Abstract
This work represents the first use of mesoporous zirconium oxide nanomaterials for highly effective and selective enrichment of phosphorylated peptides.
Reversible protein phosphorylation is a ubiquitous post-translational modification that plays a vital role in the control of many biological processes such as cellular growth, division, and signaling.1 Aberrant phosphorylation is known to be one of the underlying mechanisms for many human diseases, most notably cancer.1a,c Mass spectrometry (MS) has become the most important and powerful tool for the analysis of protein phosphorylation due to its sensitivity, speed, simplicity, separation, and specificity.2 While MS techniques have been successfully applied to determine the phosphorylation state of a single protein/peptide, MS analysis of phosphorylation on a proteome-wide scale still poses substantial challenges due to the low abundance of phosphoproteins and substoichiometric phosphorylation.3 Therefore, isolation and enrichment of the phosphoproteins/peptides are essential for MS-based phosphoproteomics.4 The affinity based method such as immobilized metal ion affinity chromatography (IMAC)5 using Ga(III), Fe(III), or other metals, has been widely used for phosphopeptides enrichment. Recently microparticles of titanium dioxide (TiO2),6a,b zirconium oxide (ZrO2),6c and other metal oxides6d–e have demonstrated higher specificity for trapping phosphate than the conventional IMAC beads since such oxides rely on specific and reversible chemisorption of phosphate groups on their amphoteric surface and have less non-specific binding. Additionally, nanoparticles, such as ZrO2, TiO2, Fe2O3, and titania-coated magnetic iron oxide (Fe3O4@TiO2) nanoparticles, have recently been explored due to their potential higher capacities than the microparticles. 7
Mesoporous materials are nanostructured materials with pore sizes typically between 2−50 nm.8 They have extremely large surface areas and have been utilized in many applications such as catalyst support and filtration. Such large surface areas, together with the many active surface sites, can be translated into even higher loading capacity for binding phosphate groups than micro- and nanoparticles.9 In addition to their well-ordered nanoscale porous structures and flow-through capacity, they are chemically stable and can be easily prepared at reasonable cost. All these attributes would make them ideal for applications in MS-based phosphoroproteomics. Herein, we report the utility of ZrO2 nanomaterials for simple and efficient enrichment of phosphopeptides with high specificity. This is the first application of mesoporous ZrO2 nanomaterials for phosphopeptide enrichment.
We chose ZrO2 metal oxide because of its known amphoteric surface properties, 10 which facilitates preferable and reversable binding and release of the phosphate groups under different pH of the solutions. We synthesized mesoporous materials using commercially available Pluronic® triblock copolymer surfactant F127 to form ordered nanoscale micellular structures in alcohol solutions to template the controlled hydrolysis of the metal precursors in a so-called evaporation induced self assembly (EISA) process.8b, 8c† The calcined materials were characterized with scanning electron microscopy (SEM, Fig. S1), transmission electron microscopy (TEM) and small angle x-ray scattering (SAXS) to examine the quality of the mesoporous structure and determine pore size and periodicity.
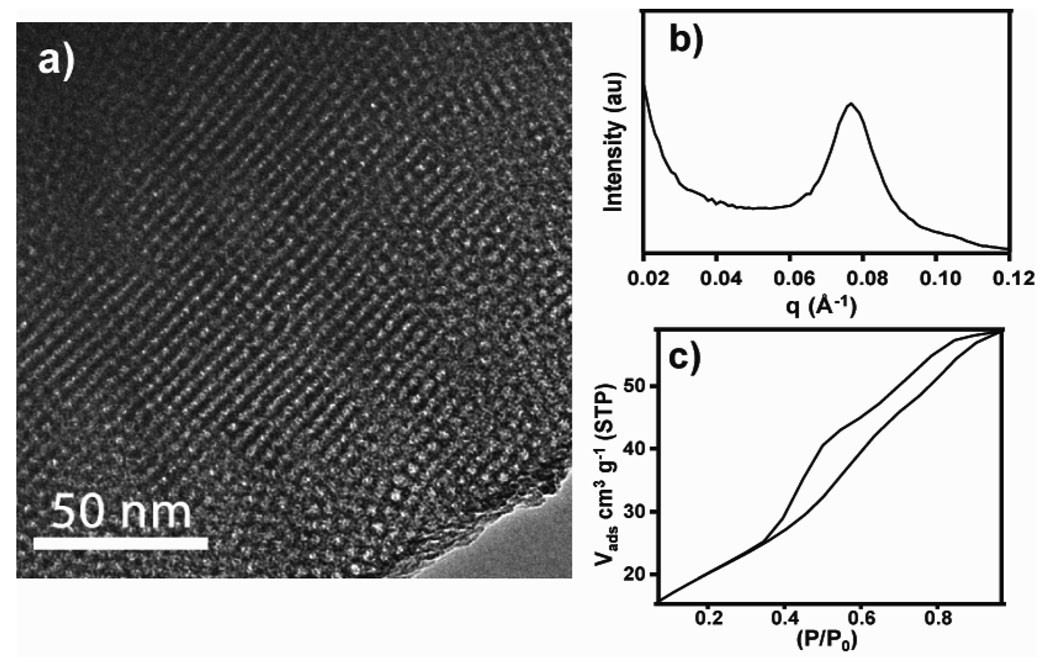
Fig. 1a clearly shows the ordered mesostructure of ZrO2. Average pore size was determined from TEM images to be 5.8 nm with an average periodicity determined from SAXS and TEM to be 8.2 nm. Brunauer-Emmett-Teller (BET) analysis of N2 absorption experiments revealed that the mesoporous ZrO2 has a high surface areas of 72 m2/g, which is in good agreement with that previously reported for meosporous ZrO2 templated with this block copolymer F127.8e The high surface area, which can be further increased when other surfactants are used,8 makes mesoporous materials good candidates for phosphopeptide enrichment.
Fig. 1.
TEM micrograph for mesoporous ZrO2 (a) with its corresponding SAXS pattern (b) and nitrogen adsorption-desorption isotherms (c).
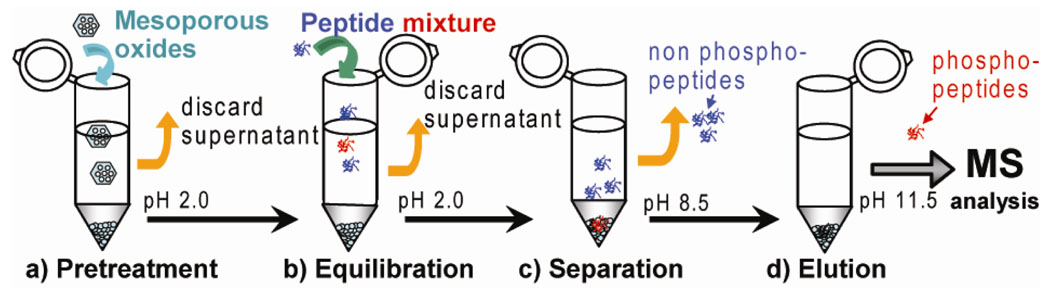
The enrichment procedures using mesoporous metal oxides (Scheme 1) include: (a) pretreatment of mesoporous oxides, (b) equilibration of the peptide mixtures with mesoporous oxides at pH 2.0, (c) separation of the unbound non phosphopeptides by removing the supernatant solutions at pH 8.5, and (d) elution of the phosphopeptides at pH 11.5.† Strong binding of the phosphate groups to ZrO2 surface allows the phosporylated peptides to remain absorbed on the mesoporous materials until eluted with a high pH solution. Non-specific binding, presumably from acidic peptides, has been minimized by optimizing the buffers used in binding, washing, and eluting steps. The best results were achieved with a binding buffer solution of 20 mg/mL phthalic acid in 0.1% trifluoroacetic acid in 50/50 water/acetonitrile (pH 2.0), washing twice with 50 mM ammounium bicarbonate in 50/50 water/acetonitrile (pH 8.5), and an eluting buffer of ammonium hydroxide (pH 11.5). The eluted phosphopeptide solutions were then adjusted properly to be analyzed by electrospray (ESI) MS. The phosphopeptides were first detected based on the facile neutral loss of phosphoric acid (H3PO4) or metaphosphoric acid (HPO3) from phosphorylated serine/threonine/tyrosine phosphopeptides generated from collisionally activated dissociation (CAD), a conventional tandem mass spectrometry (MS/MS). The sequences of the enriched phosphopeptides were further confirmed and the phosphorylation sites within phosphopeptides were unambiguously localized by both CAD and electron capture dissociation (ECD) (Fig. S2). Fragment ions were assigned with very high mass accuracy (<5 ppm) (Table S2, S3). The specificity of the enrichment enabled easy isolation of the peaks and the large trapping capacity of the mesoporous materials yielded highly abundant peaks which, upon fragmentation, gave complete or nearly complete coverage for the peptides of interest. Unlike CAD which tends to knock off the phosphate groups, ECD11 is a nonergodic MS/MS technique known to preserve labile phosphorylation making it extremely powerful for facile localization of phosphorylation sites. However, ECD requires higher signal-to-noise ratios for precursor ions thus demands efficient enrichment processes for its effective applications in phosphoproteomics.6c
Scheme 1.
Flow diagram of phosphopeptide enrichment procedure.
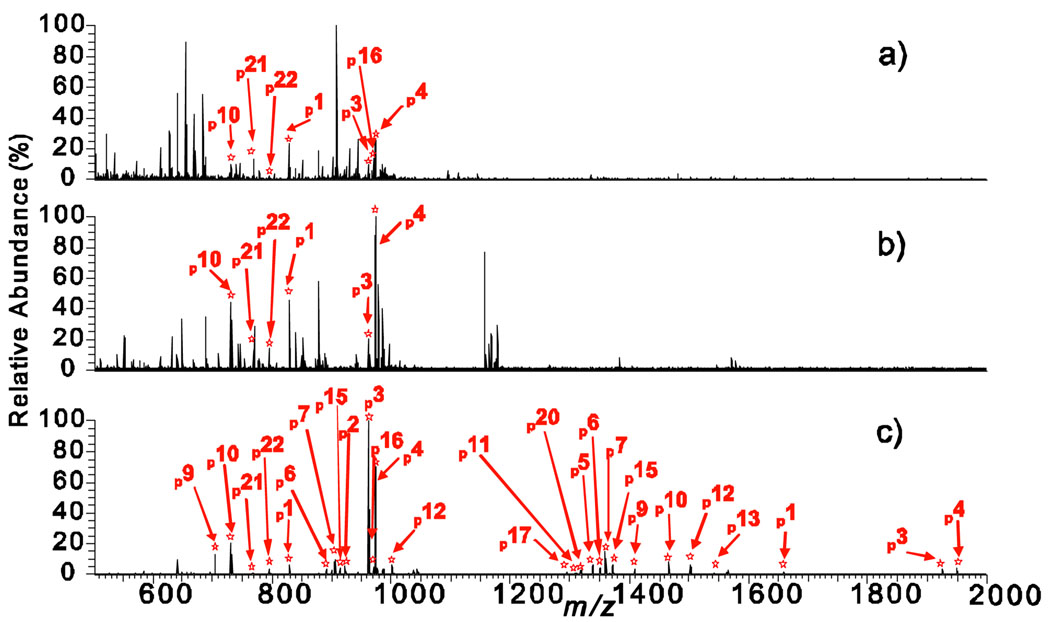
The enrichments using mesoporous ZrO2 are extremely effective as shown by the high resolution Fourier transform (FT) mass spectra of the α-casein digest before and after the enrichment (Fig. S3). Only 8 MS peaks corresponding to 6 phosphopeptides were detected before enrichment (Fig. S3a); all of which are low abundance peaks owing to ion suppression from abundant non phosphopeptides. In contrast, after enrichment with mesoporous ZrO2 (Fig. S3b), 30 multiply charged MS peaks corresponding to 20 phosphopeptides were detected in a single mass spectrum with much higher signal-to-noise ratios. When enriched with mesoporous ZrO2 nearly all of the non phosphopeptides were removed leaving only phosphorylated peaks, which substantially enhanced the signal of phosphopeptides. Furthermore, as demonstrated in a side-by-side quantitative comparison (Fig. 2), the mesoporous ZrO2 materials showed significantly higher specificity and efficiency for phosphopeptide enrichment than the leading commercial IMAC and ZrO2 nanoparticle-based phospho-enrichment methods. After enrichment with the IMAC-based enrichment product (Fig. 2a), 7 multiply charged MS peaks corresponding to 7 phosphopeptides were identified in one MS spectrum. Enrichment with the ZrO2 packed tips (Fig. 2b) revealed 6 multiply charged MS peaks corresponding to 6 phosphopeptides in one MS spectrum. In contrast, an enrichment with the mesoporous ZrO2 nanomaterials detected 27 multiply-charged MS peaks corresponding to 19 phosphopeptides (Fig. 2c).
Fig. 2.
Negative ion mode ESI/FTMS spectra of peptide mixtures digested from α-casein with trypsin acquired after enrichment with (a) a leading commercial IMAC-based product, (b) a leading commercial product of ZrO2 packed tip, and (c) the mesoporous ZrO2 nanomaterials. Phosphopeptides are labeled with numbers that are shown in Table S4.
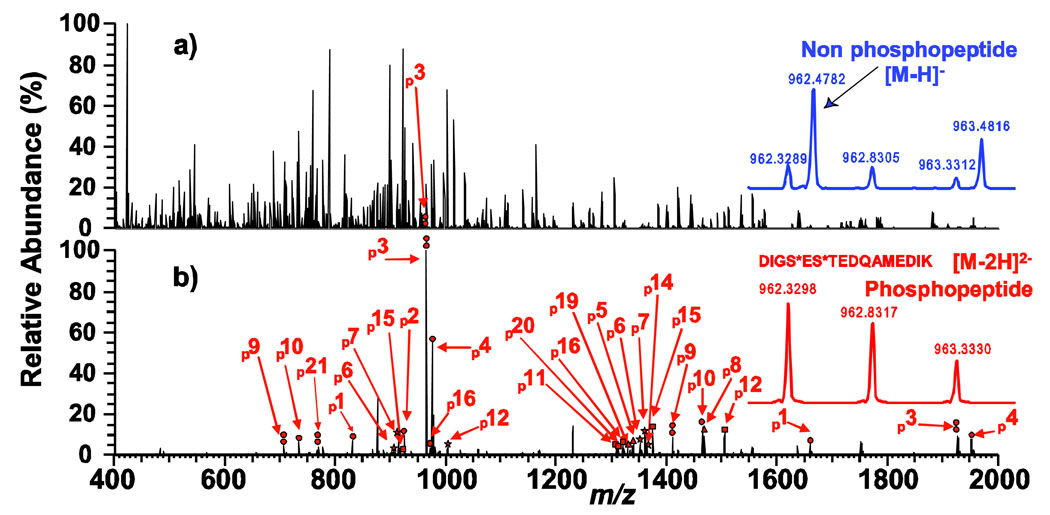
To further evaluate the specificity for phosphopeptides, we tested the mesoporous ZrO2 using a more complicated mixture with a substantial fraction of non phosphorylated proteins. 5 non phosphoproteins and 7% (by weight of the total proteins) phosphoprotein, α-casein, (Table S1) were mixed and digested with trypsin to create a complex peptide mixture. Before enrichment many non phosphopeptides in this mixture dominate the MS spectrum (Fig. 3a) so that even the most abundant phosphopeptide, p3, is severely suppressed and hardly observable. After enrichment, 28 multiply charged MS peaks corresponding to 18 phosphorylated peptides were identified (Fig. 3b). Note all of the phosphopetides and phosphorylation sites identified from the peptide mixture digested from pure α-casein, including those of very low abundance, were recovered from this highly complex peptide mixture, underlining the high specificity of this enrichment. The sequences of all the identified phosphorylated peptides in Fig. 2, 3, and S3 are summarized in Table S4. Overall, we have identified 18 unique phosphorylation sites (out of a total of 21 potential phosphorylation sites) for α-casein (s1 and s2 variants)12 from a single enrichment using mesoporous ZrO2. Such highly effective and specific enrichment of phosphopeptides out of the peptide mixtures with mesoporous ZrO2, which could almost be considered as "purification", allows a robust analysis of the phosphopeptides.
Fig. 3.
Negative ion mode ESI/FTMS spectra of peptide mixtures digested with trypsin acquired before enrichment (a), and after enrichment with mesoporous ZrO2 (b). Circle, double circle, triangle, square, and star indicate singly, doubly, triply, quadruply, and quintuply phosphorylated peptides, respectively. Phosphopeptides are labeled with numbers that are identified and shown in Table S4. Insets are expanded MS spectra at m/z 962.
In conclusion, we have demonstrated the first use of mesoprous ZrO2 nanomaterials for simple and highly effective enrichment of phosphopeptides. These materials enrich phosphopeptides with high specificity which allows a more comprehensive and efficient phosphoproteomic analysis. Proper engineering of the mesoporous materials in terms of chemical composition, porosity, surface area, and pore structures and further optimization of the enrichment procedures will enhance their performance even further. These results open up the exploitation of mesoporous metal oxide nanomaterials for their practical applications in MS-based phosphoproteomic study of complex biological samples, which are curently in progess.
Supplementary Material
Acknowledgments
This work is supported by US National Institutes of Health (NIH) CA126701 and UW-Madison IEDR and Draper TIF grants.
Footnotes
Electronic Supplementary Information (ESI) available: ZrO2 synthesis, and phosphopeptide enrichment details and results, with phosphopeptide sequences, tandem MS spectra and tables, and additional SEM of the materials. See DOI: 10.1039/b000000x/
Contributor Information
Song Jin, Email: jin@chem.wisc.edu.
Ying Ge, Email: yge@physiology.wisc.edu.
Notes and references
- 1.(a) Hunter T. Cell. 2000;100:113–127. doi: 10.1016/s0092-8674(00)81688-8. [DOI] [PubMed] [Google Scholar]; (b) Pawson T, Nash P. Science. 2003;300:445–452. doi: 10.1126/science.1083653. [DOI] [PubMed] [Google Scholar]; (c) Jumaa H, Hendricks RW, Reth MB. Annu. Rev. Immunol. 2005;23:415–445. doi: 10.1146/annurev.immunol.23.021704.115606. [DOI] [PubMed] [Google Scholar]
- 2.(a) McLafferty FW, Fridriksson EK, Horn DM, Lewis MA, Zubarev RA. Science. 1999;284:1289–1290. doi: 10.1126/science.284.5418.1289. [DOI] [PubMed] [Google Scholar]; (b) McLachlin DT, Chait BT. Curr. Opin. Chem. Biol. 2001;5:591–602. doi: 10.1016/s1367-5931(00)00250-7. [DOI] [PubMed] [Google Scholar]; (c) Huang PH, White FM. Mol. Cell. 2008;31:777–781. doi: 10.1016/j.molcel.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Carr SA, Huddleston MJ, Annan RS. Anal. Biochem. 1996;239:180–192. doi: 10.1006/abio.1996.0313. [DOI] [PubMed] [Google Scholar]
- 3.(a) Mann M, Ong SE, Gronborg M, Steen H, Jensen ON, Pandey A. Trends Biotechnol. 2002;20:261–268. doi: 10.1016/s0167-7799(02)01944-3. [DOI] [PubMed] [Google Scholar]; (b) Steen H, Jebanathirajah JA, Rush J, Morrice N, Kirschner MW. Mol. Cell. Proteomics. 2006;5:172–181. doi: 10.1074/mcp.M500135-MCP200. [DOI] [PubMed] [Google Scholar]
- 4.(a) Bodenmiller B, Mueller LN, Mueller M, Domon B, Aebersold R. Nat. Methods. 2007;4:231–237. doi: 10.1038/nmeth1005. [DOI] [PubMed] [Google Scholar]; (b) Oda Y, Nagasu T, Chait BT. Nat. Biotechnol. 2001;19:379–382. doi: 10.1038/86783. [DOI] [PubMed] [Google Scholar]; (c) Tao WA, Wollscheid B, O'Brien R, Eng JK, Li XJ, Bodenmiller B, Watts JD, Hood L, Aebersold R. Nat. Methods. 2005;2:591–598. doi: 10.1038/nmeth776. [DOI] [PubMed] [Google Scholar]
- 5.(a) Porath J, Carlsson J, Olsson I, Belfrage G. Nature. 1975;258:598–599. doi: 10.1038/258598a0. [DOI] [PubMed] [Google Scholar]; (b) Posewitz MC, Tempst P. Anal. Chem. 1999;71:2883–2892. doi: 10.1021/ac981409y. [DOI] [PubMed] [Google Scholar]; (c) Zhang X, Ye JY, Jensen ON, Roepstorff P. Mol. Cell. Proteomics. 2007;6:2032–2042. doi: 10.1074/mcp.M700278-MCP200. [DOI] [PubMed] [Google Scholar]; (d) Ficarro SB, McCleland ML, Stukenberg PT, Burke DJ, Ross MM, Shabanowitz J, Hunt DF, White FM. Nat. Biotechnol. 2002;20:301–305. doi: 10.1038/nbt0302-301. [DOI] [PubMed] [Google Scholar]; (e) Wolf-Yadlin A, Hautaniemi S, Lauffenburger DA, White FM. Proc. Natl. Acad. Sci. U.S.A. 2007;104:5860–5865. doi: 10.1073/pnas.0608638104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.(a) Pinkse MWH, Uitto PM, Hilhorst MJ, Ooms B, Heck AJR. Anal. Chem. 2004;76:3935–3943. doi: 10.1021/ac0498617. [DOI] [PubMed] [Google Scholar]; (b) Larsen MR, Thingholm TE, Jensen ON, Roepstorff P, Jorgensen TJD. Mol. Cell. Proteomics. 2005;4:873–886. doi: 10.1074/mcp.T500007-MCP200. [DOI] [PubMed] [Google Scholar]; (c) Kweon HK, Hakansson K. Anal. Chem. 2006;78:1743–1749. doi: 10.1021/ac0522355. [DOI] [PubMed] [Google Scholar]; (d) Han L, Shan Z, Chen DH, Yu XJ, Yang PY, Tu B, Zhao DY. J. Colloid. Interface Sci. 2008;318:315–321. doi: 10.1016/j.jcis.2007.10.026. [DOI] [PubMed] [Google Scholar]; (e) Ficarro SB, Parikh JR, Blank NC, Marto JA. Anal. Chem. 2008;80:4606–4613. doi: 10.1021/ac800564h. [DOI] [PubMed] [Google Scholar]
- 7.(a) Chen CT, Chen YC. Anal. Chem. 2005;77:5912–5919. doi: 10.1021/ac050831t. [DOI] [PubMed] [Google Scholar]; (b) Zhou HJ, Tian RJ, Reth MB, Xu SY, Feng S, Pan CS, Jiang XG, Li X, Zou HF. Electrophoresis. 2007;28:2201–2215. doi: 10.1002/elps.200600718. [DOI] [PubMed] [Google Scholar]
- 8.(a) Yang PD, Zhao DY, Margolese DI, Chmelka BF, Stucky GD. Nature. 1998;396:152–155. [Google Scholar]; (b) Yang PD, Zhao DY, Margolese DI, Chmelka BF, Stucky GD. Chem. Mater. 1999;11:2813–2826. [Google Scholar]; (c) Brinker CJ, Lu YF, Sellinger A, Fan HY. Advanced Materials. 1999;11:579–585. [Google Scholar]; (d) Lu YF, Ganguli R, Drewien CA, Anderson MT, Brinker CJ, Gong WL, Guo YX, Soyez H, Dunn B, Huang MH, Zink JI. Nature. 1997;389:364–368. [Google Scholar]; (e) Fan J, Boettcher SW, Stucky GD. Chem. Mater. 2006;18:6391–6396. [Google Scholar]; (f) Tian B, Yang H, Liu X, Xie S, Yu C, Fan J, Tu B. D. Zhao Chem. Comm. 2002;17:1824–1825. doi: 10.1039/b205006d. [DOI] [PubMed] [Google Scholar]
- 9.(a) Zhou H, Xu S, Ye M, Feng S, Pan C, Jiang X, Li X, Han G, Fu Y, Zou H. J. Proteome Res. 2006;5:2431–2437. doi: 10.1021/pr060162f. [DOI] [PubMed] [Google Scholar]; (b) Hu L, Zhou H, Li Y, Sun S, Guo L, Ye M, Tian X, Gu J, Yang S, Zou H. Anal. Chem. 2009;81:94–104. doi: 10.1021/ac801974f. [DOI] [PubMed] [Google Scholar]
- 10.(a) Nawrocki J, Rigney J, McCormick A, Carr PW. J. Chromatogr. A. 1993;657:229–282. doi: 10.1016/0021-9673(93)80284-f. [DOI] [PubMed] [Google Scholar]; (b) Rai D, Xia YX, Hess NJ, Strachan DM, McGrail BP. J. Solution Chem. 2001;30:949–967. [Google Scholar]
- 11.(a) Zubarev RA, Kelleher NL, McLafferty FW. J. Am. Chem. Soc. 1998;120:3265–3266. [Google Scholar]; (b) Ge Y, Lawhorn BG, ElNaggar M, Strauss E, Park JH, Begley TP, McLafferty FW. J. Am.Chem. Soc. 2002;124:672–678. doi: 10.1021/ja011335z. [DOI] [PubMed] [Google Scholar]; (c) Shi SDH, Hemling ME, Carr SA, Horn DM, Lindh I, McLafferty FW. Anal. Chem. 2001;73:19–22. doi: 10.1021/ac000703z. [DOI] [PubMed] [Google Scholar]; (d) Breuker K, McLafferty FW. Angew. Chem. Int. Ed. 2003;42:4900–4904. doi: 10.1002/anie.200351705. [DOI] [PubMed] [Google Scholar]
- 12. http://www.expasy.ch/sprot/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.