Abstract
The breast cancer resistance protein (BCRP) and the multidrug resistance-associated proteins (MRPs) have the ability to eliminate sulfate conjugates but it is not known if this constitutes one of their roles in the placenta. To determine this, the BeWo cell line was used as a model of placental trophoblast cells and we examined the mechanisms of elimination of two common sulfotransferase substrates, 4-nitrophenol and acetaminophen. At 0.5–200 μM, neither 4-nitrophenyl sulfate nor acetaminophen sulfate affected the accumulation of the BCRP substrates BODIPY FL prazosin or mitoxantrone in BeWo monolayers, indicating a lack of interaction of BCRP with the sulfates. Efflux studies and bidirectional transport studies examining the effect of BCRP/MRP inhibitors on the efflux of intracellularly generated 4-nitrophenyl sulfate and acetaminophen sulfate, indicated that one or more of the MRP isoforms play a major role in the elimination of 4-nitrophenyl sulfate and acetaminophen sulfate across the basolateral (fetal-facing) and apical (maternal-facing) membranes respectively. BCRP played a minor role in the elimination of these two sulfate conjugates across the apical membrane. Our study shows that a yet undetermined role of trophoblast efflux transporters is the elimination of sulfate conjugates.
Keywords: Trophoblast, sulfate conjugate, breast cancer resistance protein (BCRP), multidrug resistance-associated protein (MRP), mitoxantrone, BODIPY FL prazosin
1. Introduction
The placenta has the capacity of promoting elimination of drugs and natural substances by sulfation (Sodha and Schneider, 1984, Stanley et al., 2001). Since sulfate metabolites bear a negative charge at physiological pH it is likely that passive diffusion would not be the predominant mechanism of their elimination out of the placenta. This indicates transporter-mediated elimination and suggests the involvement of the membrane-bound ATP-binding cassette (ABC) family of transporters.
Literature suggests that among the efflux transporters, the multidrug resistance-associated protein (MRP) isoforms (MRP2, MRP1, MRP3, MRP4) (Zamek-Gliszczynski et al., 2006a, Deeley and Cole, 2006) and the breast cancer resistance protein (BCRP) (Zamek-Gliszczynski et al., 2006c, Adachi et al., 2005, Grube et al., 2007, Zamek-Gliszczynski et al., 2006a, Imai et al., 2003) are prime candidates to mediate the transport of sulfated metabolites. MRP isoforms and BCRP eliminate sulfate conjugates in other human tissues (Brand et al., 2008, Hu et al., 2003, Chen et al., 2005, Jeong et al., 2004), but it has not yet been examined whether they play a similar role in the placenta. However, firstly, species as well as tissue differences exist with respect to the relative contribution of the efflux transporters responsible for the elimination of sulfate conjugates (Zamek-Gliszczynski et al., 2005, Zamek-Gliszczynski et al., 2006c, Enokizono et al., 2007). Secondly, altered sulfate transport can result in toxicity. For example, impaired hepatic elimination of troglitazone sulfate, the major metabolite of the antidiabetic drug troglitazone, has the potential to reduce bile acid efflux and lead to subsequent hepatotoxicity. (Kostrubsky et al., 2001, Funk et al., 2001). Therefore, it is important to elucidate the pathways of sulfate conjugate excretion in tissues capable of sulfation, such as the liver and placenta, to understand mechanisms controlling exposure to potentially toxic metabolites.
In the placenta, excretion of sulfate conjugates is critical for reducing fetal exposure to potentially toxic metabolites and is an area of research that is largely unexplored. Substances in the maternal circulation first encounter a layer of trophoblast cells to reach the fetal circulation. At term, the trophoblast layer consists predominantly of multinucleated syncytiotrophoblast cells with few precursor mononucleated cytotrophoblast cells. It is likely that the polarized syncytiotrophoblast cells play an important role in reducing fetal exposure to substances in the maternal circulation via sulfation and sulfate metabolite elimination, as they express several sulfotransferase enzymes as well as efflux transporters. Among the efflux transporters that are known to eliminate sulfate conjugates, BCRP, MRP2, MRP3, and MRP4 are located predominantly on the syncytiotrophoblast maternal-facing membrane (Grube et al., 2007, Evseenko et al., 2007, St-Pierre et al., 2000, Azzaroli et al., 2007). MRP1 and MRP5 are expressed on both maternal-facing and fetal-facing membranes (St-Pierre et al., 2000, Atkinson et al., 2003, Meyer Zu Schwabedissen et al., 2005).
The objective of the present work was to determine the efflux transporters mediating sulfate conjugate elimination out of trophoblast cells using the BeWo cell line as a model. The BeWo cells consist predominantly of cytotrophoblast-like cells (Friedman and Skehan, 1979). In culture, they form a polarized layer of cells with the apical and basolateral membranes bearing morphological and functional similarities to the trophoblast maternal-facing and fetal-facing membranes respectively (Liu et al., 1997). We have previously found that the sulfotransferase isoforms SULT1A1 and SULT1A3 are functional in the BeWo cells (Mitra and Audus, 2009). In addition, the mRNA of BCRP and several MRP isoforms are expressed in BeWo cells, and protein expression has been determined for BCRP, MRP1, MRP2 and MRP5 (Evseenko et al., 2006, Azzaroli et al., 2007, Young, 2005, Pascolo et al., 2003). In this study, we examined the fate of two common substrates of sulfation pathways, 4-nitrophenol and acetaminophen. Acetaminophen and 4-nitrophenol were chosen because they are both substrates of the sulfotransferase isoform SULT1A1 (Falany, 1997).
2. Materials and methods
2.1. Materials
The BeWo cell line (clone b30) was obtained from Dr. Alan Schwartz (Washington University, St. Louis, MO). BODIPY FL prazosin was purchased from Invitrogen (Carlsbad, CA). MK-571 and leukotriene C4 were obtained from Biomol (Plymouth Meeting, PA). Fetal bovine serum (FBS) was purchased from Atlanta Biologicals (Norcross, GA). [3H] mitoxantrone (4 Ci/mmol) was obtained from Moravek Biochemicals Inc. (Brea, CA). All other materials and cell culture reagents were obtained either from Sigma (St. Louis, MO) or from Invitrogen (Carlsbad, CA).
2.2. BeWo cell culture
The BeWo cell line was cultured as described previously (Bode et al., 2006). Passages 29 through 45 of the cells were used in this study.
2.3. Accumulation studies
To ascertain if 4-nitrophenyl sulfate or acetaminophen sulfate interact with BCRP, their effect on the accumulation of the BCRP substrates mitoxantrone and BODIPY FL prazosin were determined. Accumulation studies were performed as published previously (Bode et al., 2006) and the relevant specifics are outlined below.
BeWo cells were plated on multiwells. At confluency, monolayers were washed in HBSS containing 25 mM glucose (HBSS-Glc), and equilibrated in HBSS-Glc for 30 minutes. For the mitoxantrone accumulation studies, solutions were prepared in HBSS-Glc. Initial studies were performed to optimize the accumulation time of mitoxantrone. BeWo cells were incubated with 20 nM [3H] mitoxantrone (final specific activity 0.02 Ci/mmol) in the absence/presence of the BCRP-specific inhibitor fumitremorgin C (5 μM) for different periods of time. The cells were then washed thrice in HBSS-Glc, lysed for 2 hours at 37°C with a solution containing 0.5% Triton X-100 in 0.2 N NaOH. The cell lysates were analyzed by liquid scintillation counting and their protein content determined by the BCA assay kit (Pierce Chemical, Rockford, IL).
For determining the effect of 4-nitrophenyl sulfate or acetaminophen sulfate on the accumulation of the BCRP substrates, BeWo cells were treated with 10 μM mitoxantrone (final specific activity 0.02 Ci/mmol) in the presence of different concentrations of 4-nitrophenyl sulfate or acetaminophen sulfate (0.5–200 μM) for 150 minutes. Cells serving as positive controls contained mitoxantrone along with 5 μM fumitremorgin C (BCRP-specific inhibitor) or 100 μM chrysin (BCRP inhibitor). The rest of the procedure was performed as outlined above.
Accumulation studies of 500 nM BODIPY FL prazosin were performed in a similar manner with the following exceptions: the dosing solutions were prepared in HBSS-Glc containing 2% BSA and the lysing solution contained 2% Triton X-100. Fluorescence was detected using a Bio-Tek FL600 Microplate Fluorescence Reader (excitation: 485 nm, emission: 535 nm).
2.4. Efflux studies
For efflux studies, cells were seeded onto multiwell plates as mentioned previously (Bode et al., 2006). BeWo cells at confluency were washed thrice in HBSS and incubated for 30 minutes in inhibitor solutions prepared in HBSS. Inhibitors used were 5 μM and 25 μM MK-571; 10 and 100 μM indomethacin; 5 μM fumitremorgin C; 200 μM chrysin; and 0.1 μM and 0.2 μM leukotriene C4. Following this, cells were freshly incubated with either 1 μM 4-nitrophenol or 250 μM acetaminophen, in the presence of inhibitors, for 2 hours and 4 hours respectively. Control cells contained vehicle in place of the inhibitors. The incubation medium was collected, centrifuged at 2,500 x g for 15 minutes, and analyzed by LC-MS/MS.
2.5. Transport studies
BeWo cells were seeded onto 0.4 μM pore size human placental collagen coated Transwell® plates (Costar Corporation, Acton, MA) at a density of 45,000 cells/cm2 (Bode et al., 2006). Experiments examining the basolateral elimination of 4-nitrophenyl sulfate were performed as published previously (Bode et al., 2006), with a few modifications. Experiments were conducted on days 6–8. BeWo cell monolayers were incubated in HBSS at 37°C with shaking (50 r.p.m.) for 20 minutes with inhibitors of ABC transporters in both apical and basolateral chambers. Inhibitors used were 25 μM MK-571, 0.2 μM leukotriene C4, or 5 μM fumitremorgin C. The preincubation solution was then aspirated off and the cells were incubated with 50 μM 4-nitrophenol solution containing inhibitors in the apical (donor) chamber. The buffer in the basolateral (receiver) chamber also contained the inhibitor. Control cells were incubated in vehicle instead of the inhibitors. Portions (200 μL) of the receiver chamber were withdrawn at predetermined intervals up to 45 minutes and replaced with fresh HBSS containing inhibitor. All samples were centrifuged at 2,500 x g for 15 minutes and a portion of the supernatant analyzed by LC-MS/MS.
2.6. Sulfotransferase enzyme assays
BeWo cell lysate was prepared and sulfotransferase activities were measured according to the procedure of Hu et al. (Hu et al., 2003) with slight modifications. BeWo cells collected in 50 mM potassium phosphate buffer (pH 7.4) were homogenized on ice for 30 seconds with a Polytron homogenizer. The homogenate was centrifuged at 14,000 r.p.m. for 15 minutes, the supernatant withdrawn and centrifuged at 14,000 r.p.m. for 15 minutes again. Protein concentration was determined using a BCA assay kit (Pierce, Rockford, IL). Sulfotransferase activities were measured as follows: BeWo cell lysate at a final concentration of 0.9 mg protein/mL was added to 100 μM 3′-phosphoadenosine-5′-phosphosulfate (PAPS) and 1 μM 4-nitrophenol/250 μM acetaminophen in a total reaction volume of 100 μL. The mixture was incubated at 37°C for 2 hours in the case of 4-nitrophenol and 4 hours in the case of acetaminophen. The reaction was stopped by the addition of 25 μL of 94% acetonitrile/6% glacial acetic acid containing the internal standards 3-methyl-4-nitrophenol or cimetidine. The samples were then centrifuged at 14,000 r.p.m. for 15 minutes and the supernatant analyzed by LC-MS/MS.
2.7. LC-MS/MS analysis of 4-nitrophenyl sulfate and acetaminophen sulfate
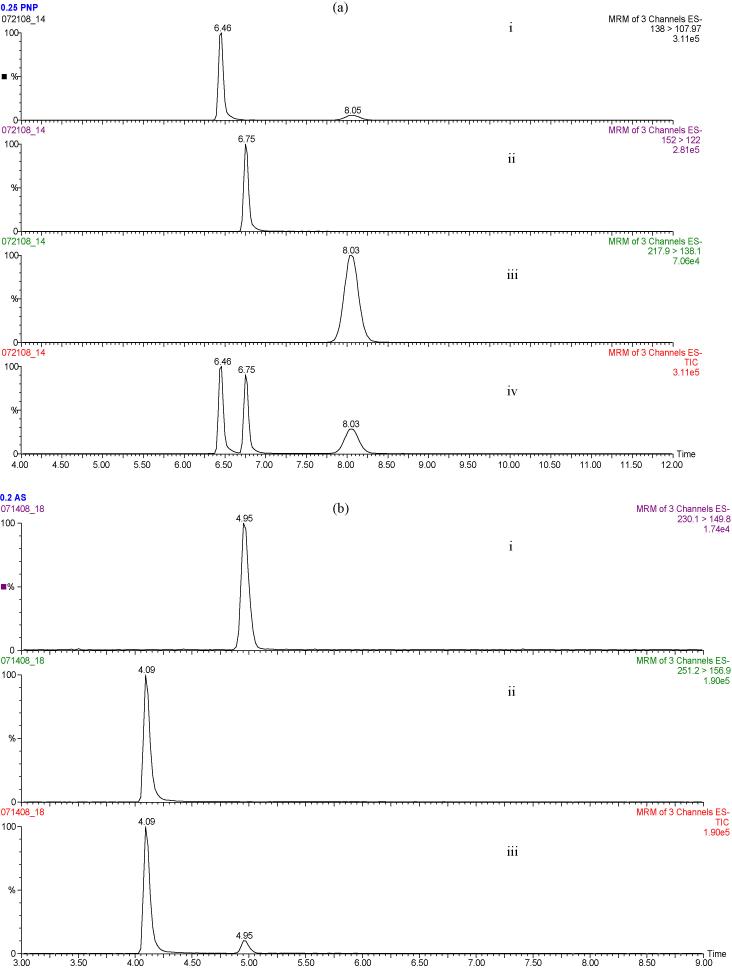
Samples from transport, efflux, and metabolism studies were analyzed by LC-MS/MS (LC coupled to Quattro Triple Quadrupole mass spectrometer). Analytes were separated on a Phenomenex Luna C18 column (2.0 × 50 mm, 5 μM pore size). 4-nitrophenol (PNP), 4-nitrophenyl sulfate (PNPS) and the internal standard 3-methyl-4-nitrophenol (MPNP) were separated using a mobile phase gradient at a flow rate of 0.3 mL/min. Solvent A was water containing 0.1% formic acid, while solvent B was acetonitrile containing 0.1% formic acid. The solvents were held at 95:5 A:B for 2 minutes, ramped to 30:70 A:B from 2 to 4.5 minutes, maintained at 30:70 A:B from 4.5 to 9.5 minutes, changed to 95:5 A:B from 9.5 to 10 minutes and then held at this composition until 15 minutes. Analytes were detected in negative ion mode using multiple reaction monitoring: PNP (138 → 108), PNPS (217.95 → 138.05), and MPNP (152 → 122) (Fig. 1a). The HPLC eluent was diverted to waste for the first 4 minutes.
Figure 1.
(a). Chromatogram demonstrating the separation of 4-nitrophenol (PNP), 4-nitrophenyl sulfate (PNPS), and the internal standard 3-methyl-4-nitrophenol (MPNP) by LC-MS/MS. The analytes were detected in negative ion mode using multiple reaction monitoring: Channel (i) represents the PNP (138 → 108) peak, channel (ii) the MPNP (152 → 122) peak, channel (iii) the PNPS (217.95 → 138.05) peak, and channel (iv) represents the complete separation. (b). Chromatogram demonstrating the separation of acetaminophen sulfate (AS) and the internal standard cimetidine. The analytes were detected in negative ion mode with multiple reaction monitoring: Channel (i) represents the AS (230.1 → 149.8) peak, channel (ii) represents the cimetidine (251.2 → 156.9) peak, and channel (iii) represents the complete separation. The Y-axis of each of the channels represents peak intensity.
Acetaminophen, acetaminophen sulfate (AS), and the internal standard cimetidine were separated using a mobile phase gradient at a flow rate of 0.3 mL/min. The solvents were held at 100:0 A:B for 1 minute, ramped to 50:50 A:B from 1 to 2.5 minutes, maintained at 50:50 A:B from 2.5 to 4.5 minutes, changed to 100:0 A:B from 4.5 to 5.5 minutes, and then held at this composition for another 3.5 minutes. AS and cimetidine were detected in negative ion mode: AS (230.1 → 149.8), cimetidine (251.2 → 156.9) (Fig. 1b). The HPLC eluent was diverted to waste for the first 3 minutes.
The sulfate conjugates were quantified from standard curves with the standards prepared in HBSS. The calibration curve of 4-ntrophenyl sulfate was linear between 3–260 ng/mL (R2 = 0.9923), while that of acetaminophen sulfate was linear between 1.3–270ng/mL (R2 = 0.9993). The lower limit of detection of acetaminophen sulfate was 1.4 ng/mL. At the lowest concentration, the S/N ratio of 4-nitrophenyl sulfate was 100.
2.8. Excretion rate calculations
Sulfate metabolite excretion rates (Vmt) were obtained by monitoring the change of metabolite concentration (Cm) in the receiver chamber as a function of time (Jeong et al., 2004, Chen et al., 2005).
Vr represents the volume of the receiver compartment.
2.9. Statistical analyses
Statistical significance was determined using the student’s t-test or one-way analysis of variance (ANOVA) followed by Dunnett’s post-comparison test (GraphPad Prism software, version 5, La Jolla, CA) as deemed appropriate. A p-value of less than 0.05 was considered to be statistically significant.
3. Results and discussion
3.1 Effect of acetaminophen sulfate and 4-nitrophenyl sulfate on the accumulation of mitoxantrone and BODIPY FL prazosin
BCRP, MRP1, MRP2, and MRP5 proteins are expressed in BeWo cells (Pascolo et al., 2003, Young, 2005, Evseenko et al., 2006), while MRP2 protein expression is weak (Young, 2005). In BeWo cells, BCRP mRNA levels were reported to be much higher than those of MRP1 or MRP2 (Serrano et al., 2007). In syncytiotrophoblast BCRP and MRP2 are localized to the maternal-facing membrane, while MRP1 and MRP5 are detected on both maternal- and fetal- facing membranes. Taking into account what is known about the expression of efflux transporters in BeWo cells, and assuming similar transporter localization in syncytiotrophoblast and BeWo cells, the initial hypothesis was that BCRP would predominantly mediate sulfate metabolite elimination across the apical (maternal-facing) trophoblast membrane. To examine this, the effects of acetaminophen sulfate and 4-nitrophenyl sulfate on the accumulation of the BCRP substrates BODIPY FL prazosin and mitoxantrone were determined. If the sulfates altered accumulation of the BCRP substrates, it would indicate an interaction of BCRP with the sulfates, and would imply a potential role of BCRP in sulfate elimination.
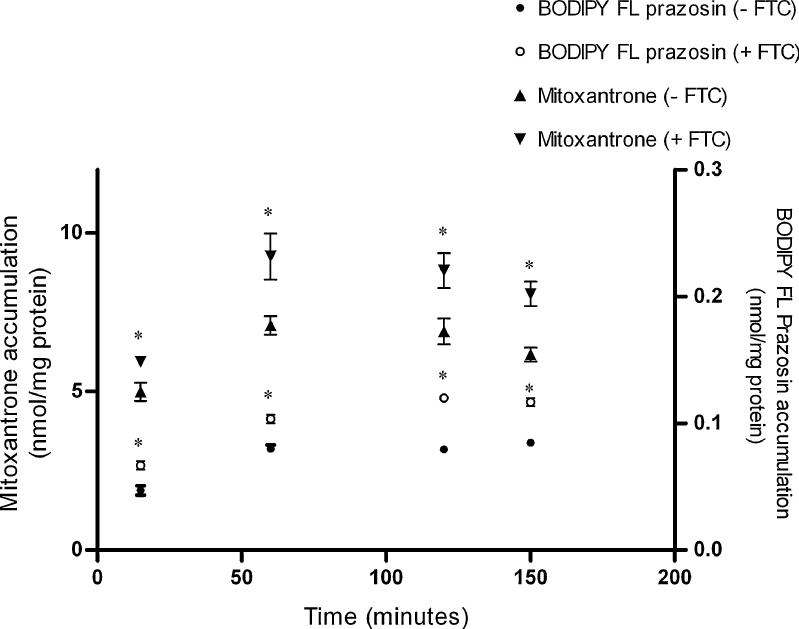
BODIPY FL prazosin and mitoxantrone were used at concentrations that are typically used for BCRP (Cerveny et al., 2006, Lee et al., 2007). The initial experiments were performed to optimize the incubation time so as to observe a maximum difference in accumulation of BODIPY FL Prazosin or mitoxantrone in the absence/presences of the BCRP-specific inhibitor fumitremorgin C (FTC). On an average, fumitremorgin C increased the accumulation of BODIPY FL prazosin by 1.5–2 fold and that of mitoxantrone by 1.3–1.5 fold. Although, accumulation of both mitoxantrone and BODIPY FL prazosin reached steady state after the first hour of incubation (Fig. 2), accumulation of both substrates in the presence/absence of FTC was on an average most significantly different at 150 minutes. Hence an incubation period of 150 minutes was utilized for all subsequent experiments.
Figure 2. Time-dependent accumulation of mitoxantrone or BODIPY FL prazosin in BeWo cells.
Accumulation of 20 nM [3H]-mitoxantrone (4 Ci/mmol) or 500 nM BODIPY FL prazosin was measured at 37°C as a function of time, in the absence or presence of the BCRP-specific inhibitor fumitremorgin C (FTC). Each experiment was repeated in triplicate with each point and bar in an individual experiment representing the mean ± S.E.M.. * indicates p < 0.05.
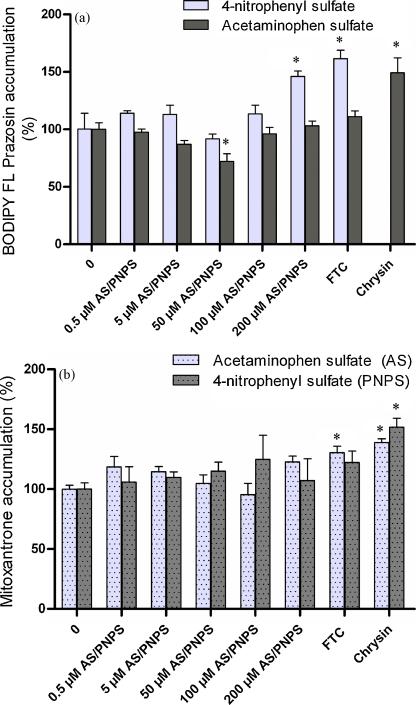
Acetaminophen sulfate (0.5–200 μM) did not affect the accumulation of mitoxantrone (Fig. 3a). It produced ~ 30% decrease in the accumulation of BODIPY FL prazosin at 50 μM which was statistically significant (Fig. 3b). However this effect was not observed at higher concentrations or lower concentrations of acetaminophen sulfate; neither did 50 μM acetaminophen sulfate affect the accumulation of mitoxantrone. Based on the accumulation of both mitoxantrone and BODIPY FL prazosin, it was concluded that acetaminophen sulfate is not interacting with BCRP. Under the same conditions, chrysin which is a nonspecific BCRP inhibitor (Gyemant et al., 2005) and fumitremorgin C (a specific BCRP inhibitor) increased accumulation by 1.5–2 fold. 4-nitrophenyl sulfate (0.5–200 μM) did not alter the accumulation of mitoxantrone (Fig. 3a). 4-nitrophenyl sulfate did not affect the accumulation of BODIPY FL prazosin either except at 200 μM where it produced a statistically significant increase in accumulation (Fig. 3b). The increase in accumulation (1.5–1.8 fold) was comparable to that produced by fumitremorgin C (1.5–2 fold). Although not as effective, this is in agreement with previous studies (Suzuki et al., 2003) where 4-nitrophenyl sulfate inhibited BCRP-mediated transport of estrone sulfate in membrane vesicles obtained from mouse lymphoma P388 cells (IC50 = 53 μM). However, 4-nitrophenyl sulfate did not change the accumulation of mitoxantrone at 200 μM. Therefore the accumulation studies do not conclusively prove that 4-nitrophenyl sulfate inhibits BCRP at 200 μM.
Figure 3.
(a). Effect of 4-nitrophenyl sulfate (PNPS) and acetaminophen sulfate (AS) on the accumulation of BODIPY FL prazosin in BeWo cells. Accumulation of 500 nM BODIPY FL prazosin was measured in the presence of 4-nitrophenyl sulfate (0.5–200 μM) or acetaminophen sulfate (0.5–200 μM) at 37°C for 150 minutes. Control cells contained BODIPY® FL prazosin alone. (b). Effect of 4-nitrophenyl sulfate (PNPS) and acetaminophen sulfate (AS) on the accumulation of BODIPY FL prazosin in BeWo cells. Accumulation of 10μM mitoxantrone (specific activity 0.02 Ci/mmol) was measured in the presence of 4-nitrophenyl sulfate (0.5–200 μM) or acetaminophen sulfate (0.5–200 μM) at 37°C for 150 minutes. Control cells contained mitoxantrone alone. BCRP inhibitors fumitremorgin C (FTC, 5μM) and chrysin (100μM) respectively, served as the positive controls. Data has been plotted as mean ± S.E.M. * indicates p < 0.05.
Overall, the accumulation of BODIPY FL Prazosin as well as mitoxantrone suggested that neither acetaminophen sulfate, nor 4-nitrophenyl sulfate inhibit BCRP. These suggested that BCRP does not interact with the sulfate metabolites and does not play a role in the elimination of these specific sulfated substrates in BeWo cells.
The membrane-bound ATP-binding cassette (ABC) family of transporters consist of trans-membrane domains and cytosolic domains. The substrate-binding sites of these transporters are usually located on the trans-membrane domains and substrates gain access to the binding site from the membrane-cytosolic interface (Sharom, 2006). It can be contended that in these experiments the charged sulfates would not be able to permeate the cells and thus would not gain access to the transporter-binding site. BeWo cells, however, have been reported to take up organic anions via temperature-sensitive mechanisms indicating carrier-mediated transport (Serrano et al., 2007). Although 4-nitrophenyl sulfate or acetaminophen sulfate uptake has not been demonstrated in BeWo cells, both sulfates are taken up by carrier-mediated processes in isolated hepatocytes (Sakuma-Sawada et al., 1997b, Sakuma-Sawada et al., 1997a). BeWo cells have the ability to produce 4-nitrophenyl sulfate intracellularly upon incubation with 4-nitrophenol (section 3.2). To assess if intracellularly-generated 4-nitrophenyl sulfate affected the accumulation of mitoxantrone in a manner different from that mentioned above, BeWo cell monolayers were incubated with 4-nitrophenol (0.5–200 μM) (data not shown). We found that under these conditions the accumulation of mitoxantrone decreased by 10–30%. As this was not dose dependent and not statistically significant at all concentrations, it indicated that intracellularly generated 4-nitrophenyl sulfate did not affect BCRP-mediated accumulation, and reaffirmed the previous conclusion that the sulfate metabolites do not interact with BCRP.
3.2. Efflux of the sulfate metabolites across the apical (maternal-facing) membrane
To identify the efflux transporters mediating sulfate elimination across the apical (maternal-facing) trophoblast membrane, BeWo monolayers grown on multiwells were incubated with 4-nitrophenol or acetaminophen. The incubation medium was analyzed for 4-nitrophenyl sulfate or acetaminophen sulfate. The amounts of 4-nitrophenyl sulfate and acetaminophen sulfate detected in the transport medium increased linearly as a function of time (data not shown). Incubation periods of 2 hours and 4 hours were selected for future experiments with 4-nitrophenol (1 μM) and acetaminophen (250 μM) respectively.
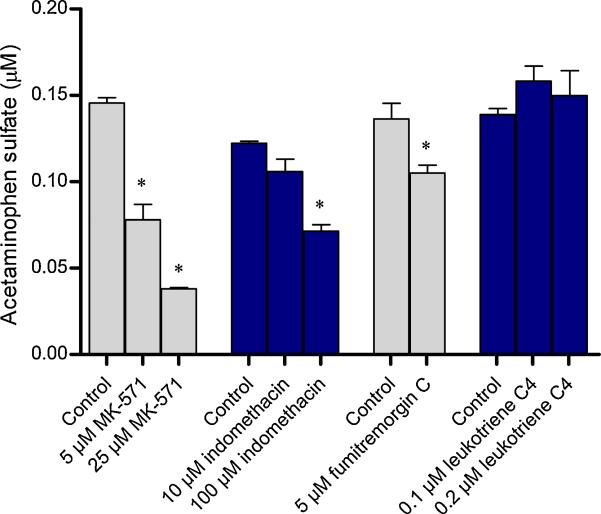
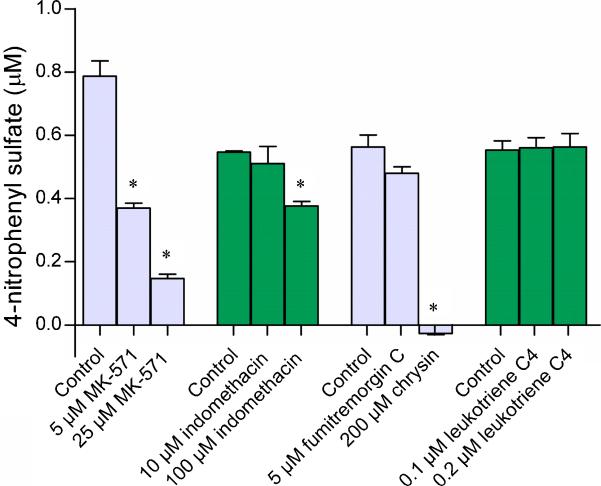
Efflux of acetaminophen sulfate across the apical membrane underwent a dose-dependent decrease in the presence of the MRP inhibitors MK-571 and indomethacin (Fig. 4). Another MRP inhibitor leukotriene C4 (LTC4) did not produce any effect. The BCRP-specific inhibitor FTC at 5 μM also produced a significant decrease in efflux; the amount of acetaminophen sulfate effluxed being ~77% of vehicle-treated controls. Compared to fumitremorgin C, much lesser amounts were eliminated at the highest concentrations of MK-571 (~26%) and indomethacin (~58%). Similar results were obtained with the apical efflux of 4-nitrophenyl sulfate (Fig. 5). The percentage of 4-nitrophenyl sulfate detected in the transport medium was ~20% in the presence of 25 μM MK-571, ~70% in the presence of 100 μM indomethacin, and ~85% in the presence of 5 μM FTC. Leukotriene C4 did not produce any effect. Interestingly, 4-nitrophenyl sulfate was undetectable in the transport medium in the presence of the nonspecific BCRP inhibitor chrysin.
Figure 4. Effect of transporter inhibitors on the efflux of acetaminophen sulfate from the apical membrane of BeWo cells.
BeWo monolayers grown on 12-well plates were incubated with 250 μM acetaminophen at 37°C for 4 hours, in the presence of MRP inhibitors (MK-571, indomethacin, and leukotriene C4) or the BCRP inhibitor (fumitremorgin C). Control cells contained the vehicle alone. At the end of 4 hours, the incubation medium was analyzed for acetaminophen sulfate. Data has been plotted as mean ± S.E.M. * indicates p < 0.05.
Figure 5. Effect of transporter inhibitors on the efflux of 4-nitrophenyl sulfate from the apical membrane of BeWo cells.
BeWo monolayers grown on 12-well plates were incubated with 1 μM 4-nitrophenol at 37°C for 2 hours, in the presence of MRP inhibitors (MK-571, indomethacin, and leukotriene C4) or BCRP inhibitors (fumitremorgin C and chrysin). At the end of 2 hours, the incubation medium was analyzed for 4-nitrophenyl sulfate. Data has been plotted as mean ± S.E.M. * indicates p < 0.05.
Inhibition by MK-571, indomethacin, FTC, and chrysin indicated that the apical efflux of 4-nitrophenyl sulfate and acetaminophen sulfate are mediated by the MRP isoforms as well as by BCRP. The MRP inhibitors utilized were not specific to a particular isoform and protein of all the MRP isoforms identified in BeWo cells so far (i.e., MRP 1, 2, and 5), are expressed on the apical syncytiotrophoblast membrane to some extent at least (St-Pierre et al., 2000, Azzaroli et al., 2007, Meyer Zu Schwabedissen et al., 2005). This along with weak/negligible expression of MRP2 in BeWo (Pascolo et al., 2003, Evseenko et al., 2006, Young, 2005) led to the conclusion that the MRP isoforms mediating apical sulfate efflux are likely MRP1 and/or MRP5.
3.3. Elimination of 4-nitrophenyl sulfate across the basolateral (fetal-facing) membrane
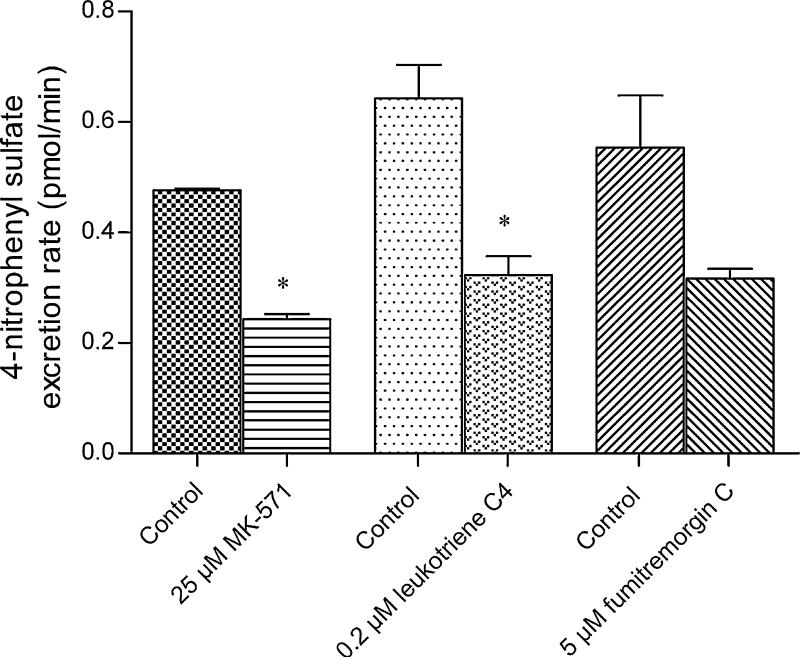
To investigate the elimination of 4-nitrophenyl sulfate across the basolateral (fetal-facing), the apical to basolateral transport of 50 μM 4-nitrophenol was determined in the presence of various inhibitors. Both MRP inhibitors MK-571 (25 μM) and LTC4 (0.2 μM) inhibited the excretion rate of 4-nitrophenyl sulfate across the basolateral membrane by approximately 50% (Fig. 6). Again, based on what is known about the expression of efflux transporters on the basolateral membrane of BeWo cells, it is likely that one or more of the MRP isoforms (likely MRP1 or MRP5) mediate the basolateral elimination of 4-nitrophenyl sulfate. Although FTC did not produce a statistically significant change in 4-nitrophenyl sulfate elimination across the basolateral membrane, it reduced the excretion rate to almost the same extent as the MRP inhibitors. BCRP is predominantly localized to syncytiotrophoblast apical membrane (Grube et al., 2007, Evseenko et al., 2007). It is likely that BCRP inhibition drives 4-nitrophenyl sulfate excretion across a basolateral membrane transporter as has been reported with hesperitin sulfate elimination in Caco-2 (Brand et al., 2008).
Figure 6. Effect of transporter inhibitors on the elimination of 4-nitrophenyl sulfate across the basolateral membrane of BeWo cells.
The apical chambers of BeWo monolayers grown on transwells were incubated with 50 μM 4-nitrophenol in the absence and presence of the different inhibitors at 37°C for 45 minutes. MK-571 and leukotriene C4 are MRP inhibitors whereas fumitremorgin C is a BCRP inhibitor. The excretion rate of 4-nitrophenyl sulfate across the basolateral membrane was obtained by determining the concentrations of 4-nitrophenyl sulfate in the basolateral chamber as a function of time. Data has been plotted as mean ± S.E.M. * indicates p < 0.05.
In our hands, when grown on transwells, BeWo monolayer integrity was compromised (i.e. the monolayers lifted off the sides of the transwell membranes) at incubation times longer than 60 minutes. Acetaminophen sulfate was undetectable in the transport medium within this period of time, and hence we were unable to determine the excretion pattern of acetaminophen sulfate across the basolateral membrane.
3.4. Effect of inhibitors on sulfate metabolite formation in BeWo cell lysate
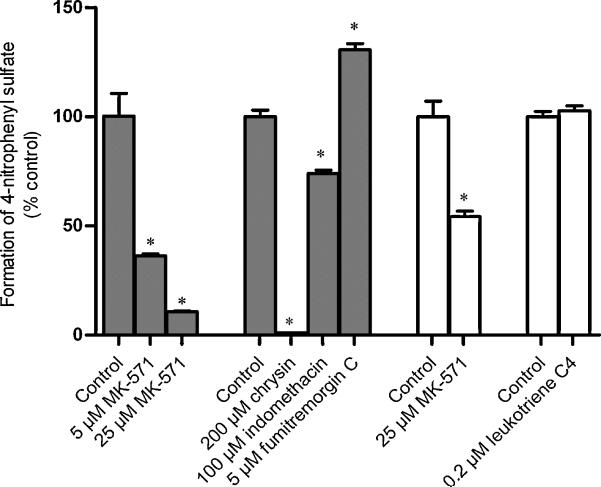
To determine if the inhibitors used in the preceding section selectively affected transport, their effect on sulfate formation in BeWo homogenate was determined. Only inhibitor concentrations that had produced a statistically significant change in the efflux or transport studies (sections 3.2 and 3.3) were used. For 1 μM 4-nitrophenol, in the presence of 5 μM MK-571, 25 μM MK-571, 100 μM indomethacin, 200 μM chrysin, and 5μM FTC, the amount of 4-nitrophenyl sulfate produced was 36%, 11%, 74%, 1%, and 131% of the vehicle-treated controls respectively (Fig. 7). The changes in all these cases were statistically significant. For 50 μM 4-nitrophenol, compared to the vehicle-treated controls, in the presence of 25 μM MK-571, 54% of 4-nitrophenyl sulfate was produced. Leukotriene C4 did not produce any change in the amount of 4-nitrophenyl sulfate produced.
Fig. 7. Effect of inhibitors on the formation of 4-nitrophenyl sulfate in BeWo cells.
BeWo cytosolic homogenate was incubated with 1μM or 50μM 4-nitrophenol and 100μM 3′-phosphoadenosine-5′-phosphosulfate (PAPS) for 2 hours at 37°C in the absence and presence of different efflux transporter inhibitors (MK-571 inhibitors: MK-571, indomethacin, leukotriene C4; BCRP inhibitors: Fumitremorgin C, chrysin). The reaction mixture was then analyzed for 4-nitrophenyl sulfate. 4-nitrophenyl sulfate formed in the presence of inhibitors was normalized to the amount produced in the vehicle-treated controls. Solid and open bars indicate that lysate was incubated with 1μM and 50μM 4-nitrophenol respectively. Data has been plotted as mean ± S.E.M. * indicates p < 0.05.
Comparing the effect of the transporter inhibitors on the formation and efflux of 4-nitrophenyl sulfate, we can see that 100 μM indomethacin inhibited metabolism and apical efflux to approximately the same extents. In the presence of 5 μM and 25 μM MK-571 respectively, only 36% and 11% of 4-nitrophenyl sulfate was formed with respect to the vehicle-treated controls, while the amount effluxed were 47% and 19% of the controls respectively. Thus no conclusion could be reached on the role of MRP isoforms in the apical efflux of 4-nitrophenyl sulfate. On the other hand, although leukotriene C4 significantly decreased the basolateral elimination of 4-nitrophenyl sulfate, it did not inhibit the formation of 4-nitrophenyl sulfate. Since out of the MRP isoforms detected on the basolateral syncytiotrophoblast membrane, MRP1 and MRP5 protein are expressed in BeWo cells, it would be reasonable to propose that either MRP1 or MRP5 mediate 4-nitrophenyl sulfate excretion across the basolateral trophoblast membrane. Chrysin, which was used as a BCRP inhibitor, almost completely inhibited both the formation and efflux of 4-nitrohenyl sulfate. Thus, the chrysin results could not be used to reach any conclusion about the role of BCRP in the apical efflux of 4-nitrophenyl sulfate. Fumitremorgin C, did not inhibit the formation of 4-nitrophenyl sulfate but increased it. However, FTC decreased the efflux of 4-nitrophenyl sulfate (~14%), and thus it can be concluded that BCRP mediates a minor portion of the apical efflux of 4-nitrophenyl sulfate.
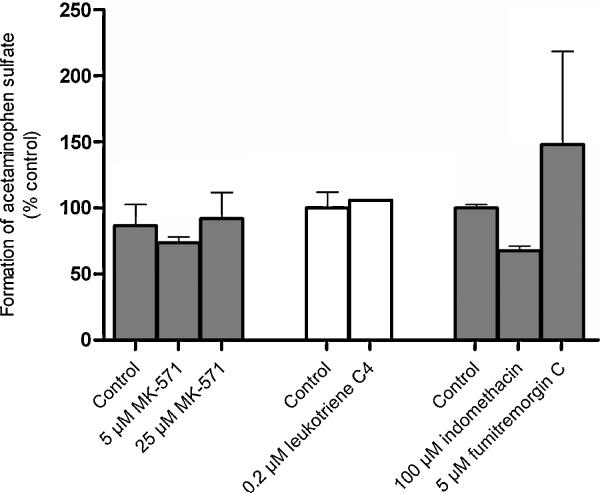
None of the inhibitors decreased the formation of acetaminophen sulfate (Fig. 8). On the other hand, MRP inhibitors as well as FTC, decreased the apical efflux of acetaminophen sulfate (Fig. 4). Therefore, it can be concluded that either MRP1 or MRP5 mediate the apical elimination of acetaminophen sulfate with a minor contribution from BCRP.
Fig. 8. Effect of inhibitors on the formation of acetaminophen sulfate in BeWo cells.
BeWo cytosolic homogenate was incubated with 250 μM acetaminophen and 100 μM 3′-phosphoadenosine-5′-phosphosulfate (PAPS) for 4 hours at 37°C in the absence and presence of different efflux transporter inhibitors (MK-571 inhibitors: MK-571, indomethacin, leukotriene C4; BCRP inhibitor: Fumitremorgin C). The reaction mixture was then analyzed for acetaminophen sulfate. Acetaminophen sulfate formed in the presence of inhibitors was normalized to the amount produced in the vehicle-treated controls. Data has been plotted as mean ± S.E.M. * indicates p < 0.05.
4. Conclusions
Efflux transporters located on the syncytiotrophoblast maternal-facing membrane, have the ability to reduce fetal exposure by pumping substances back into the maternal circulation. It is obvious that this may be one of their primary roles in the placenta. Our results demonstrate, for the first time, that another role of MRP isoforms and BCRP in trophoblast cells is sulfate conjugate elimination. We found that BCRP is responsible for a minor portion of the efflux of 4-nitrophenyl sulfate from the apical membrane of BeWo cells, while either MRP1 or MRP5 mediate its basolateral efflux. Apical elimination of acetaminophen sulfate is most likely, primarily via MRP1/MRP5, with a minor contribution from BCRP. We also concluded that 4-nitrophenyl sulfate or acetaminophen sulfate do not inhibit BCRP. Qualitatively, the mechanisms of elimination of acetaminophen sulfate and 4-nitrophenyl sulfate in BeWo cells are similar to what has been observed in rodents. In mice and rat, BCRP as well as MRP 2–4 mediate the hepatic elimination of acetaminophen sulfate (Zamek-Gliszczynski et al., 2005, Zamek-Gliszczynski et al., 2006c, Zamek-Gliszczynski et al., 2006b). Based on the hepatic excretion pattern of 4-nitrophenyl sulfate (Higaki et al., 2003) and the hepatic expression of efflux transporters (Zamek-Gliszczynski et al., 2006a) in rats, it is likely that at least a portion of 4-nitrophenyl sulfate elimination in rats is via MRP 2–4 and BCRP.
Our studies in BeWo cells suggest that in human placenta, efflux transporters located on both maternal-facing and fetal-facing syncytiotrophoblast membranes probably also eliminate sulfate conjugates. A potential physiological role of this function could be maintenance of placental estrogen balance. The placenta synthesizes increasing amounts of 17β-estradiol with advancing pregnancy (Kallen, 2004). The syncytiotrophoblast cells as well as cultured trophoblast cells express sulfotransferase isoforms that can sulfate estrogens (at least at high local estrogen concentrations). BCRP transport the sulfate conjugates of estrogens (Imai et al., 2003) and along with the sulfotransferase enzymes may play an important role in maintaining estrogen levels in the syncytiotrophoblast.
In addition to over-the-counter medicines, approximately 50% of pregnant women consume prescription medications. Common prescription drugs consumed during pregnancy include those used for the treatment of diabetes, asthma, epilepsy, psychotrpic disorders, as well as non-steroidal anti inflammatory drugs, antihistamines, antacids, decongestants etc. Some of these drugs are also substrates of the sulfotransferase enzymes (Rao and Duffel, 1991). Also, sulfate metabolites of several substrates (e.g., benzylic alcohols derived from polycyclic aromatic hydrocarbons, N-hydroxylated derivatives of homocyclic and heterocyclic amines, nitroalkanes etc.) can also produce carcinogenic DNA adducts. It is likely that a natural role of the efflux transporters located on the maternal-facing syncytiotrophoblast membrane would be reducing fetal exposure to these sulfate conjugates. On the other hand, efflux transporters located on the fetal-facing membrane could potentially enhance fetal exposure to sulfate conjugates, which underscores the importance of identifying the mechanisms of placental sulfate elimination.
Compared to other tissues, the placenta expresses high quantities of the steroid sulfatase enzyme (STS) whose physiological function in the placenta is the deconjugation of dehydroepiandrosterone sulfate (DHEAS) and 16-hydroxyl DHEAS taken up from the fetus for the de novo synthesis of estrogen in the syncytiotrophoblast (Ugele et al., 2008). It has been proposed that OATP2B1, located on the fetal-facing membrane, and BCRP located on the maternal-facing membrane, function together to eliminate dehydroepiandrosterone sulfate taken up from the fetal circulation (Grube et al., 2007). This hypothesis has been countered with the argument that high sulfatase concentrations in the syncytiotrophoblast would make this dual transport system an unlikely in vivo process (Ugele et al., 2008). The same reasoning can be extended against the role of trophoblast efflux transporters in the elimination of sulfate conjugates of small molecules. However, compared to physiological substrates such as estrone sulfate and dehydroepiandrosterone sulfate, the steroid sulfatase enzyme exhibits a lower affinity for small molecules such as 4-nitrophenylsulfate (Km = 400 μM for arylsulfatase C) (Hanson et al., 2004), whereas sulfotransferase enzymes functional in the trophoblast cells sulfate small molecules such as 4-nitrophenol at much lower concentrations (Km ~ 1 μM) (Mitra and Audus, 2009). The differential affinity indicates that in spite of the high expression of steroid sulfatase in the placenta, it is likely that the sulfotransferase enzymes and the efflux transporters act in concert to reduce unwarranted fetal exposure to at least small molecules.
Another noteworthy observation was that the transporter inhibitors affected not only efflux, but also sulfate conjugation. In several cases MK-571 at similar concentrations as used in this study or higher has been shown to inhibit sulfate efflux out of whole cells (Jeong et al., 2004, Hu et al., 2003, Zhang et al., 2007, Walle et al., 1999). In many of these cases the effect of the inhibitors on metabolism were either not examined, or were examined for time periods much shorter than those used in the efflux studies (Hu et al., 2003). Our work shows for the first time that MK-571 and indomethacin affected both formation and efflux of 4-nitrophenyl sulfate. Thus it is imperative that metabolic studies be performed alongside transport studies to tease out the effect that the inhibitors have on efflux, from that they have on sulfate formation. Further we also saw that chrysin inhibited of the formation of 4-nitrophenyl sulfate. Chrysin, a dietary flavonoid is also available as a herbal supplement and is marketed for its antioxidant and anti-inflammatory properties. It exhibits poor oral bioavailability and its plasma concentrations (10–60 nM) following oral administration are much lower than the concentration used in our studies (Walle et al., 2001). It would be interesting to study if chrysin exerts a similar effect on placental sulfotransferase enzymes at such low concentrations.
In conclusion, this study demonstrates for the first time, that efflux transporters located on the apical and basolateral membranes of the trophoblast-derived BeWo cell line have the ability to eliminate intracellularly generated sulfate conjugates. None of the inhibitors used in this study completely inhibited the efflux of either acetaminophen sulfate or 4-nitrophenyl sulfate. This suggests the involvement of other transporters such as the organic anion transporters (OATs) or the organic anion transporting polypeptides (OATPs) both of which are known to transport sulfates (Jeong et al., 2004, Hu et al., 2003, Konig et al., 2006). In placenta OATP-E/4A1 and OATP-B/2B1 are localized on apical and basolateral trophoblast membranes respectively, while OAT4 is localized on the basolateral trophoblast membrane (Young, 2005, Ugele et al., 2008). Future studies should examine if additional roles of placental organic anion transporters and organic anion transporting polypeptides include sulfate metabolite elimination.
Acknowledgements
Support for this work was received from a NICHD (HD039878) grant, Merck pre-doctoral fellowship, and Eli Lilly pre-doctoral fellowship.
The authors would like to thank Kelly Desino, Ph.D., for training PM on MS operations; and Deborah Galinis, Ph.D., for her critical comments and suggestions.
References
- ADACHI Y, SUZUKI H, SCHINKEL AH, SUGIYAMA Y. Role of breast cancer resistance protein (Bcrp1/Abcg2) in the extrusion of glucuronide and sulfate conjugates from enterocytes to intestinal lumen. Mol Pharmacol. 2005;67:923–8. doi: 10.1124/mol.104.007393. [DOI] [PubMed] [Google Scholar]
- ATKINSON DE, GREENWOOD SL, SIBLEY CP, GLAZIER JD, FAIRBAIRN LJ. Role of MDR1 and MRP1 in trophoblast cells, elucidated using retroviral gene transfer. Am J Physiol Cell Physiol. 2003;285:C584–91. doi: 10.1152/ajpcell.00418.2002. [DOI] [PubMed] [Google Scholar]
- AZZAROLI F, MENNONE A, FELETTI V, SIMONI P, BAGLIVO E, MONTAGNANI M, RIZZO N, PELUSI G, D DEA, LODATO F, FESTI D, COLECCHIA A, RODA E, BOYER JL, MAZZELLA G. Clinical trial: modulation of human placental multidrug resistance proteins in cholestasis of pregnancy by ursodeoxycholic acid. Aliment Pharmacol Ther. 2007;26:1139–46. doi: 10.1111/j.1365-2036.2007.03462.x. [DOI] [PubMed] [Google Scholar]
- BODE CJ, JIN H, RYTTING E, SILVERSTEIN PS, YOUNG AM, AUDUS KL. In vitro models for studying trophoblast transcellular transport. Methods Mol Med. 2006;122:225–39. doi: 10.1385/1-59259-989-3:225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRAND W, VAN DER WEL PA, REIN MJ, BARRON D, WILLIAMSON G, VAN BLADEREN PJ, RIETJENS IM. Metabolism and transport of the citrus flavonoid hesperetin in Caco-2 cell monolayers. Drug Metab Dispos. 2008;36:1794–802. doi: 10.1124/dmd.107.019943. [DOI] [PubMed] [Google Scholar]
- CERVENY L, PAVEK P, MALAKOVA J, STAUD F, FENDRICH Z. Lack of interactions between breast cancer resistance protein (bcrp/abcg2) and selected antiepileptic agents. Epilepsia. 2006;47:461–8. doi: 10.1111/j.1528-1167.2006.00453.x. [DOI] [PubMed] [Google Scholar]
- CHEN J, LIN H, HU M. Absorption and metabolism of genistein and its five isoflavone analogs in the human intestinal Caco-2 model. Cancer Chemother Pharmacol. 2005;55:159–69. doi: 10.1007/s00280-004-0842-x. [DOI] [PubMed] [Google Scholar]
- DEELEY RG, COLE SP. Substrate recognition and transport by multidrug resistance protein 1 (ABCC1) FEBS Lett. 2006;580:1103–11. doi: 10.1016/j.febslet.2005.12.036. [DOI] [PubMed] [Google Scholar]
- ENOKIZONO J, KUSUHARA H, SUGIYAMA Y. Involvement of breast cancer resistance protein (BCRP/ABCG2) in the biliary excretion and intestinal efflux of troglitazone sulfate, the major metabolite of troglitazone with a cholestatic effect. Drug Metab Dispos. 2007;35:209–14. doi: 10.1124/dmd.106.012567. [DOI] [PubMed] [Google Scholar]
- EVSEENKO DA, MURTHI P, PAXTON JW, REID G, EMERALD BS, MOHANKUMAR KM, LOBIE PE, BRENNECKE SP, KALIONIS B, KEELAN JA. The ABC transporter BCRP/ABCG2 is a placental survival factor, and its expression is reduced in idiopathic human fetal growth restriction. FASEB J. 2007;21:3592–605. doi: 10.1096/fj.07-8688com. [DOI] [PubMed] [Google Scholar]
- EVSEENKO DA, PAXTON JW, KEELAN JA. ABC drug transporter expression and functional activity in trophoblast-like cell lines and differentiating primary trophoblast. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1357–65. doi: 10.1152/ajpregu.00630.2005. [DOI] [PubMed] [Google Scholar]
- FALANY CN. Enzymology of human cytosolic sulfotransferases. FASEB J. 1997;11:206–16. doi: 10.1096/fasebj.11.4.9068609. [DOI] [PubMed] [Google Scholar]
- FRIEDMAN SJ, SKEHAN P. Morphological differentiation of human choriocarcinoma cells induced by methotrexate. Cancer Res. 1979;39:1960–7. [PubMed] [Google Scholar]
- FUNK C, PONELLE C, SCHEUERMANN G, PANTZE M. Cholestatic potential of troglitazone as a possible factor contributing to troglitazone-induced hepatotoxicity: in vivo and in vitro interaction at the canalicular bile salt export pump (Bsep) in the rat. Mol Pharmacol. 2001;59:627–35. [PubMed] [Google Scholar]
- GRUBE M, REUTHER S, MEYER ZU SCHWABEDISSEN H, KOCK K, DRABER K, RITTER CA, FUSCH C, JEDLITSCHKY G, KROEMER HK. Organic anion transporting polypeptide 2B1 and breast cancer resistance protein interact in the transepithelial transport of steroid sulfates in human placenta. Drug Metab Dispos. 2007;35:30–5. doi: 10.1124/dmd.106.011411. [DOI] [PubMed] [Google Scholar]
- GYEMANT N, TANAKA M, ANTUS S, HOHMANN J, CSUKA O, MANDOKY L, MOLNAR J. In vitro search for synergy between flavonoids and epirubicin on multidrug-resistant cancer cells. In Vivo. 2005;19:367–74. [PubMed] [Google Scholar]
- HANSON SR, BEST MD, WONG CH. Sulfatases: structure, mechanism, biological activity, inhibition, and synthetic utility. Angew Chem Int Ed Engl. 2004;43:5736–63. doi: 10.1002/anie.200300632. [DOI] [PubMed] [Google Scholar]
- HIGAKI K, ISHII M, ESUMI H, KANAYAMA M, OGAWARA KI, KIMURA T. Pharmacokinetic analysis of factors determining elimination pathways for sulfate and glucuronide metabolites of xenobiotics II: Studies with isolated perfused rat liver. Xenobiotica. 2003;33:1097–108. doi: 10.1080/00498250310001615771. [DOI] [PubMed] [Google Scholar]
- HU M, CHEN J, LIN H. Metabolism of flavonoids via enteric recycling: mechanistic studies of disposition of apigenin in the Caco-2 cell culture model. J Pharmacol Exp Ther. 2003;307:314–21. doi: 10.1124/jpet.103.053496. [DOI] [PubMed] [Google Scholar]
- IMAI Y, ASADA S, TSUKAHARA S, ISHIKAWA E, TSURUO T, SUGIMOTO Y. Breast cancer resistance protein exports sulfated estrogens but not free estrogens. Mol Pharmacol. 2003;64:610–8. doi: 10.1124/mol.64.3.610. [DOI] [PubMed] [Google Scholar]
- JEONG EJ, LIN H, HU M. Disposition mechanisms of raloxifene in the human intestinal Caco-2 model. J Pharmacol Exp Ther. 2004;310:376–85. doi: 10.1124/jpet.103.063925. [DOI] [PubMed] [Google Scholar]
- KALLEN CB. Steroid hormone synthesis in pregnancy. Obstet Gynecol Clin North Am. 2004;31:795–816. x. doi: 10.1016/j.ogc.2004.08.009. [DOI] [PubMed] [Google Scholar]
- KONIG J, SEITHEL A, GRADHAND U, FROMM MF. Pharmacogenomics of human OATP transporters. Naunyn Schmiedebergs Arch Pharmacol. 2006;372:432–43. doi: 10.1007/s00210-006-0040-y. [DOI] [PubMed] [Google Scholar]
- KOSTRUBSKY VE, VORE M, KINDT E, BURLIEGH J, ROGERS K, PETER G, ALTROGGE D, SINZ MW. The effect of troglitazone biliary excretion on metabolite distribution and cholestasis in transporter-deficient rats. Drug Metab Dispos. 2001;29:1561–6. [PubMed] [Google Scholar]
- LEE G, BABAKHANIAN K, RAMASWAMY M, PRAT A, WOSIK K, BENDAYAN R. Expression of the ATP-binding cassette membrane transporter, ABCG2, in human and rodent brain microvessel endothelial and glial cell culture systems. Pharm Res. 2007;24:1262–74. doi: 10.1007/s11095-007-9244-1. [DOI] [PubMed] [Google Scholar]
- LIU F, SOARES MJ, AUDUS KL. Permeability properties of monolayers of the human trophoblast cell line BeWo. Am J Physiol. 1997;273:C1596–604. doi: 10.1152/ajpcell.1997.273.5.C1596. [DOI] [PubMed] [Google Scholar]
- MEYER ZU SCHWABEDISSEN HE, GRUBE M, HEYDRICH B, LINNEMANN K, FUSCH C, KROEMER HK, JEDLITSCHKY G. Expression, localization, and function of MRP5 (ABCC5), a transporter for cyclic nucleotides, in human placenta and cultured human trophoblasts: effects of gestational age and cellular differentiation. Am J Pathol. 2005;166:39–48. doi: 10.1016/S0002-9440(10)62230-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MITRA P, AUDUS KL. Expression and functional activities of selected sulfotransferase isoforms in BeWo cells and primary cytotrophoblast cells. Biochem Pharmacol. 2009 doi: 10.1016/j.bcp.2009.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PASCOLO L, FERNETTI C, PIRULLI D, CROVELLA S, AMOROSO A, TIRIBELLI C. Effects of maturation on RNA transcription and protein expression of four MRP genes in human placenta and in BeWo cells. Biochem Biophys Res Commun. 2003;303:259–65. doi: 10.1016/s0006-291x(03)00327-9. [DOI] [PubMed] [Google Scholar]
- RAO SI, DUFFEL MW. Benzylic alcohols as stereospecific substrates and inhibitors for aryl sulfotransferase. Chirality. 1991;3:104–11. doi: 10.1002/chir.530030205. [DOI] [PubMed] [Google Scholar]
- SAKUMA-SAWADA N, IIDA S, MIZUMA T, HAYASHI M, AWAZU S. Hepatic uptake of p-nitrophenyl sulfate by transporter that acetaminophen sulfate shares for uptake: sulfate moiety as a vector for metabolite transport. Res Commun Mol Pathol Pharmacol. 1997a;97:131–8. [PubMed] [Google Scholar]
- SAKUMA-SAWADA N, IIDA S, MIZUMA T, HAYASHI M, AWAZU S. Inhibition of the hepatic uptake of paracetamol sulphate by anionic compounds. J Pharm Pharmacol. 1997b;49:743–6. doi: 10.1111/j.2042-7158.1997.tb06104.x. [DOI] [PubMed] [Google Scholar]
- SERRANO MA, MACIAS RI, BRIZ O, MONTE MJ, BLAZQUEZ AG, WILLIAMSON C, KUBITZ R, MARIN JJ. Expression in human trophoblast and choriocarcinoma cell lines, BeWo, Jeg-3 and JAr of genes involved in the hepatobiliary-like excretory function of the placenta. Placenta. 2007;28:107–17. doi: 10.1016/j.placenta.2006.03.009. [DOI] [PubMed] [Google Scholar]
- SHAROM FJ. Shedding light on drug transport: structure and function of the P-glycoprotein multidrug transporter (ABCB1) Biochem Cell Biol. 2006;84:979–92. doi: 10.1139/o06-199. [DOI] [PubMed] [Google Scholar]
- SODHA RJ, SCHNEIDER H. Sulphate conjugation of beta 2-adrenoceptor stimulating drugs by platelet and placental phenol sulphotransferase. Br J Clin Pharmacol. 1984;17:106–8. doi: 10.1111/j.1365-2125.1984.tb05009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ST-PIERRE MV, SERRANO MA, MACIAS RI, DUBS U, HOECHLI M, LAUPER U, MEIER PJ, MARIN JJ. Expression of members of the multidrug resistance protein family in human term placenta. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1495–503. doi: 10.1152/ajpregu.2000.279.4.R1495. [DOI] [PubMed] [Google Scholar]
- STANLEY EL, HUME R, VISSER TJ, COUGHTRIE MW. Differential expression of sulfotransferase enzymes involved in thyroid hormone metabolism during human placental development. J Clin Endocrinol Metab. 2001;86:5944–55. doi: 10.1210/jcem.86.12.8081. [DOI] [PubMed] [Google Scholar]
- SUZUKI M, SUZUKI H, SUGIMOTO Y, SUGIYAMA Y. ABCG2 transports sulfated conjugates of steroids and xenobiotics. J Biol Chem. 2003;278:22644–9. doi: 10.1074/jbc.M212399200. [DOI] [PubMed] [Google Scholar]
- UGELE B, BAHN A, REX-HAFFNER M. Functional differences in steroid sulfate uptake of organic anion transporter 4 (OAT4) and organic anion transporting polypeptide 2B1 (OATP2B1) in human placenta. J Steroid Biochem Mol Biol. 2008;111:1–6. doi: 10.1016/j.jsbmb.2008.04.001. [DOI] [PubMed] [Google Scholar]
- WALLE T, OTAKE Y, BRUBAKER JA, WALLE UK, HALUSHKA PV. Disposition and metabolism of the flavonoid chrysin in normal volunteers. Br J Clin Pharmacol. 2001;51:143–6. doi: 10.1111/j.1365-2125.2001.01317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALLE UK, GALIJATOVIC A, WALLE T. Transport of the flavonoid chrysin and its conjugated metabolites by the human intestinal cell line Caco-2. Biochem Pharmacol. 1999;58:431–8. doi: 10.1016/s0006-2952(99)00133-1. [DOI] [PubMed] [Google Scholar]
- YOUNG AM. Pharmaceutical Chemistry. University of Kansas; Lawrence: 2005. Characterization of efflux transporters of the human trophoblast using BeWo as a model. [Google Scholar]
- ZAMEK-GLISZCZYNSKI MJ, HOFFMASTER KA, NEZASA K, TALLMAN MN, BROUWER KL. Integration of hepatic drug transporters and phase II metabolizing enzymes: mechanisms of hepatic excretion of sulfate, glucuronide, and glutathione metabolites. Eur J Pharm Sci. 2006a;27:447–86. doi: 10.1016/j.ejps.2005.12.007. [DOI] [PubMed] [Google Scholar]
- ZAMEK-GLISZCZYNSKI MJ, HOFFMASTER KA, TIAN X, ZHAO R, POLLI JW, HUMPHREYS JE, WEBSTER LO, BRIDGES AS, KALVASS JC, BROUWER KL. Multiple mechanisms are involved in the biliary excretion of acetaminophen sulfate in the rat: role of Mrp2 and Bcrp1. Drug Metab Dispos. 2005;33:1158–65. doi: 10.1124/dmd.104.002188. [DOI] [PubMed] [Google Scholar]
- ZAMEK-GLISZCZYNSKI MJ, NEZASA K, TIAN X, BRIDGES AS, LEE K, BELINSKY MG, KRUH GD, BROUWER KL. Evaluation of the role of multidrug resistance-associated protein (Mrp) 3 and Mrp4 in hepatic basolateral excretion of sulfate and glucuronide metabolites of acetaminophen, 4-methylumbelliferone, and harmol in Abcc3−/− and Abcc4−/− mice. J Pharmacol Exp Ther. 2006b;319:1485–91. doi: 10.1124/jpet.106.110106. [DOI] [PubMed] [Google Scholar]
- ZAMEK-GLISZCZYNSKI MJ, NEZASA K, TIAN X, KALVASS JC, PATEL NJ, RAUB TJ, BROUWER KL. The important role of Bcrp (Abcg2) in the biliary excretion of sulfate and glucuronide metabolites of acetaminophen, 4-methylumbelliferone, and harmol in mice. Mol Pharmacol. 2006c;70:2127–33. doi: 10.1124/mol.106.026955. [DOI] [PubMed] [Google Scholar]
- ZHANG L, LIN G, KOVACS B, JANI M, KRAJCSI P, ZUO Z. Mechanistic study on the intestinal absorption and disposition of baicalein. Eur J Pharm Sci. 2007;31:221–31. doi: 10.1016/j.ejps.2007.04.001. [DOI] [PubMed] [Google Scholar]