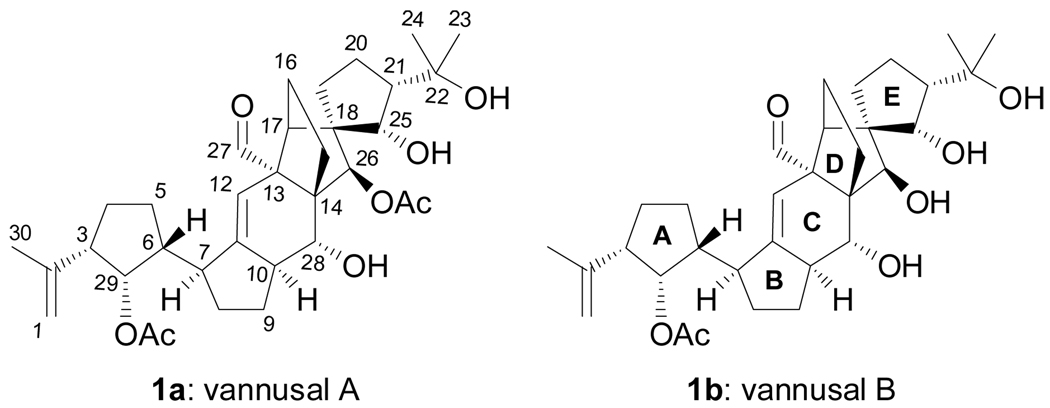
Vannusals A (1a, Figure 1) and B (1b) are two marine natural products notable for their unusual molecular architectures. Isolated from the tropical interstitial ciliate Euplotes vannus strains Si121 and BUN3, these intriguing molecules include in their C30 molecular framework seven rings and thirteen stereogenic centers, three of which are quaternary. Their structures have been assigned on the basis of mass spectrometric and NMR spectroscopic data and chemical transformations.[1, 2] Herein we report the total synthesis of structure 1b that proved that it does not represent the true structure of vannusal B.
Figure 1.
Originally assigned structures of vannusals A (1a) and B (1b).
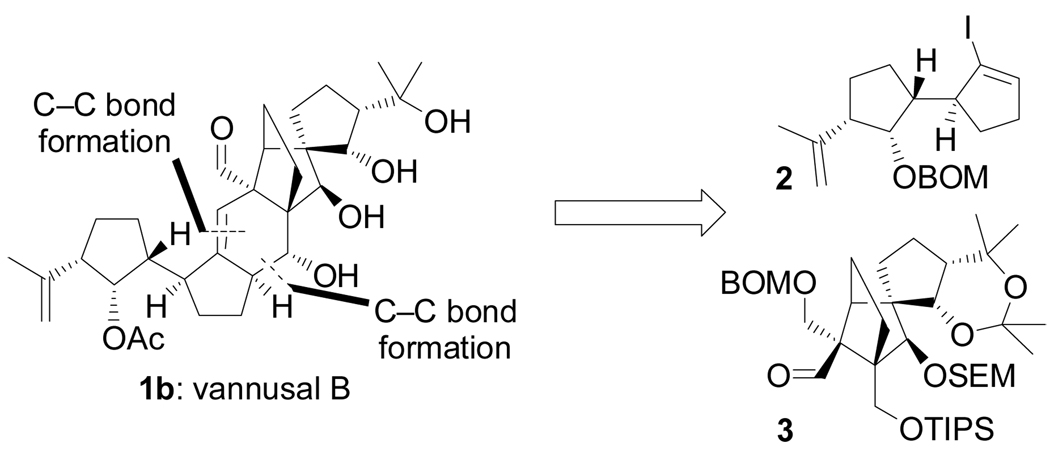
Our retrosynthetic analysis of the vannusal molecule dissected it as shown in Figure 2, revealing vinyl iodide 2 and aldehyde 3 as the key building blocks required for the projected total synthesis. The devised strategy anticipated their fusion through two carbon–carbon bond forming reactions, namely lithiation of 2 followed by addition of 3 to join them, and a samarium-induced ring closure of a subsequent intermediate to forge the final ring of the target molecule.
Figure 2.
Retrosynthetic analysis vannusal B (1b).
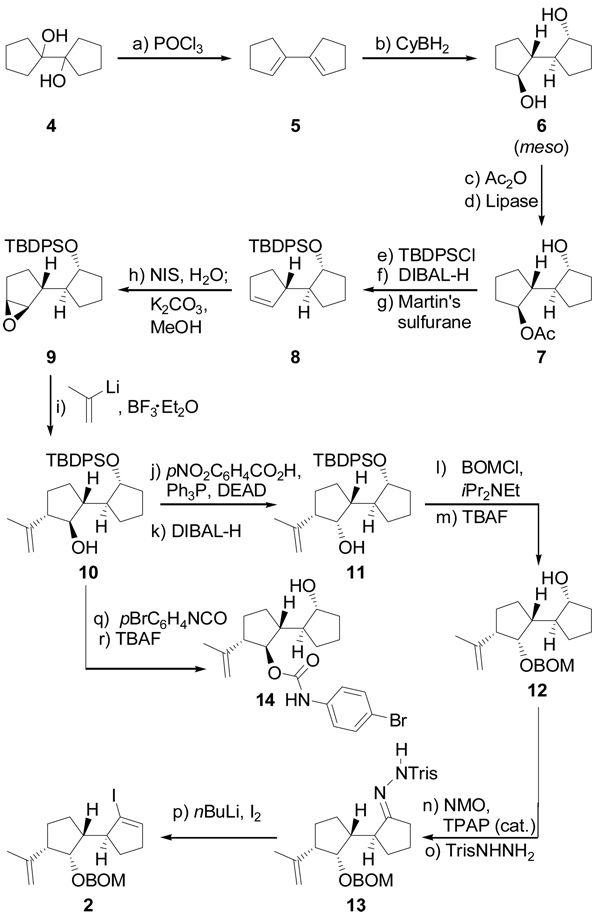
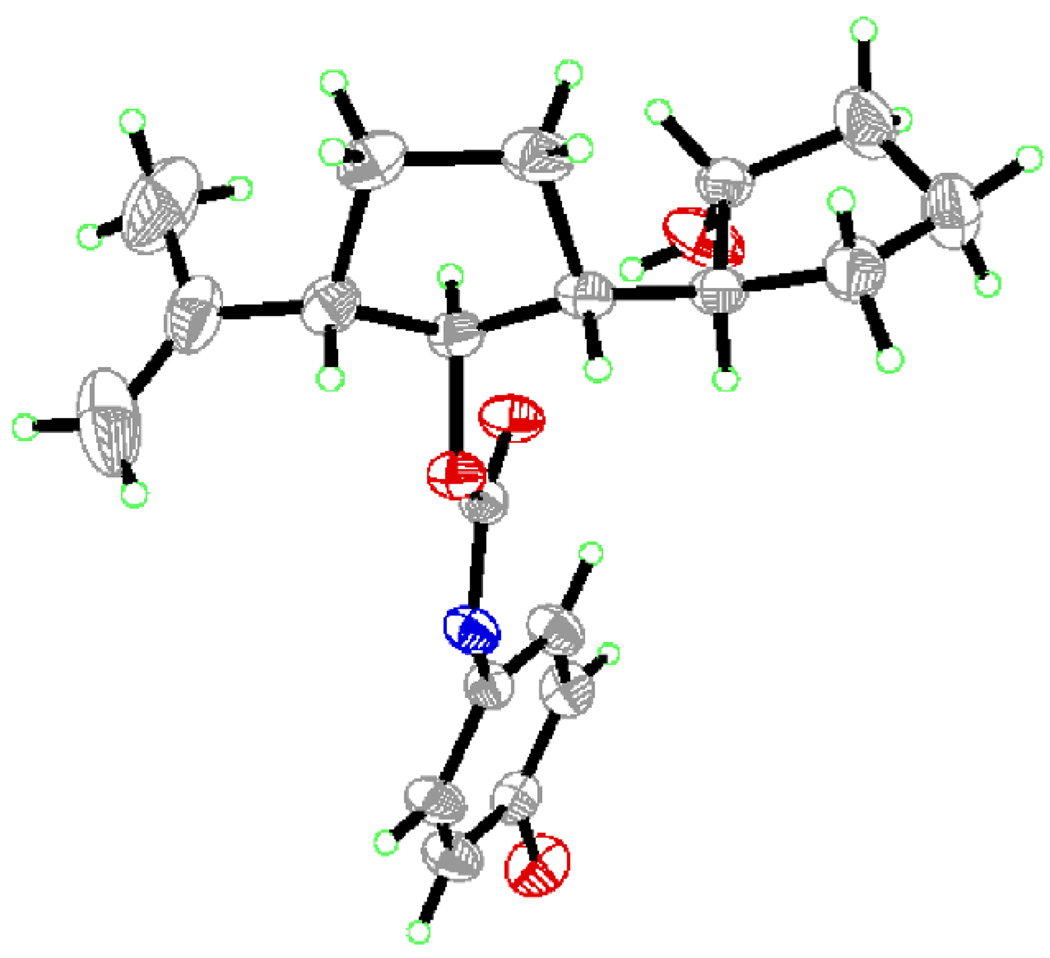
Scheme 1 summarizes the construction of vinyl iodide 2 from the commercially available meso diol 4. Thus, dehydration of 4 through the action of POCl3 (py, 90 °C) furnished conjugated diene 5 (97 % yield),[3] which was regio- and stereoselectively converted to the new meso diol 6 by a hydroboration–oxidation process (CyBH2; H2O2, NaOH, 50 % yield). The latter compound was then desymmetrized through the enantioselective hydrolytic action of Lipase Amano PS[4] on its bis-acetate (prepared in quantitative yield by reaction of 6 with Ac2O in the presence of 4-DMAP), leading to the monoacetate 7 in 100 % yield and 99 % ee (determined by Mosher ester analysis). Silylation of 7 (TBDPSCl, imid., 98 % yield), followed by acetate cleavage (DIBAL-H, 98 % yield) and treatment of the resulting alcohol with Martin’s sulfurane (Et3N, CH2Cl2, 91 % yield) afforded enantiomerically pure cyclopentene derivative 8. The planned stereoselective epoxidation of 8 was achieved through a two-step procedure that involved first iodohydrin formation (NIS, H2O), and then ring closure (K2CO3, MeOH) to give β-epoxide 9 in 90 % overall yield. This epoxide was then regio-and stereoselectively opened with 2-lithiopropene (generated from the corresponding bromide and tBuLi) in the presence of BF3•Et2O to afford hydroxy compound 10 (83 % yield), whose stereochemistry was inverted through application of a Mitsunobu (pNO2C6H4CO2H, Ph3P, DEAD)[5] / ester cleavage (DIBAL-H) protocol, leading to the desired hydroxy compound 11 in 90 % overall yield. With the proper stereochemistry now installed on 11, a BOM group was placed on the free hydroxyl group (BOMCl, iPr2NEt) and the TBDPS group was removed (TBAF, 95 % overall yield) to furnish compound 12. The newly generated hydroxyl group within 12 was then oxidized [NMO, TPAP (cat.), 90 % yield], and the resulting ketone was converted to its Tris-hydrazone 13 (TrisNHNH2, 80 % yield). Finally, the targeted vinyl iodide 2 emerged in 90 % yield through a Shapiro reaction[6] of hydrazone 13 (nBuLi, I2). The relative and absolute stereochemistry of this series of compounds was confirmed by X-ray crystallographic analysis (see ORTEP drawing, Figure 3)[7] of the crystalline p-bromophenyl carbamate 14 (m.p. 136–138 °C, EtOAc / hexanes) prepared from hydroxy compound 10 through reaction with p-bromophenyl isocyanate, followed by TBAF-induced desilylation, in 80 % overall yield as shown in Scheme 1.
Scheme 1.
Enantioselective construction of vinyl iodide 2. Reagents and conditions: (a) POCl3 (2.2 equiv), py, 90 °C, 2 h, 97 %; (b) BH3•THF (2.5 equiv), cylcohexene (2.5 equiv), −40 → 0 °C, 2 h; then 4, −40 → 25 °C, 12 h; then 50 °C, 30 min; then 30 % H2O2 / 3 N NaOH (2:1 v/v), 25 → 50 °C, 12 h, 51 %; (c) Ac2O (3.0 equiv), 4-DMAP (0.02 equiv), py, 25 °C, 3 h, 100 %; (d) Lipase Amano PS (100 wt %), acetone:phosphate buffer (pH = 7) (2:1), 25 °C, 48 h, 100 %, 99 % ee; (e) TBDPSCl (1.2 equiv), imid (3.0 equiv), CH2Cl2, 25 °C, 12 h, 99 %; (f) DIBAL-H (1.0 M in hexanes, 2.5 equiv), CH2Cl2, −78 °C, 30 min, 98 %; (g) Martin’s sulfurane (1.3 equiv), Et3N (3.0 equiv), CH2Cl2, 25 °C, 12 h, 99 %; (h) NIS (1.5 equiv), THF:H2O (4:1), 0 → 25 °C, 1.5 h; then K2CO3 (2.5 equiv), MeOH, 25 °C, 18 h, 91 %; (i) 2-bromopropene (8.5 equiv), tBuLi (1.7 M in pentane, 8.0 equiv), THF, −78 °C, 5 min; then BF3•Et2O, 2 min; 9, −78 → −20 °C, 30 min, 83 %; (j) pNO2C6H4CO2H (1.5 equiv), DEAD (1.5 equiv), Ph3P (1.8 equiv), benzene, 0 → 25 °C, 18 h, 94 %; (k) DIBAL-H (1.0 M in hexanes, 2.5 equiv), CH2Cl2, −78 °C, 30 min, 96 %; (l) BOMCl (3.0 equiv), iPr2NEt (10 equiv), PhMe, 90 °C 12 h; (m) TBAF (1.0 M in THF, 15 equiv), 70 °C, 97 % over two steps; (n) NMO (1.5 equiv), TPAP (0.03 equiv), CH2Cl2:CH3CN (9:1), 12 h, 96 %; (o) TrisNHNH2 (1.8 equiv), THF, 5 h, 80 %; (p) nBuLi (2.5 M in hexanes, 2.1 equiv) THF, −78 → −25 °C, 20 min; then I2 (2.0 equiv), −78 → −25 °C, 20 min, 90 %; (q) pBrC6H4NCO (4.0 equiv), Et3N (6.0 equiv), 25 °C; (r) TBAF, THF, 25 °C, 91 % for two steps. 4-DMAP = 4-dimethylaminopyridine, py = pyridine, TBDPS = tert-butyldiphenylsilyl, imid = imidazole, DIBAL-H = diisobutylaluminum hydride NIS = N-iodosuccinimide, DEAD = diethylazodicarboxylate, BOM = benzyloxymethyl, TBAF = tetra-n-butylammonium fluoride, NMO = N-methylmorpholine-N-oxide, TPAP = tetra-n-propylammoniumperuthenate, Tris = triisopropylsulfonyl.
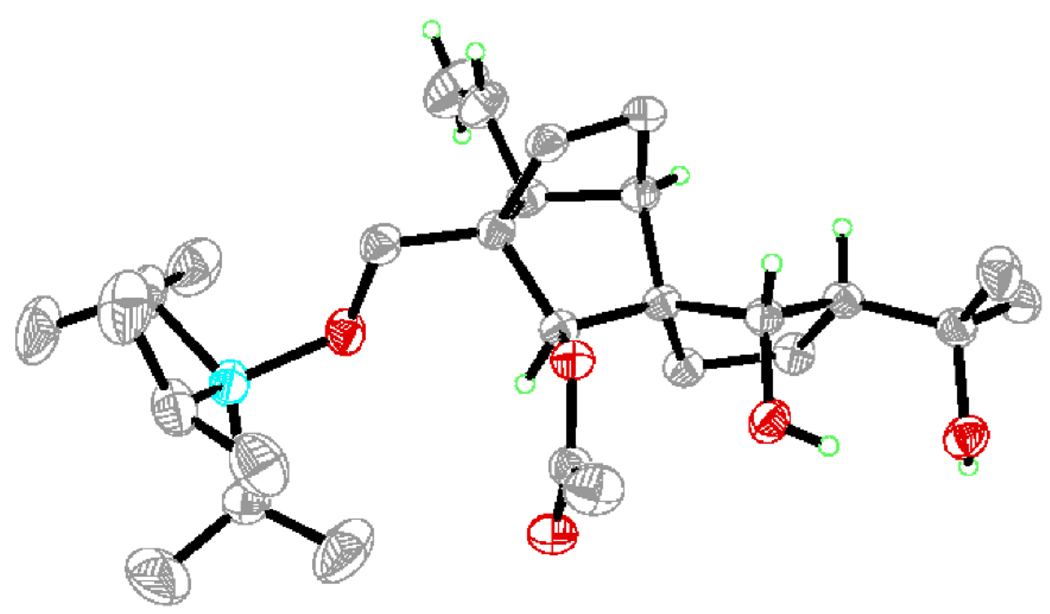
Figure 3.
X-Ray derived ORTEP drawing of 14.
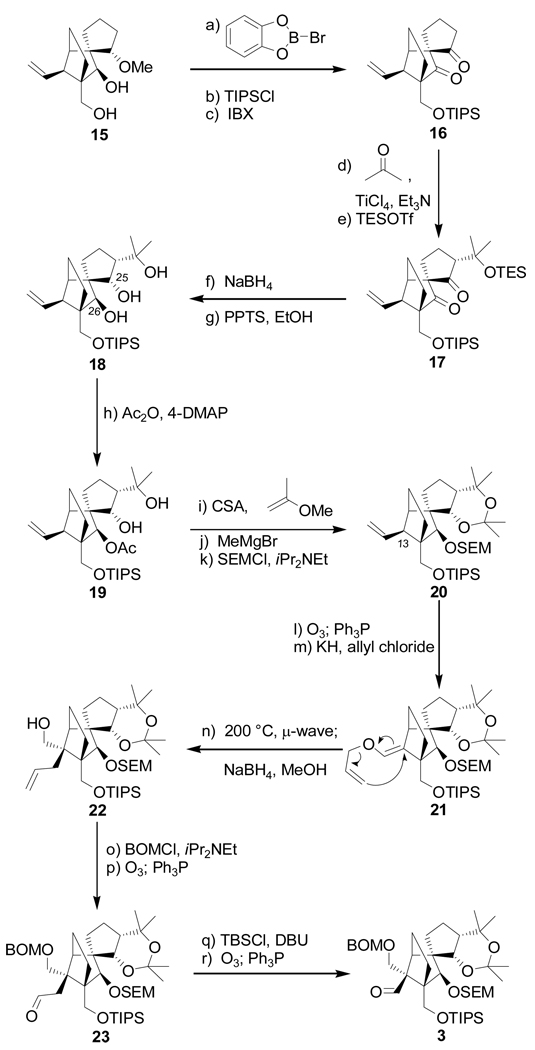
Scheme 2 outlines the construction of building block 3 starting from intermediate 15 (racemic)[8] or 15a (enantiopure).[9] Thus, exposure of dihydroxy methyl ether 15 to B-bromocatecholborane, followed by selective monosilylation of the resulting triol (TIPSCl, imid) led to the corresponding primary TIPS ether (70 % yield for the two steps), which was oxidized with IBX to afford diketone 16 (90 % yield). Generation of the titanium enolate from 16 (TiCl4, Et3N, CH2Cl2, −92 °C), followed by addition of acetone furnished the expected tertiary alcohol (ca. 9:1 dr), which (TESOTf, 2,6-lut., 78 → −40 °C) to afford bis(silyl) ether 17 (chromatographically separated) in 81 % overall yield of the desired diastereomer for the two steps. The very low temperature (−92 °C) was necessary for the good stereoselectivity observed in this aldol reaction. The next step required stereoselective reduction of the two carbonyl groups within 17 to afford the desired 25α, 26β dihydroxy compound, a prospect that, upon inspection of molecular models, looked good by virtue of the steric environment of these moieties. Indeed, exposure of 17 to NaBH4 resulted in the formation of a single diol, from which the TES group was removed under mild desilylation conditions (PPTS, EtOH) to afford triol 18 in 85 % overall yield from diketone 17. Based on intelligence gathering from other experiments that will be revealed later, and in preparation for the samarium ring closure we had in mind, a SEM group was installed at C-26, a choice that left the acetonide moiety as the obvious guardian for the other two hydroxyl groups. To this end, triol 18 was exposed to Ac2O and 4-DMAP in CH2Cl2, conditions that acetylated selectively the C-26 hydroxyl group (that turned out to be the most reactive of the three in our experience with these series of compounds), resulting in crystalline monoacetate 19 in 79 % yield (m.p. 131–133 °C, hexanes). X-Ray crystallographic analysis[10] of 19 (see ORTEP drawing, Figure 4) confirmed the relative stereochemistry of this intermediate as expected from its NMR spectroscopic data. Exposure of 19 to 2-methoxypropene in the presence of CSA afforded acetoxy acetonide (82 % yield), which after cleavage of the acetate group (MeMgBr, 50 °C, 94 % yield), was replaced with a SEM moiety leading to furnish the desired SEM intermediate 20 (SEMCl, iPr2NEt, 96 % yield). Having installed the appropriate functionalities, and in their proper configurations on our growing tricyclic system, we then turned our attention to the construction of the remaining quaternary center at C-13. To this end, a Claisen rearrangement was called upon, with allyl enol ether 21 as the substrate. This substrate was expediently prepared from 20 by ozonolysis (O3; Ph3P, 96 % yield), followed by O-allylation of the resulting aldehyde (KH, allyl chloride, 92 % yield). Pleasantly, the anticipated Claisen rearrangement of 21 proceeded smoothly upon µ-wave irradiation at 200 °C, furnishing the desired skeleton, which was swiftly reduced with NaBH4 to afford alcohol 22, in 83 % yield for the two steps. The latter compound was then protected as the BOM ether (BOMCl, iPr2NEt) and subjected to ozonolysis (O3, Ph3P) to give aldehyde 23 in 85 % yield for the two steps. Finally, truncation by one carbon was accomplished by silyl enol ether formation (TBSCl, DBU), followed by a second ozonolysis (O3, Ph3P) to furnish the targeted aldehyde 3 in 97 % overall yield for the two steps.
Scheme 2.
Construction of aldehyde 3. Reagents and conditions: (a) B-Br-catecholborane (3.5 equiv), CH2Cl2, 25 → 50 °C, 3 h; (b) TIPSCl (1.5 equiv), imid (6.0 equiv), DMF, 24 h, 70 % for two steps; (c) IBX (4.0 equiv), DMSO, 50 °C, 4 h, 90 %; (d) TiCl4 (1.0 M in CH2Cl2, 1.2 equiv), Et3N (3.0 equiv), CH2Cl2, −78 → −30 °C, 30 min; then acetone, −92 °C, 12 h (9:1 dr); (e) TESOTf (3.0 equiv), 2,6-lut. (5.0 equiv), −78 → −40 °C, 1 h, 81 % for the two steps; (f) NaBH4 (20 equiv), THF:MeOH (1:1), −10 → 25 °C, 5 h; (g) PPTS (0.2 equiv), EtOH, 25 °C, 2 h, 85 % for two steps; (h) Ac2O (30 equiv), 4-DMAP (0.1 equiv), Et3N (40 equiv), CH2Cl2, 25 °C, 18 h, 79 %; (i) 2-methoxypropene (20 equiv), CSA (1.0 equiv), CH2Cl2, −78 → −30 °C, 3 h, 82 %; (j) CH3MgBr (50 equiv), PhMe, 50 °C, 8 h, 94 %; (k) SEMCl (10 equiv), iPr2NEt (30 equiv), TBAI (1.0 equiv), CH2Cl2, 50 °C, 48 h, 96 %; (l) O3, py (1.0 equiv), CH2Cl2:MeOH (1:1), −78 °C; then Ph3P (5.0 equiv), −78 → 25 °C, 1 h, 96 %; (m) KH (10 equiv), allyl chloride (20 equiv), HMPA (5.0 equiv), DME, −10 → 25 °C, 3 h, 92 %; (n) iPr2NEt (1.0 equiv), 1,2-dichlorobenzene, 200 °C (µ-wave), 20 min; then NaBH4 (20 equiv), MeOH, 1 h, 25 °C, 88 % for two steps; (o) BOMCl (6.0 equiv), iPr2NEt (15 equiv), CH2Cl2, 50 °C 12 h; (p) O3, py (1.0 equiv), CH2Cl2:MeOH (1:1), −78 °C; then Ph3P (5.0 equiv), −78 → 25 °C, 1 h, 85 % for two steps; (q) TBSCl (10 equiv), DBU (20 equiv), CH2Cl2, 25 °C, 36 h; (r) O3, py (1.0 equiv), CH2Cl2:MeOH (1:1), −78 °C; then Ph3P (5.0 equiv), −78 → 25 °C, 1 h, 97 % for two steps. TIPS = triisopropylsilyl, TES = triethylsilyl, 2,6-lut. = 2,6-dimethylpyridine, PPTS = pyrdinium p-toluene-sulfonate, CSA = camphorsufonic acid, HMPA = hexamethylphosphoramide, DBU = 1,8-diazoicyclo[5.4.0]undec-7-ene.
Figure 4.
X-Ray derived ORTEP drawing of 19.
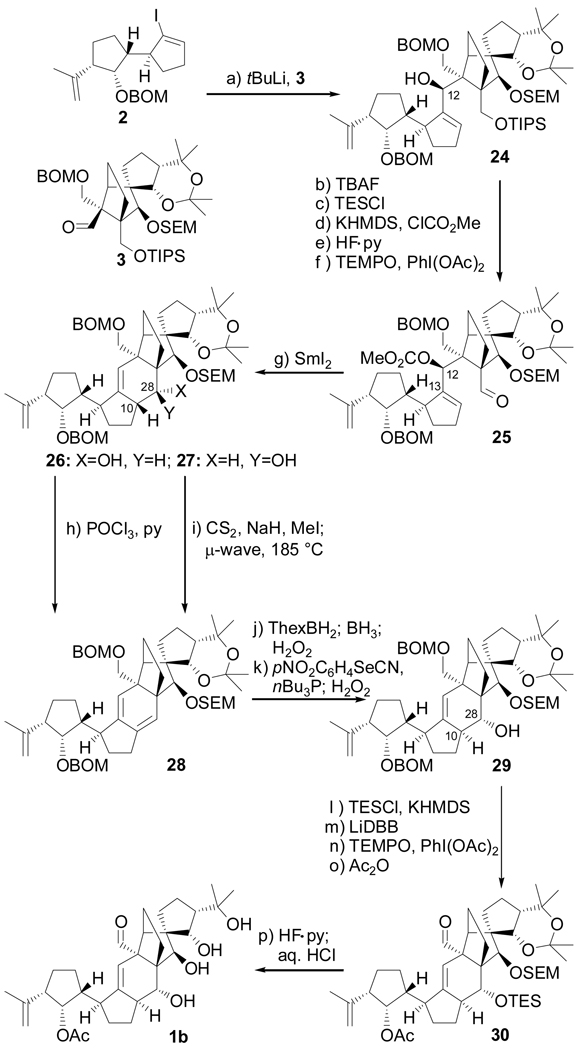
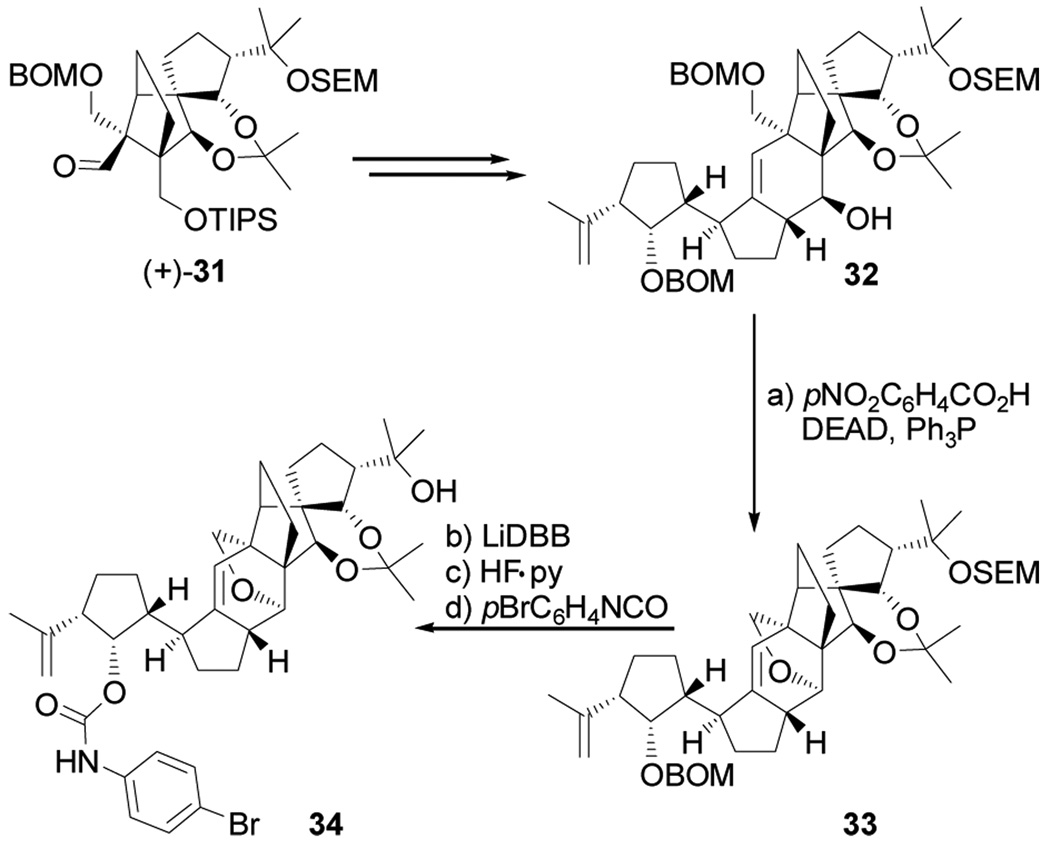
With both building blocks 2 and 3 readily available, their union and further elaboration to the reported vannusal B structure 1b became the next task. As shown in Scheme 3, lithiation of the vinyl iodide 2 (tBuLi, −78 → −40 °C), followed by addition of racemic aldehyde 3 (−40 → 0 °C) furnished a 1:1 diastereomeric mixture of products (24 and its diastereoisomer not shown) in 80 % yield. The two diastereoisomers were chromatographically separated and compared to the single (and diastereomerically desired) product obtained from the coupling reaction in which enantiopure aldehyde (+)-31 (closely related to aldehyde 3 having only the SEM and acetonide groups flipped, Scheme 4) was used to reveal the identity of the desired diastereoisomer within the above mixture. The coupling products derived from the two aldehydes [(±)-3 and (+)-31] were correlated downstream, and their structures unambiguously assigned indirectly by X-ray crystallographic analysis of a crystalline derivative (see below). This identification was important since (±)-3 was easier to obtain than its enantiopure counterpart, and was, therefore, employed at this juncture for practical reasons. The β-stereochemistry of the newly generated stereocenter in 24 (C-12) was expected on steric grounds (addition of the lithio reagent to the less hindered face of the chelated aldehyde) and was confirmed by NOE studies. At this stage, our designed strategy called for a SmI2-induced ring closure involving radical anion generation at the aldehyde site, followed by attack on the adjacent olefinic bond and expulsion of a leaving group at C-12 with concomitant migration of the double bond to the C11–C12 position.[11]
Scheme 3.
Completion of the synthesis of structure 1b. Reagents and conditions: (a) 2 (1.3 equiv), tBuLi (2.6 equiv), THF, −78 → −40 °C, 30 min; then 3 (1.0 equiv), −40 → 0 °C, 20 min, 80 %; (b) TBAF (1.0 M in THF, 5.0 equiv), THF, 25 °C, 1 h, 98 %; (c) TESCl (1.5 equiv), imid (5.0 equiv), CH2Cl2, 25 °C, 1 h, 99 %; (d) KHMDS (0.5 M in PhMe, 3.0 equiv), ClCO2Me (5.0 equiv), Et3N (5.0 equiv), THF, −78 → 25 °C, 2 h; (e) HF•py/py (1:4), 0 → 25 °C, 12 h, 92 % for two steps; (f) TEMPO (1.0 equiv), PhI(OAc)2 (3.0 equiv), CH2Cl2, 25 °C, 24 h, 98 %; (g) SmI2 (0.1 M in THF, 5.0 equiv), HMPA (15 equiv), THF, −10 → 25 °C, 30 min, 80 % (26: 28 %, 27: 52 %); (h) POCl3 (60.0 equiv), py, 60 °C, 3 h, 85 %; (i) CS2 (8.0 equiv), NaH (6.0 equiv), THF, 0 → 25 °C, 30 min; then CH3I (12 equiv), 0 → 25 °C, 3 h; then 185 °C (µ-wave), 1,2-dichlorobenzene, 15 min, 92 %; (j) ThexBH2 (5.0 equiv), THF, −10 → 25 °C, 1 h; then BH3•THF (15 equiv), 0 → 25 °C, 30 min; then 30 % H2O2/3 N NaOH (1:1), 25 → 40 °C, 1 h; 65 % (1:1.3 mix); (k) oNO2C6H4SeCN (2.0 equiv), nBu3P (6.0 equiv), py (12 equiv), THF, 25 °C; then 30 % H2O2, 0 → 25 °C, 67 %; (l) KHMDS (0.5 M in PhMe, 5.0 equiv), TESCl (5.0 equiv), Et3N (8.0 equiv), THF, −78 → 25 °C, 30 min, 94 %; (m) LiDBB (excess), THF, −78 → −50°C, 30 min, 84 %; (n) TEMPO (1.0 equiv), PhI(OAc)2 (3.0 equiv), CH2Cl2, 25 °C, 24 h, 88 %; (o) Ac2O (30 equiv), Et3N (30 equiv), 4-DMAP (1.0 equiv), CH2Cl2, 25 °C, 12 h, 100 %; (p) HF•py/THF (1:4), 25 °C, 3 h; then 3 N aq. HCl/THF (1:3), 25 °C, 6 h, 80 %. KHMDS = potassium hexamethyldisilyazide, TEMPO = 2,2,6,6-teramethyl-1-piperidinyloxy free radical, LiDBB = Lithium ditert- butylbiphenyl.
Scheme 4.
Synthesis of crystalline derivative 34. Reagents and conditions: (a) DEAD (10 equiv), Ph3P (10 equiv), pNO2C6H4CO2H (10 equiv), benzene, 60 °C, 2 h, 88 % based on 58 % conversion; (b) LiDBB, THF, −78 → −50 °C; (c) HF•py/THF, 25 °C, 4 h; (d) pBrC6H4NCO (10 equiv), py, 40 °C, 70 % for the three steps.
It was to this end that the following four-step sequence was carried out from hydroxy compound 24 to the aldehyde carbonate 25: (i) exchange of the TIPS moiety (TBAF, 25 °C, 98 % yield) for the more labile TES group (TESCl, imid, 99 % yield); (ii) carbonate formation at C-12 (KHMDS, ClCO2Me); (iii) selective removal of the TES group (HF•py, 92 % for the two steps); and (iv) oxidation of the liberated primary hydroxyl group to the aldehyde (TEMPO, PhI(OAc)2, 98 % yield). The final ring of the desired polycyclic skeleton was then forged by treatment of substrate 25 with a solution of SmI2 in THF in the presence HMPA at −10 → 25°C, yielding a mixture of two diastereomeric alcohols (differing at C-28), 26 (28 % yield) and 27 (52 % yield), which were chromatographically separated. The stereochemical assignments for these two compounds were based on NMR spectroscopic analysis, particularly NOE studies. Faced with the unpleasant stereochemical outcome of this reaction, which otherwise performed admirably, we decided to eradicate the two newly generated stereocenters (at C-10 and C-28) through dehydration, and reconstruct them in their proper configurations by exploiting the reactivity preferences of the resulting diene system. Diene 28 was secured from either isomer 26 or 27, each precursor, however, requiring its own path. Thus, treatment of isomer 26 (in which the OH group resides anti to H-10) with POCl3 (py, 60 °C) led directly to 28 (85 % yield), whereas isomer 27 (in which the OH group is syn to H-10) required conversion to its xanthate first (NaH, CS2, MeI, 0 → 25 °C), and then syn-elimination, a process that proceeded smoothly upon microwave irradiation at 185 °C (92 % overall yield).[12] The next hurdle to be overcome was the regio- and stereoselective hydration of the C10–C28 olefinic bond in the presence of the other two within intermediate 28. An expedient tactic to solve this seemingly thorny problem was devised based on the unique steric environment of each olefinic bond within this substrate. Thus, hydroboration of triene 28, first with ThexBH2 (terminal olefin, two diastereoisomers), and then with BH3•THF (C10–C28 olefin, single diastereisomer) afforded, after the usual oxidative work-up, a mixture of two diastereomeric diols (ca. 1:1.3, 65 % yield). This mixture was then dehydrated through syn-elimination (H2O2, 67 % overall yield) of the primary o-nitrophenyl selenides which were selectively generated from the diol mixture (oNO2C6H4SeCN, nBu3P; H2O2).[13] The desired configurations at C-10 and C-28 within the newly obtained hydroxy compound (29) was confirmed by NOE studies. Having reached 29 with all stereochemistry in place as required, only a short path now separated it from the targeted molecule. The requisite aldehyde and acetate moieties were installed through a short sequence [(i) KHMDS, TESCl, 100 % yield; (ii) LiDBB, THF, −78 → −50 °C, 84 % yield; (iii) TEMPO, PhI(OAc)2, 88 % yield; and (iv) Ac2O, 4-DMAP, Et3N, 100 % yield] to afford advanced intermediate 30. Global deprotection of 30 through the sequential action of HF•py and aq. HCl in the same pot furnished the coveted structure 1b in 80 % yield. The spectroscopic data of the synthesized compound, however, although similar, did not match those reported[1] for the naturally occurring vannusal B.
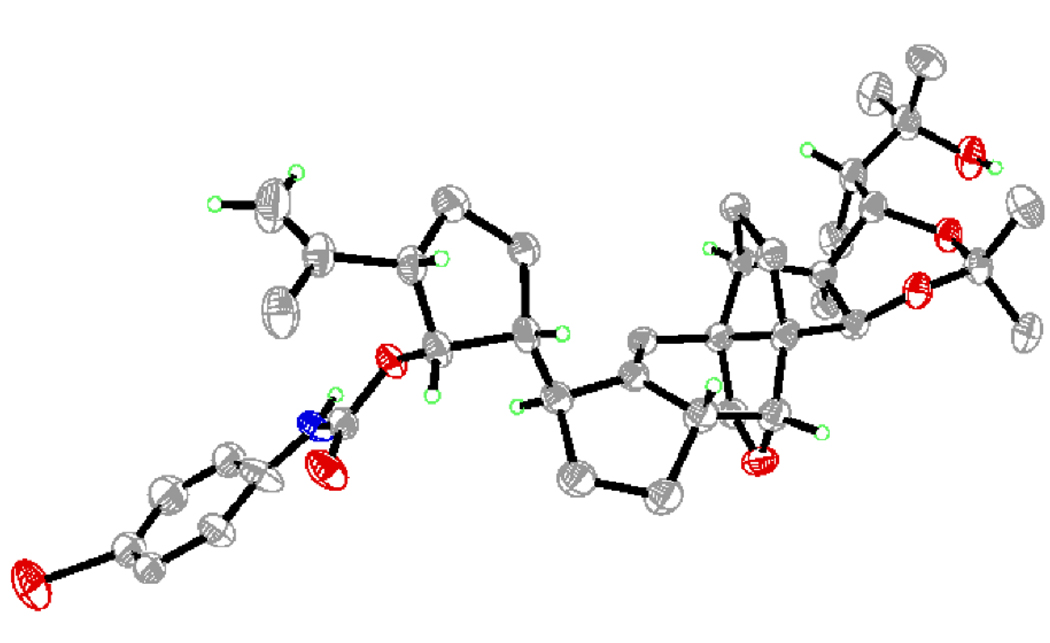
Lest there was any doubt about our synthesized structure 1b, a serendipitous discovery allowed further support for its structural identity. Thus, activation of alcohol 32 (Scheme 4) [obtained from (+)-31 through a similar sequence as that shown in Scheme 3] with the Burgess reagent or under Mitsunobu conditions (attempted for dehydration and inversion of configuration, respectively) resulted in the formation of the polycyclic compound 33 (88 % yield based on 58 % conversion). The new, and unexpected, ring within 33 was apparently formed by intramolecular attack of the OBOM group upon the initially generated reactive intermediate in each of these reactions. Proximity combined with rigidity must be responsible for this unusual, but facile process. Sequential removal of the BOM (LiDBB) and SEM (HF•py) groups gave the corresponding diol, which reacted with p-bromophenyl isocyanate to afford p-bromophenyl carbamate 34 in 81 % overall yield. The latter compound crystallized in beautiful needles from EtOAc / hexanes, m.p. 180–182 °C. X-Ray crystallographic analysis of these crystals (see ORTEP drawing, Figure 5)[14] provided unambiguous confirmation of its structure and those of its precursors.
Figure 5.
X-Ray derived ORTEP drawing of 34.
The described chemistry provides a highly convergent approach to the vannusal molecular framework, including the originally proposed structure of vannusal B (1b). It also proved that this structure (1b) does not represent the true molecular identities of the vannusals, precipitating a puzzle that still remains to be solved.
Supplementary Material
Acknowledgments
We thank Drs. D. H. Huang, G. Siuzdak and R. Chadha for NMR spectroscopic, mass spectroscopic and X-ray crystallographic assistance, respectively. Financial support for this work was provided by the NSF (fellowship to A. Ortiz), the Skaggs Institute for Research, and a grant from the National Institutes of Health (USA).
Footnotes
Supporting information for this article is available on the WWW under http://www.angewandte.org or the author.
References
- 1.Guella G, Dini F, Pietra F. Angew. Chem. 1999;111:1217–1220. doi: 10.1002/(SICI)1521-3773(19990419)38:8<1134::AID-ANIE1134>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 1999;38:1134–1136. doi: 10.1002/(SICI)1521-3773(19990419)38:8<1134::AID-ANIE1134>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 2.Guella G, Callone E, Di Giuseppe G, Frassanito R, Frontini FP, Mancini I, Dini F. Eur. J. Org. Chem. 2007:5226–5234. [Google Scholar]
- 3.Griedinger DS, Ginsburg D. J. Org. Chem. 1957;22:1406–1410. [Google Scholar]
- 4.For reviews on enzymatic desymmetrization in organic synthesis, see Garcia-Urdiales E, Alfoso I, Gotor V. Chem. Rev. 2005;105:313–354. doi: 10.1021/cr040640a. Hartung IV, Hoffmann HMR. Angew. Chem. 2004;116:1968–1984. Angew. Chem. Int. Ed. 2004;43:1934–1949. doi: 10.1002/anie.200300622.
- 5.Martin SF, Dodge JF. Tetrahedron Lett. 1991;32:3017–3020. [Google Scholar]
- 6.For use of the Shapiro reaction in organic synthesis, see Shapiro RH. Org. React. 1976;23:405–506. Shapiro RH, Heath MJ. J. Am. Chem. Soc. 1967;89:5734–5735. Adlington RM, Barrett AGM. Acc. Chem. Res. 1983;116:55–59. Nicolaou KC, Yang Z, Liu J-J, Nantermet PG, Claiborne CF, Renaud J, Guy RK, Shibayama K. J. Am. Chem. Soc. 1995;117:645–652.
- 7.CCDC-698622 contains the supplementary crystallographic data for p-bromophenyl carbamate 14. This data can be obtained free of charge from the Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif
- 8.Nicolaou KC, Jennings MP, Dagneau P. Chem. Commun. 2002:2480–2481. [Google Scholar]
- 9.Nicolaou KC, Tang W, Dagneau P, Faraoni R. Angew. Chem. 2005;117:3942–3947. doi: 10.1002/anie.200500789. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2005;44:3874–3879. doi: 10.1002/anie.200500789. [DOI] [PubMed] [Google Scholar]
- 10.CCDC-698872 contains the supplementary crystallographic data for acetoxy diol 19. This data can be obtained free of charge from the Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif
- 11.For reviews of samarium diiodide used in organic synthesis, see Edmonds D, Johnston D, Procter DJ. Chem. Rev. 2004;104:3371–3403. doi: 10.1021/cr030017a. Kagan HB. Tetrahedron. 2003;59:10351–10372. Molander GA, Harris CR. Chem. Rev. 1996;96:307–338. doi: 10.1021/cr950019y.
- 12. Chugaev L. Chem. Ber. 1899;32:3332–3335. For examples of the Chugaev elimination (xanthate pyrolysis) used in organic synthesis, see Meulemans TM, Stork GA, Macaev FZ, Jansen BJM, de Groot AJ. J. Org. Chem. 1999;64:9178–9188. Nakagawa H, Sugahara T, Ogasawara K. Tetrahedron Lett. 2001;42:4523–4526. Padwa A, Zhang H. J. Org. Chem. 2007;72:2570–2582. doi: 10.1021/jo0626111.
- 13.Grieco PA, Nishizawa MJ. J. Org. Chem. 1977;42:1717. [Google Scholar]
- 14.CCDC-698624 contains the supplementary crystallographic data p-bromophenyl carbamate 34. This data can be obtained free of charge from the Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.