Abstract
Cocoa is a rich source of dietary polyphenols. In vitro as well as cell culture data indicate that cocoa polyphenols may exhibit antioxidant and anti-inflammatory, as well as anti-atherogenic activity. Several molecular targets (e.g., nuclear factor kappa B, endothelial nitric oxide synthase, angiotensin converting enzyme) have been recently identified which may partly explain potential beneficial cardiovascular effects of cocoa polyphenols. However cocoa polyphenol concentrations, as used in many cell culture studies, are not physiologically achievable. Bioavailability studies indicate that plasma concentrations of cocoa polyphenols following dietary intake are low and in the nanomolar range. Human studies regarding the effect of cocoa polyphenols on vascular health are often underpowered and lack a rigorous study design. If dietary cocoa polyphenol intake is due to chocolate its high energy content needs to be taken into account. In order to determine potential health benefits of cocoa polyphenols large scale, long term, randomized, placebo controlled studies, (ideally with a cross-over design) as well as prospective studies are warranted.
Keywords: cocoa, chocolate, polyphenols, cardiovascular disease, endothelial function
1. Introduction
There is experimental evidence suggesting that cocoa polyphenols may mediate beneficial effects on vascular health [1–3]. The present review critically evaluates studies on cocoa polyphenols and vascular health as conducted in cultured cells, laboratory rodents and humans over the last decade. First bioavailability studies with cocoa polyphenols are summarized. Secondly potential molecular targets of cocoa polyphenols are discussed. Thirdly an overview of the human studies on cocoa polyphenols and cardiovascular disease prevention is given. Finally merits and limitations of these studies are discussed and future challenges in cocoa polyphenol research are presented.
2. Bioavailability of Polyphenols in Cocoa
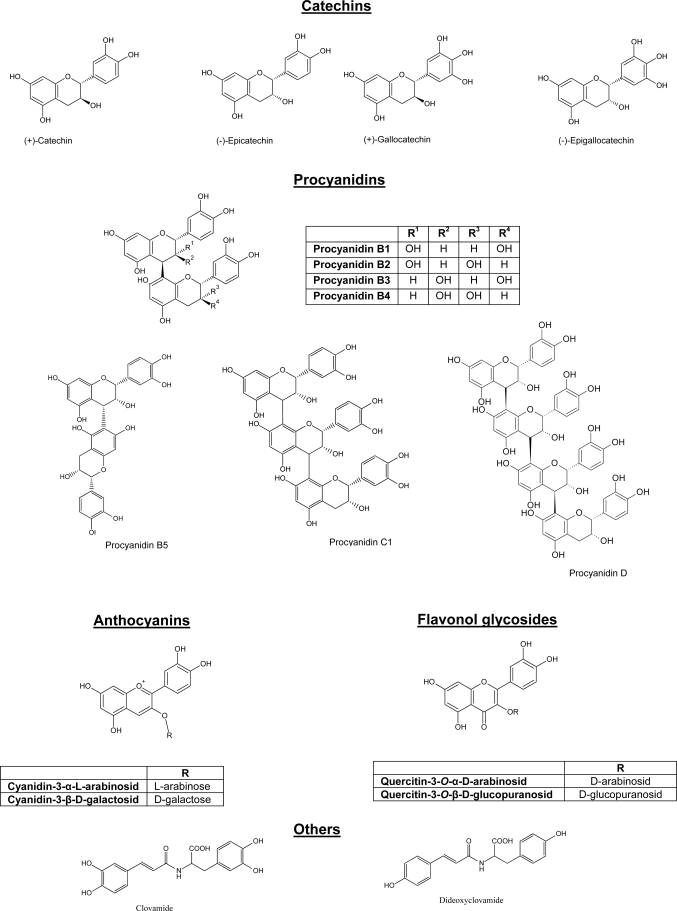
Cocoa beans (Theobroma cacao) have been used for a long period of time as major ingredient of cocoa and chocolate [4,5]. Cocoa is a rich source of polyphenols. Cocoa beans contain approximately 6–8% polyphenols by dry weight [6]. Polyphenols identified in cocoa beans and cocoa products comprise mainly catechins, flavonol glycosides, anthocyanins and procyanidins. As far as procyanidins are concerned, up to decamer ones have been identified in cocoa [7]. The chemical structure of selected cocoa polyphenols is given in Figure 1.
Figure 1.
Chemical structure of cocoa polyphenols.
The biological activity of cocoa polyphenols largely depend on their bioavailability [8]. The bioavailability of cocoa polyphenols has been measured in several studies in humans (Table 1). Polyphenols were given as cocoa rich beverages or chocolate. Monomeric flavonoids as well as dimeric and trimeric procyanidins have been detected in human plasma after consumption. The peak plasma concentration of flavonols was determined 2–3 hours after ingestion [9,10]. Plasma concentrations of cocoa polyphenols were often in the nanomolar or low micromolar range. Donovan and coworkers demonstrated that commercial chocolate samples available around the world contain a predominance of the less bioavailable (−)-catechin enantiomer as compared to the (+)-catechin which is present in most other plant derived foods. This may explain the relatively low bioavailability of catechins from chocolate and cocoa-containing products [11]. The food matrix seems to be an important factor that may affect the bioavailability of cocoa polyphenols. Cocoa powder dissolved in milk, as one of the most common ways of cocoa powder consumption, did not change the bioavailability of cocoa powder flavonoids in healthy humans [12]. Furthermore, lipid and protein rich meals did not affect the bioavailability of cocoa polyphenols. However the uptake of flavonols in humans could be increased significantly by concurrent consumption of carbohydrates [13].
Table 1.
Human bioavailability studies on cocoa polyphenols.
| No. Subjects | Age range (mean) | BMI (kg/m2) | Dietary source of polyphenols | Polyphenol content | Plasma concentration | Reference |
|---|---|---|---|---|---|---|
| 8 | 25–55 (40 ± 15) (± SD) | 23.94 ± 2.35 (± SD) | Dark chocolate: 40 g 80 g |
Epicatechin: 82 mg 164 mg |
Epicatechin: 0.383 μmol/L (t = 2 h) 0.7 μmol/L (t = 2.57 h) |
[16] |
| 20 | 20–56 | 23.8 ± 0.79 (± SEM) | Semi-sweet chocolate baking bits: 27 g 53 g 80 g |
Total procyanidins (epicatechin): 186 (46) mg 365 (90) mg 551 (136) mg |
Epicatechin (t = 2 h): 0.133 μmol/L 0.258 μmol/L 0.355 μmol/L |
[17] |
| 13 | 26–49 | 23.2 ± 1.2 (± SEM) | 105 g semi-sweet chocolate baking bits (of which 80 g procyanidin-rich chocolate) | 557 mg total procyanidins (137 mg epicatechin) | 0.257 μmol/L epicatechin (t = 2 h) | [10] |
| 5 | 30–33 (31 ± 1) (± SD) | 22.5 ± 1.3 (± SD) | 96 g chocolate 66 g cocoa |
Total polyphenols (epicatechin): 2.74 g (760 μmoL) 2.73 g (760 μmoL) |
Total epicatechin (t = 2 h): 4.77 μmol/L 4.92 μmol/L |
[18] |
| 23 | 21–62 | – | 22 g cocoa powder + 16 g dark chocolate | 466 mg total procyanidins (111 mg monomers) | 0.036 nmol/L epicatechin (t = 2 h) | [19] |
| 11 | 20–55 (39 ± 5) (± SD) | 24 ± 3 (± SD) | 37 g high- vs. low-procyanidin chocolate | Total procyanidins: 4 mg/g vs. 0.9 mg/g | 0.212 μmol/L vs. 0.011 μmol/L epicatechin (t = 2 h) | [20] |
| 16 | 22–49 | – | 300 mL cocoa beverage (18.75 g flavanol-rich cocoa powder) | 897 mg total epicatechin & procyanidins | 1.043 μmol/L epicatechin (t=2 h) | [21] |
| 5 | 23–34 | – | Cocoa beverage (0.375 g cocoa/kg bw) |
Per g cocoa: 12.2 mg monomers, 9.7 mg dimers, 28.2 mg procyanidins |
0.041 μmol/L dimer B2, 5.92 μmol/L epicatechin, 0.16 μmol/L catechin (t = 2 h) |
[22] |
| 6 | 23–39 | 23.1 ± 0.7 (± SEM) | 400 mL flavanol-rich cocoa beverage (37.5 g cocoa); 2 days |
Per g cocoa: 12.2 mg monomers, 9.7 mg dimers, 20.2 mg procyanidins |
0.08 μmol/L dimer B2, 4.11 μmol/L epicatechin, 0.4 μmol/L catechin (t = 2 h) | [23] |
| 18 | – | – | 25 g semi-sweet chocolate chips | 220 mg flavanols & procyanidins | 0.427 μmol/L epicatechin (t = 2 h) | [24] |
| 32 | 31–49 (40 ± 9) (± SD) | 26 ± 4 (± SD) | Cocoa flavanol & procyanidin supplementation for 28 days | 234 mg/d flavanols & procyanidins | 0.116 μmol/L epicatechin 0.091 nmol/L catechin (t = 28 d) |
[25] |
It is unclear if and to what extend higher oligomeric procyanidins from cocoa are absorbed. However, the biological activity of higher procyanidins may be at least partly attributed to their colonic breakdown products, including phenolic acids [14]. Data regarding the tissue distribution of cocoa polyphenols in laboratory rodents are currently missing.
Within this review we mainly focus on potential cardiovascular health benefits of cocoa polyphenols. However it needs to be taken into account that cocoa beans contain also lipids, sterols, minerals and trace elements which may also affect vascular health as reviewed by Steinberg et al. [15].
3. Putative Health Benefits of Polyphenols from Cocoa and Chocolate
3.1. Studies in Vitro and in Cultured Cells
In vitro studies, as well as studies in cultured cells, have identified several cellular and molecular targets by which cocoa polyphenols may mediate beneficial cardiovascular effects (Table 2). In fact it has been shown that cocoa polyphenols may prevent and/or inhibit the oxidation of LDL which is a key event in atherogenesis [26–30]. Furthermore cocoa polyphenols inhibit lipoxygenase activity in vitro [31]. However, comparing the 5-lipoxygenase-inhibitory effect of epicatechin (present in cocoa) with those of epigallocatechin-gallate (present in green tea) it was found that the latter flavonoid was at least one order of magnitude more potent [32]. There is experimental evidence from studies with rat liver microsomes that cocoa polyphenols may decrease NADPH-dependent lipid peroxidation and linoleic acid autoxidation [33].
Table 2.
In vitro and cell-culture studies performed with cocoa polyphenols.
| Cell type | Treatment | Outcomes | Reference |
|---|---|---|---|
| Human LDL | Cocoa powder extract (5 μmol/L GAE°) vs. pure catechin (5 μmol/L) | LDL oxidation ↓ | [26] |
| Liposomes & human LDL | Cocoa catechin monomers & procyanidin fractions (0.1–10.0 μg/mL) | LDL oxidation ↓ | [27] |
| LDL | 220 mL cocoa drink (cocoa concentration: 1.5, 2.0, 2.5, 3.0, 3.5%) | LDL oxidation ↓ dose-dependent | [28] |
| LDL | Catechin, epicatechin, procyanidin B2, procyanidin C1, cinnamtannin A2 (0.125, 0.25, 0.5, 1.0, 2.0 μg/mL) | LDL oxidizability ↓ | [29] |
| Human LDL & VLDL | Dark chocolate, cocoa, milk chocolate, hot cocoa mixes (126, 224, 52.2, 8.2 μmol/g total phenols) | Lag time of LDL & VLDL oxidation ↑ | [30] |
| LDL & VLDL | Dark chocolate & cocoa powder (containing fat or defatted) vs. cocoa butter | LDL & VLDL oxidizability ↓ | [49] |
| Rat liver microsomes | Cacao liquor | NADPH-dependent lipid peroxidation ↓ Linoleic acid autoxidation ↓ |
[33] |
| Recombinant human 5-LOX | Cocoa epicatechin & procyanidins (10 μmol/L) | 5-LOX activity ↓ Proinflammatory mediators (LTB4, LTC4, LTD4) ↓ | [32] |
| Isolated rabbit 15-LOX-1 Recombinant human platelet 12-LOX |
Cocoa procyanidins (monomers through decamers; 2.9 mg/mL) Epicatechin & procyanidin decamer |
15-LOX-1 activity ↓ dose-dependent 12-LOX activity ↓ dose-dependent |
[31] |
| Human PBMC * | Cocoa procyanidins (monomers through decamers; 25 μg/mL) | IL-1β secretion ↑ IL-2 expression ↓ IL-4 expression & secretion ↓ |
[34] |
| Human PBMC * | Cocoa procyanidins (monomers through decamers; 25 μg/mL) | IL-1β transcription & secretion ↑ (pentamers-decamers) IL-1β transcription & secretion ↓ (monomers-tetramers) |
[36] |
| Human PBMC * | Cocoa procyanidins (monomers through decamers; 25 μg/mL) | Secretory IL-4 ↑ | [35] |
| Human PBMC * | Cocoa procyanidins (monomers through decamers; 25 μg/mL) | TNF-α secretion ↑ | [37] |
| Human PBMC * | Cocoa procyanidins (monomers through decamers; 25 μg/mL) | IL-5 secretion ↑ (monomers-trimers) IL-5 secretion ↓ (hexamers-decamers) |
[38] |
| Human PBMC° | Short- (monomers-pentamers) & long-chain (hexamers-decamers) flavanol fractions (20 μg/mL) | Inflammatory mediators (IL-1β, IL-6, IL-10, TNF-α) ↑ | [53] |
| Whole blood | Purified trimeric & pentameric cocoa procyanidins (3, 10 μmol/L) | PAC-1 binding & P-selectin expression ↑ in unstimulated platelets Epinephrine-induced platelet activation ↓ |
[54] |
| Isolated rabbit aortic rings | Procyanidin-rich cocoa extracts | Endothelium-dependent relaxation ↑ NOS activity ↑ |
[45] |
| ACE from rabbit lung | Epicatechin, dimeric & hexameric procyanidins (0–500 μmol/L) | ACE activity ↓ molecular weight-dependent | [47] |
| Murine EL4BOU6 lymphocytes | Cocoa extract (5–80 μg/mL total polyphenols) vs. epicatechin (60–120 μg/mL total polyphenols) | IL-2 secretion ↓ IL-4 secretion↑ T lymphocyte activation ↓ |
[41] |
| Murine RAW264.7 macrophages # Rat NR8383 macrophages # |
Cocoa extract (5–100 μg/mL total polyphenols) vs. epicatechin (60–120 μg/mL total polyphenols) Cocoa extract (10–50 μg/mL total polyphenols) vs. epicatechin (30–60 μg/mL total polyphenols) |
inducible NO ↓ Proinflammatory mediators (TNF-α, MCP-1, IL-1α, IL-6) ↓ |
[42] |
| Jurkat T cells | Catechin, epicatechin & B-type oligomers (1.7–17.2 μmol/L) | PMA-induced NF-κB activation ↓ IL-2 expression & secretion ↓ |
[55] |
| VSMC | Cocoa procyanidins (0–100 μg/mL) & procyanidin B2 (0–100 μmol/L) | MMP-2 expression & activation ↓ VSMC invasion & migration ↓ MT1-MMP & MEk1 activities ↓ |
[46] |
| HUVEC | Epicatechin & flavanol metabolites mixture vs. control | Arginase-2 mRNA expression ↓ Arginase activity ↓ dose-dependent |
[44] |
GAE gallic acid equivalents,
° PBMC, peripheral blood mononuclear cells;
phytohemagglutinin (PHA)-stimulated;
#, lipopolysaccharide (LPS)-stimulated; MCP-1, monocyte chemoattractant protein-1;
†, 12-O-tetradecaoylphorbol-13-acetate (TPA)-stimulated; LOX, lipoxygenase; COX-2, cyclooxygenase-2; VSMC, vascular smooth muscle cells; MMP, matrix metalloproteinase; MT1, membrane type-1; MEK, mitogen-activated protein kinase kinase; HUVEC, human umbilical endothelial cells.
Further studies in cultured cells suggest that cocoa polyphenols may exhibit anti-inflammatory activity by down regulating the production of pro-inflammatory cytokines including IL1β, IL2, IL4, IL6 and TNF-α [34–38]. Activated macrophages can generate large amounts of nitric oxide from l-arginine by the action of inducible NO Synthase (iNOS). Overproduction of NO by macrophages has been associated with chronic inflammation [39]. The transcription factor NF-κB, in cooperation with AP-1, cooridnates the expression of iNOS and pro-inflammatory cytokines [40]. Cocoa polyphenols may decrease inducible nitric oxide production by inhibiting iNOS gene expression due to NFκB and AP1 dependent signal transduction pathways [41,42]. Contrary other studies in cultured RAW264.7 murine macrophages indicate that oligomeric procyanidins such as procyanidin C2, as present in cocoa, exhibit pro-inflammatory activity [43]. Mao et al. have shown that the smaller fraction of cocoa polyphenols (monomer-tetramer) consistently decreased IL1β expression in blood mononuclear cells, while the larger oligomers (pentamer-decamer) increased its expression [36].
Studies in endothelial cells suggest that cocoa polyphenols such as epicatechin inhibit arginase-2 mRNA expression and activity levels which in turn may result in a higher availability of the vaso-relaxing molecule nitric oxide in the vascular wall [44]. This finding is in line with studies using isolated rabbit aortic rings in which endothelium dependent relaxation and an increase in nitric oxide synthase has been observed. Karim and co-workers [45] studied the effect of polymeric procyanidins on endothelium dependent relaxation (EDR) in aortic rings from New Zealand White rabbits. Polymeric procyanidins (tetramers through decamers of catechins) caused EDR in aortic rings. Furthermore polymeric procyanidins significantly increased Ca2+dependent NOS activity whereas monomers, dimers and trimers of catechins exhibited no such activity.
Cocoa procyanidins inhibit metalloproteinase-2 expression and activation in smooth muscle cells which may contribute to the anti-atherosclerotic effects of cocoa [46].
Interestingly cocoa polyphenols have also been shown to inhibit ACE activity [47] which may result in a decrease of blood pressure. ACE is centrally involved in the regulation of the rennin-angiotensin system. Angiotensin causes blood vessels to constrict resulting in increased blood pressure. Thus ACE inhibition is a therapeutic approach in blood pressure regulation. As observed for the purified ACE enzyme, ACE activity in kidney membrane was inhibited by 100 μmol/L of dimer and hexamer epicatechin [47] a concentration which is not physiologically achievable.
Furthermore cocoa polyphenols circulate as conjugated metabolites whereas in most in vitro studies and studies in cultured cells non-conjugated cocoa polyphenols have been used. In this context cell culture studies using conjugated cocoa polyphenols are warranted. Furthermore the underlying mechanisms involved into the cellular uptake of cocoa polyphenols as well as its cellular concentrations and subcellular distribution need to be established.
3.2. Studies in Laboratory Animals
In vitro studies and studies in cultured cells are partly supported by studies in laboratory animals indicating that cocoa polyphenols may decrease LDL-oxidation, as well as other biomarkers of lipid peroxidation [4]. Furthermore studies in hypercholesterolemic rabbits [48] suggest that cocoa polyphenols may prolong LDL-oxidation lag time and decrease area of atherosclerotic lesions in the aorta (Table 4). Studies in hamsters report an increase in HDL as well as a decrease in LDL and triglyceride levels [49]. Furthermore cocoa procyanidins significantly reduced plasma cholesterol and increased steroid excretion in rats fed a high cholesterol diet [50]. In obese diabetic mice and rats a decrease in blood glucose as well as a decrease in 8-isoprostane levels has been observed [51]. However in other studies in rats no effect of cocoa polyphenols on biomarkers of lipid peroxidation in liver and heart has been reported [52].
Table 4.
In vivo studies with cocoa polyphenols in laboratory animals.
| Species | No. animals | Experimental duration (wk) | Treatment | Outcomes | Reference |
|---|---|---|---|---|---|
| Rats | 5 | – | Oral administration of 1 g cocoa powder/kg bw (7.8 mg epicatechin) | Lipid peroxides in plasma ↓ Oxidant-induced α-tocopherol consumption ↓ |
[4] |
| Rats | 5–6 | – | Gastric intubation with 100 mg cocoa extract | Plasma antioxidant capacity ↑ Erythrocyte hemolysis ↓ |
[56] |
| Rats | 48 | 2 | Diet containing by weight 0.5–2.0% flavanol- and procyanidin-rich cocoa (12.2 mg/g epicatechin, 2.8 mg/g catechin, 53.3 mg/g procyanidins) |
Oxidative DNA damage in testes ↓ No effect on plasma F2-isoprostane and TBARS |
[52] |
| Hypercholesterolemic rabbits | 12 | 24 | Diet containing 10% cocoa powder (0.78 g total polyphenols) | TBARS in plasma↓ No effects on plasma cholesterol, TG & phospholipids LDL oxidation lag time ↑ Area of atherosclerotic lesions in aorta ↓ |
[48] |
| Hamsters | 27 | 10 | Brownie (10 g vs. 1 g cocoa powder) | LDL & TG levels ↓ HDL ↑ dose-dependent LDL-oxidizability ↓ |
[49] |
| Obese-diabetic rats | 40 | 4 | Cocoa extract (600 mg/kg bw/d) | Blood glucose levels ↓ Plasma free fatty acids ↓ 8-isoprostane levels ↓ Superoxide dismutase activity ↑ No change in catalase activity |
[51] |
| Rats | 10 | 4 | 40 g cocoa powder per kg diet (11.0 mg/g epicatechin, 2.8 mg/g catechin, 43.0 mg/g procyanidins), vs. none | Renal arginase activity ↓ | [44] |
TG, triglycerides, TBARS: Thiobarbituric acid reactive substances.
The inhibition of arginase activity has been reported to improve endothelium vasodilating relaxation. In this context Schnorr et al. have found that cocoa flavonols lower vascular arginase activity in rat kidney. Arginase competes with endothelial nitric oxide synthase for l-arginine as the substrate. Thus an inhibition of arginase activity can be associated with elevated endothelial NO levels [44]. Potential mechanisms by which cocoa polyphenols may affect vascular health are summarized in Table 3.
Table 3.
Potential mechanisms by which polyphenols from cocoa may affect vascular health.
|
3.3. Studies in Humans
There is evidence suggesting that cocoa polyphenols may also positively affect vascular health in humans. Several studies in humans suggest that cocoa polyphenols decrease LDL-oxidation [19,57,58]. Furthermore, an improvement of plasma antioxidative status due to cocoa polyphenols has been shown [9]. Some studies indicate an increase in plasma HDL-cholesterol [19,59,60], a decrease in plasma triglyceride and a decrease of biomarkers of lipid peroxidation such as TBARS [17,61] and F2-Isoprostanes [62] following cocoa polyphenol consumption.
Importantly dietary cocoa polyphenols improve endothelial function by increasing vascular-NO-synthase activity [63–67]. This in turn may lead to a decrease in systolic and diastolic blood pressure [68–72]. The magnitude of blood pressure reduction due to cocoa polyphenols is often relatively low [73]. Blood pressure lowering effects were more evident in hypertensive than in normotensive volunteers [71,73]. However other studies did not report a beneficial effect of cocoa polyphenols on blood pressure, and flow mediated dilatation [63]. Balzer et al. [74] studied the effect of flavonol-containing cocoa on vascular function in medicated diabetic patients in a double-masked, randomized, controlled trial. The key observation was an acute effect and a later chronic effect of cocoa flavonols on endothelial function as evidenced by improvements in FMD. However, over a 6-week period, flavonol-rich cocoa did not modify vascular function in subjects with coronary artery disease. Furthermore no differences in soluble cellular adhesion molecule-concentration in plasma and forearm blood flow response to ischemia were evident [75].
Several studies report anti-aggregatory effects of cocoa polyphenols [25,61]. However the effects of cocoa polyphenols on platelet aggregation are rather modest and possibly large amounts need to be ingested to exhibit a similar anti-aggregatory effect as reported for aspirin [76]. Nevertheless in a recent study by Flammer and coworkers dark chocolate induced coronary vasodilation, improved coronary vascular function and decreased platelet adhesion 2 h after consumption of 40 g of dark chocolate containing 70% cocoa. These immediate effects were accompanied by a significant increase in plasma epicatechin levels [77].
Although there is experimental evidence that cocoa polyphenols may act as free radical scavengers in vitro, it is unlikely that cocoa polyphenols exhibit significant free radical scavenging activity in vivo. Cocoa polyphenols are present at low concentrations in the human plasma and mostly in the conjugated form [18]. Conjugation of flavonoids with glucoronic acid and sulphate blocks radical scavenging hydroxyl groups [78,79]. Furthermore the free radical scavenging activity of human plasma is mainly attributable to vitamin C, vitamin E, bilirubin and urate which occur in many times higher concentration as compared to cocoa polyphenols [80].
Hypertension is a leading risk of death in the world [87]. If a patient is diagnosed with high blood pressure anti-hypertensive drugs need to be prescribed. Functional food and their nutraceuticals may have a preventive potential, but they do not play a therapeutic role if the disease has already occurred. Although high flavonol cocoa polyphenols improved endothelial function, it did not improve the effects of exercise on body fat in obese adults [88]. From a nutritional point of view it is questionable whether one should increase the intake of dietary polyphenols due to cocoa-rich products such as chocolate, which is rich in fat and sugar and thereby high in energy. In the study by Taubert et al. consecutive daily doses of 100 g dark chocolate over two weeks increased the caloric intake by 480 kcal per day. Although systolic blood pressure was decreased by 5 mm Hg, in the long run these extra calories would probably lead to an increase in body weight and may counteract potential beneficial effects on vascular health [89]. Thus products low in sugar and fat should be preferred.
It needs to be taken into account that many human studies on cocoa polyphenols and vascular health as reported in the literature, were not placebo-controlled. Furthermore most studies regarding potential health benefits of cocoa polyphenols did include only small number of volunteers as summarized in Table 5. In fact many human intervention trials with cocoa polyphenols seem to be underpowered. Another problem with these studies is that rigorous study design is missing and compliance of the volunteers as well as plasma polyphenol concentrations have often not been reported. In order to determine health benefits of cocoa polyphenols large scale, long term randomised placebo-controlled studies (ideally with a cross-over design) are warranted [90]. Furthermore prospective studies on cocoa polyphenols need to be conducted in the future.
Table 5.
Human intervention trials with cocoa and chocolate.
| No. Subjects | Age range (mean) | BMI (kg/m2) | Intervention | Polyphenol content | Outcomes | Reference |
|---|---|---|---|---|---|---|
| 12 | 39 ± 4.0 | – | Cocoa | – | LDL oxidation ↓ | [57] |
| 15 | (32.5 ± 6.4) | 21.7 ± 2.1 | 12 g cocoa powder x3/d for 2 weeks, vs. sugar | 2610 mg total polyphenols/d (244 mg epicatechin) | LDL oxidation ↓ No change in plasma lipids or antioxidants Urinary excretion of epicatechin/metabolites ↑ |
[58] |
| 23 | 21–62 (36) | – | 22 g cocoa powder + 16 g dark chocolate/d for 4 weeks, vs. average American diet | 466 mg procyanidins/d (111 mg monomers) | LDL oxidation ↓ Serum antioxidant capacity ↑ HDL cholesterol ↑ |
[19] |
| 25 | 20–6 (32.4 ± 7.4) | 24.4 ± 3.4 | 37 g dark chocolate & 31 g cocoa powder in a drink/d for 6 weeks, vs. none | 651 mg total procyanidins/d (chocolate = 168 mg/d, cocoa = 483 mg/d) | LDL oxidizability ↓ No effect on inflammation markers, or plasma antioxidant capacity |
[81] |
| 45 | 19–49 (26) | 21.5 ± 2.9 /24.1 ± 3.5 | 75 g dark chocolate or high-phenolic dark chocolate for 3 weeks, vs. 75 g white chocolate | Total polyphenols (epicatechin): Dark = 274 (114) mg/d High = 418 (170) mg/d |
HDL cholesterol ↑ Lipid peroxidation ↓ No change in plasma antioxidant capacity |
[60] |
| 25 | (38 ± 1) | 22.1 ± 0.4 | 26 g/d cocoa powder for 12 weeks |
Per 100 g: 377 mg epicatechin, 135 mg catechin, 158 mg procyanidin B2, 96.1 mg procyanidin C1 |
LDL oxidation ↓ HDL-cholesterol ↑ |
[59] |
| 20 | 20–56 | 23.8 ± 0.79 | Semi-sweet chocolate baking bits (27, 53, 80 g), vs. none | Total procyanidins (epicatechin): 186 (46) mg 365 (90) mg 551 (136) mg |
Plasma epicatechin ↑ dose-dependent Antioxidant capacity ↑ TBARS ↓ |
[17] |
| 13 | 26–49 | 23.2 ± 1.2 | 105 g (of which 80 g chocolate) semi-sweet baking bits, vs. vanilla milk chips | 557 mg total procyanidins (137 mg epicatechin) | Plasma epicatechin ↑ Total antioxidant capacity ↑ TBARS ↓ |
[10] |
| 20 | 20–40 | – | 100 ml high- vs. low-flavanol cocoa drink | 187 mg vs. 14 mg total monomers & oligomeric procyanidins | Plasma F2-isoprostanes ↓ | [62] |
| 12 | 25–35 (32.2 ± 1.0) | 21.9 ± 0.4 | 100 g dark chocolate (with & without 200 mL milk), vs. 200 g milk chocolate | Not stated but FRAP values (147.4 μmol FE/100 g) | Plasma antioxidant capacity & epicatechin ↑, in absence of milk | [9] |
| 30 | 24–49 | – | Cocoa beverage (300 ml, 18.75 g procyanidin-rich cocoa powder), caffeinated beverage, or water | 897 mg total epicatechin & oligomeric procyanidins | Platelet activation & function ↓ | [61] |
| 18 | – | – | 25 g semi-sweet chocolate chips, vs. none | 220 mg flavanols & procyanidins | Plasma epicatechin ↑ Platelet function ↓ |
[24] |
| 32 | 40 ± 9 | 26 ± 4 | Cocoa flavanol/procyanidin tablets for 28 d, vs. placebo | 234 mg flavanols & procyanidins/d | Platelet aggregation ↓ Plasma ascorbic acid ↑ No change in oxidation status markers Plasma epicatechin & catechin ↑ |
[25] |
| 30 | 20–58 (30.6) | – | Dark (75% cocoa), vs. milk (20% cocoa) or white (no flavonoids) chocolate High polyphenol vs. low | – | Collagen–induced platelet aggregation ↓ | [82] |
| 27 | 18–72 (44 ± 3.4) | 26.9 ± 0.9 | flavanol cocoa drink (4x 230 mL/d for 4 d) | 821 mg/d total flavanols (epicatechin, catechin & related oligomers) | Improved peripheral vasodilation | [63] |
| 20 | 41 ± 14 | 25 ± 4 | 100 mL high polyphenol vs. low flavanol cocoa drink | 176 mg total flavanols (70 mg monomers, 106 mg procyanidins) | NO bioactivity ↑ Arterial FMD ↑ |
[64] |
| 10 | – | – | 200 mL high- vs. low-flavanol cocoa beverage | 985 vs. 80.4 mg total flavanols | Erythrocyte arginase activity ↓ | [44] |
| 13 | 55–64 | 21.9–26.2 | 100 g dark chocolate/d for 14 d, vs. 90 g white chocolate in hypertensive subjects | 500 mg/d total polyphenols | Systolic & diastolic blood pressure ↓ | [72] |
| 15 | (33.9 ± 7.6) | 22.6 ± 3.0 | 100 g dark chocolate, vs. 90 g white chocolate for 15 d | 500 mg total polyphenols | Insulin sensitivity ↑ Insulin resistance ↓ Systolic blood pressure ↓ |
[70] |
| 28 | – | – | 105 g/d milk chocolate for 14 d, vs. cocoa butter chocolate; hypertensive subjects | 168 mg/d flavanols (39 mg monomers, 126 mg polymers) 2.62 g procyanidins | Diastolic & mean blood pressure ↓ LDL cholesterol ↓ Oxidative stress markers ↓ |
[69] |
| 17 | 24–32 (28.9) | <27.0 | 100 g dark chocolate, vs. none | (0.54 g monomers & dimers, 0.76 g trimer-heptamers) | Improved endothelial function Vasodilation of brachial artery No change in blood pressure |
[67] |
| 20 | (43.65 ± 7.8) | 25.4 ± 1.7 | 100 g/d dark chocolate for 15 d, vs. 90 g white chocolate in hypertensive subjects | 88 mg/d flavanols (22 mg catechin, 66 mg epicatechin) | Improved insulin sensitivity Systolic & diastolic blood pressure ↓ LDL cholesterol ↓ Improved FMD |
[71] |
| 11 | (31 ± 1) | 21.8 ± 0.8 | 100 mL high- vs. low-polyphenol cocoa drink | 176–185 mg flavanols (70–74 mg monomers, 20–22 mg epicatechin, 106–111 mg procyanidins) | Circulating NO & FMD ↑ | [65] |
| 16 | 25–32 | 19–23 | 300 mL high- vs. low-flavanol cocoa drink | 917 mg flavanols (19% epicatechin) | Circulating NO species ↑ FMD response of conduit arteries ↑ Microcirculation ↑ |
[66] |
| 20 | – | – | 40 g dark chocolate, vs. white chocolate | Same brand as used for Vlachopoulos et al. (2005) | Improved FMD Platelet function ↓ Plasma total antioxidant status ↑ |
[83] |
| 34 | 18–74 (47.9 ± 3.0) | 28.0 ± 1.9/28.4 ± 1.3 | High polyphenol cocoa drink 4x 230 mL/d for 4–6 d, vs. none |
Per 100 mL: 9.2 mg epicatechin, 10.7 mg catechin, 69.3 mg flavanol oligomers (821 mg/d) |
NO synthesis ↓ FMD ↑ Pulse wave amplitude ↑ |
[84] |
| 40 | 61 ± 9 | 27.1 ± 3.9 | 48 g flavanol-rich chocolate bar + 18 g cocoa beverage/d, vs. placebo for 6 weeks in subjects with coronary artery disease | 444 vs. 19.6 mg/d total flavanols (107 vs. 4.7 mg epicatechin) | No acute or chronic changes in FMD, systemic arterial compliance, forearm blood flow, soluble cellular adhesion molecules | [75] |
| 32 | 57.7 ± 2.2/55.4 ± 1.7 | 24.9 ± 1.0/25.3 ± 0.8 | High- vs. low-flavanol cocoa beverage for 6 weeks in hypercholesterolemic subjects | 446 vs. 43 mg total flavanols | FMD ↑ Brachial artery hyperaemic blood flow ↑ VCAM-1 ↓ |
[85] |
| 11 | 22–32 (27 ± 1) | 22 ± 1 | 100 mL high-flavanol vs. low-phenolic cocoa drink x3/d for 1 week |
Per 100 ml: 59 mg epicatechin, 15 mg catechin, 232 mg flavanol oligomers (918 mg/d procyanidins) |
FMD ↑ No change in biomarkers of oxidative stress |
[86] |
| 45 | 30–75 (52.8 11.0) ± | 30.1 ± 3.3 | 74 g solid dark chocolate (22 g cocoa powder); 240 ml liquid cocoa (sugar-free vs. sugared) | 821 mg total flavanols; 805.2 & 8.5 mg total flavanols | Improved FMD Systolic & diastolic blood pressure ↓ |
[68] |
FE: Ferric equivalents; FMD: Flow-mediated dilation; FRAP: Ferric-reducing ability of plasma;
TBARS: Thiobarbituric acid reactive substances.
All data refer to healthy subjects unless otherwise stated.
4. Conclusions
There is epidemiological evidence suggesting that consumption of fruits and vegetables appear to have a protective effect against coronary heart disease [91]. Furthermore, the intake of certain flavonoid rich food items may be inversely related to coronary heart disease [92,93]. However, only few epidemiological data are currently available for cocoa polyphenols. In a cohort of elderly men, cocoa intake was inversely associated with blood pressure and 15-year cardiovascular and all-cause mortality [94]. It is suggested that before drawing conclusions, confirmation by further observational and experimental studies on cocoa polyphenols is needed. Mink et al. [95] studied flavonoid intake and cardiovascular disease mortality in a prospective study in postmenopausal women. Individual foods associated with a reduction of cardiovascular disease risk included bran, apples and pears, red wine, grapefruit, strawberries and chocolate.
Hooper and coworkers have recently conducted a meta-analysis on flavonoids, flavonoid-rich foods and cardiovascular disease risk. One hundred thirty-three trials were included into this meta-analysis. Importantly chocolate increased FMD and reduced both systolic and diastolic blood pressure [96]. Janszky et al. [97] assessed the long-term effects of chocolate consumption amongst patients with established coronary heart disease in a population based cohort study comprising >1100 non diabetic patients. Chocolate consumption was associated with a significantly reduced cardiac mortality in patients surviving the first acute myocardial infarction.
In a recent review it has been stated that most cocoa polyphenol studies in humans over the last decade have been mainly funded by industrial sponsors. Thus there may be potential for research bias [98]. Nevertheless several studies consistently reported beneficial effects of dietary polyphenols from cocoa on vascular health. However, it should be considered that the products used in controlled studies often contain much higher polyphenol contents than most of the commercially available products [94,99]. Since flavonols exhibit a bitter taste manufacturers have established processing techniques for cocoa which eliminate the bitterness together with the flavonoides [100]. As much as 90% of the flavonoids may be lost due cocoa processing [76].Thus, it needs to be established whether the consumption of products with a lower polyphenol content are associated with any health benefits in humans. Furthermore the food industry is encouraged to label the flavonoid content on their cocoa derived products.
References and Notes
- 1.Visioli F, Bernaert H, Corti R, Ferri C, Heptinstall S, Molinari E, Poli A, Serafini M, Smit HJ, Vinson JA, Violi F, Paoletti R. Chocolate, lifestyle, and health. Crit. Rev. Food Sci. Nutr. 2009;49:299–312. doi: 10.1080/10408390802066805. [DOI] [PubMed] [Google Scholar]
- 2.Galleano M, Oteiza PI, Fraga CG.Cocoa, chocolate and cardiovascular disease J Cardiovasc Pharmacol 2009. DOI: 10.1097/FJC.0b013e3181b76787. [DOI] [PMC free article] [PubMed]
- 3.Corti R, Flammer AJ, Hollenberg NK, Luscher TF. Cocoa and cardiovascular health. Circulation. 2009;119:1433–1441. doi: 10.1161/CIRCULATIONAHA.108.827022. [DOI] [PubMed] [Google Scholar]
- 4.Baba S, Osakabe N, Natsume M, Yasuda A, Takizawa T, Nakamura T, Terao J. Cocoa powder enhances the level of antioxidative activity in rat plasma. Br. J. Nutr. 2000;84:673–680. [PubMed] [Google Scholar]
- 5.Andres-Lacueva C, Monagas M, Khan N, Izquierdo-Pulido M, Urpi-Sarda M, Permanyer J, Lamuela-Raventos RM. Flavanol and flavonol contents of cocoa powder products: Influence of the manufacturing process. J. Agric. Food Chem. 2008;56:3111–3117. doi: 10.1021/jf0728754. [DOI] [PubMed] [Google Scholar]
- 6.Grassi D, Desideri G, Necozione S, Lippi C, Casale R, Properzi G, Blumberg JB, Ferri C. Blood pressure is reduced and insulin sensitivity increased in glucose-intolerant, hypertensive subjects after 15 days of consuming high-polyphenol dark chocolate. J. Nutr. 2008;138:1671–1676. doi: 10.1093/jn/138.9.1671. [DOI] [PubMed] [Google Scholar]
- 7.Hammerstone JF, Lazarus SA, Mitchell AE, Rucker R, Schmitz HH. Identification of procyanidins in cocoa (Theobroma cacao) and chocolate using high-performance liquid chromatography/mass spectrometry. J. Agric. Food Chem. 1999;47:490–496. doi: 10.1021/jf980760h. [DOI] [PubMed] [Google Scholar]
- 8.Ramiro-Puig E, Castell M. Cocoa: Antioxidant and immunomodulator. Br. J. Nutr. 2009;101:931–940. doi: 10.1017/S0007114508169896. [DOI] [PubMed] [Google Scholar]
- 9.Serafini M, Bugianesi R, Maiani G, Valtuena S, de Santis S, Crozier A. Plasma antioxidants from chocolate. Nature. 2003;424:1013. doi: 10.1038/4241013a. [DOI] [PubMed] [Google Scholar]
- 10.Rein D, Lotito S, Holt RR, Keen CL, Schmitz HH, Fraga CG. Epicatechin in human plasma: In vivo determination and effect of chocolate consumption on plasma oxidation status. J. Nutr. 2000;130:2109S–2114S. doi: 10.1093/jn/130.8.2109S. [DOI] [PubMed] [Google Scholar]
- 11.Donovan JL, Crespy V, Oliveira M, Cooper KA, Gibson BB, Williamson G. (+)-Catechin is more bioavailable than (−)-catechin: Relevance to the bioavailability of catechin from cocoa. Free Radic. Res. 2006;40:1029–1034. doi: 10.1080/10715760600868545. [DOI] [PubMed] [Google Scholar]
- 12.Roura E, Andres-Lacueva C, Estruch R, Mata-Bilbao ML, Izquierdo-Pulido M, Waterhouse AL, Lamuela-Raventos RM. Milk does not affect the bioavailability of cocoa powder flavonoid in healthy human. Ann. Nutr. Metab. 2007;51:493–498. doi: 10.1159/000111473. [DOI] [PubMed] [Google Scholar]
- 13.Schramm DD, Karim M, Schrader HR, Holt RR, Kirkpatrick NJ, Polagruto JA, Ensunsa JL, Schmitz HH, Keen CL. Food effects on the absorption and pharmacokinetics of cocoa flavanols. Life Sci. 2003;73:857–869. doi: 10.1016/s0024-3205(03)00373-4. [DOI] [PubMed] [Google Scholar]
- 14.Crozier A, Jaganath IB, Clifford MN. Dietary phenolics: Chemistry, bioavailability and effects on health. Nat. Prod. Rep. 2009;26:1001–1043. doi: 10.1039/b802662a. [DOI] [PubMed] [Google Scholar]
- 15.Steinberg FM, Bearden MM, Keen CL. Cocoa and chocolate flavonoids: Implications for cardiovascular health. J. Am. Diet. Assoc. 2003;103:215–223. doi: 10.1053/jada.2003.50028. [DOI] [PubMed] [Google Scholar]
- 16.Richelle M, Tavazzi I, Enslen M, Offord EA. Plasma kinetics in man of epicatechin from black chocolate. Eur. J. Clin. Nutr. 1999;53:22–26. doi: 10.1038/sj.ejcn.1600673. [DOI] [PubMed] [Google Scholar]
- 17.Wang JF, Schramm DD, Holt RR, Ensunsa JL, Fraga CG, Schmitz HH, Keen CL. A dose-response effect from chocolate consumption on plasma epicatechin and oxidative damage. J. Nutr. 2000;130:2115S–2119S. doi: 10.1093/jn/130.8.2115S. [DOI] [PubMed] [Google Scholar]
- 18.Baba S, Osakabe N, Yasuda A, Natsume M, Takizawa T, Nakamura T, Terao J. Bioavailability of (−)-epicatechin upon intake of chocolate and cocoa in human volunteers. Free Radic. Res. 2000;33:635–641. doi: 10.1080/10715760000301151. [DOI] [PubMed] [Google Scholar]
- 19.Wan Y, Vinson JA, Etherton TD, Proch J, Lazarus SA, Kris-Etherton PM. Effects of cocoa powder and dark chocolate on LDL oxidative susceptibility and prostaglandin concentrations in humans. Am. J. Clin. Nutr. 2001;74:596–602. doi: 10.1093/ajcn/74.5.596. [DOI] [PubMed] [Google Scholar]
- 20.Schramm DD, Wang JF, Holt RR, Ensunsa JL, Gonsalves JL, Lazarus SA, Schmitz HH, German JB, Keen CL. Chocolate procyanidins decrease the leukotriene-prostacyclin ratio in humans and human aortic endothelial cells. Am. J. Clin. Nutr. 2001;73:36–40. doi: 10.1093/ajcn/73.1.36. [DOI] [PubMed] [Google Scholar]
- 21.Pearson DA, Paglieroni TG, Rein D, Wun T, Schramm DD, Wang JF, Holt RR, Gosselin R, Schmitz HH, Keen CL. The effects of flavanol-rich cocoa and aspirin on ex vivo platelet function. Thromb. Res. 2002;106:191–197. doi: 10.1016/s0049-3848(02)00128-7. [DOI] [PubMed] [Google Scholar]
- 22.Holt RR, Lazarus SA, Sullards MC, Zhu QY, Schramm DD, Hammerstone JF, Fraga CG, Schmitz HH, Keen CL. Procyanidin dimer B2 [epicatechin-(4beta-8)-epicatechin] in human plasma after the consumption of a flavanol-rich cocoa. Am. J. Clin. Nutr. 2002;76:798–804. doi: 10.1093/ajcn/76.4.798. [DOI] [PubMed] [Google Scholar]
- 23.Steinberg FM, Holt RR, Schmitz HH, Keen CL. Cocoa procyanidin chain length does not determine ability to protect LDL from oxidation when monomer units are controlled. J. Nutr. Biochem. 2002;13:645–652. doi: 10.1016/s0955-2863(02)00215-2. [DOI] [PubMed] [Google Scholar]
- 24.Holt RR, Schramm DD, Keen CL, Lazarus SA, Schmitz HH. Chocolate consumption and platelet function. JAMA. 2002;287:2212–2213. doi: 10.1001/jama.287.17.2212. [DOI] [PubMed] [Google Scholar]
- 25.Murphy KJ, Chronopoulos AK, Singh I, Francis MA, Moriarty H, Pike MJ, Turner AH, Mann NJ, Sinclair AJ. Dietary flavanols and procyanidin oligomers from cocoa (Theobroma cacao) inhibit platelet function. Am. J. Clin. Nutr. 2003;77:1466–1473. doi: 10.1093/ajcn/77.6.1466. [DOI] [PubMed] [Google Scholar]
- 26.Waterhouse AL, Shirley JR, Donovan JL. Antioxidants in chocolate. Lancet. 1996;348:834. doi: 10.1016/S0140-6736(05)65262-2. [DOI] [PubMed] [Google Scholar]
- 27.Lotito SB, Actis-Goretta L, Renart ML, Caligiuri M, Rein D, Schmitz HH, Steinberg FM, Keen CL, Fraga CG. Influence of oligomer chain length on the antioxidant activity of procyanidins. Biochem. Biophys. Res. Commun. 2000;276:945–951. doi: 10.1006/bbrc.2000.3571. [DOI] [PubMed] [Google Scholar]
- 28.Richelle M, Tavazzi I, Offord E. Comparison of the antioxidant activity of commonly consumed polyphenolic beverages (coffee, cocoa, and tea) prepared per cup serving. J. Agric. Food Chem. 2001;49:3438–3442. doi: 10.1021/jf0101410. [DOI] [PubMed] [Google Scholar]
- 29.Osakabe N, Yasuda A, Natsume M, Takizawa T, Terao J, Kondo K. Catechins and their oligomers linked by C4 → C8 bonds are major cacao polyphenols and protect low-density lipoprotein from oxidation in vitro. Exp. Biol. Med. (Maywood) 2002;227:51–56. doi: 10.1177/153537020222700109. [DOI] [PubMed] [Google Scholar]
- 30.Vinson JA, Proch J, Zubik L. Phenol antioxidant quantity and quality in foods: Cocoa, dark chocolate, and milk chocolate. J. Agric. Food Chem. 1999;47:4821–4824. doi: 10.1021/jf990312p. [DOI] [PubMed] [Google Scholar]
- 31.Schewe T, Sadik C, Klotz LO, Yoshimoto T, Kuhn H, Sies H. Polyphenols of cocoa: Inhibition of mammalian 15-lipoxygenase. Biol. Chem. 2001;382:1687–1696. doi: 10.1515/BC.2001.204. [DOI] [PubMed] [Google Scholar]
- 32.Schewe T, Kuhn H, Sies H. Flavonoids of cocoa inhibit recombinant human 5-lipoxygenase. J. Nutr. 2002;132:1825–1829. doi: 10.1093/jn/132.7.1825. [DOI] [PubMed] [Google Scholar]
- 33.Hatano T, Miyatake H, Natsume M, Osakabe N, Takizawa T, Ito H, Yoshida T. Proanthocyanidin glycosides and related polyphenols from cacao liquor and their antioxidant effects. Phytochemistry. 2002;59:749–758. doi: 10.1016/s0031-9422(02)00051-1. [DOI] [PubMed] [Google Scholar]
- 34.Mao T, van de Water J, Keen CL, Schmitz HH, Gershwin ME. Cocoa procyanidins and human cytokine transcription and secretion. J. Nutr. 2000;130:2093S–2099S. doi: 10.1093/jn/130.8.2093S. [DOI] [PubMed] [Google Scholar]
- 35.Mao TK, Powell J, van de Water J, Keen CL, Schmitz HH, Gershwin ME. Effect of cocoa procyanidins on the secretion of interleukin-4 in peripheral blood mononuclear cells. J. Med. Food. 2000;3:107–114. doi: 10.1016/s0024-3205(00)00449-5. [DOI] [PubMed] [Google Scholar]
- 36.Mao TK, Powell J, van de Water J, Keen CL, Schmitz HH, Hammerstone JF, Gershwin ME. The effect of cocoa procyanidins on the transcription and secretion of interleukin 1 beta in peripheral blood mononuclear cells. Life Sci. 2000;66:1377–1386. doi: 10.1016/s0024-3205(00)00449-5. [DOI] [PubMed] [Google Scholar]
- 37.Mao TK, van de Water J, Keen CL, Schmitz HH, Gershwin ME. Modulation of TNF-alpha secretion in peripheral blood mononuclear cells by cocoa flavanols and procyanidins. Dev. Immunol. 2002;9:135–141. doi: 10.1080/1044667031000137601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mao TK, van de Water J, Keen CL, Schmitz HH, Gershwin ME. Effect of cocoa flavanols and their related oligomers on the secretion of interleukin-5 in peripheral blood mononuclear cells. J. Med. Food. 2002;5:17–22. doi: 10.1089/109662002753723188. [DOI] [PubMed] [Google Scholar]
- 39.Guo Q, Rimbach G, Packer L. Nitric oxide formation in macrophages detected by spin trapping with iron-dithiocarbamate complex: Effect of purified flavonoids and plant extracts. Methods Enzymol. 2001;335:273–282. doi: 10.1016/s0076-6879(01)35250-3. [DOI] [PubMed] [Google Scholar]
- 40.Saliou C, Valacchi G, Rimbach G. Assessing bioflavonoids as regulators of NF-kappa B activity and inflammatory gene expression in mammalian cells. Methods Enzymol. 2001;335:380–387. doi: 10.1016/s0076-6879(01)35260-6. [DOI] [PubMed] [Google Scholar]
- 41.Ramiro E, Franch A, Castellote C, Andres-Lacueva C, Izquierdo-Pulido M, Castell M. Effect of Theobroma cacao flavonoids on immune activation of a lymphoid cell line. Br. J. Nutr. 2005;93:859–866. doi: 10.1079/bjn20051443. [DOI] [PubMed] [Google Scholar]
- 42.Ramiro E, Franch A, Castellote C, Perez-Cano F, Permanyer J, Izquierdo-Pulido M, Castell M. Flavonoids from Theobroma cacao down-regulate inflammatory mediators. J. Agric. Food Chem. 2005;53:8506–8511. doi: 10.1021/jf0511042. [DOI] [PubMed] [Google Scholar]
- 43.Park YC, Rimbach G, Saliou C, Valacchi G, Packer L. Activity of monomeric, dimeric, and trimeric flavonoids on NO production, TNF-alpha secretion, and NF-kappaB-dependent gene expression in RAW 264.7 macrophages. FEBS Lett. 2000;465:93–97. doi: 10.1016/s0014-5793(99)01735-4. [DOI] [PubMed] [Google Scholar]
- 44.Schnorr O, Brossette T, Momma TY, Kleinbongard P, Keen CL, Schroeter H, Sies H. Cocoa flavanols lower vascular arginase activity in human endothelial cells in vitro and in erythrocytes in vivo. Arch. Biochem. Biophys. 2008;476:211–215. doi: 10.1016/j.abb.2008.02.040. [DOI] [PubMed] [Google Scholar]
- 45.Karim M, McCormick K, Kappagoda CT. Effects of cocoa extracts on endothelium-dependent relaxation. J. Nutr. 2000;130:2105S–2108S. doi: 10.1093/jn/130.8.2105S. [DOI] [PubMed] [Google Scholar]
- 46.Lee KW, Kang NJ, Oak MH, Hwang MK, Kim JH, Schini-Kerth VB, Lee HJ. Cocoa procyanidins inhibit expression and activation of MMP-2 in vascular smooth muscle cells by direct inhibition of MEK and MT1-MMP activities. Cardiovasc. Res. 2008;79:34–41. doi: 10.1093/cvr/cvn056. [DOI] [PubMed] [Google Scholar]
- 47.Actis-Goretta L, Ottaviani JI, Keen CL, Fraga CG. Inhibition of angiotensin converting enzyme (ACE) activity by flavan-3-ols and procyanidins. FEBS Lett. 2003;555:597–600. doi: 10.1016/s0014-5793(03)01355-3. [DOI] [PubMed] [Google Scholar]
- 48.Kurosawa T, Itoh F, Nozaki A, Nakano Y, Katsuda S, Osakabe N, Tsubone H, Kondo K, Itakura H. Suppressive effect of cocoa powder on atherosclerosis in Kurosawa and Kusanagi-hypercholesterolemic rabbits. J. Atheroscler. Thromb. 2005;12:20–28. doi: 10.5551/jat.12.20. [DOI] [PubMed] [Google Scholar]
- 49.Vinson JA, Proch J, Bose P, Muchler S, Taffera P, Shuta D, Samman N, Agbor GA. Chocolate is a powerful ex vivo and in vivo antioxidant, an antiatherosclerotic agent in an animal model, and a significant contributor to antioxidants in the European and American Diets. J. Agric. Food Chem. 2006;54:8071–8076. doi: 10.1021/jf062175j. [DOI] [PubMed] [Google Scholar]
- 50.Yasuda A, Natsume M, Sasaki K, Baba S, Nakamura Y, Kanegae M, Nagaoka S. Cacao procyanidins reduce plasma cholesterol and increase fecal steroid excretion in rats fed a high-cholesterol diet. Biofactors. 2008;33:211–223. doi: 10.1002/biof.5520330307. [DOI] [PubMed] [Google Scholar]
- 51.Jalil AM, Ismail A, Pei CP, Hamid M, Kamaruddin SH. Effects of cocoa extract on glucometabolism, oxidative stress, and antioxidant enzymes in obese-diabetic (ob-db) rats. J. Agric. Food Chem. 2008;56:7877–7884. doi: 10.1021/jf8015915. [DOI] [PubMed] [Google Scholar]
- 52.Orozco TJ, Wang JF, Keen CL. Chronic consumption of a flavanol- and procyanindin-rich diet is associated with reduced levels of 8-hydroxy-2′-deoxyguanosine in rat testes. J. Nutr. Biochem. 2003;14:104–110. doi: 10.1016/s0955-2863(02)00273-5. [DOI] [PubMed] [Google Scholar]
- 53.Kenny TP, Keen CL, Schmitz HH, Gershwin ME. Immune effects of cocoa procyanidin oligomers on peripheral blood mononuclear cells. Exp. Biol. Med. (Maywood) 2007;232:293–300. [PubMed] [Google Scholar]
- 54.Rein D, Paglieroni TG, Pearson DA, Wun T, Schmitz HH, Gosselin R, Keen CL. Cocoa and wine polyphenols modulate platelet activation and function. J. Nutr. 2000;130:2120S–2126S. doi: 10.1093/jn/130.8.2120S. [DOI] [PubMed] [Google Scholar]
- 55.Mackenzie GG, Carrasquedo F, Delfino JM, Keen CL, Fraga CG, Oteiza PI. Epicatechin, catechin, and dimeric procyanidins inhibit PMA-induced NF-kappaB activation at multiple steps in Jurkat T cells. FASEB J. 2004;18:167–169. doi: 10.1096/fj.03-0402fje. [DOI] [PubMed] [Google Scholar]
- 56.Zhu QY, Holt RR, Lazarus SA, Orozco TJ, Keen CL. Inhibitory effects of cocoa flavanols and procyanidin oligomers on free radical-induced erythrocyte hemolysis. Exp. Biol. Med. (Maywood) 2002;227:321–329. doi: 10.1177/153537020222700504. [DOI] [PubMed] [Google Scholar]
- 57.Kondo K, Hirano R, Matsumoto A, Igarashi O, Itakura H. Inhibition of LDL oxidation by cocoa. Lancet. 1996;348:1514. doi: 10.1016/s0140-6736(05)65927-2. [DOI] [PubMed] [Google Scholar]
- 58.Osakabe N, Baba S, Yasuda A, Iwamoto T, Kamiyama M, Takizawa T, Itakura H, Kondo K. Daily cocoa intake reduces the susceptibility of low-density lipoprotein to oxidation as demonstrated in healthy human volunteers. Free Radic. Res. 2001;34:93–99. doi: 10.1080/10715760100300091. [DOI] [PubMed] [Google Scholar]
- 59.Baba S, Osakabe N, Kato Y, Natsume M, Yasuda A, Kido T, Fukuda K, Muto Y, Kondo K. Continuous intake of polyphenolic compounds containing cocoa powder reduces LDL oxidative susceptibility and has beneficial effects on plasma HDL-cholesterol concentrations in humans. Am. J. Clin. Nutr. 2007;85:709–717. doi: 10.1093/ajcn/85.3.709. [DOI] [PubMed] [Google Scholar]
- 60.Mursu J, Voutilainen S, Nurmi T, Rissanen TH, Virtanen JK, Kaikkonen J, Nyyssonen K, Salonen JT. Dark chocolate consumption increases HDL cholesterol concentration and chocolate fatty acids may inhibit lipid peroxidation in healthy humans. Free Radic. Biol. Med. 2004;37:1351–1359. doi: 10.1016/j.freeradbiomed.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 61.Rein D, Paglieroni TG, Wun T, Pearson DA, Schmitz HH, Gosselin R, Keen CL. Cocoa inhibits platelet activation and function. Am. J. Clin. Nutr. 2000;72:30–35. doi: 10.1093/ajcn/72.1.30. [DOI] [PubMed] [Google Scholar]
- 62.Wiswedel I, Hirsch D, Kropf S, Gruening M, Pfister E, Schewe T, Sies H. Flavanol-rich cocoa drink lowers plasma F(2)-isoprostane concentrations in humans. Free Radic. Biol. Med. 2004;37:411–421. doi: 10.1016/j.freeradbiomed.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 63.Fisher ND, Hughes M, Gerhard-Herman M, Hollenberg NK. Flavanol-rich cocoa induces nitric-oxide-dependent vasodilation in healthy humans. J. Hypertens. 2003;21:2281–2286. doi: 10.1097/00004872-200312000-00016. [DOI] [PubMed] [Google Scholar]
- 64.Heiss C, Dejam A, Kleinbongard P, Schewe T, Sies H, Kelm M. Vascular effects of cocoa rich in flavan-3-ols. JAMA. 2003;290:1030–1031. doi: 10.1001/jama.290.8.1030. [DOI] [PubMed] [Google Scholar]
- 65.Heiss C, Kleinbongard P, Dejam A, Perre S, Schroeter H, Sies H, Kelm M. Acute consumption of flavanol-rich cocoa and the reversal of endothelial dysfunction in smokers. J. Am. Coll. Cardiol. 2005;46:1276–1283. doi: 10.1016/j.jacc.2005.06.055. [DOI] [PubMed] [Google Scholar]
- 66.Schroeter H, Heiss C, Balzer J, Kleinbongard P, Keen CL, Hollenberg NK, Sies H, Kwik-Uribe C, Schmitz HH, Kelm M. (−)-Epicatechin mediates beneficial effects of flavanol-rich cocoa on vascular function in humans. Proc. Natl. Acad. Sci. USA. 2006;103:1024–1029. doi: 10.1073/pnas.0510168103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vlachopoulos C, Aznaouridis K, Alexopoulos N, Economou E, Andreadou I, Stefanadis C. Effect of dark chocolate on arterial function in healthy individuals. Am J Hypertens. 2005;18:785–791. doi: 10.1016/j.amjhyper.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 68.Faridi Z, Njike VY, Dutta S, Ali A, Katz DL. Acute dark chocolate and cocoa ingestion and endothelial function: A randomized controlled crossover trial. Am. J. Clin. Nutr. 2008;88:58–63. doi: 10.1093/ajcn/88.1.58. [DOI] [PubMed] [Google Scholar]
- 69.Fraga CG. Cocoa, diabetes, and hypertension: Should we eat more chocolate? Am. J. Clin. Nutr. 2005;81:541–542. doi: 10.1093/ajcn/81.3.541. [DOI] [PubMed] [Google Scholar]
- 70.Grassi D, Lippi C, Necozione S, Desideri G, Ferri C. Short-term administration of dark chocolate is followed by a significant increase in insulin sensitivity and a decrease in blood pressure in healthy persons. Am. J. Clin. Nutr. 2005;81:611–614. doi: 10.1093/ajcn/81.3.611. [DOI] [PubMed] [Google Scholar]
- 71.Grassi D, Necozione S, Lippi C, Croce G, Valeri L, Pasqualetti P, Desideri G, Blumberg JB, Ferri C. Cocoa reduces blood pressure and insulin resistance and improves endothelium-dependent vasodilation in hypertensives. Hypertension. 2005;46:398–405. doi: 10.1161/01.HYP.0000174990.46027.70. [DOI] [PubMed] [Google Scholar]
- 72.Taubert D, Berkels R, Roesen R, Klaus W. Chocolate and blood pressure in elderly individuals with isolated systolic hypertension. JAMA. 2003;290:1029–1030. doi: 10.1001/jama.290.8.1029. [DOI] [PubMed] [Google Scholar]
- 73.Taubert D, Roesen R, Lehmann C, Jung N, Schomig E. Effects of low habitual cocoa intake on blood pressure and bioactive nitric oxide: A randomized controlled trial. JAMA. 2007;298:49–60. doi: 10.1001/jama.298.1.49. [DOI] [PubMed] [Google Scholar]
- 74.Balzer J, Rassaf T, Heiss C, Kleinbongard P, Lauer T, Merx M, Heussen N, Gross HB, Keen CL, Schroeter H, Kelm M. Sustained benefits in vascular function through flavanol-containing cocoa in medicated diabetic patients a double-masked, randomized, controlled trial. J. Am. Coll. Cardiol. 2008;51:2141–2149. doi: 10.1016/j.jacc.2008.01.059. [DOI] [PubMed] [Google Scholar]
- 75.Farouque HM, Leung M, Hope SA, Baldi M, Schechter C, Cameron JD, Meredith IT. Acute and chronic effects of flavanol-rich cocoa on vascular function in subjects with coronary artery disease: A randomized double-blind placebo-controlled study. Clin. Sci. (Lond) 2006;111:71–80. doi: 10.1042/CS20060048. [DOI] [PubMed] [Google Scholar]
- 76.Mehrinfar R, Frishman WH. Flavanol-rich cocoa: A cardioprotective nutraceutical. Cardiol. Rev. 2008;16:109–115. doi: 10.1097/CRD.0b013e31815d95e2. [DOI] [PubMed] [Google Scholar]
- 77.Flammer AJ, Hermann F, Sudano I, Spieker L, Hermann M, Cooper KA, Serafini M, Luscher TF, Ruschitzka F, Noll G, Corti R. Dark chocolate improves coronary vasomotion and reduces platelet reactivity. Circulation. 2007;116:2376–2382. doi: 10.1161/CIRCULATIONAHA.107.713867. [DOI] [PubMed] [Google Scholar]
- 78.Turner R, Baron T, Wolffram S, Minihane AM, Cassidy A, Rimbach G, Weinberg PD. Effect of circulating forms of soy isoflavones on the oxidation of low density lipoprotein. Free Radic. Res. 2004;38:209–216. doi: 10.1080/10715760310001641854. [DOI] [PubMed] [Google Scholar]
- 79.Rimbach G, Weinberg PD, de Pascual-Teresa S, Alonso MG, Ewins BA, Turner R, Minihane AM, Botting N, Fairley B, Matsugo S, Uchida Y, Cassidy A. Sulfation of genistein alters its antioxidant properties and its effect on platelet aggregation and monocyte and endothelial function. Biochim. Biophys. Acta. 2004;1670:229–237. doi: 10.1016/j.bbagen.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 80.Halliwell B.Plasma antioxidants: Health benefits of eating chocolate Nature 2003426787discussion 788. [DOI] [PubMed] [Google Scholar]
- 81.Mathur S, Devaraj S, Grundy SM, Jialal I. Cocoa products decrease low density lipoprotein oxidative susceptibility but do not affect biomarkers of inflammation in humans. J. Nutr. 2002;132:3663–3667. doi: 10.1093/jn/132.12.3663. [DOI] [PubMed] [Google Scholar]
- 82.Innes AJ, Kennedy G, McLaren M, Bancroft AJ, Belch JJ. Dark chocolate inhibits platelet aggregation in healthy volunteers. Platelets. 2003;14:325–327. doi: 10.1080/0953710031000123681. [DOI] [PubMed] [Google Scholar]
- 83.Hermann F, Spieker LE, Ruschitzka F, Sudano I, Hermann M, Binggeli C, Luscher TF, Riesen W, Noll G, Corti R. Dark chocolate improves endothelial and platelet function. Heart. 2006;92:119–120. doi: 10.1136/hrt.2005.063362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fisher ND, Hollenberg NK. Aging and vascular responses to flavanol-rich cocoa. J. Hypertens. 2006;24:1575–1580. doi: 10.1097/01.hjh.0000239293.40507.2a. [DOI] [PubMed] [Google Scholar]
- 85.Wang-Polagruto JF, Villablanca AC, Polagruto JA, Lee L, Holt RR, Schrader HR, Ensunsa JL, Steinberg FM, Schmitz HH, Keen CL.Chronic consumption of flavanol-rich cocoa improves endothelial function and decreases vascular cell adhesion molecule in hypercholesterolemic postmenopausal women J Cardiovasc Pharmacol 200647Suppl. 2S177–S186.discussion S206–S209. [DOI] [PubMed] [Google Scholar]
- 86.Heiss C, Finis D, Kleinbongard P, Hoffmann A, Rassaf T, Kelm M, Sies H. Sustained increase in flow-mediated dilation after daily intake of high-flavanol cocoa drink over 1 week. J. Cardiovasc. Pharmacol. 2007;49:74–80. doi: 10.1097/FJC.0b013e31802d0001. [DOI] [PubMed] [Google Scholar]
- 87.Mohan S, Campbell NR. Salt and high blood pressure. Clin. Sci. (Lond) 2009;117:1–11. doi: 10.1042/CS20080207. [DOI] [PubMed] [Google Scholar]
- 88.Davison K, Coates AM, Buckley JD, Howe PR. Effect of cocoa flavanols and exercise on cardiometabolic risk factors in overweight and obese subjects. Int. J. Obes. (Lond) 2008;32:1289–1296. doi: 10.1038/ijo.2008.66. [DOI] [PubMed] [Google Scholar]
- 89.Ferri C, Grassi G. Mediterranean diet, cocoa and cardiovascular disease: A sweeter life, a longer life, or both? J. Hypertens. 2003;21:2231–2234. doi: 10.1097/00004872-200312000-00006. [DOI] [PubMed] [Google Scholar]
- 90.Grassi D, Desideri G, Croce G, Tiberti S, Aggio A, Ferri C. Flavonoids, vascular function and cardiovascular protection. Curr. Pharm. Des. 2009;15:1072–1084. doi: 10.2174/138161209787846982. [DOI] [PubMed] [Google Scholar]
- 91.Joshipura KJ, Hu FB, Manson JE, Stampfer MJ, Rimm EB, Speizer FE, Colditz G, Ascherio A, Rosner B, Spiegelman D, Willett WC. The effect of fruit and vegetable intake on risk for coronary heart disease. Ann. Intern. Med. 2001;134:1106–1114. doi: 10.7326/0003-4819-134-12-200106190-00010. [DOI] [PubMed] [Google Scholar]
- 92.Hertog MG, Feskens EJ, Hollman PC, Katan MB, Kromhout D. Dietary antioxidant flavonoids and risk of coronary heart disease: The Zutphen elderly study. Lancet. 1993;342:1007–1011. doi: 10.1016/0140-6736(93)92876-u. [DOI] [PubMed] [Google Scholar]
- 93.Erdman JW, Jr, Balentine D, Arab L, Beecher G, Dwyer JT, Folts J, Harnly J, Hollman P, Keen CL, Mazza G, Messina M, Scalbert A, Vita J, Williamson G, Burrowes J. Flavonoids and heart health. In Proceedings of the ILSI North America Flavonoids Workshop, Washington, DC, USA, May 31–June 1, 2005. J. Nutr. 2007;137:718S–737S. doi: 10.1093/jn/137.3.718S. [DOI] [PubMed] [Google Scholar]
- 94.Buijsse B, Feskens EJ, Kok FJ, Kromhout D. Cocoa intake, blood pressure, and cardiovascular mortality: The Zutphen elderly study. Arch. Intern. Med. 2006;166:411–417. doi: 10.1001/archinte.166.4.411. [DOI] [PubMed] [Google Scholar]
- 95.Mink PJ, Scrafford CG, Barraj LM, Harnack L, Hong CP, Nettleton JA, Jacobs DR., Jr Flavonoid intake and cardiovascular disease mortality: A prospective study in postmenopausal women. Am. J. Clin. Nutr. 2007;85:895–909. doi: 10.1093/ajcn/85.3.895. [DOI] [PubMed] [Google Scholar]
- 96.Hooper L, Kroon PA, Rimm EB, Cohn JS, Harvey I, Le Cornu KA, Ryder JJ, Hall WL, Cassidy A. Flavonoids, flavonoid-rich foods, and cardiovascular risk: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2008;88:38–50. doi: 10.1093/ajcn/88.1.38. [DOI] [PubMed] [Google Scholar]
- 97.Janszky I, Mukamal KJ, Ljung R, Ahnve S, Ahlbom A, Hallqvist J. Chocolate consumption and mortality following a first acute myocardial infarction: The Stockholm heart epidemiology program. J. Intern. Med. 2009;266:248–257. doi: 10.1111/j.1365-2796.2009.02088.x. [DOI] [PubMed] [Google Scholar]
- 98.Cooper KA, Donovan JL, Waterhouse AL, Williamson G. Cocoa and health: A decade of research. Br. J. Nutr. 2008;99:1–11. doi: 10.1017/S0007114507795296. [DOI] [PubMed] [Google Scholar]
- 99.Mullen W, Borges G, Donovan JL, Edwards CA, Serafini M, Lean ME, Crozier A. Milk decreases urinary excretion but not plasma pharmacokinetics of cocoa flavan-3-ol metabolites in humans. Am. J. Clin. Nutr. 2009;89:1784–1791. doi: 10.3945/ajcn.2008.27339. [DOI] [PubMed] [Google Scholar]
- 100.Hollenberg NK, Fisher ND. Is it the dark in dark chocolate? Circulation. 2007;116:2360–2362. doi: 10.1161/CIRCULATIONAHA.107.738070. [DOI] [PubMed] [Google Scholar]