Abstract
Multiple Functions of Ionic Liquids in the Synthesis of 3-D Low-Connectivity Homochiral and Achiral Frameworks Dilemma for the ionic liquid: now the ionic liquid faces three different choices: complete (cations and anions), partial (cations only), or no entrapment during the self-assembly of the 3-D homochiral or achiral low-connectivity framework.
Keywords: chiral framework, metal-organic framework, template, ionic liquid, crystal structure
Low-connectivity (4- or 3-connected) of the framework building block is closely associated with the open architecture and porosity in 3-D framework materials. The importance of materials with low-connectivity is highlighted by the large-scale industrial applications of zeolites (4-connected) in catalysis, gas separation etc.[1] The synthetic development of low-connectivity frameworks with new composition and topology continues to attract much attention because applications of such materials depend on the unique composition or framework topology of each individual material.[2–6]
Despite tremendous successes in the synthesis of low-connectivity framework materials in the past several decades, 3-D chiral low-connectivity framework materials, particularly those with bulk homochirality are still rare. Traditional zeolites are typically achiral. Even those with chiral topology (e.g., zeolite β) have not been prepared in the enantiopure form. Recent progresses with metal-organic framework materials have opened up new routes toward the synthesis of homochiral solids.[7–9] However, there is a lack of generalized synthetic approach for the preparation of 3-D homochiral low-connectivity framework materials that are stoichiometrically and topologically similar to zeolites.
In this research, we seek to develop a generalized method to synthesize homochiral zeolite-like low-connectivity framework materials. While the method is equally successful with the achiral system and in molecular solvents, we focus here on the applicability of this method for the synthesis of homochiral materials and multiple roles of ionic liquids.
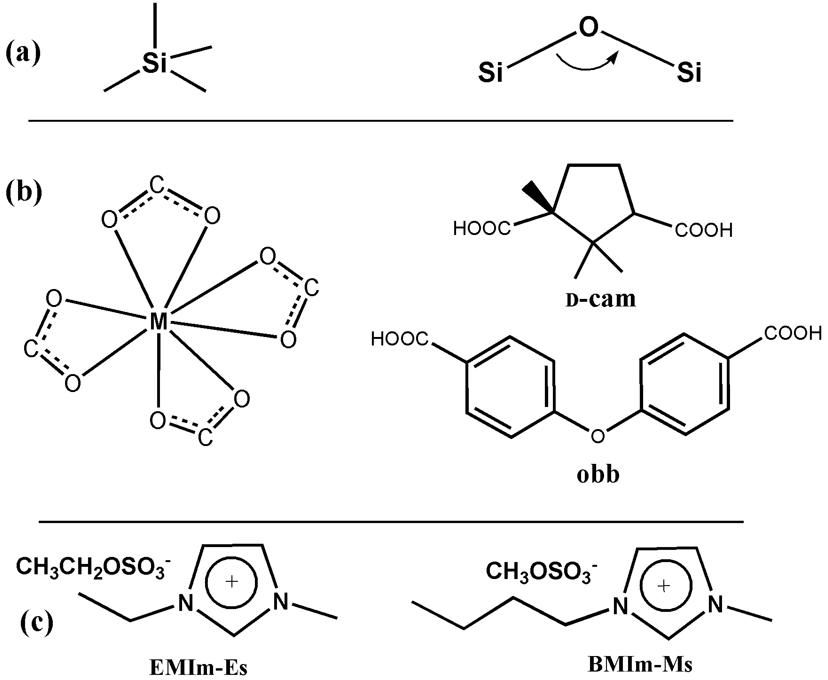
In this work, the low-connectivity (3 or 4) is generated through the formation of bidentate chelating bonds between metals and dicarboxylates (Scheme 1). Such a bonding mode is well-known in the literature. The simplest example is tin (IV) acetate, Sn(Ac)4, in which each acetate ligand chelates to a central Sn4+.[10] Other metals that exhibit such a bonding pattern include Zr4+, Cd2+, and In3+.[11, 12] However, to serve as a general synthetic method to create homochiral 3-D zeolite-like tetrahedral framework materials, the selection of metals and ligands has to be coupled with the templated synthesis approach, similar to the templated synthesis of zeolites. To this end, few examples exist in which such highly coordinated metal centers are joined into homochiral 3-D tetrahedral frameworks through templated synthesis.
Scheme 1.
A comparison of the structural building blocks in zeolites (a) with those in this work (b), and two ionic liquids used for synthesis (c).
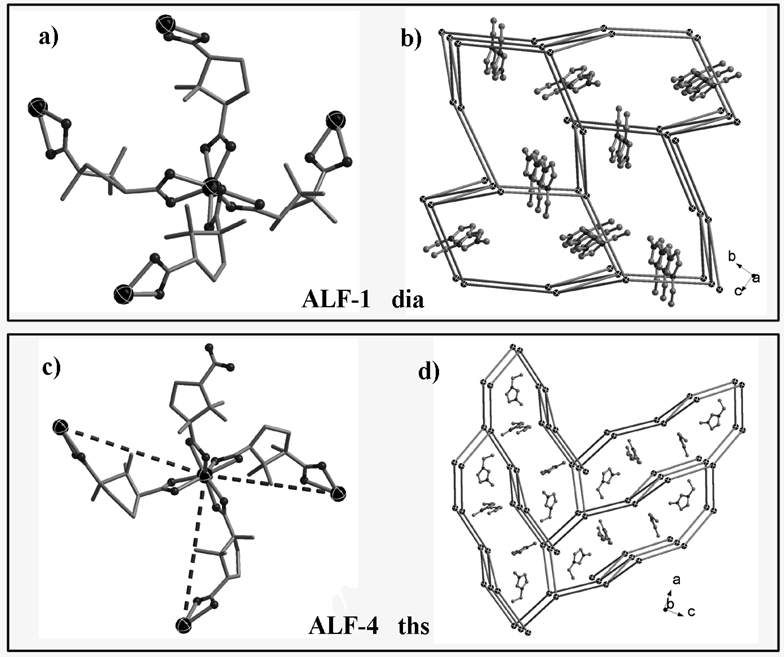
To test our synthetic method and to illustrate the generality of this method for the synthesis of both chiral and achiral materials, we employed enantiopure d-camphoric acid (= d-H2cam), as well as achiral 4,4’-oxybis(benzoic acid) (= H2obb) in two different ionic liquids for the ionothermal synthesis, which resulted in a number of open-framework materials that contain eight-coordinated In3+ sites as the tetrahedral node (Scheme 1, Figure 1, and Table 1). Both 4-connected and less common 3-connected homochiral framework materials have been made.
Figure 1.
(Top) the basic 4-connected building block (a) and diamond-type framework filled by guest EMIm cations of ALF-1 (b); (bottom) the basic 3-connected building block (c) and ths-type framework filled by guest EMIm cations of ALF-4 (d).
Table 1.
A Summary of Crystal Data and Refinement Results.
| Formula | S.G. | a (Å) | b (Å) | c (Å) | R(F) | Flack | Nets* | T/V,† nm−3 | |
|---|---|---|---|---|---|---|---|---|---|
| ALF-1 | (EMIm)[In(d-cam)2] | P212121 | 12.3398(2) | 13.4958(3) | 16.9799(3) | 0.0380 | −0.04(2) | dia | 1.41 |
| ALF-2 | (Pr4N)[In(d-cam)2] | I212121 | 10.7086(9) | 18.0006(13) | 18.0493(12) | 0.0555 | 0.01(6) | dia | 1.14 |
| ALF-2r | (Pr4N)[In(dl-cam)2]# | I-42d | 17.9054(4) | 17.9054(4) | 10.7305(5) | 0.0470 | −0.08(8) | dia | 1.14 |
| ALF-3 | (EMIm)3[In(obb)2]˙(Es)2˙(H2O)# | Cc | 22.748(2) | 15.847(1) | 15.113(1) | 0.0426 | 0.00(9) | cds | 0.73 |
| ALF-4 | (EMIm)2[In2(d-cam)3(d-Hcam)2] | P43212 | 13.7393(1) | 13.7393(1) | 37.2281(5) | 0.0798 | −0.03(7) | ths | 1.14 |
| ALF-5 | (BMIm)2[In2(d-cam)3(d-Hcam)2] | P43212 | 13.7548(2) | 13.7548(2) | 38.1612(8) | 0.0767 | −0.02(8) | ths | 1.14 |
d-H2Cam = d-camphoric acid; EMIm = 1-ethyl-3-methyl imidazolium; BMIm = 1-butyl-3-methyl imidazolium; Pr4N = tetra-n-propylammoniun; H2obb = 4,4’-oxybis(benzoic acid); Es = ethylsulfonate minus;
superscript “#” indicates non-enantiomorphous and non-centrosymmetric space group; S.G. = space group;
For definitions of three-letter abbreviations, see Reticular Chemistry Structure Resource (http://rcsr.anu.edu.au/).
T/V is the density of metal atoms per unit volume.
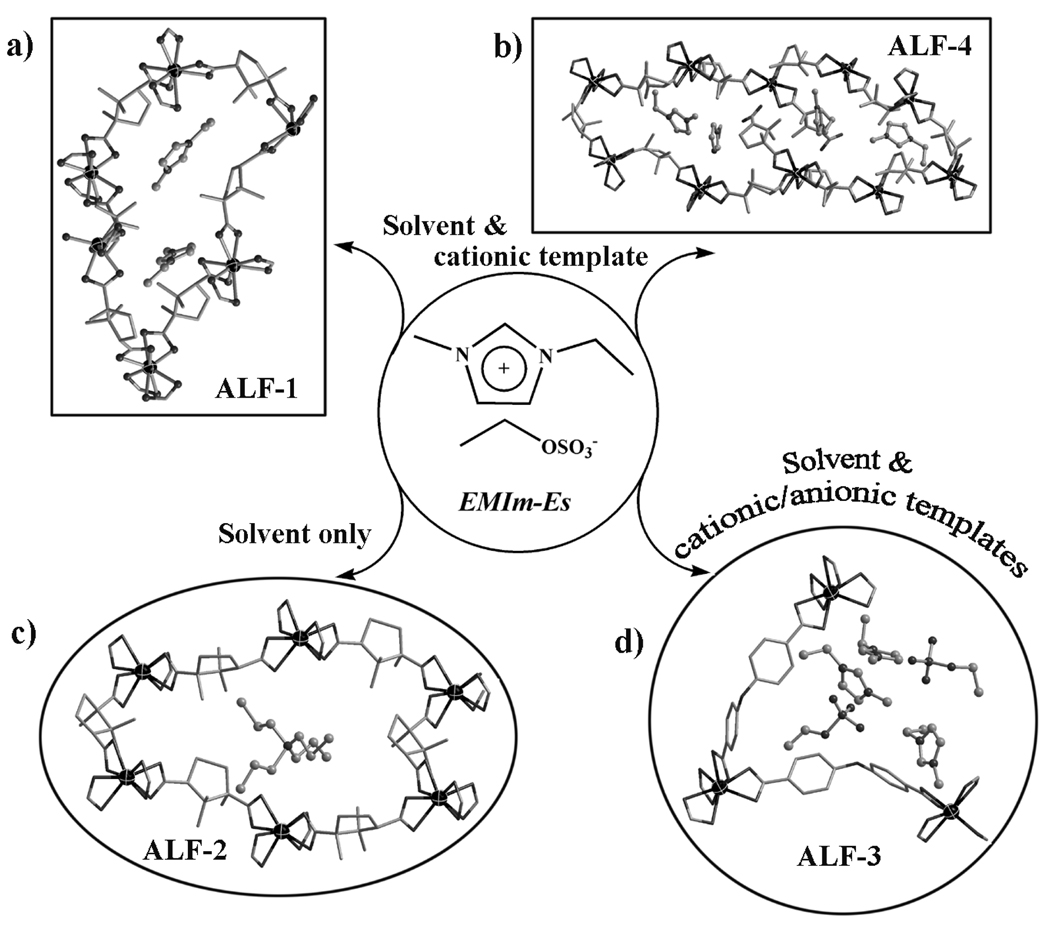
Also of interest is the demonstration the triple roles of ionic liquids (Figure 2): (1) solvent and cationic structure-directing agent, (2) solvent only, and (3) solvent and cationic/anionic structure-directing agents. The first role, as observed for ALF-1 (ALF = Anionic Low-connectivity Framework), ALF-4 and ALF-5, is to function as a solvent as well as the cationic structure-directing agent. This dual role, which is well known in the literature, particularly through the seminal work of Morris et al,[3] has the advantage of eliminating competing effects between the solvent and the separate structure-directing agent,[3], [4a] however, it has a limitation in the phase control because a new ionic liquid (can be expensive and limited in selection) would be required to exert different structure-directing effects. It can be advantageous for the ionic liquid to serve just as the solvent and use other compounds as the structure-direct agents, the choice of which would be far less restricted. This second role of the ionic liquid (solvent only) is shown here by ALF-2 and ALF-2r (r denotes racemic) in which tetrapropylammonium cations serve as the cationic structure-directing agent and suppress the structure-directing effect of the ionic liquid. Finally, a very interesting observation found in ALF-3 is the encapsulation of the whole ionic liquid (both cations and anions in the 3:2 ratio) within the cavity of a material with the 4-connected CdSO4-type topology, a feature rarely observed in the synthesis of 4-connected open-framework materials.
Figure 2.
Three different roles of the EMIm-Es ionic liquids. In (a) and (b), EMIm-Es serves as both solvent and cationic structure-directing agents, leading to 4-connected homochiral ALF-1 with the diamond-type net (shown here is the 6-membered ring.) and 3-connected homochiral ALF-4 with the ThSi-type net (shown here is the 10-membered ring.). In (c), EMIm-Es serves only as the solvent and tetrapropylammonium cations serve as the cationic structure directing agent, leading to the formation of homochiral ALF-2 with the diamond-type net (shown here is the 6- membered ring.). In (d), both cations and anions of EMIm-Es are trapped in the cavity of 4-connected framework with the CdSO4-type topology.
The structures of these anionic low-connectivity frameworks were determined from single-crystal X-ray diffraction data. In all ALFs, the negative framework charge is balanced by extra-framework organic cations (tetraalkylammonium cations, immidazolium cations). In all structures, the basic coordination chemistry at the metal site is the same. Each 8-coordinate In3+ site is bonded to four carboxylate ligands (similar to Si4+ or Al3+ in zeolites) and each carboxylate ligand bonds to two In3+ sites (similar to O2− in zeolites) (Scheme 1, Figure 1a, 1c). The framework features of ALF-1 to ALF-3 are characteristic of the 4-connected net with the AX2 formula. The simple 4-connected frameworks of ALF-1 to ALF-3 possess diamond (dia, for ALF-1, ALF-2 and 2r) (Figure 1b) or CdSO4 (cds for ALF-3) topologies.
In addition to 4-connected frameworks in ALF-1 to ALF-3, 3-D homochiral frameworks (ALF-4 and ALF-5) based on 3-connected nodes have also been preapred. This is achieved by reducing the amount of organic base, 1,4-diazabicyclo[2.2.2] octane (DABCO), in the synthesis and therefore lowering the basicity, which leads to the partial protonation of camphoric acid and decreased connectivity from 4 to 3. This demonstrates that even in the ionothermal synthesis, the acidity-basicity of the synthesis mixture can play a crucial role on the framework formation.
Both ALF-4 and ALF-5 exhibit the ths (ThSi2-type) topology. It is worth noting that while 3-connected nodes are common in layered materials (e.g., graphite), templated 3-D framework materials based on only 3-connected nodes are quite rare. In this work, the metal-ligand building block (ML4) could function as the 3-connected node when one of four ligands becomes a dangling ligand. This was achieved by using two different ionic liquids that serve as both solvent and cationic structure-directing agents. The replacement of EMIm+ in ALF-4 by BMIm+ in ALF-5 results in a slightly larger unit cell. The non-interpenetrating ths-type [In2(d-Cam)3(d-HCam) 2]n 2n− framework exhibits large channels along a or b axis that are filled by guest cations (Figure 1d).
When a separate structure-direct agent Pr4NBr was added, a new compound ALF-2 was obtained. A prominent structural feature in ALF-2 is that the Pr4N+ cations were selected into the 4-connected diamond framework, while the EMIm-Es ionic liquid only serves as the solvent. In other words, the cationic structure-directing effect of the ionic liquid is suppressed and superseded by the addition of a second structure-directing agent.
The diamond net (space group: Fd-3m) is achiral and yet ALF-1 and ALF-2 are homochiral because of the incorporation of enantiopure camphorate ligands. ALF-2r is synthesized from racemic DL-camphoric acid and has essentially the same crystal structure as ALF-2 with the only difference being the opposite handedness in 50% of the chiral ligand. This is also quite unusual because the packing of chiral molecules generally differ for pure enantiomers and for racemates as a consequence of differing symmetry requirements during the crystallization. The reason for the nearly identical packing in ALF-2 and ALF-2r is likely due to the dilution of chirality by achiral species (In3+ and extra-framework Pr4N+ here).
The low connectivity, together with the large bridging ligands in the materials reported leads to a low framework density defined as the number of tetrahedral vertices per unit volume. The concept of the framework density was introduced to characterize the openness of the zeolite-type structures. The lowest framework density is 5.2 nm−3 among the tabulated 4-connected structures.[13] The density of these ALFs ranges from 1.44 nm−3 (ALF-4) to 0.73 nm−3 (ALF-3) (Table 1).
In summary, we report here a versatile synthetic method that goes beyond the traditional limitation that relies on 4-coordinated elements (e.g., Si4+ and Al3+) to create 4- and 3-connected frameworks. The method makes it possible to create 3-D low-connectivity (3- or 4-connected) and low-density (as low as 0.73 tetrahedral nodes /nm3) frameworks from high-coordination elements (coordination number ≥ 8). Of particular significance is the application of this method for the synthesis of 4- and 3-connected homochiral framework materials. The three different functions of the ionic liquid further extend the applicability of this method and make it possible to create a diversity of homochiral and achiral framework materials with various compositions and topologies.
Experimental Section
Synthesis
(EMIm)[In(d-cam)2] (ALF-1): d-Camphoric acid (0.1173 g), 1,4-diazabicyclo[2.2.2] octane (DABCO, 0.1123 g) and In(NO3)3˙xH2O (0.0829 g) in 1-ethyl-3-methylimidazolium ethylsulfate (EMIm-Es, 1.9080 g) were mixed in a 23ml Teflon cup and the mixture was stirred for 20 min. The vessel was then sealed and heated at 160 °C for 5 days. The autoclave was subsequently allowed to cool to room temperature. The transparent colorless crystals were obtained.
(Pr4N)[In(d-cam)2] (ALF-2): d-Camphoric acid (0.1051 g), tetrapropylammonium bromide (Pr4NBr, 0.2519 g, 1 mmol), DABCO (0.0632 g) and In(NO3)3˙xH2O (0.1136 g, 0.3 mmol) in EMIm-Es, (1.8211 g) were placed in a 20 ml vial. The sample was heated at 160 °C for 5 h, and then cooled to room-temperature. After washed by ethanol and distilled water, the colorless crystals were obtained.
(Pr4N)[In(dl-cam)2] (ALF-2r): dl-Camphoric acid (0.1001 g, 0.5mmol), tetrapropylammonium bromide (Pr4NBr, 0.1234 g), DABCO (0.0632 g) and In(NO3)3˙xH2O (0.1123 g, 0.3 mmol) in EMIm-Es, (Ei-Es, 1.67 g) were placed in a 20 ml vial. The sample was heated at 120 °C for 48 h, and then cooled to room-temperature. After washed by ethanol and distilled water, the colorless crystals were obtained.
(EMIm)3[In(obb)2]˙(Es)2˙(H2O) (ALF-3): 4,4’-oxybis(benzoic acid) (0.1136 g), DABCO (0.0603 g) and In(NO3)3˙xH2O (0.0736 g) in EMIm-Es (1.4819 g) were placed in a 20 ml vial. The sample was heated at 120 °C for 4 days, and then cooled to room-temperature. After washed by ethanol and distilled water, the colorless crystals were obtained.
(EMIm)2[In2(d-cam)3(d-Hcam)2] (ALF-4): d-Camphoric acid (0.1077 g), DABCO (0.0546 g) and In(NO3)3˙xH2O (0.0917 g) in EMIm-Es (1.5635 g) were placed in a 20 ml vial. The sample was heated at 160 °C for 4 days, and then cooled to room-temperature. After washed by ethanol and distilled water, the colorless crystals were obtained.
(BMIm)2[In2(d-cam)3(d-Hcam)2] (ALF-5): d-Camphoric acid (0.109 g), DABCO (0.0602 g) and In(NO3)3˙xH2O (0.093 g) in 1-butyl-3-methylimidazolium ethylsulfate (BMIm-Es, 1.436 g) were placed in a 20 ml vial. The sample was heated at 120 °C for 3 days, and then cooled to room-temperature. After washed by ethanol and distilled water, the colorless crystals were obtained.
CCDC-671541–671543 (ALF-1, 2 and 2r), 671554 (ALF-4), 671555 (ALF-5) and 671556 (ALF-3) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/conts/retrieving.html.
Supplementary Material
Footnotes
We thank the support of this work by NIH (X. B. 2 S06 GM063119-05)
Supporting information for this article is available on the WWW under http://www.angewandte.org or from the author.
References
- 1.Flanigen EM. In: Introduction to Zeolite Science and Practice. van Bekkum H, Flanigen EM, Jansen JC, editors. New York: Elsevier; 1991. pp. 13–34. [Google Scholar]
- 2.a) Férey G. Chem. Soc. Rev. 2008;37:191. doi: 10.1039/b618320b. [DOI] [PubMed] [Google Scholar]; b) Férey G, Mellot-Draznieks C, Serre C, Millange F. Acc. Chem. Res. 2005;38:217. doi: 10.1021/ar040163i. [DOI] [PubMed] [Google Scholar]; c) Guillou N, Livage C, Drillon M, Ferey G. Angew. Chem. 2003;115:5472. doi: 10.1002/anie.200352520. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2003;42:5314. [Google Scholar]; d) Cheetham AK, Ferey G, Loiseau T. Angew. Chem., Int. Ed. 1999;38:3268. [PubMed] [Google Scholar]
- 3.a) Cooper ER, Andrews CD, Wheatley PS, Webb PB, Wormald P, Morris RE. Nature. 2004;430:1012. doi: 10.1038/nature02860. [DOI] [PubMed] [Google Scholar]; b) Parnham ER, Morris RE. Acc. Chem. Res. 2007;40:1005. doi: 10.1021/ar700025k. [DOI] [PubMed] [Google Scholar]; c) Parnham ER, Morris RE. J. Am. Chem. Soc. 2006;128:2204. doi: 10.1021/ja057933l. [DOI] [PubMed] [Google Scholar]; d) Lin Z, Slawin AMZ, Morris RE. J. Am. Chem. Soc. 2007;129:4880. doi: 10.1021/ja070671y. [DOI] [PubMed] [Google Scholar]; e) Morris RE. Angew. Chem. Int. Ed. 2008;47:442. doi: 10.1002/anie.200704888. [DOI] [PubMed] [Google Scholar]
- 4.a) Cai R, Sun M, Chen Z, Munoz R, O'Neill C, Beving DE, Yan Y. Angew. Chem. Int. Ed. 2008;47:525. doi: 10.1002/anie.200704003. [DOI] [PubMed] [Google Scholar]; b) Yaghi OM, O’Keeffe M, Ockwig NW, Chae HK, Eddaoudi M, Kim J. Nature. 2003;423:705. doi: 10.1038/nature01650. [DOI] [PubMed] [Google Scholar]
- 5.a) Tsao C-P, Sheu C-Y, Nguyen N, Lii K-H. Inorg, Chem. 2006;45:6361. doi: 10.1021/ic0603959. [DOI] [PubMed] [Google Scholar]; b) Lai YL, Lii K-H, Wang SL. J. Am. Chem. Soc. 2007;129:5350. doi: 10.1021/ja070733k. [DOI] [PubMed] [Google Scholar]; c) Sheu CY, Lee SF, Lii KH. Inorg. Chem. 2006;45:1891. doi: 10.1021/ic0518475. [DOI] [PubMed] [Google Scholar]
- 6.a) Bu XH, Feng PY, Stucky GD. Science. 1997;278:2080. doi: 10.1126/science.278.5346.2080. [DOI] [PubMed] [Google Scholar]; b) Feng PY, Bu XH, Stucky GD. Nature. 1997;388:735. [Google Scholar]; c Zheng NF, Bu XH, Wang B, Feng PY. Science. 2002;298:2366. doi: 10.1126/science.1078663. [DOI] [PubMed] [Google Scholar]; d) Feng PY, Bu XH, Zheng NF. Acc. Chem. Res. 2005;38:293. doi: 10.1021/ar0401754. [DOI] [PubMed] [Google Scholar]; e) Bu XH, Zheng NF, Feng PY. Chem. Eur. J. 2004;10:3356. doi: 10.1002/chem.200306041. [DOI] [PubMed] [Google Scholar]
- 7.a) Kesanli B, Lin W. Coord. Chem. Rev. 2003;246:305. [Google Scholar]; b) Seo JS, Whang D, Lee H, Jun SI, Oh J, Jeon YJ, Kim K. Nature. 2000;404:982. doi: 10.1038/35010088. [DOI] [PubMed] [Google Scholar]; c) Vaidhyanathan R, Bradshaw D, Rebilly J-N, Barrio JP, Gould JA, Berry NG, Rosseinsky MJ. Angew. Chem. 2006;118:6645. doi: 10.1002/anie.200602242. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2006;45:6495. [Google Scholar]; d) Cao G, Garcia ME, Alcalá M, Burgess LF, Mallouk TE. J. Am. Chem. Soc. 1992;114:7574. [Google Scholar]; e) Dybtsev DN, Nuzhdin AL, Chun H, Bryliakov KP, Talsi EP, Fedin VP, Kim K. Angew. Chem. Int. Ed. 2006;45:916. doi: 10.1002/anie.200503023. [DOI] [PubMed] [Google Scholar]; f) Xiong R-G, You X-Z, Abrahams BF, Xue Z, Che C-M. Angew. Chem. 2001;113:4554. doi: 10.1002/1521-3773(20011203)40:23<4422::aid-anie4422>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2001;40:4422. [Google Scholar]; g) Dybtsev DN, Yutkin MP, Peresypkina EV, Virovets AV, Serre C, Férey G, Fedin VP. Inorg. Chem. 2007;46:6843. doi: 10.1021/ic7009226. [DOI] [PubMed] [Google Scholar]
- 8.a) Zhang J, Bu X. Angew. Chem. Int. Ed. 2007;46:6115. doi: 10.1002/anie.200701374. [DOI] [PubMed] [Google Scholar]; b) Zhang J, Chen S, Valle H, Wong M, Austria C, Cruz M, Bu X. J. Am. Chem. Soc. 2007;129:14168. doi: 10.1021/ja076532y. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Zhang J, Liu R, Feng P, Bu X. Angew. Chem. Int. Ed. 2007;46:8388. doi: 10.1002/anie.200703008. [DOI] [PubMed] [Google Scholar]; d) Zhang J, Yao Y-G, Bu X. Chem. Mater. 2007;19:5083. [Google Scholar]; e) Zhang J, Bu X. Chem. Commun. 2008;444 doi: 10.1039/b715945e. [DOI] [PubMed] [Google Scholar]
- 9.a) Murphy DL, Malachowski MR, Campana CF, Cohen SM. Chem. Commun. 2005:5506. doi: 10.1039/b510915a. [DOI] [PubMed] [Google Scholar]; b) Garibay SJ, Stork JR, Wang Z, Cohen SM, Telfer SG. Chem. Commun. 2007:4881. doi: 10.1039/b712118k. [DOI] [PubMed] [Google Scholar]
- 10.Alcock NW, Tracy VL. Acta Cryst., Sect. B. 1979;35:80. [Google Scholar]
- 11.Sun J, Weng L, Zhou Y, Chen J, Chen Z, Liu Z, Zhao D. Angew, Chem. Int. Ed. 2002;41:4471. doi: 10.1002/1521-3773(20021202)41:23<4471::AID-ANIE4471>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 12.a) Sava DF, Kravtsov VC, Nouar F, Wojtas L, Eubank JF, Eddaoudi M. J. Am. Chem. Soc. 2008;130:3768. doi: 10.1021/ja710616j. [DOI] [PubMed] [Google Scholar]; b) Liu YL, Kravtsov VC, Larsen R, Eddaoudi M. Chem. Commun. 2006:1488. doi: 10.1039/b600188m. [DOI] [PubMed] [Google Scholar]
- 13.Baerlocher Ch, McCusker LB, Olson DH. Atlas of Zeolite Framework Types. 6th revised edition. Elsevier; 2007. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.