Abstract
The human P2Y14 receptor is potently activated by UDP-glucose (UDP-Glc), UDP-galactose (UDP-Gal), UDP-N-acetylglucosamine (UDP-GlcNAc), and UDP-glucuronic acid. Recently, cellular release of UDP-Glc and UDP-GlcNAc has been reported, but whether additional UDP-sugars are endogenous agonists for the P2Y14 receptor remains poorly defined. In the present study, we describe an assay for the quantification of UDP-Gal with sub-nanomolar sensitivity. This assay is based on the enzymatic conversion of UDP-Gal to UDP, using 1–4-β-galactosyltransferase. UDP is subsequently phosphorylated by nucleoside diphosphokinase in the presence of [γ32P]ATP and the formation of [γ32P]UTP is monitored by high performance liquid chromatography. The overall conversion of UDP-Gal to [γ32P]UTP was linear between 0.5 and 30 nM UDP-Gal. Extracellular UDP-Gal was detected on resting cultures of various cell types, and increased release of UDP-Gal was observed in 1321N1 human astrocytoma cells stimulated with the protease-activated receptor agonist thrombin. Occurrence of regulated release of UDP-Gal suggests that, in addition to its role in glycosylation reactions, UDP-Gal is an important extracellular signaling molecule.
Keywords: UDP-galactose, P2Y14 receptor, nucleotide release, galactosyltransferase
INTRODUCTORY STATEMENT
Nucleotides and nucleotide-sugars are released as extracellular signaling molecules in most tissues, effecting a broad range of physiologically important responses via activation of P2X and P2Y purinergic receptors [1;2]. P2X receptors, comprising seven species (P2X1–P2X7), are ATP-gated ion channels. P2Y receptors belong to the superfamily of G protein-coupled receptors. At least eight P2Y receptor species have been identified in humans, seven of which (P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, P2Y12, and P2Y13) are activated by adenine and/or uridine nucleoside di- and tri-phosphates [2]. The P2Y14 receptor was identified as the eighth legitimate member of the P2Y family [3;4]. Unlike other P2 receptors, the human P2Y14 receptor is not activated by di- or tri-phosphonucleotides. Instead, the P2Y14 receptor is activated with relatively similar potencies by UDP-glucose (UDP-Glc)1, UDP-galactose (UDP-Gal), UDP-N-acetylgluosamine (UDP-GlcNAc), and UDP-glucuronic acid [3;5].
P2Y14 receptor transcripts are expressed in a broad range of human tissues, including brain, stomach, intestine, adipose, lung, spleen, and heart, and also in specialized cells such as circulating neutrophils and lymphocytes [3;6–8]. UDP-sugar-promoted signaling has been reported in astrocytes and microglial cells [9;10], lung epithelial cells [11], bone marrow hematopoietic stem cells [12], and multiple types of peripheral immune cells, including neutrophils, lymphocytes, and dendritic cells [5;7;8;13;14]. These observations suggest that UDP-sugars, high-energy donor substrates in biosynthetic reactions, are released from cells in a regulated fashion to play autocrine/paracrine signaling roles.
Recently, we demonstrated that UDP-Glc and UDP-GlcNAc are released from cells under various physiological conditions [15–19], but whether other UDP-sugars are endogenous agonist(s) for the P2Y14 receptor remains to be elucidated. Several high performance liquid chromatography (HPLC) systems have been developed to resolve UDP-sugars from each other and from other nucleotides. However, a major problem in assessing nucleotides and nucleotide-sugars in extracellular solutions is that experimental conditions often require substantial dilution of the bathing medium, resulting in nucleotide concentrations that are below threshold values for standard HPLC-coupled detectors, such as UV (λ = 260 nm) or conductimetric detectors. We have recently circumvented this limitation for the measurement of UDP-Glc and UDP-GlcNAc by monitoring the UDP-Glc- and the UDP-GlcNAc-dependent conversion of [32P]PPi to [32P]UTP, using UDP-Glc pyrophosphorylase and UDP-GlcNAc pyrophophorylase (AGX2), respectively [15–19]. In the present study, we describe an enzymatic assay that quantifies UDP-Gal with sub-nanomolar sensitivity. Using this assay, we investigated the presence of UDP-Gal in extracellular solutions on resting cells as well as in cells exposed to physiologically relevant stimuli.
MATERIALS AND METHODS
Reagents
UDP-Gal, α-lactalbumin, and nucleoside diphosphokinase (NDPK, EC 2.7.4.6) from Bakers yeast were purchased from Sigma. UDP-[3H]Gal (18 Ci/mmol) [3H]glucose (22 Ci/mmol), [D-glucose-114C]lactose (57 mCi/mmol), and [γ32P]ATP (3000 Ci/mmol) were obtained from Amersham Biosciences. Bovine recombinant 1,4-β-galactosyltransferase (4βGT, E.C.2.4.1.2) was purchased from Calbiochem. All other reagents were of the highest purity available, as previously described [17;19].
Cell cultures and incubations
Polarized cultures of well-differentiated primary human bronchial epithelial (HBE) cells and Madin-Darby canine kidney (MDCK) cells were grown on 12-mm Transwell supports (Costar), as previously described [15;19]. A549 lung epithelial, 1321N1 human astrocytoma, and MCF-7 mammary carcinoma cells were grown on 24-well plastic plates (1.1 cm2 per well). Cultures were rinsed three times with Dulbecco’s modified essential medium (DMEM) containing 4.5 g/L glucose DMEM-H and allow to rest in 300 μl (DMEM-H) for 1 h at 37°C in a humidified incubator supplemented with 5% CO2 (HBE and MDCK cells were incubated in 300 μl mucosal and 500 μl basolateral DMEM-H). After the indicated incubation, 250 μl medium (mucosal medium in the case of HBE and MDCK cells) was sampled and rapidly centrifuged to remove potentially detached cells. Supernatants were heated at 95°C for 2 min, and either used immediately or stored at −20°C. For intracellular measurements, cultures were lysed with 5% trichloroacetic acid, which was subsequently extracted with ethylic ether, as described [15;19].
Galactosyltransferase reactions
The conversion of UDP-Gal to lactose was assessed in 100 μl HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid)-buffered DMEM-H (pH 7.4). Lactalbumin (0.2 mg/ml) was included in the lactose synthetase reaction, as indicated. The conversion of UDP-Gal to N-acetyllactosamine was assessed in 100 μl glucose-free HEPES-buffered DMEM (pH 7.4) supplemented with the indicated amount of N-acetylglucosamine. Lactalbumin was omitted from these incubations. All 4βGT-catalyzed reactions were performed in the presence of 5 mM MnCl2 and the indicated amount of 4βGT (typically, 0.002 U/ml) and substrates. Except as indicated otherwise, incubations were initiated by the addition of 4βGT and they were allowed for 30 min at 30°C. Incubations were terminated by heating the samples for 2 min at 95°C. Samples were transferred to ice and either analyzed immediately or stored at −20°C. Species were separated by HPLC.
UDP-sugar hydrolysis
1321N1 cells were incubated in the presence of 0.1 μCi UDP-[3H]Gal or UDP-[3H]Glc. Samples were collected at the indicated times and heated for 2 min at 95°C to inactivate enzymatic activities potentially released from cells. Radioactive species were analyzed by HPLC.
NDPK-catalyzed phosphorylation of UDP
Unless indicated otherwise, incubations (5 min at 30 °C) were performed in 100 μl DMEM-H containing 0.1 U/ml NDPK and 0.1 μC [γ32P]ATP (~200,000 cpm, 100 nM) and the indicated amount of UDP. The conversion of [32P]ATP to [32P]UTP was monitored by HPLC. To assess UDP generated from UDP-Gal released from cells, the NDPK-catalyzed reaction was preceded by a 30 min incubation of samples with or without 0.002 U/ml 4βGT, as described above. The [32P]UTP values obtained in the absence of 4βGT were used to calculate UDP levels, and were subtracted from those obtained in the presence of 4βGT to calculate UDP-Gal concentrations.
HPLC analysis
Species were separated by HPLC (Shimadzu), using the columns and solvents indicated below. The HPLC apparatus was equipped with a Radiometer Flow-One® Beta detector (Packard) and a SPD-10A UV detector (Shimadzu), and radioactivity and absorbance at λ = 260 nm were monitored on-line as described previously [19]. Briefly, the area of radioactive peaks was quantified using the Flo-One for Windows Radio-HPLC application software (Packard). [32P]- and [3H]-radioactivity were measured by Cerenkov and liquid scintillography, respectively. [32P]-peaks displaying >750 cpm (background [32P]-background <250 cpm) and [3H]-species displaying >500 cpm ([3H]-background < 150 cpm) were analyzed (signal-to-noise-ratio >3). UDP-[3H]Gal was separated from [H3]lactose or N-[H3]acetyllactosamine (4βGT reaction) or from [3H]galactose-1P and[3H]galactose (UDP-sugar hydrolysis) using a Nova-Pack C18 column (Waters) with an ion pairing mobile phase (1 ml/min) consisting of 8 mM tetrabutylammonium hydrogen sulfate and (TBEAHS) and 17 mM H2KPO4 (pH 5.3) in 10% methanol for the initial 5 min, and 8 mM TBEAHS (pH 5.3) and 100 mM H2KPO4 in 10% methanol for additional 10 min. The elution times of [3H]galactose, [3H]galactose-1P, [H3]lactose, N-[H3]acetyllactosamine, and UDP-[3H]Gal were 1.9, 2.5, 3.2, 3.6, and, 8.8 min, respectively. Using this HPLC system, UDP-[3H]Glc, [3H]glucose, and [3H]glucose-1P eluted at 9.2, 1.8, and 2.5, respectively. N-[14C]acetyglucosamine and N-[14C]acetyllactosamine were resolved from each other using a Kromasil-NH2 column (Alltech) and 75% (v/v) acetonitrile as the mobile phase (1 ml/min). The elution times were 5.8 min for N[3H]acetylglucosamine and 8.4 min for N-[3H]acetyllactosamine. [32PATP and [32P]UTP were resolved via a Hamilton PRP-X100 anion exchange column with a mobile phase (1 ml/min) consisting of 150 mM M NH4HCO3 (pH 8.5) in 30% methanol. UTP and ATP eluted at 8.4 and 13.5 min, respectively.
ATP and UDP-Glc measurements
The mass of ATP and UDP-Glc were assessed using the luciferin-luciferase assay and the UDP-Glc pyrophosphorylase reaction, respectively, as previously described [18].
Data analysis
The Sigma Plot 10.0 software was used for data analysis. The data displayed in Figures 3C and D were fitted according to a fist-order regreation line (y = b + a; where y represents the % UTP formation, x is the substrate concentration, b is the intercept of y0 on the y-axis, and a is the slope of the curve). The b, a, and regression coefficient squared (r2) values are indicated in the legend of the Figure. The half-life (t0.5) values for UDP-sugar decay (Figure 4B) were calculated as t0.5 = 0.693/k (k is the first-order rate constant), as previously described [20]. Statistical significance (by Student’s t test) was defined as p < 0.01.
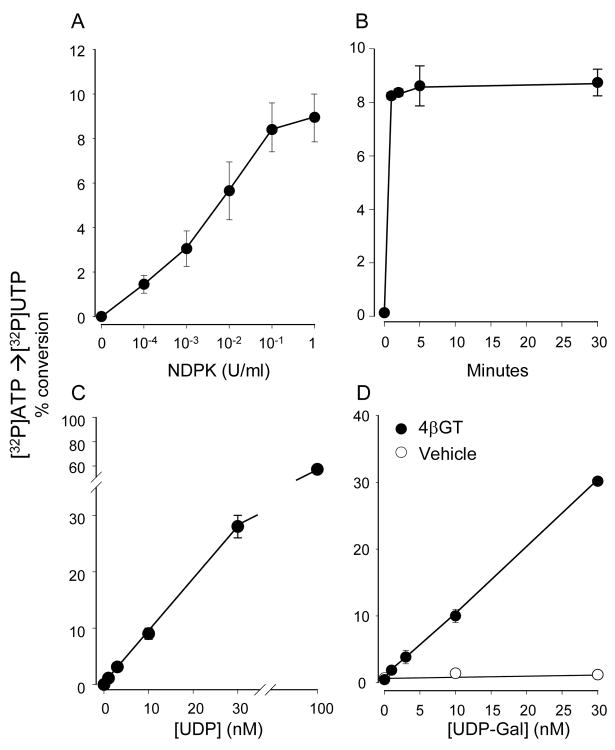
Figure 3. Assessing UDP-Gal via UDP phosphorylation.
The conversion of [γ32P]ATP to [γ32P]UTP was assessed by HPLC, as detailed in Methods. A, UDP (10 nM) was incubated in the presence of 100 nM (0.2 μCi) [γ32P]ATP and the indicated amount of NDPK. B, time course of the NDPK (0.1 U/ml)-catalyzed phosphorylation of 10 nM UDP in the presence of 100 nM (0.2 μCi) [γ32P]ATP. C, the UDP calibration curve was performed in the presence of 0.1 U/ml NDPK and 100 nM (0.2 μCi) [γ32P]ATP (5 min, 30°C). The reaction was lineal between 0–30 nM UDP (r2 = 0.9996), displaying a slope a value of 0.93784 and an intercept b value of 0.07. D, UDP-Gal (at the indicated concentration) was incubated for 30 min in the absence or presence of 0.002 U/ml 4βGT. At the end of this period, NDPK and [γ32P]ATP were added for 5 min, as detailed in C. The reaction was lineal between 0–30 nM UDP-Gal (a = 1.016, b = 0, and r2 = 0.9998). The data represent the mean ± SD, n ≥ 4. All incubations were performed in 100 μL DMEM-H supplemented with MnCl2 and α-lactalbumin, as indicated in Figure 1. Background radioactivity (i.e., observed in the absence of UDP-Gal and UDP) was subtracted from all data points to allow regression lines fitting to origin.
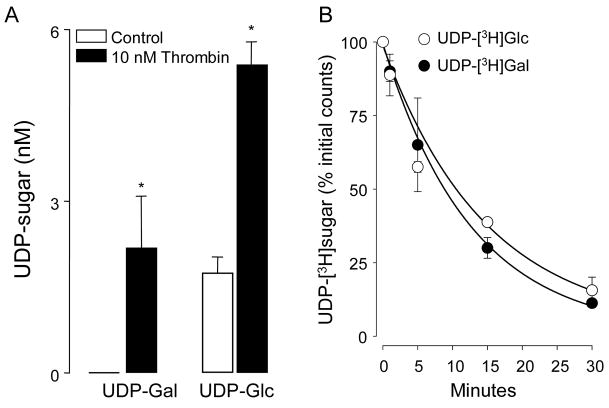
Figure 4. Enhanced UDP-Gal and UDP-Glc release from thrombin-stimulated 1321N1 human astrocytoma cells.
A, cells were rinsed and pre-incubated in 300 μl DMEM-H for 1 h, and thrombin (20 nM) was subsequently added for 5 min. UDP-Gal and UDP-Glc present in the extracellular medium were quantified, as described in Methods. The results represent the main value (± SD) from two independent experiments performed in triplicate; (*) indicates significant differences relative to control (p < 0.01, t-test). B, the stability of extracellular UDP-Gal and UDP-Glc on 1321N1 cells was assessed in cultures spiked with 0.5 μCi of the indicated radiotracer. Samples were collected at the indicated times and the resulting [3H]species were analyzed by HPLC, as described in Methods (mean ± SD, n = 4).
RESULTS
Synthesis of lactose and N-acetyllactosamine using galactosyltransferase
1,4-β-Galactosyltransferase (4βGT) from bovine milk, also known as lactose synthetase, transfers galactose from UDP-Gal to lactose, using glucose as acceptor substrate in the presence of α-lactalbumin (equation Ia). In the absence of α-lactalbumin, N-acetyglucosamine is the preferred acceptor substrate of 4βGT and lactosamine is a product of this reaction [21], as described in equation IIa.
| Ia |
| IIa |
While a number of protocols take advantage of the above reactions to assess 4βGT activity in tissues and subcellular compartments, our goal was to use purified 4βGT to quantify UDP-Gal in extracellular solutions. Based on previous measurements of extracellular nucleotides and nucleotide-sugars [19;22], UDP-Gal concentration in bulk extracellular solutions bathing cell cultures likely would be within the low nanomolar range, below the range of sensitivity of standard UV detectors. Therefore, we examined the possibility of using radiolabeled substrates to develop a 4βGT-based assay that would measure the UDP-Gal-dependent formation of radiolabeled lactose or N-acetyllactosamine, with high sensitivity. For an initial evaluation of the efficiency of 4βGT to catalyze the conversion of nanomolar amounts of UDP-Gal to either lactose or N-acetyllactosamine, UDP-[3H]Gal was used as a radiotracer, and the formation of [3H]-labeled products (Ib and IIb) was monitored by HPLC.
| Ib |
| IIb |
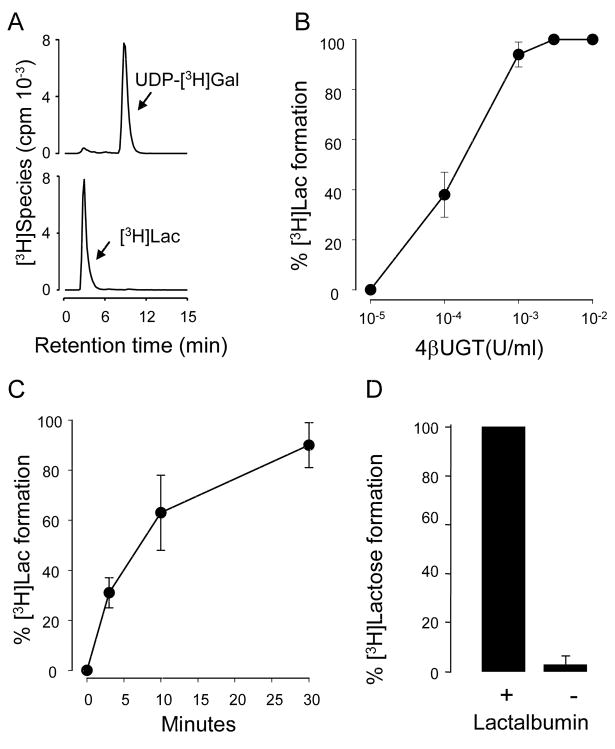
Reaction Ib was assessed in a standard glucose-containing tissue culture medium (DMEM-H) supplemented with 0.2 mg/ml α-lactalbumin and 5 mM MnCl2. A 60 min incubation of 0.002 U/ml 4βGT with 0.1 μCi (100 nM) UDP-[3H]Gal resulted in full conversion of UDP-[3H]Gal to [3H]lactose (Fig. 1A). The enzyme concentration-effect relationship for the lactose formation reaction is shown in Figure 1B and the time-course is illustrated in Figure 1C. As previously established for this reaction [21], conversion of UDP-[3H]Gal to [3H]lactose was negligible in the absence of α-lactalbumin (Fig. 1D).
Figure 1. Enzymatic conversion of UDP-[3H]Gal to [3H]lactose.
A, HPLC tracings illustrating the conversion of UDP-[3H]Gal to [3H]lactose ([3H]Lac) in the presence (bottom) or in the absence (top) of 4βGT (0.002 U/ml, 60 min, 30°C). B, incubations were for 60 min at 30°C in the presence of the indicated amount of 4βGT. C, the time-course of the reaction was examined using 0.002 U/ml 4βGT. D, the conversion of UDP-[3H]Gal to [3H]lactose was assessed in the absence (-) or presence (+) of 0.2 mg/ml α-lactalbumin. All reactions were performed in 100 μl DMEM-H containing 20 mM glucose, 100 nM (0.1 μCi) [3H]UDP-Gal, 5 mM MnCl2, and (except as indicated in D) 0.2 mg/ml α-lactalbumin. Radioactive species were separated by HPLC. The data represent the mean ± SD from at least three independent experiments performed in duplicates.
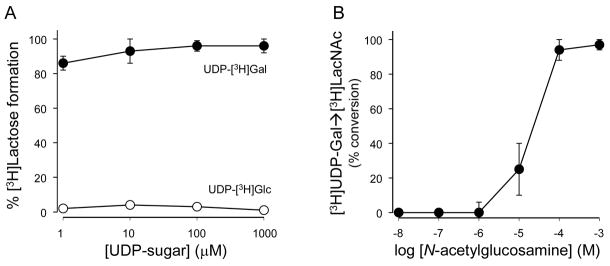
The 4 GT-catalyzed reaction was highly efficient at all UDP-Gal concentrations tested within the 1–1000 nM range (Fig. 2A). In contrast, and consistent with the high selectivity that 4 GT exhibits towards UDP-Gal over other UDP-sugars [21;23], UDP-[3H]Glc (1–1000 nM) was recovered unchanged after a 30 min incubation with 4 GT (Fig. 2A). Thus, the lactose synthetase reaction described above quantitatively transfers galactose from UDP-Gal to lactose. However, the presence of millimolar amounts of glucose in most tissue culture media and biological samples posed a limitation for assessing UDP-Gal mass with high sensitivity, using radiolabeled glucose as radiotracer. Furthermore, using conditions (enzyme concentration, incubation time, and temperature) similar to those used above for lactose synthesis, efficient conversion of UDP-[3H]Gal (100 nM) to [3H]N-acetyllactosamine was achived only at ≥ 100 μM N-acetyglucosamine (Fig. 2B). Thus, practical reasons prevented us from using high specific activity radiolabeled glucose or N-acetylglucosamine as a probe for the UDP-Gal-dependent formation of lactose or N-acetyllactosamine.
Figure 2. 4βGT-catalized formation of lactose or N-acetyllactosamine.
A, UDP-Gal but not UDP-Glc is a substrate of 4βGT. Incubations were as in Figure 1A, in the presence of the indicated concentration of UDP-[3H]Gal or UDP-[3H]Glc. B, effect of N-acetylglucosamine concentration on the conversion of 100 nM (0.1 μCi) UDP-[3H]Gal to [3H]N-acetyllactosamine. Radioactive species were separated by HPLC. The data represent the mean ± SD, n ≥ 3.
NDPK-catalyzed phosphorylation of UDP
To overcome the limitations associated with the use of radiolabeled acceptor substrate in the 4βGT reaction, we investigated the possibility of measuring the formation of UDP (rather than lactose or lactosamine) as an alternative assay for UDP-Gal mass quantification. We have previously used NDPK to synthesize UTPγS in the presence of UDP and ATPγS [24]. Based on this and other observations [20], we recently envisioned an assay that uses NDPK to quantify the UDP-dependent conversion of [γ32P]ATP to [γ32P]UTP [25]. Therefore, we reasoned that combining the 4βGT-catalyzed reaction (Ia) with the NDPK-based phosphorylation of UDP (reaction IIIa) would provide a reliable tool for the high sensitive measurement of UDP-Gal.
| Ia |
| IIIa |
To optimize the NDPK-based assay, the enzyme concentration dependence and the time course of the reaction were assessed, and a 10-fold excess of ATP mass, relative to UDP, was added to drive the reaction towards [γ32P]UTP synthesis. Figure 3A indicates that, in the presence of 100 nM [γ32P]ATP, 10 nM UDP was nearly completely converted to [γ32P]UTP, using 0.1 U/ml NDPK (5 min). Under these enzyme and substrate concentrations, the reaction reached steady state (8–9% conversion of [γ32P]ATP to [γ32P]UTP) within the first min (Fig. 3B). A calibration curve for UDP (using 0.1 U/ml NDPK, 100 nM [γ32P]ATP, 5 min at 30°C) is illustrated in Figure 3C. As little as 0.5 nM UDP could be detected with this assay (i.e., with a signal-to-noise ratio >3), and the reaction was linear to up to 30 nM UDP (Fig. 3C and data not shown). Linearity could be expanded to 100 nM UDP by increasing the concentration of [γ32P]ATP to 300 nM (not shown).
Lastly, the suitability of combining 4βGT and NDPK for the quantification of UDP-Gal was examined. Samples containing various concentrations of UDP-Gal in DMEM-H were incubated for 30 min in the absence or presence of 0.002 U/ml 4βGT, and 0.1 U/ml NDPK and 100 nM [γ32P]ATP were subsequently added for an additional 5 min. As predicted, using UDP-Gal as starting substrate, conversion of 100 nM [γ32P]ATP to [32P]UTP by NDPK was absolutely dependent on the presence of 4βGT (Fig. 3D). The overall reaction displayed a sensitivity limit of 0.5 nM UDP-Gal and linearity was observed to up 30 nM UDP-Gal. Linearity could be expanded to 100 nM UDP-Gal by increasing the concentration of [γ32P]ATP to 300 nM.
Cellular release of UDP-Gal
Having optimized conditions for the measurement of nanomolar amounts of UDP-Gal, we asked whether this UDP-sugar could be detected in the extracellular medium of culture cell models. Primary cultures of human bronchial epithelial (HBE) cells and various cell lines were rinsed and incubated undisturbed for 1 h in DMEM-H (0.3 ml/cm2). The medium was collected and samples were subsequently incubated for 30 min either with or without 4βGT, followed by a 5 min incubation with NDPK and [γ32P]ATP, as described above. UDP-Gal concentrations were calculated as the difference in [32P]UTP formation observed between samples pre-incubated with or without 4βGT. For comparison, UDP-Glc and ATP also were measured in these samples, using protocols previously described [19]. Extracellular UDP-Gal was present in detectable amounts under resting conditions on well-differentiated primary HBE cells and various cell lines, but was not detected (i.e., <0.5 nM) on resting human astrocytoma 1321N1 cells (Table 1). Differences in the mechanisms of release and extracellular metabolism likely accounted for the range of nucleotide levels observed within a given culture and among cell types.
Table 1. Extracellular nucleotide levels on resting cells.
Cells were rinsed and incubated undisturbed for 1 h in DMEM-H. The medium was collected and nucleotides measured as indicated in Methods. The data (expressed in nM) represent the mean ± SEM from at least two independent cultures with quadruplicate samples.
| Cell type | UDP-Gal | UDP | UDP-Glc | ATP |
|---|---|---|---|---|
| HBE | 1.5 ± 0.4 | 1.7 ± 0.2 | 4.6 ± 2.2 | 2.5 ± 0.5 |
| MDCK | 9.1 ± 3 | 8.2 ± 2 | 32 ± 4.2 | 1.2 ± 0.2 |
| 1321N1 | < 0.5 | 2.4 ± 0.8 | 1.5 ± 0.8 | 4.2 ± 0.5 |
| MCF-7 | 1.8 ± 0.5 | 2.7 ± 1.1 | 2.9 ± 0.4 | 6.1 ± 1.0 |
| A549 | 0.7 ± 0.3 | 3.2 ± 0.6 | 2.3 ± 1.5 | 11 ± 3.3 |
An important question regarding the extracellular accumulation of UDP-Gal is whether this nucleotide-sugar is released from cells in response to physiological stimuli. Thrombin, a serine protease, promotes release of ATP and UDP-Glc from human astrocytoma cells, via activation of protease-activated receptor-1 [17;26;27]. Therefore, we took advantage of this cell model to ask to what extent thrombin-elicited UDP-Glc release is accompanied by the release of UDP-Gal. Incubation of 1321N1 cells with thrombin (20 nM, 5 min) resulted in a enhanced accumulation of extracellular UDP-Gal, i.e., from <0.5 nM to 2.3 ± 0.8 nM (Fig. 4A). The amount of UDP-Gal release detected in response to thrombin stimulation was 690 ± 242 fmol/well (~ 7 × 105 cells), which represented approximately 0.46% of the total cellular content of this UDP-sugar (140 pmol/well). For comparison, the increase in UDP-Glc mass in thrombin-stimulated cells represented 1090 ± 165 fmoles, a 0.36% of the cellular UDP-Glc content (305 pmol/well). Thus, relative to their intracellular levels, UDP-Gal release from thrombin-stimulated 1321N1 cells was similar (although not identical) to UDP-Glc release, suggesting a mechanism of release that reflects the relative cellular abundance of these species.
It is worth nothing, however, that our measurements may have considerably underestimated the actual mass of UDP-sugar released from thrombin-stimulated cells, due to an ecto-nucleotide pyrophosphorylase (e-NPP) activity expressed on these cells [Fig. 4B and [20;28]]. Indeed, changes in extracellular levels of UDP-Glc (Fig. 4A) and ATP (1360 ± 220 pmol/well) in response to thrombin stimulation were considerably less robust than those previously assessed in the presence of the e-NPP inhibitor β, γ-methylene ATP [17;28]. Extracellular UDP levels increased not significantly after thrombin addition (control, 2.4 ± 0.6 nM; thrombin, 3.1 ± 1.2 nM). However, we have not included β,γ-methylene ATP or other ecto-nucleotidase inhibitors in our incubations due to interferences with the NDPK-based reaction used in the UDP-Gal assay (not shown). Importantly, differences in extracellular UDP-Gal vs. UDP-Glc levels unlikely reflected differences in metabolism rates between these species, since [3H]UDP-Gal and [3H]UDP-Glc were metabolized on 1321N1 cells with nearly identical half-life values (8.7 and 9.9 min, respectively, Figure 4B).
DISCUSSION
The enzymatic assay for UDP-Gal mass described here provides a highly sensitive and selective approach for the quantification of this UDP-sugar in diluted biological samples. Early works by Hull and Montgomery detected UDP-Gal in cell lysates with a sensitivity of 50 pmoles, using an ion exchange HPLC method coupled with conductimetric detection [29]. Using a UV/absorbance (λ = 260 nm) detector coupled to an ion exchange HPLC column, Tomiya et al. separated and quantified various nucleotide-sugars, including UDP-Gal, with a detection limit of approximately 1 pmol [30]. However, Kochanowski et al. illustrated that the lowest UDP-Gal concentration in cell extracts capable of giving a reliable signal-to-noise ratio is 15 pmoles [31]. The sensitivities of these previously published methods are not good enough to quantify sub-nanomolar concentrations of UDP-Gal. The assay described in the current study uses 4βGT to selectively convert UDP-Gal to UDP, and UDP is subsequently phosphorylated by NDPK using [γ32P]ATP as a radiolabeled substrate. The sensitivity of this assay (50 fmol/injection or 0.5 nM in 100 μl) is markedly greater than those previously reported. Importantly, applying the protocol described here, we quantified for the first time UDP-Gal in extracellular solutions and demonstrated the occurrence of cellular release of UDP-Gal from astrocyte-like cells in response to a physiologically relevant stimulus. Demonstration of regulated release of UDP-Gal expands previous observations with UDP-Glc and UDP-GlcNAc [17;19], providing further support for the physiological significance of the P2Y14 receptor.
Earlier studies in glycobiology have indirectly suggested that release of cellular nucleotide-sugars might occur. For example, extracellular glycosyltransferase activity [32;33] was detected and extracellular glycosylation was speculated to regulate intercellular adhesion. This idea has not been unambiguously supported by other studies [reviewed in [34]]. More recently, however, Stosell and co-workers [35] illustrated that platelet surface-associated β-1,4-galactosyltransferase produced efficient galactosylation on exposed β-GlcNAc residues when UDP-Gal was added to the platelet suspension. This observation, together with our demonstration that UDP-Glc, UDP-GlcNAc, and now UDP-Gal are released from cells in a regulated manner, reopens the question of whether extracellular UDP-sugars serve extracellular roles in addition to actions as activators of G protein-coupled receptors.
We have not addressed the issue of the mechanism of UDP-Gal release, but an attractive hypothesis is that the lumen of the secretory pathway is an important source of extracellular UDP-sugars. UDP-sugars are synthesized in the cytosol and transported to the lumen of the endoplasmic reticulum (ER) and/or Golgi apparatus to serve as sugar donors for glycosyltransferase-catalyzed reactions. ER/Golgi-resident nucleotide sugar transporters translocate cytosolic UDP-sugars to these organelles, using luminal UMP as antiporter substrate [36]. UDP-sugars imported to the ER/Golgi reach concentrations up to 20-fold higher than cytosolic levels [36]. Since ER/Golgi nucleotide-sugars are not transported back to the cytosol, potentially they can be delivered as cargo molecules and released from cells, e.g., during export/secretion of glycoconjugates. Hence, vesicular UDP-sugar release should be highly dependent on the ER/Golgi expression of the cognate transporter. Indeed, we have recently demonstrated that ER/Golgi UDP-GlcNAc transporters contribute to the release of UDP-GlcNAc from epithelial cells and yeast [19].
In conclusion, we describe a novel assay that allows the quantification of UDP-Gal with sub-nanomolar sensitivity. We have demonstrated that UDP-Gal, a potent agonist at the cell surface P2Y14 receptor, is present in the conditioned medium of various cell models, including physiologically relevant primary cultures of human bronchial epithelial cells. We also illustrated that enhanced release of UDP-Gal occurs under physiological/pathophysiological conditions, such as in thrombin-stimulated astrocytes. Given the likelihood that release of the activating P2Y14 receptor ligand may differ according to cell type, UDP-Gal predictably is an important autocrine/paracrine regulator of P2Y14 receptor activity.
Acknowledgments
We thank Catja van Heusden and Lisa Brown for tissue culture and editorial assistance of the manuscript, respectively. This work was supported by National Institute of Health grant P01-HL034322.
Footnotes
The abbreviations used are: UDP-Glc, UDP-glucose; UDP-Gal, UDP-galactose; 4βGT, 1,4-β-galactosyltransferase; NDPK, nucleoside diphosphokinase; HPLC, high performance liquid chromatography.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Burnstock G, Williams M. P2 purinergic receptors: modulation of cell function and therapeutic potential. J Pharmacol Exp Ther. 2000;295:862–869. [PubMed] [Google Scholar]
- 2.Burnstock G. Purinergic signalling. Br J Pharmacol. 2006;147(Suppl 1):S172–S181. doi: 10.1038/sj.bjp.0706429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chambers JK, Macdonald LE, Sarau HM, Ames RS, Freeman K, Foley JJ, Zhu Y, McLaughlin MM, Murdock P, McMillan L, Trill J, Swift A, Aiyar N, Taylor P, Vawter L, Naheed S, Szekeres P, Hervieu G, Scott C, Watson JM, Murphy AJ, Duzic E, Klein C, Bergsma DJ, Wilson S, Livi GP. A G protein-coupled receptor for UDP-glucose. J Biol Chem. 2000;275:10767–10771. doi: 10.1074/jbc.275.15.10767. [DOI] [PubMed] [Google Scholar]
- 4.Abbracchio MP, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, Miras-Portugal MT, King BF, Gachet C, Jacobson KA, Weisman GA, Burnstock G. Characterization of the UDP-glucose receptor (re-named here the P2Y(14) receptor) adds diversity to the P2Y receptor family. Trends Pharmacol Sci. 2003;24:52–55. doi: 10.1016/S0165-6147(02)00038-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fricks IP, Carter RL, Lazarowski ER, Harden TK. Gi-dependent cell signaling responses of the human P2Y14-receptor in model cell systems. J Pharmacol Exp Ther. 2009 doi: 10.1124/jpet.109.150730. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moore DJ, Murdock PR, Watson JM, Faull RL, Waldvogel HJ, Szekeres PG, Wilson S, Freeman KB, Emson PC. GPR105, a novel Gi/o-coupled UDP-glucose receptor expressed on brain glia and peripheral immune cells, is regulated by immunologic challenge: possible role in neuroimmune function. Brain Res Mol Brain Res. 2003;118:10–23. doi: 10.1016/s0169-328x(03)00330-9. [DOI] [PubMed] [Google Scholar]
- 7.Scrivens M, Dickenson JM. Functional expression of the P2Y(14) receptor in human neutrophils. Eur J Pharmacol. 2006;543:166–173. doi: 10.1016/j.ejphar.2006.05.037. [DOI] [PubMed] [Google Scholar]
- 8.Scrivens M, Dickenson JM. Functional expression of the P2Y(14) receptor in murine T-lymphocytes. Br J Pharmacol. 2005;146:435–444. doi: 10.1038/sj.bjp.0706322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fumagalli M, Brambilla R, D’Ambrosi N, Volonte C, Matteoli M, Verderio C, Abbracchio MP. Nucleotide-mediated calcium signaling in rat cortical astrocytes: Role of P2X and P2Y receptors. Glia. 2003;43:218–230. doi: 10.1002/glia.10248. [DOI] [PubMed] [Google Scholar]
- 10.Bianco F, Fumagalli M, Pravettoni E, D’Ambrosi N, Volonte C, Matteoli M, Abbracchio MP, Verderio C. Pathophysiological roles of extracellular nucleotides in glial cells: differential expression of purinergic receptors in resting and activated microglia. Brain Res Brain Res Rev. 2005;48:144–156. doi: 10.1016/j.brainresrev.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 11.Muller T, Bayer H, Myrtek D, Ferrari D, Sorichter S, Ziegenhagen MW, Zissel G, Virchow JC, Jr, Luttmann W, Norgauer J, Di Virgilio F, Idzko M. The P2Y14 Receptor of Airway Epithelial Cells: Coupling to Intracellular Ca2+ and IL-8 Secretion. Am J Respir Cell Mol Biol. 2005;33:601–609. doi: 10.1165/rcmb.2005-0181OC. [DOI] [PubMed] [Google Scholar]
- 12.Lee BC, Cheng T, Adams GB, Attar EC, Miura N, Lee SB, Saito Y, Olszak I, Dombkowski D, Olson DP, Hancock J, Choi PS, Haber DA, Luster AD, Scadden DT. P2Y-like receptor, GPR105 (P2Y14), identifies and mediates chemotaxis of bone-marrow hematopoietic stem cells. Genes Dev. 2003;17:1592–1604. doi: 10.1101/gad.1071503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Skelton L, Cooper M, Murphy M, Platt A. Human immature monocyte-derived dendritic cells express the G protein-coupled receptor GPR105 (KIAA0001, P2Y14) and increase intracellular calcium in response to its agonist, uridine diphosphoglucose. J Immunol. 2003;171:1941–1949. doi: 10.4049/jimmunol.171.4.1941. [DOI] [PubMed] [Google Scholar]
- 14.Shin A, Toy T, Rothenfusser S, Robson N, Vorac J, Dauer M, Stuplich M, Endres S, Cebon J, Maraskovsky E, Schnurr M. P2Y receptor signaling regulates phenotype and IFN-{alpha} secretion of human plasmacytoid dendritic cells. Blood. 2007 doi: 10.1182/blood-2007-02-071910. [DOI] [PubMed] [Google Scholar]
- 15.Lazarowski ER, Shea DA, Boucher RC, Harden TK. Release of Cellular UDP-Glucose as a Potential Extracellular Signaling Molecule. Mol Pharmacol. 2003;63:1190–1197. doi: 10.1124/mol.63.5.1190. [DOI] [PubMed] [Google Scholar]
- 16.Kreda SM, Okada SF, van Heusden CA, O’Neal W, Gabriel S, Abdullah L, Davis CW, Boucher RC, Lazarowski ER. Coordinated release of nucleotides and mucin from human airway epithelial Calu-3 cells. J Physiol. 2007;584:245–259. doi: 10.1113/jphysiol.2007.139840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kreda SM, Seminario-Vidal L, Heusden C, Lazarowski ER. Thrombin-promoted release of UDP-glucose from human astrocytoma cells. Br J Pharmacol. 2008;153:1528–1537. doi: 10.1038/sj.bjp.0707692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Esther CR, Jr, Sesma JI, Dohlman HG, Ault AD, Clas ML, Lazarowski ER, Boucher RC. Similarities between UDP-glucose and adenine nucleotide release in yeast: involvement of the secretory pathway. Biochemistry. 2008;47:9269–9278. doi: 10.1021/bi800855k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sesma JI, Esther CR, Jr, Kreda SM, Jones L, O’Neal W, Nishihara S, Nicholas RA, Lazarowski ER. ER/golgi nucelotide sugar transporters contribute to the cellular release of UDP-sugar signaling molecules. J Biol Chem. 2009;284:12572–12583. doi: 10.1074/jbc.M806759200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lazarowski ER, Boucher RC, Harden TK. Constitutive release of ATP and evidence for major contribution of ecto-nucleotide pyrophosphatase and nucleoside diphosphokinase to extracellular nucleotide concentrations. J Biol Chem. 2000;275:31061–31068. doi: 10.1074/jbc.M003255200. [DOI] [PubMed] [Google Scholar]
- 21.Fitzgerald DK, Brodbeck U, Kiyosawa I, Mawal R, Colvin B, Ebner KE. Alpha-lactalbumin and the lactose synthetase reaction. J Biol Chem. 1970;245:2103–2108. [PubMed] [Google Scholar]
- 22.Lazarowski ER, Boucher RC, Harden TK. Mechanisms of release of nucleotides and integration of their action as P2X- and P2Y-receptor activating molecules. Mol Pharmacol. 2003;64:785–795. doi: 10.1124/mol.64.4.785. [DOI] [PubMed] [Google Scholar]
- 23.Qasba PK, Ramakrishnan B, Boeggeman E. Structure and function of beta -1,4-galactosyltransferase. Curr Drug Targets. 2008;9:292–309. doi: 10.2174/138945008783954943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lazarowski ER, Watt WC, Stutts MJ, Brown HA, Boucher RC, Harden TK. Enzymatic synthesis of UTP gamma S, a potent hydrolysis resistant agonist of P-2U-purinoceptors. Br J Pharmacol. 1996;117:203–209. doi: 10.1111/j.1476-5381.1996.tb15175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tatur S, Kreda S, Lazarowski E, Grygorczyk R. Calcium-dependent release of adenosine and uridine nucleotides from A549 cells. Purinergic Signal. 2008;4:139–146. doi: 10.1007/s11302-007-9059-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blum AE, Joseph SM, Przybylski RJ, Dubyak GR. Rho-family GTPases modulate Ca(2+) -dependent ATP release from astrocytes. Am J Physiol Cell Physiol. 2008;295:C231–C241. doi: 10.1152/ajpcell.00175.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Joseph SM, Buchakjian MR, Dubyak GR. Colocalization of ATP release sites and ecto-ATPase activity at the extracellular surface of human astrocytes. J Biol Chem. 2003;278:23342–23342. doi: 10.1074/jbc.M302680200. [DOI] [PubMed] [Google Scholar]
- 28.Joseph SM, Pifer MA, Przybylski RJ, Dubyak GR. Methylene ATP analogs as modulators of extracellular ATP metabolism and accumulation. Br J Pharmacol. 2004;142:1002–1014. doi: 10.1038/sj.bjp.0705865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hull SR, Montgomery R. Separation and analysis of 4′-epimeric UDP-sugars, nucleotides, and sugar phosphates by anion-exchange high-performance liquid chromatography with conductimetric detection. Anal Biochem. 1994;222:49–54. doi: 10.1006/abio.1994.1452. [DOI] [PubMed] [Google Scholar]
- 30.Tomiya N, Ailor E, Lawrence SM, Betenbaugh MJ, Lee YC. Determination of nucleotides and sugar nucleotides involved in protein glycosylation by high-performance anion-exchange chromatography: sugar nucleotide contents in cultured insect cells and mammalian cells. Anal Biochem. 2001;293:129–137. doi: 10.1006/abio.2001.5091. [DOI] [PubMed] [Google Scholar]
- 31.Kochanowski N, Blanchard F, Cacan R, Chirat F, Guedon E, Marc A, Goergen JL. Intracellular nucleotide and nucleotide sugar contents of cultured CHO cells determined by a fast, sensitive, and high-resolution ion-pair RP-HPLC. Anal Biochem. 2006;348:243–251. doi: 10.1016/j.ab.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 32.Roth S, McGuire EJ, Roseman S. Evidence for cell-surface glycosyltransferases. Their potential role in cellular recognition. J Cell Biol. 1971;51:536–547. doi: 10.1083/jcb.51.2.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Den H, Kaufman B, McGuire EJ, Roseman S. The sialic acids. XVIII. Subcellular distribution of seven glycosyltransferases in embryonic chicken brain. J Biol Chem. 1975;250:739–746. [PubMed] [Google Scholar]
- 34.Roseman S. Reflections on glycobiology. J Biol Chem. 2001;276:41527–41542. doi: 10.1074/jbc.R100053200. [DOI] [PubMed] [Google Scholar]
- 35.Hoffmeister KM, Josefsson EC, Isaac NA, Clausen H, Hartwig JH, Stossel TP. Glycosylation restores survival of chilled blood platelets. Science. 2003;301:1531–1534. doi: 10.1126/science.1085322. [DOI] [PubMed] [Google Scholar]
- 36.Hirschberg CB, Robbins PW, Abeijon C. Transporters of nucleotide sugars, ATP, and nucleotide sulfate in the endoplasmic reticulum and Golgi apparatus. Annu Rev Biochem. 1998;67:49–69. doi: 10.1146/annurev.biochem.67.1.49. [DOI] [PubMed] [Google Scholar]