Summary
The importance of T helper type 1(Th1) immunity in host resistance to the intracellular bacterium Francisella tularensis is well established. However, the relative roles of Interleukin (IL)-12/Th1 and IL-23/T helper type 17(Th17) responses in immunity to F.tularensis have not been studied. The IL-23/Th17 pathway is critical for protective immunity against extracellular bacterial infections. In contrast, the IL-23/Th17 pathway is dispensable for protection against intracellular pathogens such as Mycobacteria. Our data show that the IL-23/Th17 pathway regulates the IL-12/Th1 pathway and is required for protective immunity against F.tularensis Live Vaccine Strain (LVS). We show that IL-17, but not IL-17F or IL-22 induces IL-12 production in dendritic cells and mediates Th1 responses. Furthermore, we show that IL-17 also induces IL-12 and IFNγ production in macrophages and mediates bacterial killing. Together, these findings illustrate a novel biological function for IL-17 in regulating IL-12/Th1 immunity and host responses to an intracellular pathogen.
Keywords: IL-23, IL-17, lung, tularemia, intracellular pathogens
Introduction
Francisella tularensis, the causative agent of tularemia is a facultative intracellular bacterium, classified by the US Center for Disease Control as a Category A agent of bioterrorism (Dennis et al., 2001). Routes of infection include contact, ingestion or inhalation, with inhalation considered the most likely route of bioterrorism due to the severe disease caused by low doses of airborne bacteria (<10 colony forming units, CFU)(Dennis et al., 2001). A F.tularensis Live Vaccine Strain (LVS) has been developed from a F. tularensis B strain and has potential for use as a vaccine, but is currently not licensed for use in humans. LVS causes disease in mice that is similar to human tularemia and is used to study the immune components required for protection against tularemia (Duckett et al., 2005).
The importance of Interferon gamma (IFNγ) and T helper type 1 (Th1) responses in immunity to F.tularensis infection is well established (Anthony et al., 1989; Duckett et al., 2005). IL-12, made up of IL-12p35 and IL-12p40 subunits is critical for the induction of IFNγ production from T and Natural Killer (NK) T cells (Trinchieri et al., 2003). IL-12p35−/− mice are susceptible to respiratory LVS infection and exogenous IL-12 treatment increases survival (Duckett et al., 2005). IL-23 is also comprised of the p40 subunit, and p19, a four-α helix molecule (Oppmann et al., 2000) and is required for maintenance of T helper type 17 (Th17) responses (Dong, 2006). Th17 cells produce IL-17A (IL-17) and the related cytokine IL-17F, and IL-21 and IL-22 (Ouyang et al., 2008) and are involved in the induction of inflammation and tissue destruction associated with models of autoimmune diseases (Langrish et al., 2005). Conversely, IL-23-dependent IL-17 responses are important for protective immunity against extracellular bacterial infections for optimal induction of chemokines, recruitment of neutrophils and bacterial killing (Happel et al., 2005; Ye et al., 2001). However, Th17 cytokines appear to be dispensable for protection against most intracellular infections examined so far. For example, mice deficient in the IL-23/Th17 pathway are resistant to Mycobacteria (Khader et al., 2005; Umemura et al., 2007; Aujla et al., 2008;), Listeria (Aujla et al., 2008) and Salmonella infections (Schulz et al., 2008). In vitro infection of human monocytes with the virulent strain of F. tularensis SCHU S4 induces IL-23 (Butchar et al., 2007). Further, pulmonary infection with LVS induces Th17 cells in the lung (Woolard et al., 2008), suggesting that Th17 cells may play a role in immunity to F.tularensis.
In light of these data, the goal of this study was to define the relative roles of IL-12/Th1 and IL-23/Th17 pathways in immunity to pulmonary LVS infection. Our data provide evidence that, unlike immunity to other intracellular pathogen infections (Khader et al., 2005; Aujla et al., 2008), the IL-23/Th17 pathway plays a unique role in protection against LVS pulmonary infection. We show that IL-17, but not IL-17F or IL-22, can induce IL-12 production in DCs and mediate protective Th1 responses. Further, we also show that IL-17 induces IL-12 and IFNγ in macrophages and enhances clearance of LVS. Our data therefore suggest that the IL-23/Th17 pathway is critical for optimal induction of Th1 responses and protection against LVS.
Results
IL-12p40 cytokine members are induced in the lung in response to LVS infection
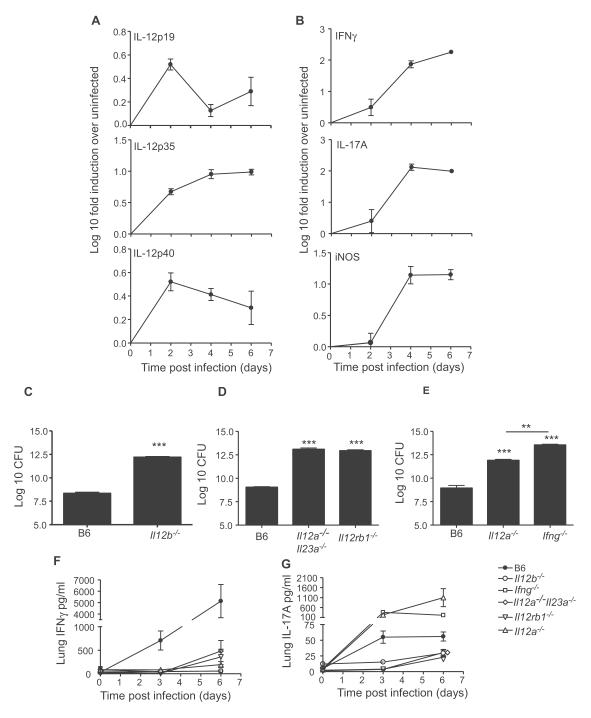
To determine whether IL-12p40 cytokines were induced in the lung during pulmonary tularemia, we infected wild type C57BL/6 (B6) mice intratracheally with LVS and found that IL-23p19 mRNA was induced at day 2, with lower induction between days 4-6 (Figure 1A). However, induction of IL-12p35 and IL-12p40 mRNA was detected at day 2 and maintained through days 4-6 (Figure 1A). Intracellular killing of LVS by activated macrophages is dependent on IFNγ-dependent inducible nitric oxide synthase (iNOS) (Lindgren et al., 2005). We found induction of IFNγ and IL-17 mRNA between days 2-4, and coincided with induction of iNOS mRNA (Figure 1B). These data indicate that LVS induces IL-12p40, IL-12 and IL-23 in the infected lung and implicate both the IL-23/Th17 and IL-12/Th1 pathway in host responses to LVS.
Figure 1. IL-12 dependent IFN-γ is critical for protection following pulmonary tularemia.
mRNA induction for IL-23p19, IL-12p35 and IL-12p40 (A) and IFNγ, IL-17 and iNOS (B) in LVS infected B6 lungs vs. uninfected lungs was determined by RT-PCR. B6 mice and IL-12p40−/− (C), B6, IL-12p35−/−IL-23p19−/−, IL-12RB1−/− (D) and B6, IL-12p35−/− and IFNγ−/− (E) were infected with LVS and the lung CFU determined and lung homogenates were assayed for IFNγ (F) or IL-17 (G) levels. Data points represent mean (±SD) from 4-5 mice (A-G). **, p ≤ 0.005, ***, p ≤0.0005. 1 experiment representative of 2 or more.
IL-12-dependent IFN-γ is critical for protection following pulmonary infection with LVS
To determine whether there was a protective role for IL-12p40-dependent cytokines in control of LVS infection, we infected B6 mice, mice lacking all IL-12p40 cytokines (IL-12p40−/−) (Figure 1C), IL-12RB1−/− mice, and mice lacking both IL-12p35 and IL-23p19 subunits (Figure 1D) with LVS and determined CFU in infected lungs. IL-12p40−/−, IL-12RB1−/− and IL-12p35−/− IL-23p19−/− mice had significantly higher CFU when compared to B6 mice (Figure 1C, D) and did not survive past day 8, whereas all B6 infected mice survived (Figure S1 and data not shown). Further, we found that both IFNγ−/− and IL-12p35−/− mice also had significantly higher lung CFU compared to B6 mice (Figure 1E). Interestingly, all IFNγ−/− mice did not survive past day 6, while IL-12p35−/− mice survived until day 8 post infection (Figure S1). Also IL-12p35−/− mice had significantly lower lung CFU when compared to IFNγ−/− mice, suggesting other IL-12p40-dependent IL-12-independent mechanisms of protection. IFNγ protein was induced in infected B6 lungs, but the absence of IL-12p40, IL-12RB1 and IL-12/IL-23 cytokines resulted in a profound defect in the induction of lung IFNγ levels (Figure 1F). IL-12p35−/− mice displayed a similar reduction in IFNγ production, indicating that of the IL-12p40 cytokine members, IL-12 is critical for generation of Th1 immunity during LVS infection. Further, MHC Class II expression on lung CD11c+ cells was up regulated in B6 infected mice, but was significantly ablated in gene-deficient infected lungs (Figure S2A,B). Also, absence of IFNγ ablated the induction of inflammatory molecules including TNF-α, IL-1α, IL-β and MIP-1α in infected IFNγ−/− lungs (Figure S2C). These data demonstrate that IL-12 is required for induction of IFNγ responses and that IFNγ responses coincide with activation of lung CD11c+ cells and control of pulmonary LVS infection.
IL-17 is induced in the lungs of LVS-infected mice and is negatively regulated by IFNγ
Since IL-17 mRNA (Figure 1B) and IL-17 protein was induced in infected B6 lungs (Figure 1G), we determined if this was dependent on IL-12p40 cytokines. As expected, the IL-17 levels were ablated in the absence of IL-12p40 and IL-12RB1, and significantly higher in the absence of IL-12 and IFNγ (Figure 1G). Further, the major producers of IL-17 in the lungs of LVS-infected mice were antigen-dependent (Figure S3A). However, we also detected IL-17-producing cells in the wells that did not receive antigen (Figure S3A), suggesting other cellular sources, such as gamma delta (γδ) T cells (Umemura et al., 2007) and NK T cells (Lee et al., 2008), as likely producers of IL-17. Although IL-12p40 and IL-12RB1 were required for the induction of IL-17-producing cells in infected mice, there was no significant increase in the total number of IL-17-producing cells detected in the IFNγ−/− and IL-12−/− infected lungs when compared to B6 lungs (Figure S3A). These data suggest that the higher levels of lung IL-17 protein in IFNγ−/− and IL-12p35−/− mice (Figure 1G) were likely due to a higher production of IL-17 on a per cell basis.
IL-17 is known to induce chemokines such as Granulocyte stimulating factor (G-CSF) and Granulocyte-Macrophage stimulating factor (GM-CSF)(Ouyang et al., 2008), and we found that G-CSF, GM-CSF, Keratinocyte chemoattractant (KC) and Monocyte Chemoattractant Protein (MCP)-1 levels were all significantly higher in IFNγ−/− infected lungs when compared to B6 mice (Figure S3B). These data demonstrate that IL-17 is induced in the lung following LVS infection and that IFNγ negatively regulates IL-17 responses during pulmonary tularemia. However, high levels of IL-17 in the absence of Th1 immunity fails to confer protection against LVS infection.
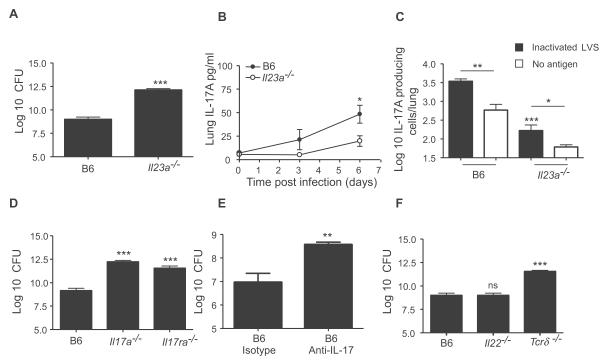
IL-23 dependent IL-17 is required for protection against pulmonary tularemia
The IL-23/IL-17 axis is dispensable for protection against studied intracellular pathogens (Khader et al., 2005; Umemura et al., 2007; Aujla et al., 2008). Interestingly, when challenged with LVS, the IL-23p19−/− mice had significantly higher lung CFU (Figure 2A), which correlated with decreased lung IL-17 levels (Figure 2B) and number of lung IL-17-producing cells (Figure 2C). Further, both IL-17−/− and IL-17R−/− mice also had significantly higher lung CFU compared to B6 mice (Figure 2D), and did not survive past day 10 post infection (Figure S1). In addition, neutralization of IL-17 in LVS–infected B6 mice also increased lung CFU when compared to isotype control-treated B6 mice (Figure 2E).
Figure 2. IL-23 dependent IL-17 is required for protection against pulmonary tularemia.
B6 and IL-23p19−/− were infected with LVS and the lung CFU determined (A). Lung IL-17 levels (B) and the number of lung IL-17 producing cells from day 4-infected B6 and IL-23p19−/− mice was determined (C). B6, IL-17−/, IL-17R−/− were infected with LVS and lung CFU determined (D). LVS infected-B6 mice were treated with either control or anti-IL-17 neutralizing antibody and lung CFU determined (E). B6, IL-22−/− and γδ−/− mice (F) were infected with LVS and lung CFU determined. Data points represent mean (±SD) from 4-5 mice (A-F). *, p ≤0.05. **, p ≤0.005, ***, p ≤0.0005. ns-not significant. 1 experiment representative of 2 or more.
IL-22, another cytokine produced by Th17 cells, induces production of anti-microbial peptides for host defense against extracellular bacteria (Aujla et al., 2008; Zheng et al., 2008). Surprisingly, IL-22−/− mice were not more susceptible than B6 mice and had comparable lung CFU to B6 mice (Figure 2F). γδ T lymphocytes are one of the major producers of IL-17 in response to intracellular pathogen infections (Umemura et al., 2007). Following infection with LVS, γδ−/− mice had significantly higher CFU (Figure 2F), and decreased induction of lung IL-17 when compared to B6 mice (B6 mice 55.8+/−14.5, γδ −/− mice 35.8+/− 7.9, p value= 0.050). These data demonstrate that IL-17 but not IL-22, is critical for protection against pulmonary tularemia and that both conventional T cells, and γδ T cells are likely cellular sources of IL-17.
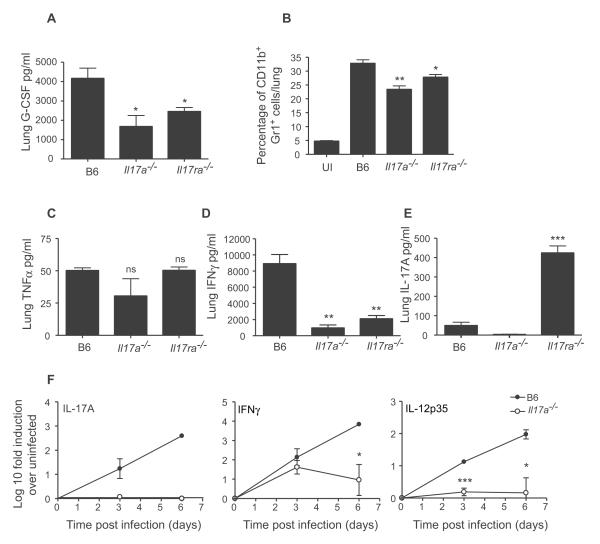
IL-17 is required for induction of IFNγ responses during pulmonary tularemia
A role for IL-17 in the induction of G-CSF and recruitment of neutrophils is well documented (Ye et al., 2001). Accordingly, G-CSF levels were significantly decreased in IL-17−/− and IL-17R−/− infected lungs (Figure 3A) which correlated with a decrease in the percentage of neutrophils (Figure 3B, S4A) and cellular infiltrates (Figure S4B,C). Nonetheless, IL-17−/− and IL-17R−/− mice still recruited significant percentages of neutrophils to the lungs when compared to uninfected lungs (Figure 3B). Therefore, we next determined whether the absence of IL-17 or IL-17R impacted induction of protective molecules such as TNFα (Leiby et al., 1992) and IFNγ (Duckett et al., 2005) in infected lungs. Although induction of TNFα was comparable (Figure 3C), IFNγ levels were significantly reduced in the lungs of infected IL-17−/− and IL-17R−/− mice (Figure 3D). As expected, IL-17 levels were elevated in the IL-17R−/− infected lungs (Smith et al., 2008) (Figure 3E). Although IFNγ and IL-12p35 mRNA are induced in the infected B6 lungs, significantly reduced induction of these transcripts was detected in the infected IL-17−/− lungs (Figure 3F). These data suggest that following LVS infection, IL-17 is important for the induction of G-CSF, recruitment of neutrophils to the lung, and generation of protective IL-12-dependent IFNγ responses.
Figure 3. IL-17 is required for induction of IFNγ responses during pulmonary tularemia.
B6, IL-17−/− and IL-17R−/− mice were infected with LVS and lung G-CSF levels determined (A). Percentage of lung neutrophils in uninfected (UI) or infected B6, IL-17−/− and IL-17R−/− mice was determined (B). Lung homogenates from day 6-infected B6, IL-17−/− and IL-17R−/− mice were assayed for TNF-α (C), IFNγ (D) and IL-17 (E). The induction of specific mRNA was determined by RT-PCR in infected B6 and IL-17−/− lungs (F). *, p ≤0.05. **, p ≤0.005. ***, p ≤0.0005. 1 experiment representative of 2 or more.
IL-17 but not IL-17F or IL-22 induces IL-12 from DCs
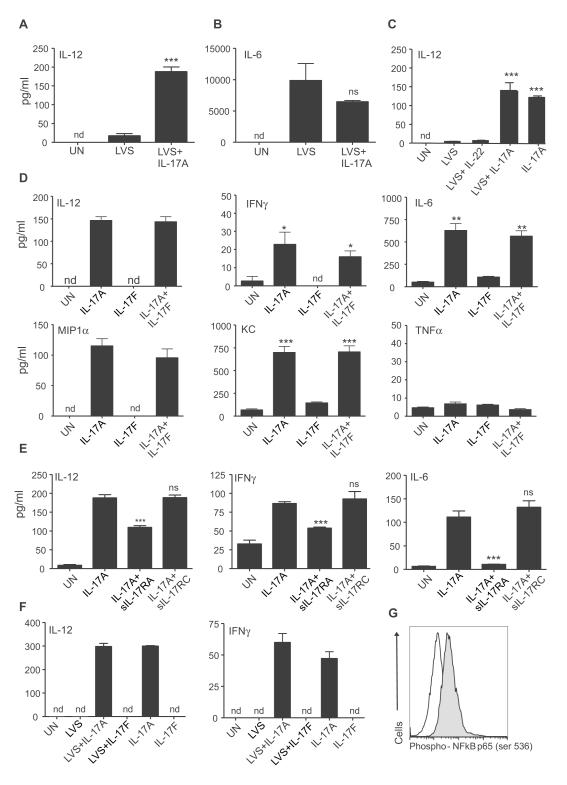
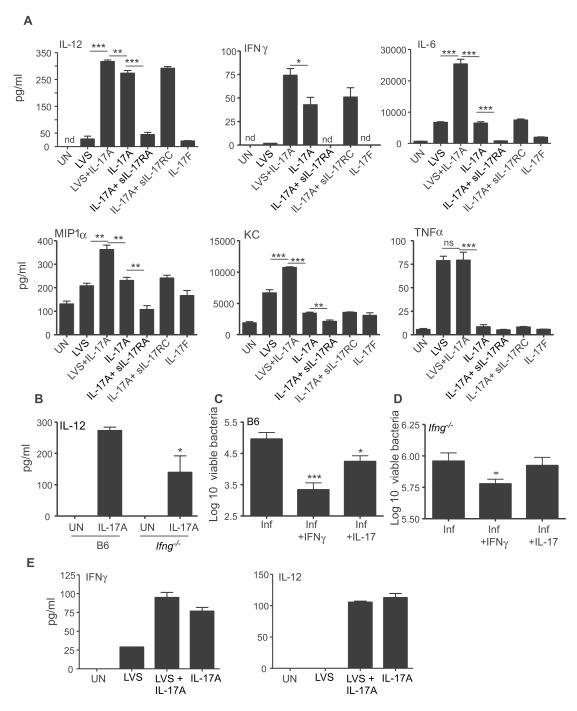
Our in vivo data suggests that IL-17 is required for induction of IL-12 and IFNγ responses following pulmonary LVS infection. DCs are one of the likely producers of IL-12 following stimulation with LVS (Hong et al., 2007). Therefore, we stimulated Bone Marrow derived Dendritic Cells (BMDCs) with LVS alone, or with IL-17, and measured IL-12 levels in supernatants. IL-17 treatment of LVS-stimulated BMDCs resulted in 10-fold induction of IL-12 in comparison to LVS alone treatment (Figure 4A). However, IL-17 treatment did not impact production of other Th17 polarizing cytokines such as IL-6 by LVS-stimulated BMDCs (Figure 4B), suggesting that LVS by itself was a strong inducer of IL-6. Interestingly, IL-17 treatment alone induced IL-12 production (Figure 4C) and up regulated reporter IL-12p40 expression in BMDCs generated from yet40 mice (Reinhardt et al., 2006) (Figure S5A). In contrast, IL-22 treatment did not impact IL-12 production by LVS-stimulated BMDCs (Figure 4C).
Figure 4. IL-17 but not IL-17F or IL-22 can induce IL-12 from BMDCs.
B6 BMDCs were left untreated (UN), stimulated with LVS alone or with IL-17 (100 ng/ml) and supernatants assayed for IL-12 (A) or IL-6 (B). B6 BMDCs were treated with LVS alone or with IL-22 or IL-17 (100 ng/ml), or IL-17 (100 ng/ml) alone and IL-12 levels determined (C). B6 BMDCs were left untreated or treated with IL-17 (100 ng/ml), IL-17F (100 ng/ml), or both IL-17 and IL-17F (100 ng/ml each) and cytokine levels determined in supernatants (D). B6 BMDCs were left untreated or treated with IL-17 (100 ng/ml) alone or with soluble IL-17RA or IL-17RC (1000 ng/ml each) and cytokine levels determined in supernatants (E). Lung CD11c+ cells from B6 mice were left untreated or treated with LVS alone or with IL-17(100 ng/ml) or IL-17F (100 ng/ml). IL-17 and IL-17F alone treated cultures were also included and supernatants assayed for IL-12 and IFNγ levels (F). BMDCs were left untreated (white histogram) or treated with IL-17 (grey histogram) and expression of Phospho-NF-κβ p65 determined. Samples were treated in triplicates (A-G). nd-not detectable, *, p ≤0.05. **, p ≤0.005. ***, p ≤0.0005, 1 experiment representative of 2 or more.
IL-17 and IL-17F share many biological activities, but also have unique biological functions (Chang and Dong, 2009), and we found that, unlike the addition of IL-17, the addition of IL-17F to BMDCs did not induce IL-12 production (Figure 4D). Moreover, IL-17 but not IL-17F induced the cytokines, IFNγ and IL-6, as well as the chemokines, MIP-1α and KC in BMDCs, but did not induce (Figure 4D). Furthermore, IL-17 induced IL-12 and IFNγ in culture supernatants within one hour of treatment (Figure S5B). Thus, IL-17 rather than IL-17F, is the primary mediator of IL-12 and IFNγ expression in DCs.
IL-17R comprised of IL-17RA subunit was initially identified as the receptor for IL-17 (Yao et al., 1995), but recently IL-17RA has been shown to partner with IL-17RC (Toy et al., 2006). Co-treatment of BMDCs with soluble IL-17RA but not IL-17RC significantly reduced the ability of IL-17 to induce IL-12, IFNγ and IL-6 (Figure 4E), as well as chemokines (data not shown). Therefore our data suggests that IL-17, but not IL-17F or IL-22 can induce the production of IL-12 and IFNγ in BMDCs and that this induction is independent of pathogen stimulation but is dependent on IL-17RA. Induction of IL-12 in macrophages is dependent on IFNγ (Flesch et al., 1995; Skeen et al., 1996), but DCs may not be subject to similar regulatory effects (Ma et al., 1997; Schulz et al., 2000). In support of this, we found that the absence of IFNγ did not impact the ability of IL-17 to induce IL-12 (Figure S5C). Further, we also found that sorted lung CD11c+ cells from B6 mice co-treated with LVS and IL-17, but not IL-17F, induced IL-12 and IFNγ (Figure 4F). Also, IL-17 induced IL-12 and IFNγ in the absence of pathogen stimulation in lung CD11c+ cells (Figure 4F).
IL-17 activates NF-κB, a transcription factor involved with inflammation (Yao et al., 1995) and we show that BMDCs treated with IL-17 resulted in activation of NF-κB (Figure 4G). Interestingly, addition of a pharmacological inhibitor of NF-κB, pyrrolidine derivative of dithiocarbamate (PTDC), during IL-17 treatment of BMDCs resulted in significant reduction in IFNγ and IL-6 but did not impact the induction of IL-12 levels (Figure S5). These data suggest that IL-17 treatment activates NF-κB in BMDCs, and that some, but not all downstream events are mediated via NF-κB signaling.
IL-17 polarizes IFNγ-producing T cells during LVS infection
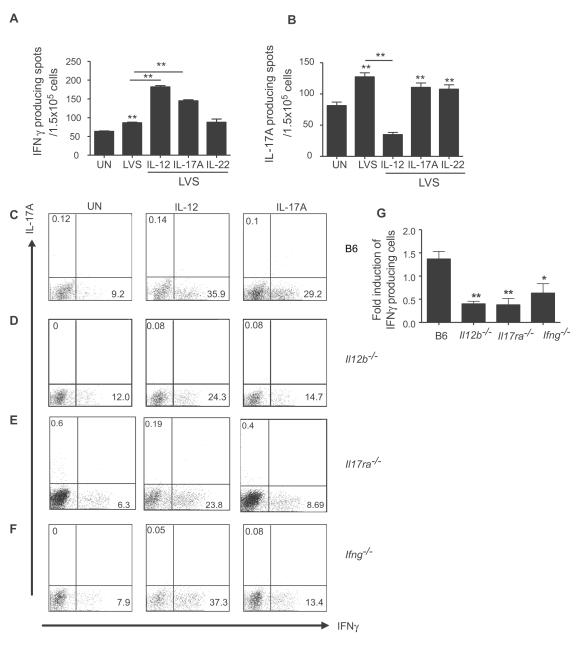
To address whether exogenous IL-17 could impact polarization of naïve T cells into IFNγ-producing cells, we cultured BMDCs with LVS and either IL-17, IL-12 or IL-22 and determined the ability of naïve CD4+ OT-II TCR Transgenic (Tg) T cells to differentiate into IFNγ or IL-17-producing T cells. LVS-stimulated BMDCs significantly increased the number of IFNγ and IL-17 producing cells when compared to uninfected BMDC cultures (Figure 5A). As expected, addition of IL-12 to LVS-stimulated BMDC cultures significantly increased IFNγ-producing cells and reduced IL-17-producing cells when compared to LVS alone treated cultures. Importantly, addition of IL-17, but not IL-22, to the LVS-stimulated BMDCs significantly increased IFNγ-producing cells (Figure 5A), when compared to LVS alone treated cultures. However, neither IL-17 nor IL-22 influenced the generation of IL-17 producing cells relative to LVS alone stimulated cultures. These data demonstrate that IL-17 can impact Th1 immunity during intracellular infections by priming of naïve CD4 T cells into IFNγ-producing cells.
Figure 5. IL-17 can induce the polarization of naïve T cells into IFNγ-producing T cells.
B6 BMDCs were stimulated with LVS alone or with IL-12 (100 ng/ml), IL-22 (100 ng/ml) or IL-17 (100 ng/ml) and naive OT-II TCR-Tg CD4+ T cells. The frequency of IFNγ (A) and IL-17 producing cells (B) were determined by ELISpot assay. BMDCs generated from B6 mice (C), IL-12p40−/− (D), IL-17R−/−(E) or IFNγ−/− (F) were cultured with naïve OT-II TCR Tg CD4+ T cells alone or with IL-12 (100 ng/ml) or IL-17(100 ng/ml) in triplicates and IL-17 and IFNγ levels determined by flow cytometry; fold induction relative to untreated control group was determined (G). *, p ≤0.05. **, p ≤0.005. ***, p ≤0.0005.
To address whether IL-17 could induce Th1 responses in a pathogen–free system, we cultured naïve OT-II Tg CD4+ cells with BMDCs, antigen and IL-17 or IL-12. Addition of IL-12 to B6 BMDCs resulted in a significant increase in IFNγ-producing cells compared to untreated wells (Figure 5C). Importantly, we show that IL-17 treatment also significantly increased the number of IFNγ–producing T cells when compared to untreated cultures (Figure 5C). We then addressed whether differentiation of IFNγ-producing cells in cultures treated with IL-17 was mediated via DC-expressed IL-12 and IL-17R. As expected, exogenous IL-12 resulted in significant induction of IFNγ-producing T cells even when IL-12p40−/− or IL-17R−/− BMDCs were used as APCs (Figure 5D, E). Importantly, addition of IL-17 to IL-12p40−/− or IL-17R−/− BMDCs resulted in significantly lower induction of IFNγ–producing cells when compared to B6 cultures (Figure 5D,E,G). Although the absence of IFNγ in BMDCs did not impact the IL-17-dependent IL-12 induction in BMDC cultures (Figure S5C), we found that IFNγ−/− BMDCs co-cultured with naïve CD4+ OT-II Tg cells and IL-17, resulted in lower induction of IFN-γ-producing cells when compared to B6 cultures (Figure 5F,G). These data demonstrate that IL-17 mediates the induction of Th1 immunity by priming of naïve CD4 T cells into IFNγ-producing cells and that APC-expressed IL-12, IL-17R and IFNγ, are required for optimal induction of IFN-γ producing cells.
IL-17 induces IL-12 and IFNγ in macrophages and enhances bacterial clearance
IL-17 was recently shown to induce IL-12 in peritoneal macrophages (Ishigame et al., 2009). We found that LVS treatment induced low levels of IL-12 in bone marrow derived macrophages(BMDMs)(Figure 6A), but addition of IL-17 to LVS-stimulated BMDMs resulted in induction of 10-fold more IL-12 compared to LVS alone-stimulated cultures (Figure 6A). Furthermore, IL-17 treatment in the absence of pathogen could also induce IL-12 production in BMDM supernatants (Figure 6A). In addition, IL-17 could also induce IFNγ and chemokines MIP-1α and KC (Figure 6A), but not TNF-α in BMDMs (Figure 6A). In contrast to the effect of IL-17 on BMDCs, co-treatment with both IL-17 and LVS resulted in synergistic induction of IL-12, IFNγ, IL-6, MIP-1α and KC in BMDMs. Further, addition of soluble IL-17RA but not IL-17RC, resulted in significant inhibition of cytokine and chemokine production (Figure 6A), suggesting that IL-17-mediated induction of these molecules in macrophages is also mediated by IL-17RA.
Figure 6. IL-17 induces IFNγ and IL-12 from macrophages and enhances bacterial clearance.
BMDMs from B6 mice were either left untreated (UN) or treated with LVS alone, IL-17 (100 ng/ml) alone, IL-17F alone (100 ng/ml) or LVS and IL-17 (100 ng/ml). Some wells received IL-17(100 ng/ml) and soluble IL-17RA or IL-17RC (1000ng/ml each) and protein levels in supernatants determined (A). BMDMs from B6 or IFNγ−/− mice were left untreated or treated with IL-17 (100 ng/ml) and IL-12 levels determined (B). BMDMs from B6 mice (C) or IFNγ−/− (D) were infected with live LVS alone, or with IFN-γ (100 ng/ml) or IL-17 (100 ng/ml) for 24 hours and intracellular CFU determined. Lung alveolar macrophages were treated with LVS alone or with IL-17, IL-17 (100 ng/ml) alone or IL-17F alone (100 ng/ml) and cytokine levels determined (E). Samples were treated in triplicates (A-E). nd-not detectable, *, p ≤0.05. **, p ≤0.005. ***, p ≤0.0005.
IFNγ is documented to enhance the ability of macrophages to produce IL-12 (Flesch et al., 1995; Skeen et al., 1996). In support of this, we found that IFNγ−/− BMDMs induced significantly lower levels of IL-12 following IL-17 treatment (Figure 6B). Since IL-17 induces IFNγ production in BMDMs, we next determined whether addition of IL-17 to BMDMs infected with live LVS would impact intracellular bacterial clearance. As expected, addition of IFNγ to LVS-infected BMDM cultures resulted in enhanced bacterial clearance (Figure 6C). Importantly, addition of IL-17 to LVS-infected BMDMs also resulted in enhanced bacterial clearance when compared to untreated LVS-infected BMDM cultures (Figure 6C) and this response was abrogated when IFNγ−/− BMDMs were used (Figure 6D). These data demonstrate that IL-17 can induce IFNγ and IL-12 production in macrophages and enhance bacterial clearance. Alveolar macrophages are amongst the first innate cells that are infected following LVS pulmonary infection (Hall et al., 2008). Co-treatment of LVS-stimulated alveolar macrophages with IL-17, but not IL-17F, resulted in induction of IL-12 and IFNγ in supernatants when compared to LVS-alone treatment (Figure 6E). These data demonstrate that in addition to lung CD11C+ cells, lung alveolar macrophages can also induce the production of IL-12 and IFNγ in response to IL-17.
Cellular sources of IL-17 in the lung following F.tularensis LVS infection
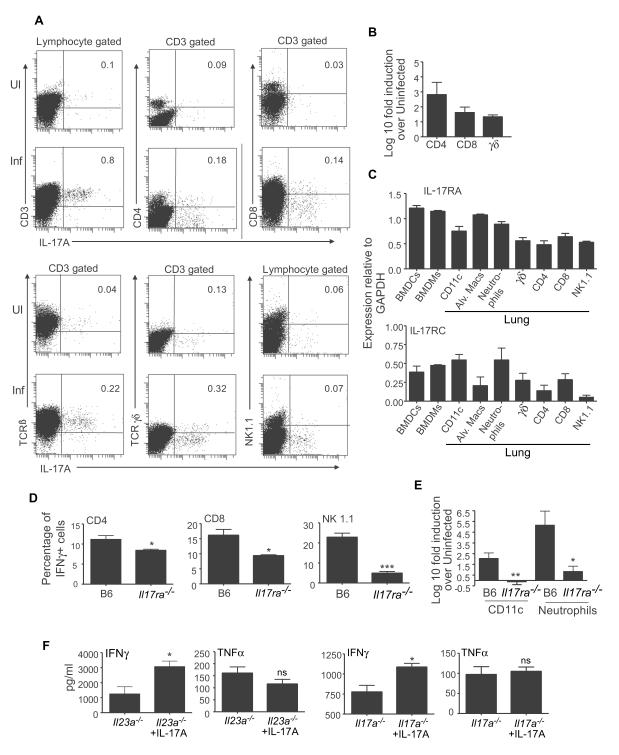
To determine the cellular sources of IL-17 in the lungs of day-6 infected B6 mice, we performed intra-cellular staining and flow cytometry. We found that one of the major producers of IL-17 in the lungs of LVS-infected mice was TCRβ+ T cells, of which the majority were CD3+ CD4+ T cells, with some IL-17–producing CD8+ T cells. Moreover, γδ T cells also produced IL-17 in B6 infected lungs when compared to uninfected lungs (Figure 7A). Furthermore, purified populations of these cells from infected B6 lungs showed significant induction of IL-17 mRNA (Figure 7B). These data suggest that innate as well as adaptive immune cells in the lung can produce IL-17 in response to LVS infection.
Figure 7. Cellular sources of IL-17 in the lung following LVS infection.
Uninfected or LVS-infected B6 (A) mice were assayed for cellular sources of IL-17 producing cells in day-6 infected lungs by flow cytometry. The percentage of cells expressing IL-17 within gated populations is shown (A). Log10 fold induction of IL-17 mRNA in sorted lung cell populations from day-6 infected B6 lungs vs. uninfected lungs was determined by RT-PCR (B). mRNA expression of IL-17RA or IL-17RC relative to GAPDH levels was determined by RT-PCR on sorted cell populations (C). The percentage of IFNγ-producing cells within each population in B6 and IL-17−/− infected lungs is shown (D). Log10 fold induction of IL-12p35 mRNA in sorted cells from infected vs. uninfected lungs was determined by RT-PCR (E). LVS-infected IL-23p19−/− or IL-17−/− mice were treated with PBS or rIL-17 and day6-infected lung homogenates assayed for IFNγ and TNF-α (F). The data points represent the mean (±SD) of values from 3-5 mice (A-F). *, p ≤0.05. **, p ≤0.005. ***, p ≤0.0005.
Since myeloid cells can respond to IL-17, we next determined the expression of IL-17RA and RC mRNA in BMDCs, BMDMs and purified lung cell populations. We found high levels of IL-17RA mRNA in myeloid cells such as DCs, macrophages and neutrophils, whereas we found lower levels of IL-17RA mRNA in γδ, CD4, CD8 T cells as well as NK1.1 cells (Figure 7C). Expression of IL-17RC mRNA was lower but detectable in both myeloid and lymphoid populations (Figure 7C).These data suggest that BMDCs, BMDMs and lung CD11c+ and alveolar macrophages express IL-17RA and IL-17RC and therefore can respond to IL-17 in vivo.
Our in vitro data shows that IL-17 could induce IL-12 and IFNγ production in innate cells such as DCs and macrophages and elicit IL-12-dependent Th1 responses in CD4 T cells (Figures 4-6). However, the reduced IFNγ protein seen in the IL-17R−/− infected lungs could reflect reduced IFNγ production in several cell populations. We found that CD4, CD8 T cells and NK1.1 cells produced IFNγ in the B6 infected lungs, and IFNγ production in these populations was significantly reduced in the lungs of infected IL-17R−/− mice (Figure 7D). These data support our findings that IL-17 is required for the induction of IFNγ in CD4 T cells and also suggests that IL-17 could play a critical role in eliciting IFNγ expression in other cell types, such as CD8 and NK1.1 cells. Furthermore, CD11c+ cells and neutrophils isolated from IL-17R−/− infected lungs expressed lower levels of IL-12p35 mRNA when compared to cells sorted from B6 infected lungs (Figure 7E). Since IFNγ was reduced in the lungs of infected IL-17−/− mice when compared to infected B6 lungs (Figure 3D), we determined whether exogenous delivery of IL-17 would rescue IFNγ responses in vivo in IL-23p19−/− and IL-17−/− mice. We found that intratracheal delivery of rIL-17 into infected IL-23−/− and IL-17−/− mice resulted in a significant increase of IFNγ but not TNFα, when compared to PBS treatment (Figure 7F). These data suggest that IL-17 is required in vivo for the induction of IFNγ from several cell populations and exogenous delivery of IL-17 can rescue IFNγ levels in infected lungs.
Discussion
IL-23/Th17 pathway is critical for protective immunity against extracellular bacterial infections (Happel et al., 2005; Ye et al., 2001). However, the IL-23/Th17 axis is thought to be dispensable for protection against intracellular pathogens (Khader et al., 2005; Schulz et al., 2008; Aujla et al., 2008). We show that absence of IL-23 and IL-17 but not IL-22 results in increased susceptibility to pulmonary LVS infection. We also show that IL-17 can induce IL-12 and IFNγ from DCs and mediate Th1 responses, as well as induce IL-12 and IFNγ from macrophages and mediate bacterial clearance. Thus, we demonstrate a novel biological function for IL-17 in regulating IL-12/Th1 immunity in protection against an intracellular pathogen.
Our data shows that LVS effectively induces the production of IL-12p40, IL-12 and IL-23 in the lungs of infected mice. Similar to other pulmonary infection models (Happel et al., 2005), early induction of IL-23p19 and the more sustained production of IL-12p35 in the LVS-infected lungs suggests that IL-23 functions during the early immune response. Induction of IL-23 and IL-17 between days 2-4 may contribute to the sustained IL-12 mRNA levels detected in infected B6 lungs. Both Th1 and Th17 responses are induced in the lungs of LVS-infected mice, and are dependent on IL-12p40 cytokines. Specifically, IL-12 is required for the generation of Th1 responses while IL-23 is required for the generation of Th17 responses. Further, IL-12 and IFNγ negatively regulate Th17 responses in vivo during LVS infection. However, the failure of high levels of IL-17 or its inducible chemokines to protect in the absence of IL-12/Th1 axis suggests that Th17 pathway alone cannot confer protection against pulmonary tularemia.
Our data shows that the IL-17 response is antigen-driven (Figure S3A) and a majority of the IL-17-producers were TCRB+ CD3+ and CD4+ T cells, with a smaller population of CD8 cells (Figure 7A,B). A proportion of IL-17-producing cells were detected in wells that did not receive antigen (Figure S3A) and are produced by γδ T cells in LVS infected lungs (Figure7A,B). Furthermore, that γδ−/− mice have reduced levels of IL-17 and are susceptible to pulmonary tularemia suggests that IL-17 produced by innate cells may serve as a defense mechanism until the adaptive immune cells are recruited to control the infection.
Although depletion of neutrophils resulted in reduced protection against systemic Francisella infection (Sjostedt et al., 1994), Francisella can evade neutrophil-killing (Allen and McCaffrey, 2007). Therefore, although decreased recruitment of neutrophils in the lungs of infected IL-17/IL-17R−/− mice may impact protective immunity against LVS, there is nonetheless significant recruitment of neutrophils in absence of the IL-17/IL-17R pathway. This suggests that the increased susceptibility seen in mice lacking IL-17/IL-17R is likely due to the markedly reduced Th1 responses. We show that IL-17, but not IL-17F or IL-22 can enhance the ability of LVS-stimulated DCs to produce IL-12. This extends the recent finding that IL-17 can induce the IL-12 production in peritoneal macrophages (Ishigame et al., 2009), to include BMDCs, BMDMs, lung alveolar macrophages and lung CD11C+ cells. Importantly, since IL-17 can induce the production of IL-12 and IFN-γ within one hour of treatment, we conclude that IL-17 dependent induction of IL-12 in APCs is a rapid event that can impact downstream T cell events. This pathway may provide the basis for the plasticity of conversion of Th17 to Th1 responses seen in vivo (Lee et al., 2009), where it is possible that IL-17 produced by Th17 cells can impact the induction of IL-12 in APCs and induce the conversion of Th17 to Th1 cells.
Until recently it was thought that the primary responses to IL-17 occurred in non-hematopoietic cells such as fibroblast and epithelial cells (Shen and Gaffen, 2008). However, our data, as well as data using peritoneal macrophages (Ishigame et al., 2009), suggest that myeloid cells such as DCs and macrophages express significant levels of IL-17RA mRNA and respond to IL-17 by production of cytokines and chemokines. That this response is mediated primarily through IL-17RA but not IL-17RC, is consistent with studies that show that murine IL-17RC can only bind to murine IL-17F but not IL-17, whereas human IL-17RC can bind to both human IL-17 and IL-17F (Kuestner et al., 2007). IL-17 is known to activate NF-κβ and our studies show that IL-17 treatment of BMDCs results in activation of NF-κβ. However, co-treatment with an NF-κβ inhibitor results in loss of some IL-17-induced cytokines such as IFNγ and IL-6, but not others such as IL-12. That NF-κβ inhibitor PTDC did not completely repress TLR driven IL-12 production in DCs (Bohnenkamp et al., 2007) suggests that other pathways such as mitogen activated protein kinases (MAPK) are involved. Additional experiments using pathway specific inhibitors will delineate the signaling pathways involved in IL-17 mediated responses in APCs.
An important facet of our findings is that IL-17 can induce IL-12 and IFNγ production and enhance LVS bacterial clearance in macrophages. In a Bordetella pertussis model, it was shown that IL-17 treatment of macrophages enhanced bacterial clearance (Higgins et al., 2006) and was thought to be mediated by direct macrophage activation. Our data suggest that IL-17-dependent activation of macrophages and bacterial killing is mediated through induction of IFNγ, since IFNγ−/− BMDMs could not mediate IL-17-dependent control of bacteria. This suggests that IL-17 can modulate the innate responses and contribute to immunity and bacterial clearance until the arrival of adaptive immune cells to the site of infection.
Given that IL-17 can induce IL-12 and IFNγ production from APCs in the absence of pathogen stimulation suggests that IL-17 acts downstream of the initial pathogen-APC interaction. Although it is the Th1 response that controls intracellular bacteria, the IL-17 pathway provides critical “help” for induction of the Th1 pathway. This is evident from the reduced IFNγ levels in the lungs of infected IL-17−/− and IL-17R−/− mice and the increased IFNγ responses in infected IL-23p19−/− and IL-17−/− mice treated with exogenous IL-17. Further proof for IL-17 in generating Th1 responses is reflected by the decreased frequencies of IFNγ-producing cells and decreased induction of IL-12p35 mRNA in CD11c+ cells and neutrophils in IL-17R−/− infected lungs. That IFNγ production in CD8 T cells and NK 1.1 cells is also decreased in IL-17R−/− infected lungs, suggests an even broader role for IL-17 in maintenance of Th1 immune responses in the lung. Recent work shows that IL-17 inhibits T-bet expression and Th1 differentiation in anti-CD3 anti-CD28 stimulated Th1 cultures (O’Connor et al., 2009). However, our data shows that Th cultures generated in the presence of APC and IL-17 can induce Th1 responses via induction of IL-12, suggesting that differential outcomes can be expected depending on whether the Th1 differentiation is mediated in the presence or absence of DCs.
Absence of the IL-23/Th17 axis did not impact IFNγ responses during M.tuberculosis (Khader et al., 2005) or L.monocytogenes (Aujla et al., 2008) infections, but resulted in reduced IFNγ responses following M.bovis BCG infection (Umemura et al., 2007). The unique requirement for the IL-23/Th17 pathway in induction of Th1 responses during LVS and M. bovis BCG, but not other intracellular infections is intriguing. Induction of IL-12 by LVS infection is dependent on TLR-2 signaling (Hong et al., 2007), while induction of IL-12p40 by a heat shock protein of Francisella is dependent on TLR-4 (Ashtekar et al., 2008). M.bovis BCG lipomannans induce IL-12p40 through TLR2 (Quesniaux et al., 2004). In contrast, IL-12 production by DCs following M.tuberculosis infection can take place in the absence of either TLR-2 and TLR-4 (Jang et al., 2004) and requires signals from both TLR2 and TLR9 (Bafica et al., 2005).This suggests that different intracellular bacteria may stimulate differential TLRs on APCs and produce distinct polarizing cytokines that impact host immune response to infection. It is likely that some intracellular bacterial infections can effectively induce IL-12/IFNγ responses in the host, while other pathogens require the IL-23/IL-17 pathway for effective induction of host IL-12/IFNγ responses for pathogen control.
In summary, we show that IL-23 dependent IL-17 is induced during LVS infection and is required for induction of IL-12, optimal induction of Th1 responses and host resistance to infection. Further studies to understand the unique requirement for IL-17 in protection against LVS will advance our understanding of the fundamental requirements for protective immunity to intracellular pathogens.
Experimental Procedures
Animals
C57BL/6 (B6), IL-12p40−/−, IL-12p35−/−, IFNγ−/−, γδ−/−, yet40 reporter mice were purchased from The Jackson Laboratory (Bar Harbor, ME). IL-12RB1−/−(Piccotti et al., 1999), IL-23p19−/− (Ghilardi et al., 2004), IL-12p35−/− IL-23p19−/− (Khader et al., 2005), IL-17−/− (Nakae et al., 2002), IL-17R−/− (Ye et al., 2001), IL-22−/− (Zheng et al., 2008) on the B6 background were used in accordance with University of Pittsburgh IACUC guidelines.
Experimental infection, cytokine and antibody treatment
LVS(BEI Resources) was grown in Mueller–Hinton (MH) broth or agar (Duckett et al., 2005). For pulmonary infections, mice were infected intratracheally with 1000 CFU LVS. Heat inactivated LVS stocks were prepared by incubating bacteria at 60° C for 1 hour. Some mice received recombinant IL-17(R and D Systems) intratracheally (1.5 μg) on day 3 post infection. Some B6 mice received 100 μg of isotype control antibody (501040) or anti-IL-17 (54447, R and D Systems) intraperitoneally on days 2 and 4 post infection. Serial dilutions of homogenized infected lungs were plated on day 6 to determine lung CFUs.
Cell Preparation
Lung cell suspensions were prepared as described before (Khader et al., 2005) and used for ELISpot, flow cytometric analyses or for sorting purified populations.
Detection of cytokine producing cells by ELISpot assay
Detection of antigen-specific IFNγ- and IL-17-producing cells was carried out using an ELISpot assay (Khader et al., 2005). LVS heat inactivated antigen (0.5μg/ml, BEI Resources) or OVA323-339 peptide (5μM) was used to stimulate cells from LVS-infected or OT-II αβTCR Tg mice respectively.
Generation and stimulation of BMDMs and BMDCs
BMDMs and BMDCs were generated from the bone marrow cells (Khader et al., 2005) by culturing in cDMEM containing GM-CSF (PeproTech). On day 7, nonadherent cells were used as BMDCs, while adherent cells were used as BMDMs. Lung alveolar macrophages were obtained by bronchoalveolar lavage. Cells were stimulated with heat inactivated LVS (MOI 1:100) alone or in combination with IL-17, IL-17F or IL-22 (100 ng/ml each) and either soluble IL-17RA Fc chimera or soluble IL-17RC (1000ng/ml each; R and D Systems). In some experiments, the BMDCs were pretreated with a NF-κβ inhibitor PTDC (100uM) for 45 mins, followed by co-treatment with IL-17 for 45 mins. Culture supernatants were analyzed by luminex assays. For macrophage killing assays, BMDMs were infected with live LVS (MOI 1:10) for 24 hours in the presence of either IFNγ or IL-17 (both at 100 ng/ml) in antibiotic-free cDMEM. BMDMs were washed extensively, lysed with sterile water, serially diluted and CFU was determined.
Determination of protein levels
IL-17, IL-12, IFNγ, IL-6, G-CSF, GM-CSF, KC, MIP-1α, MCP-1, IL-1α, IL-1β protein levels were measured in lung homogenates and cell culture supernatants using a 22-plex mouse Luminex assay (Linco/Millipore).
Naive CD4+ T cell isolation and in vitro effector generation
Naïve CD4+ T cells were isolated from OT-II αβTCR Tg mice using magnetic CD4+ beads (GK1.5) (Miltenyi Biotec) and purified based on CD62L high and CD25 low expression on a BD FACSAria (> 98%). Naive OT-II CD4+ T cells (1 × 106 cells/ml) were cultured with LVS-stimulated or unstimulated BMDCs (1 × 106 cells/ml), OVA323–339 peptide (5 μM) for 6 days. In some wells IL-12, IL-17 or IL-22 (100 ng/ml) were added. At the end of the culture period, the numbers of IFNγ- or IL-17-producing CD4+ T cells was determined by ELISpot assay or intracellular staining.
Flow cytometry and cell sorting
Single cell suspensions were stained with fluorochrome-labeled antibodies specific for CD3 (17A2), CD4 (RM4-5), CD8 (53-6.7), TCRβ (H57-597), TCRγδ(GL3), NK1.1(PK136), Gr1(RB6-8C5), CD11b (M1/70), CD11c (HL3), and MHC class II I-Ab (AF6-120.1). For intracellular analyses of cells, cells stimulated with Phorbol myristate acetate (50ng/ml), ionomycin (750 ng/ml; Sigma Aldrich) and Golgistop (BD Pharmingen), were surface stained, permeabilized with Cytofix/Cytoperm solution (BD Pharmingen) and stained with anti-IFNγ (XMG1.2) and anti-IL-17(TC11-18H10). To determine activation of NF-κB using the Phospho-NF-kB p65 (3H1, Cell signaling), cells were stained according to manufacturer’s instructions. To sort for purified lung population, stained cells were sorted on BD FACS Aria flow cytometer as CD3+ CD4+ (>94%), CD3+ CD8+ (>96%) and CD11b+Gr1+ neutrophils (>95%). For other sorts, CD11C+ (> 91%), γδ cells (>84%) and NK1.1 (>93%) cells were sorted based on respective staining. For analysis, FlowJo (Tree Star Inc, CA) was used.
Real Time PCR (RT-PCR)
RNA was extracted (Khader et al., 2005), reverse transcribed and amplified using FAM-labeled probe and primers on the ABI Prism 7700 detection system. Fold increase in signal over that derived from uninfected samples was determined using the ΔΔct calculation. The primer and probes sequences have been previously published (Khader et al., 2005) or were commercially purchased (ABI Biosystems).
Statistical Analysis
Differences between the means of groups were analyzed using the two tailed Student’s t-test in GraphPad Prism 4 (La Jolla, CA). Inherently logarithmic data from bacterial growth and RT-PCR were transformed for statistical analyses.
Supplementary Material
Acknowledgements
This work was supported by Children’s Hospital Of Pittsburgh and AI075106 to SAK, HL69409 to TDR and AR054389 to SLG. Authors thank L.Bauer and A.Magill for technical help and Drs. G.Kirimanjeswara and D.Metzger, Albany Medical College for LVS infection protocols. We also thank Drs. N.Ghilardi and O.Wenjun, Genentech Inc, for providing IL-23p19−/− and IL-22−/− mice respectively. IL-17R−/− mice were a kind gift from Amgen Inc. The authors thank Dr. J.Piganelli for critical reading of the manuscript. SLG has received travel reimbursement, honoraria and a research grant from Amgen Inc. All authors have no other conflicting financial interests.
Abbreviations
- Th
T (helper)
- (LVS)
Live vaccine strain
- (BMDC)
Bone-marrow Dendritic Cells
- (BMDMs)
Bone marrow derived macrophages
- (CFU)
Colony Forming units
- (IL)
Interleukin
- (IFNγ)
Interferon gamma
- (NK)
Natural Killer
- (IL-12RB1)
Interleukin-12 Receptor beta 1
- (IL-17R)
Interleukin 17 Receptor
- (TNFα)
Tumor Necrosis Factor alpha
- (IL-1α)
Interleukin 1 alpha
- (IL-1β)
Interleukin 1 beta
- (MIP-1α)
Macrophage inflammatory protein 1 alpha
- (G-CSF)
Granulocyte Colony stimulating factor
- (GM-CSF)
Granulocyte-Macrophage Colony stimulating factor
- (KC)
Keratinocyte chemoattractant
- (MCP-1)
Monocyte Chemoattractant Protein 1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Allen LA, McCaffrey RL. To activate or not to activate: distinct strategies used by Helicobacter pylori and Francisella tularensis to modulate the NADPH oxidase and survive in human neutrophils. Immunol Rev. 2007;219:103–117. doi: 10.1111/j.1600-065X.2007.00544.x. [DOI] [PubMed] [Google Scholar]
- Anthony LS, Ghadirian E, Nestel FP, Kongshavn PA. The requirement for gamma interferon in resistance of mice to experimental tularemia. Microb Pathog. 1989;7:421–428. doi: 10.1016/0882-4010(89)90022-3. [DOI] [PubMed] [Google Scholar]
- Ashtekar AR, Zhang P, Katz J, Deivanayagam CC, Rallabhandi P, Vogel SN, Michalek SM. TLR4-mediated activation of dendritic cells by the heat shock protein DnaK from Francisella tularensis. J Leukoc Biol. 2008;84:1434–1446. doi: 10.1189/jlb.0308215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aujla SJ, Chan YR, Zheng M, Fei M, Askew DJ, Pociask DA, Reinhart TA, McAllister F, Edeal J, Gaus K, et al. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat Med. 2008;14:275–281. doi: 10.1038/nm1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bafica A, Scanga C, Feng C, Leifer C, Cheever A, Sher A. TLR9 regulates Th1 responses and cooperates with TLR2 in mediating optimal resistance to Mycobacterium tuberculosis. Journal of Experimental Medicine. 2005;202:1715–1724. doi: 10.1084/jem.20051782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnenkamp HR, Papazisis KT, Burchell JM, Taylor-Papadimitriou J. Synergism of Toll-like receptor-induced interleukin-12p70 secretion by monocyte-derived dendritic cells is mediated through p38 MAPK and lowers the threshold of T-helper cell type 1 responses. Cell Immunol. 2007;247:72–84. doi: 10.1016/j.cellimm.2007.07.008. [DOI] [PubMed] [Google Scholar]
- Butchar JP, Rajaram MV, Ganesan LP, Parsa KV, Clay CD, Schlesinger LS, Tridandapani S. Francisella tularensis induces IL-23 production in human monocytes. J Immunol. 2007;178:4445–4454. doi: 10.4049/jimmunol.178.7.4445. [DOI] [PubMed] [Google Scholar]
- Chang SH, Dong C. IL-17F: regulation, signaling and function in inflammation. Cytokine. 2009;46:7–11. doi: 10.1016/j.cyto.2008.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis DT, Inglesby TV, Henderson DA, Bartlett JG, Ascher MS, Eitzen E, Fine AD, Friedlander AM, Hauer J, Layton M, et al. Tularemia as a biological weapon: medical and public health management. Jama. 2001;285:2763–2773. doi: 10.1001/jama.285.21.2763. [DOI] [PubMed] [Google Scholar]
- Dong C. Diversification of T-helper-cell lineages: finding the family root of IL-17-producing cells. Nat Rev Immunol. 2006;6:329–333. doi: 10.1038/nri1807. [DOI] [PubMed] [Google Scholar]
- Duckett NS, Olmos S, Durrant DM, Metzger DW. Intranasal interleukin-12 treatment for protection against respiratory infection with the Francisella tularensis live vaccine strain. Infect Immun. 2005;73:2306–2311. doi: 10.1128/IAI.73.4.2306-2311.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flesch IE, Hess JH, Huang S, Aguet M, Rothe J, Bluethmann H, Kaufmann SH. Early interleukin 12 production by macrophages in response to mycobacterial infection depends on interferon gamma and tumor necrosis factor alpha. Journal of Experimental Medicine. 1995;181:1615–1621. doi: 10.1084/jem.181.5.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghilardi N, Kljavin N, Chen Q, Lucas S, Gurney AL, De Sauvage FJ. Compromised humoral and delayed-type hypersensitivity responses in IL-23-deficient mice. J Immunol. 2004;172:2827–2833. doi: 10.4049/jimmunol.172.5.2827. [DOI] [PubMed] [Google Scholar]
- Hall JD, Woolard MD, Gunn BM, Craven RR, Taft-Benz S, Frelinger JA, Kawula TH. Infected-host-cell repertoire and cellular response in the lung following inhalation of Francisella tularensis Schu S4, LVS, or U112. Infect Immun. 2008;76:5843–5852. doi: 10.1128/IAI.01176-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Happel KI, Dubin PJ, Zheng M, Ghilardi N, Lockhart C, Quinton LJ, Odden AR, Shellito JE, Bagby GJ, Nelson S, Kolls JK. Divergent roles of IL-23 and IL-12 in host defense against Klebsiella pneumoniae. J Exp Med. 2005;202:761–769. doi: 10.1084/jem.20050193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins SC, Jarnicki AG, Lavelle EC, Mills KH. TLR4 mediates vaccine-induced protective cellular immunity to Bordetella pertussis: role of IL-17-producing T cells. J Immunol. 2006;177:7980–7989. doi: 10.4049/jimmunol.177.11.7980. [DOI] [PubMed] [Google Scholar]
- Hong KJ, Wickstrum JR, Yeh HW, Parmely MJ. Toll-like receptor 2 controls the gamma interferon response to Francisella tularensis by mouse liver lymphocytes. Infect Immun. 2007;75:5338–5345. doi: 10.1128/IAI.00561-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishigame H, Kakuta S, Nagai T, Kadoki M, Nambu A, Komiyama Y, Fujikado N, Tanahashi Y, Akitsu A, Kotaki H, et al. Differential Roles of Interleukin-17A and -17F in Host Defense against Mucoepithelial Bacterial Infection and Allergic Responses. Immunity. 2009;30:108–119. doi: 10.1016/j.immuni.2008.11.009. [DOI] [PubMed] [Google Scholar]
- Jang S, Uematsu S, Akira S, Salgame P. IL-6 and IL-10 induction from dendritic cells in response to Mycobacterium tuberculosis is predominantly dependent on TLR2-mediated recognition. J Immunol. 2004;173:3392–3397. doi: 10.4049/jimmunol.173.5.3392. [DOI] [PubMed] [Google Scholar]
- Khader SA, Pearl JE, Sakamoto K, Gilmartin L, Bell GK, Jelley-Gibbs DM, Ghilardi N, deSauvage F, Cooper AM. IL-23 compensates for the absence of IL-12p70 and is essential for the IL-17 response during tuberculosis but is dispensable for protection and antigen-specific IFN-gamma responses if IL-12p70 is available. J Immunol. 2005;175:788–795. doi: 10.4049/jimmunol.175.2.788. [DOI] [PubMed] [Google Scholar]
- Kuestner RE, Taft DW, Haran A, Brandt CS, Brender T, Lum K, Harder B, Okada S, Ostrander CD, Kreindler JL, et al. Identification of the IL-17 receptor related molecule IL-17RC as the receptor for IL-17F. J Immunol. 2007;179:5462–5473. doi: 10.4049/jimmunol.179.8.5462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KA, Kang MH, Lee YS, Kim YJ, Kim DH, Ko HJ, Kang CY. A distinct subset of natural killer T cells produces IL-17, contributing to airway infiltration of neutrophils but not to airway hyperreactivity. Cell Immunol. 2008;251:50–55. doi: 10.1016/j.cellimm.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Lee YK, Turner H, Maynard CL, Oliver JR, Chen D, Elson CO, Weaver CT. Late developmental plasticity in the T helper 17 lineage. Immunity. 2009;30:92–107. doi: 10.1016/j.immuni.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiby DA, Fortier AH, Crawford RM, Schreiber RD, Nacy CA. In vivo modulation of the murine immune response to Francisella tularensis LVS by administration of anticytokine antibodies. Infect Immun. 1992;60:84–89. doi: 10.1128/iai.60.1.84-89.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindgren H, Stenman L, Tarnvik A, Sjostedt A. The contribution of reactive nitrogen and oxygen species to the killing of Francisella tularensis LVS by murine macrophages. Microbes Infect. 2005;7:467–475. doi: 10.1016/j.micinf.2004.11.020. [DOI] [PubMed] [Google Scholar]
- Ma X, Neurath M, Gri G, Trinchieri G. Identification and characterization of a novel Ets-2-related nuclear complex implicated in the activation of the human interleukin-12 p40 gene promoter. J Biol Chem. 1997;272:10389–10395. doi: 10.1074/jbc.272.16.10389. [DOI] [PubMed] [Google Scholar]
- Nakae S, Komiyama Y, Nambu A, Sudo K, Iwase M, Homma I, Sekikawa K, Asano M, Iwakura Y. Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity. 2002;17:375–387. doi: 10.1016/s1074-7613(02)00391-6. [DOI] [PubMed] [Google Scholar]
- O’Connor W, Jr., Kamanaka M, Booth CJ, Town T, Nakae S, Iwakura Y, Kolls JK, Flavell RA. A protective function for interleukin 17A in T cell-mediated intestinal inflammation. Nat Immunol. 2009;10:603–609. doi: 10.1038/ni.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppmann B, Lesley R, Blom B, Timans J, Xu Y, Hunte B, Vega F, Yu N, Wang J, Singh K, et al. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000;13:715–725. doi: 10.1016/s1074-7613(00)00070-4. [DOI] [PubMed] [Google Scholar]
- Ouyang W, Kolls JK, Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity. 2008;28:454–467. doi: 10.1016/j.immuni.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccotti J, Li K, Chan S, Eichwald E, Bishop D. Interleukin-12 (IL-12)-driven alloimmune responses in vitro and in vivo: requirement for beta1 subunit of the IL-12 receptor. Transplantation. 1999;67:1453–1460. doi: 10.1097/00007890-199906150-00011. [DOI] [PubMed] [Google Scholar]
- Quesniaux VJ, Nicolle DM, Torres D, Kremer L, Guerardel Y, Nigou J, Puzo G, Erard F, Ryffel B. Toll-like receptor 2 (TLR2)-dependent-positive and TLR2-independent-negative regulation of proinflammatory cytokines by mycobacterial lipomannans. J Immunol. 2004;172:4425–4434. doi: 10.4049/jimmunol.172.7.4425. [DOI] [PubMed] [Google Scholar]
- Reinhardt R, Hong S, Kang S, Wang Z, Locksley R. Visualization of IL-12/23p40 In Vivo Reveals Immunostimulatory Dendritic Cell Migrants that Promote Th1 Differentiation. Journal of Immunology. 2006;177:1618–1627. doi: 10.4049/jimmunol.177.3.1618. [DOI] [PubMed] [Google Scholar]
- Schulz O, Edwards AD, Schito M, Aliberti J, Manickasingham S, Sher A, Reis e Sousa C. CD40 triggering of heterodimeric IL-12 p70 production by dendritic cells in vivo requires a microbial priming signal. Immunity. 2000;13:453–462. doi: 10.1016/s1074-7613(00)00045-5. [DOI] [PubMed] [Google Scholar]
- Schulz SM, Kohler G, Schutze N, Knauer J, Straubinger RK, Chackerian AA, Witte E, Wolk K, Sabat R, Iwakura Y, et al. Protective immunity to systemic infection with attenuated Salmonella enterica serovar enteritidis in the absence of IL-12 is associated with IL-23-dependent IL-22, but not IL-17. J Immunol. 2008;181:7891–7901. doi: 10.4049/jimmunol.181.11.7891. [DOI] [PubMed] [Google Scholar]
- Shen F, Gaffen SL. Structure-function relationships in the IL-17 receptor: Implications for signal transduction and therapy. Cytokine. 2008 doi: 10.1016/j.cyto.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjostedt A, Conlan JW, North RJ. Neutrophils are critical for host defense against primary infection with the facultative intracellular bacterium Francisella tularensis in mice and participate in defense against reinfection. Infect Immun. 1994;62:2779–2783. doi: 10.1128/iai.62.7.2779-2783.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skeen MJ, Miller MA, Shinnick TM, Ziegler HK. Regulation of murine macrophage IL-12 production. Activation of macrophages in vivo, restimulation in vitro, and modulation by other cytokines. J Immunol. 1996;156:1196–1206. [PubMed] [Google Scholar]
- Smith E, Stark MA, Zarbock A, Burcin TL, Bruce AC, Vaswani D, Foley P, Ley K. IL-17A inhibits the expansion of IL-17A-producing T cells in mice through “short-loop” inhibition via IL-17 receptor. J Immunol. 2008;181:1357–1364. doi: 10.4049/jimmunol.181.2.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toy D, Kugler D, Wolfson M, Vanden Bos T, Gurgel J, Derry J, Tocker J, Peschon J. Cutting edge: interleukin 17 signals through a heteromeric receptor complex. J Immunol. 2006;177:36–39. doi: 10.4049/jimmunol.177.1.36. [DOI] [PubMed] [Google Scholar]
- Trinchieri G, Pflanz S, Kastelein R. The IL-12 family of heterodimeric cytokines: new players in the regulation of T cell responses. Immunity. 2003;19:641–644. doi: 10.1016/s1074-7613(03)00296-6. [DOI] [PubMed] [Google Scholar]
- Umemura M, Yahagi A, Hamada S, Begum MD, Watanabe H, Kawakami K, Suda T, Sudo K, Nakae S, Iwakura Y, Matsuzaki G. IL-17-mediated regulation of innate and acquired immune response against pulmonary Mycobacterium bovis bacille Calmette-Guerin infection. J Immunol. 2007;178:3786–3796. doi: 10.4049/jimmunol.178.6.3786. [DOI] [PubMed] [Google Scholar]
- Woolard MD, Hensley LL, Kawula TH, Frelinger JA. Respiratory Francisella tularensis live vaccine strain infection induces Th17 cells and prostaglandin E2, which inhibits generation of gamma interferon-positive T cells. Infect Immun. 2008;76:2651–2659. doi: 10.1128/IAI.01412-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Z, Fanslow WC, Seldin MF, Rousseau AM, Painter SL, Comeau MR, Cohen JI, Spriggs MK. Herpesvirus Saimiri encodes a new cytokine, IL-17, which binds to a novel cytokine receptor. Immunity. 1995;3:811–821. doi: 10.1016/1074-7613(95)90070-5. [DOI] [PubMed] [Google Scholar]
- Ye P, Rodriguez FH, Kanaly S, Stocking KL, Schurr J, Schwarzenberger P, Oliver P, Huang W, Zhang P, Zhang J, et al. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med. 2001;194:519–527. doi: 10.1084/jem.194.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Valdez PA, Danilenko DM, Hu Y, Sa SM, Gong Q, Abbas AR, Modrusan Z, Ghilardi N, de Sauvage FJ, Ouyang W. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat Med. 2008;14:282–289. doi: 10.1038/nm1720. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.