Summary
Cyclophosphamide, a drug that has not previously had an important role in whole-organ transplantation, was given as a primary immunosuppressant to one liver and eleven kidney recipients, in combination with prednisone and horse antilymphocyte globulin. One of the patients died despite good renal-graft function. Two kidneys from a common cadaveric donor failed. The other nine patients have excellent function of their homografts after 2–3 months. Cyclophosphamide was substituted for azathioprine in one hepatic and five renal recipients who were suspected of having liver toxicity from azathioprine 3 months to almost 8 years post-transplantation. Graft function was maintained after this change, and the evidence of liver injury subsided.
INTRODUCTION
Since its clinical introduction in 1962, azathioprine has been considered the cornerstone agent in both the double-drug 1-4 and triple-drug 5 immunosuppressive regimens that have received worldwide trial. In the first regimen azathioprine was given with prednisone and in the second it was combined with prednisone and heterologous antilymphocyte globulin (a.l.g.).
We have shown azathioprine to be dispensable by replacing it from the beginning with cyclophosphamide in twelve organ recipients who were managed in other respects by the triple-drug programme. In addition, six patients who were treated at first with azathioprine and who were suspected of having hepatoxicity from this agent had cyclophosphamide substituted from 3 months to almost 8 years after renal (five cases) or liver (one case) transplantation.
Although the follow-ups are still short, we are convinced that cyclophosphamide is a major neglected immunosuppressant—one that is at least as potent and practical as azathioprine for whole-organ transplantation and one that may permit substantial improvements in patient care.
METHODS
Cyclophosphamide from Outset
Using standard surgical techniques,6,7 eleven patients received kidney grafts and one was given an orthotopic liver for the indications listed in table I. Three of the eleven renal patients were undergoing transplantation for the second time after rejecting their first kidneys hyperacutely (case 10), acutely (case 2), or by a chronic process (case 3), despite therapy with the old triple-drug regimen that included azathioprine. A fourth renal patient (case 6) hyperacutely rejected a maternal homograft during treatment with the cyclophosphamide-containing triple-drug programme. The transplant was immediately removed. The recipient had performed cytotoxic antibodies against the mother's lymphocytes but not against those of the cadaver, which donated a functioning kidney 1½ days later.
TABLE I.
TRANSPLANT DETAILS
| Case no. | Recipient |
Kind of graft |
Donor | Time of follow-up (mo.) |
No. of antigen groups |
Histocompatibility grade |
Dose of cyclophosphamide/kg. |
Present graft function |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | Disease | Mismatched | Missing | Loading per 24 hr. |
Average per day postoperatively |
|||||||
| Cadaveric donor: | ||||||||||||
| 1 | 15 | Wilson's disease | Orthotopic liver | Cadaver | 3 | 1 | .. | C | 4·4 | 1·1 | Normal | |
| 2 | 20 | C.P.N. | 2nd kidney | Cadaver | 3 | 4 | .. | D | 1·0 | 1·3 | Normal | |
| 3 | 36 | C.G.N. | 2nd kidney | Cadaver | 3 | 3 | .. | D | 2·7 | 0·8 | Patient dead | |
| 4 | 35 | C.G.N. | Kidney 2nd kidney |
Cadaver Cadaver |
|
3 3 |
1 .. |
D D |
2·9 .. |
.. 1·2 |
Failed Normal |
|
| 5 | 51 | C.G.N. | Kidney | Cadaver | 2 | 1 | D | 3·7 | 1·3 | Failed | ||
|
|
6a | 27 | C.P.N. | Kidney | Mother | .. | 1 | .. | C, *F | 4·8 | .. | Hyperacute rejection |
| 6b | .. | .. | 2nd kidney | Cadaver | 2 | 2 | 2 | D | .. | 1·3 | Normal | |
| 7 | 40 | C.G.N. | Kidney | Cadaver | 2 | 1 | 1 | C | 2·8 | 1·4 | Normal | |
| 8 | 40 | C.G.N. | Kidney | Cadaver | 2 | 3 | 1 | D | 3·4 | 0·9 | Normal | |
| Related donor: | ||||||||||||
| 9 | 41 | C.G.N. | Kidney | Brother | 3 | 0 | .. | A | 5·8 | 1·6 | Normal | |
| 10 | 31 | C.G.N. | Kidney | Sister | 2 | 1 | D | 2·4 | 0·8 | Normal | ||
| 11 | 27 | C.P.N. | Kidney | Sister | 2 | 0 | .. | A | 4·3 | 1·5 | Normal | |
| 12 | 48 | C.G.N. | Kidney | Sister | 2 | 0 | 2 | B | 4·6 | 1·1 | Normal | |
Positive cross-match with donor.
C.P.N. = Chronic pyelonephritis.
C.G.N. = Chronic glomerulonephritis.
Cadaveric organs provided the definitive homografts in eight of the twelve cases. Within this group of donor/recipient pairs there were no good HL-A matches—indeed a “full house” mismatch was either proved or else possible in half of these cases. When the transplanted organ came from a relative the quality of matching ranged from excellent to poor. A summary grade (table I) of the tissue match was given by the A–D, F designations of Terasaki and colleagues,8 the A–D spectrum going from the best to the worst compatibility, and the F denoting a positive cross-match.
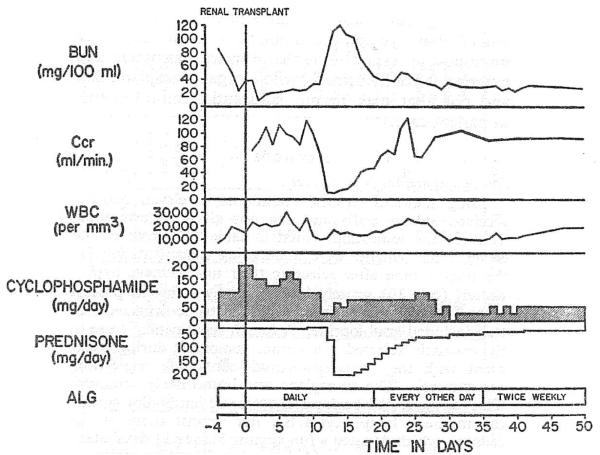
The treatment schedule was adapted from the triple-drug regimen used at our institution since 1966, except that cyclophosphamide was administered in place of azathioprine. The patients receiving consanguineous kidneys were given cyclophosphamide orally for 4–9 days before operation (average 5·3), starting with 1·4–2·1 mg. per kg. per day and concluding with loading doses the day before and the day of transplantation of 2·45–5·8 mg. per kg. per day. During the same pretreatment interval daily intramuscular injections were given of horse a.l.g. that had leucoagglutinin and lymphocytotoxin titres of 1/8000 and 1/16,000, respectively, and a protein concentration of 5·1–7·2 g. per 100 ml. Finally, prednisone was started orally in preoperative doses of 0·6–0·7 mg. per kg. per day. A typical schedule of treatment for intrafamilial cases is shown in fig. 1.
Fig. 1.
A moderately severe but completely reversed rejection crisis 10 days after renal transplantation from a sibling to an HL-A matched recipient who was treated with cyclophosphamide, prednisone, and A.L.G. b.u.n. = Blood-urea-nitrogen. Ccr = Creatinine clearance. w.b.c. = White-blood-cell count.
For the cadaveric cases, pretreatment was not feasible, and immunosuppression was usually started on the day of operation. Under these conditions (fig. 2), the initial dose of cyclophosphamide was 1·0–4·8 mg. per kg. by intravenous infusion over 3 hours, usually during the operation.
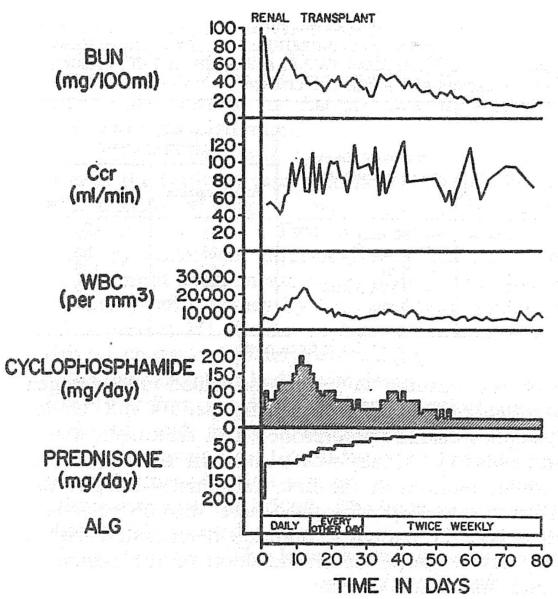
Fig. 2.
Case 2: uncomplicated early course of the recipient of a cadaveric kidney.
After transplantation, drug therapy was by the general triple-drug format previously described,5,7 except that cyclophosphamide instead of azathioprine was used (figs. 1 and 2). The protocol called for a decreasing number of a.l.g. injections after 2 weeks and for adjustments in prednisone dosage, especially after the first few weeks, according to the difficulty encountered with rejection. The average daily doses of cyclophosphamide used postoperatively were usually slightly more than 1 mg. per kg. per day (table I). These doses were adjusted carefully, a primary objective being the avoidance of leucopenia (figs. 1 and 2). If for any reason the patient was not eating, the cyclophosphamide or prednisone was switched from the oral to the intravenous route.
Late Cyclophosphamide Substitution
Renal recipients
Five renal recipients had liver-function abnormalities consisting most prominently in four cases of elevated s.g.o.t. and s.g.p.t. These four recipients were not jaundiced, but the fifth patient had become deeply icteric (bilirubin 13 mg. per 100 ml.) and was approaching the terminal stages of liver disease. Multiple sera from the five recipients were studied by three precipitation methods as well as by complement-fixation to determine if the Australia (Au) antigen was or had ever been detectable in the postoperative period. Evidence of the Au antigen was present in only one of the cases. In all five, it was considered that azathioprine hepatotoxicity was a possible factor. Consequently, azathioprine was stopped from 3 months to almost 8 years post-transplantation (table II) and cyclophosphamide was substituted at comparable doses per kg. Hepatic and renal function were monitored both before and after this change.
TABLE II.
S.G.O.T. IN FIVE RENAL HOMOGRAFT RECIPIENTS DURING TREATMENT WITH AZATHIOPRINE AND CYCLOPHOSPHAMIDE, RESPECTIVELY (THE MEAN VALUES ARE GIVEN OF 2–5 DETERMINATIONS BEFORE AND AFTER THE CHANGE)
| Case no. |
Time of change post-transplantation |
S.G.O.T. (I.U.) during treatment with: |
|
|---|---|---|---|
| Azathioprine | Cyclo- phosphamide |
||
| 1 | 10 mo. | 256 | 312 |
| 2 | 7 yr., 9 mo. | 425 | 62 |
| 3 | 8 mo. | 142 | 105 |
| 4 | 3 yr., 3 mo. | 607 | 193 |
| 5* | 3 mo. | 91 | 22 |
Au-antigen positive.
Hepatic recipient
Cyclophosphamide was substituted for azathioprine in the recipient of an orthotopic liver homograft who had persistent transaminase elevations many months after operation (see Results).
RESULTS
Cyclophosphamide from Outset
Mortality
One of the twelve patients (case 3) died 62 days after retransplantation. In January, 1969, she had received a cadaveric kidney which slowly failed over the ensuing 2 years, the blood-urea-nitrogen eventually rising to about 100 mg. per 100 ml. Under cyclophosphamide/prednisone/a.l.g. treatment, a second cadaveric kidney was placed on the other side without removal of the first transplant. Despite the sparing use of prednisone, there was no rejection. However, the patient's condition deteriorated with a very severe herpes-zoster infection of the perineum which was made almost unmanageable by intractable diarrhœa. At necropsy, a giant ulcer of the cæcum and ascending colon was found, involving most of the bowel circumference and causing transmural necrosis. Both transplanted kidneys were examined by Prof. K. A. Porter, of St. Mary's Hospital and Medical School, London. The first homograft had a fresh thrombosis of the renal artery with multiple cortical infarctions. The renal homograft which had been protected by the new immunosuppressive regimen was almost normal. The only abnormalities were a light, scattered infiltration with mononuclear cells and atrophy of a few tubules.
Graft loss
The hyperacutely rejected maternal kidney will not be mentioned further in this report since it was immediately removed and could not be considered for the evaluation of treatment. Of the twelve definitive organs, there were two renal homo-grafts, both from the same cadaveric donor, which failed. One of these organs (case 4) never functioned well and was unable to reduce the blood-urea-nitrogen below 64 mg. per 100 ml. during the first 5 postoperative weeks. A second cadaveric kidney was then placed on the other side without removal of the first one, with excellent function during the ensuing 6 weeks. A recent intravenous pyelogram displayed the second transplant but not the first one. The contralateral kidney from the same donor had fair initial function (case 5) which first improved and later deteriorated so that return to dialysis was necessary 9½ weeks after transplantation.
Rejection
There was no evidence of rejection of seven of the twelve organs that have been followed up for 2 months or longer; these included six kidneys (fig. 2) and the liver. Two other homografts (cases 6 and 8) underwent equivocal rejection for which minor steroid adjustments were made. One patient, who was given a kidney by her sister with whom she had a perfect HL-A match, had a severe rejection crisis which was completely reversed (fig. 1).
The liver recipient had a completely uncomplicated recovery at first, but 6 weeks after operation jaundice developed, and serum transaminases and alkaline-phosphatase levels rose. Coincident with these changes, the complement-fixation test for Au antigen became positive with a titre of 1/5000. The findings of hepatitis subsequently receded.
Graft function
The kidneys and liver remaining all have normal or nearly normal function after 2 to more than 3 months.
Toxicity
Most cases have had no evidence of bone-marrow depression at any time (figs. 1 and 2). However, three of the twelve patients had significant leucopenia as defined by the need to omit cyclophosphamide for at least 3 consecutive days. There was only one example of leucopenia for as long as a week. Thrombocytopenia was occasionally seen, but it seemed related more to a.l.g. therapy than to cyclophosphamide. Some of the classical complications of cyclophosphamide therapy were never observed, including hemorrhagic cystitis and alopecia. Early in the series, cyclophosphamide was given orally in the early evening, some time after the evening meal. Anorexia, nausea, vomiting, and diarrhoea were common. However, when the medication was given with the evening meal, these symptoms were avoided in all but case 3. At necropsy, the explanation for the intractable diarrhoea in this patient was a giant cecal and right colonic ulcer.
Late Cyclophosphamide Substitution
Renal recipients
Renal recipients (cases 2–5, table II), who had elevated serum-transaminases as the predominant finding of post-transplantation liver after the change to cyclophosphamide. In the fifth patient (case 1, table II), with hepatic failure and jaundice, the raised transaminase levels did not improved immediately; however, the serum-bilirubin slowly fell from 13 mg. per 100 ml. to normal and she eventually recovered completely. In all five recipients, renal homograft function remained unchanged or else improved after the change to cyclophosphamide.
Hepatic recipient
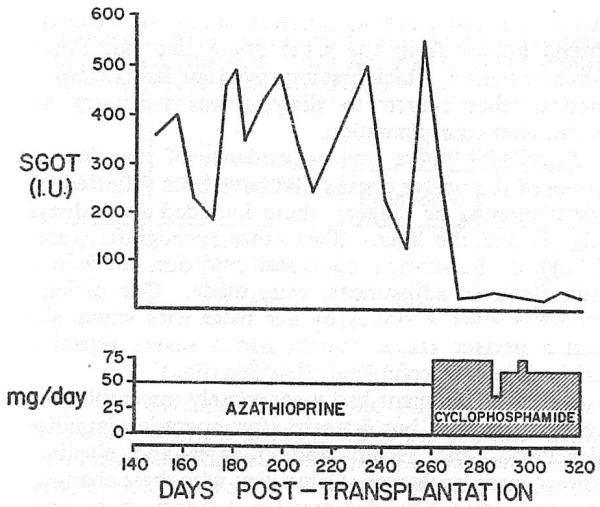
This patient had undergone liver replacement 7 months earlier for the treatment of chronic aggressive hepatitis, Au-antigen positive. Au antigen disappeared for almost 2 months but then reappeared. At the same time, all hepatic-function tests deteriorated. The problem was diagnosed and treated as acute hepatitis. Many of the manifestations of the serum-hepatitis, including jaundice, subsided, but the Au antigen remained positive and there were persistent deviations of s.g.o.t. and s.g.p.t. After substitution of cyclophosphamide for azathioprine, because of the suspicion of drug hepatotoxicity, the transaminase values promptly fell (fig. 3) despite continuing Au antigenæmia.
Fig. 3.
S.G.O.T. values (normal range 4-13 I.U.) in a patient who received an orthotopic liver homograft 9 months previously; azathioprine was replaced by cyclophosphamide.
DISCUSSION
Until now, consistent recovery after whole-organ transplantation in man has been well documented only with drug regimens that included azathioprine. In this investigation, cyclophosphamide replaced azathioprine, and was given in combination with prednisone and horse a.l.g. The cyclophosphamide doses per kg. were smaller than those that would have been predicted for azathioprine, but they were similar enough, so that the substitution of cyclophosphamide for azathioprine required little mental adjustment.
We looked for the well-known adverse reactions of cyclophosphamide seen in cancer chemotherapy—including alopecia, bone-marrow depression, and serious gastrointestinal disturbances. In the dose range used, these toxic effects were generally either not troublesome or not observed at all. In one patient, a cæcal ulcer was the most important factor in causing death, but this kind of complication has been seen with all steroid-containing immunosuppressive regimens. Leucopenia, presumably from bone-marrow depression, was observed in only three patients, and it was a transient finding that did not necessitate prolonged withdrawal of cyclophosphamide treatment.
The results of therapy with this triple-drug programme have been so encouraging that we believe that cyclophosphamide should make possible major improvements in the care of organ-transplant recipients. Rejection has never been diagnosed in seven of the twelve recipients. It was of a very minor nature in two others and it was severe but completely reversible in one more. Two kidneys eventually failed, but under circumstances that may have been extenuating. Both these organs came from the same cadaver; one never functioned properly and the other provided acceptable function for a short time only.
The success of treatment was especially striking since most of the cases were unfavourable by well-accepted criteria. Two-thirds of the organs were from cadavers; almost half of the recipients were relatively old; four of them were undergoing retransplantation with its risk of prior sensitisation; one was a liver recipient; and poor HL-A matches were present in nine of the twelve cases (although it must be conceded from our own experience that this is not necessarily an adverse factor 9).
In retrospect, it is almost unbelievable that cyclophosphamide has played such a trivial role in the transplantation of whole human organs, since the drug has been thought for a decade to possess strong immunosuppressive properties. 10-12 Moreover, the animal work in favour of this contention has been frequently reviewed and updated.13-19 Essentially all of the encouraging laboratory investigations were done in mice, rats or other rodents, or in rabbits. Unfortunately, when cyclophosphamide was tested in the dog kidney or intestinal transplantation models as an intermediate step to clinical application, no prolongation of graft survival was obtained, or else the effect was a minor one.20-22 It may now be suggested that the dampening influence of the discouraging canine experiments was based upon a species difference that made the dog an inappropriate animal to evaluate cyclophosphamide for human immunosuppression.
Despite the experience in dogs, there has been evidence in man supporting the propriety of testing cyclophosphamide for clinical whole-organ transplantation. Some of this information came from efforts to promote tolerance to bone-marrow grafts, as proposed by Santos and colleagues 23,24 and subsequently carried out by several other workers. Prodigious doses of cyclophosphamide (45–100 mg. per kg. per day) were given, but only for a few days in close temporal approximation to infusion of the homologous bone-marrow. Although such efforts represent an essentially different therapeutic approach to ours, it is worth emphasising that Santos' data on several immunosuppressive drugs have indicated that, in man, cyclophosphamide is superior to most other agents, including azathioprine.23,25
Immunosuppression with cyclophosphamide has been reported in a few cases of kidney transplantation. Almost 10 years ago Goodwin et al. treated a renal recipient with cyclophosphamide plus prednisone; good kidney function was maintained during much of the 144 days of post-transplantation life.26 Shortly afterwards, Parsons, Fox, and others reported four cases of cadaveric renal transplantation in which cyclophosphamide was given as the sole therapy.16,27 One patient died after a technical surgical accident, and a second died from infection after 33 days. The other two recipients lived for 8 and 23 months, a remarkable feat which in our experience can be achieved only rarely with azathioprine or any other single agent after renal transplantation from a non-relative.6 In a follow-up of these two patients, and four more who survived for only a few days, Parsons et al. pessimistically advised against further clinical trials of cyclophosphamide.28
It is regrettable that these early efforts at renal transplantation under cyclophosphamide therapy were made when the conditions were not more propitious. Specifically, it was then believed that the deliberate production of leucopenia was desirable, whereas now we believe such a policy to be dangerous and unnecessary. Moreover, as already implied, the importance of combination drug therapy was not yet fully appreciated. We were able to use cyclophosphamide with two other potent immunosuppressants, prednisone and a.l.g., and against a background of considerable experience with multiple drug treatment.
The impact that cyclophosphamide will have on clinical transplantation is still speculative, since few cases have been so managed and follow-up has been limited. At the least, however, cyclophosphamide is a potent drug to which one can turn if azathioprine therapy is stopped, as was demonstrated in the six patients with probable azathioprine hepatotoxicity described here. Moreover, it is conceivable that improvements in other aspects of routine immunosuppression may be possible using cyclophosphamide as a component of the triple-drug regimen (as described here), in combination with prednisone or a.l.g. alone, or in conjunction with azathioprine or other agents that have not yet been fully evaluated.
Acknowledgments
This work was supported by research grants from the Veterans Administration, by grants RR-00051 and RR-00069 from the general clinical research centres programme of the Division of Research Resources, National Institutes of Health, and by grants AI-10176-01, AI-AM-08898, AM-07772, GM-01686, and HE-09110 of the U.S. Public Health Service.
REFERENCES
- 1.Hume DM, Magee JH, Kauffman HM, Jr., Rittenbury MS, Prout GR., Jr Ann. Surg. 1963;158:608. doi: 10.1097/00000658-196310000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murray JE, Merrill JP, Harrison JH, Wilson RE, Dammin GJ. New Engl. J. Med. 1963;268:1315. doi: 10.1056/NEJM196306132682401. [DOI] [PubMed] [Google Scholar]
- 3.Starzl TE, Marchioro TL, Waddell WR. Surgery Gynec. Obstet. 1963;117:385. [PMC free article] [PubMed] [Google Scholar]
- 4.Woodruff MFA, Robson JS, Nolan B, Lambie AT, Wilson TI, Clark JG. Lancet. 1963;ii:675. doi: 10.1016/s0140-6736(63)90465-3. [DOI] [PubMed] [Google Scholar]
- 5.Starzl TE, Marchioro TL, Porter KA, Iwasaki Y, Cerilli GJ. Surgery Gynec. Obstet. 1967;124:301. [PMC free article] [PubMed] [Google Scholar]
- 6.Starzl TE. Experience in Renal Transplantation. Philadelphia: 1964. [Google Scholar]
- 7.Starzl TE. Experience in Hepatic Transplantation. Philadelphia: 1969. [Google Scholar]
- 8.Mickey MR, Kreisler M, Albert ED, Tanaka N, Terasaki PI. Tissue Antigens. 1971;1:57. doi: 10.1111/j.1399-0039.1971.tb00079.x. [DOI] [PubMed] [Google Scholar]
- 9.Starzl TE, Porter KA, Halgrimson CG, Andres G, Hurwitz R, Giles G, Terasaki PI, Penn I, Lilly J, Starkie SJ, Schroter GPJ, Putnam CW. Ann. Surg. 1970;172:437. doi: 10.1097/00000658-197009000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stender HS, Ringleb D, Strauch D, Winter H. Strahlenther. 1959;43(suppl):392. [PubMed] [Google Scholar]
- 11.Maguire HC, Jr., Maibach HI. J. invest. Derm. 1961;37:427. doi: 10.1038/jid.1961.138. [DOI] [PubMed] [Google Scholar]
- 12.Sutton WR, Griffith HB, Preston FW. Surg. Forum. 1961;12:117. [PubMed] [Google Scholar]
- 13.Santos GW, Owens AH., Jr Fedn Proc. 1962;21:25. [Google Scholar]
- 14.Jones JW, Brody GL, Oneal RM, Haines RF. J. surg. Res. 1963;3:189. doi: 10.1016/s0022-4804(63)80057-8. [DOI] [PubMed] [Google Scholar]
- 15.Berenbaum ML, Brown IN. Nature. 1963;200:84. doi: 10.1038/200084a0. [DOI] [PubMed] [Google Scholar]
- 16.Fox M. Transplantation. 1964;2:475. doi: 10.1097/00007890-196407000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Brody GL, Jones JW, Haines RF. J. Am. med. Ass. 1965;191:297. [PubMed] [Google Scholar]
- 18.Frisch AW, Davies GH. Cancer Res. 1965;25:745. [PubMed] [Google Scholar]
- 19.Owens AH, Jr., Santos GW. Transplantation. 1971;11:378. doi: 10.1097/00007890-197104000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Reams GB. Nature. 1963;197:713. doi: 10.1038/197713a0. [DOI] [PubMed] [Google Scholar]
- 21.Zukoski CF, Callaway JM, Rhea WR. Transplantation. 1963;1:293. doi: 10.1097/00007890-196301030-00004. [DOI] [PubMed] [Google Scholar]
- 22.Preston FW, Macalalad F, Wachowski TJ, Randolph DA, Apostol JV. Surgery, St. Louis. 1966;60:1203. [PubMed] [Google Scholar]
- 23.Santos GW, Burke PF, Sensenbrenner LL, Owens AH., Jr . In: Pharmacological Treatment in Organ and Tissue Transplantation. Bertelli A, Monaco AP, editors. Amsterdam: 1970. p. 24. [Google Scholar]
- 24.Santos GW, Sensenbrenner LL, Burke PJ, Colvin M, Owens AH, Jr., Bias WB, Slavin RE. Transplant. Proc. 1971;3:400. [PubMed] [Google Scholar]
- 25.Santos GW, Owens AH, Jr., Sensenbrenner LL. Ann. N.Y. Acad. Sci. 1964;114:404. doi: 10.1111/j.1749-6632.1964.tb53594.x. [DOI] [PubMed] [Google Scholar]
- 26.Goodwin WE, Kaufman JJ, Mims MM, Turner RD, Glassock R, Goldman R, Maxwell MM. J. Urol. 1963;89:13. doi: 10.1016/S0022-5347(17)64491-4. [DOI] [PubMed] [Google Scholar]
- 27.Parsons FM, Raper FP, Fox M, Markland AC, Anderson CK. Transplantation. 1964;2:162. [Google Scholar]
- 28.Parsons FM, Fox M, Anderson CK, Markland C, Clark PB, Raper FP. Br. J. Urol. 1966;38:673. doi: 10.1111/j.1464-410x.1966.tb09776.x. [DOI] [PubMed] [Google Scholar]