Abstract
Atrial fibrillation is the most common cardiac arrhythmias, and a major cause of morbidity and mortality due to cardioembolic stroke. The left atrial appendage is the major site of thrombus formation in non-valvular atrial fibrillation. Loss of atrial systole in atrial fibrillation and increased relative risk of associated stroke point strongly toward a role for stasis of blood in left atrial thrombosis, although thrombus formation is multifactorial, and much more than blood flow irregularities are implicated. Oral anticoagulation with vitamin-K-antagonists is currently the most effective prophylaxis for stroke in atrial fibrillation. Unfortunately, this treatment is often contraindicated, particularly in the elderly, in whom risk of stroke is high. Moreover, given the risk of major bleeding, there is reason to be skeptical of the net benefit when warfarin is used in those patients. This work reviews the pathophysiology of cardioembolic stroke and critically spotlights the current status of preventive anticoagulation therapy. Various techniques to exclude the left atrial appendage from circulation were discussed as a considerable alternative for stroke prophylaxis.
Keywords: Atrial appendage, Atrial fibrillation, Thromboembolism, Stroke, Prostheses and implants, Prognosis
Introduction
Atrial fibrillation (AF) is the most common cardiac rhythm disturbance observed in clinical practice, and has numerous potential complications; of these, stroke is the most serious and life threatening. Prevalence of AF increases with age, up to 15% in octogenerians, and continues to grow rapidly due to the increasing proportion of aging in the population. Moreover, the elderly population of today has a higher prevalence of predisposing conditions for AF, such as diabetes, heart failure, hypertension, and coronary heart disease.1-6) Lifetime risk for development of AF is 1 in 4 for men and women 40 years of age and older.7)
Absence of a regular contraction of the fibrillating atria leads to an increase of atrial pressure and dilatation, which, together with hemoconcentration,8),9) endothelial dysfunction, and a prothrombotic state, is prerequisite for thrombus formation.10) Echocardiography and autopsy studies have shown that more than 90% of all thrombi in patients with AF originating in the left atrium form in the left atrial appendage (LAA).11-15) Consequently, LA thrombi are responsible for at least one fourth of ischemic stroke, which is more frequently associated with persistent and severe disability, compared to ischemic events attributable to vascular disease.16-19) Associated mortality is 27% in 12 months, and a five year recurrence rate of up to 34%.6) Socio-economical dimensions of this disease, therefore, are relevant.
The main risk of an embolic event in patients with atrial fibrillation is lack of adequate oral anticoagulation (OAC). Vitamin-K-antagonists (VKA) are highly effective for stroke prevention in patients with AF, but a substantial number of patients are not eligible for chronic therapy with coumadines due to their narrow therapeutic range and bleeding complications that are potentially fatal. Even eligible patients may have reservations about long-term OAC with VKA, due to the need for continuous laboratory monitoring and possible interactions with food, drugs, and individual lifestyle. Patients at high risk of embolic stroke, but with contraindications for OAC are in a need of an alternative approach that is not associated with long-term risk of hemorrhage or other attendant circumstances. This is particularly true for those who have survived intracranial hemorrhage but remain at high risk for cardiogenic embolism. A reasonable alternative may be exclusion of the LAA cavity from circulation, using either surgical or percutaneous catheter-based procedures. Currently, excision of the LAA at the time of mitral valve surgery is recommended for reduction of future stroke risk.20) Efficacy of LAA exclusion in patients undergoing elective coronary artery bypass graft surgery was shown in the LAA Occlusion Study (LAAOS).21)
In this review, we discuss pathophysiology of thrombogenesis in the LAA, and its consequential role in AF-related embolic stroke. Further emphasis is put on technical and clinical data from different percutaneous transcatheter devices for occlusion of the LAA for protection of cardiogenic embolism.
Anatomy and Function of the Left Atrial Appendage
The LAA is a long, tubular, often multilobed and trabeculated stucture, a remnant of the embryonic left atrium (LA), whereas the smooth LA cavity is derived from an outgrowth of the pulmonary veins.22) The orifice of the LAA is oval in shape, located between the left ventricle and the left upper pulmonary vein. The body of the appendage extends over the atrioventricular groove and the surface of the left ventricle, towards the left circumflex artery and the great cardiac vein in an anterior direction.23-25) LAA structure was initially characterized from 1,842 autopsy hearts.26) Anatomy of the LAA is complex and varies considerably in terms of volume (0.7-19.2 mL), length (16-51 mm), and size of the orifice (5-40 mm).25),27),28) The structure of the cardiac muscle cells of the LAA appears similar to that of the surrounding myocardium.29) The muscular mass of the LAA contains the majority of cardiac atrial natriuretic factor (ANF).30) ANF increases sodium excretion, diuresis, and natriuresis, and, thus, opposes the sodium-conserving actions of the renin-angiotensin-aldosterone system.31) Tabata et al.32) found that the most important factor increasing plasma concentration of ANF in patients with left-sided cardiac dysfunction is distension of the LAA wall. Moreover, stretch sensitive receptors within the LAA are involved in regulation of heart rate.33)
After primary ex vivo characterization of LAA anatomy, use of transoesophageal echocardiography (TOE) has made clear in vivo imaging of the LAA possible, so that its size, shape, flow patterns, and content can be assessed in health and disease.34-37) However, due to its variable and complex anatomy, standard definitions of tomographic imaging planes are nearly impossible. On the other hand, assessment of LAA function by Doppler echocardiography and detection of LAA spontaneous echocardiographic contrast (SEC) is of considerable clinical relevance. New insights into LAA anatomy, in terms of angulation and motility, have recently been provided by computed tomography, which also confirmed the echocardiographic finding of a broad interindividual variation in LAA morphology.38-40) Also, using magnetic resonance imaging, three dimensional analysis of the LAA is possible and will be of considerable interest in the future, due to avoidance of radiation for the patient.41)
In sinus rhythm, the LAA shortens to a greater extent than the rest of the left atrium and has a distinct pattern of contraction,34) although this seems to be of minor importance for overall cardiac performance.42) Considering emptying and filling waves, TOE Doppler flow within the LAA was described as quadriphasic in the majority of healthy subjects with sinus rhythm.43),44) In AF without thrombus, the LAA appears to empty passively and fill with multiple small fibrillatory contractions that do not contribute to LV filling. LA pressure is the essential determinant of LAA flow. Hence, the magnitude of LAA filling and emptying is influenced primarily by both left ventricular function and heart rate.43),44) Therefore, in a heart disease state with both increased atrial and left ventricular end diastolic pressure, the LAA may compensate for consequent volume overload due to its distensibility.45),46)
Thrombogenesis in Atrial Fibrillation
The LAA is the site most commonly associated with thrombus formation, particularly in patients with non-valvular AF.11-15),47),48) A multivariable analysis of cohorts followed prospectively in clinical trials and other care settings revealed that thrombi have been identified using TOE in 15-20% of patients with AF who have clinical risk factors for ischemic stroke.12),48) Pathogenesis of LAA thrombus formation has not been fully elucidated, but the precondition is likely to result from a hypercoagulable state explained by Virchow's triad of thrombogenesis i. e., abnormal changes of the vessel wall, blood flow, and blood constituents.49),50) Nowadays, this is translated as follows: "Abnormal blood flow" refers to reduced flow up to stasis due to lack of contraction in combination with increased volume and size of the LAA; "abnormal blood constituents" are represented by activated coagulation factors and platelets, and "abnormal vessel wall" in this case refers to structural and functional changes of endothelial or endocardial cells.
Abnormal blood flow
Volume and size of the LAA increases in atrial fibrillation in what is termed as atrial remodeling.34),38),48),51) Larger LA and LAA sizes are associated with lower LAA flow velocity47) and risk of ischemic stroke.52),53) Diminished contractility of the appendage understandably leads to reduction of blood flow as well,34),42) which is associated with increased thrombogenicity within this trabeculated blind spot.50),54),55) Notably, risk of ischemic stroke due to diminished LAA flow appears not to be related to the underlying cardiac rhythm which led to the consideration of LAA velocity as a surrogate parameter for risk stratification.56) Under conditions of low LAA blood flow, SEC may occur on TOE47),48),50),56-59) in strong association with LAA thrombus formation and systemic embolism.15),52),60-64) SEC is thought to be related to an intensified interaction between fibrinogen and erythrocytes,65) but a low level of hemoglobin is not associated with lower prevalence of SEC when controlled for clinical and echocardiographic variables.66) Density of SEC increases and LAA velocities show significant and progressive decline together with accumulation of clinical risk factors for stroke, as evaluated by the CHADS2 score (see below).67) Furthermore, there is a high likelyhood of cerebral embolism and death, despite anticoagulant therapy, in patients with low LAA emptying velocity and dense SEC.68),69) It is important to note that anticoagulation does not influence the presence of SEC because it does not change underlying hemodynamic abnormalities. In chronic congestive heart failure (CHF), there is a negative correlation between LAA emptying velocity, LV ejection fraction, and LV end-diastolic pressure, possibly explaining the increased incidence of stroke in patients with atrial fibrillation and CHF.70-74) Furthermore, independent predictors for the presence of thrombus and dense SEC included left ventricular ejection fraction <40% and left atrial dimension >50 mm.75)
Abnormal blood constituents
Presence of a LA thrombus is a result of a dynamic process of coagulation activation and fibrinolysis. Therefore, a high fibrin turnover is a cause rather than a consequence of cardiogenic embolism in patients with AF, in whom laboratory markers of both activated coagulation and impaired fibrinolysis can be found.49) Prothrombin fragments 1+250),76) and thrombin-antithrombin-complexes (TAT),50),57) as a measure of thrombin generation, as well as markers for platelet activation,50) are increased in AF. Elevated D-dimer plasma levels indicating increased fibrin formation and degradation50),57),76-79) are independently predictive of the presence of LAA thrombi on TOE,80) and, most importantly, are associated with future thromboembolic events.72),81) Initiation of OAC reduces D-dimer values;79),82) however, in patients with thromboembolism, these values remain at a higher level compared to those without events.72) This led to the assumption that, comparable to the diagnostic algorithm in deep vein thrombosis/pulmonary embolism, D-dimers could be helpful in predicting the absence of LAA thrombi in patients with AF.80),83),84) Activation of coagulation is directly related to diminished LAA function, in that an inverse correlation was shown between both LAA flow velocities and LAA diameter, and TAT as well as D-dimer.57),85),86) Tissue factor expression induced by local inflammation is involved in pathogenesis of thrombosis in patients with nonvalvular atrial fibrillation.87) Levels of coagulation activation markers also rise, together with the number of risk factors for stroke.88-90) SEC visible on TOE shows a significant correlation to prothrombin fragments 1+2, D-dimer, and TAT.91)
Studies on the fibrinolytic system in AF are few, and conflicting results regarding clinical relevance have been reported, which may be due to the natural course of the fibrinolytic response to coagulation activation. Hyperfibrinolysis as a consequence of a strong coagulation stimulus is more likely in early inflammatory states than in later or chronic states, where predominance of inhibitors leads to hypofibrinolysis.92) Tissue type plasminogen activator (tPA) plays a crucial role in inititation of fibrinolysis and can be found in elevated levels in AF.93) Plasmin is the most important component of the fibrinolytic system generated in response to thrombin formation, but it is rapidly inactivated by α2-antiplasmin. The activator-inhibitor-complex (plasmin-α2-antiplasmin; PAP) has a short half-life in plasma, and therefore indicates recent fibrinolytic activity.94) In a subgroup of patients enrolled in the Stroke Prevention in Atrial Fibrillation (SPAF) III study, PAP levels in patients with AF were associated with clinical characteristics predictive of thromboembolism, including older age and reduced LV function.95) PAP levels are also elevated in acute stroke96) and myocardial infarction,97) and may therefore indicate nonspecific cell damage, inflammation, and endothelial dysfunction. The same is true for both tissue plasminogen activator (t-PA) and its inhibitor (PAI-1),72),98) which can be correlated with severity of inflammatory disorders.99)
The significance of platelet activation in atrial fibrillation is uncertain and may also be seen in the light of a nonspecific inflammatory reaction. Procoagulant membrane vesicles derived from activated platelets, known as microparticles, are elevated in patients with conditions that are associated with atrial fibrillation (e. g., hypertension, coronary artery disease, diabetes, stroke).100) Soluble sP-selectin is well known as a marker of platelet activation and is elevated in the plasma of patients with AF.50),74),101-105) Recently, Choudhury et al.100) found that both AF patients and disease control subjects had significantly higher levels of platelet derived microparticles and sP-selectin compared to healthy control subjects; however, there was no difference between AF patients and disease control subjects. β-thromboglobulin is another platelet-specific protein that indicates activation and another examplary representative of flow-dependent hemostatic activation, as it was found to be highest in patients with the lowest LAA flow velocities and the greatest atrial dilatation.85) Plasma levels of β-thromboglobulin are dependent on duration of AF, and showed a significant increase 12 hours after onset of paroxysmal AF.106) It is worthy of mention that results concerning the effect of antithrombotic treatment on markers of platelet activation in AF have been uncertain. In studies by Kamath et al.107),108) treatment with warfarin or aspirin either failed to demonstrate any significant benefit on platelet activation (β-thromboglobulin, sP-selectin), or showed an effect in favor of aspirin on the absolute amount of P-selectin per platelet in patients with AF.108) The clinical significance of this finding remains to be elucidated. Likewise, the prognostic relevance of platelet activation in terms of future thromboembolic events is ambivalent. A substudy of the SPAF III trial revealed no association,103) whereas the Rotterdam study found plasma levels of sP-selectin to be predictive of clinical adverse outcomes in AF.104) On the whole, evidence of platelet activation in AF patients seems most likely due to underlying cardiovascular disease, rather than arrhythmia per se.
Abnormal endothelium and endocardium
Further insight into the hypercoagulable state in atrial fibrillation is provided by studies of von Willebrand factor (vWF), a hemostatic mediator involved in platelet aggregation and clot stabilization, derived from endothelial cells and thrombocytes.109) Endothelial activation or disturbance is indexed by elevated plasma levels of vWF.110) Increased plasma vWF can be found in patients with AF,50),76),78),79),86),105),111) and tends to increase further with concomitant heart failure.74) Most important, the level of vWF is intimately related to recognized independent risk factors for stroke (heart failure, age, diabetes, previous stroke) and might itself be predictive of future stroke.102),112),113) As expected, increased plasma levels of vWF were found to be associated with presence of LAA thrombus, visible by TOE,50) whereas Fukuchi et al.114) found a significant correlation between degree of endocardial expression of vWF and extent of platelet adhesion/thrombus formation in the LAA. Endocardial overexpression of vWF may occur during the process of atrial structural remodeling in chronic AF.115),116) These structural changes also affect the extracellular matrix117),118) and, therefore, the system of matrix metalloproteinases (MMP). There are hints to a link between the MMP system and a prothrombic state.119)
Increased levels of circulating endothelial cells (CEC) have been demonstrated in conditions associated with endothelial damage.120) Freestone et al.111) found that CEC levels in patients with AF and an acute cardiovascular or cerebrovascular event were significantly elevated compared to patients with stable, chronic AF. Therefore, in the interim balance, it can be stated that AF itself is the major contributory factor to thrombogenesis, which implies activation of the coagulation cascade, rather than platelets, and is the key to excess thromboembolic risk in AF.
Stroke-Risk Asessment in Atrial Fibrillation
Risk of stroke varies considerably among patients with AF. On the one hand, individual risk for stroke must be considered prior to prescription of VKA for anticoagulation; on the other hand, risk of stroke must be outweighted against the risk of bleeding and burden on the patient due to the need for continuous laboratory INR monitoring or possible interactions associated with food, drugs, and individual lifestyle or preferences.121) Several stroke risk stratification schemes are available to help clinicians with this decision.68),122-125) Two useful resources stand out in clinical practice: The Framingham risk score, derived by Wang et al.,68) and the CHADS2 score published by Gage et al.123) Both use a five step calculation to predict the risk for stroke in patients with AF. The former considers age, gender, systolic blood pressure, diabetes, and prior stroke or TIA. Each category is assigned different grading, and predicts 5-year stroke risk in the absence of anticoagulation. Concerning the latter, the C stands for recent congestive heart failure, the H for hypertension, the A for age 75 or older, the D for diabetes, and the S for prior stroke or TIA. Each category is assigned one point, except for stroke or TIA, which receives two points due to high association with subsequent stroke. A high score on this index correlates with raised annual stroke rate. The CHADS2 score may be easier to use, but is less precise.126)
The Effect of Cardioversion
One of the theoretic benefits of cardioversion in patients with AF is the assumption that restoring normal atrial electromechanical activity may diminish the risk of cardiogenic thromboembolism and, therefore, spare the need for anticoagulation. However, there is a body of evidence to demonstrate that a temporary worsening of LAA function ("stunning") after cardioversion is responsible for development of new clots.127),128) The hallmark of LAA stunning is reduction of post cardioversion LAA flow velocity in sinus rhythm compared to those in AF, regardless of iatrogenic (electrical or pharmocological) or spontaneous cardioversion.129),130) Even after succesful restoration of sinus rhythm, SEC can be seen in up to 37% of patients after 3 months,131) indicating the persistence of a hypercoagulable state after cardioversion.78),132) The strategy of rhythm control has been directly compared with simple rate control in several randomized clinical trials. In a pooled analysis, frequency of ischemic stroke in the group of patients assigned to rate control was comparable to that of patients assigned to rhythm control.133) It is amazing, therefore, that Cox et al.134) reported on 306 patients who underwent the maze procedure for treatment of medically refractory atrial fibrillation. Only 2 perioperative strokes occurred, and in 265 patients followed up to 11.5 years after the maze procedure, there was only one late minor stroke, which has since been completely resolved. On the one hand, this good result may be explained by predominance of sinus rhythm, and, on the other hand, by the absence of the LAA and restoration of left atrial mechanical function.135)
Hypothetically, restoring mechanical activity to the LAA with cardioversion may also result in systemic embolism due to wash out of pre-existing LAA thrombi, which may explain the occurence of stroke shortly after treatment.136),137) The Assessment of Cardioversion Using Transesophageal Echocardiography (ACUTE) trial138) compared a TOE-guided strategy combined with short-term anticoagulation using a conventional 3-week oral anticoagulation pre cardioversion strategy. Although there was significant difference in the composite end point of major and minor bleeding and a shorter time to cardioversion, there was no difference in the composite end point of stroke, transient ischemic attack, and peripheral embolism. Therefore, current guidelines for cardioversion in patients with atrial fibrillation lasting longer than 48 hours recommend coumadin treatment for at least three weeks prior to cardioversion, and for a minimum of four weeks afterward. Alternatively, performance of a cardioversion without anticoagulation is justifiable if direct previous TOE shows no thrombi present in the LAA.121)
Cardiac Imaging for Thrombus Detection
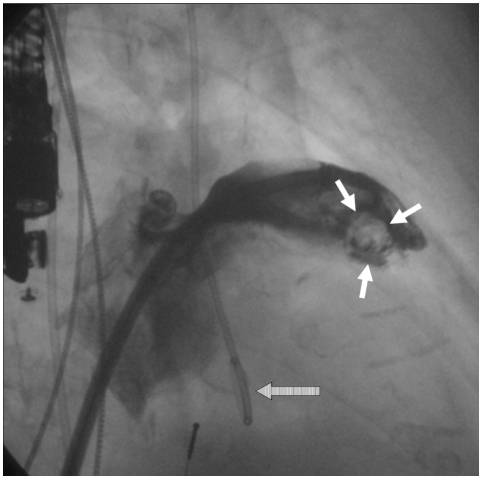
During the past thirty years, TOE has become a valuable tool for diagnosis of thrombus within the appendage by allowing semi-invasive, highly accurate imaging of the LAA.139) It is, therefore, an essential part of the guidelines for management of AF,121) and the modality of choice for detecting LA or LAA thrombi with a sensitivity and specificity of approximately 95% to 100%.127) Nevertheless, detection of LAA thrombus is prone to misdiagnosis because clots may remain hidden due to the three-dimensional complexity of the LAA, and a false-positive diagnosis of thrombus may stem from false interpretation of a prominent pectinate muscle. The aforementioned sensitivity and specificity regarding thrombus detection by TOE was found in comparison with intraoperative observations;13) however, no comparison has been made using direct left atrial angiography. Sensitivity for thrombus detection during the left ventricular phase of pulmonary angiogram was found to be 100% compared to later surgical inspection.140) In our own experience with transcatheter LAA occlusion and by means of direct angiography, we detected LAA thrombi not seen with simultaneously performed TOE in approximately 10% of patients (Fig. 1).141) Furthermore, we revealed additional LAA lobes in some patients during repeated LAA angiography. The "collapsed" lobes seem to be "blown up" by contrast injections into the LAA. Consequently, in these cases, the procedure was halted and postponed, causing inconvenience and additional risk for patients, as well as increased overall treatment costs. Furthermore, this finding strengthens the hypothesis of cardiac embolism due to wash out of pre-existing LAA thrombi after successful cardioversion. Hence, an imaging modality with higher accuracy of preprocedural LAA thrombus detection would be very helpful for planning of LAA occlusion procedures. Further technical development of echocardiography seems promising. Recent introduction of an RT-m3D TOE transducer permits virtually instantaneous 3D imaging while preserving spatial and temporal resolution, thereby significantly enhancing visualization of complex 3D structures such as the LAA.142) Further improvement of B-mode image quality of 3D-TOE will be available in the near future. The combination of tissue doppler imaging and use of contrast agents may help to better characterize thrombogenic structures within the LAA.58) Also, with respect to LAA imaging, few studies using MRI and spiral CT scan have been reported on detection of thrombus and SEC.38),39),143-146) Regarding LAA occlusion procedures, the advantages of cardiac CT/MRA include the following: 1) preprocedural imaging of the anatomical characteristics of the LAA and neighbouring atrial structures; 2) assessment of the anatomical relationship of the LA, esophagus, and adjacent vascular structures; 3) postprocedural detection of structural and functional changes of the LA and LAA.147)
Fig. 1.
Angiographic contrast filling defect in the contast shadow of the LAA (white arrows) indicating a spherical thrombus, which was not diagnosed by TOE. Note the TOE probe at the left margin and the loop of the pigtail catheter (striped arrow), indicating the position of the aortic valve. LAA: left atrial appendage, TOE: transoesophageal echocardiography.
Oral Anticoagulation: Therapeutic Gold Standard in Atrial Fibrillation
Of the VKAs, warfarin is the most widely used and investigated coumarin derivative, exerting its anticoagulant effect through modulation of c-carboxylation of glutamic acid (GLA) residues of the vitamin K-dependent coagulation factors II, VII, IX, and X, resulting in production of coagulation factors with reduced coagulant capacity.148),149) Dose adjusted (INR 2.0-3.0) OAC is well established in patients with nonvalvular AF, and has been associated with a decreased risk of cardioembolic events of greater than 60%.150),151) Effectiveness of OAC depends on intensity of treatment,77) in that insufficient doses of anticoagulants reduce the therapeutic benefit, while excessive anticoagulation increases the risk of bleeding.152) The benefit of dose adjusted OAC is confirmed by a significant decrease of markers of coagulation activation,72),79) whereas fixed low-dose warfarin or aspirin-warfarin combination treatment did not substantially reduce markers of thrombogenesis.153) Despite the proven benefit, OAC with warfarin or other VKA remains underused in clinical practice,154) although underutilization implies that a decision not to use warfarin is reached after assessment of the balance between benefit (prevention of thromboembolic stroke) and risk (bleeding), which suggests a net benefit. With regard to anti-thrombotic therapy, there are two groups of AF patients: Those with no contraindications for therapy who never received warfarin, and those who are on therapy, but with an INR below or above the range of 2.0-3.0, having increased risk for either stroke or bleeding. Overall, 55% of warfarin-eligible patients actually use the drug, with the lowest rates seen in the oldest patients-those at highest risk of stroke.155),156) In the United States, AF patients on warfarin spend only about one-half of the time within therapeutic INR range,157-159) and of those patients admitted to hospital with a stroke while receiving warfarin therapy, most have subtherapeutic international normalized ratios.160),161) Leckey et al.162) found that only 13% of patients with ischemic stroke and known AF before stroke were taking warfarin. Considering the worst case scenario, that half of patients are untreated and the other half are out of range 50 percent of the time, only one fourth receive optimal treatment.
Review of the literature has identified several barriers to prescription of VKA, which are related to the patient, the physician, and the health care system.163) One of the strongest patient related predictors of warfarin withholding is age. Warfarin use increases with a history of ischemic stroke, and decreases with age >80 years. The most important physician related reasons not to anticoagulate include 1) the perception of benefit vs. risk of therapy, insofar as the risk for embolism, relative to hemorrhage, is judged to be lower, and 2) the relative contraindication to therapy due to lack of patient reliability or patient noncompliance as a reason for difficulties in monitoring the prothrombin ratio.163)
Acetylsalicylic acid (ASA, Aspirin) as an anticoagulant has some advantages over warfarin. These include substantially less potential for drug-drug or drug-diet interactions, a wider therapeutic index, and no need for INR-monitoring. For AF patients with increased risk of bleeding, it is often prescribed instead of warfarin on the presumption that it is safer. A meta-analysis of six randomized controlled trials suggests that ASA does reduce the risk for ischemic stroke in AF (22% relative risk reduction for ASA compared with placebo).12),164) It is noteworthy that only one of these trials {the Stroke Prevention in Atrial Fibrillation (SPAF) study165)} reported a statistically significant difference. Recently, the Birmingham Atrial Fibrillation Treatment of the Aged Study, BAFTA,166) reaffirmed that warfarin is superior to ASA in stroke prevention. On the other hand, the risk of major hemorrhage, including hemorrhagic stroke, was similar in both aspirin (2.0% per year) and warfarin (1.9% per year) treated patients. The authors recommended use of anticoagulation (warfarin) for all people over 75 years who have atrial fibrillation, unless there are contraindications, or the patient decides that the size of the benefit is not worth the inconvenience of the treatment.165)
Bleeding Risk Under Oral Anticoagulation
The emphasis on avoidance of hemorrhagic stroke or traumatic intracranial hemorrhage and other iatrogenic events may cause physicians and patients to choose therapy that minimizes side effects, rather than therapy that maximizes benefit. In association with anticoagulation, bleeding is the complication of greatest concern; therefore, selection of appropriate therapy should weigh advantages and disadvantages carefully.167) In anticoagulated patients, annual risk of major bleeding, meaning a hemorrhage requiring >2 units of blood or requiring hospitalization, ranges between 1.1-1.7% and 0.3-0.6% for intracranial hemorrhage.124),168) However, this low level of risk has rarely been replicated in contemporary studies, and then, perhaps, only in patients who are stable on long-term anticoagulation. Higher rates of hemorrhage were found in the Stroke Prevention in Atrial Fibrillation II Study, which consisted of 2 parallel trials of patients aged >75 years and ≤75 years.169) Annual rates of major bleeding were 4.2% and 1.7%, respectively. Hylek and coworkers170) studied a cohort of 472 patients of whom one third were ≥80 years of age, compared with a total of 20 patients >75 years in the pooled analysis of 5 randomized trials that proved efficacy of anticoagulation,150) and 91% had ≥1 stroke risk factor. Cumulative incidence of major hemorrhage for patients ≥80 years of age was 13.1 per 100 person-years and 4.7 for those <80 years of age (p=0.009). Thus, the increasing risk of bleeding with increasing age is clearly demonstrated in this study. Moreover, during the first 90 days of warfarin treatment, age ≥80 years and international normalized ratio (INR) ≥4.0 were associated with increased risk, despite trial-level anticoagulation control. Within the first year, 26% of patients who were ≥80 years of age stopped taking warfarin. Perceived safety issues accounted for 81% of them. The most obvious results to be emphasized from this study are that the risk of major hemorrhage increases by a factor of 10-fold between CHADS2 scores of 0 and ≥4, and also that warfarin termination was highest among patients with CHADS2 scores ≥3.170) The finding that bleeding risk is highest within the first year of therapy was confirmed by results from the ACTIVE-W study: annual risk of major hemorrhage over the duration of the study is quoted as 2.6% and 2.0% for warfarin-naive and warfarin-experienced patients, respectively. In contrast, the risks are more like 6% to 7% and 4%, respectively, in the first year.171) Moreover, an ancillary analysis from the ACTIVE-W trial reports that risk of major bleeding during OAC was lower among patients with a CHADS2 score of 1 (1.36% per year) compared with CHADS2 >1 (2.75% per year).172)
The following patient characteristics are considered risk factors for anticoagulation-related bleeding complications: Advanced age, uncontrolled hypertension, history of myocardial infarction or ischemic heart disease, cerebrovascular disease, anemia or a history of bleeding, and concomittant use of other drugs, such as antiplatelet agents.173) Moreover, primary clinical and health-related problems associated with typical geriatric syndrome in older adults, and which make the decision regarding use VKA more complex, include functional decline, frailty and falls, polypharmacy, nutritional deficiencies, and cognitive dysfunction.167) All of these conditions are often cited as reasons to preclude the elderly from anticoagulation.174) A prospective observational study of 207 AF patients in an acute-care setting hospital, assessing frail and non-frail patients, demonstrated that frail patients were less likely to receive warfarin, both on hospital admission and on discharge. Notably, such patients at the same time were at greater risk of experiencing embolic stroke (12.3% vs. 3.9% in frail and non-frail patients). Aronow et al.175) described 312 AF patients with an average age of 84 years residing in a chronic care facility. Rates of stroke over three years in those not anticoagulated were 56% in those with no prior history of thromboembolism and 81% in those with prior history of thromboembolism. These frail patients also had a tendency to sustain a greater risk of major/severe hemorrhage, as well as greater mortality.176)
Comparable to existing stroke risk stratification schemes, there are bleeding risk models:177-182) Beyth and coworkers178) identified four independent risk factors for bleeding: Age ≥65 years, history of GI bleeding, history of stroke, and one or more of four specific comorbid conditions. They found a cumulative incidence of major bleeding at 48 months of 53% in high-risk paitients (three or four risk factors), 12% in middle-risk patients (one or two risk factors), and 3% in low-risk patients (no risk factors). Kuijer and colleagues179) developed another prediction model based on age, gender, and presence of malignancy. In patients classified at high, middle, and low risk, frequency of major bleeding was 7%, 4%, and 1%, respectively, after 3 months of therapy. Comparable bleeding rates for comparable risk classes were found by Shireman et al.181) (5.4%, 2.0%, and 0.9%, respectively, after 90 days of treatment) using the following criteria: Age >70 years, gender, remote bleeding, recent bleeding, alcohol/drug abuse, diabetes, anemia, and antiplatelet use. Gage et al.182) gave 2 points for a prior bleed and 1 point for each of 10 further stroke risk factors, and claimed the highest accuracy of all other bleed prediction schemes. Nevertheless, none of these risk prediction schemes has been fully validated in large prospective AF patient cohorts.
The outlined clinical complexity of pathophysiology and prevention of thromboembolism in AF patients is most impressively illustrated by the fact that those at highest risk of stroke, and, therefore with the greatest need for antithrombotic therapy, also experience the most bleeding. Thus, an alternative that combines high efficacy in stroke prevention with low risk of bleeding is warranted.
Left Atrial Appendage Occlusion for Stroke Risk Reduction
The frequency of thrombus formation in the LAA of patients with AF and its suspected role as a source of embolism led to the hypothesis that resection or obliteration of the LAA might reduce the risk of stroke. Hellerstein et al.183) was the first to show the feasibility of LAA resection in dogs. The first resection of the LAA for prophylaxis of recurrent arterial embolism in men was performed in 1949 by Madden,184) followed by Beal et al.185) in 1950. Johnson and coworkers performed atrial appendectomies in 437 patients during cardiac surgery. No strokes were attributed to AF, and no patients were found to have atrial clots on TOE during follow-up.4) Odell and coworkers186) demonstrated in dogs and human cadavers that thoracoscopic exclusion of the LAA using either a stapler or an endoloop is also feasible and effective; Blackshear and colleagues187) evaluated left atrial appendage obliteration in 14 high-risk patients with atrial fibrillation who had clinical risk factors for stroke and an absolute contraindication to or failure of prior thrombosis prevention with warfarin. One fatal stroke occurred 55 months after surgery, and one non-disabling stroke occurred three months after surgery. For the LAA Occlusion Study,21),188) 77 patients with risk factors for stroke were randomized to either LAA occlusion or control (52 patients for LAA occlusion, 25 patients in the control group) at the time of coronary artery bypass graft (CABG). Two patients (2.6%), both randomized to the LAA occlusion group, had perioperative thromboembolic events: One had an intraoperative ischemic stroke, and the other a TIA occurring on the third postoperative day. The former patient was in AF and had echocardiographic evidence of a patent foramen ovale and bilateral carotid stenoses. After a mean follow-up of 13±7 months, no further strokes or TIAs occured in the LAA occlusion group.21) Nonetheless, surgical or thoracoscopic LAA closure, other than as an adjunctive procedure, as recommended by ACC guidelines20) in patients undergoing mitral valve surgery, has not been enthusiastically accepted due to its invasive nature. Thus, based on surgical experience, development of a less invasive percutaneous approach to close the LAA by implantation of a mechanical device was a logical consequence.189),190)
The Percutaneous Left Atrial Appendage Transcatheter Occlusion system
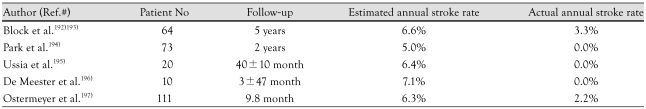
The Percutaneous Left Atrial Appendage Transcatheter Occlusion (PLAATO) (EV3, Inc., Plymouth, MN, USA) device was the first to be successfully deployed for use in humans,191) but was withdrawn from the market by the manufacturer in 2006. The PLAATO system consists of an implant and a delivery catheter. The implant is a self-expanding nitinol cage covered with an occlusive expanded polytetrafluoroethylene membrane. The expanded membrane has intimate contact with the inner wall of the appendage, so that complete closure of the ostium can be achieved. The diameter of the nitinol cage ranges from 15 to 32 mm. Small hooklets along the struts and passing through the membrane assist with device anchoring. The device was delivered through a custom 12 Fr transseptal sheath curved to point at the left atrial appendage.
Several reports demonstrate efficiacy in stroke prevention using PLAATO (Table 1).
Table 1.
Currently published data for stroke prevention using the PLAATO system
PLAATO: Percutaneous Left Atrial Appendage Transcatheter Occlusio
However, related serious adverse events occurred in every patient cohort procedure; these included vessel perforation during vascular access and cardiac tamponade after transseptal puncture. Some patients experienced pericardial effusions, which were mostly uneventful, but also lead to pericardiocentesis and a prolonged hospital stay. The worst case in our series was a periprocedural death due to device embolization, resulting in acute occlusion of the left ventricular outflow tract. Implant anchoring in another patient appeared unstable in LA angiography, so that the device was explanted, and the LAA of the patient was occluded by open heart surgery on the catheter table.194) Our LAA occlusion program was halted following these disastrous cases, and extensive revision was undertaken for all 73 prior implantations. As a result, we discovered a rotation of the device at the moment of release from the delivery system in a significant number of cases which resulted in loss of contact between parts of the anchor rows and the LAA wall (unpublished observation). One further theoretical concern was de novo formation of thrombi on the atrial surface of the implant which, to the best of our knowledge, has never been reported on the basis of clinical data. Nevertheless, there are conflicting results concerning formation of neo-endothelium on the device, which is the prerequisite to absence of thrombogenicity. Post mortem analysis by Omran et al.198) demonstrated a PLAATO device completely covered by neoendothelium on the atrial surface one year after implantation, whereas we found no endothelialization of the luminal side 2.5 years after LAA occlusion.199)
The Watchman Left Atrial Appendage system
The second device specifically designed for percutaneous transcatheter LAA exclusion is the Watchman Left Atrial Appendage System (Atritech Inc., Plymouth, MN, USA). This three-part system consists of a transseptal sheath, a delivery catheter, and an implantable device. The implant is a selfexpanding nitinol frame structure with fixation barbs and a permeable polyester fabric that covers the atrial side, and is available in diameters ranging from 21-33 mm. The device has been implanted since 2002 in Europe and since 2003 in the United States.200) Two patients experienced device embolization; both implants were successfully retrieved percutaneously. Five pericardial effusions (two of them needing pericardiocentesis) and one major air embolism occurred without long-term sequelae. The WATCHMAN Left Atrial Appendage System for Embolic PROTECTion in Patients With Atrial Fibrillation (PROTECT AF) study was designed to demonstrate safety, efficacy, and non-inferiority of the WATCHMAN device against chronic warfarin therapy in patients with nonvalvular atrial fibrillation who are eligible for long-term OAC.201),202) Of 707 patients enrolled, 463 were randomly assigned to LAA closure and 244 to warfarin therapy. The device was successfully implanted in 91% of the patients in whom it was attempted. Patients were followed for an aggregate of 1,065 patient-years. After 6 months, 355 (92%) of patients with an implanted device were able to discontinue warfarin therapy. For the control group, plasma warfarin concentration was in the therapeutic INR range (between 2.0 and 3.0) 66% of the time. Rate of ischemic stroke was higher in the intervention group than in the control group. Five patients had periprocedural events, mainly air embolism. After the periprocedural timeframe, ischemic stroke occurred in nine patients in the intervention group, compared with six patients in the control group. In both groups, all ischemic strokes that had INR measurements available at the time of the event occurred at a subtherapeutic INR level. Hemorrhagic strokes were less frequent in the intervention group than in the control group. Five of the six hemorrhagic strokes in the control group were fatal, and all occurred in patients with therapeutic INR levels. Device embolisation occurred in three patients; one was noted during the procedure and two were discovered by TEE on day 45. One device embolisation was removed percutaneously by use of a vascular snare; the other two patients underwent surgery, one of whom had concomitant aortic valve replacement. Endpoint data of adverse events are shown in Table 2.202)
Table 2.
Results of the PROTECT-AF-Study202): adverse events
*Defined as the need for percutaneous or surgical drainage. †Major bleeding is defined as a bleeding event that required at least 2 units of packed red blood cells or surgery to correct. ‡Of the seven hemorhagic strokes, six resulted in death (intervention group, n=1; control group, n=5). §An oesophageal tear and a procedurerelated arrhythmia
Nevertheless, this initial study shows substantial drawbacks: 12.3% of patients had serious procedural complications and 2.2% of attempted implantations resulted in cardiovascular surgical intervention due to device-related complications. Therefore, a substantial learning curve must be considered in association with device implantation. The primary efficiacy estimate of the PROTECT-AF-study is less precise, due to the small number of participants, and nearly 30% of patients receiving devices had a CHADS2 score of 1, and were therefore candidates for aspirin therapy without warfarin, even without LAA occlusion.203)
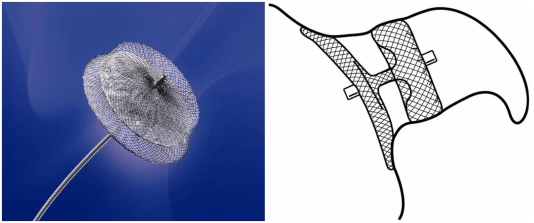
The AMPLATZER Cardiac Plug system
The AMPLATZER Cardiac Plug (ACP) is a transcatheter, self-expanding device constructed from a nitinol mesh and polyester patch (Fig. 2). The ACP consists of a lobe and a disc connected by a central waist. It is available in eight diameter sizes (16, 18, 20, 22, 24, 26, 28, and 30 mm). The lobe has stabilizing wires to improve device placement and retention. The device has threaded screw attachments at each end for connection to the delivery and loading cable. Radio-opaque markers at each end and at the stabilizing wires assist with fluoroscopic positioning. The ACP is a further development based on the AMPLATZER double-disk septal occluder, which was designed for closure of atrial septal defects and patent foramen ovale. In principle, this device can also be used for occlusion of the LAA; however, results of a feasibility trial were disappointing, as an embolization occured in one of 16 patients.204) The currently unpublished initial experience with ACP is encouraging. Its implantation is rather demanding technically, however, the disadvantages and risks associated with use of the PLAATO system are apparently eliminated (Fig. 3). A multicenter prospective registry trial to evaluate technical and short term success is approaching.
Fig. 2.
The AMPLATZER Cardiac Plug (ACP). On the right, the ideal position within the LAA is sketched. The lobe of the device is anchored in the "landing zone" 1-2 cm distal of the LAA orifice, while the disc fully covers the outer shape and enables endothelialization from the surrounding atrial wall. These images were provided by, and are property of AGA, Inc., Minneapolis, MN, USA. LAA: left atrial appendage.
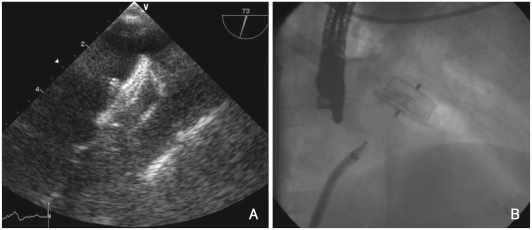
Fig. 3.
Images of the AMPLATZER Cardiac Plug (ACP) in situ. A: TOE. B: fluoroscopy after implantation.
Concerns Over Left Atrial Appendage Closure
Controversy persists in regard to the risks and benefits of LAA occlusion for prevention of embolic stroke. Adverse hemodynamic and physiological effects may result from LAA obliteration.205) Fluid retention is a potential late complication of LAA occlusion. Human atrial appendages contain 30% of total cardiac ANF.30) Experimental data have shown that bilateral appendagectomy in dogs eliminates ANF release and blunts renal excretion of sodium and water during acute volume load.206) Clinically evident postoperative fluid retention after the maze procedure with bilateral appendagectomy has been reported.207) Another study of the maze procedure reported on diminished ANF secretion accompanied by increased need for postoperative diuretics and dopamine.208) However, this effect was abolished when the right atrial appendage was preserved.209) There are clues, however, that in the natural course of permanent atrial fibrillation, atrial degeneration may at least lead to decreased ANF secretion.210) However, to date, many cases of surgical LAA exclusion with long term follow-up have not shown deleterious results. Pathophysiological consequences of implanting a foreign body into the LAA remain to be fully elucidated. Small iatrogenic atrial septal defects can be created after transseptal puncture. They usually disappear within 6 months of the procedure. Furthermore, every implanted foreign material bears the risk of infection.211)
Conclusion
AF is known to confer a risk of stroke; however, this risk is not homogeneous. Chronic oral anticoagulation as the prophylactic measure of choice has a number of major limitations associated with its narrow therapeutic range. A great deal of overlap evidently exists in thromboembolic stroke risk and risk of bleeding. Such overlapping risk creates a difficult management problem. Given the demonstrated risk of major bleeding, there is reason to be skeptical about net benefit when warfarin is used in some elderly patients with AF. Whether or not the needs of high-risk patients can be met by newer pharmacological and nonpharmacological antithrombotic/antiembolic therapies remains to be determined. It would seem that risk stratification schemes must be refined to incorporate data available from imaging studies that enhance predictive value for ischemic events and risk factors for bleeding. Occlusion of the LAA orifice, therefore, offers a theoretically appealing way to reduce incidence of stroke in patients who cannot be anticoagulated, or who developed stroke despite being on OAC. Nevertheless, there are limitations to this approach in that it cannot be easily applied prophylactically to large numbers of patients. Concerns about procedural safety and need for long-term follow up should be addressed before this potentially important technology is widely deployed.
References
- 1.The Stroke Prevention in Atrial Fibrillation Investigators. Predictors of thromboembolism in atrial fibrillation: I. clinical features of patients at risk. Ann Intern Med. 1992;116:1–5. doi: 10.7326/0003-4819-116-1-1. [DOI] [PubMed] [Google Scholar]
- 2.Falk RH. Atrial fibrillation. N Engl J Med. 2001;344:1067–1078. doi: 10.1056/NEJM200104053441407. [DOI] [PubMed] [Google Scholar]
- 3.Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the Anticoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 4.Johnson WD, Ganjoo AK, Stone CD, Srivyas RC, Howard M. The left atrial appendage: our most lethal human attachment!: surgical implications. Eur J Cardiothorac Surg. 2000;17:718–722. doi: 10.1016/s1010-7940(00)00419-x. [DOI] [PubMed] [Google Scholar]
- 5.Kannel WB, Benjamin EJ. Status of the epidemiology of atrial fibrillation. Med Clin North Am. 2008;92:17–40. doi: 10.1016/j.mcna.2007.09.002. ix. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lloyd-Jones D, Adams R, Carnethon M, et al. Heart disease and stroke statistics - 2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:e21–e181. doi: 10.1161/CIRCULATIONAHA.108.191261. [DOI] [PubMed] [Google Scholar]
- 7.Lloyd-Jones DM, Wang TJ, Leip EP, et al. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation. 2004;110:1042–1046. doi: 10.1161/01.CIR.0000140263.20897.42. [DOI] [PubMed] [Google Scholar]
- 8.Yamada H, Sugiyama T, Ashida T, Fujii J. Sustained hemoconcentration in patients with chronic atrial fibrillation, a potential risk for stroke and thromboembolic complications: a retrospective study. Jpn Heart J. 1998;39:715–720. doi: 10.1536/ihj.39.715. [DOI] [PubMed] [Google Scholar]
- 9.Kamath S, Blann AD, Chin BS, Lip GY. Platelet activation, haemorheology and thrombogenesis in acute atrial fibrillation: a comparison with permanent atrial fibrillation. Heart. 2003;89:1093–1095. doi: 10.1136/heart.89.9.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Al-Saady NM, Obel OA, Camm AJ. Left atrial appendage: structure, function, and role in thromboembolism. Heart. 1999;82:547–554. doi: 10.1136/hrt.82.5.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aberg H. Atrial fibrillation: I. a study of atrial thrombosis and systemic embolism in a necropsy material. Acta Med Scand. 1969;185:373–379. [PubMed] [Google Scholar]
- 12.Blackshear JL, Odell JA. Appendage obliteration to reduce stroke in cardiac surgical patients with atrial fibrillation. Ann Thorac Surg. 1996;61:755–759. doi: 10.1016/0003-4975(95)00887-X. [DOI] [PubMed] [Google Scholar]
- 13.Manning WJ, Weintraub RM, Waksmonski CA, et al. Accuracy of transesophageal echocardiography for identifying left atrial thrombi: a prospective, intraoperative study. Ann Intern Med. 1995;123:817–822. doi: 10.7326/0003-4819-123-11-199512010-00001. [DOI] [PubMed] [Google Scholar]
- 14.Tsai LM, Chen JH, Lin LJ, Yang YJ. Role of transesophageal echocardiography in detecting left atrial thrombus and spontaneous echo contrast in patients with mitral valve disease or non-rheumatic atrial fibrillation. J Formos Med Assoc. 1990;89:270–274. [PubMed] [Google Scholar]
- 15.Leung DY, Black IW, Cranney GB, Hopkins AP, Walsh WF. Prognostic implications of left atrial spontaneous echo contrast in nonvalvular atrial fibrillation. J Am Coll Cardiol. 1994;24:755–762. doi: 10.1016/0735-1097(94)90025-6. [DOI] [PubMed] [Google Scholar]
- 16.Lin HJ, Wolf PA, Kelly-Hayes M, et al. Stroke severity in atrial fibrillation: the Framingham Study. Stroke. 1996;27:1760–1764. doi: 10.1161/01.str.27.10.1760. [DOI] [PubMed] [Google Scholar]
- 17.Hylek EM, Go AS, Chang Y, et al. Effect of intensity of oral anticoagulation on stroke severity and mortality in atrial fibrillation. N Engl J Med. 2003;349:1019–1026. doi: 10.1056/NEJMoa022913. [DOI] [PubMed] [Google Scholar]
- 18.Anderson DC, Kappelle LJ, Eliasziw M, Babikian VL, Pearce LA, Barnett HJ. Occurrence of hemispheric and retinal ischemia in atrial fibrillation compared with carotid stenosis. Stroke. 2002;33:1963–1967. doi: 10.1161/01.str.0000023445.20454.a8. [DOI] [PubMed] [Google Scholar]
- 19.Spratt N, Wang Y, Levi C, Ng K, Evans M, Fisher J. A prospective study of predictors of prolonged hospital stay and disability after stroke. J Clin Neurosci. 2003;10:665–669. doi: 10.1016/j.jocn.2002.12.001. [DOI] [PubMed] [Google Scholar]
- 20.Bonow RO, Carabello BA, Kanu C, et al. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): developed in collaboration with the Society of Cardiovascular Anesthesiologists: endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. Circulation. 2006;114:e84–e231. doi: 10.1161/CIRCULATIONAHA.106.176857. [DOI] [PubMed] [Google Scholar]
- 21.Healey JS, Crystal E, Lamy A, et al. Left Atrial Appendage Occlusion Study (LAAOS): results of a randomized controlled pilot study of left atrial appendage occlusion during coronary bypass surgery in patients at risk for stroke. Am Heart J. 2005;150:288–293. doi: 10.1016/j.ahj.2004.09.054. [DOI] [PubMed] [Google Scholar]
- 22.Brown NA, Anderson RH. Symmetry and laterality in the human heart: developmental implications. In: Harvey RP, Rosenthal N, editors. Heart Development. San Diego: Academic Press; 1999. pp. 447–461. [Google Scholar]
- 23.Veinot JP, Harrity PJ, Gentile F, et al. Anatomy of the normal left atrial appendage: a quantitative study of age-related changes in 500 autopsy hearts: implications for echocardiographic examination. Circulation. 1997;96:3112–3115. doi: 10.1161/01.cir.96.9.3112. [DOI] [PubMed] [Google Scholar]
- 24.Kerut EK. Anatomy of the left atrial appendage. Echocardiography. 2008;25:669–673. doi: 10.1111/j.1540-8175.2008.00637.x. [DOI] [PubMed] [Google Scholar]
- 25.Su P, McCarthy KP, Ho SY. Occluding the left atrial appendage: anatomical considerations. Heart. 2008;94:1166–1170. doi: 10.1136/hrt.2006.111989. [DOI] [PubMed] [Google Scholar]
- 26.Sharma S, Devine W, Anderson RH, Zuberbuhler JR. The determination of atrial arrangement by examination of appendage morphology in 1842 heart specimens. Br Heart J. 1988;60:227–231. doi: 10.1136/hrt.60.3.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ernst G, Stollberger C, Abzieher F, et al. Morphology of the left atrial appendage. Anat Rec. 1995;242:553–561. doi: 10.1002/ar.1092420411. [DOI] [PubMed] [Google Scholar]
- 28.Stollberger C, Ernst G, Bonner E, Finsterer J, Slany J. Left atrial appendage morphology: comparison of transesophageal images and postmortem casts. Z Kardiol. 2003;92:303–308. doi: 10.1007/s00392-003-0903-x. [DOI] [PubMed] [Google Scholar]
- 29.Lannigan RA, Zaki SA. Ultrastructure of the myocardium of the atrial appendage. Br Heart J. 1966;28:796–807. doi: 10.1136/hrt.28.6.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chapeau C, Gutkowska J, Schiller PW, et al. Localization of immunoreactive synthetic atrial natriuretic factor (ANF) in the heart of various animal species. J Histochem Cytochem. 1985;33:541–550. doi: 10.1177/33.6.3158698. [DOI] [PubMed] [Google Scholar]
- 31.Peterson TV, Benjamin BA. The heart and control of renal excretion: neural and endocrine mechanisms. FASEB J. 1992;6:2923–2932. doi: 10.1096/fasebj.6.11.1386584. [DOI] [PubMed] [Google Scholar]
- 32.Tabata T, Oki T, Yamada H, Abe M, Onose Y, Thomas JD. Relationship between left atrial appendage function and plasma concentration of atrial natriuretic peptide. Eur J Echocardiogr. 2000;1:130–137. doi: 10.1053/euje.2000.0019. [DOI] [PubMed] [Google Scholar]
- 33.Kappagoda CT, Linden RJ, Mary DA. Gradation of the reflex response from atrial receptors. J Physiol. 1975;251:561–567. doi: 10.1113/jphysiol.1975.sp011108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pollick C, Taylor D. Assessment of left atrial appendage function by transesophageal echocardiography: implications for the development of thrombus. Circulation. 1991;84:223–231. doi: 10.1161/01.cir.84.1.223. [DOI] [PubMed] [Google Scholar]
- 35.Li YH, Hwang JJ, Ko YL, et al. Left atrial spontaneous echo contrast in patients with rheumatic mitral valve disease in sinus rhythm: implication of an altered left atrial appendage function in its formation. Chest. 1995;108:99–103. doi: 10.1378/chest.108.1.99. [DOI] [PubMed] [Google Scholar]
- 36.Valocik G, Kamp O, Mihciokur M, et al. Assessment of the left atrial appendage mechanical function by three-dimensional echocardiography. Eur J Echocardiogr. 2002;3:207–213. doi: 10.1053/euje.2002.0149. [DOI] [PubMed] [Google Scholar]
- 37.Trambaiolo P, Salustri A, Tanga M, Tonti G, Fedele F, Palamara A. Assessment of left atrial appendage wall velocities by transesophageal tissue Doppler echocardiography: a clinical study in patients with sinus rhythm. J Am Soc Echocardiogr. 2002;15:425–432. doi: 10.1067/mje.2002.115886. [DOI] [PubMed] [Google Scholar]
- 38.Lacomis JM, Goitein O, Deible C, et al. Dynamic multidimensional imaging of the human left atrial appendage. Europace. 2007;9:1134–1140. doi: 10.1093/europace/eum227. [DOI] [PubMed] [Google Scholar]
- 39.Budge LP, Shaffer KM, Moorman JR, Lake DE, Ferguson JD, Mangrum JM. Analysis of in vivo left atrial appendage morphology in patients with atrial fibrillation: a direct comparison of transesophageal echocardiography, planar cardiac CT, and segmented three-dimensional cardiac CT. J Interv Card Electrophysiol. 2008;23:87–93. doi: 10.1007/s10840-008-9281-7. [DOI] [PubMed] [Google Scholar]
- 40.Wongcharoen W, Tsao HM, Wu MH, et al. Morphologic characteristics of the left atrial appendage, roof, and septum: implications for the ablation of atrial fibrillation. J Cardiovasc Electrophysiol. 2006;17:951–956. doi: 10.1111/j.1540-8167.2006.00549.x. [DOI] [PubMed] [Google Scholar]
- 41.Heist EK, Refaat M, Danik SB, Holmvang G, Ruskin JN, Mansour M. Analysis of the left atrial appendage by magnetic resonance angiography in patients with atrial fibrillation. Heart Rhythm. 2006;3:1313–1318. doi: 10.1016/j.hrthm.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 42.Zhang LT, Gay M. Characterizing left atrial appendage functions in sinus rhythm and atrial fibrillation using computational models. J Biomech. 2008;41:2515–2523. doi: 10.1016/j.jbiomech.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 43.Donal E, Yamada H, Leclercq C, Herpin D. The left atrial appendage, a small, blind-ended structure: a review of its echocardiographic evaluation and its clinical role. Chest. 2005;128:1853–1862. doi: 10.1378/chest.128.3.1853. [DOI] [PubMed] [Google Scholar]
- 44.Agmon Y, Khandheria BK, Gentile F, Seward JB. Echocardiographic assessment of the left atrial appendage. J Am Coll Cardiol. 1999;34:1867–1877. doi: 10.1016/s0735-1097(99)00472-6. [DOI] [PubMed] [Google Scholar]
- 45.Hoit BD, Shao Y, Tsai LM, Patel R, Gabel M, Walsh RA. Altered left atrial compliance after atrial appendectomy: influence on left atrial and ventricular filling. Circ Res. 1993;72:167–175. doi: 10.1161/01.res.72.1.167. [DOI] [PubMed] [Google Scholar]
- 46.Davis CA, 3rd, Rembert JC, Greenfield JC., Jr Compliance of left atrium with and without left atrium appendage. Am J Physiol. 1990;259:H1006–H1008. doi: 10.1152/ajpheart.1990.259.4.H1006. [DOI] [PubMed] [Google Scholar]
- 47.Li YH, Lai LP, Shyu KG, Hwang JJ, Kuan P, Lien WP. Clinical implications of left atrial appendage flow patterns in nonrheumatic atrial fibrillation. Chest. 1994;105:748–752. doi: 10.1378/chest.105.3.748. [DOI] [PubMed] [Google Scholar]
- 48.Thambidorai SK, Murray RD, Parakh K, et al. Utility of transesophageal echocardiography in identification of thrombogenic milieu in patients with atrial fibrillation (an ACUTE ancillary study) Am J Cardiol. 2005;96:935–941. doi: 10.1016/j.amjcard.2005.05.051. [DOI] [PubMed] [Google Scholar]
- 49.Choudhury A, Lip GY. Atrial fibrillation and the hypercoagulable state: from basic science to clinical practice. Pathophysiol Haemost Thromb. 2003-2004;33:282–289. doi: 10.1159/000083815. [DOI] [PubMed] [Google Scholar]
- 50.Heppell RM, Berkin KE, McLenachan JM, Davies JA. Haemostatic and haemodynamic abnormalities associated with left atrial thrombosis in non-rheumatic atrial fibrillation. Heart. 1997;77:407–411. doi: 10.1136/hrt.77.5.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Subramaniam B, Riley MF, Panzica PJ, Manning WJ. Transesophageal echocardiographic assessment of right atrial appendage anatomy and function: comparison with the left atrial appendage and implications for local thrombus formation. J Am Soc Echocardiogr. 2006;19:429–433. doi: 10.1016/j.echo.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 52.The Stroke Prevention in Atrial Fibrillation Investigators. Predictors of thromboembolism in atrial fibrillation: II. Echocardiographic features of patients at risk. Ann Intern Med. 1992;116:6–12. doi: 10.7326/0003-4819-116-1-6. [DOI] [PubMed] [Google Scholar]
- 53.Di Tullio MR, Sacco RL, Sciacca RR, Homma S. Left atrial size and the risk of ischemic stroke in an ethnically mixed population. Stroke. 1999;30:2019–2024. doi: 10.1161/01.str.30.10.2019. [DOI] [PubMed] [Google Scholar]
- 54.Narumiya T, Sakamaki T, Sato Y, Kanmatsuse K. Relationship between left atrial appendage function and left atrial thrombus in patients with nonvalvular chronic atrial fibrillation and atrial flutter. Circ J. 2003;67:68–72. doi: 10.1253/circj.67.68. [DOI] [PubMed] [Google Scholar]
- 55.Shively BK, Gelgand EA, Crawford MH. Regional left atrial stasis during atrial fibrillation and flutter: determinants and relation to stroke. J Am Coll Cardiol. 1996;27:1722–1729. doi: 10.1016/0735-1097(96)00049-6. [DOI] [PubMed] [Google Scholar]
- 56.Handke M, Harloff A, Hetzel A, Olschewski M, Bode C, Geibel A. Left atrial appendage flow velocity as a quantitative surrogate parameter for thromboembolic risk: determinants and relationship to spontaneous echocontrast and thrombus formation - a transesophageal echocardiographic study in 500 patients with cerebral ischemia. J Am Soc Echocardiogr. 2005;18:1366–1372. doi: 10.1016/j.echo.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 57.Igarashi Y, Kashimura K, Makiyama Y, Sato T, Ojima K, Aizawa Y. Left atrial appendage dysfunction in chronic nonvalvular atrial fibrillation is significantly associated with an elevated level of brain natriuretic peptide and a prothrombotic state. Jpn Circ J. 2001;65:788–792. doi: 10.1253/jcj.65.788. [DOI] [PubMed] [Google Scholar]
- 58.Donal E, Sallach JA, Murray RD, et al. Contrast-enhanced tissue Doppler imaging of the left atrial appendage is a new quantitative measure of spontaneous echocardiographic contrast in atrial fibrillation. Eur J Echocardiogr. 2008;9:5–11. doi: 10.1016/j.euje.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 59.Ozer N, Kilic H, Arslan U, et al. Echocardiographic predictors of left atrial appendage spontaneous echocontrast in patients with stroke and atrial fibrillation. J Am Soc Echocardiogr. 2005;18:1362–1365. doi: 10.1016/j.echo.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 60.The Stroke Prevention in Atrial Fibrillation Investigators Committee on Echocardiography. Transesophageal echocardiographic correlates of thromboembolism in high-risk patients with nonvalvular atrial fibrillation. Ann Intern Med. 1998;128:639–647. doi: 10.7326/0003-4819-128-8-199804150-00005. [DOI] [PubMed] [Google Scholar]
- 61.Kamp O, Verhorst PM, Welling RC, Visser CA. Importance of left atrial appendage flow as a predictor of thromboembolic events in patients with atrial fibrillation. Eur Heart J. 1999;20:979–985. doi: 10.1053/euhj.1998.1453. [DOI] [PubMed] [Google Scholar]
- 62.Zabalgoitia M, Halperin JL, Pearce LA, Blackshear JL, Asinger RW, Hart RG. Transesophageal echocardiographic correlates of clinical risk of thromboembolism in nonvalvular atrial fibrillation: Stroke Prevention in Atrial Fibrillation III Investigators. J Am Coll Cardiol. 1998;31:1622–1626. doi: 10.1016/s0735-1097(98)00146-6. [DOI] [PubMed] [Google Scholar]
- 63.Tsai LM, Chao TH, Chen JH. Association of follow-up change of left atrial appendage blood flow velocity with spontaneous echo contrast in nonrheumatic atrial fibrillation. Chest. 2000;117:309–313. doi: 10.1378/chest.117.2.309. [DOI] [PubMed] [Google Scholar]
- 64.Bernhardt P, Schmidt H, Hammerstingl C, Luderitz B, Omran H. Patients at high risk with atrial fibrillation: a prospective and serial follow-up during 12 months with transesophageal echocardiography and cerebral magnetic resonance imaging. J Am Soc Echocardiogr. 2005;18:919–924. doi: 10.1016/j.echo.2005.01.028. [DOI] [PubMed] [Google Scholar]
- 65.Rastegar R, Harnick DJ, Weidemann P, et al. Spontaneous echo contrast videodensity is flow-related and is dependent on the relative concentrations of fibrinogen and red blood cells. J Am Coll Cardiol. 2003;41:603–610. doi: 10.1016/s0735-1097(02)02898-x. [DOI] [PubMed] [Google Scholar]
- 66.Cavalcante JL, Al Mallah M, Arida M, Garcia-Sayan E, Chattahi J, Ananthasubramaniam K. The relationship between spontaneous echocontrast, transesophageal echocardiographic parameters, and blood hemoglobin levels. J Am Soc Echocardiogr. 2008;21:868–872. doi: 10.1016/j.echo.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 67.Ohara K, Hirai T, Fukuda N, et al. Relation of left atrial blood stasis to clinical risk factors in atrial fibrillation. Int J Cardiol. 2009;132:210–215. doi: 10.1016/j.ijcard.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 68.Wang TJ, Massaro JM, Levy D, et al. A risk score for predicting stroke or death in individuals with new-onset atrial fibrillation in the community: the Framingham Heart Study. JAMA. 2003;290:1049–1056. doi: 10.1001/jama.290.8.1049. [DOI] [PubMed] [Google Scholar]
- 69.Bernhardt P, Schmidt H, Hammerstingl C, Luderitz B, Omran H. Patients with atrial fibrillation and dense spontaneous echo contrast at high risk: a prospective and serial follow-up over 12 months with transesophageal echocardiography and cerebral magnetic resonance imaging. J Am Coll Cardiol. 2005;45:1807–1812. doi: 10.1016/j.jacc.2004.11.071. [DOI] [PubMed] [Google Scholar]
- 70.Ito T, Suwa M, Kobashi A, Yagi H, Hirota Y, Kawamura K. Influence of altered loading conditions on left atrial appendage function in vivo. Am J Cardiol. 1998;81:1056–1059. doi: 10.1016/s0002-9149(98)00011-3. [DOI] [PubMed] [Google Scholar]
- 71.Hondo T, Okamoto M, Yamane T, et al. The role of the left atrial appendage: a volume loading study in open-chest dogs. Jpn Heart J. 1995;36:225–234. doi: 10.1536/ihj.36.225. [DOI] [PubMed] [Google Scholar]
- 72.Vene N, Mavri A, Kosmelj K, Stegnar M. High D-dimer levels predict cardiovascular events in patients with chronic atrial fibrillation during oral anticoagulant therapy. Thromb Haemost. 2003;90:1163–1172. doi: 10.1160/TH03-06-0363. [DOI] [PubMed] [Google Scholar]
- 73.Cioffi G, Pozzoli M, Forni G, et al. Systemic thromboembolism in chronic heart failure: a prospective study in 406 patients. Eur Heart J. 1996;17:1381–1389. doi: 10.1093/oxfordjournals.eurheartj.a015073. [DOI] [PubMed] [Google Scholar]
- 74.Lip GY, Pearce LA, Chin BS, Conway DS, Hart RG. Effects of congestive heart failure on plasma von Willebrand factor and soluble P-selectin concentrations in patients with non-valvar atrial fibrillation. Heart. 2005;91:759–763. doi: 10.1136/hrt.2004.036160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kleemann T, Becker T, Strauss M, Schneider S, Seidl K. Prevalence and clinical impact of left atrial thrombus and dense spontaneous echo contrast in patients with atrial fibrillation and low CHADS2 score. Eur J Echocardiogr. 2009;10:383–388. doi: 10.1093/ejechocard/jen256. [DOI] [PubMed] [Google Scholar]
- 76.Kahn SR, Solymoss S, Flegel KM. Nonvalvular atrial fibrillation: evidence for a prothrombotic state. CMAJ. 1997;157:673–681. [PMC free article] [PubMed] [Google Scholar]
- 77.Lip GY, Lip PL, Zarifis J, et al. Fibrin D-dimer and beta-thromboglobulin as markers of thrombogenesis and platelet activation in artrial fibrillation: effects of introducing ultra-low-dose warfarin and aspirin. Circulation. 1996;94:425–431. doi: 10.1161/01.cir.94.3.425. [DOI] [PubMed] [Google Scholar]
- 78.Marin F, Roldan V, Climent VE, et al. Plasma von Willebrand factor, soluble thrombomodulin, and fibrin D-dimer concentrations in acute onset non-rheumatic atrial fibrillation. Heart. 2004;90:1162–1166. doi: 10.1136/hrt.2003.024521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lip GY, Lowe GD, Rumley A, Dunn FG. Increased markers of thrombogenesis in chronic atrial fibrillation: effects of warfarin treatment. Br Heart J. 1995;73:527–533. doi: 10.1136/hrt.73.6.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Habara S, Dote K, Kato M, et al. Prediction of left atrial appendage thrombi in non-valvular atrial fibrillation. Eur Heart J. 2007;28:2217–2222. doi: 10.1093/eurheartj/ehm356. [DOI] [PubMed] [Google Scholar]
- 81.Di Napoli M, Papa F. Inflammation, hemostatic markers, and antithrombotic agents in relation to long-term risk of new cardiovascular events in first-ever ischemic stroke patients. Stroke. 2002;33:1763–1771. doi: 10.1161/01.str.0000019124.54361.08. [DOI] [PubMed] [Google Scholar]
- 82.Nozawa T, Inoue H, Iwasa A, et al. Effects of anticoagulation intensity on hemostatic markers in patients with non-valvular atrial fibrillation. Circ J. 2004;68:29–34. doi: 10.1253/circj.68.29. [DOI] [PubMed] [Google Scholar]
- 83.Tayebjee MH, Lip GY. Fibrin D-dimer levels in atrial fibrillation as an index of thrombogenesis: a possible test to exclude left atrial thrombus? Am J Cardiol. 2003;92:47–49. doi: 10.1016/s0002-9149(03)00463-6. [DOI] [PubMed] [Google Scholar]
- 84.Somloi M, Tomcsanyi J, Nagy E, Bodo I, Bezzegh A. D-dimer determination as a screening tool to exclude atrial thrombi in atrial fibrillation. Am J Cardiol. 2003;92:85–87. doi: 10.1016/s0002-9149(03)00476-4. [DOI] [PubMed] [Google Scholar]
- 85.Shinohara H, Fukuda N, Soeki T, et al. Relationship between flow dynamics in the left atrium and hemostatic abnormalities in patients with nonvalvular atrial fibrillation. Jpn Heart J. 1998;39:721–730. doi: 10.1536/ihj.39.721. [DOI] [PubMed] [Google Scholar]
- 86.Mondillo S, Sabatini L, Agricola E, et al. Correlation between left atrial size, prothrombotic state and markers of endothelial dysfunction in patients with lone chronic nonrheumatic atrial fibrillation. Int J Cardiol. 2000;75:227–232. doi: 10.1016/s0167-5273(00)00336-3. [DOI] [PubMed] [Google Scholar]
- 87.Nakamura Y, Nakamura K, Fukushima-Kusano K, et al. Tissue factor expression in atrial endothelia associated with nonvalvular atrial fibrillation: possible involvement in intracardiac thrombogenesis. Thromb Res. 2003;111:137–142. doi: 10.1016/s0049-3848(03)00405-5. [DOI] [PubMed] [Google Scholar]
- 88.Varughese GI, Patel JV, Tomson J, Lip GY. The prothrombotic risk of diabetes mellitus in atrial fibrillation and heart failure. J Thromb Haemost. 2005;3:2811–2813. doi: 10.1111/j.1538-7836.2005.01694.x. [DOI] [PubMed] [Google Scholar]
- 89.Inoue H, Nozawa T, Okumura K, Jong-Dae L, Shimizu A, Yano K. Prothrombotic activity is increased in patients with nonvalvular atrial fibrillation and risk factors for embolism. Chest. 2004;126:687–692. doi: 10.1378/chest.126.3.687. [DOI] [PubMed] [Google Scholar]
- 90.Ohara K, Inoue H, Nozawa T, et al. Accumulation of risk factors enhances the prothrombotic state in atrial fibrillation. Int J Cardiol. 2008;126:316–321. doi: 10.1016/j.ijcard.2007.06.020. [DOI] [PubMed] [Google Scholar]
- 91.Sakurai K, Hirai T, Nakagawa K, et al. Left atrial appendage function and abnormal hypercoagulability in patients with atrial flutter. Chest. 2003;124:1670–1674. doi: 10.1378/chest.124.5.1670. [DOI] [PubMed] [Google Scholar]
- 92.Esmon CT, Fukudome K, Mather T, et al. Inflammation, sepsis, and coagulation. Haematologica. 1999;84:254–259. [PubMed] [Google Scholar]
- 93.Kahn SR, Solymoss S, Flegel KM. Increased tissue plasminogen activator levels in patients with nonvalvular atrial fibrillation. CMAJ. 1997;157:685–689. [PMC free article] [PubMed] [Google Scholar]
- 94.Mustonen P, Lepantalo M, Lassila R. Physical exertion induces thrombin formation and fibrin degradation in patients with peripheral atherosclerosis. Arterioscler Thromb Vasc Biol. 1998;18:244–249. doi: 10.1161/01.atv.18.2.244. [DOI] [PubMed] [Google Scholar]
- 95.Feinberg WM, Macy E, Cornell ES, et al. Plasmin-alpha2-antiplasmin complex in patients with atrial fibrillation: Stroke Prevention in Atrial Fibrillation Investigators. Thromb Haemost. 1999;82:100–103. [PubMed] [Google Scholar]
- 96.Takano K, Yamaguchi T, Uchida K. Markers of a hypercoagulable state following acute ischemic stroke. Stroke. 1992;23:194–198. doi: 10.1161/01.str.23.2.194. [DOI] [PubMed] [Google Scholar]
- 97.Hoffmeister HM, Jur M, Wendel HP, Heller W, Seipel L. Alterations of coagulation and fibrinolytic and kallikrein-kinin systems in the acute and postacute phases in patients with unstable angina pectoris. Circulation. 1995;91:2520–2527. doi: 10.1161/01.cir.91.10.2520. [DOI] [PubMed] [Google Scholar]
- 98.Roldan V, Marin F, Marco P, Martinez JG, Calatayud R, Sogorb F. Hypofibrinolysis in atrial fibrillation. Am Heart J. 1998;136:956–960. doi: 10.1016/s0002-8703(98)70149-8. [DOI] [PubMed] [Google Scholar]
- 99.Leithäuser B, Matthias FR, Nicolai U, Voss R. Hemostatic abnormalities and the severity of illness in patients at the onset of clinically defined sepsis: possible indication of the degree of endothelial cell activation? Intensive Care Med. 1996;22:631–636. doi: 10.1007/BF01709738. [DOI] [PubMed] [Google Scholar]
- 100.Choudhury A, Chung I, Blann AD, Lip GY. Elevated platelet microparticle levels in nonvalvular atrial fibrillation: relationship to p-selectin and antithrombotic therapy. Chest. 2007;131:809–815. doi: 10.1378/chest.06-2039. [DOI] [PubMed] [Google Scholar]
- 101.Lim HS, Blann AD, Lip GY. Soluble CD40 ligand, soluble P-selectin, interleukin-6, and tissue factor in diabetes mellitus: relationships to cardiovascular disease and risk factor intervention. Circulation. 2004;109:2524–2528. doi: 10.1161/01.CIR.0000129773.70647.94. [DOI] [PubMed] [Google Scholar]
- 102.Conway DS, Pearce LA, Chin BS, Hart RG, Lip GY. Prognostic value of plasma von Willebrand factor and soluble P-selectin as indices of endothelial damage and platelet activation in 994 patients with nonvalvular atrial fibrillation. Circulation. 2003;107:3141–3145. doi: 10.1161/01.CIR.0000077912.12202.FC. [DOI] [PubMed] [Google Scholar]
- 103.Feinberg WM, Pearce LA, Hart RG, et al. Markers of thrombin and platelet activity in patients with atrial fibrillation: correlation with stroke among 1531 participants in the stroke prevention in atrial fibrillation III study. Stroke. 1999;30:2547–2553. doi: 10.1161/01.str.30.12.2547. [DOI] [PubMed] [Google Scholar]
- 104.Heeringa J, Conway DS, van der Kuip DA, et al. A longitudinal population-based study of prothrombotic factors in elderly subjects with atrial fibrillation: the Rotterdam Study 1990-1999. J Thromb Haemost. 2006;4:1944–1949. doi: 10.1111/j.1538-7836.2006.02115.x. [DOI] [PubMed] [Google Scholar]
- 105.Li-Saw-Hee FL, Blann AD, Gurney D, Lip GY. Plasma von Willebrand factor, fibrinogen and soluble P-selectin levels in paroxysmal, persistent and permanent atrial fibrillation: effects of cardioversion and return of left atrial function. Eur Heart J. 2001;22:1741–1747. doi: 10.1053/euhj.2000.2531. [DOI] [PubMed] [Google Scholar]
- 106.Sohara H, Amitani S, Kurose M, Miyahara K. Atrial fibrillation activates platelets and coagulation in a time-dependent manner: a study in patients with paroxysmal atrial fibrillation. J Am Coll Cardiol. 1997;29:106–112. doi: 10.1016/s0735-1097(96)00427-5. [DOI] [PubMed] [Google Scholar]
- 107.Kamath S, Blann AD, Chin BS, et al. A study of platelet activation in atrial fibrillation and the effects of antithrombotic therapy. Eur Heart J. 2002;23:1788–1795. doi: 10.1053/euhj.2002.3259. [DOI] [PubMed] [Google Scholar]
- 108.Kamath S, Blann AD, Caine GJ, Gurney D, Chin BS, Lip GY. Platelet P-selectin levels in relation to plasma soluble P-selectin and beta-thromboglobulin levels in atrial fibrillation. Stroke. 2002;33:1237–1242. doi: 10.1161/01.str.0000013739.82306.7f. [DOI] [PubMed] [Google Scholar]
- 109.Ruggeri EM, Ware J. Von Willebrand factor. FASEB J. 1993;7:308–316. doi: 10.1096/fasebj.7.2.8440408. [DOI] [PubMed] [Google Scholar]
- 110.Makin AJ, Blann AD, Chung NA, Silverman SH, Lip GY. Assessment of endothelial damage in atherosclerotic vascular disease by quantification of circulating endothelial cells: relationship with von Willebrand factor and tissue factor. Eur Heart J. 2004;25:371–376. doi: 10.1016/j.ehj.2003.04.001. [DOI] [PubMed] [Google Scholar]
- 111.Freestone B, Lip GY, Chong AY, Nadar S, Lee KW, Blann AD. Circulating endothelial cells in atrial fibrillation with and without acute cardiovascular disease. Thromb Haemost. 2005;94:702–706. doi: 10.1160/TH05-02-0093. [DOI] [PubMed] [Google Scholar]
- 112.Conway DS, Pearce LA, Chin BS, Hart RG, Lip GY. Plasma von Willebrand factor and soluble P-selectin as indices of endothelial damage and platelet activation in 1321 patients with nonvalvular atrial fibrillation: relationship to stroke risk factors. Circulation. 2002;106:1962–1967. doi: 10.1161/01.cir.0000033220.97592.9a. [DOI] [PubMed] [Google Scholar]
- 113.Lip GY, Lane D, van Walraven C, Hart RG. Additive role of plasma von Willebrand factor levels to clinical factors for risk stratification of patients with atrial fibrillation. Stroke. 2006;37:2294–2300. doi: 10.1161/01.STR.0000236840.00467.84. [DOI] [PubMed] [Google Scholar]
- 114.Fukuchi M, Watanabe J, Kumagai K, et al. Increased von Willebrand factor in the endocardium as a local predisposing factor for thrombogenesis in overloaded human atrial appendage. J Am Coll Cardiol. 2001;37:1436–1442. doi: 10.1016/s0735-1097(01)01125-1. [DOI] [PubMed] [Google Scholar]
- 115.Kumagai K, Fukuchi M, Ohta J, et al. Expression of the von Willebrand factor in atrial endocardium is increased in atrial fibrillation depending on the extent of structural remodeling. Circ J. 2004;68:321–327. doi: 10.1253/circj.68.321. [DOI] [PubMed] [Google Scholar]
- 116.Uemura T, Kaikita K, Yamabe H, et al. Changes in plasma von Willebrand factor and ADAMTS13 levels associated with left atrial remodeling in atrial fibrillation. Thromb Res. 2009;124:28–32. doi: 10.1016/j.thromres.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 117.Boldt A, Wetzel U, Lauschke J, et al. Fibrosis in left atrial tissue of patients with atrial fibrillation with and without underlying mitral valve disease. Heart. 2004;90:400–405. doi: 10.1136/hrt.2003.015347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Frustaci A, Chimenti C, Bellocci F, Morgante E, Russo MA, Maseri A. Histological substrate of atrial biopsies in patients with lone atrial fibrillation. Circulation. 1997;96:1180–1184. doi: 10.1161/01.cir.96.4.1180. [DOI] [PubMed] [Google Scholar]
- 119.Marin F, Roldan V, Climent V, Garcia A, Marco P, Lip GY. Is thrombogenesis in atrial fibrillation related to matrix metalloproteinase-1 and its inhibitor, TIMP-1? Stroke. 2003;34:1181–1186. doi: 10.1161/01.STR.0000065431.76788.D9. [DOI] [PubMed] [Google Scholar]
- 120.Haubitz M, Woywodt A. Circulating endothelial cells and vasculitis. Intern Med. 2004;43:660–667. doi: 10.2169/internalmedicine.43.660. [DOI] [PubMed] [Google Scholar]
- 121.Fuster V, Ryden LE, Cannom DS, et al. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation--executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation) J Am Coll Cardiol. 2006;48:854–906. doi: 10.1016/j.jacc.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 122.Hart RG, Pearce LA, McBride R, Rothbart RM, Asinger RW. Factors associated with ischemic stroke during aspirin therapy in atrial fibrillation: analysis of 2012 participants in the SPAF I-III clinical trials. The Stroke Prevention in Atrial Fibrillation (SPAF) Investigators. Stroke. 1999;30:1223–1229. doi: 10.1161/01.str.30.6.1223. [DOI] [PubMed] [Google Scholar]
- 123.Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001;285:2864–2870. doi: 10.1001/jama.285.22.2864. [DOI] [PubMed] [Google Scholar]
- 124.Risk factors for stroke and efficacy of antithrombotic therapy in atrial fibrillation: analysis of pooled data from five randomized controlled trials. Arch Intern Med. 1994;154:1449–1457. [PubMed] [Google Scholar]
- 125.van Walraven C, Hart RG, Wells GA, et al. A clinical prediction rule to identify patients with atrial fibrillation and a low risk for stroke while taking aspirin. Arch Intern Med. 2003;163:936–943. doi: 10.1001/archinte.163.8.936. [DOI] [PubMed] [Google Scholar]
- 126.Stroke Risk in Atrial Fibrillation Working Group. Comparison of 12 risk stratification schemes to predict stroke in patients with nonvalvular atrial fibrillation. Stroke. 2008;39:1901–1910. doi: 10.1161/STROKEAHA.107.501825. [DOI] [PubMed] [Google Scholar]
- 127.Klein AL, Murray RD, Grimm RA. Role of transesophageal echocardiography-guided cardioversion of patients with atrial fibrillation. J Am Coll Cardiol. 2001;37:691–704. doi: 10.1016/s0735-1097(00)01178-5. [DOI] [PubMed] [Google Scholar]
- 128.Melduni RM, Ammash NM, Callahan MJ, Malouf JF, Chandrasekaran K, Gersh BJ. Images in cardiovascular medicine: severe left atrial appendage stunning after electrical cardioversion of atrial fibrillation. Circulation. 2008;118:e699–e700. doi: 10.1161/CIRCULATIONAHA.108.787374. [DOI] [PubMed] [Google Scholar]
- 129.Omran H, Jung W, Rabahieh R, et al. Left atrial chamber and appendage function after internal atrial defibrillation: a prospective and serial transesophageal echocardiographic study. J Am Coll Cardiol. 1997;29:131–138. doi: 10.1016/s0735-1097(96)00439-1. [DOI] [PubMed] [Google Scholar]
- 130.Mazzone C, Pandullo C, Scardi S, et al. Left atrial and appendage mechanical function after pharmacological or electrical cardioversion in patients with chronic atrial fibrillation: a multicenter, randomized study. Ital Heart J. 2000;1:128–136. [PubMed] [Google Scholar]
- 131.Wang YC, Lin JL, Hwang JJ, et al. Left atrial dysfunction in patients with atrial fibrillation after successful rhythm control for >3 months. Chest. 2005;128:2551–2556. doi: 10.1378/chest.128.4.2551. [DOI] [PubMed] [Google Scholar]
- 132.Sakurai K, Hirai T, Nakagawa K, et al. Prolonged activation of hemostatic markers following conversion of atrial flutter to sinus rhythm. Circ J. 2004;68:1041–1044. doi: 10.1253/circj.68.1041. [DOI] [PubMed] [Google Scholar]
- 133.de Denus S, Sanoski CA, Carlsson J, Opolski G, Spinler SA. Rate vs rhythm control in patients with atrial fibrillation: a meta-analysis. Arch Intern Med. 2005;165:258–262. doi: 10.1001/archinte.165.3.258. [DOI] [PubMed] [Google Scholar]
- 134.Cox JL, Ad N, Palazzo T. Impact of the maze procedure on the stroke rate in patients with atrial fibrillation. J Thorac Cardiovasc Surg. 1999;118:833–840. doi: 10.1016/s0022-5223(99)70052-8. [DOI] [PubMed] [Google Scholar]
- 135.Song BG, Cho SJ, Lee SY, et al. Atrial mechanical function after maze procedure for atrial fibrillation concomitant with mitral valve surgery. Korean Circ J. 2008;38:606–611. [Google Scholar]
- 136.Black IW, Fatkin D, Sagar KB, et al. Exclusion of atrial thrombus by transesophageal echocardiography does not preclude embolism after cardioversion of atrial fibrillation: a multicenter study. Circulation. 1994;89:2509–2513. doi: 10.1161/01.cir.89.6.2509. [DOI] [PubMed] [Google Scholar]
- 137.Bernhardt P, Schmidt H, Hammerstingl C, Luderitz B, Omran H. Incidence of cerebral embolism after cardioversion of atrial fibrillation: a prospective study with transesophageal echocardiography and cerebral magnetic resonance imaging. J Am Soc Echocardiogr. 2005;18:649–653. doi: 10.1016/j.echo.2004.09.022. [DOI] [PubMed] [Google Scholar]
- 138.Asher CR, Klein AL. Transesophageal echocardiography to guide electrical cardioversion in atrial fibrillation: Assessment of Cardioversion Using Transesophageal Echocardiography. Cleve Clin J Med. 2002;69:713–718. doi: 10.3949/ccjm.69.9.713. [DOI] [PubMed] [Google Scholar]
- 139.Aschenberg W, Siglow V, Kremer P, Schluter M, Bleifeld W. Thrombi in the left atrial appendage in mitral defects despite adequate anticoagulation: the advantages of transesophageal echocardiography. Dtsch Med Wochenschr. 1987;112:663–668. doi: 10.1055/s-2008-1068117. [DOI] [PubMed] [Google Scholar]
- 140.Sharma S, Kumar MV, Reddy VM, Kaul U, Rajani M, Venugopal P. Comparison of left coronary and laevo-phase pulmonary angiograms in detecting left atrial thrombi in rheumatic mitral stenosis. Clin Radiol. 1991;44:27–30. doi: 10.1016/s0009-9260(05)80222-3. [DOI] [PubMed] [Google Scholar]
- 141.Leithäuser B, Gerk U, Vransky M, Park JW, Jung F. Transesophageal echocardiography overlooks thrombi inside left atrial appendage in patients with atrial fibrillation. Comparison with LA angiography. Eur Heart J. 2008;29(Suppl 1):84. Abstract. [Google Scholar]
- 142.Mizuguchi KA, Burch TM, Bulwer BE, Fox AA, Rizzo RJ, Shernan SK. Thrombus or bilobar left atrial appendage?: diagnosis by real-time three-dimensional transesophageal echocardiography. Anesth Analg. 2009;108:70–72. doi: 10.1213/ane.0b013e3181857e53. [DOI] [PubMed] [Google Scholar]
- 143.Ohyama H, Hosomi N, Takahashi T, et al. Comparison of magnetic resonance imaging and transesophageal echocardiography in detection of thrombus in the left atrial appendage. Stroke. 2003;34:2436–2439. doi: 10.1161/01.STR.0000090350.73614.0F. [DOI] [PubMed] [Google Scholar]
- 144.Mohrs OK, Nowak B, Petersen SE, et al. Thrombus detection in the left atrial appendage using contrast-enhanced MRI: a pilot study. AJR Am J Roentgenol. 2006;186:198–205. doi: 10.2214/AJR.04.1504. [DOI] [PubMed] [Google Scholar]
- 145.Patel A, Au E, Donegan K, et al. Multidetector row computed tomography for identification of left atrial appendage filling defects in patients undergoing pulmonary vein isolation for treatment of atrial fibrillation: comparison with transesophageal echocardiography. Heart Rhythm. 2008;5:253–260. doi: 10.1016/j.hrthm.2007.10.025. [DOI] [PubMed] [Google Scholar]
- 146.Martinez MW, Lin G, Williamson EE, Brady PA. Dual source computed tomography with delayed imaging for left atrial appendage thrombus compared with transoesophageal echocardiography. Heart. 2009;95:460. doi: 10.1136/hrt.2008.158402. [DOI] [PubMed] [Google Scholar]
- 147.Schwartzman D, Katz WE, Smith AJ, Anderson WD. Malpositioning of a left atrial appendage occlusion device?: a case with implications for percutaneous transcatheter left atrial appendage occlusion device therapy. Heart Rhythm. 2007;4:648–650. doi: 10.1016/j.hrthm.2006.11.018. [DOI] [PubMed] [Google Scholar]
- 148.Friedman PA, Rosenberg RD, Hauschka PV, Fitz-James A. A spectrum of partially carboxylated prothrombins in the plasmas of coumarin-treated patients. Biochim Biophys Acta. 1977;494:271–276. doi: 10.1016/0005-2795(77)90155-6. [DOI] [PubMed] [Google Scholar]
- 149.Ansell J, Hirsh J, Hylek E, Jacobson A, Crowther M, Palareti G. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th edition) Chest. 2008;133(6 Suppl):160S–198S. doi: 10.1378/chest.08-0670. [DOI] [PubMed] [Google Scholar]
- 150.Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146:857–867. doi: 10.7326/0003-4819-146-12-200706190-00007. [DOI] [PubMed] [Google Scholar]
- 151.van Walraven C, Hart RG, Singer DE, et al. Oral anticoagulants vs aspirin in nonvalvular atrial fibrillation: an individual patient meta-analysis. JAMA. 2002;288:2441–2448. doi: 10.1001/jama.288.19.2441. [DOI] [PubMed] [Google Scholar]
- 152.Lopes RD, Piccini JP, Hylek EM, Granger CB, Alexander JH. Antithrombotic therapy in atrial fibrillation: guidelines translated for the clinician. J Thromb Thrombolysis. 2008;26:167–174. doi: 10.1007/s11239-008-0272-4. [DOI] [PubMed] [Google Scholar]
- 153.Li-Saw-Hee FL, Blann AD, Lip GY. Effects of fixed low-dose warfarin, aspirin-warfarin combination therapy, and dose-adjusted warfarin on thrombogenesis in chronic atrial fibrillation. Stroke. 2000;31:828–833. doi: 10.1161/01.str.31.4.828. [DOI] [PubMed] [Google Scholar]
- 154.Cohen N, Almoznino-Sarafian D, Alon I, et al. Warfarin for stroke prevention still underused in atrial fibrillation: patterns of omission. Stroke. 2000;31:1217–1222. doi: 10.1161/01.str.31.6.1217. [DOI] [PubMed] [Google Scholar]
- 155.Wittkowsky AK. Effective anticoagulation therapy: defining the gap between clinical studies and clinical practice. Am J Manag Care. 2004;10(10 Suppl):S297–S306. discussion S312-7. [PubMed] [Google Scholar]
- 156.Waldo AL, Becker RC, Tapson VF, Colgan KJ. Hospitalized patients with atrial fibrillation and a high risk of stroke are not being provided with adequate anticoagulation. J Am Coll Cardiol. 2005;46:1729–1736. doi: 10.1016/j.jacc.2005.06.077. [DOI] [PubMed] [Google Scholar]
- 157.Baker WL, Cios DA, Sander SD, Coleman CI. Meta-analysis to assess the quality of warfarin control in atrial fibrillation patients in the United States. J Manag Care Pharm. 2009;15:244–252. doi: 10.18553/jmcp.2009.15.3.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Rose AJ, Ozonoff A, Henault LE, Hylek EM. Warfarin for atrial fibrillation in community-based practise. J Thromb Haemost. 2008;6:1647–1654. doi: 10.1111/j.1538-7836.2008.03075.x. [DOI] [PubMed] [Google Scholar]
- 159.Ansell J, Hollowell J, Pengo V, Martinez-Brotons F, Caro J, Drouet L. Descriptive analysis of the process and quality of oral anticoagulation management in real-life practice in patients with chronic non-valvular atrial fibrillation: the international study of anticoagulation management (ISAM) J Thromb Thrombolysis. 2007;23:83–91. doi: 10.1007/s11239-006-9022-7. [DOI] [PubMed] [Google Scholar]
- 160.Albers GW, Bittar N, Young L, et al. Clinical characteristics and management of acute stroke in patients with atrial fibrillation admitted to US university hospitals. Neurology. 1997;48:1598–1604. doi: 10.1212/wnl.48.6.1598. [DOI] [PubMed] [Google Scholar]
- 161.Brass LM, Krumholz HM, Scinto JM, Radford M. Warfarin use among patients with atrial fibrillation. Stroke. 1997;28:2382–2389. doi: 10.1161/01.str.28.12.2382. [DOI] [PubMed] [Google Scholar]
- 162.Leckey R, Aguilar EG, Phillips SJ. Atrial fibrillation and the use of warfarin in patients admitted to an acute stroke unit. Can J Cardiol. 2000;16:481–485. [PubMed] [Google Scholar]
- 163.Bungard TJ, Ghali WA, Teo KK, McAlister FA, Tsuyuki RT. Why do patients with atrial fibrillation not receive warfarin? Arch Intern Med. 2000;160:41–46. doi: 10.1001/archinte.160.1.41. [DOI] [PubMed] [Google Scholar]
- 164.Hart RG, Benavente O, McBride R, Pearce LA. Antithrombotic therapy to prevent stroke in patients with atrial fibrillation: a meta-analysis. Ann Intern Med. 1999;131:492–501. doi: 10.7326/0003-4819-131-7-199910050-00003. [DOI] [PubMed] [Google Scholar]
- 165.Stroke prevention in atrial fibrillation study: final results. Circulation. 1991;84:527–539. doi: 10.1161/01.cir.84.2.527. [DOI] [PubMed] [Google Scholar]
- 166.Mant J, Hobbs FD, Fletcher K, et al. Warfarin versus aspirin for stroke prevention in an elderly community population with atrial fibrillation (the Birmingham Atrial Fibrillation Treatment of the Aged Study, BAFTA): a randomised controlled trial. Lancet. 2007;370:493–503. doi: 10.1016/S0140-6736(07)61233-1. [DOI] [PubMed] [Google Scholar]
- 167.Levine MN, Raskob G, Beyth RJ, Kearon C, Schulman S. Hemorrhagic complications of anticoagulant treatment: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126(3 Suppl):287S–310S. doi: 10.1378/chest.126.3_suppl.287S. [DOI] [PubMed] [Google Scholar]
- 168.Gullov AL, Koefoed BG, Petersen P. Bleeding during warfarin and aspirin therapy in patients with atrial fibrillation: the AFASAK (Atrial Fibrillation Aspirin and Anticoagulation) 2 study. Arch Intern Med. 1999;159:1322–1328. doi: 10.1001/archinte.159.12.1322. [DOI] [PubMed] [Google Scholar]
- 169.Stroke Prevention in Atrial Fibrillation II Study. Warfarin versus aspirin for prevention of thromboembolism in atrial fibrillation. Lancet. 1994;343:687–691. [PubMed] [Google Scholar]
- 170.Hylek EM, Evans-Molina C, Shea C, Henault LE, Regan S. Major hemorrhage and tolerability of warfarin in the first year of therapy among elderly patients with atrial fibrillation. Circulation. 2007;115:2689–2696. doi: 10.1161/CIRCULATIONAHA.106.653048. [DOI] [PubMed] [Google Scholar]
- 171.Hohnloser SH, Pajitnev D, Pogue J, et al. Incidence of stroke in paroxysmal versus sustained atrial fibrillation in patients taking oral anticoagulation or combined antiplatelet therapy: an ACTIVE W Substudy. J Am Coll Cardiol. 2007;50:2156–2161. doi: 10.1016/j.jacc.2007.07.076. [DOI] [PubMed] [Google Scholar]
- 172.Healey JS, Hart RG, Pogue J, et al. Risks and benefits of oral anticoagulation compared with clopidogrel plus aspirin in patients with atrial fibrillation according to stroke risk: the atrial fibrillation clopidogrel trial with irbesartan for prevention of vascular events (ACTIVE-W) Stroke. 2008;39:1482–1486. doi: 10.1161/STROKEAHA.107.500199. [DOI] [PubMed] [Google Scholar]
- 173.Hughes M, Lip GY. Risk factors for anticoagulation-related bleeding complications in patients with atrial fibrillation: a systematic review. QJM. 2007;100:599–607. doi: 10.1093/qjmed/hcm076. [DOI] [PubMed] [Google Scholar]
- 174.Fang MC, Chen J, Rich MW. Atrial fibrillation in the elderly. Am J Med. 2007;120:481–487. doi: 10.1016/j.amjmed.2007.01.026. [DOI] [PubMed] [Google Scholar]
- 175.Aronow WS, Ahn C, Kronzon I, Gutstein H. Incidence of new thromboembolic stroke in persons 62 years and older with chronic atrial fibrillation treated with warfarin versus aspirin. J Am Geriatr Soc. 1999;47:366–368. doi: 10.1111/j.1532-5415.1999.tb03004.x. [DOI] [PubMed] [Google Scholar]
- 176.Perera V, Bajorek BV, Matthews S, Hilmer SN. The impact of frailty on the utilisation of antithrombotic therapy in older patients with atrial fibrillation. Age Ageing. 2009;38:156–162. doi: 10.1093/ageing/afn293. [DOI] [PubMed] [Google Scholar]
- 177.Palareti G, Leali N, Coccheri S, et al. Bleeding complications of oral anticoagulant treatment: an inception-cohort, prospective collaborative study (ISCOAT). Italian Study on Complications of Oral Anticoagulant Therapy. Lancet. 1996;348:423–428. doi: 10.1016/s0140-6736(96)01109-9. [DOI] [PubMed] [Google Scholar]
- 178.Beyth RJ, Quinn LM, Landefeld CS. Prospective evaluation of an index for predicting the risk of major bleeding in outpatients treated with warfarin. Am J Med. 1998;105:91–99. doi: 10.1016/s0002-9343(98)00198-3. [DOI] [PubMed] [Google Scholar]
- 179.Kuijer PM, Hutten BA, Prins MH, Buller HR. Prediction of the risk of bleeding during anticoagulant treatment for venous thromboembolism. Arch Intern Med. 1999;159:457–460. doi: 10.1001/archinte.159.5.457. [DOI] [PubMed] [Google Scholar]
- 180.Landefeld CS, McGuire E, 3rd, Rosenblatt MW. A bleeding risk index for estimating the probability of major bleeding in hospitalized patients starting anticoagulant therapy. Am J Med. 1990;89:569–578. doi: 10.1016/0002-9343(90)90174-c. [DOI] [PubMed] [Google Scholar]
- 181.Shireman TI, Mahnken JD, Howard PA, Kresowik TF, Hou Q, Ellerbeck EF. Development of a contemporary bleeding risk model for elderly warfarin recipients. Chest. 2006;130:1390–1396. doi: 10.1378/chest.130.5.1390. [DOI] [PubMed] [Google Scholar]
- 182.Gage BF, Yan Y, Milligan PE, et al. Clinical classification schemes for predicting hemorrhage: results from the National Registry of Atrial Fibrillation (NRAF) Am Heart J. 2006;151:713–719. doi: 10.1016/j.ahj.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 183.Hellerstein HK, Sinaiko E, Dolgin M. Amputation of the canine atrial appendages. Proc Soc Exp Biol Med. 1947;66:337. doi: 10.3181/00379727-66-16084p. [DOI] [PubMed] [Google Scholar]
- 184.Madden JL. Resection of the left auricular appendix: a prophylaxis for recurrent arterial emboli. J Am Med Assoc. 1949;140:769–772. doi: 10.1001/jama.1949.02900440011003. [DOI] [PubMed] [Google Scholar]
- 185.Beal JM, Longmire WP, Jr, Leake WH. Resection of the auricular appendages. Ann Surg. 1950;132:517–530. doi: 10.1097/00000658-195009000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Odell JA, Blackshear JL, Davies E, et al. Thoracoscopic obliteration of the left atrial appendage: potential for stroke reduction? Ann Thorac Surg. 1996;61:565–569. doi: 10.1016/0003-4975(95)00885-3. [DOI] [PubMed] [Google Scholar]
- 187.Blackshear JL, Johnson WD, Odell JA, et al. Thoracoscopic extracardiac obliteration of the left atrial appendage for stroke risk reduction in atrial fibrillation. J Am Coll Cardiol. 2003;42:1249–1252. doi: 10.1016/s0735-1097(03)00953-7. [DOI] [PubMed] [Google Scholar]
- 188.Crystal E, Lamy A, Connolly SJ, et al. Left Atrial Appendage Occlusion Study (LAAOS): a randomized clinical trial of left atrial appendage occlusion during routine coronary artery bypass graft surgery for long-term stroke prevention. Am Heart J. 2003;145:174–178. doi: 10.1067/mhj.2003.44. [DOI] [PubMed] [Google Scholar]
- 189.Nakai T, Lesh MD, Gerstenfeld EP, Virmani R, Jones R, Lee RJ. Percutaneous Left Atrial Appendage Occlusion (PLAATO) for preventing cardioembolism: first experience in canine model. Circulation. 2002;105:2217–2222. doi: 10.1161/01.cir.0000015605.30810.51. [DOI] [PubMed] [Google Scholar]
- 190.Nakai T, Gerstenfeld EP, Lesh MD, Lee RJ. Assessment of Transcatheter Left Atrial Appendage Occlusion Device for Prevention of Thromboembolism. Am J Cardiol. 2001;88(Suppl 5A):199G. Abstract. [Google Scholar]
- 191.Sievert H, Lesh MD, Trepels T, et al. Percutaneous left atrial appendage transcatheter occlusion to prevent stroke in high-risk patients with atrial fibrillation: early clinical experience. Circulation. 2002;105:1887–1889. doi: 10.1161/01.cir.0000015698.54752.6d. [DOI] [PubMed] [Google Scholar]
- 192.El Chami MF, Grow P, Eilen D, Lerakis S, Block PC. Clinical outcomes three years after PLAATO implantation. Catheter Cardiovasc Interv. 2007;69:704–707. doi: 10.1002/ccd.21026. [DOI] [PubMed] [Google Scholar]
- 193.Block PC, Burstein S, Casale PN, et al. Percutaneous left atrial appendage occlusion for patients in atrial fibrillation suboptimal for warfarin therapy: 5-year results of the PLAATO (Percutaneous Left Atrial Appendage Transcatheter Occlusion) Study. JACC Cardiovasc Interv. 2009;2:594–600. doi: 10.1016/j.jcin.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 194.Park JW, Leithäuser B, Gerk U, Vransky M, Jung F. Percutaneous Left Atrial Appendage Transcatheter Occlusion (PLAATO) for stroke prevention in atrial fibrillation: 2-years outcome. J Invasive Cardiol. 2009;21:446–450. [PubMed] [Google Scholar]
- 195.Ussia GP, Mule M, Cammalleri V, et al. Percutaneous closure of left atrial appendage to prevent embolic events in high-risk patients with chronic atrial fibrillation. Catheter Cardiovasc Interv. 2009;74:217–222. doi: 10.1002/ccd.22099. [DOI] [PubMed] [Google Scholar]
- 196.De Meester P, Thijs V, Van Deyk K, Budts W. Prevention of stroke by percutaneous left atrial appendage closure: short term follow-up. Int J Cardiol. 2008 doi: 10.1016/j.ijcard.2008.11.112. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 197.Ostermayer SH, Reisman M, Kramer PH, et al. Percutaneous Left Atrial Appendage Transcatheter Occlusion (PLAATO system) to prevent stroke in high-risk patients with non-rheumatic atrial fibrillation: results from the international multi-center feasibility trials. J Am Coll Cardiol. 2005;46:9–14. doi: 10.1016/j.jacc.2005.03.042. [DOI] [PubMed] [Google Scholar]
- 198.Omran H, Schmidt H, Hardung D, et al. Post mortem analysis of a left atrial appendage occlusion device (PLAATO) in a patient with permanent atrial fibrillation. J Interv Card Electrophysiol. 2005;14:17–20. doi: 10.1007/s10840-005-3489-6. [DOI] [PubMed] [Google Scholar]
- 199.Park JW, Gerk U, Franke RP, Jung F. Post-Mortem Analysis of a Left Atrial Appendage Occlusion Device (PLAATO) in a patient with permanent atrial fibrillation. Cardiology. 2008;112:205–208. doi: 10.1159/000149574. [DOI] [PubMed] [Google Scholar]
- 200.Sick PB, Schuler G, Hauptmann KE, et al. Initial worldwide experience with the WATCHMAN left atrial appendage system for stroke prevention in atrial fibrillation. J Am Coll Cardiol. 2007;49:1490–1495. doi: 10.1016/j.jacc.2007.02.035. [DOI] [PubMed] [Google Scholar]
- 201.Fountain RB, Holmes DR, Chandrasekaran K, et al. The PROTECT AF (WATCHMAN Left Atrial Appendage System for Embolic PROTECTion in Patients with Atrial Fibrillation) trial. Am Heart J. 2006;151:956–961. doi: 10.1016/j.ahj.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 202.Holmes DR, Reddy VY, Turi ZG, et al. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non-inferiority trial. Lancet. 2009;374:534–542. doi: 10.1016/S0140-6736(09)61343-X. [DOI] [PubMed] [Google Scholar]
- 203.Maisel WH. Left atrial appendage occlusion: closure or just the beginning? N Engl J Med. 2009;360:2601–2603. doi: 10.1056/NEJMp0903763. [DOI] [PubMed] [Google Scholar]
- 204.Meier B, Palacios I, Windecker S, et al. Transcatheter left atrial appendage occlusion with Amplatzer devices to obviate anticoagulation in patients with atrial fibrillation. Catheter Cardiovasc Interv. 2003;60:417–422. doi: 10.1002/ccd.10660. [DOI] [PubMed] [Google Scholar]
- 205.Stollberger C, Schneider B, Finsterer J. Elimination of the left atrial appendage to prevent stroke or embolism?: anatomic, physiologic, and pathophysiologic considerations. Chest. 2003;124:2356–2362. doi: 10.1378/chest.124.6.2356. [DOI] [PubMed] [Google Scholar]
- 206.Stewart JM, Dean R, Brown M, et al. Bilateral atrial appendectomy abolishes increased plasma atrial natriuretic peptide release and blunts sodium and water excretion during volume loading in conscious dogs. Circ Res. 1992;70:724–732. doi: 10.1161/01.res.70.4.724. [DOI] [PubMed] [Google Scholar]
- 207.Ad N, Tian YY, Verbalis J, Imahara SD, Cox JL. The effect of the maze procedure on the secretion of arginine-vasopressin and aldosterone. J Thorac Cardiovasc Surg. 2003;126:1095–1100. doi: 10.1016/s0022-5223(03)00975-9. [DOI] [PubMed] [Google Scholar]
- 208.Yoshihara F, Nishikimi T, Kosakai Y, et al. Atrial natriuretic peptide secretion and body fluid balance after bilateral atrial appendectomy by the maze procedure. J Thorac Cardiovasc Surg. 1998;116:213–219. doi: 10.1016/s0022-5223(98)70119-9. [DOI] [PubMed] [Google Scholar]
- 209.Yoshihara F, Nishikimi T, Sasako Y, et al. Preservation of the right atrial appendage improves reduced plasma atrial natriuretic peptide levels after the maze procedure. J Thorac Cardiovasc Surg. 2000;119:790–794. doi: 10.1016/S0022-5223(00)70015-8. [DOI] [PubMed] [Google Scholar]
- 210.Yoshihara F, Nishikimi T, Sasako Y, et al. Plasma atrial natriuretic peptide concentration inversely correlates with left atrial collagen volume fraction in patients with atrial fibrillation: plasma ANP as a possible biochemical marker to predict the outcome of the maze procedure. J Am Coll Cardiol. 2002;39:288–294. doi: 10.1016/s0735-1097(01)01719-3. [DOI] [PubMed] [Google Scholar]
- 211.Khumri TM, Thibodeau JB, Main ML. Transesophageal echocardiographic diagnosis of left atrial appendage occluder device infection. Eur J Echocardiogr. 2008;9:565–566. doi: 10.1016/j.euje.2007.06.005. [DOI] [PubMed] [Google Scholar]