Abstract
Background and Objectives
Triple anti-platelet therapy may produce more potent inhibition of platelet aggregation in patients undergoing coronary stent implantation. We tested whether this effect could be maintained in diabetic patients, where platelet reactivity is increased and the risk of stent thrombosis is higher.
Subjects and Methods
Fifty five type 2 diabetic patients who had undergone drug-eluting stent (DES) implantation and chronic anti-platelet therapy (>1 month) were stratified according to the status of anti-platelet therapy. Platelet aggregation after adenosine diphosphate (ADP; 10 µmol/L and 20 µmol/L) stimulation was compared using light transmittance aggregometry between dual (aspirin plus clopidogrel, n=34) and triple therapy (aspirin, clopidogrel plus cilostazol, n=21) groups.
Results
The 2 groups had similar clinical and procedural characteristics. Maximal ADP-induced platelet aggregation was significantly lower in the triple therapy group than the dual therapy group (ADP 10 µmol/L, 37.1±15.4 vs. 28.3±11.8, p=0.03; ADP 20 µmol/L, 63.1±15.0 vs. 49.1±15.1, p=0.01), but there were no differences in diabetic treatment (oral hypoglycemic agent vs. insulin) or diabetic control {hemoglobin Alc (HbA1c) ≤7 vs. HbA1c >7}.
Conclusion
Triple anti-platelet therapy showed more potent inhibition of maximal ADP induced platelet aggregation in type 2 diabetic patients receiving chronic anti-platelet therapy. This finding suggests that triple antiplatelet therapy may be more effective in preventing thrombotic complications after DES implantation in type 2 diabetic patients.
Keywords: Antiplatelet drugs, Diabetes mellitus, Platelet aggregation, Stents
Introduction
Type 2 diabetic patients have approximately 2 to 4-fold higher incidence of coronary artery diseases than non-diabetic patients, and restenosis commonly occurs after coronary stent implantation.1),2) Type 2 diabetes mellitus is also a risk factor for stent thrombosis after the implantation of drug-eluting stents (DES).3) Functional changes in platelets can increase cardiovascular complications in type 2 diabetic patients4-6) and decrease the response to aspirin,7),8) which increases the risk of developing coronary artery disease.9-11) Platelet aggregation is higher in diabetic patients after a loading-dose of clopidogrel, and platelet aggregation and activity are also higher after the administration of aspirin and clopidogrel for more than 1 month.12) Thus, the anti-platelet effects of clopidogrel and aspirin are decreased in diabetic patients. The triple anti-platelet therapy with aspirin, clopidogrel, and cilostazol is more effective in preventing stent thrombosis than dual anti-platelet therapy with aspirin, clopidogrel, or ticlopidine.13) Triple therapy also inhibits platelet aggregation and activity more than dual therapy after the drug-induced stimulation of platelets.14)
To date, however, there is no data comparing dual and triple therapy in type 2 diabetic patients. Therefore, we tested platelet aggregation after double and triple therapy using optical aggregometry in type 2 diabetic patients that received anti-platelet drugs after DES implantation.
Subjects and Methods
Subjects
We recruited 55 patients with type 2 diabetes mellitus who had received a DES at least one month earlier that then visited the outpatient clinic between March and May, 2008. Patients were taking anti-platelet agents without any notable clinical events. Patients were divided into a dual therapy group, with aspirin and clopidogrel, or a triple therapy group, with aspirin, clopidogrel, and cilostazol. The selection of anti-platelet agents was wholly based on physician discretion. Exclusion criteria were: patients with impaired glucose tolerance not taking oral hypoglycemic agents, patients taking oral anti-coagulation agents, patients taking non-steroidal anti-inflammatory agents before the platelet aggregation test, patients who abused other drugs or alcohol, patients whose platelet counts were less than 100,000/µL, patients whose hematocrit was less than 25%, patients whose serum creatinine was higher than 2.5 mg/dL, and patients whose serum liver enzymes were 2 times higher than normal. An institutional review committee approved this study and all subjects gave informed consent.
Platelet aggregation test
The brachial vein was directly punctured with a 22-gauge needle and blood was drawn. The first 2- to 3 mL blood to avoid the spontaneous activation of platelets. The remaining blood was placed in a test tube containing 3.8% trisodium citrate. Within an hour following blood sampling, the degree of maximal platelet aggregation was measured using optical aggregometry (Chronolog Aggregometer, Norbis, Stuttgart, Germany) after platelet stimulation with adenosine diphosphate (ADP), 10 µmol/L and 20 µmol/L. Centrifugation was performed at 800 r.p.m. for 10 minutes, and supernatant was harvested to prepare platelet-rich plasma. Using autologous plasma, platelet counts were set at 250,000/µL. Platelet-poor plasma was generated by a secondary centrifugation at 2,500 r.p.m. for 10 minutes. The light transmittance of platelet-rich plasma was set at 0% and that of platelet-poor plasma was set at 100%. The curve of blood coagulation was recorded for six minutes at each measurement. The degree of platelet aggregation was expressed as the percent changes in the light transmittance of platelet-rich plasma at 37℃ following ADP stimulation based on platelet-poor plasma (100%). At each measurement, the maximal platelet aggregation was measured.
Statistical analysis
Continuous variables were expressed as mean±standard deviation and categorical variables are expressed as the frequency and its percentage. Continuous variables following a normal distribution were analyzed using Student's t-test, non-normal data were analyzed using Mann-Whitney test. Categorical variables were analyzed using the Chi-square test or Fisher's exact test. A value of p<0.05 was considered statistically significant. All the statistical analyses were performed using Statistical Package for Social Science (SPSS) 15.0 (SPSS Inc. Chicago, IL, USA).
Results
Clinical characteristics
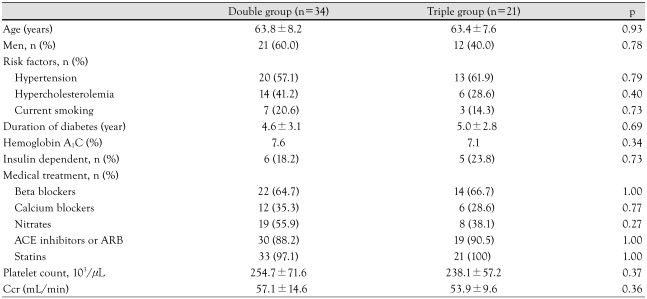
We enrolled 34 patients receiving dual therapy and 21 patients receiving triple therapy. All patients were taking 100 mg aspirin and 75 mg clopidogrel a day. Patients on triple therapy also took 200 mg cilostazol a day. There were no differences in the duration of diabetes mellitus, cardiovascular risk factors, creatinine clearance as calculated using the methods of Cockcroft and Gault, treatment agents other than anti-platelet agents, or hematologic tests (Table 1). In the dual therapy group, 30 patients were treated with 39 sirolimus-eluting stents (SES) and 4 patients were treated with 6 paclitaxel-eluting stents (PES). In the triple therapy group, 18 patients were treated with 22 SESs and 3 patients were treated with 4 PESs. Anti-platelet agents had been administered after DES implantation for 224.2±93.2 days in the dual therapy group and 181.2±121.2 days in the triple therapy group (p=0.18).
Table 1.
Clinical characteristics of patients (n=55)
ACE: angiotensin converting enzyme, ARB: angiotensin receptor blocker, Ccr: creatinine clearance calculated by Cockcroft and Gault method
Platelet aggregation test
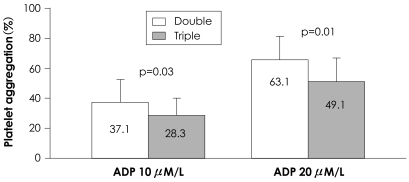
Platelet aggregation was lower in the triple therapy group than the dual therapy group after 10 µmol/L ADP (28.3±11.8% vs. 37.1±15.4%, p=0.03) and 20 µmol/L ADP (49.1±15.1% vs. 63.1±15.0%, p=0.01) (Fig. 1). Maximum platelet aggregation was not different in the insulin or oral hypoglycemic groups (Table 2). Maximum platelet aggregation was not different based on diabetic control status, as measured by hemoglobin levels of A1c 7.0 (Table 3).
Fig. 1.
Maximal platelet aggregation following ADP (10 and 20 µM) in type 2 diabetic patients according to anti-platelet drugs (Double, aspirin plus clopidogrel; Triple, aspirin, clopidogrel plus cilostazol). Numbers in the box denote the mean value of platelet aggregation. ADP: adenosine diphosphate.
Table 2.
Maximal platelet aggregation following 20 µM/L ADP according to hypoglycemic therapy modalities
*Double: aspirin plus clopidogrel, †Triple: aspirin, clopidogrel plus cilostazol. ADP: adenosine diphosphate, OHA: oral hypoglycemic agents
Table 3.
Maximal platelet aggregation following 20 µM/L ADP according to diabetic control
*Double: aspirin plus clopidogrel, †Triple: aspirin, clopidogrel plus cilostazol. ADP: adenosine diphosphate, HbA1c: hemoglobin A1c
Discussion
We found that triple therapy with aspirin, clopidogrel, and cilostazol inhibited ADP-induced platelet aggregation better than dual therapy with aspirin and clopidogrel in type 2 diabetic patients after DES implantation. Similarly, Lee et al.14) found that triple therapy inhibited platelet aggregation better than dual therapy after DES implantation, although that study included non-diabetic and diabetic patients.
Type 2 diabetes mellitus is equivalent to coronary artery disease and presents higher thrombogenic potential that increases cardiovascular risk. Vascular endothelial dysfunction, abnormal fibrinolysis, increased levels of coagulation factors, alterations of platelet function, and the decreased response to anti-platelet agents are closely associated with the formation of blood clots.4-8) Platelet responses to aspirin can be decreased even though thromboxane A2 in diabetics, but clopidogrel can also help prevent cardiovascular diseases in type 2 diabetic patients.15) Dual therapy with aspirin and clopidogreal would be more effective than individual treatments, but is less effective in type 2 diabetic patients.12)
Cilostazol and clopidogrel suppress ADP-induced platelet aggregation, but they work differently. Clopidogrel blocks the ADP receptor to inhibit adenylate cyclase, whereas cilostazol suppresses phosphodiesterase III activity. Both drugs therefore elevate the intracellular concentration of cyclic adenosine monophosphate (cAMP),16),17) which suppresses platelet aggregation better than either drug alone. Clinical studies have demonstrated that the triple therapy could lower the incidence of stent thrombosis more than dual therapy.13),18)
Enhanced platelet activation may be a consequence of more prevalent atherosclerotic lesions or reflect the influence of metabolic disturbances on platelet biochemistry and function. Decreased responses to anti-platelet agents may result from increased ADP receptors on the platelet surface, oxidative stress resulting in aspirin-insensitive thromboxane biosynthesis, and altered structure of the platelet membrane and enhanced protein glycation reducing interaction with drug target.19),20) Because protein glycation can decrease the efficacy of anti-platelet agents, diabetic treatment modalities, either hypoglycemic agents or insulin, or diabetic control, may influence anti-platelet activity. However, we did not find differences in treatment modalities or diabetic status in either therapy group. However, the small number of patients on insulin and the large number of patients with good diabetic control limit the strength of this conclusion. The degree of platelet aggregation was somewhat higher in patients with insulin treatment or hemoglobin A1c (HbA1c) of >7.0, suggesting that triple therapy would be effective for these patients.
Greater suppression of platelet aggregation in triple therapy might increase the incidence of complications including hemorrhage, and could increase medication expenses. Large-scale clinical studies did not show differences for the two therapies in the incidence of complications such as bleeding or gastrointestinal complications.13),21),22) The lack of established criteria for treatment periods for anti-platelet agents implies the need for individual treatment plans. The results of this study suggests that triple therapy may be useful in type 2 diabetic patients with left main coronary artery lesion, bifurcation lesions, or long lesions with high risk of stent thrombosis. DES can also produce later stent thrombosis than baremetal stents, indicating that triple therapy may be indicated long after stent implantation. Cilostazol can also reduce restenosis,21) which has significant clinical benefit.
There are some limitations of the current study. First, we used a retrospective analysis of medical records based on a single-center experience in a small number of patients, which could have selection bias. Second, we enrolled patients in an outpatient clinic without major cardiac events after DES implantation. Third, there were some discrepancies in the period of the use of anti-platelet agents. These limitations prevent the results of the current study from being generalized. Further prospective, randomized studies that are currently planned at our institution in patients with type 2 diabetes mellitus could overcome these limitations. Finally, we used optical aggregometry and did not measure clinical outcomes of inhibiting platelet aggregation. Further large-scale clinical studies are warranted to clarify differences in dual and triple therapy in diabetic patients.
In conclusion, triple therapy inhibited platelet aggregation more than dual therapy in type 2 diabetic patients taking anti-platelet agents. The current study provides an experimental basis for the selective use of triple therapy in patients with stents at high risk for thrombosis.
Acknowledgments
This work was supported by a Grant from Inje University, 2006.
References
- 1.Beckman JA, Creager MA, Libby P. Diabetes and atherosclerosis: epidemiology, pathophysiology, and management. JAMA. 2002;287:2570–2581. doi: 10.1001/jama.287.19.2570. [DOI] [PubMed] [Google Scholar]
- 2.Kim W, Jeong MH, Kim KH, et al. Long-term clinical and angiographic results of coronary stenting in diabetic patients. Korean Circ J. 2001;31:24–30. [Google Scholar]
- 3.Iakovou I, Schmidt T, Bozizzoni E, et al. Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. JAMA. 2005;293:2126–2130. doi: 10.1001/jama.293.17.2126. [DOI] [PubMed] [Google Scholar]
- 4.Colwell JA, Nesto RW. The platelet in diabetes: focus on prevention of ischemic events. Diabetes Care. 2003;26:2181–2188. doi: 10.2337/diacare.26.7.2181. [DOI] [PubMed] [Google Scholar]
- 5.Vinik AI, Erbas T, Park TS, Nolan R, Pittenger GL. Platelet dysfunction in type 2 diabetes. Diabetes Care. 2001;24:1476–1485. doi: 10.2337/diacare.24.8.1476. [DOI] [PubMed] [Google Scholar]
- 6.Ferroni P, Basili S, Falco A, Davi G. Platelet activation in type 2 diabetes mellitus. J Thromb Haemost. 2004;2:1282–1291. doi: 10.1111/j.1538-7836.2004.00836.x. [DOI] [PubMed] [Google Scholar]
- 7.Watala C, Golanski J, Pluta J, et al. Reduced sensitivity of platelets from type 2 diabetic patients to acetylsalicylic acid (aspirin): its relation to metabolic control. Thromb Res. 2004;113:101–113. doi: 10.1016/j.thromres.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 8.Mori TA, Vandongen R, Douglas AJ, McCulloch RK, Burke V. Differential effect of aspirin on platelet aggregation in IDDM. Diabetes. 1992;41:261–266. doi: 10.2337/diab.41.3.261. [DOI] [PubMed] [Google Scholar]
- 9.Gum PA, Jottke-Marchant K, Welsh PA, White J, Topol EJ. A prospective, blinded determination of the natural history of aspirin resistance among stable patients with cardiovascular disease. J Am Coll Cardiol. 2003;41:961–965. doi: 10.1016/s0735-1097(02)03014-0. [DOI] [PubMed] [Google Scholar]
- 10.Eikelboom JW, Hirsh J, Weitz JI, Johnston M, Yi Q, Yusuf S. Aspirin-resistant thromboxane biosynthesis and the risk of myocardial infarction, stroke, or cardiovascular death in patients at high risk for cardiovascular events. Circulation. 2002;105:1650–1655. doi: 10.1161/01.cir.0000013777.21160.07. [DOI] [PubMed] [Google Scholar]
- 11.Grotemeyer KH, Scharafinski HW, Husstedt IW. Two-year follow-up of aspirin responder and aspirin non responder: a pilot-study including 180 post-stroke patients. Thromb Res. 1993;71:397–403. doi: 10.1016/0049-3848(93)90164-j. [DOI] [PubMed] [Google Scholar]
- 12.Angiolillo DJ, Fernandez-Ortiz A, Bernardo E, et al. Platelet function profiles in patients with type 2 diabetes and coronary artery disease on combined aspirin and clopidogrel treatment. Diabetes. 2005;54:2430–2435. doi: 10.2337/diabetes.54.8.2430. [DOI] [PubMed] [Google Scholar]
- 13.Lee SW, Park SW, Hong MK, et al. Triple versus dual antiplatelet therapy after coronary stenting: impact on stent thrombosis. J Am Coll Cardiol. 2005;46:1833–1837. doi: 10.1016/j.jacc.2005.07.048. [DOI] [PubMed] [Google Scholar]
- 14.Lee BK, Lee SW, Park SW, et al. Effects of triple antiplatelet therapy (aspirin, clopidogrel, and cilostazol) on platelet aggregation and P-selectin expression in patients undergoing coronary artery stent implantation. Am J Cardiol. 2007;100:610–614. doi: 10.1016/j.amjcard.2007.03.070. [DOI] [PubMed] [Google Scholar]
- 15.Bhatt DL, Marso SP, Hirsch AT, Ringleb PA, Hacke W, Topol EJ. Amplified benefit of clopidogrel versus aspirin in patients with diabetes mellitus. Am J Cardiol. 2002;90:625–628. doi: 10.1016/s0002-9149(02)02567-5. [DOI] [PubMed] [Google Scholar]
- 16.Ryningen A, Olav Jensen B, Holmsen H. Elevation of cyclic AMP decreases phosphoinositide turnover and inhibits thrombin-induced secretion in human platelets. Biochim Biophys Acta. 1998;1394:235–248. doi: 10.1016/s0005-2760(98)00106-4. [DOI] [PubMed] [Google Scholar]
- 17.Defreyn G, Gachet C, Savi P, Driot F, Cazenave JP, Maffrand JP. Ticlopidine and clopidogrel (SR 25990C) selectively neutralize ADP inhibition of PGE1-activated platelet adenylate cylcase in rats and rabbits. Thromb Haemost. 1991;65:186–190. [PubMed] [Google Scholar]
- 18.Ahn JC, Song WH, Kwon JA, et al. Effects of cilostazol on platelet activation in coronary stenting patients who already treated with aspirin and clopidogrel. Korean J Intern Med. 2004;19:230–236. doi: 10.3904/kjim.2004.19.4.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cipollone F, Ciabattoni G, Patrignani P, et al. Oxidative stress and aspirin-insensitive thromboxane biosynthesis in severe unstable angina. Circulation. 2000;102:1007–1013. doi: 10.1161/01.cir.102.9.1007. [DOI] [PubMed] [Google Scholar]
- 20.Winocour PD, Watala C, Perry DW, Kinlough-Rathbone RL. Decreased platelet membrane fluidity due to glycation or acetylation of membrane proteins. Thromb Haemost. 1992;68:577–582. [PubMed] [Google Scholar]
- 21.Lee SW, Park SW, Kim YH, et al. Drug-eluting stenting followed by cilostazol treatment reduces late restenosis in patients with diabetes mellitus the DECLARE-DIABETES Trial. J Am Coll Cardiol. 2008;51:1181–1187. doi: 10.1016/j.jacc.2007.11.049. [DOI] [PubMed] [Google Scholar]
- 22.Park JS, Kim YJ. The clinical effects of cilostazol on atherosclerotic vascular disease. Korean Circ J. 2008;38:441–445. [Google Scholar]