Abstract
Objectives
In a matched analysis, we investigated clinical, histopathological, and survival characteristics of small (≤2 cm) pancreatic cancer (PaC) as compared to large PaC.
Methods
From the Mayo pathology database, we identified 41 consecutive patients with small PaC and 94 matched controls with margin-negative PaC >2 cm. Two experienced pathologists, who were blinded to survival data, independently reviewed tumor stage and differentiation. Kaplan-Meier survival analysis and Cox proportional hazards models were applied for data analyses.
Results
In patients with localized disease (stages I and II), survival was similar in small and large PaC but survival was significantly better in small PaC with regional nodal metastasis (stage III) as compared to similar stage large PaC (5-year survival 44 vs. 7%, median survival 58 vs.18 months, p < 0.001). Well-differentiated small and large PaC had similar median survival (76 vs. 74 months, p = NS). In multivariate analysis, tumor differentiation, not tumor size, was the only independent factor predicting survival in PaC (risk ratio, RR, for moderate vs. well- differentiated: 2.6, 95% confidence interval, CI, 1.5–4.5, and RR for poorly differentiated vs. well-differentiated: 5.0, 95% CI 2.4–10.1).
Conclusion
Tumor differentiation may be a better predictor of survival in resectable PaC than tumor stage.
Key Words: Small pancreatic cancer, Tumor stage, Tumor differentiation, Survival prediction
Introduction
Pancreatic ductal adenocarcinoma (PaC) with a diameter of ≤2 cm (small PaC) is considered an early cancer based on the TNM [1] and Japan Pancreas Society classifications [2], both of which define a T1 tumor as ≤2 cm in size. Tumor size is an important prognostic factor as small PaCs have been reported to have better prognosis and survival after surgery than large PaCs [3,4,5,6,7,8,9], but resected PaCs are usually not small. Of 1,459 resected PaCs collected in 8 series [3,4,5,6,7,8,9,10], only 347 (24%) were small. Most survival data on small PaC are derived from either large surgical series analyzing all resected PaCs [3,4,5,6,7,8,9,10,11] or collected reviews limited to small PaCs [12,13,14,15,16,17]. Most of the large surgical series did not specifically explore the characteristics of the small PaC group in detail. Case series of small PaC, although including more patients, are limited by heterogeneity of data collected from different hospitals using questionnaires and the lack of a comparison group (i.e. large PaC).
In order to gain further insights into the prognosis of small PaC, we compared 41 consecutive patients with small PaCs (≤2cm) resected at the Mayo Clinic between 1985–2001 with a group of 94 patients with large (>2 cm) PaCs who underwent surgery at our institution during the same time period and who were matched for age, gender, tumor location, type of surgery and, if possible, the surgeon. We confined the analysis to subjects who underwent curative resection, i.e. had negative margins. In addition to tumor size and stage, we compared histologic and prognostic markers such as tumor differentiation, angiolymphatic invasion, and perineural invasion.
Material and Methods
Patient Selection
This study was approved by the Mayo Foundation Institutional Review Board. From the Mayo Clinic Surgical Pathology Database, we selected all patients who underwent curative resection for PaC from January 1985 to July 2001. Tumors with the largest diameters reported as ≤2 cm in the gross pathological report were defined as small PaC. All histopathologic sections were independently re-reviewed by two experienced pathologists (T.C.S. and A.O.) to confirm PaC diagnosis, assign tumor stage, evaluate tumor differentiation, and exclude other diagnoses. We excluded patients for whom histopathologic materials were not available for review, patients with positive resection margins, either gross or microscopic, those dying within 1 month of surgery, and patients with simultaneous resection or diagnosis of a second cancer. Both pathologists were blinded to the survival data on these patients.
For every case of small PaC, we selected, blinded to the patients’ data on survival and tumor stage, 2 patients with margin-negative pancreatic ductal adenocarcinoma with a tumor size of >2 cm. Large and small PaCs were matched for age (±5 years), gender, tumor location, type of surgery (pancreaticoduodenectomy vs. distal pancreatectomy), date of surgery (±2 years), and, if possible, pancreatic surgeon. Exclusion criteria similar to those used for small PaC were applied to large PaC. All histopathologic sections of selected large PaC patients were re-reviewed for confirmation of diagnosis, staging, and differentiation by both pathologists independently (T.C.S. and A.O.).
Clinical and Survival Data
Medical records were reviewed to obtain demographic data, presenting symptoms, and date of last contact or death. Follow-up information was obtained from questionnaires sent annually by the Mayo Tumor Registry. If necessary, notice of death was also obtained from Social Security Death Index. Postoperative survival was determined from the date of surgery to the date of last contact or death. Patients who died within 30 days of surgery were excluded. PaC was staged according to the TNM Classification [1].
Histopathologic Data
Tumor differentiation was classified according to the WHO classification as well differentiated, moderately differentiated, and poorly differentiated [18]. The presence of angiolymphatic and perineural invasion was noted.
Statistical Analysis
Continuous variables are presented as mean ± standard deviation and were compared using Student's t test. Categorical variables were compared using the χ2 test or Fisher's exact test (if small number). Survival analyses were performed by Kaplan-Meier analysis and compared by log-rank test. Survival data are presented as 5-year survival and median survival. Multivariate analyses were performed using Cox proportional hazards model and presented as hazard ratios and 95% confidence intervals. Statistical significance was considered when p < 0.05. Statistical analyses were performed using SAS v8.0 (SAS Institute, Inc., Cary, N.C., USA).
Results
Between 1985 and 2001, we identified 85 patients with resected small PaC and negative margins. Eleven patients with small PaC were excluded due to the lack of availability of histopathologic sections for review. After review of the 74 cases, an additional 33 patients were excluded due to revision of diagnosis (ampullary carcinoma (n = 14), intraductal papillary mucinous neoplasm (n = 8), presence of multiple primary cancers (n = 2), positive resection margins (n = 7), and postoperative death (n = 2). Thus, 41 patients with margin-negative small PaC (≤2 cm) were included in the final analysis. We selected 94 patients with large PaC (>2 cm) who were matched to the small PaC patients. There was an excellent correlation for tumor staging and differentiation among the two pathologists, who independently reviewed both small and large PaC.
The small and large PaC groups were similar in terms of mean age, gender, location of tumor, presenting symptoms, and duration of symptoms (table 1). Data on adjuvant chemoradiation were available in 37 and 85 patients with small and large PaC, respectively. Ninety-four percent of small PaC and 89% of large PaC received adjuvant chemoradiation (p = NS). Compared to large PaCs, small PaCs were more likely to be stage I (32 vs. 12%, p = 0.005) and less likely to be stage IVa (2 vs. 14%, p = 0.05; table 1). However, the two groups were similar in the proportion of patients with stage II and stage III disease and positive lymph nodes. When compared to large PaCs, small PaCs were more likely to be well differentiated (44 vs. 15%, p = 0.001) and less likely to be poorly differentiated (0 vs. 27%), p < 0.0001. Large PaC was more likely to have perineural invasion (65 vs. 46%, p = 0.04), whereas angiolymphatic invasion was similar in the two groups (46 vs. 65%, p = NS).
Table 1.
Demographic and clinical characteristics of small (≤2 cm) and large (>2 cm) PaC groups
| Small PaC (n = 41) | Large PaC (n = 94) | p value | |
|---|---|---|---|
| Age at surgery | 62.0812.1 | 62.2811.1 | NS |
| Male, % | 61 | 52 | NS |
| Tumor size, cm | 1.6980.35 | 3.3980.92 | <0.01 |
| Location of tumor | |||
| Head | 40 (98%) | 91 (97%) | NS |
| Body or tail | 1 (2%) | 3 (3%) | |
| Presenting symptoms | |||
| Jaundice | 35 (85%) | 70 (75%) | NS |
| Abdominal pain | 4 (10%) | 19 (20%) | NS |
| Others | 2 (5%) | 5 (5%) | NS |
| Duration of symptoms before surgery, weeks | 9.2±13.6 (0–80) | 9.3±8.7 (0–41) | NS |
| TNM staging | |||
| Stage I | 13 (32%) | 11 (12%) | 0.005 |
| Stage II | 9 (22%) | 24 (25%) | NS |
| Stage III | 18 (44%) | 46 (49%) | NS |
| Stage IVa | 1 (2%) | 13 (14%) | 0.05 |
| Positive lymph nodes | 18 (44%) | 52 (55%) | NS |
Values for age at surgery, tumor size and duration of symptoms before surgery are expressed as mean ± SD.
Small PaC had better overall 5-year actual survival compared to large PaC (41 vs. 14%, median survival 38 vs. 17 months, p = 0.004; table 2). In patients with localized disease (stage I and II), survival was not different between small and large PaC. In contrast, survival of small PaC patients with regional node metastasis (stage III) was significantly better than that of patients with large PaC (5-year survival 44 vs. 6%, median survival 60 vs. 16 months, p = 0.002; table 2).
Table 2.
Effect of tumor stage and lymph node status on survival in small (≤2 cm) and large (>2 cm) PaC
| Parameter | Small PaC (n = 41) |
Large PaC (n = 94) |
pc | ||||
|---|---|---|---|---|---|---|---|
| 5-year survival | median survival, months | pa | 5-year survival | median survival, months | pb | ||
| All cases | 17 (41%) | 38 | 14 (14%) | 17 | <0.01 | ||
| Tumor stage | |||||||
| I | 21 (52%) | 22 | 17 (18%) | 17 | NS | ||
| II | 11 (27%) | 27 | NS | 24 (26%) | 20 | NS | NS |
| III | 18 (44%) | 60 | 6 (6%) | 16 | <0.01 | ||
| IVa | 1 (0%) | 6 | 22 (23%) | 18 | 0.02 | ||
| Lymph nodes | |||||||
| Negative | 16 (39%) | 27 | NS | 24 (25%) | 20 | 0.02 | NS |
| Positive | 18 (44%) | 60 | 5 (5%) | 16 | <0.01 | ||
| Adjuvant chemoradiation | |||||||
| Yes | 19 (45%) | 45 | <0.01 | 8 (9%) | 16 | NS | <0.01 |
| No | 1 (0%) | 12 | 36 (38%) | 18 | NS | ||
Between subgroups of small PaC.
Between subgroups of large PaC.
Between small and large PaC.
Among patients with small PaC, survival was not different among patients with tumor stages I, II, and III, and between lymph node-negative and lymph node-positive patients. In contrast, survival of large PaC was significantly reduced when lymph nodes were positive (5-year survival 25 vs. 5%, median survival 20 vs. 16 months, p = 0.02).
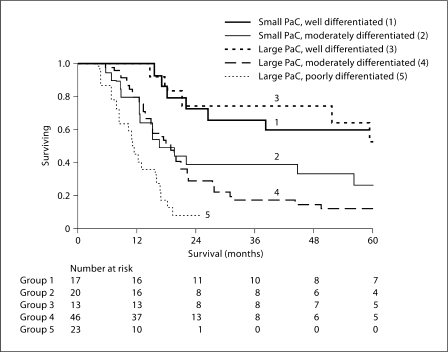
On univariate analysis, the only histopathologic factor affecting survival of small PaC was tumor differentiation, whereas in large PaC, tumor differentiation, presence of angiolymphatic invasion and perineural invasion all affected survival (table 3). Multivariate analysis indicated that tumor differentiation was the only independent factor that determined survival of PaC (table 4). Tumor size (small vs. large), lymph node status, angiolymphatic and perineural invasion and adjuvant chemoradiation were not independent factors affecting survival. For the same degree of tumor differentiation, survival of small and large PaC was not different (fig. 1).
Table 3.
Effect of histopathologic features on survival of small (≤2 cm) and large (>2 cm) PaC
| Parameter | Small PaC (n = 41) |
Large PaC (n = 94) |
pc | ||||
|---|---|---|---|---|---|---|---|
| 5-year survival | median survival, months | pa | 5-year survival | median survival, months | pb | ||
| Tumor differentiation | |||||||
| Well | 23 (56%) | 72 | 0.05 | 45 (48%) | 59 | NS | |
| Moderate | 12 (29%) | 20 | 10 (11%) | 17 | <0.001 | NS | |
| Poor | – | – | 0 (0%) | 13 | – | ||
| Angiolymphatic invasion | |||||||
| No | 20 (49%) | 45 | NS | 17 (18%) | 19 | 0.01 | 0.02 |
| Yes | 9 (23%) | 20 | 7 (8%) | 15 | NS | ||
| Perineural invasion | |||||||
| No | 19 (46%) | 60 | NS | 22 (23%) | 21 | 0.01 | NS |
| Yes | 16 (37%) | 20 | 9 (10%) | 15 | 0.05 | ||
Between subgroups of small PaC.
Between subgroups of large PaC.
Between small and large PaC.
Table 4.
Multivariate analysis of factors influencing survival in PaC
| Variables | Hazard ratio | 95% CI | p value |
|---|---|---|---|
| Tumor size >2 cm | 1.27 | 0.72–2.30 | NS |
| Lymph node status | 0.99 | 0.61–1.64 | NS |
| Tumor differentiation | |||
| Well | Reference group | ||
| Moderate | 2.89 | 1.49–6.07 | <0.01 |
| Poor | 6.53 | 2.81–15.91 | <0.01 |
| Angiolymphatic invasion | 1.37 | 0.84–2.22 | NS |
| Perineural invasion | 1.50 | 0.91–2.53 | NS |
| No adjuvant chemoradiation | 0.91 | 0.43–2.21 | NS |
Fig. 1.
Effect of tumor differentiation on survival after resection of small (≤2 cm) and large PaC (>2 cm). Survival was similar between small and large PaCs with the similar degree of tumor differentiation (p = NS), but was progressively worse with the decreasing degree of tumor differentiation (p = 0.05 for small PaC and p < 0.01 for large PaC).
Discussion
In this study, we confirm the previously noted findings that small PaCs have a better prognosis than large PaCs and are more likely to be confined to the pancreas (stage I) than larger tumors. However, only a third of small PaCs are confined to the pancreas and the better prognosis of small PaCs despite lymph node metastases appears to be related to better differentiation.
Tumor size is an important prognostic factor for PaC. Large surgical series, which included both small and large PaC, reveal that 5-year survival of small PaC (20–32%) and median survival (23–30 months) were better compared to 5-year survival (1–20%) and median survival (10–15 months) of large PaC [3,4,5,6,7,8,9]. The most recent collective review of small PaC by Tsunoda et al. [16], including 302 patients from fifteen reports and seven previous collective studies, noted that the average 5-year survival for small PaC is in the range of 30–40%. These results suggest better survival for small PaC compared to the historically reported survival rates for PaC.
The better prognosis of small PaC may be attributed to detection at an earlier stage. Indeed, in our study, when compared to large PaCs, small PaCs were more likely to be confined to the pancreas, i.e. stage I (32 vs. 12%) and less likely to have distant metastases, i.e. stage IVa. These results are also similar to those of Tsunoda et al. [16], who reported that 40% of small PaCs were stage I and 60% had no lymph node involvement. However, the better differentiation and improved survival of small PaCs compared to large PaCs of similar stage or lymph node status may indicate that small PaCs represent a more indolent subset of PaC rather than being a precursor to large PaC. Support for this argument comes from key observations of our study. Small PaCs were better differentiated than large tumors. While 44% of small PaCs were well differentiated and none were poorly differentiated, only 12% of large PaCs were well differentiated and 28% were poorly differentiated. These observations are similar to those of Manabe et al. [19], who reported a series of 12 small PaCs, of which 9 were well differentiated, 2 were moderately differentiated, and only one was poorly differentiated.
The other pointer towards small PaCs being more indolent is the observation that small PaC patients, even with metastatic disease to lymph nodes (stage III), do remarkably well. In contrast, large PaC patients show a significant drop off in long-term survival once the cancer spreads to lymph nodes. In multivariate analyses, tumor differentiation was the only factor predicting survival in both small and large PaC, further lending support to the importance of biologic behavior of the tumor in determining survival in resectable PaC. Tumor stage, lymph node status, or other histopathologic findings did not affect survival of small PaC. These findings are similar to the results of the collective studies of small PaC by Tsuchiya et al. [12], and Satake et al. [14], who found no difference in 5-year survival between different stages of small PaC and between lymph node positive and negative small PaC. Interestingly, we found that even in large PaC, the only independent prognostic factor was tumor differentiation. Tumor size (small vs. large) itself was not the prognostic factor.
In PaC, tumor differentiation has been found to be a prognostic factor in some studies [3, 7, 20] but not in others [5, 10, 11]. The subjective nature of the assessment of tumor differentiation may partly explain why it is not consistently the predominant prognostic indicator. A recent study showed that tumor grading in the hands of expert pathologists using refined WHO criteria is an important independent prognostic factor [21]. Criteria that relate to cellular and structural differentiation seemed to be more predictive than those related to proliferation. In our study, we had an excellent correlation of tumor staging and differentiation when slides were reviewed independently by experienced gastrointestinal pathologists, suggesting that, in expert hands, evaluation of tumor stage and differentiation are reliable. However, one of the limitations of our study is that we were not able to study the immunohistochemical characteristics of these tumors to further probe their biologic behavior. In addition, our study is also limited by a relatively small sample size, and therefore the finding that small PaCs are in general, better differentiated than large PaCs needs to be confirmed in a larger cohort of patients.
In conclusion, our study confirmed that small PaC has a better prognosis than large PaC. The better differentiation and excellent survival of small PaC, even when metastatic, suggests that small PaCs may be more indolent. Future studies should be directed towards identifying factors that contribute to differences in biologic behavior among small and large PaC.
Acknowledgements
Dr. Chari's research was funded by grants from NIH (R01 CA 100685) and the Mayo Clinic Pancreas Cancer SPORE (P50 CA 10270). Dr. Petersen's research was funded by grants from NIH (R01 CA 100685 and P50 CA 10270).
References
- 1.International Union Against Cancer . TNM Classification of Malignant Tumors. New York: Wiley-Liss; 2002. [Google Scholar]
- 2.Japan Pancreas Society . Classification of Pancreatic Carcinoma. Tokyo: Kanehara; 2003. [PubMed] [Google Scholar]
- 3.Geer RJ, Brennan MF. Prognostic indicators for survival after resection of pancreatic adenocarcinoma. Am J Surg. 1993;165:68–72. doi: 10.1016/s0002-9610(05)80406-4. [DOI] [PubMed] [Google Scholar]
- 4.Nitecki SS, Sarr MG, Colby TV, van Heerden JA. Long-term survival after resection for ductal adenocarcinoma of the pancreas. Is it really improving? Ann Surg. 1995;221:59–66. doi: 10.1097/00000658-199501000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yeo CJ, Cameron JL, Lillemoe KD, Sitzmann JV, Hruban RH, Goodman SN, Dooley WC, Coleman J, Pitt HA. Pancreaticoduodenectomy for cancer of the head of the pancreas. 201 patients. Ann Surg. 1995;221:721–731. doi: 10.1097/00000658-199506000-00011. discussion 731–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brennan MF, Moccia RD, Klimstra D. Management of adenocarcinoma of the body and tail of the pancreas. Ann Surg. 1996;223:506–511. doi: 10.1097/00000658-199605000-00006. discussion 511–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meyer W, Jurowich C, Reichel M, Steinhäuser B, Wünsch PH, Gebhardt C. Pathomorphological and histological prognostic factors in curatively resected ductal adenocarcinoma of the pancreas. Surg Today. 2000;30:582–587. doi: 10.1007/s005950070096. [DOI] [PubMed] [Google Scholar]
- 8.Pantalone D, Ragionieri I, Nesi G. Improved survival in small pancreatic cancer. Dig Surg. 2001;18:41–46. doi: 10.1159/000050095. [DOI] [PubMed] [Google Scholar]
- 9.Lim JE, Chien MW, Earle CC. Prognostic factors following curative resection for pancreatic adenocarcinoma: a population-based, linked database analysis of 396 patients. Ann Surg. 2003;237:74–85. doi: 10.1097/00000658-200301000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cameron JL, Crist DW, Sitzmann JV, Hruban RH, Boitnott JK, Seidler AJ, Coleman J. Factors influencing survival after pancreaticoduodenectomy for pancreatic cancer. Am J Surg. 1991;161:120. doi: 10.1016/0002-9610(91)90371-j. [DOI] [PubMed] [Google Scholar]
- 11.Ahmad NA, Lewis JD, Ginsberg GG, Haller DG, Morris JB, Williams NN, Rosato EF, Kochman ML. Long term survival after pancreatic resection for pancreatic adenocarcinoma. Am J Gastroenterol. 2001;96:2609–2615. doi: 10.1111/j.1572-0241.2001.04123.x. [DOI] [PubMed] [Google Scholar]
- 12.Tsuchiya R, Noda T, Harada N, Miyamoto T, Tomioka T, Yamamoto K, Yamaguchi T, Izawa K, Tsunoda T, Yoshino R, et al. Collective review of small carcinomas of the pancreas. Ann Surg. 1986;203:77–81. doi: 10.1097/00000658-198601000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsuchiya R, Tsunoda T. Tumor size as a predictive factor. Int J Pancreatol. 1990;7:117–123. doi: 10.1007/BF02924227. [DOI] [PubMed] [Google Scholar]
- 14.Satake K, Nishiwaki H, Yokomatsu H, Kawazoe Y, Kim K, Haku A, Umeyama K, Miyazaki I. Surgical curability and prognosis for standard versus extended resection for T1 carcinoma of the pancreas. Surg Gynecol Obstet. 1992;175:259–265. [PubMed] [Google Scholar]
- 15.Furukawa H, Okada S, Saisho H, Ariyama J, Karasawa E, Nakaizumi A, Nakazawa S, Murakami K, Kakizoe T. Clinicopathologic features of small pancreatic adenocarcinoma. A collective study. Cancer. 1996;78:986–990. doi: 10.1002/(SICI)1097-0142(19960901)78:5<986::AID-CNCR7>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 16.Tsunoda T, Yamamoto Y, Kimoto M, Imai H, Iwamoto S, Kawasaki S, Kawashima K, Tadaoka Y, Majima T, Onuma E, Iki K, Kubozoe T, Eto T. Staging and treatment for patients with pancreatic cancer. How small is an early pancreatic cancer? J Hepatobiliary Pancreat Surg. 1998;5:128–132. doi: 10.1007/s005340050022. [DOI] [PubMed] [Google Scholar]
- 17.Tsunoda T, Tsuchiya R. Classification of pancreatic cancer staging revisited. Clinical comparison of UICC and Japanese classifications. Japanese experience. Int J Pancreatol. 1994;16:105–108. [Google Scholar]
- 18.Hamilton S, Aaltonen L. (eds): Tumours of the Exocrine Pancreas. Lyon: IARC Press; 2000. [Google Scholar]
- 19.Manabe T, Miyashita T, Ohshio G, Nonaka A, Suzuki T, Endo K, Takahashi M, Tobe T. Small carcinoma of the pancreas: clinical and pathologic evaluation of 17 patients. Cancer. 1988;62:135–141. doi: 10.1002/1097-0142(19880701)62:1<135::aid-cncr2820620123>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 20.van Geenen RC, van Gulik TM, Offerhaus GJ, de Wit LT, Busch OR, Obertop H, Gouma DJ. Survival after pancreaticoduodenectomy for periampullary adenocarcinoma: an update. Eur J Surg Oncol. 2001;27:549–557. doi: 10.1053/ejso.2001.1162. [DOI] [PubMed] [Google Scholar]
- 21.Lüttges J, Schemm S, Vogel I, Hedderich J, Kremer B, Klöppel G. The grade of pancreatic ductal carcinoma is an independent prognostic factor and is superior to the immunohistochemical assessment of proliferation. J Pathol. 2000;191:154–161. doi: 10.1002/(SICI)1096-9896(200006)191:2<154::AID-PATH603>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]