Abstract
Vitamin D and calcium insufficiencies are risk factors for multiple chronic diseases. Data from 46 recent studies from Europe, North America, South-East Asia and the South Pacific area clearly indicate that a low vitamin D status and inadequate calcium nutrition are highly prevalent in the general population (30–80%), affecting both genders. The extent of insufficiencies is particularly high in older populations, and in some geographical areas, also in children and in young women of child-bearing age, in ethnic minorities and immigrants, as well as in people of low socio-economic status. Enrichment of cereal grain products with vitamin D and calcium would be a viable approach to increase consumption and improve health outcomes in the general population worldwide.
Keywords: vitamin D status; calcium intake; 25-hydroxyvitamin D; 1,25-dihydroxyvitamin D; calcium-sensing receptor; osteoporosis; colorectal cancer; breast cancer; prevention; food fortification
1. Introduction
It is common knowledge that an inadequate supply of vitamin D and calcium has negative effects on bone health at all ages, inasmuch as it causes rickets in infants, retards acquisition of an adequate bone mass during skeletal development in adolescents, and is finally responsible for accelerated bone loss in adulthood in both women and men, leading to the development of osteoporosis. Importantly, there is also evidence from epidemiological studies, clinical intervention trials as well as from studies with animal models of human diseases that a compromised vitamin D status and inadequate calcium nutrition are predisposing conditions for a great number of other diseases, including various types of cancer, chronic infectious, inflammatory and autoimmune diseases, metabolic disorders, as well as hypertension and cardiovascular diseases (Table 1; for details, [1–3]).
Table 1.
Rating of evidence for association of vitamin D and/or calcium insufficiency with frequent chronic diseases (for details, [1,3]).
| Vitamin D insufficiency | Nutritional Calcium deficit |
|---|---|
| A. Convincing evidence from multiple epidemiological (prospective, cross-sectional, retrospective) large cohort studies, interventional trials and experimental studies | |
| Osteoporosis | Osteoporosis |
| Cancer (colorectal, breast) | Cancer (colorectal, breast) |
| B. Good evidence from >3 observational studies and/or interventional trials | |
| Cancer (renal, prostate, endometrial, ovarian, esophageal, gastric, pancreatic, bladder) Hodgkin’s and non-Hodgkin’s lymphoma | Cancer (renal) |
| Cardiovascular disease | Cardiovascular disease |
| Hypertension | |
| Neuromuscular dysfunctions | Neuromuscular dysfunctions |
| Diabetes mellitus Type I | |
| Tuberculosis | |
| Gingivitis | |
| Periodontal disease, tooth loss | Periodontal disease, tooth loss |
| C. Emerging evidence from observational studies | |
| Hypertension | |
| Metabolic Syndrome | Metabolic Syndrome |
| Diabetes mellitus Type II | Diabetes mellitus Type II |
| D. Evidence mainly from studies with animal models of the respective human disease | |
| Inflammatory bowel disease | Inflammatory bowel disease |
| Multiple Sclerosis | Multiple Sclerosis |
2. Why a Low Vitamin D Status and a Nutritional Calcium Deficit are Risk Factors for many Chronic Diseases
Vitamin D comes from two sources in humans, it could either be synthesized in form of vitamin D3 (cholecalciferol) under the influence of solar UV-B radiation in the epidermis, or be absorbed from the diet or from supplements and food additives, which in some countries may contain vitamin D2 (ergocalciferol). In any form, vitamin D is transferred to the liver, where it is metabolized to 25-hydroxyvitamin D (25-(OH)D). The term 25-(OH)D is used to denote the sum of 25-(OH)D3 and 25-(OH)D2. Thus, the plasma level of this metabolite reflects the sum of vitamin D from endogenous synthesis and from dietary intake, and is therefore a reliable indicator of an individual’s vitamin D status.
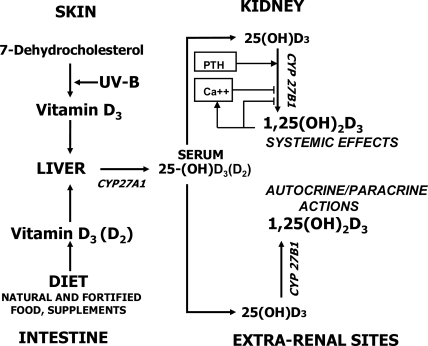
Conversion of 25-(OH)D3 to the biologically most active metabolite, 1,25-dihydroxyvitamin D3 (1,25-(OH)2D3), is catalyzed by the CYP27B1-encoded enzyme, 25-(OH)D-1α-hydroxylase, and takes place predominantly in the kidney, but also at many extra-renal sites [4] (see Figure 1): Examples are normal and neoplastic epithelial cells of the skin [5], of the gastrointestinal tract [6,7] and of female and male reproductive organs [8–10] as well as osteoblasts and osteoclasts [11,12] and cells of the vascular [13], the central nervous [14] and the immune system [15,16].
Figure 1.
Synthesis, absorption and metabolism of vitamin D.
1,25-(OH)2D3 bound to the nuclear high-affinity vitamin D receptor (VDR) functions as a transactivating regulator of gene expression. Circulating 1,25-(OH)2D3, which is produced by up to 90% in the kidney, plays a key role in systemic calcium and phosphate homeostasis by regulating ion fluxes in its classical target organs, i.e., small intestine, kidney and bone. 1,25-(OH)2D3 is also an important local regulator of cellular proliferation, differentiation and function in many organs and cell systems that express the 25-(OH)D-1α-hydroxylase [4] (Figure 1). The extent of intracellular synthesis of 1,25-(OH)2D3 at extra-renal sites depends largely on ambient 25-(OH)D3 levels and is not associated with circulating 1,25-(OH)2D3 concentrations (e.g., [17]). Therefore, at low serum levels of 25-(OH)D, 25-(OH)D-1α-hydroxylase activity may not be sufficient to maintain tissue concentrations of 1,25-(OH)2D3 necessary for efficient autocrine/paracrine regulation of cellular growth and function. This explains why many chronic diseases, as listed in Table 1, show significant negative associations with serum 25-(OH)D [1,3,18]. Importantly, low serum 25-(OH)D has been shown to be a reliable predictor of all-cause mortality [19].
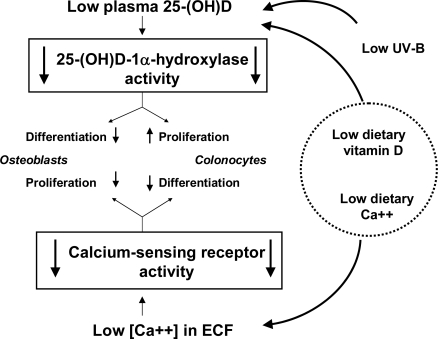
There is evidence from many human and animal studies for a significant inverse relationship between dietary calcium and risk of multiple chronic diseases [1]. This is difficult to understand because the effect of even large variations in calcium intake levels on extracellular calcium concentrations [Ca2+]o is attenuated by the systemic actions of calcium-regulating hormones allowing physiological variations in [Ca2+]o to occur only within a narrow range. However, many types of cells express a calcium-sensing receptor (CaR), which senses even minute changes in [Ca2+]o and thus allows Ca2+ to function as a “first messenger” for various cellular responses [20]. An important feature of the CaR is the high cooperativity between multiple Ca2+-binding sites in its extracellular domain. This results in amplification of signals from extracellular Ca2+, which, by cell-specific coupling to stimulatory and inhibitory G proteins, are transduced into various intracellular signalling pathways. Expression of a functioning CaR thus allows cell-specific reactions to physiological changes in [Ca2+]o. The CaR not only controls PTH secretion from parathyroid gland cells but plays key roles in normal cartilage and bone formation [21–23], as well as in limitation of cellular growth of normal and neoplastic cells [2,24]. Conversely, low dietary calcium causes hyperparathyroidism by impairment of CaR activity and, by the same token, can be linked to the development of not only osteoporosis and various malignancies, but possibly other calcium-insufficiency-related chronic diseases (Table 1) (for details, [1,3]; see also Figure 2).
Figure 2.
Tissue-specific consequences of low vitamin D and calcium status on cellular proliferation and differentiation. ECF, extracellular fluid.
Relevant for our understanding how vitamin D and calcium status interact in the pathogenesis of chronic diseases, is the observation that most cell types jointly express the 25-(OH)D-1α-hydroxylase and the CaR. Therefore, cell-specific cooperative signalling from 1,25-(OH)2D3/VDR and CaR, which is necessary to maintain normal cell functions (as detailed in [1,3]), is impaired under conditions of vitamin D and calcium insufficiency (Figure 2). This has been shown particularly for osteoporosis and many malignancies, particularly colorectal and breast cancer (as detailed in the following). Low serum 25-(OH)D and inadequate calcium intake reportedly are associated with cardiovascular risk factors such as hypertension [25–27], obesity [28–30], metabolic syndrome and diabetes mellitus type II [31,32]. Vitamin D and calcium insufficiencies have also been correlated with incident cardiovascular symptoms, including angina, coronary insufficiency, myocardial infarction, transient ischemic attack, and stroke [33], as well as with greater mortality from chronic cardiovascular disease [19,34,35].
3. Vitamin D Insufficiency: A World-Wide Phenomenon
3.1. Definition of Vitamin D Insufficiency
The vitamin D status of an individual is a composite of UV-B mediated synthesis of vitamin D3 in the epidermis and of intake and absorption from the gut. Outright vitamin D deficiency is indicated by plasma 25-(OH)D levels below 10–15 nM [36]. In this situation, 1,25-(OH)2D production in the kidney is severely limited because of substrate depletion, causing a decrease in intestinal calcium absorption with rickets or osteomalacia as a consequence. At 25-(OH)D serum concentrations above 15 nM, the kidney produces enough 1,25-(OH)2D3 to maintain systemic mineral ion homeostasis [37], but availability of 25-(OH)D for intracellular production of 1,25-(OH)2D3 at extra-renal sites may be insufficient for autocrine/paracrine control of cellular functions [1]. The definition of vitamin D insufficiency is still a matter of debate. At one time, a serum 25-(OH)D concentration of ~30 nM was thought to be the delimitation between vitamin D insufficiency and adequate vitamin D supply [38]. Now there is growing agreement that serum 25(OH)D should be at least 50 nM [39] (see also Tables 2–4). Even higher cut-offs, e.g. 60–100 nM, are supported by studies on optimal health outcomes [40].
Table 2.
Prevalence of vitamin D insufficiency in healthy adults in selected countries.
| Country | Population segment |
% Vitamin D insufficiency |
Study | ||
|---|---|---|---|---|---|
| with upper reference limit at | |||||
| Age (yr) | Gender | 25–30 nM | 50 nM | ||
|
Europe | |||||
| Austria | 19–79 | M + F | 26 | 60 | Kudlacek et al. [44] |
| Denmark | 45–58 | F | 7 | 40 | Brot et al. [45] |
| Finland | 31–43 | M + F | 27 | Lamberg–A. et al.[47] | |
| France | 35–65 | M + F | 14 | Chapuy et al. [38] | |
| Germany | 18–79 | M + F | 58 | Hintzpeter et al. [26] | |
| UK | 45 | M + F | 16 | 47 | Hypponen & Power [46] |
|
North America | |||||
| Canada (Alberta) | 27–89 | M + F | 61 | Rucker et al. [52] | |
| USA | 20–49 | M + F | 5 | 32 | Looker et al. [51] |
|
South–East Asia | |||||
| Bangladesh | 16–40 | F | 12 – 17 | Islam et al. [48] | |
| Japan | 30–66 | F | 10 | Nakamura et al. [50] | |
|
South Pacific | |||||
| Australia (Queensland) | 17–65 | M + F | 8 a) | 23 | McGrath et al. [49] |
| Australia (Queensland) | <60 | M + F | 40 | Van der Mei et al. [54] | |
| Australia (Victoria) | 20–92 | F | 11 | 43 | Pasco et al. [53] |
| Australia (Geelong) | <60 | F | 37 | Van der Mei et al. [54] | |
| Australia (Tasmania) | <60 | M + F | 67 | Van der Mei et al. [54] | |
| New Zealand | 15–65+ | M + F | 48 | Rockell et al. [55] | |
Serum 25-(OH)-D <38 nM
Table 4.
Prevalence of vitamin D insufficiency in children, adolescents and young adults.
| Country | Population segment |
% Vitamin D insufficiency |
Study | ||
|---|---|---|---|---|---|
| with upper reference limit of serum 25–(OH)D at | |||||
| Age (yrs) | Gender | 25–30 nM | 50 nM | ||
|
Europe | |||||
| Germany | 3–17 | M | 18 | 62 | Hintzpeter et al.[64] |
| Germany | 3–17 | F | 18 | 65 | Hintzpeter et al. [64] |
| France | 13–16 | M | 78 | Guillemant et al. [65] | |
| Denmark | 12–13 | F | 51 | 93 | Andersen et al. [60] |
| Finland | 12–13 | F | 37 | 97 | Andersen et al. [60] |
| Ireland | 12–13 | F | 26 | 89 | Andersen et al. [60] |
| Poland | 12–13 | F | 33 | 87 | Andersen et al. [60] |
|
North America | |||||
| USA | 12–19 | M | 10 | 24 | Looker et al. [51] |
| USA | 12–19 | F | 16 | 31 | Looker et al. [51] |
| Canada | 18–35 | F | >15 | Vieth et al. [66] | |
|
South–East Asia | |||||
| India (Delhi) | 6 – 18 | F | 91 | Puri et al. [68] | |
| China | 20–35 | F | 18–40 | >90 | Woo et al. [70] |
| China | 15 | F | 31 | 89 | Foo et al. [69] |
| Indonesia | 18–40 | F | 63 | Green et al. [67] | |
| Malaysia | 18–40 | F | 60 | Green et al. [67] | |
| Japan | 19–30 | F | 42 | Nakamura et al. [50] | |
|
South Pacific | |||||
| New Zealand | 15–18 | M | 55 | Rockell et al. [55] | |
| New Zealand | 19–24 | F | 52 | Rockell et al. [55] | |
3.2. Epidemiology of Vitamin D Insufficiency
Considering reasons for vitamin D insufficiency, one has to take into account that cutaneous UV-B-mediated production of vitamin D3 is affected by many factors, such as time of the day, season of the year, latitude, altitude, skin pigmentation or use of sunscreens. Also aging can markedly reduce the capacity of the skin to produce vitamin D3 [41]. Geographical differences in vitamin D status result from varying contributions to the vitamin D supply from exposure to solar UV-B but also from intake of dietary and supplemental vitamin D [42,43]. Table 2 lists the results of the available nationally representative studies on prevalence of vitamin D insufficiency in the normal adult population in Europe, North America, East Asia and in the South Pacific area.
In Europe, 7–27% of the adult population have a serum 25-(OH)D concentration below 25–30 nM [26,38,44–47]. In South-East Asia and in Australia, incidence of vitamin D insufficiency varies between 8–17% [48–50]. A relatively low value of 5% has been reported for the USA [51]. However, if 50 nM 25-(OH)D is considered the upper reference limit, on the average one-half of the adult population in Europe [26,38,44–47], Western Canada [52], Australia [53,54] and New Zealand [55] presents with vitamin D insufficiency, whereas only one-third is afflicted in the USA [51] (Table 2).
It must be noted that the proportion of the general population with serum 25-(OH)D below the desirable level of 70–80 nM [40,56] is 73% in the USA [51], 84–87% in Europe and in the South Pacific area [44,46,55] and up to 97% in Canada [52].
3.3. Vitamin D Insufficiency in Different Population Segments
Elderly people: It has been known for decades that vitamin D insufficiency is common in people, who are immobilized because of chronic diseases or are housebound due to old age. However, recent nationally representative data show that vitamin D insufficiency is present in a substantial portion of old age ambulant people worldwide (Table 3): For example, the European SENECA Study on diet and health of elderly people from 19 towns in 12 European countries revealed that overall 36% of men and 47% of women had serum 25-(OH)D concentrations below 30 nM [57]. In the Netherlands, 50% of people aged 65 years and older had serum levels of 25-(OH)D below 50 nM [58]. Similar values were reported for elderly women in Belgium [59]. According to the OPTIFORD Study [60], 50–92 % of elderly women in Denmark, Finland, Ireland and Poland had wintertime 25-(OH)D concentrations lower than 50 nM. On the average, prevalence of vitamin D insufficiency in elderly people in North America [51,61], Australia [62], New Zealand [55] and Japan [63] seems to be lower than in Europe (Table 3).
Table 3.
Prevalence of vitamin D insufficiency in elderly people.
| Country | Population segment |
% Vitamin D insufficiency |
Study | ||
|---|---|---|---|---|---|
| with upper reference limit of serum 25-(OH)D at | |||||
| Age (yr) | Gender | 25–30 nM | 50 nM | ||
|
Europe | |||||
| Belgium | 76.5 ± 7.5 | F | 16 | 43 | Neuprez et al. [59] |
| Denmark | 70–75 | F | 17 | 55 | Andersen et al. [60] |
| Finland | 70–75 | F | 10 | 57 | Andersen et al. [60] |
| Ireland | 70–75 | F | 14 | 60 | Andersen et al. [60] |
| Italy | 75–80 | F | 92 | v. d. Wielen et al. [57] | |
| Netherlands | 65+ | M + F | 13 | 52 | Wicherts et al. [58] |
| Poland | 70–75 | F | 25 | 92 | Andersen et al. [60] |
| Switzerland | 75–80 | M | 12 | v. d. Wielen et al. [57] | |
|
North America | |||||
| USA | 55+ | F | 4 | 14 | Lappe et al. [61] |
| USA | 50–70+ | M | 10 | 27 | Looker et al. [51] |
|
East Asia | |||||
| Japan | 46–82 | F | 5 | 28 | Nakamura et al. [63] |
|
South Pacific | |||||
| Australia | 75+ | F | 22 | Flicker et al. [62] | |
| New Zealand | 65+ | M | 41 | Rockell et al. [55] | |
Children, adolescents and young adults: In some European countries such as Denmark, Finland, Ireland and Poland [60], 37% of 12-year old girls had 25-(OH)D concentrations lower than 25 nM (Table 4). By using a broader definition, i.e., <50 nM, 92% had to be considered vitamin Dinsufficient. Comparative values are much lower in Germany [64], but considerably higher in France [65].
In the USA, the proportion of male and female adolescents between 12–19 years, who had 25-(OH)D values below 50 nM, ranged from 24 to 31%, respectively [51]. Incidence of vitamin D insufficiency in younger women is low in Canada [66], compared to Indonesia, Malaysia [67] or Japan [50]. Alarmingly high rates were found in older girls and young women at child-bearing age in India [68] and China [69,70] (Table 4).
Pregnant women and neonates: In Europe and in the USA, a poor vitamin D status is observed with increasing frequency in pregnant women [71] and consequently in their neonates causing a high risk of not only rickets, but also non-skeletal diseases in later life, e.g. type 1 diabetes [72].
Obesity deserves a special note as a condition frequently associated with vitamin D and calcium insufficiency. A number of earlier studies have well documented a high prevalence of vitamin D insufficiency in morbidly obese women. Now evidence is emerging that in otherwise healthy women and men, body fat mass is frequently inversely associated with vitamin D insufficiency [29,73]. Sequestration of vitamin D in the subcutaneous fat, which alters its release into the circulation, could be one by which obesity could contribute to vitamin D insufficiency [74].
Ethnic groups: The immigrant population is at high risk for vitamin D insufficiency in many European countries such as Denmark, Norway and Great Britain [75–77]. In Germany, the proportion of vitamin D inadequacy in children and adolescents, aged 2–17 years, is higher in immigrant than in non-immigrant girls and boys at any time. Notably, after termination of vitamin D supplementation for prophylaxis of rickets between the age of 1–2 years, serum 25-(OH)-D levels fell rapidly below 50 nM in both groups [64]. In the USA, prevalence of vitamin D insufficiency in Mexican American and Non-Hispanic black people is higher than in Non-Hispanic White individuals [51]. Analogous skin pigmentation and degree of vitamin D inadequacy has been reported for three ethnic groups in New Zealand, i.e., Pacific people, Maori and people of European origin [55]. In Australia, dark-skinned and “veiled” women, particularly when pregnant, belong to the group with the highest risk for vitamin D insufficiency [78].
4. Inadequate Calcium Intake: A World-Wide Problem
4.1. Recommended Calcium Intake Levels
Different intake levels for calcium are recommended by FAO/WHO experts for infants, children and adults [79] to assure optimal whole body calcium retention and consequently adequate development and maintenance of bone mass and mineral density. For children and adolescents between 10–18 years of age, consumption of 1,300 mg per day is recommended, while 1,000 mg per day apply for men between 25–50 years of age and also for women in the same age group, except when higher intake is necessary during pregnancy or after menopause. Recommended calcium allowance per day for males over 65 years and postmenopausal women is 1,300 mg [79].
4.2. Epidemiology of Calcium Intake
Findings listed in Table 5 indicate that in Europe daily calcium intake from nutrient sources is consistently low. For example, 84% of the adult population in Austria fail to meet recommended intake levels [44]. The situation is apparently better in Germany [80,81], with one study reporting daily calcium intake even at recommended levels [26]. This seems to be also the case in Great Britain [82].
Table 5.
Nutritional calcium deficit in selected countries.
|
Country |
Age (yrs) | DRI a) (mg/day) |
Calcium intake (mg/day) |
Study | |
|---|---|---|---|---|---|
|
Gender |
|||||
| M | F | ||||
|
Europe | |||||
| Austria | 19–79 | >1,000 | 561 (±290) b) | 576 (±309) b) | Kudlacek et al. [44] |
| <40 | 1,000 | 604 (±345) b) | 560 (±299) b) | Kudlacek et al. [44] | |
| 40–60 | >1,000 | 590 (±318) b) | 561 (±287) b) | Kudlacek et al. [44] | |
| Germany | 18–79 | >1,000 | 1,181 (902–1,535) | 1,082 (849–1,379) | Hintzpeter et al. [26] |
| Adults | 1,000 | 619 (213–1,025) | 705 (313–1,094) | Anke [80] | |
| 40–64 | >1,000 | 774 (334–1,330) c) | 707 (287–1,225) c) | Schulze et al. [81] | |
| UK f) | 45–55 | 1,000 | 1,133 (950–1,316) | 1,063 (931–1,195) | Vyas et al. [82] |
|
North America | |||||
| USA | 19–50 | 1,000 | 812 (788–837) | 626 (596–659) | Ma et al. [83] |
|
South–East Asia | |||||
| Bangladesh | 16 – 40 | 1,000 | 180 e) | Islam et al. [86] | |
| Indonesia | 18–40 | 1,000 | 270 (239–302) d) | Green et al. [67] | |
| Malaysia | 18–40 | 1,000 | 386 (353–420) d) | Green et al. [67] | |
|
South Pacific | |||||
| Australia | 20–94 | >1,000 | 643 (±340) b) | Pasco et al. [84] | |
| M + F | |||||
| New Zealand | 40–64 | >1,000 | 794 (8–1,580) e) | Metcalf et al. [85] | |
Daily Recommended Intake by FAO/WHO [79];
mean (±SD);
median (90% CI);
median (95% CI);
mean (range);
white, urban population
In contrast, 40% of the population does not meet adequacy in the USA [83]. Similar values probably pertain for Australia [84] and New Zealand [85]. Special consideration must be given to the nutritional calcium deficit in South-East Asian countries such as Indonesia, Malaysia [67] and Bangladesh [86]: The situation is particularly alarming in Bangladesh, where 47% of premenopausal women in the higher socio-economic brackets failed to meet a daily allowance of 400–500 mg calcium, and 63% of women of low socio-economic standing had calcium intake even lower than 200 mg/day [86].
4.3. Population Segments with Low Habitual Calcium Intake
Chronically ill people: A chronically negative calcium balance due to malabsorption develops, for example, in the many individuals suffering worldwide from lactose intolerance or from inflammatory bowel disease (Crohn’s disease, ulcerative colitis). In addition, calcium malabsorption must be reckoned with in all cases of vitamin D insufficiency resulting from intestinal, hepatic, renal or endocrine disorders as well as in the group of bariatric surgery patients who increase in numbers as a result of the obesity epidemic in the affluent parts of the world.
Individuals with reduced physical activity: It must be noted that immobilization even for a short period, e.g. 1–2 weeks of bed rest leads to mobilization of calcium from bone and consequently to net calcium loss [87]. Therefore not only patients in geriatric, psychiatric or neurological care, but also healthy individuals with low habitual physical activity have an increased risk of calcium insufficiency.
Elderly people: The data collated in Table 6 confirm the long-standing assumption that particularly the elderly ingest significantly less calcium in their diet than the recommended amount, which is currently considered 1,300 mg per day for this age group [79]. In the European SENECA Study [88], the overall mean calcium intake by elderly people was 894 mg per day, with variations from 600–1,100 mg between different study sites. In the OPTIFORD Study, the median calcium intake among elderly women was 632 mg per day, being lowest in Poland (325 mg) and highest in Finland (925 mg) [60]. In the USA, the mean intake of calcium in women after age 55 is only ~600 mg/day [61]. Daily consumption of ~500 mg by elderly Japanese women [89] is far below a recommended level of 1,200 mg, although daily calcium requirements of East Asian populations may be lower for ethnic reasons [79].
Table 6.
Nutritional calcium deficit in elderly people.
| Country | Calcium intake (mg/day) | Study | |||
|---|---|---|---|---|---|
| Age (yrs) | DRI (mg/d)a) | M | F | ||
|
Europe | |||||
| Austria | >60 | 1,300 | 503 (±221) b) | 569 (±287) b) | Kudlacek et al. [44] |
| Belgium | 75–80 | 1,300 | 748 (324–1,166) c) | 676 (287–1,101) c) | Amorim Cruz et al. [88] |
| Denmark | 70–75 | 1,300 | 544 (127–1,812)d) | Andersen et al. [60] | |
| Finland | 70–75 | 1,300 | 975 (404–2,313) d) | Andersen et al. [60] | |
| France | 75–80 | 1,300 | 620 (402–1,010) c) | 635 (428–944) c) | Amorim Cruz et al. [88] |
| Ireland | 70–75 | 1,300 | 824 (339–1,669) d) | Andersen et al. [60] | |
| Netherlands | 75–80 | 1,300 | 1,036 (725–1,447) c) | 1,010(612–1,616) c) | Amorim Cruz et al. [88] |
| Poland | 70–75 | 1,300 | 325 (86–851) d) | Andersen et al. [60] | |
|
North America | |||||
| USA | >55 | 1,300 | 611 (381–892) c) | Lappe et al. [61] | |
|
South–East Asia | |||||
| Japan | 65–75 | 1,300 | 527 (±195) b) | Nakamura et al. [89] | |
Children, adolescents and young adults: In the European OPTIFORD study the median daily calcium intake of girls at a mean age of ~13 years was 823 mg, ranging from 524 mg in Poland to 1,092 mg in Finland [60] (Table 7). Data from the USA indicate that after the age of 10, calcium malnutrition is a common phenomenon. For example, average daily calcium intake in a group of young adolescents (12.7 ± 1.0 yr of age) was found to be 906 mg [90]. Grossly inadequate calcium intake was observed also in young adults in Canada [91]. Average daily calcium intake by schoolgirls in India between 400–500 mg [68], though corresponding to recommended daily allowances for Indians, nevertheless is far below current FAO/WHO recommendations of 1,000–1,300 mg/day [79].
Table 7.
Nutritional calcium deficit in children, adolescents and young adults.
| Country | Calcium intake (mg/day) | Study | |||
|---|---|---|---|---|---|
| Gender | |||||
| Age (yr) | DRI a) (mg/day) | M + F | F | ||
|
Europe | |||||
| Denmark | 12.6 | 1,300 | 831 (260–2,475) b) | Andersen et al. [60] | |
| Finland | 12.6 | 1,300 | 1,092 (546–2,452) b) | Andersen et al. [60] | |
| Ireland | 12.6 | 1,300 | 728 (54–2,259) b) | Andersen et al. [60] | |
| Poland | 12.6 | 1,300 | 524 (117–1,580) b) | Andersen et al. [60] | |
|
North America | |||||
| USA | 12.7 | 1,300 | 906 (417–1,616) c) | Abrams et al. [90] | |
| Canada | 18–35 | 1,000 | 562 (0–2,630) c) | Rubin et al. [91] | |
|
South-East Asia | |||||
| India (Delhi) | 6–18 | 700–1,300 | 575 (±219) d) | Puri et al. [68] | |
Ethnic groups: It has to be borne in mind that not only vitamin D deficiency but also a nutritional calcium deficit is an important cause of rickets [92]. So-called calcium deficiency rickets are prevalent in Middle Eastern and many sub-tropical and tropical countries, such as Nigeria, Ethiopia, South Africa, India and Bangladesh, despite the fact that such countries have ample sunlight [93–95]. Under this condition, the disease is attributable to low dietary calcium intake from mainly cereal-based diets.
5. Strategies for disease prevention
Studies on the vitamin D intake in different parts of the world cannot be directly compared because results may be confounded to some extent by differences in life style and clothing habits, consumption of traditional foods or supplement intake. Exact determination of the extent of calcium malnutrition is also difficult, because different methods are used for evaluation of daily calcium intake from nutrient sources and, in addition, for ethnic, dietary and geographical reasons different recommendations apply for different parts of the world [79]. However, combined evidence from all the studies that are included in the present survey clearly indicates that vitamin D insufficiency and calcium malnutrition are common in both genders worldwide, not only in elderly people as previously believed but also in younger adults. Importantly, the highest rates of insufficiencies are found in children and adolescents as well as in women of child bearing age.
5.1. Need to Increase Combined Intakes to Daily 800 IU Vitamin D and 1,200 mg Calcium
Vitamin D: A recent survey on world-wide vitamin D intake [96] clearly indicates that in many countries vitamin D supply from nutrient sources is too low to sustain mean 25-(OH)D levels in the general population between 40–100 nM, which are considered sufficiently high to achieve a better health outcome [97]. Cashman et al. [98] calculated that in 20–40 yr old adults, depending on the extent of sun exposure in summer, daily intakes of vitamin D between 300 and 1,600 IU are required to maintain an adequate vitamin D status in wintertime. Notably, Nelson et al. [56] reported that daily doses of 800 IU vitamin D3 were sufficient to sustain “optimal” 25-(OH)D serum concentrations (≥75nM) in 80% of a group of pre-menopausal women. With approximately the same daily dose of vitamin D3, serum 25-(OH)D levels could be maintained at 50 nM in 97.5% of a group of elderly people in the absence of sufficient sun exposure [99]. The beneficial effects of 800 IU supplemental vitamin D for various health outcomes are well documented. Daily intake of 700–800 IU vitamin D3 maintains normal bone turnover in healthy men at wintertime [100], and reduces the risk for colorectal or breast cancer by 50% [101,102].
Calcium: The suggestion to raise daily consumption of calcium to an average of 1,200 mg per day is based not only on physiological considerations [103] but can be deduced also from considerations of optimal health outcomes: For example, daily doses of 1,200 mg calcium effectively prevent osteoporotic bone loss and fractures in people aged 50 years or older [104], and cause a 40–50% risk reduction of colorectal cancer in men and of breast cancer in premenopausal women [105,106].
5.2. Rationale for Advocating Combined Intake of Vitamin D and Calcium
Simultaneous correction of nutritional vitamin D and calcium deficits for prevention or amelioration of many chronic diseases is necessary for two reasons: First, dietary intakes of vitamin D and calcium are strongly associated [107] and therefore vitamin D insufficiency is frequently associated with low calcium intake [1,44]. Second, because vitamin D and calcium interact positively in modulation of cellular proliferation, differentiation and function, as detailed before [3,18], it can be expected that an adequate vitamin D status is required to achieve the nutritional benefits of calcium and vice versa.
Osteoporosis: Combined supplementation with daily 800 IU vitamin D and 1,200 mg calcium is the essential basis for pharmacological prevention and treatment of osteoporosis. From a meta-analysis of 10 randomized controlled trials of oral vitamin D with or without calcium supplementation vs placebo/no treatment on the risk of hip fracture in elderly people, Boonen et al. [108] concluded that oral vitamin D appears to reduce the risk of hip fractures only when calcium supplementation is added.
Cancer: Lappe et al. [109] reported evidence from a randomized placebo-controlled trial that in post-menopausal women combined high-dose calcium and vitamin D3 supplementation, i.e., 1,400–1,500 mg calcium plus 1,100 IU vitamin D3, reduced the cumulative risk of cancer of the breast, lung, colon, uterus, lymphoid and myeloid system to 0.232 after four years of trial. Cho et al. [110] concluded from an analysis of pooled primary data from 10 cohort studies with a follow-up of more than half a million individuals for 6–16 years, that optimal risk reduction for colorectal cancer necessitates high intake levels of both vitamin D and calcium. This notion was shown to be valid not only for Western but also for Asian populations [111]. In pre-menopausal women, Bérubé et al. [112] found highly significant inverse relations between total intakes of vitamin D and calcium and breast density, which is a surrogate marker for breast cancer risk. It is noteworthy, that higher intake of one nutrient was related to lower breast density only in the presence of higher intake of the other nutrient.
6. What can be Done?
Simultaneous supplementation of vitamin D and calcium represents a safe and inexpensive strategy for prevention of osteoporosis, colorectal and breast cancer and possibly of many other chronic diseases (Table 1). It must be emphasized that daily consumption of 800 IU vitamin D and 1,200 mg calcium is well below the currently accepted tolerable upper intake levels of 2,000 IU (=50 μg) vitamin D3 and 3,000 mg calcium [79].
6.1. Supplementation by Fixed Vitamin D/Calcium Combination Tablets
Osteoporotic fractures can be effectively prevented at relatively low cost by combined supplementation with 1,200 mg/d calcium and 800 IU/d vitamin D3. However, a significant effect of vitamin D/calcium treatment is only seen in cohorts with at least 80% compliance [104]. Low compliance and lack of adherence seen with any long-term medication certainly will limit the usefulness of combined vitamin D and calcium supplementation for correction of respective insufficiencies in the general population. Therefore, combined vitamin D and calcium supplementation should be promoted specifically for disease prevention in high risk groups, i.e., individuals, who otherwise are unable to attain a normal vitamin D and calcium status due to specific living conditions (immobilization, physical incapacitation, advanced age, chronic diseases), traditional or personal nutritional habits, preferred lifestyle (lack of physical activity, indoor dwelling) etc.
6.2. Vitamin D and Calcium Enrichment in Single Foodstuffs
Fortified foods are an important source of vitamin D for those who consume them [113]. In a recent survey on the efficacy of food fortification on serum 25-(OH)D concentrations [114], dose-effect relations for vitamin D from food sources were found equivalent to those reported for vitamin D supplements. Vitamin D-fortified milk has been found to be a safe, effective and acceptable method of administering vitamin D, particularly to the elderly, community-based population. Orange juice fortified with vitamin D2 (1,000 IU/240 ml) was tested for its suitability to serve as an alternative for vitamin D-fortified milk. Fortification with vitamin D3 of wheat and rye bread is technically easy, and stability and bioavailability of vitamin D is good [115]. Consumption of bread fortified with 5,000 IU vitamin D3 and 320 mg calcium per daily serving for 12 months improved the vitamin D status of sun-deprived nursing home residents. Together with suppression of secondary hyperparathyroidism this apparently caused a significant increase in bone mineral density at the lumbar spine and the hip [116].
Calcium fortification is in use all over the world: Staples and food stuffs that are enriched with calcium include flour, cereals, milk, orange juice, mineral waters, soymilk etc. [117]. Calcium- and vitamin D-fortified milk, when providing 800 IU vitamin D and 1,000 mg calcium per day, has a significant positive effect on bone mass and strength in older men [118,119].
It is apparent that fortification of traditional and widely consumed foodstuffs (milk and milk products, bread, orange juice etc.) will guarantee a minimum additional supply of vitamin D and calcium.
6.3. Vitamin D and Calcium Addition to Cereal Grain Products
At present, cereal grain products, such as flour, corn meal, noodles and the like, are enriched with vitamin D and calcium in some countries in Europe and in the USA. Newmark et al. [117] summarized the rationale, data, efficacy, safety, cost and practicality of the addition of both calcium and vitamin D to cereal grain products to reduce the risk of osteoporosis and colon cancer. The authors estimate that, if cereal grain products were uniformly enriched with 90 IU/100g vitamin D, average daily intake of vitamin D could increase by up to 200 IU vitamin D. Enrichment of cereal grain products with 1,200–1,800 mg/kg calcium could raise dietary intake by about 200–400 mg/day. A conservative estimate suggests that through these measures at least a 20% reduction of the rate of osteoporotic fractures and of colorectal incidence can be achieved [117].
Enrichment of cereal grain products with vitamin D and calcium at indicated levels would be possible within current legal regulations in the USA [117]. It also conforms to legislation introduced in the European Union as of 2007. However, some important member states such as Germany have not yet changed their national law accordingly.
In summary, enrichment with vitamin D and calcium of cereal grain products is an effective and safe measure at very low cost to broaden the range of commonly consumed foods as dietary sources of vitamin D and calcium. This will guarantee at least some modest improvement in both vitamin D and calcium nutrition at the same time without necessitating a change in traditional eating habits. To fulfill individual needs for vitamin D and calcium however, additional consumption of particularly vitamin D- and calcium-rich food and food products or even supplement use is certainly indicated.
Acknowledgments
We thank Sandra E. Guggino, Johns Hopkins University Medical School (Baltimore, USA) for critical reading of the manuscript.
References
- 1.Peterlik M, Cross HS. Vitamin D and calcium deficits predispose for multiple chronic diseases. Eur. J. Clin. Invest. 2005;35:290–304. doi: 10.1111/j.1365-2362.2005.01487.x. [DOI] [PubMed] [Google Scholar]
- 2.Peterlik M, Grant WB, Cross HS. Calcium, vitamin D and cancer. Anticancer Res. 2009;29:3687–3698. [PubMed] [Google Scholar]
- 3.Peterlik M, Cross HS.Vitamin D and calcium insufficiency-related chronic diseases: molecular and cellular pathophysiology Eur J Clin Nutr 2009. doi:10.1038/ejcn.2009.105 [DOI] [PubMed]
- 4.Zehnder D, Bland R, Williams MC, McNinch RW, Howie AJ, Stewart PM, Hewison M. Extrarenal expression of 25-hydroxyvitamin D3-1 alpha-hydroxylase. J. Clin. Endocrinol. Metab. 2001;86:888–894. doi: 10.1210/jcem.86.2.7220. [DOI] [PubMed] [Google Scholar]
- 5.Pillai S, Bikle DD, Elias PM. 1,25-Dihydroxyvitamin D production and receptor binding in human keratinocytes varies with differentiation. J. Biol. Chem. 1988;263:5390–5395. [PubMed] [Google Scholar]
- 6.Cross HS, Peterlik M, Reddy GS, Schuster I. Vitamin D metabolism in human colon adenocarcinoma-derived Caco-2 cells: expression of 25-hydroxyvitamin D3-1alpha-hydroxylase activity and regulation of side-chain metabolism. J. Steroid Biochem. Mol. Biol. 1997;62:21–28. doi: 10.1016/s0960-0760(97)00020-4. [DOI] [PubMed] [Google Scholar]
- 7.Schwartz GG, Eads D, Rao A, Cramer SD, Willingham MC, Chen TC, Jamieson DP, Wang L, Burnstein KL, Holick MF, Koumenis C. Pancreatic cancer cells express 25-hydroxyvitamin D-1 alpha-hydroxylase and their proliferation is inhibited by the prohormone 25-hydroxyvitamin D3. Carcinogenesis. 2004;25:1015–1026. doi: 10.1093/carcin/bgh086. [DOI] [PubMed] [Google Scholar]
- 8.Becker S, Cordes T, Diesing D, Diedrich K, Friedrich M. Expression of 25-hydroxyvitamin D3-1alpha-hydroxylase in human endometrial tissue. J. Steroid Biochem. Mol. Biol. 2007;103:771–775. doi: 10.1016/j.jsbmb.2006.12.075. [DOI] [PubMed] [Google Scholar]
- 9.Schwartz GG, Whitlatch LW, Chen TC, Lokeshwar BL, Holick MF. Human prostate cells synthesize 1,25-dihydroxyvitamin D3 from 25-hydroxyvitamin D3. Cancer Epidemiol. Biomarkers Prev. 1998;7:391–395. [PubMed] [Google Scholar]
- 10.Friedrich M, Rafi L, Mitschele T, Tilgen W, Schmidt W, Reichrath J. Analysis of the vitamin D system in cervical carcinomas, breast cancer and ovarian cancer. Recent Results Cancer Res. 2003;164:239–246. doi: 10.1007/978-3-642-55580-0_17. [DOI] [PubMed] [Google Scholar]
- 11.van Driel M, Koedam M, Buurman CJ, Hewison M, Chiba H, Uitterlinden AG, Pols HA, van Leeuwen JP. Evidence for auto/paracrine actions of vitamin D in bone: 1alphahydroxylase expression and activity in human bone cells. FASEB J. 2006;20:2417–2419. doi: 10.1096/fj.06-6374fje. [DOI] [PubMed] [Google Scholar]
- 12.Atkins GJ, Anderson PH, Findlay DM, Welldon KJ, Vincent C, Zannettino AC, O’Loughlin PD, Morris HA. Metabolism of vitamin D3 in human osteoblasts: evidence for autocrine and paracrine activities of 1alpha,25-dihydroxyvitamin D3. Bone. 2007;40:1517–1528. doi: 10.1016/j.bone.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 13.Zehnder D, Bland R, Chana RS, Wheeler DC, Howie AJ, Williams MC, Stewart PM, Hewison M. Synthesis of 1,25-dihydroxyvitamin D3 by human endothelial cells is regulated by inflammatory cytokines: a novel autocrine determinant of vascular cell adhesion. J. Am. Soc. Nephrol. 2002;13:621–629. doi: 10.1681/ASN.V133621. [DOI] [PubMed] [Google Scholar]
- 14.Eyles DW, Smith S, Kinobe R, Hewison M, McGrath JJ. Distribution of the vitamin D receptor and 1 alpha-hydroxylase in human brain. J. Chem. Neuroanat. 2005;29:21–30. doi: 10.1016/j.jchemneu.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 15.Hewison M, Freeman L, Hughes SV, Evans KN, Bland R, Eliopoulos AG, Kilby MD, Moss PA, Chakraverty R. Differential regulation of vitamin D receptor and its ligand in human monocyte-derived dendritic cells. J. Immunol. 2003;170:5382–5390. doi: 10.4049/jimmunol.170.11.5382. [DOI] [PubMed] [Google Scholar]
- 16.Sigmundsdottir H, Pan J, Debes GF, Alt C, Habtezion A, Soler D, Butcher EC. DCs metabolize sunlight-induced vitamin D3 to ‘program’ T cell attraction to the epidermal chemokine CCL27. Nat. Immunol. 2007;8:285–293. doi: 10.1038/ni1433. [DOI] [PubMed] [Google Scholar]
- 17.Anderson PH, O’Loughlin PD, May BK, Morris HA. Modulation of CYP27B1 and CYP24 mRNA expression in bone is independent of circulating 1,25(OH)2D3 levels. Bone. 2005;36:654–662. doi: 10.1016/j.bone.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 18.Cross HS, Kallay E. Regulation of the colonic vitamin D system for prevention of tumor progression: an update. Future Oncol. 2009;5:493–507. doi: 10.2217/fon.09.22. [DOI] [PubMed] [Google Scholar]
- 19.Dobnig H, Pilz S, Scharnagl H, Renner W, Seelhorst U, Wellnitz B, Kinkeldei J, Boehm BO, Weihrauch G, Maerz W. Independent association of low serum 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D levels with all-cause and cardiovascular mortality. Arch. Intern. Med. 2008;168:1340–1349. doi: 10.1001/archinte.168.12.1340. [DOI] [PubMed] [Google Scholar]
- 20.Tfelt-Hansen J, Brown EM. The calcium-sensing receptor in normal physiology and pathophysiology: a review. Crit. Rev. Clin. Lab. Sci. 2005;42:35–70. doi: 10.1080/10408360590886606. [DOI] [PubMed] [Google Scholar]
- 21.Dvorak MM, Siddiqua A, Ward DT, Carter DH, Dallas SL, Nemeth EF, Riccardi D. Physiological changes in extracellular calcium concentration directly control osteoblast function in the absence of calciotropic hormones. Proc. Natl. Acad. Sci. U S A. 2004;101:5140–5145. doi: 10.1073/pnas.0306141101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang W, Tu C, Chen TH, Bikle D, Shoback D. The extracellular calcium-sensing receptor (CaSR) is a critical modulator of skeletal development. Sci Signal. 2008;1:ra1. doi: 10.1126/scisignal.1159945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brown EM, Lian JB. New insights in bone biology: unmasking skeletal effects of the extracellular calcium-sensing receptor. Sci Signal. 2008;1:pe40. doi: 10.1126/scisignal.135pe40. [DOI] [PubMed] [Google Scholar]
- 24.Rodland KD. The role of the calcium-sensing receptor in cancer. Cell Calcium. 2004;35:291–295. doi: 10.1016/j.ceca.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 25.McCarron DA, Reusser ME. Finding consensus in the dietary calcium-blood pressure debate. J. Am. Coll. Nutr. 1999;18:398S–405S. doi: 10.1080/07315724.1999.10718904. [DOI] [PubMed] [Google Scholar]
- 26.Hintzpeter B, Mensink GB, Thierfelder W, Muller MJ, Scheidt-Nave C. Vitamin D status and health correlates among German adults. Eur. J. Clin. Nutr. 2008;62:1079–1089. doi: 10.1038/sj.ejcn.1602825. [DOI] [PubMed] [Google Scholar]
- 27.Judd SE, Nanes MS, Ziegler TR, Wilson PW, Tangpricha V. Optimal vitamin D status attenuates the age-associated increase in systolic blood pressure in white Americans: results from the third National Health and Nutrition Examination Survey. Am. J. Clin. Nutr. 2008;87:136–141. doi: 10.1093/ajcn/87.1.136. [DOI] [PubMed] [Google Scholar]
- 28.Davies KM, Heaney RP, Recker RR, Lappe JM, Barger-Lux MJ, Rafferty K, Hinders S. Calcium intake and body weight. J. Clin. Endocrinol. Metab. 2000;85:4635–4638. doi: 10.1210/jcem.85.12.7063. [DOI] [PubMed] [Google Scholar]
- 29.Arunabh S, Pollack S, Yeh J, Aloia JF. Body fat content and 25-hydroxyvitamin D levels in healthy women. J. Clin. Endocrinol. Metab. 2003;88:157–161. doi: 10.1210/jc.2002-020978. [DOI] [PubMed] [Google Scholar]
- 30.Alemzadeh R, Kichler J, Babar G, Calhoun M. Hypovitaminosis D in obese children and adolescents: relationship with adiposity, insulin sensitivity, ethnicity, and season. Metabolism. 2008;57:183–191. doi: 10.1016/j.metabol.2007.08.023. [DOI] [PubMed] [Google Scholar]
- 31.Pittas AG, Lau J, Hu FB, Dawson-Hughes B. The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 2007;92:2017–2029. doi: 10.1210/jc.2007-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reis JP, von Muhlen D, Kritz-Silverstein D, Wingard DL, Barrett-Connor E. Vitamin D, parathyroid hormone levels, and the prevalence of metabolic syndrome in community-dwelling older adults. Diabetes Care. 2007;30:1549–1555. doi: 10.2337/dc06-2438. [DOI] [PubMed] [Google Scholar]
- 33.Wang TJ, Pencina MJ, Booth SL, Jacques PF, Ingelsson E, Lanier K, Benjamin EJ, D’Agostino RB, Wolf M, Vasan RS. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117:503–511. doi: 10.1161/CIRCULATIONAHA.107.706127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bostick RM, Kushi LH, Wu Y, Meyer KA, Sellers TA, Folsom AR. Relation of calcium, vitamin D, and dairy food intake to ischemic heart disease mortality among postmenopausal women. Am. J. Epidemiol. 1999;149:151–161. doi: 10.1093/oxfordjournals.aje.a009781. [DOI] [PubMed] [Google Scholar]
- 35.Hagstrom E, Hellman P, Larsson TE, Ingelsson E, Berglund L, Sundstrom J, Melhus H, Held C, Lind L, Michaelsson K, Arnlov J. Plasma parathyroid hormone and the risk of cardiovascular mortality in the community. Circulation. 2009;119:2765–2771. doi: 10.1161/CIRCULATIONAHA.108.808733. [DOI] [PubMed] [Google Scholar]
- 36.Need AG, O’Loughlin PD, Morris HA, Coates PS, Horowitz M, Nordin BE. Vitamin D metabolites and calcium absorption in severe vitamin D deficiency. J. Bone Miner. Res. 2008;23:1859–1863. doi: 10.1359/jbmr.080607. [DOI] [PubMed] [Google Scholar]
- 37.Hansen KE, Jones AN, Lindstrom MJ, Davis LA, Engelke JA, Shafer MM. Vitamin D insufficiency: disease or no disease? J. Bone Miner. Res. 2008;23:1052–1060. doi: 10.1359/JBMR.080230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chapuy MC, Preziosi P, Maamer M, Arnaud S, Galan P, Hercberg S, Meunier PJ. Prevalence of vitamin D insufficiency in an adult normal population. Osteoporos. Int. 1997;7:439–443. doi: 10.1007/s001980050030. [DOI] [PubMed] [Google Scholar]
- 39.Whiting SJ, Calvo MS. Dietary recommendations for vitamin D: a critical need for functional end points to establish an estimated average requirement. J. Nutr. 2005;135:304–309. doi: 10.1093/jn/135.2.304. [DOI] [PubMed] [Google Scholar]
- 40.Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am. J. Clin. Nutr. 2006;84:18–28. doi: 10.1093/ajcn/84.1.18. [DOI] [PubMed] [Google Scholar]
- 41.Holick MF. Vitamin D: A millenium perspective. J. Cell. Biochem. 2003;88:296–307. doi: 10.1002/jcb.10338. [DOI] [PubMed] [Google Scholar]
- 42.Ovesen L, Andersen R, Jakobsen J. Geographical differences in vitamin D status, with particular reference to European countries. Proc. Nutr. Soc. 2003;62:813–821. doi: 10.1079/PNS2003297. [DOI] [PubMed] [Google Scholar]
- 43.Kuchuk NO, van Schoor NM, Pluijm SM, Chines A, Lips P. Vitamin D status, parathyroid function, bone turnover, and BMD in postmenopausal women with osteoporosis: global perspective. J. Bone Miner. Res. 2009;24:693–701. doi: 10.1359/jbmr.081209. [DOI] [PubMed] [Google Scholar]
- 44.Kudlacek S, Schneider B, Peterlik M, Leb G, Klaushofer K, Weber K, Woloszczuk W, Willvonseder R. Assessment of vitamin D and calcium status in healthy adult Austrians. Eur. J. Clin. Invest. 2003;33:323–331. doi: 10.1046/j.1365-2362.2003.01127.x. [DOI] [PubMed] [Google Scholar]
- 45.Brot C, Vestergaard P, Kolthoff N, Gram J, Hermann AP, Sorensen OH. Vitamin D status and its adequacy in healthy Danish perimenopausal women: relationships to dietary intake, sun exposure and serum parathyroid hormone. Br. J. Nutr. 2001;86:S97–103. doi: 10.1079/bjn2001345. [DOI] [PubMed] [Google Scholar]
- 46.Hypponen E, Power C. Hypovitaminosis D in British adults at age 45 y: nationwide cohort study of dietary and lifestyle predictors. Am. J. Clin. Nutr. 2007;85:860–868. doi: 10.1093/ajcn/85.3.860. [DOI] [PubMed] [Google Scholar]
- 47.Lamberg-Allardt CJ, Outila TA, Karkkainen MU, Rita HJ, Valsta LM. Vitamin D deficiency and bone health in healthy adults in Finland: could this be a concern in other parts of Europe? J. Bone Miner. Res. 2001;16:2066–2073. doi: 10.1359/jbmr.2001.16.11.2066. [DOI] [PubMed] [Google Scholar]
- 48.Islam MZ, Lamberg-Allardt C, Karkkainen M, Outila T, Salamatullah Q, Shamim AA. Vitamin D deficiency: a concern in premenopausal Bangladeshi women of two socio-economic groups in rural and urban region. Eur. J. Clin. Nutr. 2002;56:51–56. doi: 10.1038/sj.ejcn.1601284. [DOI] [PubMed] [Google Scholar]
- 49.McGrath JJ, Kimlin MG, Saha S, Eyles DW, Parisi AV. Vitamin D insufficiency in south-east Queensland. Med. J. Aust. 2001;174:150–151. doi: 10.5694/j.1326-5377.2001.tb143195.x. [DOI] [PubMed] [Google Scholar]
- 50.Nakamura K, Nashimoto M, Matsuyama S, Yamamoto M. Low serum concentrations of 25-hydroxyvitamin D in young adult Japanese women: a cross sectional study. Nutrition. 2001;17:921–925. doi: 10.1016/s0899-9007(01)00662-1. [DOI] [PubMed] [Google Scholar]
- 51.Looker AC, Pfeiffer CM, Lacher DA, Schleicher RL, Picciano MF, Yetley EA. Serum 25-hydroxyvitamin D status of the US population: 1988–1994 compared with 2000–2004. Am. J. Clin. Nutr. 2008;88:1519–1527. doi: 10.3945/ajcn.2008.26182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rucker D, Allan JA, Fick GH, Hanley DA. Vitamin D insufficiency in a population of healthy western Canadians. CMAJ. 2002;166:1517–1524. [PMC free article] [PubMed] [Google Scholar]
- 53.Pasco JA, Henry MJ, Nicholson GC, Sanders KM, Kotowicz MA. Vitamin D status of women in the Geelong Osteoporosis Study: association with diet and casual exposure to sunlight. Med. J. Aust. 2001;175:401–405. doi: 10.5694/j.1326-5377.2001.tb143643.x. [DOI] [PubMed] [Google Scholar]
- 54.van der Mei IA, Ponsonby AL, Engelsen O, Pasco JA, McGrath JJ, Eyles DW, Blizzard L, Dwyer T, Lucas R, Jones G. The high prevalence of vitamin D insufficiency across Australian populations is only partly explained by season and latitude. Environ. Health Perspect. 2007;115:1132–1139. doi: 10.1289/ehp.9937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rockell JE, Skeaff CM, Williams SM, Green TJ. Serum 25-hydroxyvitamin D concentrations of New Zealanders aged 15 years and older. Osteoporos. Int. 2006;17:1382–1389. doi: 10.1007/s00198-006-0118-x. [DOI] [PubMed] [Google Scholar]
- 56.Nelson ML, Blum JM, Hollis BW, Rosen C, Sullivan SS. Supplements of 20 microg/d cholecalciferol optimized serum 25-hydroxyvitamin D concentrations in 80% of premenopausal women in winter. J. Nutr. 2009;139:540–546. doi: 10.3945/jn.108.096180. [DOI] [PubMed] [Google Scholar]
- 57.van der Wielen RP, Lowik MR, van den Berg H, de Groot LC, Haller J, Moreiras O, van Staveren WA. Serum vitamin D concentrations among elderly people in Europe. Lancet. 1995;346:207–210. doi: 10.1016/s0140-6736(95)91266-5. [DOI] [PubMed] [Google Scholar]
- 58.Wicherts IS, van Schoor NM, Boeke AJ, Visser M, Deeg DJ, Smit J, Knol DL, Lips P. Vitamin D status predicts physical performance and its decline in older persons. J. Clin. Endocrinol. Metab. 2007;92:2058–2065. doi: 10.1210/jc.2006-1525. [DOI] [PubMed] [Google Scholar]
- 59.Neuprez A, Bruyere O, Collette J, Reginster JY. Vitamin D inadequacy in Belgian postmenopausal osteoporotic women. BMC Public Health. 2007;7:1–9. doi: 10.1186/1471-2458-7-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Andersen R, Molgaard C, Skovgaard LT, Brot C, Cashman KD, Chabros E, Charzewska J, Flynn A, Jakobsen J, Karkkainen M, Kiely M, Lamberg-Allardt C, Moreiras O, Natri AM, O’Brien M, Rogalska-Niedzwiedz M, Ovesen L. Teenage girls and elderly women living in northern Europe have low winter vitamin D status. Eur. J. Clin. Nutr. 2005;59:533–541. doi: 10.1038/sj.ejcn.1602108. [DOI] [PubMed] [Google Scholar]
- 61.Lappe JM, Davies KM, Travers-Gustafson D, Heaney RP. Vitamin D status in a rural postmenopausal female population. J. Am. Coll. Nutr. 2006;25:395–402. doi: 10.1080/07315724.2006.10719551. [DOI] [PubMed] [Google Scholar]
- 62.Flicker L, Mead K, MacInnis RJ, Nowson C, Scherer S, Stein MS, Thomasx J, Hopper JL, Wark JD. Serum vitamin D and falls in older women in residential care in Australia. J. Am. Geriatr. Soc. 2003;51:1533–1538. doi: 10.1046/j.1532-5415.2003.51510.x. [DOI] [PubMed] [Google Scholar]
- 63.Nakamura K, Nashimoto M, Hori Y, Yamamoto M. Serum 25-hydroxyvitamin D concentrations and related dietary factors in peri- and postmenopausal Japanese women. Am. J. Clin. Nutr. 2000;71:1161–1165. doi: 10.1093/ajcn/71.5.1161. [DOI] [PubMed] [Google Scholar]
- 64.Hintzpeter B, Scheidt-Nave C, Muller MJ, Schenk L, Mensink GB. Higher prevalence of vitamin D deficiency is associated with immigrant background among children and adolescents in Germany. J. Nutr. 2008;138:1482–1490. doi: 10.1093/jn/138.8.1482. [DOI] [PubMed] [Google Scholar]
- 65.Guillemant J, Le HT, Maria A, Allemandou A, Peres G, Guillemant S. Wintertime vitamin D deficiency in male adolescents: effect on parathyroid function and response to vitamin D3 supplements. Osteoporos. Int. 2001;12:875–879. doi: 10.1007/s001980170040. [DOI] [PubMed] [Google Scholar]
- 66.Vieth R, Cole DE, Hawker GA, Trang HM, Rubin LA. Wintertime vitamin D insufficiency is common in young Canadian women, and their vitamin D intake does not prevent it. Eur. J. Clin. Nutr. 2001;55:1091–1097. doi: 10.1038/sj.ejcn.1601275. [DOI] [PubMed] [Google Scholar]
- 67.Green TJ, Skeaff CM, Rockell JE, Venn BJ, Lambert A, Todd J, Khor GL, Loh SP, Muslimatun S, Agustina R, Whiting SJ. Vitamin D status and its association with parathyroid hormone concentrations in women of child-bearing age living in Jakarta and Kuala Lumpur. Eur. J. Clin. Nutr. 2008;62:373–378. doi: 10.1038/sj.ejcn.1602696. [DOI] [PubMed] [Google Scholar]
- 68.Puri S, Marwaha RK, Agarwal N, Tandon N, Agarwal R, Grewal K, Reddy DH, Singh S. Vitamin D status of apparently healthy schoolgirls from two different socioeconomic strata in Delhi: relation to nutrition and lifestyle. Br. J. Nutr. 2008;99:876–882. doi: 10.1017/S0007114507831758. [DOI] [PubMed] [Google Scholar]
- 69.Foo LH, Zhang Q, Zhu K, Ma G, Trube A, Greenfield H, Fraser DR. Relationship between vitamin D status, body composition and physical exercise of adolescent girls in Beijing. Osteoporos. Int. 2009;20:417–425. doi: 10.1007/s00198-008-0667-2. [DOI] [PubMed] [Google Scholar]
- 70.Woo J, Lam CW, Leung J, Lau WY, Lau E, Ling X, Xing X, Zhao XH, Skeaff CM, Bacon CJ, Rockell JE, Lambert A, Whiting SJ, Green TJ. Very high rates of vitamin D insufficiency in women of child-bearing age living in Beijing and Hong Kong. Br. J. Nutr. 2008;99:1330–1334. doi: 10.1017/S0007114507844382. [DOI] [PubMed] [Google Scholar]
- 71.Hollis BW, Wagner CL. Vitamin D deficiency during pregnancy: an ongoing epidemic. Am. J. Clin. Nutr. 2006;84:273. doi: 10.1093/ajcn/84.1.273. [DOI] [PubMed] [Google Scholar]
- 72.Hypponen E, Laara E, Reunanen A, Jarvelin MR, Virtanen SM. Intake of vitamin D and risk of type 1 diabetes: a birth-cohort study. Lancet. 2001;358:1500–1503. doi: 10.1016/S0140-6736(01)06580-1. [DOI] [PubMed] [Google Scholar]
- 73.Hypponen E, Power C. Vitamin D status and glucose homeostasis in the 1958 British birth cohort: the role of obesity. Diabetes Care. 2006;29:2244–2246. doi: 10.2337/dc06-0946. [DOI] [PubMed] [Google Scholar]
- 74.Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am. J. Clin. Nutr. 2000;72:690–693. doi: 10.1093/ajcn/72.3.690. [DOI] [PubMed] [Google Scholar]
- 75.Andersen R, Molgaard C, Skovgaard LT, Brot C, Cashman KD, Jakobsen J, Lamberg-Allardt C, Ovesen L. Pakistani immigrant children and adults in Denmark have severely low vitamin D status. Eur. J. Clin. Nutr. 2008;62:625–634. doi: 10.1038/sj.ejcn.1602753. [DOI] [PubMed] [Google Scholar]
- 76.Meyer HE, Falch JA, Sogaard AJ, Haug E. Vitamin D deficiency and secondary hyperparathyroidism and the association with bone mineral density in persons with Pakistani and Norwegian background living in Oslo, Norway, The Oslo Health Study. Bone. 2004;35:412–417. doi: 10.1016/j.bone.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 77.Roy DK, Berry JL, Pye SR, Adams JE, Swarbrick CM, King Y, Silman AJ, O’Neill TW. Vitamin D status and bone mass in UK South Asian women. Bone. 2007;40:200–204. doi: 10.1016/j.bone.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 78.Nowson CA, Margerison C. Vitamin D intake and vitamin D status of Australians. Med. J. Aust. 2002;177:149–152. doi: 10.5694/j.1326-5377.2002.tb04702.x. [DOI] [PubMed] [Google Scholar]
- 79.Anonymous Human vitamin and mineral requirements Report of a Joint FAO/WHO Expert Consultation. Food and Agriculture Organization, Rome, Italy, 2002
- 80.Anke M.Lebensnotwendigkeit, Toxizität, Versorgung, scheinbare Absorption und Bilanz der Alkali- und Erdalkalimetalle beim Menschen Sitzungsberichte der Mathematisch-Naturwissenschaftlichen Klasse der Akademie gemeinnütziger Wissenschaften, Verlag der Akademie gemeinnütziger Wissenschaften, Erfurt, Germany, 2006139–58. [Google Scholar]
- 81.Schulze MB, Linseisen J, Kroke A, Boeing H. Macronutrient, vitamin, and mineral intakes in the EPIC-Germany cohorts. Ann. Nutr. Metab. 2001;45:181–189. doi: 10.1159/000046727. [DOI] [PubMed] [Google Scholar]
- 82.Vyas A, Greenhalgh A, Cade J, Sanghera B, Riste L, Sharma S, Cruickshank K. Nutrient intakes of an adult Pakistani, European and African-Caribbean community in inner city Britain. J. Hum. Nutr. Diet. 2003;16:327–337. doi: 10.1046/j.1365-277x.2003.00461.x. [DOI] [PubMed] [Google Scholar]
- 83.Ma J, Johns RA, Stafford RS. Americans are not meeting current calcium recommendations. Am. J. Clin. Nutr. 2007;85:1361–1366. doi: 10.1093/ajcn/85.5.1361. [DOI] [PubMed] [Google Scholar]
- 84.Pasco JA, Henry MJ, Nicholson GC, Brennan SL, Kotowicz MA. Behavioural and physical characteristics associated with vitamin D status in women. Bone. 2009;44:1085–1091. doi: 10.1016/j.bone.2009.02.020. [DOI] [PubMed] [Google Scholar]
- 85.Metcalf PA, Scragg RK, Tukuitonga CF, Dryson EW. Dietary intakes of middle-aged European, Maori and Pacific Islands people living in New Zealand. N. Z. Med. J. 1998;111:310–313. [PubMed] [Google Scholar]
- 86.Islam MZ, Lamberg-Allardt C, Karkkainen M, Ali SM. Dietary calcium intake in premenopausal Bangladeshi women: do socio-economic or physiological factors play a role? Eur. J. Clin. Nutr. 2003;57:674–680. doi: 10.1038/sj.ejcn.1601597. [DOI] [PubMed] [Google Scholar]
- 87.Whedon GD. Recent advances in management of osteoporosis. In: Massry SG, Ritz E, Jahn H, editors. Phosphate and Mineral in Health and Disease. Plenum Press; New York, USA: 1980. pp. 600–613. [Google Scholar]
- 88.Amorim Cruz JA, Moreiras O, Brzozowska A. Longitudinal changes in the intake of vitamins and minerals of elderly Europeans. SENECA Investigators. Eur. J. Clin. Nutr. 1996;50:S77–85. [PubMed] [Google Scholar]
- 89.Nakamura K, Saito T, Yoshihara A, Ishikawa M, Tsuchiya Y, Oshiki R, Kobayashi R, Maruyama K, Hyodo K, Nashimoto M, Tsugawa N, Okano T, Oyama M, Yamamoto M.Low calcium intake is associated with increased bone resorption in postmenopausal Japanese women: Yokogoshi Study Public Health Nutr 2009. Epub ahead of print, doi:10.1017/S1368980009005084 [DOI] [PubMed]
- 90.Abrams SA, Griffin IJ, Hawthorne KM, Gunn SK, Gundberg CM, Carpenter TO. Relationships among vitamin D levels, parathyroid hormone, and calcium absorption in young adolescents. J. Clin. Endocrinol. Metab. 2005;90:5576–5581. doi: 10.1210/jc.2005-1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rubin LA, Hawker GA, Peltekova VD, Fielding LJ, Ridout R, Cole DE. Determinants of peak bone mass: clinical and genetic analyses in a young female Canadian cohort. J. Bone Miner. Res. 1999;14:633–643. doi: 10.1359/jbmr.1999.14.4.633. [DOI] [PubMed] [Google Scholar]
- 92.Pettifor JM. Nutritional rickets: deficiency of vitamin D, calcium, or both? Am. J. Clin. Nutr. 2004;80:1725S–1729S. doi: 10.1093/ajcn/80.6.1725S. [DOI] [PubMed] [Google Scholar]
- 93.Thacher TD, Fischer PR, Pettifor JM, Lawson JO, Isichei CO, Reading JC, Chan GM. A comparison of calcium, vitamin D, or both for nutritional rickets in Nigerian children. N. Engl. J. Med. 1999;341:563–568. doi: 10.1056/NEJM199908193410803. [DOI] [PubMed] [Google Scholar]
- 94.Graff M, Thacher TD, Fischer PR, Stadler D, Pam SD, Pettifor JM, Isichei CO, Abrams SA. Calcium absorption in Nigerian children with rickets. Am. J. Clin. Nutr. 2004;80:1415–1421. doi: 10.1093/ajcn/80.5.1415. [DOI] [PubMed] [Google Scholar]
- 95.Balasubramanian K, Rajeswari J, Govil YC, Agarwal AK, Kumar A, Bhatia V. Varying role of vitamin D deficiency in the etiology of rickets in young children vs. adolescents in northern India. J. Trop. Pediatr. 2003;49:201–206. doi: 10.1093/tropej/49.4.201. [DOI] [PubMed] [Google Scholar]
- 96.Calvo MS, Whiting SJ, Barton CN. Vitamin D intake: a global perspective of current status. J. Nutr. 2005;135:310–316. doi: 10.1093/jn/135.2.310. [DOI] [PubMed] [Google Scholar]
- 97.Yetley EA, Brule D, Cheney MC, Davis CD, Esslinger KA, Fischer PW, Friedl KE, Greene-Finestone LS, Guenther PM, Klurfeld DM, L’Abbe MR, McMurry KY, Starke-Reed PE, Trumbo PR. Dietary reference intakes for vitamin D: justification for a review of the 1997 values. Am. J. Clin. Nutr. 2009;89:719–727. doi: 10.3945/ajcn.2008.26903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cashman KD, Hill TR, Lucey AJ, Taylor N, Seamans KM, Muldowney S, Fitzgerald AP, Flynn A, Barnes MS, Horigan G, Bonham MP, Duffy EM, Strain JJ, Wallace JM, Kiely M. Estimation of the dietary requirement for vitamin D in healthy adults. Am. J. Clin. Nutr. 2008;88:1535–1542. doi: 10.3945/ajcn.2008.26594. [DOI] [PubMed] [Google Scholar]
- 99.Cashman KD, Wallace JM, Horigan G, Hill TR, Barnes MS, Lucey AJ, Bonham MP, Taylor N, Duffy EM, Seamans K, Muldowney S, Fitzgerald AP, Flynn A, Strain JJ, Kiely M. Estimation of the dietary requirement for vitamin D in free-living adults ≥64 y of age. Am. J. Clin. Nutr. 2009;89:1366–1374. doi: 10.3945/ajcn.2008.27334. [DOI] [PubMed] [Google Scholar]
- 100.Viljakainen HT, Vaisanen M, Kemi V, Rikkonen T, Kroger H, Laitinen E, Rita H, Lamberg-Allardt C. Wintertime vitamin D supplementation inhibits seasonal variation of calcitropic hormones and maintains bone turnover in healthy men. J. Bone Miner. Res. 2009;24:346–352. doi: 10.1359/jbmr.081009. [DOI] [PubMed] [Google Scholar]
- 101.Gorham ED, Garland CF, Garland FC, Grant WB, Mohr SB, Lipkin M, Newmark HL, Giovannucci E, Wei M, Holick MF. Vitamin D and prevention of colorectal cancer. J. Steroid Biochem. Mol. Biol. 2005;97:179–194. doi: 10.1016/j.jsbmb.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 102.Garland CF, Gorham ED, Mohr SB, Grant WB, Giovannucci EL, Lipkin M, Newmark H, Holick MF, Garland FC. Vitamin D and prevention of breast cancer: Pooled analysis. J. Steroid Biochem. Mol. Biol. 2007;103:708–711. doi: 10.1016/j.jsbmb.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 103.Weaver CM. Age related calcium requirements due to changes in absorption and utilization. J. Nutr. 1994;124:1418S–1425S. doi: 10.1093/jn/124.suppl_8.1418S. [DOI] [PubMed] [Google Scholar]
- 104.Tang BM, Eslick GD, Nowson C, Smith C, Bensoussan A. Use of calcium or calcium in combination with vitamin D supplementation to prevent fractures and bone loss in people aged 50 years and older: a meta-analysis. Lancet. 2007;370:657–666. doi: 10.1016/S0140-6736(07)61342-7. [DOI] [PubMed] [Google Scholar]
- 105.Shin MH, Holmes MD, Hankinson SE, Wu K, Colditz GA, Willett WC. Intake of dairy products, calcium, and vitamin D and risk of breast cancer. J. Natl. Cancer Inst. 2002;94:1301–1311. doi: 10.1093/jnci/94.17.1301. [DOI] [PubMed] [Google Scholar]
- 106.Slattery ML, Sorenson AW, Ford MH. Dietary calcium intake as a mitigating factor in colon cancer. Am. J. Epidemiol. 1988;128:504–514. doi: 10.1093/oxfordjournals.aje.a114998. [DOI] [PubMed] [Google Scholar]
- 107.Berube S, Diorio C, Verhoek-Oftedahl W, Brisson J. Vitamin D, calcium, and mammographic breast densities. Cancer Epidemiol. Biomarkers Prev. 2004;13:1466–1472. [PubMed] [Google Scholar]
- 108.Boonen S, Lips P, Bouillon R, Bischoff-Ferrari HA, Vanderschueren D, Haentjens P. Need for additional calcium to reduce the risk of hip fracture with vitamin D supplementation: evidence from a comparative metaanalysis of randomized controlled trials. J. Clin. Endocrinol. Metab. 2007;92:1415–1423. doi: 10.1210/jc.2006-1404. [DOI] [PubMed] [Google Scholar]
- 109.Lappe JM, Travers-Gustafson D, Davies KM, Recker RR, Heaney RP. Vitamin D and calcium supplementation reduces cancer risk: results of a randomized trial. Am. J. Clin. Nutr. 2007;85:1586–1591. doi: 10.1093/ajcn/85.6.1586. [DOI] [PubMed] [Google Scholar]
- 110.Cho E, Smith-Warner SA, Spiegelman D, Beeson WL, van den Brandt PA, Colditz GA, Folsom AR, Fraser GE, Freudenheim JL, Giovannucci E, Goldbohm RA, Graham S, Miller AB, Pietinen P, Potter JD, Rohan TE, Terry P, Toniolo P, Virtanen MJ, Willett WC, Wolk A, Wu K, Yaun SS, Zeleniuch-Jacquotte A, Hunter DJ. Dairy foods, calcium, and colorectal cancer: a pooled analysis of 10 cohort studies. J. Natl. Cancer Inst. 2004;96:1015–1022. doi: 10.1093/jnci/djh185. [DOI] [PubMed] [Google Scholar]
- 111.Ishihara J, Inoue M, Iwasaki M, Sasazuki S, Tsugane S. Dietary calcium, vitamin D, and the risk of colorectal cancer. Am. J. Clin. Nutr. 2008;88:1576–1583. doi: 10.3945/ajcn.2008.26195. [DOI] [PubMed] [Google Scholar]
- 112.Berube S, Diorio C, Masse B, Hebert-Croteau N, Byrne C, Cote G, Pollak M, Yaffe M, Brisson J. Vitamin D and calcium intakes from food or supplements and mammographic breast density. Cancer Epidemiol. Biomarkers Prev. 2005;14:1653–1659. doi: 10.1158/1055-9965.EPI-05-0068. [DOI] [PubMed] [Google Scholar]
- 113.Lamberg-Allardt C. Vitamin D in foods and as supplements. Prog. Biophys. Mol. Biol. 2006;92:33–38. doi: 10.1016/j.pbiomolbio.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 114.O’Donnell S, Cranney A, Horsley T, Weiler HA, Atkinson SA, Hanley DA, Ooi DS, Ward L, Barrowman N, Fang M, Sampson M, Tsertsvadze A, Yazdi F. Efficacy of food fortification on serum 25-hydroxyvitamin D concentrations: systematic review. Am. J. Clin. Nutr. 2008;88:1528–1534. doi: 10.3945/ajcn.2008.26415. [DOI] [PubMed] [Google Scholar]
- 115.Natri AM, Salo P, Vikstedt T, Palssa A, Huttunen M, Karkkainen MU, Salovaara H, Piironen V, Jakobsen J, Lamberg-Allardt CJ. Bread fortified with cholecalciferol increases the serum 25-hydroxyvitamin D concentration in women as effectively as a cholecalciferol supplement. J. Nutr. 2006;136:123–127. doi: 10.1093/jn/136.1.123. [DOI] [PubMed] [Google Scholar]
- 116.Mocanu V, Stitt PA, Costan AR, Voroniuc O, Zbranca E, Luca V, Vieth R. Long-term effects of giving nursing home residents bread fortified with 125 microg (5000 IU) vitamin D3 per daily serving. Am. J. Clin. Nutr. 2009;89:1132–1137. doi: 10.3945/ajcn.2008.26890. [DOI] [PubMed] [Google Scholar]
- 117.Newmark HL, Heaney RP, Lachance PA. Should calcium and vitamin D be added to the current enrichment program for cereal-grain products? Am. J. Clin. Nutr. 2004;80:264–270. doi: 10.1093/ajcn/80.2.264. [DOI] [PubMed] [Google Scholar]
- 118.Daly RM, Bass S, Nowson C. Long-term effects of calcium-vitamin-D3-fortified milk on bone geometry and strength in older men. Bone. 2006;39:946–953. doi: 10.1016/j.bone.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 119.Daly RM, Brown M, Bass S, Kukuljan S, Nowson C. Calcium- and vitamin D3-fortified milk reduces bone loss at clinically relevant skeletal sites in older men: a 2-year randomized controlled trial. J. Bone Miner. Res. 2006;21:397–405. doi: 10.1359/JBMR.051206. [DOI] [PubMed] [Google Scholar]