Abstract
In mammals, climbing fiber axons compete for sole innervation at each Purkinje cell. At the same time, synapses disappear from Purkinje somata and appear in great numbers on the dendrites. In this issue of Neuron, Hashimoto et al. show that, by the time climbing fibers ascend the dendrites, the winner and losers are already decided.
In mammals, large numbers of synaptic partnerships are broken during early postnatal life as a means of refining neural circuits. A role for neural activity in this process is well established. But how and why some connections are maintained while others are eliminated is not fully understood. This process, known as synapse elimination, has been found in both the central and peripheral nervous system. Studies in most parts of the brain have been limited by the complexity, variability, and inaccessibility of central synapses. An exception, however, is the cerebellum, where the emergence of the one-to-one climbing fiber-to-Purkinje cell association has long been appreciated.
In early life, climbing fiber axons form highly branched collaterals with weak perisomatic connections onto hundreds of Purkinje cells (Sugihara, 2006). Each Purkinje cell receives polyneuronal input from a number of different climbing fibers. This arrangement is transient with the removal of all but one of the climbing fibers over the first several postnatal weeks in rodents (Crepel et al., 1976). The transition to single innervation occurs in stages. By the end of the first postnatal week, climbing fibers focus their synapses in “nests” around a subset of their initial Purkinje cell targets. On each Purkinje cell, one of these synaptic nests becomes 2- to 3-fold more powerful than the others (Hashimoto and Kano, 2003). This skewing becomes more extreme in the second postnatal week because weaker inputs completely disconnect. At roughly the same time, however, other excitatory (parallel fibers) and inhibitory inputs (basket cells) make their first synapses onto Purkinje cells (Hashimoto et al., 2009b; Sotelo, 2008). The second postnatal week is also the period when climbing fibers earn their name by growing upward along the proximal dendritic shafts of Purkinje cells to extend their synaptic territory. Finally, in the third postnatal week, synapse elimination is complete, and only one climbing fiber remains. It establishes hundreds of synapses distributed along the proximal dendritic tree, while virtually no climbing fiber synapses remain on the soma (Cesa and Strata, 2009).
Despite the details outlined above, relatively little is known concerning the kinds of interactions that occur between competing climbing fibers. For example, do climbing fibers contend for the same synaptic sites? Do they occupy spatially segregated territories? Do all the inputs climb the dendrites? To address the latter question, Hashimoto et al. (2009a) developed new methods to assay positional information about the synaptic sites of individual axons.
To assess the distance of each climbing fiber from the soma, the authors measured rise times and delays of quantal excitatory postsynaptic currents. At postnatal days 11–14, these measures are correlated for single axons, suggesting that some inputs were on average farther from the soma than others. Moreover, the strongest climbing fiber input appeared to have the strongest dendritic presence. This physiological conclusion was confirmed by reconstructing the extent of the dendritic projection of single climbing fibers labeled with fluorescent protein.
Surprisingly, by looking at earlier stages the authors found that the weaker inputs never appear to establish a significant number of synapses on dendrites. Moreover, they found that when the axons are competing on the cell soma—prior to dendritic translocation—one input becomes dominant. This selective strengthening appears as an increase in the number of somatic contacts the strong axon makes. From serial electron-microscopic data, the authors estimate that at the beginning of the translocation stage (postnatal day 9), the strong input has approximately three times as many synaptic puncta as any other single competitor. Thus, inter-axonal competition on the cell soma seems to establish the identity of the one axon that will take over the dendrite region and hence become the one lasting connection. Importantly, this work suggests that synaptic competition has a role not only in removing synapses of losing axons but also in the elaboration of synapses by the winning one. Why no other input gains access to and grows along the proximal dendrite remains unknown.
One value of the present work is that it links a physiological assessment of circuit changes to a structural one. While such physiology/anatomy relationships have been evaluated in two parts of the peripheral nervous system (the neuromuscular junction and the parasympathetic submandibular ganglion), they are rare in the mammalian CNS. Now that they have been done, it is clear that there are similarities between synapse elimination at all three sites.
The first major similarity is that inputs are removed permanently during development in these systems. In muscle and cerebellum, this leaves the postsynaptic cell innervated by one motor axon or one climbing fiber. In the submandibular ganglion, it leaves the target cell with one suprathreshold input and a very small number of subthreshold inputs (Lichtman, 1977). It is not that the synapses of the weaker axons are functionally silenced; rather, all structural and physiological evidence suggests that they have been physically removed. One hypothesis is that physical removal allows an axon to concentrate its resources at other postsynaptic targets, and consequently increase its efficacy at its remaining competitions. In any case, this removal is an irreversible termination that marks the end of a “critical period” of development. Inputs that are removed do not ordinarily ever reinnervate these targets.
A second similarity is that in all three systems there are major alterations in the synaptic inputs that survive the elimination phase. In each case, a large increase in synaptic contact number by the remaining axon assures that it becomes a reliable driver of postsynaptic activity without the necessity for synaptic integration. Interestingly, ultimately in each system there is exactly one suprathreshold input. Why two or more separate strong inputs are unable to sustain innervation to the same postsynaptic cell is not understood, but one popular idea is that the most stable innervation pattern would be one where the pre- and postsynaptic activity patterns are highly concordant. Thus, when all the synaptic release sites originate from the same axon, then every synapse is assured to have an activity pattern that matches the postsynaptic cell's activity pattern. A number of experiments in cerebellum and neuromuscular junction support this “Hebbian” view (Busetto et al., 2003; Andjus et al., 2003). What governs which input will win and which will lose is an even more perplexing question for which there is yet no answer.
A final similarity to note is that in all three developmental situations it appears that the location of axonal branches affects their fate. In the present study, synapses on the somata were fated for loss whereas synapses of the dendrites were not. In muscle, synapses near a competing input were fated for quick removal, whereas synapses located only slightly farther away were more stable and only lost at the very last stage of synapse elimination (Walsh and Lichtman, 2003). In both muscle and cerebellum, it also appears that the addition of synapses by the remaining axon is temporally correlated with the loss of synapses. Thus, as one axon adds synapses by translocating to dendrites (climbing fibers) or taking over synaptic sites (motor axons), competing axons are losing synaptic contact sites. Moreover, it appears that even the winning axon inevitably loses parts of its original territory as synapse elimination ensues. In cerebellum, all climbing fibers are booted off the soma, including the ultimate victor. The authors interpret this as a “nonselective elimination stage” distinct from the competitive era when it is determined which axon moves to the dendrites. Another interpretation would be that there is some form of activity-dependent competition (perhaps between climbing fibers and other classes of input) that causes synapse loss from the soma. One interesting question for future studies would be to determine if climbing fibers segregate their synaptic territories on the soma prior to the translocation event. If so, this would suggest a potential precondition that helps select the axon that will occupy the dendrites. Such a segregation step would furthermore denote a potential shared mechanism for synaptic strengthening among these diverse systems (see Gan and Lichtman, 1998).
It is surprising that synapses on muscle fibers, autonomic ganglion cells, and Purkinje cells should have any similarities given the enormous functional, structural, and biochemical differences between these systems. Nonetheless, as shown in Figure 1, both neuromuscular and cerebellar systems seem to be going through analogous stages as postsynaptic cells transform from multiply innervated to singly innervated targets. As more is discovered about alterations in developing circuits, it will become clearer whether the essential processes at work during early postnatal refinement are indeed driven by common mechanisms throughout the nervous system. Will principles emerge that transcend the peculiarities of each system? We think this article makes a case for optimism.
Figure 1. A Comparative View of Synapse Elimination.
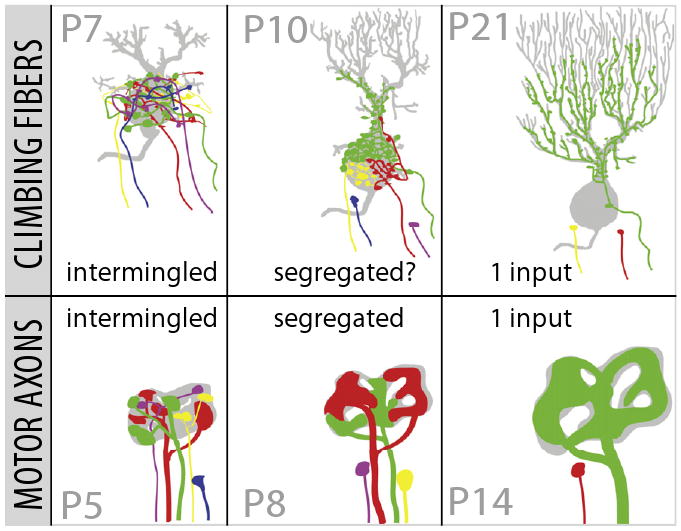
Development of the climbing fiber-Purkinje synapse (top row) parallels the changes observed in the motor axon-muscle fiber synapse (bottom row). At early stages (left column), each synapse is innervated by inputs of varying synaptic strength that are spatially intermingled. As development proceeds (middle column), the weakest inputs are permanently disconnected, others weaken but stay attached, and the remaining strengthen by increasing their synaptic representation. Motor axons at this age segregate into spatial territories. One climbing fiber segregates its inputs to the dendrite; whether or not the remaining somatic synapses also have synaptic territories is not known. At the end of synapse elimination (right column), only one input maintains its connection, while all others are physically disconnected. The winning input—in both cases—greatly enlarges the number and strength of its synapses. Figure adapted from Sugihara, 2006.
References
- Andjus PR, Zhu L, Cesa R, Carulli D, Strata P. Neuroscience. 2003;121:563–572. doi: 10.1016/s0306-4522(03)00556-6. [DOI] [PubMed] [Google Scholar]
- Busetto G, Buffelli M, Cangiano L, Cangiano A. J Neurocytol. 2003;32:795–802. doi: 10.1023/B:NEUR.0000020624.48032.ed. [DOI] [PubMed] [Google Scholar]
- Cesa R, Strata P. Neuroscience. 2009 doi: 10.1016/j.neuroscience.2009.02.061. in press. [DOI] [PubMed] [Google Scholar]
- Crepel F, Mariani J, Delhaye-Bouchaud N. J Neurobiol. 1976;7:567–578. doi: 10.1002/neu.480070609. [DOI] [PubMed] [Google Scholar]
- Gan WB, Lichtman JW. Science. 1998;282:1508–1511. doi: 10.1126/science.282.5393.1508. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Kano M. Neuron. 2003;38:785–796. doi: 10.1016/s0896-6273(03)00298-8. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Ichikawa R, Kitamura K, Watanabe M, Kano M. Neuron. 2009a;63(this issue):106–118. doi: 10.1016/j.neuron.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Yoshida T, Sakimura K, Mishina M, Watanabe M, Kano M. Neuroscience. 2009b doi: 10.1016/j.neuroscience.2008.12.037. in press. [DOI] [PubMed] [Google Scholar]
- Lichtman JW. J Physiol. 1977;273:155–177. doi: 10.1113/jphysiol.1977.sp012087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotelo C. J Comp Neurol. 2008;506:240–262. doi: 10.1002/cne.21501. [DOI] [PubMed] [Google Scholar]
- Sugihara I. Cerebellum. 2006;5:15–22. doi: 10.1080/14734220500527385. [DOI] [PubMed] [Google Scholar]
- Walsh MK, Lichtman JW. Neuron. 2003;37:67–73. doi: 10.1016/s0896-6273(02)01142-x. [DOI] [PubMed] [Google Scholar]


