, 2005]. However, the above conclusions should be regarded as tentative until further work reveals the specific contribution of the premotor cortex to dual‐tasking limitations.
Posterior pLPFC
The final region activated across all four sensorimotor tasks was the pLPFC. This region, which straddled the frontal and prefrontal cortex, included the anterior portion of lateral BA6 and posterior sections of BA9. The prefrontal cortex has been frequently implicated in response selection (Bunge, 2004; Bunge et al., 2002b; Hester et al., 2007; Jiang and Kanwisher, 2003a; Schumacher et al., 2003; van Eimeren et al., 2006). This pLPFC ROI overlaps with the inferior frontal junction (IFJ) area believed to be involved in response conflict, task switching, dual‐tasking, and cognitive control [Brass et al., 2005; Derfuss et al., 2005; Dux et al., 2006; Hester et al., 2007]. The anterior portion of the pLPFC ROI also overlaps with posterior regions of the prefrontal cortex implicated in stimulus‐response compatibility [Schumacher and D'Esposito, 2002; Stelzel et al., 2006], dual‐task coordination [Szameitat et al., 2006], and executive control [Duncan and Owen, 2001; Duncan, 2001; Miller and Cohen, 2001]. It has been suggested that the posterior prefrontal cortex does not subserve a single function, but that it uses an adaptive coding mechanism to encode any task‐relevant information and operations for the purpose of meeting current behavioral goals [Duncan and Owen, 2001; Duncan, 2001]. Moreover, a recent time‐resolved fMRI study has provided direct evidence that this brain region plays a role in dual‐tasking limitations [Dux et al., 2006]. It may even be recruited under both perceptual and response‐related forms of dual‐task limitations [Marois and Ivanoff, 2005]. Thus, both the present findings, and previous studies, are consistent with the view that the pLPFC may have central, amodal functions in response selection, and dual‐task studies.
Summary
When considering both this study and prior neuroimaging, neurophysological, and neuropsychological work, the pLPFC, together with the anterior insula, are the most likely candidates for neural foci of a central, amodal bottleneck of information processing. Both regions have been observed in response selection tasks and in dual‐task studies, and both are relatively insensitive to the sensory or motor modality of the task. Interestingly, despite the fact that our experiment was designed to isolate any brain regions activated across sensorimotor tasks from stimulus perception to response execution, the regions that showed conjunction of activity generally corresponded to regions previously associated with response selection [cf. Hester et al., 2007; Jiang and Kanwisher, 2003a; Marois et al., 2006; Schumacher et al., 2003; van Eimeren et al., 2006]. It is worth noting, however, that no region of the parietal cortex was conjointly activated across all sensorimotor tasks, a finding that seems inconsistent with studies that have implicated this brain region in response selection [e.g., Jiang and Kanwisher, 2003a; Schumacher et al., 2003]. There are at least three reasons that we did not identify any parietal regions as a potential hub of response selection. First, those studies that have identified parietal regions as neural loci of response selection used compatibility manipulations, often within a restricted set of sensorimotor modalities. Here, we have used a broader approach of isolating any brain regions involved in sensorimotor processing irrespective of the sensory or motor modality. Second, given that response selection may not be a singular cognitive process [Hester et al., 2007; Schumacher and Jiang, 2003], it is possible that parietal involvement is only necessary for some, but not all, forms of sensorimotor response selection. Last, the parietal cortex may not have been considered to include conjunction‐related activation because of the stringent exclusionary criteria that we employed in the present study relative to previous studies [Jiang and Kanwisher, 2003a; Schumacher et al., 2003].
Implications for Models of Dual‐Task Interference
Although several neural models have been proposed to account for dual‐task limitations [Klingberg, 1998; Marois and Ivanoff, 2005], the central bottleneck model of dual‐tasking has received extensive support from the behavioral literature [Pashler, 1994]. Here, we attempted to rigorously assess a key neural assumption of this model, which is that all sensorimotor tasks rely on a common set of brain regions to process information at a central, amodal stage of response selection. Our results provide neuroanatomical evidence that is consistent with this structural bottleneck account, as specific frontal, prefrontal, and possibly subcortical areas were found to be commonly activated by all sensorimotor tasks irrespective of the sensory or motor modality of these tasks. These regions may therefore serve as amodal, convergence zones of neural information processing that limit our ability to carry out more than one task at a time. As such, this study sets the stage for future functional neuroimaging studies aimed at testing the involvement of these different brain regions in dual‐task limitations.
Contributor Information
Jason Ivanoff, Email: Jason.Ivanoff@smu.ca.
René Marois, Email: rene.marois@vanderbilt.edu.
REFERENCES
- Adcock RA, Constable RT, Gore JC, Golman‐Rakic PS ( 2000): Functional neuroanatomy of executive processes involved in dual‐task performance. Proc Natl Acad Sci 97: 3567–3572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agresti A ( 2002): Categorical Data Analysis. Hoboken, NJ: Wiley. [Google Scholar]
- Barch DM, Braver TS, Akbudak E, Conturo T, Ollinger J, Snyder A ( 2001): Anterior cingulate cortex and response conflict: Effects of response modality and processing domain. Cereb Cortex 11: 837–848. [DOI] [PubMed] [Google Scholar]
- Binder JR, Rao SM, Hammeke TA, Yetkin FZ, Jesmanowicz A, Bandettini PA, Wong EC, Estkowski LD, Goldstein MD, Haughton VM ( 1994): Functional magnetic resonance imaging of human auditory cortex. Ann Neurol 35: 662–672. [DOI] [PubMed] [Google Scholar]
- Binder JR, Liebenthal E, Possing ET, Medler DA, Ward BD ( 2004): Neural correlates of sensory and decision processes in auditory object identification. Nat Neurosci 7: 295–301. [DOI] [PubMed] [Google Scholar]
- Boussaoud D ( 2001): Attention versus intention in the primate premotor cortex. Neuroimage 14: S40–S45. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS ( 2004): Conflict monitoring and anterior angulate cortex: An update. Trends Cogn Sci 8: 539–546. [DOI] [PubMed] [Google Scholar]
- Boynton GM, Engel SA, Glover GH, Heeger DJ ( 1996): Linear systems analysis of functional magnetic resonance imaging in human V1. J Neurosci 16: 4207–4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brass M, Derfuss J, Forstmann B, von Cramon DY ( 2005): The role of the inferior frontal junction area in cognitive control. Trends Cogn Sci 9: 314–316. [DOI] [PubMed] [Google Scholar]
- Brass M, von Cramon DY ( 2002): The role of the frontal cortex in task preparation. Cereb Cortex 12: 908–914. [DOI] [PubMed] [Google Scholar]
- Buccino G, Binkofski F, Fink GR, Fadiga L, Fogassi L, Gallese V, Seitz RJ, Zilles K, Rizzolatti G, Freund H‐J ( 2001): Action observation activates premotor and parietal areas in a somatopic manner: An fMRI study. Eur J Neurosci 13: 400–404. [PubMed] [Google Scholar]
- Bunge SA ( 2004): How we use rules to select actions: A review of evidence from cognitive neuroscience. Cogn, Affect, Behav Neurosci 4: 564–579. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Klingberg T, Jacobsen RB, Gabrieli JDE ( 2000): A resource model of the neural basis of executive working memory. Proc Natl Acad Sci 97: 3573–3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge SA, Dudukovic NM, Thomason ME, Vaidya CJ, Gabrieli JDE ( 2002a): Immature frontal lobe contributions to cognitive control in children: Evidence from fMRI. Neuron 33: 301–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge SA, Halzetine E, Scanlon MD, Rosen AC, Gabrieli JDE ( 2002b): Dissociable contributions of prefrontal and parietal cortices to response selection. NeuroImage 17: 1562–1571. [DOI] [PubMed] [Google Scholar]
- Burle B, Vidal F, Tandonnet C, Hasbrouq T ( 2004): Physiological evidence for response inhibition in choice reaction time tasks. Brain Cogn 56: 153–164. [DOI] [PubMed] [Google Scholar]
- Bushara KO, Hanakawa T, Immisch I, Kansaku K, Hallett M ( 2003): Neural correlates of cross‐modal binding. Nat Neurosci 6: 190–195. [DOI] [PubMed] [Google Scholar]
- Calvert GA ( 2001): Crossmodal processing in the human brain: Insights from functional neuroimaging studies. Cereb Cortex 11: 1110–1123. [DOI] [PubMed] [Google Scholar]
- Calvert GA, Bullmore ET, Brammer MJ, Campbell R, Williams SC, McGuire PK, Woodruff OW, Iversen SD, David AS ( 1997): Activation of auditory cortex during silent lipreading. Science 276: 593–596. [DOI] [PubMed] [Google Scholar]
- Carrier LM, Pashler H ( 1995): Attentional limits in memory retrieval. J Exp Psychol: Learn Mem Cogn 21: 1339–1348. [DOI] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD ( 1998): Anterior cingulated cortex, error detection, and the online monitoring of performance. Science 280: 747–749. [DOI] [PubMed] [Google Scholar]
- Clopper CJ, Pearson ES ( 1934): The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika 26: 404–413. [Google Scholar]
- Collette F, Olivier L, Van der Linden M, Laureys S, Delfiore G, Luxen A, Salmon E ( 2005): Involvement of both prefrontal and inferior parietal cortex in dual‐task performance. Cogn Brain Res 24: 237–231. [DOI] [PubMed] [Google Scholar]
- Corfield DR, Murphy K, Josephs O, Fink GR, Frackowiak RSJ, Guz A, Adams L, Turner R ( 1999): Cortical and subcortical control of tongue movement in humans: A functional neuroimaging study using fMRI. J Appl Physiol 86: 1468–1477. [DOI] [PubMed] [Google Scholar]
- Cunnington R, Windischberger C, Deecke L, Moser E ( 2002): The preparation and execution of self‐initiated and externally triggered movement : A study of event‐related fMRI. Neuroimage 15: 373–385. [DOI] [PubMed] [Google Scholar]
- Dale AM ( 1999): Optimal experimental design for event‐related fMRI. Human Brain Mapp 8: 109–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dassonville P, Lewis SM, Zhu X‐H, Ugurbil K, Kim S‐G, Ashe J ( 2001): The effect of stimulus‐response compatibility on cortical motor activation. Neuroimage 13: 1–14. [DOI] [PubMed] [Google Scholar]
- Deiber M‐P, Honda M, Ibañez V, Sadato N, Hallett M ( 1999): Mesial motor areas in self‐initiated versus externally triggered movements examined with fMRI: Effect of movement type and rate. J Neurophysiol 81: 3065–3077. [DOI] [PubMed] [Google Scholar]
- Derfuss J, Brass M, Neumann J, von Cramon DY ( 2005): Involvement of the inferior frontal junction in cognitive control: Meta‐analyses of switching and Stroop studies. Human Brain Mapp 25: 22–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Esposito M, Detre JA, Alsop DC, Shin RK, Atlas S, Grossman M ( 1995): The neural basis of the central executive system of working memory. Nature 378: 279–281. [DOI] [PubMed] [Google Scholar]
- Desmond JE, Gabrieli JDE, Glover GH ( 1998): Dissociation of frontal and cerebellar activity in a cognitive task: Evidence for a distinction between selection and search. Neuroimage 7: 368–376. [DOI] [PubMed] [Google Scholar]
- Doesnbach NUF, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HC, Burgund ED, Grimes AL, Schlaggar BL, Petersen SE ( 2006): A core system for the implementation of task sets. Neuron 50: 799–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreher J‐C, Grafman J ( 2003): Dissociating the roles of the rostral anterior cingulated and the lateral prefrontal cortices in performing two tasks simultaneously or successively. Cereb Cortex 13: 329–339. [DOI] [PubMed] [Google Scholar]
- Dum RP, Stick PL ( 2002): Motor areas in the frontal lobe of the primate. Physiol Behav 77: 677–682. [DOI] [PubMed] [Google Scholar]
- Duncan J ( 2001): An adaptive coding model of neural function in prefrontal cortex. Nat Rev Neurosci 2: 820–829. [DOI] [PubMed] [Google Scholar]
- Duncan J, Owen AM ( 2001): Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci 23: 475–483. [DOI] [PubMed] [Google Scholar]
- Dux PE, Ivanoff J, Asplund CL, Marois R ( 2006): Isolation of a central bottleneck of information processing with time‐resolved fMRI. Neuron 52: 1109–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdler M, Windischberger C, Lanzenberger R, Vinod E, Gartus A, Deeckke L, Beisteiner R ( 2001): Dissociation of supplementary motor area and primary motor cortex in human subjects when comparing index and little finger movements with functional magnetic resonance imaging. Neurosci Lett 313: 5–8. [DOI] [PubMed] [Google Scholar]
- Erickson KI, Colcombe SJ, Wadhwa R, Bherer L, Peterson MS, Scalf PE, Kramer AF ( 2005): Neural correlates of dual‐task performance after minimizing task‐preparation. NeuroImage 28: 967–979. [DOI] [PubMed] [Google Scholar]
- Erickson KI, Colcombe SJ, Wadhwa R, Bherer L, Peterson MS, Scalf PE, Kim JS, Alvardo M, Kramer AF ( 2006): Training induced functional changes in dual‐task processing: An fMRI study. Cereb Cortex 17: 192–204. [DOI] [PubMed] [Google Scholar]
- Falchier A, Clavagnier S, Barone P, Kennedy H ( 2002): Anatomical evidence of multimodal integration in primate striate cortex. J Neurosci 22: 5749–5759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink GR, Frackowiak RSJ, Pietrzyk U, Passingham RE ( 1997): Multiple nonprimary motor areas in the human cortex. J Neurophysiol 77: 2164–2174. [DOI] [PubMed] [Google Scholar]
- Fitts PM, Seeger CM ( 1953): SR compatibility: Spatial characteristics of stimulus and response codes. J Exp Psychol 46: 199–210. [DOI] [PubMed] [Google Scholar]
- Galaburda A, Sanides F ( 1980): Cytoarchitectonic organization of the human auditory cortex. J Comp Neurol 190: 597–610. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Laza NA, Nichol T ( 2002): Thresholding of statistical maps in functional neuroimaging using the false discovery rate. NeuroImage 15: 870–878. [DOI] [PubMed] [Google Scholar]
- Hazeltine E, Ruthruff E ( 2006): Modality pairing effects and the response selection bottleneck. Psychol Res 70: 504–513. [DOI] [PubMed] [Google Scholar]
- Herath P, Klingberg T, Young J, Amunts K, Roland P ( 2001): Neural correlates of dual task interference can be dissociated from those of divided attention: An fMRI study. Cereb Cortex 11: 796–805. [DOI] [PubMed] [Google Scholar]
- Hester R, D'Esposito M, Cole MW, Garavan H ( 2006): Neural mechanisms for response selection: Comparing selection of responses and items from working memory. NeuroImage 34: 446–454. [DOI] [PubMed] [Google Scholar]
- Hick WE ( 1952): On the rate of gain of information. Quart J Exp Psychol 4: 11–26. [Google Scholar]
- Indovina I, Sanes JN ( 2001): On somatotopic representation centers for finger movements in human primary motor cortex and supplementary motor areas. Neuroimage 13: 1027–1034. [DOI] [PubMed] [Google Scholar]
- Ivry RB, Franz EA, Kingstone A, Johnston JC ( 1998): The psychological refractory period effect following callosotomy: Uncoupling of lateralized response codes. J Exp Psychol: Hum Percep Perform 24: 463–480. [DOI] [PubMed] [Google Scholar]
- Jaeggi SM, Seewer R, Nirkko AC, Eckstein D, Schroth G, Groner R, Gutbrod K ( 2003): Does excessive memory load attenuate activation in the prefrontal cortex? Load‐dependent processing in single and dual tasks: Functional magnetic resonance imaging study. NeuroImage 19: 210–225. [DOI] [PubMed] [Google Scholar]
- Jiang Y ( 2004): Resolving dual‐task interference: An fMRI study. Neuroimage 22: 748–754. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Kanwisher K ( 2003a): Common neural substrates for response selection across modalities and mapping paradigms. J Cogn Neurosci 15: 1080–1094. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Kanwisher K ( 2003b): Common neural mechanisms for response selection and perceptual processing. J Cogn Neurosci 15: 1095–1110. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Saxe R, Kanwisher N ( 2004): Functional magnetic resonance imaging provides new constraints on theories of the psychological refractory period. Psychol Sci 15: 390–396. [DOI] [PubMed] [Google Scholar]
- Johnson JA, Zatorre RJ ( 2005): Attention to simultaneous unrelated auditory and visual events: Behavioral and neural correlates. Cereb Cortex 15: 1609–1620. [DOI] [PubMed] [Google Scholar]
- Just MA, Carpenter PA, Keller TA, Emery L, Zajac H, Thulborn KR ( 2001): Independence of nonoverlapping cortical systems in dual cognitive tasks. NeuroImage 14: 417–426. [DOI] [PubMed] [Google Scholar]
- Kamitani Y, Tong F ( 2005): Decoding the visual and subjective contents of the human brain. Nat Neurosci 8: 679–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingberg T ( 1998): Concurrent performance of two working memory tasks: Potential mechanisms of interference. Cereb Cortex 8: 593–601. [DOI] [PubMed] [Google Scholar]
- Kuypers HGJM ( 1981): Anatomy of descending pathways In: Vernon BB, editor.Handbook of Physiology. The Nervous System. Motor Control. Sect. I, Vol II American Physiological Society: Bethesda, MD: pp 597–666. [Google Scholar]
- Lau HC, Rogers RD, Ramnani N, Passingham RE ( 2004): Willed action and attention to the selection of action. NeuroImage 21: 1407–1415. [DOI] [PubMed] [Google Scholar]
- Lau H, Rogers RD, Passingham RE ( 2006): Dissociating response selection and conflict in the medial frontal surface. NeuroImage 29: 446–451. [DOI] [PubMed] [Google Scholar]
- Lee K‐M, Chang K‐H, Roh J‐K ( 1999): Subregions within the supplementary motor area activated at different stages of movement preparation and execution. NeuroImage 9: 117–123. [DOI] [PubMed] [Google Scholar]
- Lewis JW, Beauchamp MS, DeYope EA ( 2000): A comparison of visual and auditory motion processing in human cerebral cortex. Cereb Cortex 10: 873–888. [DOI] [PubMed] [Google Scholar]
- Logan GD, Schulkind MD ( 2000): Parallel memory retrieval in dual‐task situations. I. Semantic memory. J Exp Psychol: Hum Percep Perform 26: 1072–1090. [DOI] [PubMed] [Google Scholar]
- Marois R, Ivanoff J ( 2005): Capacity limits of information processing in the brain. Trends Cogn Sci 9: 296–305. [DOI] [PubMed] [Google Scholar]
- Marois R, Larson JM, Chun MM, Shima D ( 2006): Response‐specific sources of dual‐task interference in human pre‐motor cortex. Psychol Res 70: 436–447. [DOI] [PubMed] [Google Scholar]
- Matsumoto R, Ikeda A, Ohara S, Matsuhashi M, Baba K, Yamane F, Hori T, Mihara T, Nagamine T, Shibasaki H ( 2003): Motor‐related function subdivisions of human lateral premotor cortex: Epicortical recording in conditional visuomotor task. Clin Neurophysiol 114: 1102–1115. [DOI] [PubMed] [Google Scholar]
- McLeod P, Posner MI ( 1984): Privileged loops from percept to act In Bouma H, Bouwhuis DG, editors. Attention and Performance X: Control of Language Processes. London: Erlbaum; pp 55–66. [Google Scholar]
- Michael GA, Buron L ( 2005): The human pulvinar and stimulus‐driven attentional control. Behav Neurosci 119: 1353–1367. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD ( 2001): An integrative theory of prefrontal cortex function. Annu Rev Neurosci 24: 167–202. [DOI] [PubMed] [Google Scholar]
- Nichols T, Brett M, Andersson J, Wager T, Poline J‐P ( 2005): Valid conjunction inference with the minimum statistic. Neuroimage 25: 653–660. [DOI] [PubMed] [Google Scholar]
- Pashler H ( 1994a): Dual‐task interference in simple tasks: Data and theory. Psychol Bull 116: 220–244. [DOI] [PubMed] [Google Scholar]
- Pashler H ( 1994b): Graded capacity‐sharing in dual‐task interference? J Exp Psychol: Hum Percep Perform 20: 330–342. [DOI] [PubMed] [Google Scholar]
- Pashler H, Johnston JC ( 1998): Attentional limitations in dualtask performance In Pashler H, editor. Attention. Hove, England: Psychology Press; pp 155–189. [Google Scholar]
- Pashler H, Luck SJ, Hillyard SA, Mangun GR, O'Brien S, Gazzaniga MS ( 1994): Sequential operation of disconnected cerebral hemispheres in split‐brain patients. NeuroReport 5: 2381–2384. [DOI] [PubMed] [Google Scholar]
- Passingham D, Sakai K ( 2004): The prefrontal cortex and working memory: Physiology and brain imaging. Curr Opin Neurobiol 14: 163–168. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Feinstein JS, Leland D, Simmons AN ( 2005): Superior temporal gyrus and insula provide response and outcome‐dependent information during assessment and action selection in a decision‐making situation. Neuroimage 25: 607–615. [DOI] [PubMed] [Google Scholar]
- Paus T ( 2001): Primate anterior cingulate cortex: Where motor control, drive, and cognition interface. Nat Rev Neurosci 2: 417–424. [DOI] [PubMed] [Google Scholar]
- Paus T, Petrides M, Evans AC, Meyer E ( 1993): Role of the human anterior cingulated cortex in the control of oculomotor, manual, and speech responses: A positron emission tomography study. J Neurophysiol 70: 453–469. [DOI] [PubMed] [Google Scholar]
- Paus T, Koski L, Caramanos Z, Westbury C ( 1998): Regional differences in the effects of task difficulty and motor output on blood flow response in the human anterior cingulated cortex: A review of 107 PET activation studies. NeuroReport 9: R37–R47. [DOI] [PubMed] [Google Scholar]
- Picard N, Strick P ( 2001): Imaging the premotor areas. Curr Opin Neurobiol 11: 663–672. [DOI] [PubMed] [Google Scholar]
- Poldrack RA ( 2000): Imaging brain plasticity: Conceptual and methodological issues—A theoretical review. NeuroImage 12: 1–13. [DOI] [PubMed] [Google Scholar]
- Posner MI, Petersen S, Fox P, Raichle ME ( 1988): Localization of cognitive operations in the human brain. Science 240: 1627–1631. [DOI] [PubMed] [Google Scholar]
- Reiner A, Yekutieli D, Benjammini Y ( 2003): Identifying differentially expressed genes using false discovery rate controlling procedures. Bioinformatics 19: 368–375. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fogassi L, Gallese V ( 2002): Motor and cognitive functions of the ventral premotor cortex. Curr Opin Neurobiol 12: 149–154. [DOI] [PubMed] [Google Scholar]
- Rushworth MFS, Walton ME, Kennerley SW, Bannerman DM ( 2004): Action sets and decisions in the medial frontal cortex. Trends Cogn Sci 8: 410–417. [DOI] [PubMed] [Google Scholar]
- Rushworth MFS, Buckley MJ, Behrens TE, Walton ME, Bannerman DM ( 2007): Fucntion organization of the medial frontal cortex. Curr Opin Neurobiol 17: 220–227. [DOI] [PubMed] [Google Scholar]
- Ruthruff E, Miller J, Lachmann T ( 1995): Does mental rotation require central mechanisms? J Exp Psychol: Hum Percep Perform 21: 552–570. [DOI] [PubMed] [Google Scholar]
- Sakai K, Hikosaka O, Takino R, Miyauchi S, Nielsen M, Tamada T ( 2000): What and when: Parallel and convergent processing in motor control. J Neurosci 20: 2691–2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanfrey AG, Rilling JK, Aronson JA, Nystrom LE, Cohen JD ( 2003): The neural basis of economic decision‐making in the ultimatum game. Science 300: 1755–1758. [DOI] [PubMed] [Google Scholar]
- Schubert T, Szameitat AJ ( 2003): Functional neuroanatomy of interference in overlapping dual tasks: An fMRI study. Cogn Brain Res 17: 733–746. [DOI] [PubMed] [Google Scholar]
- Schubotz RI, von Cramon DY ( 2001): Functional organization of the lateral premotor cortex: fMRI reveals different regions activated by anticipation of object properties, location and speed. Cogn Brain Res 11: 97–112. [DOI] [PubMed] [Google Scholar]
- Schubotz RI, von Cramon DY ( 2003): Functional‐anatomical concepts of human premotor cortex: Evidence from fMRI and PET studies. NeuroImage 20: S210–S131. [DOI] [PubMed] [Google Scholar]
- Schumacher EH, D'Esposito M ( 2002): Neural implementation of response selection in humans as revealed by localized effects of stimulus‐response compatibility on brain activation. Human Brain Mapp 17: 193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher EH, Jiang Y ( 2003): Neural mechanisms for response selection: Representation specific or modality independent? J Cogn Neurosci 15: 1077–1079. [DOI] [PubMed] [Google Scholar]
- Schumacher EH, Elston PA, D'Esposito M ( 2003): Neural evidence for representation‐specific response selection. J Cogn Neurosci 15: 1111–1121. [DOI] [PubMed] [Google Scholar]
- Sherman SM, Guillery RW ( 1996): Functional organization of thalamocortical relays. J Neurophysiol 76: 1367–1395. [DOI] [PubMed] [Google Scholar]
- Shimojo S, Shams L ( 2001): Sensory modalities are not separate modalities: Plasticity and interactions. Curr Opin Neurobiol 11: 505–509. [DOI] [PubMed] [Google Scholar]
- Sigman M, Dehaene S ( 2008): Brain mechanisms of serial and parallel processing during dual‐task performance. J Neurosci 28: 7585–7598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon SR, Meunier M, Piettre L, Berardi AM, Segebarth CM, Boussaoud D ( 2002): Spatial attention and memory versus motor preparation: Premotor cortex involement as revealed by fMRI. J Neurophysiol 88: 2047–2057. [DOI] [PubMed] [Google Scholar]
- Stelzel C, Schumacher EH, Schubert T, D'Esposito M ( 2006): The neural effect of stimulus‐response modality compatibility on dual‐task performance: An fMRI study. Psychol Res 70: 514–525. [DOI] [PubMed] [Google Scholar]
- Stelzel C, Kraft A, Brandt SA, Schubert T ( 2008): Dissociable neural effects of task order control and task set maintenance during dual‐task processing. J Cogn Neurosci 20: 613–628. [DOI] [PubMed] [Google Scholar]
- Szameitat AJ, Schubert T, Müller K, von Cramon DY ( 2002): Localization of executive functions in dual‐task performance with fMRI. J Cogn Neurosci 14: 1184–1199. [DOI] [PubMed] [Google Scholar]
- Szameitat AJ, Lepsien J, von Cramon DY, Sterr A, Schubert T ( 2006): Task‐order coordination in dual‐task performance and the lateral prefrontal cortex. Psychol Res 70: 541–552. [DOI] [PubMed] [Google Scholar]
- Thach WT ( 1998): What is the role of the cerebellum in motor learning and cognition? Trends Cogn Sci 2: 331–337. [DOI] [PubMed] [Google Scholar]
- Turken AU, Swick D ( 1999): Response selection in the human anterior cingulate cortex. Nat Neurosci 2: 920–924. [DOI] [PubMed] [Google Scholar]
- Ullsberger M, von Cramon DY ( 2001): Subprocesses of performance monitoring: A dissociation of error processing and response competition revealed by event‐related fMRI and ERPs. Neuroimage 14: 1387–1401. [DOI] [PubMed] [Google Scholar]
- van Eimeren T, Wolbers T, Münchau A, Büchel C, Weiller C, Sieber HR ( 2006): Implementation of visuospatial cues in response selection. Neuroimage 29: 286–294 ( 2006). [DOI] [PubMed] [Google Scholar]
- Wager TD, Sylvester C.‐YC, Lacey SC, Nee DE, Franklin M, Jonides J ( 2005): Common and unique components of response inhibition revealed by fMRI. NeuroImage 27: 323–340. [DOI] [PubMed] [Google Scholar]
- Wallace MT, Ramachandran R, Stein BE ( 2004): A revised view of sensory cortical parcellation. Proc Natl Acad Sci USA 101: 2167–2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickens CD ( 1984): Processing resources in attention In Parasuraman R, Davies DR, editors. Varieties of Attention. Orlando: Academic Press; pp 63–102. [Google Scholar]
- Weissman DH, Gopalakrishnan A, Hazlett CJ, Woldorff MG ( 2005): Dorsal anterior cingulated cortex resolves conflict from distracting stimuli by boosting attention toward relevant events. Cereb Cortex 15: 229–237. [DOI] [PubMed] [Google Scholar]
- Wilson SM, Saygin AP, Sereno MI, Iacoboni M ( 2004): Listening to speech activates motor areas involved in speech production. Nat Neurosci 7: 701–702. [DOI] [PubMed] [Google Scholar]
- Wise SP ( 1985): The primate premotor cortex: Past, present, and preparatory. Annu Rev Neurosci 8: 1–19. [DOI] [PubMed] [Google Scholar]
- Woo S‐H, Lee K‐M ( 2007): Effect of the number of response alternatives on brain activity in response selection. Hum Brain Mapp 28: 950–958. [DOI] [PMC free article] [PubMed] [Google Scholar]


