Abstract
Purpose of review
The intent of this evidence-based review is to analyze the role of folate in chronic diseases, focusing on cancer and cardiovascular disease.
Recent findings
Low folate status has been shown to be a risk factor for cancer and cardiovascular disease. While epidemiological data suggest an inverse association between folate status and disease risk, intervention studies give equivocal results, suggesting the response to folate intake does not follow a linear continuum. Moreover, recent folate intervention trials raise concern about possible adverse effects of folate supplementation and suggest that too much folate in inopportune settings may be potentially harmful in individuals at higher risk for cardiovascular disease and cancer.
Summary
Although folate intake at sufficient levels appears to be an effective cancer chemopreventive strategy, high dose supplementation of folate has not been effective in reducing recurrence of cardiovascular events or colorectal adenomas in clinical intervention trials. Although controversial, high folate status achieved through folate fortification or supplementation may increase the risk of certain chronic diseases among certain individuals, possibly by interfering with the homeostasis of one-carbon metabolism. Further research is urgently needed to accurately define the relationship between supraphysiological intake of folate and chronic diseases.
Keywords: folate, folic acid fortification, colorectal cancer, cardiovascular disease
Introduction
Low folate status is regarded as a risk factor for cancer and cardiovascular disease. Observational studies report that high dietary or plasma folate levels may possess chemopreventive properties for cancer and cardiovascular disease. However, recent folate intervention trials suggest that folate supplementation may instead pose an increased risk for individuals at a higher risk of these chronic diseases.
Epidemiologic evidence for the role of folate in chronic diseases
Adequate folate intake is associated with a number of health benefits, such as the reduction of neural tube defects, lower risk of cardiovascular disease and prevention of the development of certain cancers [1].
To date, the overwhelming consensus of epidemiologic evidence indicates that high folate status, measured either by high dietary intake or blood concentrations, is associated with a reduced risk of colorectal cancer (CRC) [2-7]. Moreover, a growing literature suggests that folate might also convey protection against cancers of other tissues such as the breast, and cervix [8]. However, an interesting secondary analysis of two clinical trials that examined adenoma recurrence (that were not originally designed to examine the effect of folate) suggested that the protective effect of the vitamin does not increase indefinitely with increasing levels of folate intake; rather, the protective effect appeared to saturate beyond a certain level of folate intake [7]. More specifically, the incremental rise in the degree of protection that was conveyed by higher folate status was confined to those subjects who were not habitual users of multivitamins. In other words, those who were chronic consumers of multivitamins did not seem to benefit from further increases in folate intake above that which they were already receiving from their multivitamin.
Consistent with a saturable effect of protection are the observations of van Guelpen et al. [9], who observed a bell-shaped association between plasma folate and CRC risk in a Swedish cohort in which a decreased CRC risk was present among subjects with moderate folate status, with higher risk present at each of the extremes of intake. Similarly, another prospective study reported that higher folate plasma concentrations were associated with an increased risk of premenopausal breast cancer. The authors concluded that B vitamins may confer little or no reduction of breast cancer risk [10*]. Moreover, in a large prospective cohort study the risk of post-menopausal breast cancer was also increased among those who consumed the highest quintile of folate (> 850 micrograms per day) [11].
Epidemiological data also suggest that low folate levels pose a risk for cardiovascular disease. Biologically, this has been ascribed to the fact that inadequate intake of folate, vitamin B-6, vitamin B-12, or a combination of B vitamins increases homocysteine levels, an independent biomarker for cardiovascular disease and stroke [12]. Additionally, observational studies have shown that individuals with high plasma concentrations or dietary intake of folate and vitamin B-6 have a decreased risk of coronary heart disease [13, 14].
Due to the protection conveyed against neural tube defects, it is recommended that women of reproductive age consume 400 mcg of folate daily, but this level of intake is generally not achieved. Therefore, in 1998, nationwide fortification of flour and other uncooked cereal grain products with folic acid, an oxidized form of folate, was introduced in the United States and Canada. This measure increased plasma concentrations of folate by 100%, decreased plasma homocysteine and resulted in a 20-50% reduction in neural tube defects [15-17]. However, population-based observations on data sets from the United States and Canada have shown a close chronological relationship between a reversal of the downward trend of the CRC incidence and the marked increase in systemic folate status that accompanied the introduction of mandatory folic acid fortification [18**]. These observations do not prove a causal link between folic acid fortification and increasing rates of CRC, but do raise concerns. It is estimated that 35-50 % of Americans over the age of 50 harbor colorectal adenomas [19], and there is a possibility that excessive intake of folic acid could have promoted the growth of preneoplastic lesions, or small cancers that had not yet been detected [20, 21].
Effects of folic acid supplementation in human trials
Folate intervention trials have generally shown favorable effects on biochemical and molecular biomarkers for colorectal carcinogenesis; whereas data from a trial utilizing adenoma recurrence as the primary endpoint did not demonstrate salutary effects (see Table 1). This is of particular concern since the colonic adenoma is the only validated biomarker of CRC risk.
Table 1.
Overview about human folic acid intervention trials
| Intervention | Participants | Duration | Outcome | Reference |
|---|---|---|---|---|
| cardiovascular endpoints | ||||
| 0.8 mg FA, 0.4 mg vitamin B12, and 40 mg vitamin B6; 0.8 mg FA and 0.4 mg vitamin B12; 40 mg of vitamin B6; or placebo | 3749 men and women who have had acute myocardial infarction | 3.3 years | Homocysteine level was lowered by 27% among patients given FA plus vitamin B12; Treatment with B vitamins did not lower the risk of recurrent cardiovascular disease, there was a trend toward an increased risk | Bonaa et al. 2006 [22] |
| 2.5 mg FA, 50 mg vitamin B6, and 1 mg vitamin B12 per day or placebo | 5522 patients who have had vascular disease or diabetes | 5 years | Plasma homocysteine levels decreased; active treatment did not significantly decrease the risk of major cardiovascular events in patients with vascular disease | Lonn et al. 2006 [23] |
| 2.5 mg FA, 50 mg vitamin B6, and 1 mg vitamin B12 per day or placebo | 5442 women with a history of CVD or 3 or more coronary risk factors | 7.3 years | Plasma homocysteine level was decreased by 18.5%; total cardiovascular events among high-risk women were not lowered | Albert et al. 2008 [24] |
| cancer endpoints | ||||
| 1 mg/d of FA or placebo | 1021 men and women with a recent history of colorectal adenomas | 3 years + 3 or 5 years | During the first 3 years FA at 1 mg/d does not reduce colorectal adenoma risk; after the 2nd follow-up FA was associated with higher risks of having 3 or more adenomas and of noncolorectal cancers | Cole et al. 2007 [25] |
| 0.5 mg FA/day | 945 patients with removed colorectal adenomas | 3 years | Folate supplementation had no effect on colorectal adenoma recurrence | Logan et al. 2008 [26] |
FA=folic acid; CVD=cardiovascular disease
Recently, in the Aspirin/Folate Polyp Prevention Study, adverse effects of folic acid supplementation were reported. In this double-blind, placebo-controlled randomized trial on the use of folic acid as a chemopreventive agent for the recurrence of colorectal polyps subjects were divided into different groups receiving folic acid with or without aspirin [25**]. At a second follow-up, approximately 5 years after the initiation of the trial, more advanced lesions and multiple adenomas were observed in individuals supplemented with 1 mg/d of folic acid [25**]. The study also found a higher rate of invasive prostate cancer among participants in the folic acid group [25**]. These results suggest that people with prior adenomas are at a greater risk of developing multiple, or advanced polyps, as a result of folic acid supplementation.
A second trial investigating colorectal adenoma recurrence also reported no benefit of folic acid supplementation (0.5 mg/d) on preventing the recurrence of adenoma or advanced lesions [26*], although the abovementioned adverse effects were not observed.
Three recent randomized trials have examined the impact of folate in combination with other B vitamins on cardiovascular disease. Although each has reported a marked reduction in homocysteine levels, no beneficial effects on the prevention of the recurrence of cardiovascular endpoints in populations at high risk for cardiovascular disease by folic acid supplementation have been observed [22-24*]. One of these studies even reported harmful results [22] and another reported a marginal increase in the occurrence of colon and prostate cancer in the intervention group [23]. The most recent randomized trial also reported that after 7.3 years of treatment and follow-up, total cardiovascular events among high-risk women were not reduced by vitamin supplementation [24*]. A meta-analysis came to similar conclusions: no reduction in the risk of cardiovascular disease or all-cause mortality among participants with a history of vascular disease were observed with folic acid supplementation [27]. However, another meta-analysis of eight randomized trials of folic acid supplementation [28**] concluded that there was a significantly reduced risk in one category of cardiovascular disease—stroke--among individuals with no prior history of stroke. Thus, folic acid is feasibly effective in the primary prevention of stroke although evidence for its efficacy in secondary prevention is lacking. The potential importance of dose is underscored by a study in non-folate-fortified patients with coronary artery disease: low-dose folic acid supplementation (400 mcg/d) improved a variety of biochemical and physiological indicators of vascular function, whereas high-dose folic acid supplementation (5 mg/d) provided no additional benefit [29*].
In summary, in contrast to conclusions drawn from observational studies, high doses of folic acid in clinical intervention trials have generally not been effective in preventing neoplasms or cardiovascular disease, with the possible exception of stroke.
Dietary folate versus folic acid from supplements
Folic acid intake derived from fortified foods was significantly higher than originally anticipated in the early era of fortification probably due to the practice of overage [30] of the mandated amount of folic acid. In combination with the use of multivitamin supplements, certain segments of the population probably far exceeded a folic acid intake of 1 mg/d, which is the tolerable upper intake level defined by the Dietary Reference Intakes [31].
Naturally occurring folates differ from oxidized folic acid which is used in supplements and fortification. Natural folates are unstable and can readily lose their activity in foods depending on the food source and preparation method. Folic acid (pteroylmonoglutamic acid) is inexpensive, more stable and has greater bioavailability than the naturally occurring polyglutamate folates which need to be hydrolyzed to the monoglutamate form at the intestinal wall before absorption [32].
The intake of folic acid from fortified foods and concurrent supplement use can result in very high chronic intake of folic acid, such as serum folate levels of 40 nmol/L [33] and detectable levels of unmetabolized folic acid in the plasma [34*]. It has been shown that the oral intake of folic acid at physiological levels of 400 mcg saturates the enzymatic reduction to 5-methyl THF in cells of the intestinal mucosa [34*].
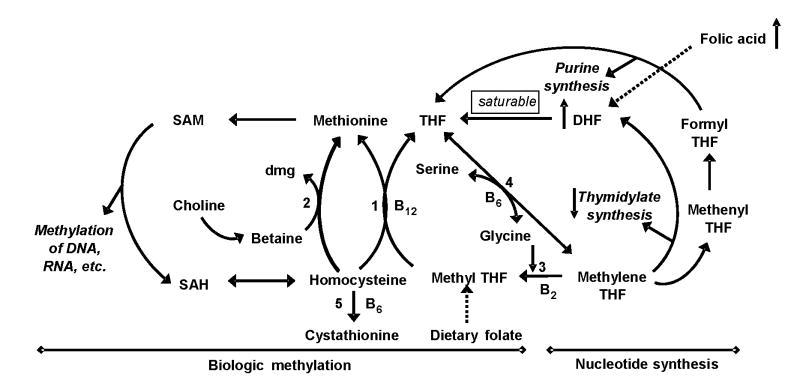
Although unproven, high intake of folic acid feasibly could exert antagonistic effects due to an accumulation of dihydrofolate (DHF, the co-enzymatic form of folate through which folic acid enters one-carbon metabolism) since it inhibits thymidylate synthase and hence the synthesis of thymidylate. DHF also inhibits methylenetetrahydrofolate reductase (MTHFR) by which high concentrations of folic acid could inhibit the formation of 5-methyl tetrahydrofolate (5-methyl THF) (see Figure 1).
Figure 1.
A potential mechanism for the inhibitory effect of high folic acid intake on one-carbon metabolism that controls biological methylation and nucleotide synthesis
High intake of folic acid can induce an accumulation of dihydrofolate (DHF) because a high oral dose of folic acid bypasses the normal folate absorption mechanism, an enzymatic reduction to 5-methyl THF in cells of the intestinal mucosa, and is directly absorbed as a folic acid which can convert to DHF in tissues. Increased DHF in one-carbon metabolism can inhibit the activities of both thymidylate synthase and methylenetetrahydrofolate reductase.
(DHF = Dihydrofolate; dmg = dimethylglycine; SAH = S-adenosylhomocysteine; SAM = S-adenosylmethionine; THF = tetrahydrofolate; 1 = methionine synthase; 2 = betaine:homocysteine methyltransferase; 3 = methylenetetrahydrofolate reductase; 4 = serine hydroxymethyltransferase; 5 = cystathionine β-synthase)
Although folate is generally regarded as safe, with the exception of those with compromised B12 status, comprehensive data about possible antagonistic effects of folic acid on metabolism are lacking. A summary of recent mechanistic studies on folic acid is shown in Table 2.
Table 2.
Recent studies on the impact of folic acid supplementation on metabolic and epigenetic endpoints
| Folic acid level | Study model | Endpoint | Outcome | Reference |
|---|---|---|---|---|
| metabolic studies | ||||
| Diet low in folate (<233 μg/d), folate-rich diet plus supplements >400 μg/d | 105 healthy, postmenopausal women | Immune function, natural killer cell (NK) cytotoxicity | A folate-rich diet (>400 μg/d) including FA supplements was associated with reduced NK cytotoxicity | Troen et al. 2006 [35] |
| 40 mg FA/kg diet | Weanling (3-week-old) male rats | Effect of FA on methionine cycle | FA did not alter SAM/SAH ratio, DNA methylation, enzymatic activities or concentrations of vitamins in methionine cycle | Achón et al. 2007 [36] |
| Oversupplemented (100 μmol FA/L) or sufficient FA (0.25 and 9 μmol FA/L) | Human-derived intestinal Caco-2, renal HK-2 epithelial cells | Intestinal and renal folate uptake processes | Long-term oversupplementation with FA down-regulates intestinal and renal folate uptake | Ashokkumar et al. 2007 [37] |
| Enriched pellet diet containing 200 mg FA/kg diet | Sprague–Dawley rats | Xenobiotic metabolising enzymes (XME) in liver | FA supplementation impaired rodent hepatic metabolism; FA inactivated Phase-I XME | Canistro et al. 2008 [38] |
| Chronic supplemental (100 ng/ml), normal (10 ng/ml) level of FA | HT29 human colon cancer cells | Cell turnover, differentiation | At 100 ng/ml, cell showed higher proliferation and apoptosis, decreased differentiation | Pellis et al. 2008 [39] |
| epigenetic endpoints | ||||
| Folate intake in the upper (>212 μg/d) or lower (<183 μg/d) tertile | Adenoma tissue of patients with colorectal adenoma (n=149) and controls (n=286) from a case-control study | Promoter methylation of tumor suppressor and DNA repair genes | Folate was inversely associated with promoter methylation and positively associated with the occurrence of adenomas without promoter methylation | van den Donk et al. 2007a [40] |
| Daily supplementation with 5 mg folic acid and 1.25 mg vitamin B-12 for 6 months | Rectal biopsies of 86 subjects with a history of colorectal adenoma | Uracil misincorporation, promoter methylation | Supplementation with high doses of FA and vitamin B-12 did not favorably influence uracil incorporation and promoter methylation | van den Donk et al. 2007b [41] |
| 0, 4.5, or 18 μmol folate/kg diet | Colon tissue of old and young male C57BL/6 mice | Genomic DNA methylation, p16 promoter methylation | Folate supplementation increased genomic DNA methylation and p16 promoter methylation in old mice | Keyes et al. 2007 [42] |
| Dietary exposure before and after mandatory folic acid fortification | 101 tissue specimens of the pre- and 96 of the postfortification period of cervical intraepithelial neoplasia | DNA methylase activity | Dnmt-1 expression was higher in all lesion types in the postfortification | Piyathilake et al. 2008 [43] |
FA=folic acid; SAH = S-adenosylhomocysteine; SAM = S-adenosylmethionine; XME=xenobiotic metabolizing enzymes
The observations of Troen et al. are consistent with the concerns about excess intake of the pharmaceutical form of folate, folic acid, although the study was cross-sectional in design and therefore could not provide definitive evidence of causality. These investigators reported detectable levels of folic acid in the blood of 78% of a study population of free-living women, of whom 54% were daily users of a folate supplement. Among the elder participants, the authors observed that increasing concentrations of plasma folic acid, but not total folate, were inversely associated with the cytotoxicity of circulating natural killer cells in those individuals [35]. This population of lymphocytes plays a role in cell-mediated immunosurveillance and is thought to be instrumental in the destruction of arising clones of neoplastic cells.
Recent preclinical studies have pointed out some previously unexplored effects of chronic exposure to supraphysiological doses of folic acid. A recent study by Canistro et al. [38*] reported that folic acid supplementation has a major impact on the activity of xenobiotic metabolizing enzymes in the liver of rats, underscoring that the spectrum of toxicological characteristics of supraphysiological doses of folic acid is not fully understood yet. An in vitro study pointed out that long-term supplementation with high doses of folic acid might also affect intestinal and renal folate uptake processes by down-regulating folate carriers [37*].
Pellis et al. (2008) observed in a colon cancer cell line that exposure to supplemental levels of folic acid decreased markers of cell differentiation and increased cell turnover, both of which would be consistent with a promoting effect of high doses of folate on neoplastic cells [39*]. An in vivo study examined the effects of supranormal folic acid supplementation on methionine metabolism and DNA methylation and reported that supplementation for 4 weeks with a supraphysiological dose of 40 mg/kg diet did not cause biochemical evidence of hepatic or renal damage in male rats [36]. The authors concluded that under the studied conditions, extreme high folic acid intake did not have adverse effects on one-carbon metabolism in rodents, although the spectrum of endpoints here was very limited, providing only a narrow degree of reassurance [36*].
Some human studies have also raised concern regarding the overly abundant intake of supplemental folic acid. Although two large prospective cohort studies found that total folate intake was not associated with pancreatic cancer risk, a comprehensive meta-analysis demonstrated a protective effect of dietary folates against this cancer [44]. In contrast, multivitamin supplement users in the two prospective cohorts had a non-significant higher risk of pancreatic cancer [45]. Moreover, the results of a large prospective study on postmenopausal women show a positive association between total folate and supplemental folic acid intake and breast cancer, but no association between dietary folate intake and cancer risk [11]. In this study, there was a 19% higher risk of developing breast cancer in the subjects using folic acid supplements. This result contrasts with other studies that have generally demonstrated a protection against postmenopausal breast cancer with generous intake of folate [46]. The apparent discrepancy is possibly explained by the fact that the high-risk group in the Stolzenberg-Solomon study [11] was ingesting more than twice the level of total folate than the ‘high folate intake’ group in other studies.
The role of folate in carcinogenesis and epigenetic implications
Folate as a critical cofactor in one-carbon metabolism has an important function in biologic methylation and de novo nucleotide synthesis pathways [47, 48]. Adequate folate intake appears to help sustain normal patterns of DNA methylation and minimize DNA damage [48]. In contrast, inadequate folate availability impairs DNA synthesis in rapidly dividing tissues such as the epithelium of the gastrointestinal tract, and leads to misincorporation of uracil into DNA due to inadequate thymidylate production [49, 50] producing genomic instability. Therefore, adequate folate status is crucial for accurate DNA synthesis and cell division.
Methylation of DNA and histones are epigenetic modifications which are important determinants of gene transcription and silencing [51]. Folate plays an essential role in the synthesis of the methyl donor S-adenosylmethionine (SAM). The methyl groups donated by SAM are used to methylate specific cytosine residues in DNA or arginine and lysine residues of histones.
Epigenetic modifications are also perceived to be crucial for other disease-related processes. As reviewed in [52], aberrant methylation may also contribute to increased atherosclerosis among older individuals by modulating the expression of a variety of genes controlling proliferation and migratory capabilities: the change of quiescent, contractile smooth muscle cells to proliferative and migratory cells is a key process in atherosclerosis.
Methylation of DNA has been shown to be modulated by folate. In rodents, low folate status can diminish genomic DNA methylation in certain tissues [53] whereas folate supplementation may increase genomic DNA methylation [54]. Moreover, folate availability alters promoter methylation of the tumor suppressor gene p16 in the colon of aged mice [42*].
Several case-control and intervention trials have examined the potential effects of folate status on genomic and gene-specific methylation in the human colon (Table 2). An intervention study with high doses of folic acid and vitamin B-12 in subjects with previous colorectal adenomas observed that uracil misincorporation and promoter methylation were not favorably influenced in rectal mucosa DNA [41*]. Contrarily, folate intake was inversely associated with promoter methylation of tumor suppressor and DNA repair genes in adenomas and normal tissue specimens from a case-control study [40]. Piyathilake et al. (2008) has shown in a study of tissue specimens before and after the introduction of folic acid fortification that Dnmt-1 expression seemed to be influenced by folate status [43*]. Insufficient observations have accrued to date to conclude whether excessive folic acid may change promoter-specific methylation patterns in DNA or in histones in humans.
Future directions of attention
Further research is needed to determine the long-term effects of chronic exposure to very high levels of folate, and in particular, the effects of unmetabolized folic acid on intracellular folate metabolism. Future studies need to explore whether a causal relationship exists between supraphysiological levels of folate intake and colorectal carcinogenesis, the development of cancers at other sites (e.g. prostate, pancreas, and breast) and whether there might be effects that are particular to the pharmaceutical form of folate, folic acid. In parallel, we need to understand the impact of the overly abundant delivery of folate on occlusive vascular disease. By defining how folate modulates a spectrum of genetic and epigenetic factors, we may better determine the role of folate in the prevention or promotion of chronic diseases.
Considerable evidence indicates that genetic polymorphisms of enzymes involved in one-carbon metabolism are an important co-determinant in these matters and they will undoubtedly need to be incorporated into the complex picture of folate in the prevention of disease [55, 56].
Conclusions
There is growing concern that detrimental effects may be produced when folate is administered in inappropriate doses, particularly to individuals who may be particularly susceptible to excessive doses of the vitamin. Therefore a thorough understanding of both the mechanistic and clinical phenomena is needed if we are to continue to safely implement mandatory fortification programs for the reduction of NTDs and if we are to continue to explore whether there are any circumstances under which supplemental folate intake can be used for the prevention of cardiovascular disease or cancer. As we have learned with other nutrients, the physiological and health effects exerted by folate do not necessarily operate on a linear continuum and do not necessarily impact on all individuals in the population in an equal manner.
Acknowledgments
This material is based upon work supported by the U.S. Department of Agriculture, under agreement No. 581950-9-001. Any opinions, findings, conclusion, or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the view of the U.S. Dept of Agriculture. Authors do not have any competing interest. This project has been supported in part by the National Institute of Health Grants R21 AA16681 and R01 AG25834 (SWC) and the National Institute of Health Grants U54 CA10097 and K05 CA100048 (JBM).
References
- 1.Ulrich CM, Potter JD. Folate supplementation: too much of a good thing? Cancer Epidemiol Biomarkers Prev. 2006 Feb;15(2):189–93. doi: 10.1158/1055-9965.EPI-152CO. [DOI] [PubMed] [Google Scholar]
- 2.Kim YI. Folate and DNA methylation: a mechanistic link between folate deficiency and colorectal cancer? Cancer Epidemiol Biomarkers Prev. 2004 Apr;13(4):511–9. [PubMed] [Google Scholar]
- 3.Giovannucci E. Epidemiologic studies of folate and colorectal neoplasia: a review. J Nutr. 2002 Aug;132(8 Suppl):2350S–5S. doi: 10.1093/jn/132.8.2350S. [DOI] [PubMed] [Google Scholar]
- 4.Ma J, Stampfer MJ, Giovannucci E, et al. Methylenetetrahydrofolate Reductase Polymorphism, Dietary Interactions, and Risk of Colorectal Cancer. Cancer Res. 1997 March 15;57(6):1098–102. [PubMed] [Google Scholar]
- 5.Su LJ, Arab L. Nutritional status of folate and colon cancer risk: evidence from NHANES I epidemiologic follow-up study. Ann Epidemiol. 2001 Jan;11(1):65–72. doi: 10.1016/s1047-2797(00)00188-5. [DOI] [PubMed] [Google Scholar]
- 6.Sanjoaquin MA, Allen N, Couto E, et al. Folate intake and colorectal cancer risk: a meta-analytical approach. Int J Cancer. 2005 Feb 20;113(5):825–8. doi: 10.1002/ijc.20648. [DOI] [PubMed] [Google Scholar]
- 7.Martinez ME, Giovannucci E, Jiang R, et al. Folate fortification, plasma folate, homocysteine and colorectal adenoma recurrence. Int J Cancer. 2006 Sep 15;119(6):1440–6. doi: 10.1002/ijc.21978. [DOI] [PubMed] [Google Scholar]
- 8.McCullough ML, Giovannucci EL. Diet and cancer prevention. Oncogene. 2004 Aug 23;23(38):6349–64. doi: 10.1038/sj.onc.1207716. [DOI] [PubMed] [Google Scholar]
- 9.Van Guelpen B, Hultdin J, Johansson I, et al. Low folate levels may protect against colorectal cancer. Gut. 2006 Oct;55(10):1461–6. doi: 10.1136/gut.2005.085480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *10.Lin J, Lee IM, Cook NR, et al. Plasma folate, vitamin B-6, vitamin B-12, and risk of breast cancer in women. Am J Clin Nutr. 2008 Mar;87(3):734–43. doi: 10.1093/ajcn/87.3.734. [DOI] [PubMed] [Google Scholar]; This study reports that plasma concentrations of folate were not associated with overall breast cancer risk and confer little or no reduction in overall risk of developing breast cancer.
- 11.Stolzenberg-Solomon RZ, Chang SC, Leitzmann MF, et al. Folate intake, alcohol use, and postmenopausal breast cancer risk in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. Am J Clin Nutr. 2006 Apr;83(4):895–904. doi: 10.1093/ajcn/83.4.895. [DOI] [PubMed] [Google Scholar]
- 12.Homocysteine Lowering Trialists C. Dose-dependent effects of folic acid on blood concentrations of homocysteine: a meta-analysis of the randomized trials. Am J Clin Nutr. 2005 October 1;82(4):806–12. doi: 10.1093/ajcn/82.4.806. [DOI] [PubMed] [Google Scholar]
- 13.Folsom AR, Nieto FJ, McGovern PG, et al. Prospective Study of Coronary Heart Disease Incidence in Relation to Fasting Total Homocysteine, Related Genetic Polymorphisms, and B Vitamins : The Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 1998 July 21;98(3):204–10. doi: 10.1161/01.cir.98.3.204. [DOI] [PubMed] [Google Scholar]
- 14.Robinson K, Arheart K, Refsum H, et al. Low Circulating Folate and Vitamin B6 Concentrations : Risk Factors for Stroke, Peripheral Vascular Disease, and Coronary Artery Disease. Circulation. 1998 February 10;97(5):437–43. doi: 10.1161/01.cir.97.5.437. [DOI] [PubMed] [Google Scholar]
- 15.Honein MA, Paulozzi LJ, Mathews TJ, et al. Impact of folic acid fortification of the US food supply on the occurrence of neural tube defects. JAMA. 2001 Jun 20;285(23):2981–6. doi: 10.1001/jama.285.23.2981. [DOI] [PubMed] [Google Scholar]
- 16.Jacques PF, Selhub J, Bostom AG, et al. The effect of folic acid fortification on plasma folate and total homocysteine concentrations. N Engl J Med. 1999 May 13;340(19):1449–54. doi: 10.1056/NEJM199905133401901. [DOI] [PubMed] [Google Scholar]
- 17.Ray JG. Folic acid food fortification in Canada. Nutr Rev. 2004 Jun;62(6 Pt 2):S35–9. doi: 10.1111/j.1753-4887.2004.tb00072.x. [DOI] [PubMed] [Google Scholar]
- **18.Mason JB, Dickstein A, Jacques PF, et al. A temporal association between folic acid fortification and an increase in colorectal cancer rates may be illuminating important biological principles: a hypothesis. Cancer Epidemiol Biomarkers Prev. 2007 Jul;16(7):1325–9. doi: 10.1158/1055-9965.EPI-07-0329. [DOI] [PubMed] [Google Scholar]; The authors present the hypothesis that the institution of folic acid fortification may have been responsible for the observed increase in CRC rates in the mid-1990s. Although these observations do not prove causality the authors encourage deliberating about the institution of fortification programs.
- 19.Lieberman DA, Smith FW. Screening for colon malignancy with colonoscopy. Am J Gastroenterol. 1991 Aug;86(8):946–51. [PubMed] [Google Scholar]
- 20.Song J, Medline A, Mason JB, et al. Effects of dietary folate on intestinal tumorigenesis in the apcMin mouse. Cancer Res. 2000 Oct 1;60(19):5434–40. [PubMed] [Google Scholar]
- 21.Song J, Sohn KJ, Medline A, et al. Chemopreventive effects of dietary folate on intestinal polyps in Apc+/-Msh2-/- mice. Cancer Res. 2000 Jun 15;60(12):3191–9. [PubMed] [Google Scholar]
- 22.Bonaa KH, Njolstad I, Ueland PM, et al. Homocysteine lowering and cardiovascular events after acute myocardial infarction. N Engl J Med. 2006 Apr 13;354(15):1578–88. doi: 10.1056/NEJMoa055227. [DOI] [PubMed] [Google Scholar]
- 23.Lonn E, Yusuf S, Arnold MJ, et al. Homocysteine lowering with folic acid and B vitamins in vascular disease. N Engl J Med. 2006 Apr 13;354(15):1567–77. doi: 10.1056/NEJMoa060900. [DOI] [PubMed] [Google Scholar]
- *24.Albert CM, Cook NR, Gaziano JM, et al. Effect of folic acid and B vitamins on risk of cardiovascular events and total mortality among women at high risk for cardiovascular disease: a randomized trial. JAMA. 2008 May 7;299(17):2027–36. doi: 10.1001/jama.299.17.2027. [DOI] [PMC free article] [PubMed] [Google Scholar]; In this randomized trial it was tested if a combination of folic acid and other B vitamins lowers the risk of CVD among high-risk women. The supplementation did not reduce total cardiovascular events among high-risk women.
- **25.Cole BF, Baron JA, Sandler RS, et al. Folic acid for the prevention of colorectal adenomas: a randomized clinical trial. Jama. 2007 Jun 6;297(21):2351–9. doi: 10.1001/jama.297.21.2351. [DOI] [PubMed] [Google Scholar]; This is the first double-blind, placebo-controlled, randomized clinical trial showing that folic acid was associated with higher risks of having 3 or more adenomas and of prostate cancer. The authors conclude that folic acid at 1 mg/d does not reduce colorectal adenoma risk.
- *26.Logan RF, Grainge MJ, Shepherd VC, et al. Aspirin and folic acid for the prevention of recurrent colorectal adenomas. Gastroenterology. 2008 Jan;134(1):29–38. doi: 10.1053/j.gastro.2007.10.014. [DOI] [PubMed] [Google Scholar]; The results of this multicenter, randomized, double-blind trial of folic acid supplements (0.5 mg/day) to prevent colorectal adenoma recurrence show that folate supplementation had no effect on adenoma recurrence.
- 27.Bazzano LA, Reynolds K, Holder KN, et al. Effect of folic acid supplementation on risk of cardiovascular diseases: a meta-analysis of randomized controlled trials. JAMA. 2006 Dec 13;296(22):2720–6. doi: 10.1001/jama.296.22.2720. [DOI] [PubMed] [Google Scholar]
- **28.Wang X, Qin X, Demirtas H, et al. Efficacy of folic acid supplementation in stroke prevention: a meta-analysis. Lancet. 2007 Jun 2;369(9576):1876–82. doi: 10.1016/S0140-6736(07)60854-X. [DOI] [PubMed] [Google Scholar]; This meta-analysis assessed the efficacy of folic acid supplementation in the prevention of stroke in relevant randomized trials. This analysis revealed that folic acid supplementation can reduce the risk of stroke in primary prevention.
- *29.Shirodaria C, Antoniades C, Lee J, et al. Global improvement of vascular function and redox state with low-dose folic acid: implications for folate therapy in patients with coronary artery disease. Circulation. 2007 May 1;115(17):2262–70. doi: 10.1161/CIRCULATIONAHA.106.679084. [DOI] [PubMed] [Google Scholar]; This study presents that a low-dose folic acid intervention improves vascular function whereas a high-dose folic acid treatment provides no additional benefit.
- 30.Rader JI, Weaver CM, Angyal G. Total folate in enriched cereal-grain products in the United States following fortification. Food Chemistry. 2000;70(3):275–89. [Google Scholar]
- 31.Institute of Medicine (U.S.) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes IoMUSPoF, Other B Vitamins, and Choline. Institute of Medicine (U.S.), Subcommittee on Upper Reference Levels of Nutrients. Dietary reference intakes for thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12, pantothenic acid, biotin, and choline. Washington, D.C.: National Academy Press; 1998. [PubMed] [Google Scholar]
- 32.Shaw S, Jayatilleke E, Herbert V, et al. Cleavage of folates during ethanol metabolism. Role of acetaldehyde/xanthine oxidase-generated superoxide. Biochem J. 1989 Jan 1;257(1):277–80. doi: 10.1042/bj2570277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pfeiffer CM, Caudill SP, Gunter EW, et al. Biochemical indicators of B vitamin status in the US population after folic acid fortification: results from the National Health and Nutrition Examination Survey 1999-2000. Am J Clin Nutr. 2005 Aug;82(2):442–50. doi: 10.1093/ajcn.82.2.442. [DOI] [PubMed] [Google Scholar]
- *34.Sweeney MR, McPartlin J, Scott J. Folic acid fortification and public health: report on threshold doses above which unmetabolised folic acid appear in serum. BMC Public Health. 2007;7(147):41. doi: 10.1186/1471-2458-7-41. [DOI] [PMC free article] [PubMed] [Google Scholar]; A supplementation of 400 microg folic acid/d leads to detectable unmetabolized folic acid in serum.
- 35.Troen AM, Mitchell B, Sorensen B, et al. Unmetabolized folic acid in plasma is associated with reduced natural killer cell cytotoxicity among postmenopausal women. J Nutr. 2006 Jan;136(1):189–94. doi: 10.1093/jn/136.1.189. [DOI] [PubMed] [Google Scholar]
- *36.Achon M, Alonso-Aperte E, Ubeda N, et al. Supranormal dietary folic acid supplementation: effects on methionine metabolism in weanling rats. Br J Nutr. 2007 Sep;98(3):490–6. doi: 10.1017/S0007114507721499. [DOI] [PubMed] [Google Scholar]; This rat study presents that a 4 week supplementation of folic acid did not exert adverse effects on one-carbon metabolism under the studied conditions.
- *37.Ashokkumar B, Mohammed ZM, Vaziri ND, et al. Effect of folate oversupplementation on folate uptake by human intestinal and renal epithelial cells. Am J Clin Nutr. 2007 Jul;86(1):159–66. doi: 10.1093/ajcn/86.1.159. [DOI] [PubMed] [Google Scholar]; This in vitro study examined the effects of over supplementation of folic acid on intestinal and renal folate uptake processes and showed that these processes are down-regulated.
- *38.Canistro D, Pozzetti L, Sapone A, et al. Perturbation of rat hepatic metabolising enzymes by folic acid supplementation. Mutat Res. 2008 Jan 1;637(1-2):16–22. doi: 10.1016/j.mrfmmm.2007.06.007. [DOI] [PubMed] [Google Scholar]; This rat study analyzed the potential of folate to modulate xenobiotic metabolizing enzymes. These results point at the requirement to clarify the toxicological implications of folic acid for humans.
- *39.Pellis L, Dommels Y, Venema D, et al. High folic acid increases cell turnover and lowers differentiation and iron content in human HT29 colon cancer cells. Br J Nutr. 2008 Apr;99(4):703–8. doi: 10.1017/S0007114507824147. [DOI] [PubMed] [Google Scholar]; The results of this study using human colon cancer cells with high folic acid supplementation corroborate the promoting effect of folic acid supplementation on transformed cells.
- 40.van den Donk M, van Engeland M, Pellis L, et al. Dietary folate intake in combination with MTHFR C677T genotype and promoter methylation of tumor suppressor and DNA repair genes in sporadic colorectal adenomas. Cancer Epidemiol Biomarkers Prev. 2007 Feb;16(2):327–33. doi: 10.1158/1055-9965.EPI-06-0810. [DOI] [PubMed] [Google Scholar]
- *41.van den Donk M, Pellis L, Crott JW, et al. Folic acid and vitamin B-12 supplementation does not favorably influence uracil incorporation and promoter methylation in rectal mucosa DNA of subjects with previous colorectal adenomas. J Nutr. 2007 Sep;137(9):2114–20. doi: 10.1093/jn/137.9.2114. [DOI] [PubMed] [Google Scholar]; In this study a supplementation of high doses of folic acid and vitamin B-12 did not favorably influence biomarkers of normal DNA synthesis and methylation pattern.
- *42.Keyes MK, Jang H, Mason JB, et al. Older age and dietary folate are determinants of genomic and p16-specific DNA methylation in mouse colon. J Nutr. 2007 Jul;137(7):1713–7. doi: 10.1093/jn/137.7.1713. [DOI] [PubMed] [Google Scholar]; In this mice study it was shown that the epigenetic determinants, genomic DNA methylation and promoter methylation which are associated with carcinogenesis, are dependent on the level of dietary folate.
- *43.Piyathilake CJ, Celedonio JE, Macaluso M, et al. Mandatory fortification with folic acid in the United States is associated with increased expression of DNA methyltransferase-1 in the cervix. Nutrition. 2008 Jan;24(1):94–9. doi: 10.1016/j.nut.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study analyzed archived cervical neoplasia specimens before (1990-1992) and after (2000-2002) mandatory folic acid fortification and showed an increased expression of DNA methyltransferase-1, an enzyme which controls normal DNA methylation.
- 44.Larsson SC, Giovannucci E, Wolk A. Folate intake, MTHFR polymorphisms, and risk of esophageal, gastric, and pancreatic cancer: a meta-analysis. Gastroenterology. 2006 Oct;131(4):1271–83. doi: 10.1053/j.gastro.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 45.Skinner HG, Michaud DS, Giovannucci EL, et al. A prospective study of folate intake and the risk of pancreatic cancer in men and women. Am J Epidemiol. 2004 Aug 1;160(3):248–58. doi: 10.1093/aje/kwh214. [DOI] [PubMed] [Google Scholar]
- 46.Ericson U, Sonestedt E, Gullberg B, et al. High folate intake is associated with lower breast cancer incidence in postmenopausal women in the Malmo Diet and Cancer cohort. Am J Clin Nutr. 2007 Aug;86(2):434–43. doi: 10.1093/ajcn/86.2.434. [DOI] [PubMed] [Google Scholar]
- 47.Choi SW, Mason JB. Folate and carcinogenesis: an integrated scheme. J Nutr. 2000;130(2):129–32. doi: 10.1093/jn/130.2.129. [DOI] [PubMed] [Google Scholar]
- 48.Choi SW, Mason JB. Folate status: effects on pathways of colorectal carcinogenesis. J Nutr. 2002;132(8 Suppl):2413S–8S. doi: 10.1093/jn/132.8.2413S. [DOI] [PubMed] [Google Scholar]
- 49.Duthie SJ, Narayanan S, Blum S, et al. Folate deficiency in vitro induces uracil misincorporation and DNA hypomethylation and inhibits DNA excision repair in immortalized normal human colon epithelial cells. Nutr Cancer. 2000;37(2):245–51. doi: 10.1207/S15327914NC372_18. [DOI] [PubMed] [Google Scholar]
- 50.Duthie SJ, Grant G, Narayanan S. Increased uracil misincorporation in lymphocytes from folate-deficient rats. Br J Cancer. 2000 Dec;83(11):1532–7. doi: 10.1054/bjoc.2000.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fuks F. DNA methylation and histone modifications: teaming up to silence genes. Curr Opin Genet Dev. 2005 Oct;15(5):490–5. doi: 10.1016/j.gde.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 52.Dong C, Yoon W, Goldschmidt-Clermont PJ. DNA methylation and atherosclerosis. J Nutr. 2002 Aug;132(8 Suppl):2406S–9S. doi: 10.1093/jn/132.8.2406S. [DOI] [PubMed] [Google Scholar]
- 53.Friso S, Choi SW. Gene-nutrient interactions and DNA methylation. J Nutr. 2002;132(8 Suppl):2382S–7S. doi: 10.1093/jn/132.8.2382S. [DOI] [PubMed] [Google Scholar]
- 54.Choi SW, Friso S, Keyes MK, Mason JB. Folate supplementation increases genomic DNA methylation in the liver of elder rats. Br J Nutr. 2005 Jan;93(1):31–5. doi: 10.1079/bjn20041283. [DOI] [PubMed] [Google Scholar]
- 55.Kono S, Chen K. Genetic polymorphisms of methylenetetrahydrofolate reductase and colorectal cancer and adenoma. Cancer Sci. 2005 Sep;96(9):535–42. doi: 10.1111/j.1349-7006.2005.00090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Friso S, Choi SW. Gene-nutrient interactions in one-carbon metabolism. Curr Drug Metab. 2005 Feb;6(1):37–46. doi: 10.2174/1389200052997339. [DOI] [PubMed] [Google Scholar]