Abstract
The effect of inhibiting nitric oxide synthase (NOS) on the visual responses of mouse retinal ganglion cells (RGCs) was studied under light adaptation by using patch-clamp recordings. The results demonstrated that NOS inhibitor, L-NAME, reduced the sensitivity of RGCs to light under light adaptation at different ambient light conditions. These observations were seen in all cells that recordings were made from. L-NAME diminished the excitatory synaptic currents (EPSCs), rather than increasing the inhibitory synaptic currents, of RGCs to reduce the sensitivity of RGCs to light. Cones may be the sites that L-NAME acted to diminish the EPSCs of RGCs.
Keywords: L-NAME, visual response, IPSC, EPSC, mouse retina
INTRODUCTION
Nitric oxide (NO) is synthesized from L-arginine by nitric oxide synthase (NOS; (Deguchi & Yoshioka, 1982, Palmer, Rees, Ashton & Moncada, 1988) and has been shown to be synthesized and released by nearly every cell type in the retina (Eldred & Blute, 2005). Despite the diffusible nature of nitric oxide, it is believed to be tightly regulated by being bound to intracellular sinks, such as thiols in the retina (Blute, Lee & Eldred, 2000, Eldred & Blute, 2005). The cell-specific nature of NO is further evidenced by the uneven distribution of soluble guanylyl cyclase (sGC) throughout the retinal layers. For instance, sGC expression is strong in the inner retina (Ahmad & Barnstable, 1993), and sGC staining is particularly strong in amacrine and bipolar cells (Blute, Velasco & Eldred, 1998, Ding & Weinberg, 2007). Furthermore, sGC is preferentially expressed in cone rather than rod bipolar cells, and GABAergic rather than glycinergic amacrine cells (Ding & Weinberg, 2007). Direct imaging of NO production has shown NO present in the photoreceptors as well (Blute et al., 2000, Eldred & Blute, 2005).
NO has been reported to modulate voltage-gated ion channels in rods and cyclic-nucleotide-gated channels in both rods and cones (Kurenny, Moroz, Turner, Sharkey & Barnes, 1994, Rieke & Schwartz, 1994, Savchenko, Barnes & Kramer, 1997). This is in line with the finding that light stimulation induces NO release in the retina (Neal, Cunningham & Matthews, 1998). NO has also been found to decrease electrical coupling in horizontal cells (DeVries & Schwartz, 1989, Miyachi, Murakami & Nakaki, 1990), to modulate cGMP levels in bipolar cells (Nawy & Jahr, 1990, Nawy & Jahr, 1991, Shiells & Falk, 1990), and to reduce coupling between AII amacrine cells and On-cone bipolar cells (Mills & Massey, 1995). In ganglion cells, NO donors have been shown to modulate cGMP-gated conductances (Ahmad, Leinders-Zufall, Kocsis, Shepherd, Zufall & Barnstable, 1994, Kawai & Sterling, 2002) and to increase the amplitude of N-type calcium currents (Hirooka, Kourennyi & Barnes, 2000). We have found that NO selectively blocked the APB sensitive rod Off-pathway while it has decreased most On responses in the dark-adapted retina (Wang, Liets & Chalupa, 2003). Additionally, we demonstrated that lack of nNOS reduced the sensitivity of RGCs to light under dark adaptation (Wang, van der List, Nemargut, Coombs & Chalupa, 2007).
The activity of NOS has been shown to be dependent on the state of ambient illumination (Zemel, Eyal, Lei & Perlman, 1996), thereby suggesting that NO could play a role in light adaptation. In fact, NO has been found to be synthesized and released in the retina during photopic stimulation (Levy, Twig & Perlman, 2004, Neal et al., 1998, Sekaran, Cunningham, Neal, Hartell & Djamgoz, 2005). Furthermore, endogenous NO appears to control cone contractions that occur in lower vertebrates as a part of the light adaptation process (Angotzi, Hirano, Vallerga & Djamgoz, 2002, Greenstreet & Djamgoz, 1994). However, nothing is known about how NO modulates the visual responses of retinal ganglion cells in the light-adapted retina.
In the current study, patch-clamp recordings were made from RGCs in light-adapted retinas. We found that Nω-Nitro-L-arginine methyl ester (L-NAME), a NOS inhibitor, reduced the sensitivity of RGCs to photopic stimuli in light-adapted mouse retinas. The results presented in this study suggest that endogenous NO is important to maintain the sensitivity of RGCs to light stimuli under light adaptation by diminishing excitatory neurotransmission in the retina.
EXPERIMENTAL PROCEDURES
The basic methods used in this study were similar to those used previously (Bai, Zhu, Yang, Savoie & Wang, 2009, Nemargut, Zhu, Savoie & Wang, 2009, Wang et al., 2007). All procedures were in compliance with National Institutes of Health guidelines and were approved by the campus animal use committees of Tulane University. Animals were dark-adapted overnight prior to the experiments and all procedures, including animal surgery, dissection of retinas, and recordings from cells were made in complete darkness. Infrared goggles were used to visualize the tissue on the dissecting and recording microscopes and to maneuver in the recording room. LEDs (850 nm) were used to provide light to the dissecting microscope while the illumination from the recording microscope was passed through an 850 nm cut off-filter.
Retinal preparation
Retinas were obtained from three to four month old mice (C57BL/6 from Charles River Farm CA). Following a lethal dose of barbiturate (Beuthanasia-D 360 mg/kg i.p.), the eyes were removed and placed in oxygenated L15 (Sigma, L1518, ingredients listed online) at 37 C for 12 min. The retinas were then carefully peeled from the eyecup and stored at room temperature in Minimal Essential Medium Eagle (MEME, Mediatech, Inc., 15-010-CM), and continuously bubbled with 95% O2 and 5% CO2. A small piece of retina was placed ganglion cell layer up in the recording chamber and stabilized with an overlying piece of filter paper. A 2 mm hole in the filter paper provided access for the recording electrode. Cells were visualized through a 40× objective mounted on an upright epifluorescence microscope (Nikon).
During recordings the retina was perfused continuously with MEME (1.5 mL/min) through a gravity fed line, heated with a dual channel temperature controller (Warner Instruments), and continuously bubbled with 95% O2 and 5% CO2. A calibrated thermocouple monitored the temperature in the recording chamber, maintained at 35 C. Recordings from each individual cell usually lasted 30–120 minutes, and retinal segments from which recordings were made typically remained viable for 8–12h. For current-clamp recordings, patch electrodes are filled with the solution containing (in mM): K-gluconate, 110; KCl, 10; MgCl2, 1; CaCl2, 0.5; HEPES, 10; EGTA, 5; 0.5 mg/ml Nystatin; 2.5 mg/ml Pluronic F-68; 0.5% Lucifer Yellow; 6%; pH 7.4; osmolarity, 290 mOsm. For voltage-clamp recordings, patch electrodes were filled with a solution containing (in mM): Cesium methanesulfonate, 118; CsCl, 12; CaCl2, 0.5; MgCl2, 0.5; HEPES, 10; EGTA, 5; 0.5 mg/ml Nystatin; 2.5 mg/ml Pluronic F-68; 0.5% Lucifer Yellow; QX-314, 5; pH 7.4; osmolarity, 290 mOsm. The chloride equilibrium potential, ECl, with this internal solution was approximately −57mV. 5mM QX-314 was included in the electrode solution to eliminate sodium currents in the recorded cell. There were no differences in the results obtained with and without Nystatin and Pluronic, although the use of these chemicals permitted stable recordings for longer time periods (Bai et al., 2009, Robinson & Chalupa, 1997). Using fluorescent microscopy, we found that Nystatin and Pluronic facilitated the formation of whole-cell configuration. With Nystain and Pluronic in the electrode solution, the soma is usually filled with Lucifier Yellow within five minutes after the formation of the high resistant seal, indicating whole-cell configuration was obtained. All recordings were made with the whole-cell configuration. By the end of the experiment the soma and the dendritic arborizations were usually completely filled. Once complete filling was achieved, the retina was removed and fixed in 4% paraformaldehyde for 6–8 hours at 4 C.
Morphological identification
Following fixation, as described above, the retinas were mounted on a slide and viewed initially with a fluorescent microscope (Nikon Eclipse E600-FN) equipped with DIC optics to determine how well the cell was filled with Lucifer Yellow. Subsequently, using a Leica TCS SP2 confocal microscope (Leica Microsystems, Heidelberg GMbH), high-resolution three dimensional images were made of each cell. Scans were taken at 0.25–0.7 μm intervals along the z axis depending on the objective used. The dendritic stratifications of RGCs in the inner plexiform layer (IPL) were determined by rotating the confocal stack image 90°. DAPI was used to label the nuclei of the ganglion cell layer and the inner nuclear layer (INL). The depth of the IPL was defined as the area between the ganglion cell layer and the inner border of the INL.
Electrophysiology
Whole-cell patch-clamp recordings were made from retinal ganglion cells in light-adapted retinas. Patch pipettes with a tip resistance between 3 and 7 MΩ were pulled from thick-walled 1.5 mm-OD borosilicate glass on a Sutter Instruments puller (P-97). Whole-cell patch-clamp recordings were made with a MultiClamp 700B patch-clamp amplifier. The data were low-pass filtered at rates between 1 and 2 kHz and digitized at rates 5 kHz before storage on a computer for subsequent off-line analysis. To attain whole cell access, the vitreous and the limiting membrane overlying the recording area were removed by gently brushing the retinal surface with the tip of a glass pipette. Recordings were obtained by patching onto cells with clear, non-granular cytoplasm. High-resistance seals were obtained by moving the patch electrode onto the cell membrane and applying gentle suction. After formation of a high-resistance seal between the electrode and the cell membrane, transient currents caused by pipette capacitance were electronically compensated by the circuit of the MultiClamp 700B patch-clamp amplifier. Recordings from cells with a seal resistance < 1 GΩ were discarded. The series resistance was 7–16 MΩ. Recordings were terminated whenever significant increases (>20%) in series resistance occurred. After attaining a whole-cell configuration the resting membrane potential was read off the amplifier. The value of the resting potential was monitored regularly throughout the recording, and if significant changes were observed, the recording was terminated. The sudden or gradual changes in the resting potential were considered significant if the changes were over 15% of the original values (positive or negative) and lasted longer than 10 minutes, when no electrical, light or chemical stimulations were applied.
Recording excitatory and inhibitory postsynaptic currents
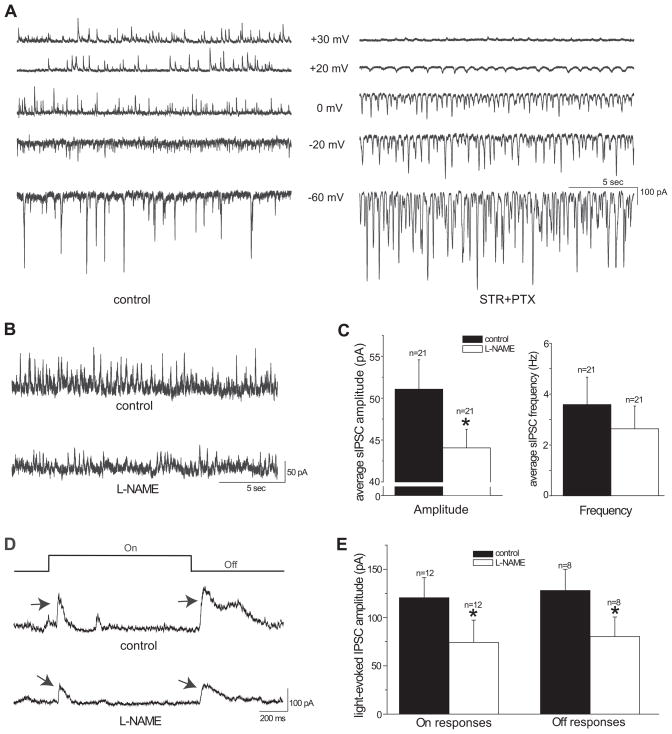
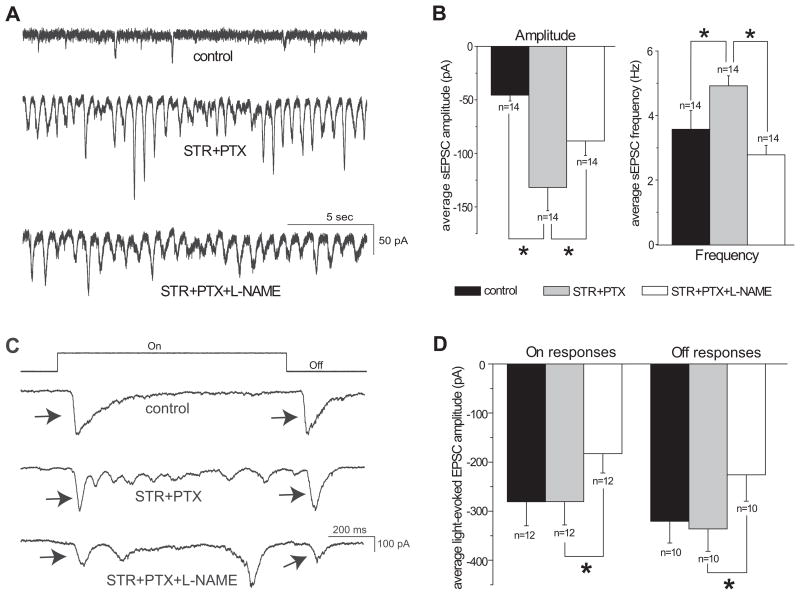
Spontaneous and light-evoked excitatory postsynaptic currents (EPSCs) were recorded at −60mV, approximately the chloride reversal potential of the RGCs. The holding potential used for recording spontaneous and light-evoked inhibitory postsynaptic currents (IPSCs) has been determined to be the potential where the driving force of the EPSCs is near 0 (Gao & Wu, 1998). To determine where the driving force was near 0, the spontaneous postsynaptic currents were recorded at various membrane potentials from −80 mV to +40 mV after blocking the inhibitory neurotransmission (Figure 4A right panel). Inhibitory neurotransmission was blocked by applying glycine receptor antagonist strychnine (STR, 5μM) and GABAA and GABAC receptor antagonist picrotoxin (PTX, 100μM). All of the IPSCs were eliminated after applying STR and PTX to the bath solution, as seen in Figure 4A. The reversal potential of the EPSCs with STR and PTX was approximately +20mV, which was used for the holding potential to record the spontaneous and light-evoked IPSCs. This is higher than the 0 mV previously reported in the salamander retina, possibly due to a species-related difference (Gao & Wu, 1998, Gao & Wu, 1999).
Figure 4.
L-NAME did not enhance the inhibitory synaptic input to ganglion cells under light adaptation with the background light intensity at 3000 photons/μm2/sec. A: Spontaneous postsynaptic currents from an On-Off RGC at various holding potentials with bath solution (left panel). Bath application of 5μM strychnine (STR) and 100μM picrotoxin (PTX) blocked outward currents at holding values of 0 mV and lower (right panel). Spontaneous EPSCs reversed at approximately +20mV with STR and PTX. B: Spontaneous IPSCs of an On-Off ganglion cell recorded at +20mV in bath solution (upper trace). Bath application of 100μM L-NAME decreased the IPSCs of this cell (lower trace). C: L-NAME significantly reduced the average amplitude (left panel), but not the frequency (right panel) of spontaneous IPSCs of recorded ganglion cells. D: Light-evoked On and Off IPSCs from an On-Off ganglion cell, indicated by arrows, under control conditions and after application of L-NAME. The stimulus light intensity for this cell was 1.6 × 105 photons/μm2/sec and the contrast was 0.68. L-NAME decreased the amplitude of light-evoked On and Off IPSCs. E: L-NAME significantly reduced the average amplitude of light-evoked On and Off IPSCs of recorded ganglion cells (*p<0.05 paired t-test).
The frequency and amplitude of spontaneous postsynaptic currents (sPSCs) were measured by Clampfit 9 (Molecular Devices, Inc) and Minianalysis (Synaptosoft, Inc), according to procedures used by Gao and Wu (1998, 1999). Individual sPSCs were detected by the computer with a detection threshold ±10 pA from the center of the baseline noise. For sPSCs with multiple peaks, subsequent peaks were counted as separate events only if the preceding peak had returned for >50% from its peak and the subsequent peak was >10 pA and a rise time <10 ms. sPSC peak amplitudes were measured by the computer at the rising phase of each event. The average sPSC amplitude at each holding potential was calculated by the ratio of the sum of peak amplitudes to the total number of events. sPSC events were counted, and the number of events during 3 minute intervals were used to calculate the frequency of the sPSCs.
Light stimulus
Light-evoked responses were obtained by delivering square wave spots of light to the retina from a one-inch-diameter computer monitor, with a green (P43, 545 nm light) phosphor (Lucivid MR1-103; MicroBrightField, Colchester, VT), through the camera port of the microscope (Demb, Haarsma, Freed & Sterling, 1999). The sizes of the spots of light were varied from 200 to 500 μm in diameters in different cells. For each cell, different sized spots were used to evoke light responses before the functional properties were tested. The size of the spot that evoked the optimal light-evoked response for this cell was selected and used to test the functional properties. The spots of light were always centered on the soma. The stimuli were programmed in Matlab (Math Works, Natick, MA), using the Psychophysics Toolbox extensions (Brainard, 1997, Pelli, 1997). The intensity of a spot of light was calibrated with a spectroradiometer/photometer (UDT instruments, S350/268R) and expressed in term of photons per μm2 per second (photons/μm2/s). The instrument was calibrated relative to standards of the National Institute of Standards and Technology.
We have successfully established a recording procedure to record from the same ganglion cell under dark and different levels of light adaptation. With this procedure, whole-cell patch-clamp recordings were made from a ganglion cell first under the dark-adapted condition, then a background light was be delivered to the retina to induce light adaptation, and the recording was be continued from the same cell. For these studies, background lights of constant brightness in the photopic range, of either 3000 or 2.9 × 104 photons/μm2/s, were provided full-field by the computer controlled one-inch-diameter monitor (Lucivid) for 10 minutes to allow the transition from dark adaptation to light adaptation. Both of these background light intensities completely inactivate rods. After exposing these cells to the background light at these intensities, they were temporarily unable to respond to dim light, validating that the rods were inactivated. With these intensities of background light, we found solid, consistent and reliable light-evoked responses could be recorded at least for two hours after the transition from dark to light adaptation.
Light stimuli with intensities greater than that of the background light were used in the light-adapted retina to evoke light responses from ganglion cells. For each cell, different intensities, ranging from 3.2 × 103 to 1.6 × 104 photons/μm2/s for the 3000 photons/μm2/s background and 6.25 × 104 to 6.72 × 106 photons/μm2/s for the 2.9 × 104 photons/μm2/s background, were used to evoke light responses. The optimal response level was characterized as the largest synaptic current amplitude (in voltage-clamp mode) or highest average peak firing rate (in current-clamp mode) of visual responses of each cell.
The contrast of the stimuli was calculated by using the Michelson contrast equation: contrast=(F−B)/(F+B), where F is the light intensity of the spots of light, and B is the steady background intensity (Burkhardt & Gottesman, 1987). The lowest intensity required to evoke optimal responses was used to stimulate the retinas and to test the effects of L-NAME on the light-evoked On and Off excitatory and inhibitory pathways. Their related contrasts varied from 0.54 to 0.88 under the 3000 photons/μm2/s background and 0.95 to 0.99 under the 2.9 × 104 photons/μm2/s background. The intensities and the contrasts used are indicated in the figure legends. Within the intensity range studied, we found consistent and reliable spontaneous and light-evoked visual responses for each given cell.
Measurements of the averaged peak firing rates of On and Off responses
The magnitude of On and Off responses to each light stimulation in RGCs are depicted as “average peak firing rates” at light onset or offset. For each stimulus, the average peak firing rate was calculated by counting the number of spikes within a window that encompassed the highest firing rate, which occurred initially following the light onset or offset, and dividing the spike number by the duration of the window. The window was determined by the time period that most clustered spikes occurred and varied from cell to cell (Nemargut et al., 2009, Wang et al., 2003, Wang et al., 2007). The window endpoints corresponded to the time point where the frequency dropped by 15% of its highest frequency.
Determining sensitivity
The sensitivity of the light-adapted RGCs was determined by the distributions of On and Off responses as a function of their light intensities, as described previously in our laboratory (Wang et al., 2007). The average peak firing rates and peak amplitudes of each cell were normalized to their optimal responses and plotted as a function of light intensity. The data points were then fitted with the Michaelis-Menten equation (Baylor, Hodgkin & Lamb, 1974, Naka & Rushton, 1966, Thibos & Werblin, 1978) as follows:
where R represents the measured response, Rmax represents the maximum response (optimal response), I indicates stimulus intensity, σ indicates the intensity that evokes a half-maximal response, and N is the Hill coefficient. The intensity (σ) that evoked a half-maximal response of each cell was obtained from the fitting.
Drug application
Nω-Nitro-L-arginine methyl ester (L-NAME, 100μM), strychnine (STR, 5 μM), picrotoxin (PTX, 100μM), and hydroxylamine (HA, 20mM) were freshly dissolved in MEME on the day of the experiment and administered through a gravity fed line. The pH was adjusted to 7.4 using 5M Tris buffer. All drugs were purchased from Sigma and prepared and stored in accordance with the manufacturer’s recommendations and applied in concentrations normally utilized in mammalian retinas.
5 μM of strychnine and 100μM of picrotoxin was sufficient to block the glycine, GABAA and GABAC receptors in the retina (Shen & Jiang, 2007, Wang & Slaughter, 2005). The solutions were heated with a dual channel temperature controller (Warner Instruments) and continuously bubbled with 95% O2 and 5% CO2. A six-position rotary valve (Western Analytical Products) was used to switch between bath and drug solutions. The effects of L-NAME were observed after a perfusion time of 10 minutes and continued throughout the duration of the L-NAME perfusion for each of the L-NAME experiments (Li & Hatton, 2000). L-NAME, a NOS inhibitor, has been shown to decrease endogenous NO in the retina (Cimini, Strang, Wotring, Keyser & Eldred, 2008, Kono, Chisato, Ebisawa, Asama, Sugawara, Ayabe, Kohgo, Kasai, Yoneda & Takahashi, 2004).
RESULTS
Effects of L-NAME on light-evoked responses of RGCs under light adaptation
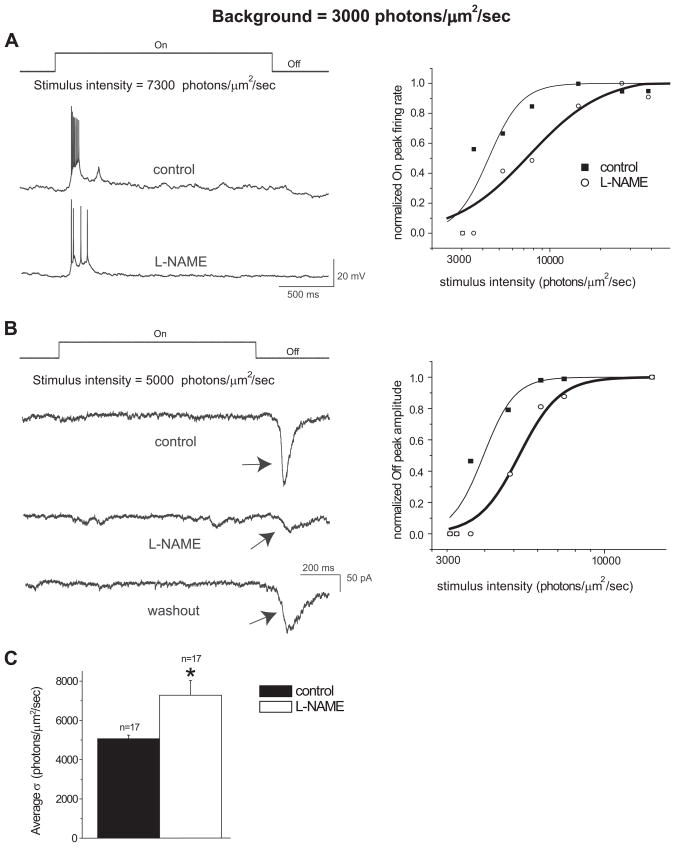
Recordings were initially made from RGCs under the background light with intensity at 3000 photons/μm2/sec, and intensities that are higher than the background light were used to evoke responses. With current-clamp recordings, bath application of L-NAME, a NOS inhibitor, decreased the peak firing rate of all cells (n=13) from which recordings were made when exposed to the same intensity light stimulus. These results are similar to our previous findings in the dark-adapted retina with L-NAME and in nNOS knockout mice (Wang et al., 2007). Examples of recordings from an On cell before and after L-NAME at a given light stimulus intensity are shown in Figure 1A. Similar findings were seen in Off as well as On-Off cells. The suppressive effect of L-NAME on the light-evoked responses was also validated with voltage-clamp recordings in 4 cells, as seen in an Off cell in Figure 1B.
Figure 1.
L-NAME reduced the sensitivity of RGCs to light stimuli under light adaptation with the background light intensity at 3000 photons/μm2/sec. A: Visual responses obtained by whole-cell current-clamp recordings from an On ganglion cell. When stimulated with the same light intensity, L-NAME (100μM) reduced the average peak firing rate of the On response. The light onset and offset are indicated above the recording traces. The normalized response-intensity curves from the On cell in the left panel is represented in the right panel. The data sets were fitted with the Michaelis-Menten equation. B: Visual responses obtained by whole-cell voltage-clamp recordings from an Off ganglion cell. L-NAME reduced the peak amplitude of the Off response, as indicated by arrows, and after a 20 minute washout of L-NAME partial recovery of the peak amplitude of the Off response was obtained. The normalized response-intensity curves from the Off cell in the left panel is represented in the right panel. The data sets were fitted with the Michaelis-Menten equation. C: The average intensity that evoked a half-maximal response (σ) was significantly higher following the L-NAME treatment from the 17 cells tested (*p<0.05, paired t-test). Error bars represent ± SE.
The effect of L-NAME diminishing light-evoked responses of RGCs was not only observed at a given stimulus light intensity, but also found with a range of stimulus light intensities. However, for a given cell, the same amplitude of optimal responses as that before L-NAME were evoked by higher light intensity after application of L-NAME. To further quantify the effects of L-NAME on the light-evoked responses of RGCs, the response-intensity relations of the recorded RGCs were established, as described in the methods section. For current-clamp recordings, the average peak firing rates of the responses were normalized to their optimal responses and plotted as a function of light intensity, and the data points were fitted with the Michaelis-Menten equation (Figure 1A, right panel). For voltage-clamp recordings, the response peak amplitudes were normalized to their optimal responses and also fitted with the Michaelis-Menten equation (Figure 1B, right panel). L-NAME shifted the response-intensity curve of each cell to the right of its control curve under current- and voltage-clamp modes. The intensities that evoked half-maximal responses (σ) from the intensity-response curves of each cell before and after L-NAME application were compared. The average σ of the control with the bath solution was 5064 ± 183 photons/μm2/sec (mean±SE) and significantly increased to 7283 ± 743 photons/μm2/sec after applying L-NAME (Figure 1C, p<0.05 paired t-test, n=17), indicating that L-NAME decreased the sensitivity of RGCs to light under the 3000 photons/μm2/sec ambient light condition. The average hill coefficients for the control and L-NAME conditions were 5.42 ± 0.89 and 5.57 ± 1.27, respectively. These findings are not attributed to the running down of the recordings over time, because the same amplitude of optimal responses of a given cell were evoked with higher intensity light stimuli after application of L-NAME; and following a 20 minute washout of L-NAME, partial recovery of responses were seen for a given cell with the same intensity light stimuli, as shown in Figure 1B.
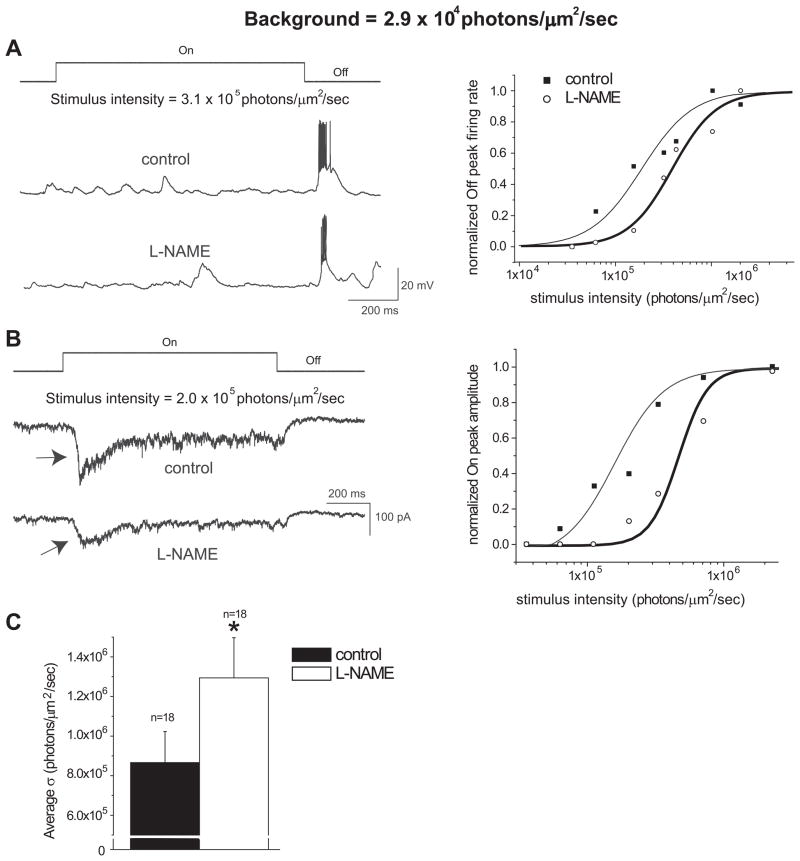
To determine how L-NAME affects the light-evoked responses of RGCs under different ambient light intensities, recordings were made from RGCs under a higher background light intensity of 2.9 × 104 photons/μm2/sec, and intensities higher than the background light were used to evoke responses. Interestingly, similar results as described above were also found under the ambient light condition with the intensity at 2.9 × 104 photons/μm2/sec. Both current- (n=7) and voltage-clamp (n=11) recordings showed that L-NAME decreased the light-evoked responses of RGCs (Figure 2A&B). An example of recordings from an Off cell before and after L-NAME at a given light stimulus intensity is shown in Figure 2A. L-NAME shifted the response-intensity curve to the right of its control curve (Figure 2A, right panel). Similar results were seen in voltage-clamp recordings, as shown in Figure 2B. The average σ with the bath solution was 8.65 × 105 ± 1.57 × 104 photons/μm2/sec and significantly increased to 1.29 × 106 ± 2.03 × 104 photons/μm2/sec after applying L-NAME (Figure 2C, p<0.05, paired t-test, n=18). The average hill coefficients for the control and L-NAME conditions were 3.57 ± 1.19 and 3.55 ± 0.87, respectively. These findings indicate that inhibition of NOS decreases the sensitivity of RGCs to light under light adaptation at different background light intensities.
Figure 2.
L-NAME reduced the sensitivity of RGCs to light stimuli under light adaption with the background light intensity at 2.9 × 104 photons/μm2/sec. A: Visual responses obtained by whole-cell current-clamp from an Off ganglion cell. When exposed to the same intensity light stimulus, L-NAME (100μM) reduced the average peak firing rate of the Off response. The normalized response-intensity curves of this cell are represented in the right panel. B: Visual responses obtained by whole-cell voltage-clamp recordings from an On ganglion cell. L-NAME reduced the peak amplitude of the On response, as indicated by arrows. The normalized response-intensity curves from the On cell in the left panel is represented in the right panel. C: The average intensity that evoked a half-maximal response (σ) was significantly higher following the L-NAME treatment for the 18 cells tested (*p<0.05, paired t-test).
Effects of L-NAME on ganglion cell types
The suppressive effect of L-NAME on RGCs was seen in all cells that recordings were made from. The cells included On, Off, On-Off cells as well as transient and sustained cells. A total of 35 cells were included in the sensitivity experiments: 10 On, 8 Off, and 17 On-Off cells, which includes 14 transient and 21 sustained cells.
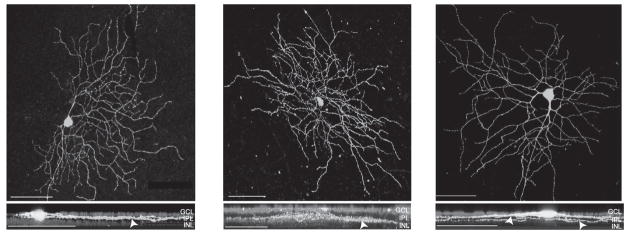
Recorded cells were filled with Lucifer Yellow during the course of the recording, and the cell class was determined based on confocal images and was classified according to the study by Doi and colleagues (1995), Coombs et al. (2006), and Sun et al. (2002). The examples of confocal images from three recorded ganglion cells are shown in Figure 3. The top panels show the top views of the dendritic branching patterns and the lower panels show the side views of the dendritic stratification patterns of On, Off, and On-Off ganglion cells. A total of 32 cells were obtained with morphological identifications. They included 13 Type I, 13 Type II, and 6 Type III cells according to Doi and colleagues (1995); 4 M3, 1 M4, 4 M6, 2 M7, 1 M8, 2 M9, 5 M10, 1 M11, 4 M12, 4 M13, and 4 M14 cells according to Coombs et al. (2006); and 3 RGA1, 3 RGA2, 1 RGB1, 4 RGB3, 1 RGB4, 4 RGC2, 4 RGC3, 5 RGD1, and 7 RGD2 cells according to Sun et al. (2002). Our findings here indicate that L-NAME decreases the sensitivity of RGCs in various subgroups.
Figure 3.
Confocal reconstructions of three morphologically-identified ganglion cells from which recordings were made. The cells were filled with Lucifer Yellow during the course of recording. The top views and the 90° rotated confocal stacked images of these cells are shown in the upper and lower panels, respectively. Arrowheads indicate the dendritic stratifications of these cells in the IPL. Left panels: An On cell classified as a Type I/M8/RGC2 ganglion cell. Middle panels: An Off cell classified as a Type III/M4/RGC2 ganglion cell. Right panels: A bistratified cell classified as a Type I/M12/RGD2 ganglion cell. The nuclei of the ganglion cell layer and inner nuclear layer were stained with DAPI. Scale bars = 75μm. GCL = ganglion cell layer INL = inner nuclear layer IPL = inner plexiform layer.
L-NAME did not enhance the inhibitory synaptic inputs to ganglion cells under light adaptation
The mechanism that underlies the suppressive effect of L-NAME on light sensitivity of RGCs under light adaptation is unknown. One possible mechanism is that L-NAME enhances the inhibitory synaptic inputs to RGCs and consequently reduces the sensitivity of RGCs to light. To test this possibility, we determined how L-NAME affects the inhibitory postsynaptic currents (IPSCs) of RGCs under light adaptation. A background light with intensity at 3000 photons/μm2/sec was used in the experiments to induce light adaptation. If L-NAME enhances the inhibitory synaptic inputs to RGCs, one would expect that L-NAME increases the frequency and/or amplitude of IPSCs of RGCs. On the contrary, we found that L-NAME decreased the amplitude of the spontaneous and light-evoked IPSCs of RGCs (Figure 4B–E). The average amplitude of the spontaneous IPSCs (sIPSCs) was 51.1 ± 3.5 pA in the control condition, and 44.1 ± 2.2 pA after application of L-NAME (Figure 4C left panel, n=21, p<0.05 paired t-test). The frequency of sIPSCs was 3.59 ± 1.08 Hz in the control condition, and 2.64 ± 0.89 Hz following the L-NAME treatment (Figure 4C right panel, n=21, p>0.05 paired t-test). A reduction in the light-evoked IPSCs was also seen (Figure 4D&E). The average amplitude of light-evoked On IPSCs was 120.5 ± 20.8 pA in the control condition and 74.1 ± 23.2 pA after L-NAME (Figure 4E, n=12, p<0.05 paired t-test); the average amplitude of light-evoked Off IPSCs was 128.0 ± 22.0 pA for the control and 80.3 ± 20.2 pA after the application of L-NAME (Figure 4E, n=8, p<0.05 paired t-test). The reduction of IPSCs of RGCs caused by L-NAME was also evidenced in 4 cells which recordings were made from with the background light at 2.9 × 104 photons/μm2/sec. Although the mechanism that underlies the effects of L-NAME reducing IPSCs of RGCs under light adaptation remains unknown and needs to be established in the future, our findings here do not support the hypothesis that L-NAME enhances the inhibitory synaptic inputs to RGCs and consequently reduces the sensitivity of RGCs to light.
L-NAME diminished the excitatory synaptic inputs to ganglion cells under light adaptation
Another possible mechanism is that L-NAME reduces the excitatory synaptic inputs to RGCs and consequently reduces the sensitivity of RGCs to light. To test this possibility, the effects of L-NAME on the excitatory postsynaptic currents (EPSCs) of RGCs were determined under light adaptation with the background light at 2.9 × 104 photons/μm2/sec. STR and PTX were used to eliminate the inhibitory synaptic inputs from glycine and γ-aminobutyric acid (GABA) to RGCs, respectively, and isolate the excitatory neurotransmission. Application of STR and PTX enhanced the amplitude and frequency of the spontaneous EPSCs (sEPSCs) in all of the cells recorded (Figure 5A&B). The average sEPSC amplitude was −45.6 ± 5.4 pA in the control condition, which significantly enhanced to −131.8 ± 21.8 pA after perfusion of STR and PTX; the average sEPSC frequency was significantly enhanced from 3.57 ± 0.58 Hz in the bath solution to 4.92 ± 0.31 Hz after application of STR and PTX (Figure 5B, n=14, p<0.05 paired t-test). These observations are in line with those of previous reports (Ariel & Adolph, 1985).
Figure 5.
L-NAME reduced the excitatory synaptic input to ganglion cells under light adaptation with the background light intensity at 2.9 × 104 photons/μm2/sec. A: Spontaneous EPSCs recorded at −60mV from an On-Off ganglion cell in bath solution (top trace), during application of 5μM STR and 100μM PTX (middle trace), and during application of STR, PTX and 100μM L-NAME (bottom trace). STR and PTX increased the EPSCs, while L-NAME decreased them in this cell. B: STR and PTX significantly increased the average amplitude (left panel) and frequency (right panel) of spontaneous EPSCs of recorded cells. L-NAME significantly reduced the average amplitude and frequency of spontaneous EPSCs, compared with STR and PTX alone. C: Light-evoked On and Off EPSCs from an On-Off ganglion cell, indicated by arrows, under control conditions (top trace), with STR and PTX (middle trace), and with L-NAME applied with STR and PTX (bottom trace). The stimulus light intensity for this cell was 6.7 × 106 photons/μm2/sec and the contrast was 0.99. STR and PTX had little effect on the light-evoked On and Off EPSCs, but L-NAME decreased them in this cell. D: L-NAME significantly reduced the average amplitude of light-evoked On and Off EPSCs from the cells that recording were made from, compared with STR and PTX applied alone (*p<0.05, paired t-test).
There was no significant difference in the amplitude of light-evoked optimal On and Off EPSCs when STR and PTX were applied. The average optimal On EPSC amplitude was −280.8 ± 49.0 pA and −280.5 ± 47.6 pA during the bath solution and STR and PTX applications, respectively. The average Off EPSC amplitude was −320.5 ± 44.5 pA under the control conditions, and −336.0 ± 45.8 pA with STR and PTX (Figure 5D, p>0.05, paired t-test).
If L-NAME reduces the excitatory synaptic inputs to RGCs, one would expect that L-NAME reduces the frequency and/or amplitude of EPSCs of RGCs. Indeed, we found that L-NAME significantly decreased the amplitude of the spontaneous and light-evoked EPSCs of RGCs following STR and PTX treatment (Figure 5). The average amplitude of the sEPSCs was −131.8 ± 21.8 pA and −88.4 ± 13.7 pA before and after L-NAME, respectively (Figure 5B left panel, n=14, p<0.05, paired t-test). L-NAME also significantly reduced the frequency of sEPSCs from 4.92 ± 0.31 Hz to 2.78 ± 0.29 Hz before and after L-NAME, respectively (Figure 5B right panel, n=14, p<0.05, paired t-test). The amplitude of the light-evoked On and Off EPSCs were reduced following application of L-NAME, as seen in Figure 5C. The average optimal On response amplitude was −280.5 ± 47.6 pA during STR and PTX application, and significantly reduced to −182.9 ± 39.5 pA during application of STR, PTX, and L-NAME (Figure 5D, n=12, p<0.05 paired t-test). The average Off response amplitude was −336.0 ± 45.8 pA with STR and PTX, and significantly reduced to −226.1 ± 53.5 pA after L-NAME (Figure 5D, n=10, p<0.05 paired t-test). The reduction of EPSCs of RGCs caused by L-NAME was also found in 3 cells which recordings were made from with the background light at 3000 photons/μm2/sec. The results that L-NAME decreased the EPSCs of RGCs under light adaptation support the hypothesis that L-NAME reduces the excitatory synaptic inputs to RGCs and consequently reduces the sensitivity of RGCs to light.
Bleaching photoreceptors eliminated the effects of L-NAME on EPSCs of RGCs under light adaptation
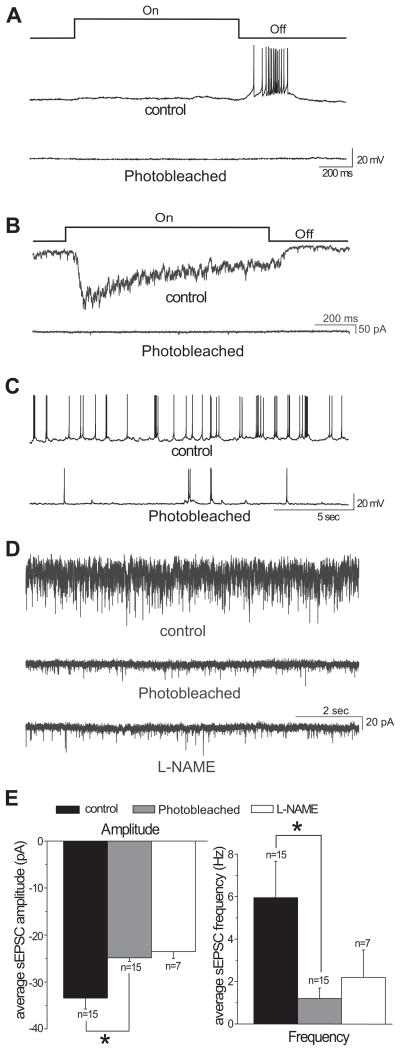
Under light adaptation, the excitatory visual information flows from the cones to the cone bipolar cells, then to the RGCs (Sterling & Demb, 2004). Whether L-NAME acted on cones, cone bipolar cells, or ganglion cells to reduce the excitatory synaptic inputs of RGCs is not clear. To better understand how L-NAME acts on the cone-driven excitatory circuitry in the retina to diminish EPSCs, we used hydroxylamine (HA) to photobleach cones and record the EPSCs of RGCs under light adaptation.
HA acts upon the Metarhodopsin II molecule to inhibit the phototransduction cascade in photoreceptors (Hofmann, Emeis & Schnetkamp, 1983, Yamazaki, Yamazaki, Yamazaki & Usukura, 2006). Photobleaching has been shown to dramatically reduce the dark current in both rods and cones (Leibrock & Lamb, 1997, Rieke & Baylor, 2000, Sampath & Baylor, 2002), and consequently decrease glutamate release from photoreceptors.
To photobleach the cones, HA was perfused for 7 minutes, and then was washed out for 10 minutes to avoid any possible cytotoxic effects (Leibrock & Lamb, 1997). Then, the recordings were continued without HA while the cones remained bleached. A recent study has shown that washout of HA after brief exposure cannot fully restore the light responses of cones (Holcman & Korenbrot, 2005). We found that after washout of HA, the light responses of RGCs under light adaptation could not be evoked for at least for 90 minutes, as was demonstrated in current- and voltage-clamp modes (Figure 6A&B). However, the RGCs were able to spontaneously fire action potentials (Figure 6C), indicating the procedural application of HA is not creating cytotoxic side effects of HA. Under 2.9 × 104 photons/μm2/sec background illumination, after washout of HA, the spontaneous EPSCs of RGCs were significantly lower compared with those before HA application. Thus, the average sEPSC amplitude was −33.4 ± 2.3 pA and −24.9 ± 0.81 pA, and the average sEPSC frequency was 5.95 ± 1.72 Hz and 1.20 ± 0.49 Hz before and after HA application, respectively. The differences are significant (Figure 6E, n=15, p<0.05, paired t-test). These results indicate that HA treatment acts on cones to eliminate their photoresponses.
Figure 6.
A&B: Recordings from two RGCs showing that photobleaching by hydroxylamine (HA) treatment eliminated the light-evoked responses of RGCs. The stimulus intensity was 6.7 × 106 photons/μm2/sec and the contrast was 0.99. A: Current-clamp recordings from an Off ganglion cell before and after photobleaching. B: Voltage-clamp recordings from an On ganglion cell before and after photobleaching. C: Recordings from an Off RGC depicting that photobleaching decreased the spontaneous firing activity of the RGC. D: Voltage-clamp recordings from an On-Off RGC demonstrating that L-NAME did not change the EPSCs of the cell after photobleaching cones by HA. Spontaneous EPSCs were recorded in bath solution (top trace), after photobleaching (middle trace), and when L-NAME was applied following the photobleaching (bottom trace). E: Photobleaching significantly reduced the average amplitude (left panel) and frequency (right panel) of sEPSCs (n=15, *p<0.05 paired t-test). L-NAME had little effect on the EPSCs after photobleaching cones by HA (n=7). Recordings were made under the background light intensity 2.9 × 104 photons/μm2/sec.
After washing out HA with the photoreceptors remaining bleached, bath application of L-NAME had little effect on the EPSCs of RGCs (Figure 6D). The average sEPSC amplitude was −24.3 ± 1.0 pA and −23.6 ± 1.4 pA, and the average sEPSC frequency was 1.30 ± 1.0 Hz and 2.20 ± 1.29 Hz before and after L-NAME application, respectively. These differences are not significant (Figure 6E, n=7, p>0.05, paired t-test). These findings suggest that photobleaching cones eliminates the suppressive effects of L-NAME on EPSCs of RGCs under light adaptation.
DISCUSSION
Extensive studies have documented that NO modulates the membrane properties of various retinal neurons, yet little is known about how NO modulates visual responses of RGCs under different ambient light conditions. In previous studies, we demonstrated that under dark adaptation, increasing NO selectively blocked the APB sensitive rod Off-pathways (Wang et al., 2003) and decreasing NO reduced the sensitivity of RGCs to light stimulation (Wang et al., 2007). The results of the present study demonstrate that under light adaptation, inhibiting NOS by L-NAME reduced the sensitivity of RGCs to photopic light stimulation. This desensitization was seen for a range of light intensities and under different ambient light conditions (Figures 1&2). Moreover, the reduction in sensitivity of RGCs induced by inhibiting NOS was seen in all cells that recordings were made from. This effect was seen in every physiological and morphological cell type studied. These findings provide the first direct evidence that under light adaptation, endogenous NO can provide powerful modulation on visual responses of RGCs. Taking together our findings in this study and those under dark adaptation (Wang et al., 2007), we identified that endogenous NO plays a critical role in maintaining the sensitivity of RGCs to light stimulation under both dark- and light-adapted conditions.
The mechanism that underlies the effects of inhibition of NOS by L-NAME desensitizing RGCs to light
To determine the mechanisms that underlie the effects of L-NAME reducing the sensitivity of RGCs to light, we first focused on how L-NAME affects the inhibitory synaptic inputs to RGCs. In the retina, GABA and glycine are the major inhibitory neurotransmitters. NO has been shown to inhibit glycine release in the retina (Neal, Cunningham & Matthews, 1997) via GABA (Yu & Eldred, 2005a). Our results showed that blocking inhibitory inputs increased the spontaneous excitatory postsynaptic currents (EPSCs, Figure 4A right panel & Figure 5A), indicating that inhibitory inputs play an important role in inhibiting EPSCs of ganglion cells. Therefore, our working hypothesis was that inhibition of NOS by L-NAME may enhance the inhibitory synaptic inputs to RGCs and consequently reduces the sensitivity of RGCs to light. To test this hypothesis, we determined how L-NAME affects the inhibitory postsynaptic currents (IPSCs) of RGCs under light adaptation. We found that L-NAME decreased the amplitude of the spontaneous and light-evoked IPSCs of RGCs (Figure 4B–E). Although the mechanism that underlies the effects of L-NAME reducing IPSCs of RGCs under light adaptation remains unknown, our findings here do not support the hypothesis that inhibition of NOS by L-NAME enhances the inhibitory synaptic inputs to RGCs and consequently reduces the sensitivity of RGCs to light.
We have not attempted to establish the mechanisms that underlie the effects of L-NAME reducing IPSCs of RGCs under light adaptation, but the available literature suggests several possibilities that would be worth pursuing in future studies. For example, NO has been shown to stimulate GABA release (Yu & Eldred, 2005a). Inhibition of NOS by L-NAME could reduce GABA release and consequently decrease the IPSCs of ganglion cells. Alternatively, NO transiently converts synaptic inhibition to excitation in retinal amacrine cells; this conversion might result in an enhancement of inhibitory output onto RGCs to suppress the activity of RGCs (Hoffpauir, McMains & Gleason, 2006); therefore inhibition of NOS by L-NAME may reduce the inhibitory output from amacrine cells to ganglion cells, as a result, the IPSCs of ganglion cell may reduced. It is also possible that L-NAME inhibits NOS in ganglion cells to reduce their IPSCs, as we found L-NAME decreased the amplitude of IPSCs. The amplitude of IPSCs usually reflects the postsynaptic activity, which is ganglion cell activity in this study.
Another possible mechanism is that L-NAME reduces the excitatory synaptic inputs to RGCs and consequently reduces the sensitivity of RGCs to light. Nitric oxide has been shown to act on several excitatory synapses in the retina. For instance, glutamate release from the cones and interactions with glutamate receptors on horizontal cells is NO-sensitive (Levy et al., 2004). A source of NO production in the retina that modulates photoreceptor calcium channels is the photoreceptors themselves (Kourennyi, Liu, Hart, Mahmud, Baldridge & Barnes, 2004) and it is has been hypothesized that NO from the ellipsoid of cones acts mainly upon the cone outer segments (Levy et al., 2004). Furthermore, NO donors and cGMP analogs were found to reduce the KA and AMPA-type responses of hybrid bass horizontal cells (McMahon & Ponomareva, 1996, McMahon & Schmidt, 1999). Also, the mGluR6 receptors from rod bipolar and On cone bipolar cells are potentiated following NO donors or cGMP analogs (Shiells & Falk, 1992, Snellman & Nawy, 2004, Snellman, Zenisek & Nawy, 2009).
To isolate the impact of L-NAME on excitatory neurotransmission, GABA and glycine receptors in the light-adapted retina were initially blocked with STR and PTX. Our results showed that STR and PTX increased the frequency and amplitude of spontaneous EPSCs of every ganglion cell under light adaptation (Figure 5). This is in line with previous research showing that STR and PTX increases the spontaneous activity of turtle RGCs under light adaptation (Ariel & Adolph, 1985). The findings validate that glycine and GABA are the dominant inhibitory neurotransmitters which play an important role in the retina under light adaptation.
The main purpose of using strychnine and picrotoxin in this study was to block the inhibitory synaptic pathways and focus on how L-NAME affects the excitatory pathways. A reciprocal relationship of GABA and glycine with NO has been demonstrated by Yu and Eldred (Yu & Eldred, 2003, Yu & Eldred, 2005b). It is possible that application of strychnine and picrotoxin would elevate the level of NO in retina. Since blocking GABA and glycine receptors increases the level of NO via NOS (Yu & Eldred, 2003), L-NAME could reduce the elevated level of NO by inhibiting NOS. Our results, that blocking GABA and glycine receptors did not alter the amplitude of the light-evoked EPSCs of ganglion cells in the light-adapted retina, suggesting that the elevated NO level by blocking GABA and glycine receptors may not play an important role in the light-evoked EPSCs of ganglion cells. However, reducing NO by L-NAME decreased the light-evoked EPSCs of ganglion cells (Figure 5).
To test if L-NAME is acting on the excitatory neurotransmission in the retina, the excitatory currents were measured under light adaptation. If L-NAME reduces the excitatory synaptic inputs to RGCs, one would expect that L-NAME reduces the frequency and/or amplitude of EPSCs of RGCs. Indeed, we found that L-NAME significantly decreased the amplitude of the spontaneous and light-evoked EPSCs and frequency of sEPSCs of RGCs after blocking inhibitory currents (Figure 5). This was seen in all cell types tested. This correlates to previous research demonstrating that NO stimulates glutamate release (Prast & Philippu, 2001). These results strongly support the hypothesis that L-NAME reduces endogenous NO in the retina to reduce the excitatory synaptic inputs to RGCs and consequently reduces their sensitivity to light.
Possible sites for inhibition of NOS to diminish excitatory synaptic currents of RGCs
L-NAME may act on cones, cone bipolar cells, or ganglion cells in the light-adapted retina to cause the reductions in EPSCs of RGCs. Although the precise site of L-NAME action on the visual responses of ganglion cells remains to be established, our results showed that photobleaching cones eliminated the effects of L-NAME on EPSCs of RGCs under light adaptation, suggesting cones may be the sites that L-NAME acts to reduce the sensitivity of RGCs to light under light adaptation.
Photobleaching dramatically reduced the sEPSC frequency and amplitude of RGCs (Figure 6). Along the Off-cone pathway, Off cone bipolar cells are hyperpolarized via the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and kainate (KA) receptors in response to removal of glutamate (DeVries, 2000, DeVries & Schwartz, 1999). Therefore photobleaching likely hyperpolarized the Off cone bipolar cells to reduce their glutamate release onto Off ganglion cells. Our data also show that these reductions in sEPSCs also occur along the On-cone pathway in each On cell. The mechanism that underlines the reductions in sEPSCs caused by photobleaching is unclear. It is unlikely due to the cytotoxic side effects of HA because our results showed that after washout HA the RGCs were able to spontaneously fire action potentials (Figure 6C). The possible mechanism may due to the fact that On bipolar cells display desensitization in response to withdraw of transmitter (Nawy, 2004, Shiells, 1999, Snellman, Kaur, Shen & Nawy, 2008).
After photobleaching cones, L-NAME had little effect on the EPSCs of RGCs. These findings indicate that photobleaching cones eliminate the effects of L-NAME reducing EPSCs under light adaptation, emphasizing the importance of endogenous NO in photoreceptor glutamate transmission. These results also support the hypothesis that L-NAME inhibits NOS in cones to reduce excitatory synaptic currents and the sensitivity of ganglion cells to light in the light-adapted mouse retina. Further research is needed to directly test how inhibition of NOS affects the sensitivity of cones to light under light adaptation.
In photoreceptors, endogenous NO is necessary to modulate cyclic nucleotide-gated (CNG) channels and calcium currents in rod and cone terminals (Kurenny et al., 1994, Rieke & Schwartz, 1994, Savchenko et al., 1997). These CNG channels may be activated by steady-state levels of cGMP in cones and contribute to glutamate release by allowing calcium influx even at hyperpolarized potentials (Savchenko et al., 1997). The NO-mediated modulation of CNG channels, as seen under light adaptation, allows for cones to extend their voltage range of exocytosis and increase their gain (Kourennyi et al., 2004, Rieke & Schwartz, 1994).
Other mechanisms could also be involved in the effect of L-NAME diminishing the sensitivity of RGCs under light adaptation. For instance, L-NAME may act by diminishing the cGMP-gated currents on ganglion cells to reduce their excitability (Kawai & Sterling, 2002). Alternatively, L-NAME may promote changes in glutamate transmission by acting on glutamate uptake, as demonstrated in the rat spinal cord (Liaw, Stephens, Binns, Chu, Sepkuty, Johns, Rothstein & Tao, 2005) or dopamine uptake, as has been shown in the rat striatum (Kiss, Zsilla & Vizi, 2004).
Collectively, our data support the hypothesis that regulation of glutamate transmission by endogenous nitric oxide is responsible for maintaining the sensitivity of RGCs to light stimuli in the light-adapted retina.
Acknowledgments
We thank Wei Huang for technical support. This research was supported by grant EY13301 from the National Eye Institute, Tulane Research Enhancement Fund, and the Tulane New Faculty Startup Fund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmad I, Barnstable C. Differential laminar expression of particulate and soluble guanylate cyclase genes in rat retina. Exp Eye Res. 1993;56(1):51–62. doi: 10.1006/exer.1993.1008. [DOI] [PubMed] [Google Scholar]
- Ahmad I, Leinders-Zufall T, Kocsis J, Shepherd G, Zufall F, Barnstable C. Retinal ganglion cells express a cGMP-gated cation conductance activatable by nitric oxide donors. Neuron. 1994;12(1):155–165. doi: 10.1016/0896-6273(94)90160-0. [DOI] [PubMed] [Google Scholar]
- Angotzi AR, Hirano J, Vallerga S, Djamgoz MB. Role of nitric oxide in control of light adaptive cone photomechanical movements in retinas of lower vertebrates: a comparative species study. Nitric Oxide. 2002;6(2):200–204. doi: 10.1006/niox.2001.0401. [DOI] [PubMed] [Google Scholar]
- Ariel M, Adolph AR. Neurotransmitter inputs to directionally sensitive turtle retinal ganglion cells. J Neurophysiol. 1985;54(5):1123–1143. doi: 10.1152/jn.1985.54.5.1123. [DOI] [PubMed] [Google Scholar]
- Bai X, Zhu J, Yang J, Savoie BT, Wang GY. Mechanisms that limit the light stimulus frequency following through the APB sensitive and insensitive rod Off-pathways. Neuroscience. 2009 doi: 10.1016/j.neuroscience.2009.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor DA, Hodgkin AL, Lamb TD. Reconstruction of the electrical responses of turtle cones to flashes and steps of light. J Physiol. 1974;242(3):759–791. doi: 10.1113/jphysiol.1974.sp010733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blute T, Velasco P, Eldred W. Functional localization of soluble guanylate cyclase in turtle retina: modulation of cGMP by nitric oxide donors. Vis Neurosci. 1998;15(3):485–498. doi: 10.1017/s0952523898153075. [DOI] [PubMed] [Google Scholar]
- Blute TA, Lee MR, Eldred WD. Direct imaging of NMDA-stimulated nitric oxide production in the retina. Vis Neurosci. 2000;17(4):557–566. doi: 10.1017/s0952523800174061. [DOI] [PubMed] [Google Scholar]
- Brainard DH. The psychophysics toolbox. Spatial Vision. 1997;10(4):433–436. [PubMed] [Google Scholar]
- Burkhardt DA, Gottesman J. Light adaptation and responses to contrast flashes in cones of the walleye retina. Vision Res. 1987;27(9):1409–1420. doi: 10.1016/0042-6989(87)90151-9. [DOI] [PubMed] [Google Scholar]
- Cimini BA, Strang CE, Wotring VE, Keyser KT, Eldred WD. Role of acetylcholine in nitric oxide production in the salamander retina. J Comp Neurol. 2008;507(6):1952–1963. doi: 10.1002/cne.21655. [DOI] [PubMed] [Google Scholar]
- Coombs J, van der List D, Wang GY, Chalupa LM. Morphological properties of mouse retinal ganglion cells. Neuroscience. 2006;140(1):123–136. doi: 10.1016/j.neuroscience.2006.02.079. [DOI] [PubMed] [Google Scholar]
- Deguchi T, Yoshioka M. L-Arginine identified as an endogenous activator for soluble guanylate cyclase from neuroblastoma cells. J Biol Chem. 1982;257(17):10147–10151. [PubMed] [Google Scholar]
- Demb J, Haarsma L, Freed M, Sterling P. Functional circuitry of the retinal ganglion cell’s nonlinear receptive field. J Neurosci. 1999;19(22):9756–9767. doi: 10.1523/JNEUROSCI.19-22-09756.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVries SH. Bipolar cells use kainate and AMPA receptors to filter visual information into separate channels. Neuron. 2000;28(3):847–856. doi: 10.1016/s0896-6273(00)00158-6. [DOI] [PubMed] [Google Scholar]
- DeVries SH, Schwartz EA. Modulation of an electrical synapse between solitary pairs of catfish horizontal cells by dopamine and second messengers. J Physiol. 1989;414:351–375. doi: 10.1113/jphysiol.1989.sp017692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVries SH, Schwartz EA. Kainate receptors mediate synaptic transmission between cones and ‘Off’ bipolar cells in a mammalian retina. Nature. 1999;397(6715):157–160. doi: 10.1038/16462. [DOI] [PubMed] [Google Scholar]
- Ding JD, Weinberg RJ. Distribution of soluble guanylyl cyclase in rat retina. J Comp Neurol. 2007;502(1):734–745. [PubMed] [Google Scholar]
- Doi M, Uji Y, Yamamura H. Morphological classification of retinal ganglion cells in mice. J Comp Neurol. 1995;356(3):368–386. doi: 10.1002/cne.903560305. [DOI] [PubMed] [Google Scholar]
- Eldred WD, Blute TA. Imaging of nitric oxide in the retina. Vision Res. 2005;45(28):3469–3486. doi: 10.1016/j.visres.2005.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao F, Wu S. Characterization of spontaneous inhibitory synaptic currents in salamander retinal ganglion cells. J Neurophysiol. 1998;80(4):1752–1764. doi: 10.1152/jn.1998.80.4.1752. [DOI] [PubMed] [Google Scholar]
- Gao F, Wu S. Multiple types of spontaneous excitatory synaptic currents in salamander retinal ganglion cells. Brain Res. 1999;821(2):487–502. doi: 10.1016/s0006-8993(99)01067-7. [DOI] [PubMed] [Google Scholar]
- Greenstreet EH, Djamgoz MB. Nitric oxide induces light-adaptive morphological changes in retinal neurones. Neuroreport. 1994;6(1):109–112. doi: 10.1097/00001756-199412300-00029. [DOI] [PubMed] [Google Scholar]
- Hirooka K, Kourennyi D, Barnes S. Calcium channel activation facilitated by nitric oxide in retinal ganglion cells. J Neurophysiol. 2000;83(1):198–206. doi: 10.1152/jn.2000.83.1.198. [DOI] [PubMed] [Google Scholar]
- Hoffpauir B, McMains E, Gleason E. Nitric oxide transiently converts synaptic inhibition to excitation in retinal amacrine cells. J Neurophysiol. 2006;95(5):2866–2877. doi: 10.1152/jn.01317.2005. [DOI] [PubMed] [Google Scholar]
- Hofmann KP, Emeis D, Schnetkamp PP. Interplay between hydroxylamine, metarhodopsin II and GTP-binding protein in bovine photoreceptor membranes. Biochim Biophys Acta. 1983;725(1):60–70. doi: 10.1016/0005-2728(83)90224-4. [DOI] [PubMed] [Google Scholar]
- Holcman D, Korenbrot JI. The limit of photoreceptor sensitivity: molecular mechanisms of dark noise in retinal cones. J Gen Physiol. 2005;125(6):641–660. doi: 10.1085/jgp.200509277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai F, Sterling P. cGMP modulates spike responses of retinal ganglion cells via a cGMP-gated current. Vis Neurosci. 2002;19(3):373–380. [PubMed] [Google Scholar]
- Kiss JP, Zsilla G, Vizi ES. Inhibitory effect of nitric oxide on dopamine transporters: interneuronal communication without receptors. Neurochem Int. 2004;45(4):485–489. doi: 10.1016/j.neuint.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Kono T, Chisato N, Ebisawa Y, Asama T, Sugawara M, Ayabe T, Kohgo Y, Kasai S, Yoneda M, Takahashi T. Impaired nitric oxide production of the myenteric plexus in colitis detected by a new bioimaging system. J Surg Res. 2004;117(2):329–338. doi: 10.1016/j.jss.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Kourennyi D, Liu X, Hart J, Mahmud F, Baldridge W, Barnes S. Reciprocal modulation of calcium dynamics at rod and cone photoreceptor synapses by nitric oxide. J Neurophysiol. 2004;92(1):477–483. doi: 10.1152/jn.00606.2003. [DOI] [PubMed] [Google Scholar]
- Kurenny DE, Moroz LL, Turner RW, Sharkey KA, Barnes S. Modulation of ion channels in rod photoreceptors by nitric oxide. Neuron. 1994;13(2):315–324. doi: 10.1016/0896-6273(94)90349-2. [DOI] [PubMed] [Google Scholar]
- Leibrock CS, Lamb TD. Effect of hydroxylamine on photon-like events during dark adaptation in toad rod photoreceptors. J Physiol. 1997;501(Pt 1):97–109. doi: 10.1111/j.1469-7793.1997.00097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy H, Twig G, Perlman I. Nitric oxide modulates the transfer function between cones and horizontal cells during changing conditions of ambient illumination. Eur J Neurosci. 2004;20(11):2963–2974. doi: 10.1111/j.1460-9568.2004.03758.x. [DOI] [PubMed] [Google Scholar]
- Li Z, Hatton GI. Histamine suppresses non-NMDA excitatory synaptic currents in rat supraoptic nucleus neurons. J Neurophysiol. 2000;83(5):2616–2625. doi: 10.1152/jn.2000.83.5.2616. [DOI] [PubMed] [Google Scholar]
- Liaw WJ, Stephens RL, Jr, Binns BC, Chu Y, Sepkuty JP, Johns RA, Rothstein JD, Tao YX. Spinal glutamate uptake is critical for maintaining normal sensory transmission in rat spinal cord. Pain. 2005;115(1–2):60–70. doi: 10.1016/j.pain.2005.02.006. [DOI] [PubMed] [Google Scholar]
- McMahon D, Ponomareva L. Nitric oxide and cGMP modulate retinal glutamate receptors. J Neurophysiol. 1996;76(4):2307–2315. doi: 10.1152/jn.1996.76.4.2307. [DOI] [PubMed] [Google Scholar]
- McMahon D, Schmidt K. Horizontal cell glutamate receptor modulation by NO: mechanisms and functional implications for the first visual synapse. Vis Neurosci. 1999;16(3):425–433. doi: 10.1017/s0952523899163041. [DOI] [PubMed] [Google Scholar]
- Mills SL, Massey SC. Differential properties of two gap junctional pathways made by AII amacrine cells. Nature. 1995;377(6551):734–737. doi: 10.1038/377734a0. [DOI] [PubMed] [Google Scholar]
- Miyachi E, Murakami M, Nakaki T. Arginine blocks gap junctions between retinal horizontal cells. Neuroreport. 1990;1(2):107–110. doi: 10.1097/00001756-199010000-00006. [DOI] [PubMed] [Google Scholar]
- Naka KI, Rushton WA. S-potentials from luminosity units in the retina of fish (Cyprinidae) J Physiol. 1966;185(3):587–599. doi: 10.1113/jphysiol.1966.sp008003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawy S. Desensitization of the mGluR6 transduction current in tiger salamander On bipolar cells. J Physiol. 2004;558(Pt 1):137–146. doi: 10.1113/jphysiol.2004.064980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawy S, Jahr CE. Suppression by glutamate of cGMP-activated conductance in retinal bipolar cells. Nature. 1990;346(6281):269–271. doi: 10.1038/346269a0. [DOI] [PubMed] [Google Scholar]
- Nawy S, Jahr CE. cGMP-gated conductance in retinal bipolar cells is suppressed by the photoreceptor transmitter. Neuron. 1991;7(4):677–683. doi: 10.1016/0896-6273(91)90380-i. [DOI] [PubMed] [Google Scholar]
- Neal M, Cunningham J, Matthews K. Nitric oxide enhancement of cholinergic amacrine activity by inhibition of glycine release. Invest Ophthalmol Vis Sci. 1997;38(8):1634–1639. [PubMed] [Google Scholar]
- Neal M, Cunningham J, Matthews K. Selective release of nitric oxide from retinal amacrine and bipolar cells. Invest Ophthalmol Vis Sci. 1998;39(5):850–853. [PubMed] [Google Scholar]
- Nemargut J, Zhu J, Savoie B, Wang G. Differential effects of charybdotoxin on the activity of retinal ganglion cells in the dark- and light-adapted mouse retina. Vision Res. 2009;49(3):388–397. doi: 10.1016/j.visres.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer R, Rees D, Ashton D, Moncada S. L-arginine is the physiological precursor for the formation of nitric oxide in endothelium-dependent relaxation. Biochem Biophys Res Commun. 1988;153(3):1251–1256. doi: 10.1016/s0006-291x(88)81362-7. [DOI] [PubMed] [Google Scholar]
- Pelli D. The VideoToolbox software for visual psychophysics: transforming numbers into movies. Spat Vis. 1997;10(4):437–442. [PubMed] [Google Scholar]
- Prast H, Philippu A. Nitric oxide as modulator of neuronal function. Prog Neurobiol. 2001;64(1):51–68. doi: 10.1016/s0301-0082(00)00044-7. [DOI] [PubMed] [Google Scholar]
- Rieke F, Baylor DA. Origin and functional impact of dark noise in retinal cones. Neuron. 2000;26(1):181–186. doi: 10.1016/s0896-6273(00)81148-4. [DOI] [PubMed] [Google Scholar]
- Rieke F, Schwartz EA. A cGMP-gated current can control exocytosis at cone synapses. Neuron. 1994;13(4):863–873. doi: 10.1016/0896-6273(94)90252-6. [DOI] [PubMed] [Google Scholar]
- Robinson DW, Chalupa LM. The intrinsic temporal properties of alpha and beta retinal ganglion cells are equivalent. Curr Biol. 1997;7(6):366–374. doi: 10.1016/s0960-9822(06)00184-9. [DOI] [PubMed] [Google Scholar]
- Sampath AP, Baylor DA. Molecular mechanism of spontaneous pigment activation in retinal cones. Biophys J. 2002;83(1):184–193. doi: 10.1016/S0006-3495(02)75160-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savchenko A, Barnes S, Kramer RH. Cyclic-nucleotide-gated channels mediate synaptic feedback by nitric oxide. Nature. 1997;390(6661):694–698. doi: 10.1038/37803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekaran S, Cunningham J, Neal MJ, Hartell NA, Djamgoz MB. Nitric oxide release is induced by dopamine during illumination of the carp retina: serial neurochemical control of light adaptation. Eur J Neurosci. 2005;21(8):2199–2208. doi: 10.1111/j.1460-9568.2005.04051.x. [DOI] [PubMed] [Google Scholar]
- Shen W, Jiang Z. Characterization of glycinergic synapses in vertebrate retinas. J Biomed Sci. 2007;14(1):5–13. doi: 10.1007/s11373-006-9118-2. [DOI] [PubMed] [Google Scholar]
- Shiells R, Falk G. Retinal on-bipolar cells contain a nitric oxide-sensitive guanylate cyclase. Neuroreport. 1992;3(10):845–848. doi: 10.1097/00001756-199210000-00006. [DOI] [PubMed] [Google Scholar]
- Shiells RA. Ca(2+)-induced light adaptation in retinal ON-bipolar cells. Keio J Med. 1999;48(3):140–146. doi: 10.2302/kjm.48.140. [DOI] [PubMed] [Google Scholar]
- Shiells RA, Falk G. Glutamate receptors of rod bipolar cells are linked to a cyclic GMP cascade via a G-protein. Proc Biol Sci. 1990;242(1304):91–94. doi: 10.1098/rspb.1990.0109. [DOI] [PubMed] [Google Scholar]
- Snellman J, Kaur T, Shen Y, Nawy S. Regulation of ON bipolar cell activity. Prog Retin Eye Res. 2008;27(4):450–463. doi: 10.1016/j.preteyeres.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snellman J, Nawy S. cGMP-dependent kinase regulates response sensitivity of the mouse on bipolar cell. J Neurosci. 2004;24(29):6621–6628. doi: 10.1523/JNEUROSCI.1474-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snellman J, Zenisek D, Nawy S. Switching between transient and sustained signaling at the rod bipolar-AII amacrine cell synapse of the mouse retina. J Physiol. 2009 doi: 10.1113/jphysiol.2008.165241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterling P, Demb J. Retina. In: Shepherd G, editor. Synaptic Organization of the Brain. New York: Oxford University Press; 2004. pp. 163–169. [Google Scholar]
- Sun W, Li N, He S. Large-scale morphological survey of mouse retinal ganglion cells. J Comp Neurol. 2002;451(2):115–126. doi: 10.1002/cne.10323. [DOI] [PubMed] [Google Scholar]
- Thibos LN, Werblin FS. The response properties of the steady antagonistic surround in the mudpuppy retina. J Physiol. 1978;278:79–99. doi: 10.1113/jphysiol.1978.sp012294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Liets L, Chalupa L. Nitric oxide differentially modulates ON and OFF responses of retinal ganglion cells. J Neurophysiol. 2003;90(2):1304–1313. doi: 10.1152/jn.00243.2003. [DOI] [PubMed] [Google Scholar]
- Wang GY, van der List DA, Nemargut JP, Coombs JL, Chalupa LM. The sensitivity of light-evoked responses of retinal ganglion cells is decreased in nitric oxide synthase gene knockout mice. J Vis. 2007;7(14):7.1–13. doi: 10.1167/7.14.7. [DOI] [PubMed] [Google Scholar]
- Wang P, Slaughter MM. Effects of GABA receptor antagonists on retinal glycine receptors and on homomeric glycine receptor alpha subunits. J Neurophysiol. 2005;93(6):3120–3126. doi: 10.1152/jn.01228.2004. [DOI] [PubMed] [Google Scholar]
- Yamazaki A, Yamazaki M, Yamazaki RK, Usukura J. Illuminated rhodopsin is required for strong activation of retinal guanylate cyclase by guanylate cyclase-activating proteins. Biochemistry. 2006;45(6):1899–1909. doi: 10.1021/bi0519396. [DOI] [PubMed] [Google Scholar]
- Yu D, Eldred W. GABA(A) and GABA(C) receptor antagonists increase retinal cyclic GMP levels through nitric oxide synthase. Vis Neurosci. 2003;20(6):627–637. doi: 10.1017/s0952523803206052. [DOI] [PubMed] [Google Scholar]
- Yu D, Eldred W. Nitric oxide stimulates gamma-aminobutyric acid release and inhibits glycine release in retina. J Comp Neurol. 2005a;483(3):278–291. doi: 10.1002/cne.20416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu D, Eldred WD. Gycine and GABA interact to regulate the nitric oxide/cGMP signaling pathway in the turtle retina. Vis Neurosci. 2005b;22(6):825–838. doi: 10.1017/S0952523805226123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemel E, Eyal O, Lei B, Perlman I. NADPH diaphorase activity in mammalian retinas is modulated by the state of visual adaptation. Vis Neurosci. 1996;13(5):863–871. doi: 10.1017/s0952523800009111. [DOI] [PubMed] [Google Scholar]