Abstract
We have investigated the effect of benzo[a]pyrene (B[a]P), a carcinogen of tobacco smoke and an agonist for the aryl hydrocarbon receptor (AHR), on hypoxia-induced angiogenesis. Ischemia was induced by femoral artery ligation in wild-type and AHR-null mice, and the animals were subjected to oral administration of B[a]P (125 mg/kg) once a week. Exposure to B[a]P up-regulated the expression of metallothionein in the ischemic hindlimb and markedly inhibited ischemia-induced angiogenesis in wild-type mice. The amounts of interleukin-6 and of vascular endothelial growth factor (VEGF) mRNA in the ischemic hindlimb of wild-type mice were reduced by exposure to B[a]P. These various effects of B[a]P were markedly attenuated in AHR-null mice. Our observations suggest that the loss of the inhibitory effect of B[a]P on ischemia-induced angiogenesis apparent in AHR-null mice may be attributable to maintenance of interleukin-6 expression and consequent promotion of angiogenesis through up-regulation of VEGF expression.
Keywords: Angiogenesis, Hypoxia, Smoking, Benzo[a]pyrene, Peripheral vascular disease, Metallothionein, Interleukin-6
Cigarette smoke is a major risk factor for ischemic heart disease, peripheral vascular disease, and chronic obstructive pulmonary disease [1,2]. It has adverse effects on vascular biology, inducing endothelial dysfunction and arterial stiffness [3], and it inhibits angiogenesis by pulmonary artery endothelial cells in the setting of severe vascular obstruction and lung tissue ischemia [4]. Angiogenesis, the development of new blood vessels from existing vessels, is a tightly controlled physiological process [5]. Vascular endothelial growth factor (VEGF) is a key angiogenic factor produced by ischemic tissue and growing tumors [6], and up-regulation of VEGF expression at the transcriptional level is thought to be responsible for the progressive development of the collateral circulation [7]. Although the association between smoking and vascular disease is well established, the mechanism by which cigarette smoke inhibits angiogenesis has remained unclear.
The effects of benzo[a]pyrene (B[a]P) in cigarette smoke and of other polycyclic and halogenated aromatic hydrocarbons in the environment are mediated by the aryl hydrocarbon receptor (AHR) [8]. The ligand-bound form of the AHR and AHR nuclear translocator (ARNT), which belongs to the Per-Arnt-Sim (PAS) family of basic helix-loop-helix transcription factors, form a heterodimeric transcription factor [9] that binds to xenobiotic response elements (XREs) in the promoter regions of target genes such as that encoding cytochrome P4501A1 (CYP1A1) [10]. Activation of the VEGF gene is mediated by the binding of another basic helix-loop-helix transcription factor, hypoxia-inducible factor-1α (HIF-1α) [11], which also dimerizes with ARNT [12]. We previously showed that AHR-ARNT signaling plays an important role in the regulation of ischemia-induced angiogenesis [13]. Given that smoking is a risk factor for ischemic heart disease and peripheral vascular disease, we hypothesized that B[a]P might impair angiogenesis and that AHR signaling might contribute to such an effect. We have now investigated this hypothesis with the use of AHR-null mice and an animal model in which ischemia is induced surgically in a hindlimb.
Materials and methods
Animals
Twelve-week-old male wild-type (WT) or AHR-null mice backcrossed with C57BL/6N mice for more than eight generations were studied. AHR-null mice were obtained from the National Cancer Institute colony [14] and maintained by the Animal Resource Facility at Nagoya University Graduate School of Medicine. Animals were subjected to left femoral artery ligation as described previously [13]. All experimental procedures were performed in accordance with Institutional Guidelines for Animal Research, and the study was approved by the Animal Ethics Committee of Nagoya University Graduate School of Medicine.
Systolic blood pressure and laser Doppler perfusion imaging
Systolic blood pressure of conscious mice was measured by the tail-cuff method (BP-98A, MCP-1; Softron, Tokyo, Japan) immediately before and each week after arterial ligation. Evidence for ischemia-induced functional changes in vascularization was obtained by laser Doppler perfusion imaging with a laser Doppler blood flow analyzer (Moor LDI; Moor Instruments, Axminster, UK) immediately as well as each week after surgery, as previously described [13]. After scanning, the stored images were analyzed to quantify blood flow, and the average flows of the ischemic and nonischemic hindlimbs were calculated. To avoid interference from ambient light and body temperature, we expressed blood flow in the ischemic leg relative to that in the nonischemic leg.
Oral exposure to B[a]P
The effect of B[a]P treatment on angiogenesis induced by femoral artery ligation was examined in WT and AHR-null mice. B[a]P (Sigma–Aldrich Japan, Tokyo, Japan) was first administered to WT mice by oral gavage in corn oil (10 ml/kg) at 125, 25, or 5 mg/kg once per week as described [15], with the first dose being administered immediately before surgery. Ischemia-induced functional changes in vascularization were evaluated by laser Doppler perfusion imaging immediately as well as 7, 14, and 21 days after surgery. Given that B[a]P at only the dose of 125 mg/kg per week induced significant inhibition of the recovery of blood flow in the ischemic hindlimb of WT mice, the effects of B[a]P were compared between WT and AHR-null mice at this dose.
RT-PCR and immunoblot analyses
Total RNA was extracted from ischemic or nonischemic thigh muscles isolated 3 days after surgery and was subjected to quantitative reverse transcription (RT) and polymerase chain reaction (PCR) analysis with primers and probes specific for HIF-1α, ARNT, AHR, CYP1A1, VEGF, VEGF receptor (Flt-1, Flk-1), Ang-1, Ang-1 receptor (Tie2), metallothionein (MT)-1, MT-2, and interleukin (IL)-6 mRNAs. The mRNA for β-actin was used as an internal standard. Protein extracts of ischemic or nonischemic thigh muscles isolated 1 week after surgery were subjected to immunoblot analysis with rabbit polyclonal antibodies to mouse VEGF (Santa Cruz Biotechnology, Santa Monica, CA), to mouse Ang-1 (Alpha Diagnostic, San Antonio, TX), and to human MT (Santa Cruz Biotechnology) at dilutions of 1:500, 1:1000, and 1:200, respectively, as well as with a mouse monoclonal antibody to mouse β-actin (Sigma, St. Louis, MO) at a dilution of 1:2000. Immune complexes were detected with enhanced chemiluminescence (ECL) reagents (GE Healthcare Bio-Science, Piscataway, NJ).
Assay of IL-6
Protein extracts prepared from ischemic or nonischemic thigh muscles isolated 1 week after surgery were assayed for IL-6 with an enzyme-linked immunosorbent assay (ELISA) kit (Pierce/Endogen, Rockford, IL) as described [16]. The assay was performed in duplicate, and absorbance at 450 nm was measured with a microtiter plate reader. The tissue content of IL-6 was expressed as picograms per milligram of protein.
Statistical analysis
Data are presented as means ± SEM. Statistical significance of differences was evaluated by one-way analysis of variance followed by Dunnett's post hoc test for comparisons among four or eight means. A P value of <0.05 was considered statistically significant.
Results
Body weight and systolic blood pressure
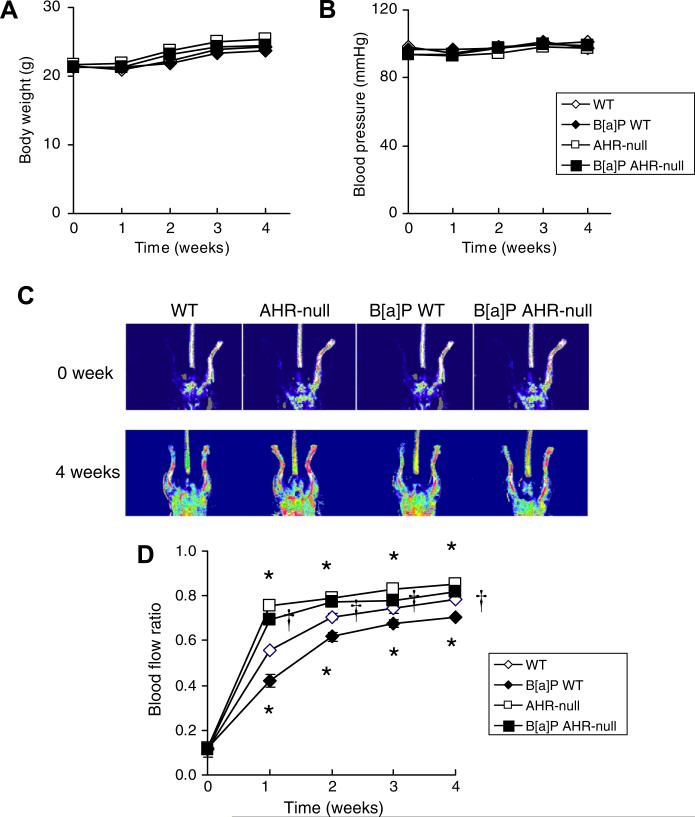
Body weight did not differ between AHR-null and WT mice either before or for up to 4 weeks after arterial ligation to induce hypoxia in the left hindlimb (Fig. 1A). Weekly oral administration of B[a]P (125 mg/kg) beginning immediately before surgery did not affect body weight in either mouse strain (Fig. 1A). Systolic blood pressure remained at basal levels for up to 4 weeks after surgery and also did not differ between AHR-null and WT mice (Fig. 1B). Exposure to B[a]P did not affect systolic blood pressure in AHR-null or WT mice (Fig. 1B).
Fig. 1.
Changes in body weight, systolic blood pressure, and blood flow ratio after arterial ligation in the left hindlimb of AHR-null or WT mice exposed to B[a]P or vehicle. (A,B) Body weight (A) and systolic blood pressure (B) before (time 0) and for up to 4 weeks after surgery and the onset of weekly oral administration of B[a]P (125 mg/kg) or vehicle. (C) Laser Doppler perfusion imaging of blood flow immediately and 4 weeks after surgery and the onset of weekly administration of B[a]P or vehicle. (D) The ratio of blood flow in the ischemic (left) hindlimb to that in the normal (right) hindlimb measured immediately as well as each week after surgery and the onset of weekly administration of B[a]P or vehicle. All quantitative data are means ± SEM of values from eight animals per group. *P < 0.05 versus corresponding value for vehicle-treated WT mice; †P < 0.05 versus corresponding value for B[a]P-treated WT mice.
Blood flow ratio
The ischemic/nonischemic blood flow ratio of AHR-null mice was significantly greater than that of WT mice at 1 week after surgery, and this difference remained apparent for up to 4 weeks (Fig. 1C and D). Blood flow recovered in the ischemic hindlimb of WT mice exposed to B[a]P. However, 1 week after surgery, the ischemic/nonischemic blood flow ratio of WT mice exposed to B[a]P was significantly smaller than that of vehicle-treated WT mice, and this difference also remained apparent for up to 4 weeks (Fig. 1C and D). Furthermore, the blood flow ratio was significantly greater in AHR-null mice exposed to B[a]P than in WT animals exposed to this agent.
Expression of VEGF, Ang-1, and their receptors
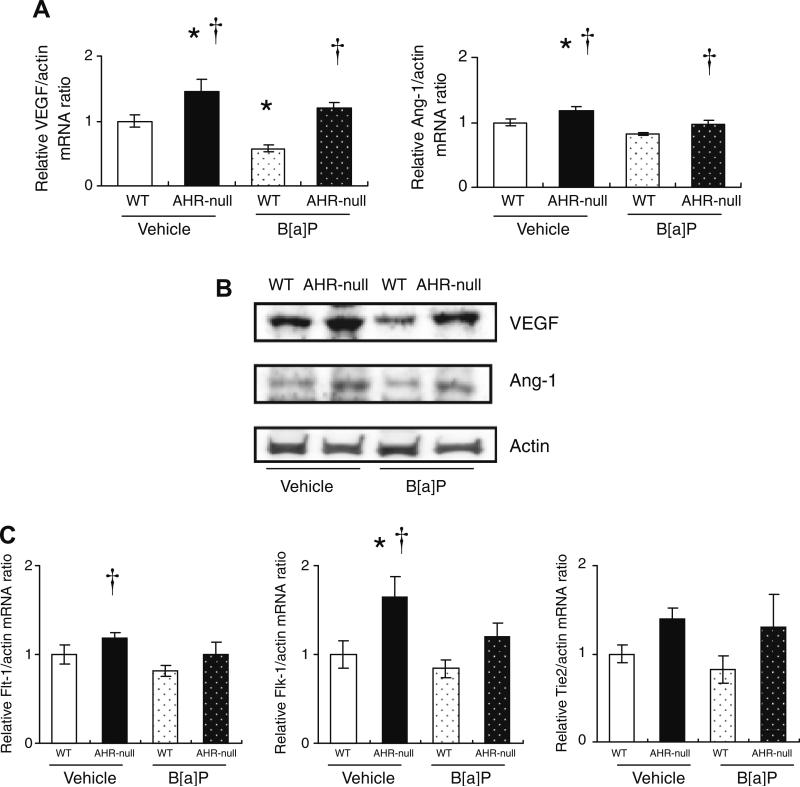
The amounts of mRNAs for the angiogenic factors, VEGF and Ang-1, in the ischemic hindlimb were significantly greater for AHR-null mice than for WT mice at 3 days after surgery (Fig. 2A). The abundance of VEGF mRNA in the ischemic hindlimb was also significantly reduced in WT mice exposed to B[a]P compared with that in vehicle-treated WT mice. The amounts of VEGF and Ang-1 mRNAs in the ischemic hindlimb were significantly greater for AHR-null mice exposed to B[a]P than for WT animals exposed to this agent. Immunoblot analysis revealed similar changes in the abundance of VEGF and Ang-1 proteins at 1 week after surgery (Fig. 2B). With regard to the expression of genes encoding receptors for VEGF (Flt-1, Flk-1) or for Ang-1 (Tie2), the amount of Flk-1 mRNA in the ischemic hindlimb was significantly greater for AHR-null mice than for WT mice (Fig. 2C). However, B[a]P had no effect on the abundance of Flt-1, Flk-1, or Tie2 mRNAs in the ischemic hindlimb of either mouse strain, and there were no differences in the amounts of these mRNAs in the ischemic hindlimb between AHR-null or WT mice exposed to B[a]P (Fig. 2C).
Fig. 2.
Expression of VEGF, Ang-1, and their receptors in the ischemic hindlimb of AHR-null or WT mice exposed to B[a]P or vehicle. (A) Quantitative RT-PCR analysis of VEGF and Ang-1 mRNAs in ischemic skeletal muscle isolated 3 days after surgery and oral administration of B[a]P or vehicle. Data are normalized by the abundance of β-actin mRNA. (B) Representative immunoblot analysis of VEGF and Ang-1 in ischemic skeletal muscle isolated 1 week after surgery and administration of B[a]P or vehicle. (C) Quantitative RT-PCR analysis of Flt-1, Flk-1, and Tie2 mRNAs in ischemic skeletal muscle isolated 3 days after surgery and administration of B[a]P or vehicle. All quantitative data are means ± SEM of values from eight mice per group. *P < 0.05 versus corresponding value for vehicle-treated WT mice; †P < 0.05 versus corresponding value for B[a]P-treated WT mice.
Expression of HIF-1α, ARNT, AHR, and CYP1A1
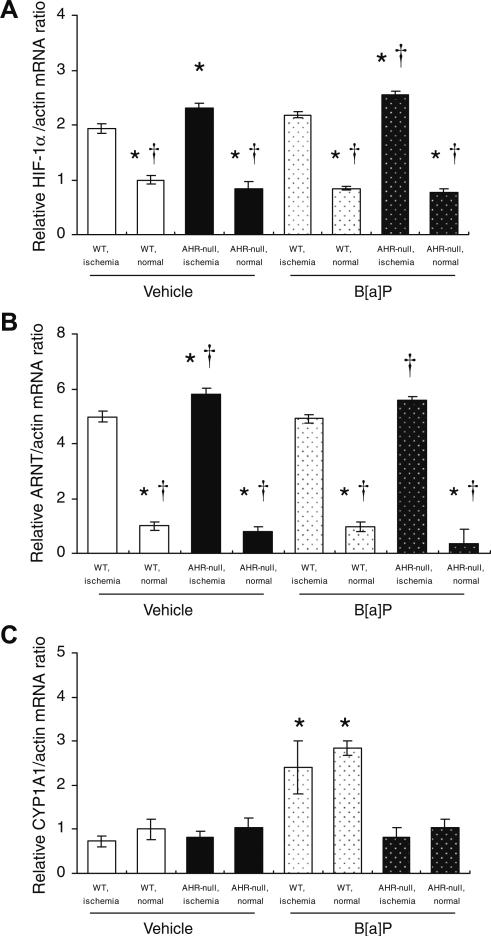
Quantitative RT-PCR analysis revealed that, whereas the amounts of both HIF-1α and ARNT mRNAs were significantly greater for the ischemic hindlimb than for the nonischemic hind-limb of AHR-null or WT mice at 3 days after surgery, they were also significantly greater for the ischemic hindlimb of AHR-null mice than for that of WT mice (Fig. 3A and B). Exposure to B[a]P did not affect the amounts of HIF-1α or ARNT mRNAs in AHR-null or WT mice. The amount of AHR mRNA in skeletal muscle of WT mice did not differ between the ischemic and nonischemic hindlimbs and was also not affected by exposure to B[a]P (data not shown). There was no difference in the abundance of CYP1A1 mRNA between the ischemic and nonischemic hindlimbs of either mouse strain (Fig. 3C). Exposure to B[a]P induced significant increases in the amount of CYP1A1 mRNA only in WT mice (Fig. 3C).
Fig. 3.
Expression of HIF-1a, ARNT, and CYP1A1 in the ischemic and nonischemic hindlimbs of AHR-null or WT mice exposed to B[a]P or vehicle. The amounts of HIF-1α (A), ARNT (B), and CYP1A1 (C) mRNAs in tissue isolated 3 days after surgery and administration of B[a]P or vehicle were determined by quantitative RT-PCR analysis. Data are normalized by the abundance of β-actin mRNA and are means ± SEM of values from eight mice per group. *P < 0.05 versus corresponding value for the ischemic hindlimb of vehicle-treated WT mice; †P < 0.05 versus corresponding value for the ischemic hindlimb of B[a]P-treated WT mice.
Expression of MT-1 and MT-2
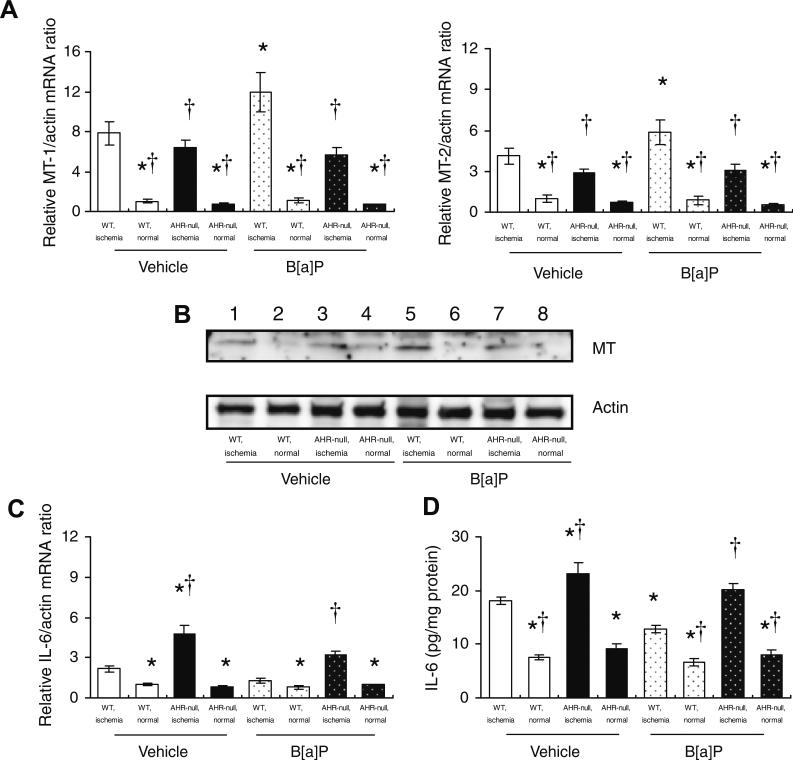
The amounts of MT-1 and MT-2 mRNAs were increased in the ischemic hindlimb of both AHR-null and WT mice compared with those in the corresponding nonischemic hindlimb at 3 days after surgery (Fig. 4A). Exposure to B[a]P resulted in significant increases in the abundance of MT-1 and MT-2 mRNAs in the ischemic hindlimb of WT mice but not in that of AHR-null mice (Fig. 4A). Immunoblot analysis also revealed similar changes in the abundance of MT at 1 week after surgery (Fig. 4B).
Fig. 4.
Expression of MT and abundance of IL-6 mRNA and IL-6 immunoreactivity in the ischemic and nonischemic hindlimbs of AHR-null or WT mice exposed to B[a]P or vehicle. (A) Quantitative RT-PCR analysis of MT-1 and MT-2 mRNAs in tissue isolated 3 days after surgery and administration of B[a]P or vehicle. Data are normalized by the abundance of β-actin mRNA. (B) Representative immunoblot analysis of MT in tissue isolated 1 week after surgery and administration of B[a]P or vehicle. (C) Quantitative RT-PCR analysis of IL-6 mRNA in tissue isolated 3 days after surgery and administration of B[a]P or vehicle. (D) IL-6 immunoreactivity in tissue isolated 1 week after surgery. All data are means ± SEM of values from eight mice per group. *P < 0.05 versus corresponding value for the ischemic hindlimb of vehicle-treated WT mice; †P < 0.05 versus corresponding value for the ischemic hindlimb of B[a]P-treated WT mice.
Expression of IL-6
The amount of IL-6 mRNA was increased in the ischemic hindlimb of both AHR-null and WT mice compared with that in the corresponding nonischemic hindlimb at 3 days after surgery (Fig. 4C). This effect of ischemia was more pronounced in AHR-null mice than in WT mice. The abundance of IL-6 mRNA in the ischemic hindlimb of AHR-null mice exposed to B[a]P was also significantly greater than that for WT mice exposed to this agent. The amount of IL-6 immunoreactivity was also increased in the ischemic hindlimb of both AHR-null and WT mice compared with that in the corresponding nonischemic hindlimb at 1 week after surgery (Fig. 4D). IL-6 immunoreactivity in the ischemic hindlimb of WT mice exposed to B[a]P was significantly reduced compared with that for vehicle-treated WT mice. It was also significantly greater in the ischemic hindlimb of AHR-null mice exposed to B[a]P than in that of WT animals exposed to this agent.
Discussion
Exposure to cigarette smoke has a negative effect on endothelial function, serum lipid profile, and hemostatic factors [17]. Smoking plays a central role in the initiation and progression of Buerger's disease, a nonatherosclerotic condition that most commonly affects small and medium-sized arteries and veins as well as nerves of the extremities [18]. To examine the effects of cigarette smoke on angiogenesis, we treated mice with B[a]P, one of the polycyclic and halogenated aromatic hydrocarbons found in tobacco smoke. Oral exposure to B[a]P resulted in significant inhibition of the increase in blood flow induced by hypoxia in WT mice. Furthermore, we found that this inhibition of angiogenesis by B[a]P was associated with inhibition of hypoxia-induced up-regulation of VEGF expression. Cigarette smoke was previously shown to inhibit secretion of the soluble form of the VEGF receptor Flt-1 in a dose-dependent manner [19]. Inhibition of the VEGF receptor Flk-1 was also shown to augment the vascular and endothelial dysfunction induced by cigarette smoke [20]. However, we detected no significant effect of B[a]P on the abundance of Flt-1 or Flk-1 mRNAs in ischemic hindlimb of WT mice in the present study.
MTs are cysteine-rich metal-binding proteins with diverse physiological functions, including protection against metal toxicity and oxidants [21]. Furthermore, MT expression has been shown to be increased at the transcriptional level by hypoxia acting through metal response elements in the proximal promoter region of MT genes [22]. In the present study, we detected a marked increase in the expression of MT-1 and MT-2 in the ischemic hindlimb of both AHR-null and WT mice. Moreover, B[a]P induced a further marked increase in MT expression in the ischemic hindlimb of WT mice. This observation is consistent with previous studies showing that MT expression was induced in response to B[a]P exposure [23]. Such an increase in MT expression may reflect a protective response to B[a]P-induced oxidative stress. We also found that B[a]P induced a decrease in the amount of IL-6 in the ischemic hindlimb of WT mice. IL-6 has been shown to induce MT expression by binding to IL-6-responsive elements in the gene promoter [24]. On the other hand, the lipopolysaccharide-induced increases in the circulating concentration of IL-6 as well as in the expression of IL-6 in lung, kidney, and liver were markedly greater in mice deficient in MT-1 and MT-2 than in WT mice [25]. Furthermore, overexpression of MT-1 inhibited up-regulation of ectopic IL-6 expression in astrocytes of transgenic mice [26]. IL-6 has also been shown to support angiogenesis by up-regulating the expression of VEGF [27]. The increase in MT expression in the ischemic hindlimb of WT mice induced by B[a]P in the present study may thus result in down-regulation of IL-6 expression, which in turn may lead to impairment of hypoxia-induced angiogenesis.
AHR-null mice are relatively unaffected by the potent AHR ligand 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) at doses that induce severe toxic and pathological effects in WT mice [28]. Moreover, genotoxic and carcinogenic responses to B[a]P or dibenzo[a,l]pyrene are greatly diminished in AHR-null mice [29]. In the present study, the increase in the amount of CYP1A1 mRNA induced by B[a]P in WT mice was not apparent in AHR-null mice, consistent with our finding that AHR deficiency attenuated the inhibition of ischemia-induced angiogenesis by B[a]P. The expression of MT induced by B[a]P in the ischemic hindlimb of WT mice was not detected in that of AHR-null mice. The amount of IL-6 in the ischemic hindlimb of AHR-null mice was not affected by exposure to B[a]P, consistent with the lack of an effect of B[a]P on MT expression and possibly contributing to the diminution of the impairment of ischemia-induced angiogenesis by B[a]P in these mice.
In conclusion, oral exposure to B[a]P resulted in a marked impairment of angiogenesis in response to surgically induced hindlimb ischemia. Furthermore, the impairment of ischemia-induced angiogenesis by B[a]P was greatly reduced in AHR-null mice. Our observations suggest that the loss of the inhibitory effect of B[a]P on ischemia-induced angiogenesis apparent in AHR-null mice may be attributable to maintenance of IL-6 expression and consequent promotion of angiogenesis through up-regulation of VEGF expression.
Acknowledgments
This study was supported in part by grants (to S.I.) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (No. 18590553).
References
- 1.Celermajer D, Sorensen K, Georgakopoulos D, Bull C, Thomas O, Robinson J, Deanfield J. Cigarette smoking is associated with dose-related and potentially reversible impairment of endothelium-dependent dilation in healthy young adults. Circulation. 1993;88:2149–2155. doi: 10.1161/01.cir.88.5.2149. [DOI] [PubMed] [Google Scholar]
- 2.Sethi JM, Rochester CL. Smoking and chronic obstructive pulmonary disease. Clin. Chest Med. 2000;21:67–86. doi: 10.1016/s0272-5231(05)70008-3. [DOI] [PubMed] [Google Scholar]
- 3.Celermajer DS, Adams MR, Clarkson P, Robinson J, McCredie R, Donald A, Deanfield JE. Passive smoking and impaired endothelium-dependent arterial dilatation in healthy young adults. N. Engl. J. Med. 1996;334:150–154. doi: 10.1056/NEJM199601183340303. [DOI] [PubMed] [Google Scholar]
- 4.Su Y, Cao W, Han Z, Block ER. Cigarette smoke extract inhibits angiogenesis of pulmonary artery endothelial cells: the role of calpain. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004;287:L794–L800. doi: 10.1152/ajplung.00079.2004. [DOI] [PubMed] [Google Scholar]
- 5.Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438:932–936. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- 6.Thomas KA. Vascular endothelial growth factor, a potent and selective angiogenic agent. J. Biol. Chem. 1996;271:603–606. doi: 10.1074/jbc.271.2.603. [DOI] [PubMed] [Google Scholar]
- 7.Takeshita S, Zheng LP, Brogi E, Kearney M, Pu L-Q, Bunting S, Ferrara N, Symes JF, Isner JM. Therapeutic angiogenesis: a single intraarterial bolus of vascular endothelial growth factor augments revascularization in a rabbit ischemic hind limb model. J. Clin. Invest. 1994;93:662–670. doi: 10.1172/JCI117018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmidt JV, Bradfield CA. Ah receptor signaling pathways. Annu. Rev. Cell Dev. Biol. 1996;12:55–89. doi: 10.1146/annurev.cellbio.12.1.55. [DOI] [PubMed] [Google Scholar]
- 9.Reyes H, Reisz-Porszansz S, Hankinson O. Identification of the Ah receptor nuclear translocator protein (Arnt) as a component of the DNA binding form of the Ah receptor. Science. 1992;256:1193–1195. doi: 10.1126/science.256.5060.1193. [DOI] [PubMed] [Google Scholar]
- 10.Dong L, Ma Q, Whitlock JP., Jr. DNA binding by the heterodimeric Ah receptor: relationship to dioxin-induced CYP1A1 transcription in vivo. J. Biol. Chem. 1996;271:7942–7948. doi: 10.1074/jbc.271.14.7942. [DOI] [PubMed] [Google Scholar]
- 11.Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, Semenza GL. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol. Cell. Biol. 1996;16:4604–4613. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wiesener MS, Turley H, Allen WE, Willam C, Eckardt KU, Talks KL, Wood SM, Gatter KC, Harris AL, Pugh CW, Ratcliffe PJ, Maxwell PH. Induction of endothelial PAS domain protein-1 by hypoxia: characterization and comparison with hypoxia-inducible factor-1α. Blood. 1998;92:2260–2268. [PubMed] [Google Scholar]
- 13.Ichihara S, Yamada Y, Ichihara G, Nakajima T, Li P, Kondo T, Gonzalez FJ, Murohara T. A role for the aryl hydrocarbon receptor in regulation of ischemia-induced angiogenesis. Arterioscler. Thromb. Vasc. Biol. 2007;27:1297–1304. doi: 10.1161/ATVBAHA.106.138701. [DOI] [PubMed] [Google Scholar]
- 14.Fernandez-Salguero P, Pineau T, Hilbert DM, McPhail T, Lee SS, Kim S, Nebert DW, Rudikoff S, Ward JM, Gonzalez FJ. Immune system impairment and hepatic fibrosis in mice lacking the dioxin-binding Ah receptor. Science. 1995;268:722–726. doi: 10.1126/science.7732381. [DOI] [PubMed] [Google Scholar]
- 15.Uno S, Dalton TP, Derkenne S, Curran CP, Miller ML, Shertzer HG, Nebert DW. Oral exposure to benzo[a]pyrene in the mouse: detoxification by inducible cytochrome P450 is more important then metabolic activation. Mol. Pharmacol. 2004;65:1225–1237. doi: 10.1124/mol.65.5.1225. [DOI] [PubMed] [Google Scholar]
- 16.Ajuwon KM, Spurlock ME. Adiponectin inhibits LPS-induced NF-κB activation and IL-6 production and increases PPARγ2 expression in adipocytes. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005;288:R1220–R1225. doi: 10.1152/ajpregu.00397.2004. [DOI] [PubMed] [Google Scholar]
- 17.Villablanca AC, McDonald JM, Rutledge JC. Smoking and cardiovascular disease. Clin. Chest Med. 2000;21:159–172. doi: 10.1016/s0272-5231(05)70015-0. [DOI] [PubMed] [Google Scholar]
- 18.Olin JW. Thromboangiitis obliterans (Buerger's disease) N. Engl. J. Med. 2000;343:864–869. doi: 10.1056/NEJM200009213431207. [DOI] [PubMed] [Google Scholar]
- 19.Mehendale R, Hibbard J, Fazleabas A, Leach R. Placental angiogenesis markers sFlt-1 and PIGF: response to cigarette smoke. Am. J. Obstet. Gynecol. 2007;197:363.e1–363.e5. doi: 10.1016/j.ajog.2007.06.025. [DOI] [PubMed] [Google Scholar]
- 20.Edirisinghe I, Yang SR, Yao H, Rajendrasozhan S, Caito S, Adenuga D, Wong C, Rahman A, Phipps RP, Jin ZG, Rahman I. VEGFR-2 inhibition augments cigarette smoke-induced oxidative stress and inflammatory responses leading to endothelial dysfunction. FASEB J. 2008;22:2297–2310. doi: 10.1096/fj.07-099481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haq F, Mahoney M, Koropatnick J. Signaling events for metallothionein induction. Mutat. Res. 2003;533:211–226. doi: 10.1016/j.mrfmmm.2003.07.014. [DOI] [PubMed] [Google Scholar]
- 22.Gasull T, Giralt M, Hernandez J, Martinez P, Bremner I, Hidalgo J. Regulation of metallothionein concentrations in rat brain: effect of glucocorticoids, zinc, copper, and endotoxin. Am. J. Physiol. 1994;266:E760–E767. doi: 10.1152/ajpendo.1994.266.5.E760. [DOI] [PubMed] [Google Scholar]
- 23.Lee SS, Yang SF, Ho YC, Tsai CH, Chang YC. The upregulation of metallothionein-1 expression in areca quid chewing-associated oral squamous cell carcinomas. Oral Oncol. 2008;44:180–186. doi: 10.1016/j.oraloncology.2007.01.019. [DOI] [PubMed] [Google Scholar]
- 24.Kobayashi K, Kuroda J, Shibata N, Hasegawa T, Seko Y, Satoh M, Tohyama C, Takano H, Imura N, Sakabe K, Fujishiro H, Himeno S. Induction of metallothionein by manganese is completely dependent on interleukin-6 production. J. Pharmacol. Exp. Ther. 2007;320:721–727. doi: 10.1124/jpet.106.112912. [DOI] [PubMed] [Google Scholar]
- 25.Inoue K, Takano H, Shimada A, Wada E, Yanagisawa R, Sakurai M, Satoh M, Yoshikawa T. Role of metallothionein in coagulatory disturbance and systemic inflammation induced by lipopolysaccharide in mice. FASEB J. 2006;20:533–535. doi: 10.1096/fj.05-3864fje. [DOI] [PubMed] [Google Scholar]
- 26.Molinero A, Penkowa M, Hernandez J, Camats J, Giralt M, Lago N, Carrasco J, Campbell IL, Hidalgo J. Metallothionein-I overexpression decreases brain pathology in transgenic mice with astrocyte-targeted expression of interleukin-6. J. Neuropathol. Exp. Neurol. 2003;62:315–328. doi: 10.1093/jnen/62.3.315. [DOI] [PubMed] [Google Scholar]
- 27.Rega G, Kaun C, Demyanets S, Pfaffenberger S, Rychli K, Hohensinner PJ, Kastl SP, Speidl WS, Weiss TW, Breuss JM, Furnkranz A, Uhrin P, Zaujec J, Zilberfarb V, Frey M, Roehle R, Maurer G, Huber K, Wojta J. Vascular endothelial growth factor is induced by the inflammatory cytokines interleukin-6 and oncostatin M in human adipose tissue in vitro and in murine adipose tissue in vivo. Arterioscler. Thromb. Vasc. Biol. 2007;27:1587–1595. doi: 10.1161/ATVBAHA.107.143081. [DOI] [PubMed] [Google Scholar]
- 28.Fernandez-Salguero PM, Hilbert DM, Rudikoff S, Ward JM, Gonzalez FJ. Aryl-hydrocarbon receptor-deficient mice are resistant to 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced toxicity. Toxicol. Appl. Pharmacol. 1996;140:173–179. doi: 10.1006/taap.1996.0210. [DOI] [PubMed] [Google Scholar]
- 29.Shimizu Y, Nakatsuru Y, Ichinose M, Takahashi Y, Kume H, Mimura J, Fujii-Kuriyama Y, Ishikawa T. Benzo[a]pyrene carcinogenicity is lost in mice lacking the aryl hydrocarbon receptor. Proc. Natl. Acad. Sci. USA. 2000;97:779–782. doi: 10.1073/pnas.97.2.779. [DOI] [PMC free article] [PubMed] [Google Scholar]